Abstract
Objective:
Molecular based predictive biomarkers have been developed but still unaffordable in developing countries. The leukocyte ratio is known as a promising, affordable and practical biomarker. However, the evidence to support their application is still lacking, especially from developing countries. Therefore, this study aimed to evaluate the association between leukocytes count ratios as predictive markers of metastasis in luminal type breast cancer.
Methods:
A retrospective cross-sectional study was conducted using breast cancer patient data obtained at Sanglah General Hospital (2016-2020). Complete blood count (CBC) and histopathological records of the patients were collected and the basophil-to-lymphocyte ratio (BLR), neutrophil-to-lymphocyte ratio (NLR), monocyte-to-lymphocyte ratio (MLR), and platelet-to-lymphocyte ratio (PLR) were calculated. Tumor stadium was classified into early (I-II) and advance (III-IV) stage while distant metastasis was classified into M0 and M1. Data were then analyzed using ROC curve and then followed by chi square and logistic regression analysis to obtain OR value.
Results:
Two hundred eighty-three luminal breast cancer patient data were used in this study with mean age 49.27 ± 9.451. Most of the patient had advanced disease (177 patients; 62.5%) while metastatic disease accounted for 54 patients (19.1%) of all patients. Patients with metastatic disease had higher median of BLR, MLR, NLR and PLR (0.043 ± 0.025, p=0.034; 0.289 ± 0.285, p=0.008; 3.489 ± 5.027, p=0.044; 159.538 ± 127.79, p=0.008) than patients without metastasis. The AUC (sensitivity and specificity) of BLR, MLR, NLR and PLR in predicting metastasis were 0.593 (51%; 65%), 0.616 (35%; 89%), 0.588 (46%; 75%) and 0.615 (40%; 81%), respectively. In multivariate risk analysis model, patients with metastasis were found in high BLR (Adjusted OR: 2.045; 95%CI=1.123-3.723; p=0.019), MLR (Adjusted OR: 4.862; 95%CI=2.401-9.844; p<0.001), NLR (Adjusted OR: 2.727; 95%CI=1.475-5.044; p=0.001) and PLR (Adjusted OR: 3.061; 95%CI=1.618-5.792; p=0.001).
Conclusion:
Pretreatment leukocyte ratios are potential predictive markers for metastasis. However, these findings need to be validated in larger and prospective studies with more comprehensive design.
Key Words: Luminal type, breast cancer, leukocyte count ratio, metastases, predictive
Introduction
Breast cancer is one of the most common type of cancer that occurs in women with high incidence and mortality rate worldwide. According to GLOBOCAN in 2018, the number of breast cancer cases were 2,088, 849 or 11.6% and the mortality cases were 626,679 or 6,6%) (Ambroise et al., 2011). Breast cancer is multifactorial and complex disease consists of many subtypes that has different characteristics and patterns of clinical outcomes and response of treatment in patients (Azab et al., 2013). Breast cancer was classified into some subtypes according to its molecular profiling and gene expression that can be divided into some intrinsic subtypes, one of a kind was luminal type breast cancer. Mostly, the subtype was arisen from luminal epithelial cell of the duct of mammary gland and it was known to have better prognosis compared to other subtypes (non-luminal). In addition, this subtype tended to be more sensitive of hormonal therapy since its characteristic was hormone receptor-positive subtypes. This subtype represents about 60% of all breast cancer.6 The luminal type breast cancer can be divided into luminal A and luminal B which represent different patterns and also clinical outcomes in breast cancer patients.
Luminal A type breast cancer is the most common subtype that can be found in breast cancer patients.Characteristic of luminal A subtypes was higher level of ER and/or PR with low Ki67 and negative Her2. It also has low mitotic activity, low grade of histological aspects and low proliferation (Azab et al., 2013; Chantharakhit and Sujaritvanichpong, 2020). Ga et al reported that luminal A was Ki67 <14%, ER positive (ER+), PR 20%, HER2 negative, and had a low risk of recurrence based on gene- based assays (Chen et al., 2015). In Egypt, it also showed that luminal A is the most frequent type of breast cancer patients with 113 cases or 41.2% and followed by Her-2, luminal B, and triple-negative subtypes.8 Meanwhile, luminal B breast cancer is defined as one of the luminal type breast cancer that has lower expression of ER, PR, and high grade of histological aspects. It also tended to have worse prognosis than luminal A type and share similar prognosis result as non-luminal type breast cancer (Cihan et al., 2014; Coffelt et al., 2016; Ding et al., 2019).
Recently, systemic inflammation response in the tumor microenvironment has important role on cancer development and progression.12,13 In previous study, it was shown that host response in systemic inflammation is an independent factor to predict outcome in cancer patients. It is also well established that systemic inflammation is associated with alteration of peripheral blood component such as leukocytes and erythrocyte. The peripheral blood cell test was a routine and simple test that can be applied to breast cancer patients and it was able to determine the severity of the systemic inflammatory response in patients (Elesawy et al., 2014; Ethier et al., 2017; Franco et al., 2015).
These hematological markers specifically were neutrophil-to-lymphocyte ratio (NLR), platelet-to- lymphocyte ratio (PLR), monocyte-to-lymphocyte ratio (MLR), and basophil-to-lymphocyte ratio (BLR). Earlier studies have found that high NLR has associated with worse prognosis compared with low NLR in stage IV advanced gastric cancer (Gago-Dominguez et al., 2020). In previous study, it was found that high NLR was significantly correlated with poor prognosis and considered as an independent predictor of mortality in breast cancer patients. Similar to NLR, high PLR is also associated with higher risk of mortality in breast cancer patients (Gao & Swain, 2018). Another studies showed that elevated PLR was correlated with breast cancer independently. It shows that PLR is a considerable prognostic predictor in breast cancer patients (Goto et al., 2018).
In previous study, it was stated that monocyte also contributed to cancer progression. It was found that high MLR are correlated with poor overall survival in breast cancer patients who did not receive their treatment such as trastuzumab (Gago-Dominguez et al., 2020). Meanwhile, currently there is little information about the multifunctional role of basophil in cancer. In earlier studies, it was found that pre-treatment basophil count was the independent prognostic factor in colorectal carcinoma in stage II in multivariate analysis (Guthrie et al., 2013). Another studies showed that low basophil count was associated with poor survival and may affect the clinical outcomes in colorectal cancer patients (Hashmi et al., 2018). However, there were no prognostic value of basophil count and breast cancer stage I-III patients (He et al., 2015).
Given the importance of identifying better predictive markers with simple, practical, and affordable test for breast cancer patients, the aim of present study was to evaluate the association between leukocytes count ratios as predictive markers in metastasis in luminal type breast cancer.
Materials and Methods
Study Design and Subject Selection
The study was conducted using 283 luminal type breast cancer patients from Sanglah General Hospital, Bali, Indonesia over a five-year period (2016-2020). The data of the patients who admitted with luminal type breast cancer were collected from medical record. Histopathology of the patients were divided into invasive lobular carcinoma and invasive ductal carcinoma.
Staging of cancer was categorized using FIGO stage classification into early stage (I-II) and advanced stage (III-IV). The present of distant metastasis was divided into M0 (non-metastasis) and M1 (metastasis). Complete blood count (CBC) of the patients prior to any treatment provided were recorded. Patients were excluded from the study if they had already received any breast cancer treatment.
Data Mining
Patient’s demographic data were collected such as age of diagnosed, number of parity, menstrual status and educational status. CBC data such as white blood cells (WBC), absolute basophil count (ABC), absolute monocyte count (AMC), absolute eosinophil count (AEC), absolute neutrophil count (ANC), absolute leukocyte count (ALC), absolute platelet count (APC) were analyzed as hematological parameters of interest. The basophil-to- lymphocyte ratio (BLR) was defined as the ratio between ABC to ALC. The monocyte-to-lymphocyte ratio (MLR) was defined as the ratio between AMC to ALC. The eosinophil-to-lymphocyte ratio (ELR) was defined as the ratio between AEC to ALC. The neutrophil-to-lymphocyte ratio (NLR) was defined as the ratio between ANC to ALC. The platelet-to-lymphocyte ratio (PLR) was defined as the ratio between APC to ALC. The neutrophil-to-monocyte ratio (NMR) was defined as the ratio between ANC to AMC.
Statistical Analysis and Ethical Approval
Data analysis were performed in SPSS ver. 25 with α-value 0.05. Data distribution was examined by using the Kolmogorov-Smirnov test. If the data were found normally distributed, the variables were described with mean ± standard deviation and if the data were found not normally distributed, variables were described with median ± standard deviation. The cut-off values of BLR, MLR, ELR, NLR, PLR and NMR were determined using Receiver Operating Curve (ROC) analysis with Area Under the Curve (AUC) for both metastasis and advanced stage. The cut-off values were used to define the sensitivity, specificity, positive predictive values and negative predictive values of each variable and risk model in chi square and logistic regression analysis to compare the risk of metastasis. The study was approved by the Ethics Committee of Udayana University Faculty of Medicine and the teaching hospital, Sanglah General Hospital (1309/UN14.2.2.VII.14/LT/2021).
Results
Subject Characteristic
Subject’s mean age of diagnosed were 49.27 ± 9.451. There was no significant difference found in age of diagnosed between metastasis group (48.5 ± 10.229) and non-metastasis group (48±9.283). There was no significant difference in the menstrual status, number of parity, educational status and histopathology for metastasis (M1 and M0). In the other hand, laboratory parameters such as AMC, APC, BLR, MLR, NLR and PLR were significantly higher in metastasis group compared to the non-metastasis group (p<0.05) (Table 1).
Table 1.
Baseline Characteristics of the Patients
| Parameter | Non-Metastasis (229) |
Metastasis (54) |
p |
|---|---|---|---|
| Age Diagnosed | 48 ± 9.283 | 48.5 ± 10.229 | 0.852 |
| ≤50 | 140 (80.5%) | 34 (19.5%) | 0.804 |
| >50 | 89 (81.7%) | 20 (18.3%) | |
| Educational Status | |||
| Low Grade | 182 (80.2%) | 45 (19.8%) | 0.523 |
| High Grade | 47 (83.9%) | 9 (16.1) | |
| Menstrual Status | |||
| Pre-menopause | 119 (80.4%) | 29 (19.6%) | 0.818 |
| Post-menopause | 110 (81.5%) | 25 (18.5) | |
| Parity | |||
| Null (0) | 37 (75.5%) | 12 (24.5%) | 0.321 |
| Low (1-3) | 172 (81.9%) | 38 (18.1%) | |
| Grand (>3) | 20 (83.3%) | 4 (16.7%) | |
| Histological Type | |||
| ILC | 41 (83.7%) | 8 (16.3%) | 0.59 |
| IDC | 188 (80.3%) | 46 (19.7%) | |
| WBC (109/L) | 7.52 ± 3.776 | 8.005 ± 3.899 | 0.286 |
| Basophil (109/L) | 0.079 ± 0.128 | 0.077 ± 0.04 | 0.399 |
| Monocyte (109/L) | 0.49 ± 0.301 | 0.6 ± 0.269 | 0.014* |
| Eosinophil (109/L) | 0.25 ± .,43 | 0.198 ± 0.178 | 0.368 |
| Neutrophil (109/L) | 4.49 ± 3.303 | 4.915 ± 3.515 | 0.135 |
| Lymphocyte (109/L) | 2.03 ± 0.738 | 2.035 ± 0.744 | 0.264 |
| Platelet (109/L) | 274.8 ± 124.936 | 311.65 ± 111.909 | 0.02* |
| BLR | 0.039 ± 0.066 | 0.043 ± 0.025 | 0.034* |
| MLR | 0.226 ± 0.152 | 0.289 ± 0.285 | 0.008* |
| ELR | 0.115 ± 0.166 | 0.092 ± 0.064 | 0.626 |
| NLR | 2.531 ± 2.015 | 3.489 ± 5.027 | 0.044* |
| PLR | 138.715 ± 72.288 | 159.538 ± 127.79 | 0.008* |
| NMR | 9.303 ± 8.608 | 9.41 ± 7.865 | 0.585 |
*Statistically significant (p<0.05); ILC, invasive lobular carcinoma; IDC, invasive ductal carcinoma; BLR, basophil-to-lymphocyte ratio; MLR, monocyte-to-lymphocyte ratio; ELR, eosinophil-to-lymphocyte ratio; NLR, neutrophil-to-lymphocyte ratio; PLR, platelet-to-lymphocyte ratio; NMR, neutrophil-to-monocyte ratio.
Predictive model of BLR, MLR, ELR, NLR, PLR and NMR as hematological markers in the luminal type breast cancer distant metastasis
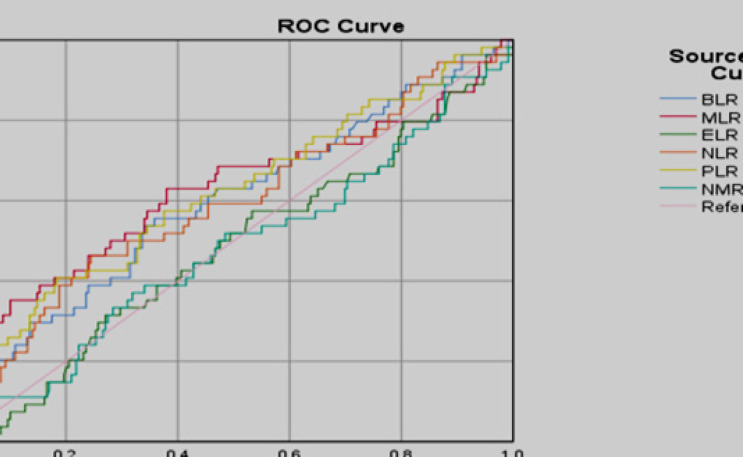
Predictive models were performed through ROC curve analysis on BLR, MLR, ELR, NLR, PLR and NMR parameters against advanced stage and metastasis of luminal type breast cancer as an outcome in the study (Figure 1). There were no feasible parameters to be used as predictive model because they have an area under the curve <0.7.
Figure 1.
ROC Analysis of BLR, MLR, ELR, NLR, PLR, and NMR as Predictive Value in the Metastatic Luminal Subtype Breast Cancer
Risk analysis model of hematological markers as predictive value in the advance stage and metastasis group of luminal type breast cancer
Risk analysis model is based on the cut-off values of OC analysis. BLR, MLR, ELR, NLR, PLR and NMR values that above the cut-off point will be classified as high level ratio and values under the cut-off point will be classified into normal level ratio. In univariate risk analysis model, high value of BLR, MLR, NLR and PLR were significantly associated with risk factor for metastasis group luminal type breast cancer (OR> 1; 0<0.05). In multivariate risk analysis model after adjustment age of diagnosed, menstrual status and number of parity, we found that BLR, MLR, NLR and PLR were significantly associated with risk factor for metastasis group luminal type breast cancer (Adjusted OR> 1; 0<0.05) (Table 2).
Table 2.
Risk Analysis Model of Hematological Markers as Predictive Value in the Advance Stage and Metastasis Group of Luminal Type Breast Cancer
| Parameters | Level | Early | Advance | S.E. | Adjusted OR | 95% CI | p (bivariate) | p (multivariate) |
|---|---|---|---|---|---|---|---|---|
| BLR | High | 79 (73.8%) | 28 (26.2%) | 0.306 | 2.045 | 1.123-3.723 | 0.018* | 0.019* |
| Normal | 150 (85.2%) | 26 (14.8%) | ||||||
| MLR | High | 23 (54.8%) | 19 (45.2%) | 0.36 | 4.862 | 2.401-9.844 | <0.001* | <0.001* |
| Normal | 206 (85.5%) | 35 (14.5%) | ||||||
| ELR | High | 121 (79.6%) | 31 (20.4%) | 0.305 | 1.203 | 0.661-2.189 | 0.545 | 0.545 |
| Normal | 108 (82.4%) | 23 (17.5%) | ||||||
| NLR | High | 55 (68.8%) | 25 (31.3%) | 0.314 | 2.727 | 1.475-5.044 | 0.001* | 0.001* |
| Normal | 174 (85.7%) | 29 (14.3%) | ||||||
| PLR | High | 42 (65.6%) | 22 (34.4%) | 0.325 | 3.061 | 1.618-5.792 | <0.001* | 0.001* |
| Normal | 187 (85.4%) | 32 (14.6%) | ||||||
| NMR | High | 72 (78.3%) | 20 (21.7%) | 0.316 | 1.283 | 0.691-2.381 | 0.43 | 0.43 |
| Normal | 157 (82.2%) | 34 (17.8%) |
*Statistically significant (p<0.05); BLR, basophil-to-lymphocyte ratio; MLR, monocyte-to-lymphocyte ratio; ELR, eosinophil-to-lymphocyte ratio; NLR, neutrophil-to-lymphocyte ratio; PLR, platelet-to-lymphocyte ratio; NMR, neutrophil-to-monocyte ratio; AUC, area under the curve; CI, confidence interval.
Discussion
Tumor immunology and hematology are fast growing sciences in the science of cancer. This field of science has a vital role in breast cancer metastasis because the prognosis of cancer is related to the functional status of the immune system (Huszno & Kolosza, 2019). In addition, with regard to hematology, various white blood cells and platelet indices have been reported as potential markers for disease progression, predicting recurrence, and overall survival in several types of cancer, including metastases breast cancer (Jia et al., 2015).
Setyawati et al., (2018) study showed there was no significant difference for lymph node metastasis (p= 0.540) (Setyawati et al., 2018) These results were similar to studies by Ambroise et al., (2011), Tamaki et al., (2013), and Jones et al., (2013). However, other studies have found some association between lymph node status and breast cancer subtypes (Cihan et al., 2014; Koh et al., 2015; Liu et al., 2020; Mantas et al., 2016; Nakamura et al., 2018; Nakasone et al., 2018; Shaul & Fridlender, 2017; Si et al., 2014). These inconsistent results suggest that lymph node status cannot be an independent prognostic factor for breast cancer. Another prognostic factor used is lymphovascular invasion. Luminal B cancer has a higher incidence of lymphovascular invasion so that recurrence of disease in post-treatment luminal B cancer can reduce the survival rate in these patients and make them more difficult to treat (Nakasone et al., 2018).
Luminal type breast cancer is a subtype of breast cancer characterized by increased expression of estrogen hormone receptors. Luminal subtype breast cancer is divided into two, namely luminal A and luminal B, where luminal B has a worse prognosis than luminal A. Luminal subtype breast cancer can be treated using hormone-targeting therapies, including selective estrogen receptor modulators (SERMs), aromatase inhibitors (AIs), selective estrogen receptor down-regulators (SERDs), and luteinizing hormone-releasing hormone analogs (LHRH analogs). Although luminal breast cancer has a good prognosis compared to other subtypes, there are still differences in responses and outcomes of hormone- targeting therapy given to patients (Sepideh Siadati et al., 2015). In addition, the findings of distant metastases in patients with luminal subtype breast cancer indicate the presence of other factors that play a role in the progression of cancer cells to the metastatic stage. This is most likely due to the involvement of the immune system of patients with luminal subtype breast cancer, so the researchers tried to conduct studies related to the involvement of the immune system (leukocytes ratio) as a predictor of metastasis in luminal subtype breast cancer.
The immune system response is one parameter that can be used to see and predict the progression, chemotherapy responses and recurrence of cancer cells. Leukocytes, especially monocytes and neutrophils, are cells that will respond to cytokines secreted by cancer cells. In term of tumor progression and recurrence, immune cells, especially neutrophils play a role in the progression of cancer cells that are dormant or remaining after therapy. Neutrophils can be divided into two, namely N1 (anti- tumor) and N2 (pro-tumor). Neutrophils will transform into N2 phenotype when exposed to granulocytes colony stimulating factor (G-CSF) and transforming growth factor (TGF-β). The N2 phenotype then secretes transforming growth factor (TGF-β) in the tumor microenvironment, causing more pro-tumor immune cells (Sun et al., 2016; Tamaki et al., 2013). This will lead to an adjustment of the number and composition of the components of white blood cells through a decrease in the lymphocyte count and an increase in the neutrophil and monocyte counts in response to received cytokine signaling (Chen et al., 2015; Wei et al., 2016). This is supported by the results of research by Huszno et al., (2019) which found an increase in the results of monocyte and neutrophil counts compared to the results of the lymphocyte count, where NLR was greater than 2 and MLR was greater than 0.25. This finding is in line with the results of our study in which we found higher neutrophil, monocyte and lymphocyte counts in the metastatic group compared to the non-metastatic group. Neutrophil-to-lymphocyte ratios (NLR) in our study showed a higher cut off value compared to previous studies (cut off value=2.696). In a study conducted by Huszno et al., (2019), the NLR cut off value was 2.65.5 This is in line with the study of Noh et al., (2013) which showed a cut off value of 2.5 with the finding that a higher NLR value indicates a disease-specific survival rate for all subtypes and is significantly associated with a poor prognosis in patients with breast cancer. type luminal A (Noh et al., 2013). Similar results were also obtained in a study conducted by Gago-Dominguez et al., (2020). In a case control study conducted, it was found that an increase in the absolute number of neutrophils was associated with an increased risk of breast cancer and an increase in NLR in breast cancer patients was found to be dominant in patients with luminal cancer type A (Gago-Dominguez et al., 2020). However, in a study conducted by Rubio et al., (2019) involving 263 metastatic breast cancer patients, it was found that NLR was not significantly associated as a predictor of OS on multivariate analysis (HR=1.12; 95% CI=0.80-1.56), even though at the results of univariate analysis showed significant results (HR=1.36; 95% CI=1.00-1.83) (Ivars Rubio et al., 2019). This shows that the baseline increase of NLR with patient OS however is most likely influenced by the relationship with other clinical prognosis factors so that further research is needed. Furthermore, increased monocyte-to-lymphocyte ratios (MLR) values were also found in patients with a poor prognosis. In a study conducted by Goto et al., (2018) showed that a decrease in lymphocyte-to-monocyte ratios (LMR) (in reverse to MLR) showed a worse DSF in all patient groups (p=0.005). It was also found that low LMR was an independent risk factor for DFS (HR=2.245; p=0.008) (Goto et al., 2018). Similar results were also found in the study by Ni et al., (2014). In their research it was found that higher LMRs were significantly related to favorable DFS, both in the univariate and multivariate analyzes (p=0.009 and p=0.011, respectively) (Ni et al., 2014). However, this study used patients who had received therapy in the form of neo-adjuvant chemotherapy (NAC) so that this is likely to be a biasing factor in this finding.
The use of hematological parameters in determining cancer prognosis, both in pre-treatment and post- treatment conditions has become an increasingly frequent examination by clinicians (Parida and Mandal, 2014; Prabawa et al., 2019; Sengal et al., 2017). Based on a meta-analysis conducted by Wei et al., (2016) involving 12 studies, it shows that higher baseline of NLR is associated with poor patient prognosis. Patients with higher NLR had a shorter DFS (HR=1.46; 95% CI=1.12-1.90; p=0.044) and OS (HR=2.03; 95% CI=1.41-2.93; p <0.001). However, in this study there was no significant relationship between higher NLR and poor prognosis in luminal A and luminal B breast cancer patients (Wei et al., 2016). The results of this study are in line with the meta-analysis conducted by Ethier et al., (2017) involving 15 studies with a total of 8563 patients. In this meta-analysis it was found that higher NLR was associated with worse OS (HR=2.56; 95% CI=1.96- 3.35; p <0.001) and DFS (HR=1.74; 95% CI=1.47-2.07; p<0.001) (Ethier et al., 2017).
The proliferation, angiogenesis, invasion and metastasis of tumor cells are influenced by the host inflammatory response and immune response in the tumor microenvironment. Of all the parameters, platelet-to- lymphocyte ratios (PLR) is one of the parameters that depends on the systemic inflammatory response which is influenced by the response from cancer and also the inflammatory conditions that occur (Prabawa et al., 2019) In response to inflammatory conditions that occur, platelets could secrete cellular growth factors such as vascular endothelial growth factor (VEGF), platelet-derived growth factor, platelet factor 4 and transforming growth factor beta (TGF-β) which play a role in stimulating tumor cell growth and angiogenesis and suppress the cytolytic activity of NK cells. Platelets also contribute to the process of adhesion of tumor cells to the endothelium and in the transmigration of tumor cells out of the vascular system. In addition, platelets can also increase stromal formation of tumor cells by promoting the migration process of inflammatory cells. Based on the results of our study, it was found that the cut off value in predicting distant metastasis from the PLR was 183,814. In addition, a higher median of PLR was also found in the metastatic than non-metastatic group. Although not many studies have discussed PLR in predicting metastasis in breast cancer patients, in general, higher PLR is associated with poor prognosis and worse clinical outcome. Based on research conducted by Wiranata et al., (2020), the higher median of PLR was found in a group of patients with advanced stage of cancer compared to early stage. Univariate and multivariate analyzes also showed significant results (OR = 3.949; 95% CI = 1.679-9,287; p = 0.001 and OR = 2.737; 95% CI = 1.092-6,859; p = 0.032, respectively) (Wiranata et al., 2020). This result is also in line with the meta-analysis conducted by Guo et al., (2019) involving 39 studies and 17,079 breast cancer patients, which found a significant relationship between higher PLR and worse patient prognosis, where higher PLR was associated with shorter DFS (HR=1.43; 95% CI=1.09-1.86; p=0.009) (Guo et al., 2019). In addition to playing a role in maintaining the integrity of the blood barrier and hemostasis, platelets also have an involvement in the progression of cancer cells through the secretion of several proteins, nucleotides and bioactive lipids that can induce inflammatory processes and the development of cancer cells, one of which progresses to the metastatic stage involving complex mechanisms, such as changes in cell adhesion, invasion and access to blood and lymph vessels that allow cancer cells to spread systemically (Yersal et al., 2017). Research on basophil-to-lymphocyte ratios (BLR) is still very limited to date. Prabawa et al.,’s (2019) study involving 282 patients with cervical cancer showed that BLR (r=0.362) only had a moderate positive correlation with the stage of cervical cancer. The results of the multivariate analysis found that BLR was not significant after adjustment so that further research was needed (Prabawa et al., 2019). The BLR and MLR are novel parameters that are still underdeveloped in predicting the presence of metastases and determining the prognosis of breast cancer patients. We believe that our study is the first to discuss BLR and MLR as predictors of metastasis in luminal subtype breast cancer. In our study, there was a significant association between BLR and MLR with distant metastasis. The higher median of BLR and MLR were also found in patients with metastases compared with the group of non-metastatic patients. In univariate analysis, we found a significant association between increased NLR, BLR, MLR and PLR and the risk of metastases in luminal subtype breast cancer patients. A significant association was also found in the multivariate risk analysis model after adjusting for age at diagnosis, parity and menstrual status. In conclusion, the NLR, BLR, MLR, and PLR are associated with the incidence of metastasis in luminal type breast cancer. Therefore, these parameters have a significant degree of potential in predicting the presence of distant metastasis in luminal subtype breast cancer patients. In addition, all four parameters were also found to be independently associated with the risk of metastatic events in patients. Therefore, it is necessary to do a more comprehensive study with the combination existing parameters with leukocytes ratio to test the feasibility of these parameters before they are applied in a clinical setting.
Author Contribution Statement
All of the authors have contributed in this study. Study conception and design: I Gede Wikania Wira Wiguna, I Gede Putu Supadmanaba, Desak Made Wihandani, Putu Anda Tusta Adiputra; data collection: Ni Putu Sri Indrani Remitha, Sinta Wiranata, I Gusti Ayu Stiti Sadvika, I Wayan Ardyan Sudartha Putra; analysis and interpretation of result: I Gede Wikania Wira Wiguna, I Gede Putu Supadmanaba; draft manuscript preparation: Ni Putu Sri Indrani Remitha, Sinta Wiranata, I Gusti Ayu Stiti Sadvika. All authors reviewed the results and approved the final version of the manuscript.
Acknowledgements
We thank every authors for their amazing contributions in this research.
References
- Ambroise M, Ghosh M, Mallikarjuna VS, Kurian A. Immunohistochemical profile of breast cancer patients at a tertiary care hospital in South India. Asian Pac J Cancer Prev. 2011;12:625–9. [PubMed] [Google Scholar]
- Azab B, Shah N, Radbel J, et al. Pretreatment neutrophil/lymphocyte ratio is superior to platelet/lymphocyte ratio as a predictor of long-term mortality in breast cancer patients. Med Oncol. 2013;30:1. doi: 10.1007/s12032-012-0432-4. [DOI] [PubMed] [Google Scholar]
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- Casbon AJ, Reynau D, Park C, et al. Invasive breast cancer reprograms early myeloid differentiation in the bone marrow to generate immunosuppressive neutrophils. Proc Nat Acad Sci U S A. 2015;112:566–75. doi: 10.1073/pnas.1424927112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chantharakhit C, Sujaritvanichpong N. Pretreatment Absolute Neutrophil-to-Lymphocyte Ratio (NLR) Predict the Risk for Febrile Neutropenia in the First Cycle Adjuvant Chemotherapy for Breast Cancer. Asian Pac J Cancer Biol. 2020;5:81–7. [Google Scholar]
- Chen J, Deng Q, Pan Y, et al. Prognostic value of neutrophil-to-lymphocyte ratio in breast cancer FEBS Open Bio. Federation of European Biochemical Societies. 2015;5:502–7. doi: 10.1016/j.fob.2015.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cihan YB, Arslan A, Cetindag MF, Mutlu H. Lack of prognostic value of blood parameters in patients receiving adjuvant radiotherapy for breast cancer. Asian Pac J Cancer Prev. 2014;15:4225–31. doi: 10.7314/apjcp.2014.15.10.4225. [DOI] [PubMed] [Google Scholar]
- Coffelt SB, Wellenstein MD, De Visser KE. Neutrophils in cancer: Neutral no more. Nat Rev Cancer. 2016;16:431–46. doi: 10.1038/nrc.2016.52. [DOI] [PubMed] [Google Scholar]
- Ding N hua, Liu C fan, Hu C, et al. Prognostic Factors for Luminal B-like Breast Cancer. Curr Med Sci. 2019;39:396–402. doi: 10.1007/s11596-019-2049-8. [DOI] [PubMed] [Google Scholar]
- Elesawy BH, Abd El Hafez A, Shawky AEA, Arafa M. 2014), Immunohistochemistry-based subtyping of breast carcinoma in Egyptian women: A clinicopathologic study on 125 patients. Ann Diagn Pathol. 18:21–6. doi: 10.1016/j.anndiagpath.2013.10.005. [DOI] [PubMed] [Google Scholar]
- Ethier JL, Desautels D, Templeton A, Shah PS, Amir E. Prognostic role of neutrophil-to-lymphocyte ratio in breast cancer: A systematic review and meta-analysis. Breast Cancer Res. 2017;19:1–13. doi: 10.1186/s13058-016-0794-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco AT, Corken A, Ware J. Platelets at the interface of thrombosis, inflammation, and cancer. Blood. 2015;126:582–8. doi: 10.1182/blood-2014-08-531582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gago-Dominguez M, Matabuena M, Redondo CM, et al. Neutrophil to lymphocyte ratio and breast cancer risk: analysis by subtype and potential interactions. Sci Rep Nat Publishing Group UK. 2020;10:1–11. doi: 10.1038/s41598-020-70077-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao JJ, Swain SM. Luminal A Breast Cancer and Molecular Assays: A Review. Oncologist. 2018;23:556–65. doi: 10.1634/theoncologist.2017-0535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto W, Kashiwagi S, Asano Y, et al. Predictive value of lymphocyte-to-monocyte ratio in the preoperative setting for progression of patients with breast cancer. BMC Cancer. 2018;18:1–9. doi: 10.1186/s12885-018-5051-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo W, Lu X, Liu Q, et al. Prognostic value of neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio for breast cancer patients: An updated meta-analysis of 17079 individuals. Cancer Med. 2019;8:4135–48. doi: 10.1002/cam4.2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guthrie GJK, Charles KA, Roxburgh CSD, et al. The systemic inflammation-based neutrophil-lymphocyte ratio: Experience in patients with cancer. Critical Rev Oncol Hematol. 2013;88:218–30. doi: 10.1016/j.critrevonc.2013.03.010. [DOI] [PubMed] [Google Scholar]
- Hashmi AA, Aijaz S, Khan SM, et al. Prognostic parameters of luminal A and luminal B intrinsic breast cancer subtypes of Pakistani patients. World J Surg Oncol. 2018;16:1–6. doi: 10.1186/s12957-017-1299-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He ZY, Wu SG, Yang Q, et al. Breast cancer subtype is associated with axillary lymph node metastasis. Medicine (United States) 2015;94:1–7. doi: 10.1097/MD.0000000000002213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huszno J, Kolosza Z. Prognostic value of the neutrophil-lymphocyte, platelet-lymphocyte and monocyte-lymphocyte ratio in breast cancer patients. Oncol Lett. 2019;18:6275–83. doi: 10.3892/ol.2019.10966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivars Rubio A, Yufera JC, de la Morena P, et al. Neutrophil-lymphocyte ratio in metastatic breast cancer is not an independent predictor of survival, but depends on other variables. Sci Rep. 2019;9:1–9. doi: 10.1038/s41598-019-53606-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia W, Wu J, Jia H, et al. The peripheral blood neutrophil-to-lymphocyte ratio is superior to the lymphocyte-to-monocyte ratio for predicting the long-term survival of triple-negative breast cancer patients. PLoS One. 2015;10:1–13. doi: 10.1371/journal.pone.0143061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh CH, Bhoo-Pathy N, Ng KL, et al. Utility of pre-treatment neutrophil-lymphocyte ratio and platelet-lymphocyte ratio as prognostic factors in breast cancer. Br J Cancer. 2015;113:150–8. doi: 10.1038/bjc.2015.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Luo D, Cai S, Li Q, Li X. Circulating basophil count as a prognostic marker of tumor aggressiveness and survival outcomes in colorectal cancer. Clin Transl Med. 2020:9. doi: 10.1186/s40169-019-0255-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantas D, Kostakis ID, Machairas N, Markopoulos C. White blood cell and platelet indices as prognostic markers in patients with invasive ductal breast carcinoma. Oncol Lett. 2016;12:1610–4. doi: 10.3892/ol.2016.4760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K, Nakayama K, Tatsumi N, et al. Prognostic significance of pretreatment neutrophiltolymphocyte and platelettolymphocyte ratios in nonsurgically treated uterine cervical carcinoma. Mol Clin Oncol. 2018;2018:138–44. doi: 10.3892/mco.2018.1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakasone ES, Hurvitz SA, McCann KE. Harnessing the immune system in the battle against breast cancer. Drugs Context. 2018;7:1–21. doi: 10.7573/dic.212520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni XJ, Zhang XL, Ou-Yang QW, et al. An elevated peripheral blood lymphocyte-to-monocyte ratio predicts favorable response and prognosis in locally advanced breast cancer following neoadjuvant chemotherapy. PLoS One. 2014:9 7–12. doi: 10.1371/journal.pone.0111886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noh H, Eomm M, Han A. Usefulness of pretreatment neutrophil to lymphocyte ratio in predicting disease-specific survival in breast cancer patients. J Breast Cancer. 2013;16:55–9. doi: 10.4048/jbc.2013.16.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parida S, Mandal M. Inflammation induced by human papillomavirus in cervical cancer and its implication in prevention. Eur J Cancer Prev. 2014;23:432–48. doi: 10.1097/CEJ.0000000000000023. [DOI] [PubMed] [Google Scholar]
- Prabawa IPY, Bhargah A, Liwang F, et al. Pretreatment neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR) as a predictive value of hematological markers in cervical cancer. Asian Pac J Cancer Prev. 2019;20:863–8. doi: 10.31557/APJCP.2019.20.3.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengal AT, Haj-Mukhtar NS, Elhaj AM, et al. Immunohistochemistry defined subtypes of breast cancer in 678 Sudanese and Eritrean women; hospitals based case series. BMC Cancer. 2017;17:1–9. doi: 10.1186/s12885-017-3805-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sepideh S, Majid S, Nikbakhsh N, Ghaemian N. Correlation of ER, PR and HER-2/Neu with other Prognostic Factors. Iran J Pathol. 2015;10:221–6. [PMC free article] [PubMed] [Google Scholar]
- Setyawati Y, Rahmawati Y, Widodo I, Ghozali A, Purnomosari D. The association between molecular subtypes of breast cancer with histological grade and lymph node metastases in Indonesian woman. Asian Pac J Cancer Prev. 2018;19:1263–8. doi: 10.22034/APJCP.2018.19.5.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaul ME, Fridlender ZG. Neutrophils as active regulators of the immune system in the tumor microenvironment. J Leukocyte Biol. 2017;102:343–9. doi: 10.1189/jlb.5MR1216-508R. [DOI] [PubMed] [Google Scholar]
- Si C, Jin Y, Wang H, Zou Q. Association between molecular subtypes and lymph node status in invasive breast cancer. Int J Clin Exp Pathol. 2014;7:6800–06. [PMC free article] [PubMed] [Google Scholar]
- Sun P, Zhang F, Chen C, et al. The ratio of hemoglobin to red cell distribution width as a novel prognostic parameter in esophageal squamous cell carcinoma: A retrospective study from southern China. Oncotarget. 2016;7:42650–60. doi: 10.18632/oncotarget.9516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamaki M, Kamio T, Kameoka S, Kojimahara N, Nishikawa T. The relevance of the intrinsic subtype to the clinicopathological features and biomarkers in Japanese breast cancer patients. World J Surg Oncol. 2013;11:1–13. doi: 10.1186/1477-7819-11-293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei B, Yao M, Xing C, et al. The neutrophil lymphocyte ratio is associated with breast cancer prognosis: An updated systematic review and meta-analysis. Onco Targets Ther. 2016;9:5567–75. doi: 10.2147/OTT.S108419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiranata S, Anjani IAW, Saputra IPGS, et al. Pretreatment neutrophil-to-lymphocyte ratio and platelet-tolymphocyte ratio as a stage determination in breast cancer. Open Access Macedonian J Med Sci. 2020;8:1058–63. [Google Scholar]
- Yersal Ö, Çetinkünar S, Aktimur R, et al. Neutrophil/lymphocyte and platelet/lymphocyte ratios are not different among breast cancer subtypes. Asian Pac J Cancer Prev. 2017;18:2227–31. doi: 10.22034/APJCP.2017.18.8.2227. [DOI] [PMC free article] [PubMed] [Google Scholar]