Abstract
Background:
Tumor cell autophagy can influence cellular immunity by participation in the recognition and modification of tumor-related antigens.
Objectives:
The objective of this study was to evaluate the immunohistochemical expression of the autophagy-related marker; light chain 3B (LC3B) in tumor cells and the assessment of T lymphocytes by a cluster of differentiation 3 (CD3) in gliomas, and to correlate them with the available clinic-pathological variables in glioma patients.
Materials and methods:
Immunohistochemical staining for LC3B and CD3 was performed on 60 paraffin-embedded glioma tissue. LC3B immunoreactivity score of 0-6 was designated negative, and those scoring 7-12 were considered positive. The median level of CD3 positive T lymphocytes was calculated for both high and low-grade gliomas. In low-grade gliomas, the CD3 expressing T lymphocytes equal to or more than 2.6, were considered positive while in high-grade gliomas, those equal to or more than 16 were considered positive.
Results:
LC3B expression in tumor cells was detected in 24/60 (40%) of gliomas. Expression of LC3B was significantly more frequent in high-grade gliomas (23/33, 69.7%) compared to low grade ones (1/27, 5%), (p value= 0.000). LC3B expression was correlated with the patients age (P value= 0.047) & histological variants (P value= 0.000). CD3 positive T lymphocytes were significantly more prominent in high-grade gliomas (25/33 , 41.7%) than low-grade ones (2/27, 3.3%), (P value= 0.001). A significant association was noted between CD3 expression and the patients age (P value= 0.003), sex (P value: 0.035) and histological variants (P value= 0.001). LC3B expression in tumor cells was significantly correlated with CD3 positive T lymphocytes (P value: 0.000).
Conclusion:
Autophagic activity of tumor cells and T lymphocyte infiltrates were reported more in high-grade gliomas compared to low-grade ones, giving high-grade gliomas the chance in autophagy target therapy & immunotherapy.
Key Words: Glioma, LC3B, CD3, T lymphocytes, Immunohistochemistry
Introduction
Gliomas represent a group of diverse primary brain tumors that affect multiple age groups (Ferlay et al., 2018).
In Egypt, gliomas rank first among the primary central nervous system (CNS) tumors, accounting for 35% of all neoplasms. The most common gliomas are astrocytic tumors and glioblastoma represents 44.1% of this category (Hewedi et al., 2018).
Despite the recent treatment modalities of glioma which combines surgery with neoadjuvant radiotherapy and chemotherapy; gliomas are still the most lethal intracranial tumors with a high incidence of relapse. Immunotherapy has currently emerged as a potential method for cancer management with a low recurrence rate and minor adverse effects (Kohrt et al., 2016).
Autophagy is a special process in which lysosomes destroy the impaired cell organelles in addition to macromolecules. Autophagy has an essential role in cellular metabolism and homeostasis (Vessoni et al., 2013). LC3 encompasses a soluble LC3 I and a lipidated structure named LC3 II. LC3 II is enrolled into autophagosomes (Schulte et al., 2020). Multiple stressors enhance the conjugation of LC3 I to phosphatidylethanolamine to regulate the autophagosome-specific LC3 II, which is regarded as a consistent marker of autophagy (Tanida et al.,2008; Arani et al.,2021).
Exploring the role and the relationship between the autophagy process and cell-mediated immunity in gliomas is of potential value for the application of immunotherapy (Cj et al., 2018).
Microtubule-associated protein 1 light chain 3 (MAP1LC3 or LC3) is a basic protein of autophagosomal membranes. The family of the LC3 gene constitutes three members, LC3A, LC3B, and LC3C (He et al., 2003). LC3B is one of the necessary proteins involved in autophagy and is important for its execution; therefore, we aimed in this study to evaluate the expression of LC3B in gliomas, in addition to studying the infiltration of immune T lymphocytes (Barth et al., 2010).
Materials and Methods
Retrieval of Cases
This cross-sectional study was applied on 60 retrieved formalin-fixed paraffin-embedded glioma sections, obtained from the Pathology Department, Faculty of Medicine, Cairo University in the period from April 2017 till April 2019.
Histopathological Assessment
Three 4 μm thick sections were cut from each glioma paraffin block then one section was mounted on a glass slide, stained by routine hematoxylin and eosin (H&E) for histopathological assessment and the other two sections were prepared on charged slides for immunophenotyping.
LC3B and CD3 Immunohistochemical Staining and Evaluation
Immunohistochemical staining was done using LC3B, rabbit, monoclonal antibody (Cell Signaling Technology, Beverly, United States, 1:500), and CD3 antibody (Abcam, ab203034, Cambridge, UK, 1:200). Steam heating of the charged slides was applied for antigen retrieval. Afterward, blocking of endogenous biotin was done, followed by staining through an automated immunostainer (Dako). Detection was done using a streptavidin-biotin detection system (Dako). Positive control sections were applied in each run according to the manufacturer’s recommended protocol; neurons in normal brain tissue for LC3B and T lymphocytes in normal skin for CD3.
The intensity of LC3B cytoplasmic staining was assessed as follows: negative staining, 0 points; faint yellow, 1 point; tan, 2 points; dark brown, 3 points. The percentage of tumor cells with positive LC3B expression was assessed as follows: 0%-5% positive cells, 0 points; 6%-25% positive cells, 1 point; 26%-50% positive cells, 2 points; 51%-75% positive cells, 3 points; 76%-100% positive cells, 4 points. A final immunostaining score was recorded by multiplying the staining intensity score by the score depending on the percentage of positive tumor cells. Tumors with a final immunostaining score of 0-6 were regarded as negative, and those with scores ranging from 7 to 12 were regarded as positive (Zhang et al., 2018).
CD3 was expressed as membranous and cytoplasmic staining of T lymphocytes. The number of CD3 positive T lymphocytes were counted per 10 high-magnification (400×) in each field. The average count of CD3 positive T lymphocytes was applied as the measure of expression. The median level was calculated for both high and low-grade glioma cases and used as cutoffs (Zhang et al., 2018). So average CD3 positive T lymphocytes equal to or more than 15 and 2.6 in high and low-grade gliomas respectively, were regarded as positive in the study.
Statistical Analysis
The studied demographic, pathological, and immunohistochemical data were then enrolled in the SPSS Software program, version 26 (Armonk, New York, United States). Simple descriptive statistical methods (mean and standard deviation) were recorded for summarization of quantitative data and frequencies were recorded for qualitative data. The unpaired T-student test was applied for comparing two groups. Chi-square (χ2) test was applied to compare categorical variables. A P-value less than 0.05 was considered significant. The correlations between the immunohistochemical expression of LC3B in tumor cells and the count of CD3 positive T lymphocytes were analyzed by Spearman correlation. Spearman’s correlation coefficient (r) values were analyzed as {(r) 0: no detected correlation, (r) more than 0 but less than 1: positive correlation, (r) less than zero but more than -1: negative correlation}.
Results
This study included 60 cases of glioma. Their age ranged from 6 to 68 years with a mean of 37.3 years. Regarding gender, the studied cases showed near equal distribution among both sexes (48% females and 52 % males).
LC3B scoring was done and showed positive cytoplasmic expression (score 7-12) in 40% of glioma cases. Expression of LC3B was significantly more frequent in high-grade gliomas (23/33, 69.7%) compared to low-grade ones (1/27, 5%), (Table 1), with a statistically significant difference (p value= 0.000).
Table 1.
Distribution of Glioma Cases Regarding LC3B Staining Score
| Score | 0+ | 1+ | 2+ | 3+ | 4+ | 6 | 8 | 9 | 12 | Total |
|---|---|---|---|---|---|---|---|---|---|---|
| Number | 8 | 1 | 11 | 5 | 6 | 5 | 4 | 16 | 4 | 60 |
| Percent (%) | 13.3 | 1.7 | 18.3 | 8.3 | 10 | 8.3 | 6.7 | 26.7 | 6.7 | 100 |
| LC3B expression | Negative LC3B Expression | Positive LC3B Expression | ||||||||
There was a significant association between LC3B expression and the age of patients (P value= 0.047) and histological variants (P value= 0.000). However, there was no significant correlation between LC3B expression and either sex (p=0.172), site of the tumor (p=0.,158), or history of recurrence (p=0.480).
CD3 positive T lymphocytes were detected in twenty-seven (45%) glioma cases. CD3 positive T lymphocytes were significantly more prominent in high-grade gliomas (25/33, 41.7%) than low-grade ones (2/27, 3.3%), with a statistically significant difference (P value= 0.001). There was a significant association between CD3 expression and the age of patients (P value= 0.003), sex of patients (P-value: 0.035) and glioma histological variants (P value= 0.001). However, there was no significant correlation between CD3 positive T lymphocytes and either site of the tumor (p=0.109) or history of recurrence (p=0.728).
Immunostaining of low and high-grade gliomas for LC3B and CD3 labeled T lymphocytes are shown in Figures 1 and 2 respectively
Figure 1.
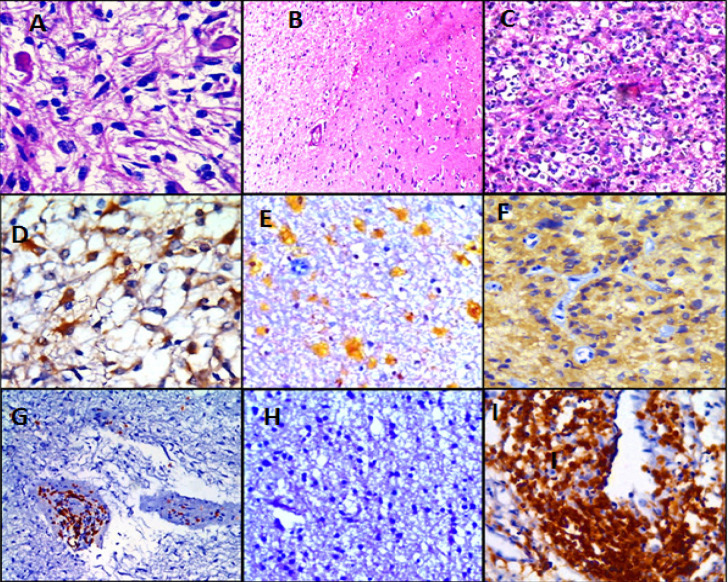
Different Cases of Low Grade Gliomas; Figures (A, B &C): Pilocytic astrocytoma, WHO grade I, Diffuse astrocytoma, WHO grade II & Oligodendroglioma, WHO grade II respectively (H&E; (A)X400, (B)X100 & (C)X200 original magnification). Figures (D, E & F): Pilocytic astrocytoma shows LC3B brown cytoplasmic staining in about 50% of tumor cells, Score 6+; Oligodendroglioma shows LC3B yellowish cytoplasmic staining in about 50% of tumor cells, Score 2+ and oligodendroglioma shows LC3B tan cytoplasmic staining in about 90% of tumor cells, Score 8+ respectively (IHC; (D)X400, (E)X100 & (F)X200 original magnification). Figures (G, H & I): Pilocytic astrocytoma shows CD3 positive T lymphocytes arranged mainly around blood vessels with few of them scattered in between tumor cells, Oligodendroglioma shows absent CD3 positive T lymphocytes in tumor cells (low power) and higher magnification of CD3 positive T lymphocytic infiltrates around blood vessels respectively (IHC; (G)X100, (H)X100 & (I)X400 original magnification)
Figure 2.
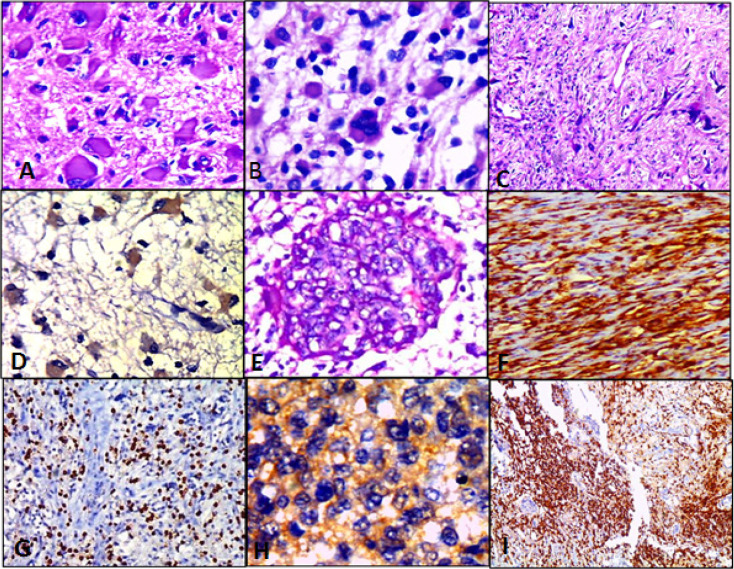
Different Cases of High Grade Gliomas; Figures (A, B, E &C); Anaplastic astrocytoma with neoplastic gemistiocytes, WHO grade III; Glioblastoma, WHO grade IV; Micovascular proliferation & Gliosarcoma, WHO grade IV respectively (H&E; (A)X400, (B)X400, (E)X 200 & (C)X100 original magnification). Figures (D, H & F); Anaplastic astrocytoma shows LC3B brown cytoplasmic staining in about 70% of tumor cells, Score 9+; Glioblastoma shows LC3B tan cytoplasmic staining in about 80% of tumor cells, Score 8+ and Gliosarcoma shows LC3B brown cytoplasmic staining in about 90% of tumor cells, Score 12+ respectively (IHC; (D)X400, (H)X400 & (F)X200 original magnification). Figures (G & I); High grade gliomas show dense CD3 positive lymphocytes scattered between tumor cells (IHC; (G)X200 & (I)X100 original magnification)
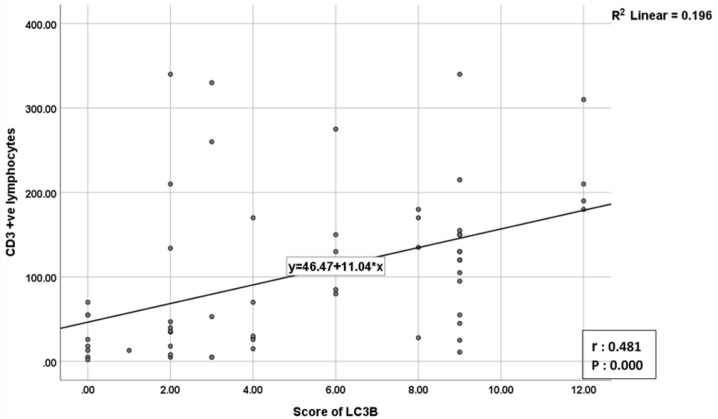
The correlation of LC3B and CD3 expression with various pathological characteristics among studied cases is summarized (Table 2). The correlation between LC3B positive tumor cells and CD3 positive T lymphocytes achieved a statistical significance (P-value: 0.000) (Table 3), where there was a strong positive relationship (r: 0.481) between CD3 and LC3B scores. The more the LC3B score, the more the number of CD3 positive lymphocytes (Figure 3).
Table 2.
Correlation of LC3B and CD3 Expression with Various Pathological Characteristics among the Studied Glioma Cases
| Pathological characteristics | LC3B positive (%) |
LC3B negative (%) |
P value | CD3 positive (%) |
CD3 negative (%) |
P value | |
|---|---|---|---|---|---|---|---|
| Age (years) | ≥50 | 12 (57) | 9 (43) | 0.000* | 15 (71.4) | 6 (28.6) | 0.003* |
| ˂50 | 12 (30.7) | 27 (69.3) | 12 (30.8) | 27 (69.2) | |||
| Gender | Male | 9 (31) | 20 (69) | 0.172 | 9 (31) | 20 (69) | 0.035* |
| Female | 15 (48.3) | 16 (51.7) | 18 (58) | 13 (42) | |||
| Tumor Size | ≤3cm | 4 (28.5) | 10 (71.5) | 0.319 | 5 (35.7) | 9 (64.3) | 0.544 |
| >3cm | 20 (43.5) | 26 (56.5) | 22 (47.8) | 24 (52.2) | |||
| Tumor Location |
Frontal | 3 (20 ) | 12 (80) | 0.158 | 4 (26.7) | 11 (73.3) | 0.109 |
| Parietal | 9 (56.3) | 7 (43.7) | 9 (56.3) | 7 (43.7) | |||
| Temporal | 5 (45.5) | 6 (54.5) | 6 (54.5) | 5 (45.5) | |||
| Posterior | 3 (60) | 2 (40) | 4 (80) | 1 (20) | |||
| Cerebellum | 0 (0) | 4 (100) | 0 (0) | 4 (100) | |||
| Others | 4 (44.4) | 5 (55.6) | 4 (44.4) | 5 (55.6) | |||
| Recurrence | Yes | 5 (50) | 5 (50) | 0.48 | 5 (50) | 5 (50) | 0.728 |
| No | 19 (38) | 31 (62) | 22 (44) | 28 (56) | |||
| Histological type | Pilocytic astrocytoma | 0 (0) | 12 (100) | 0.000* | 1 (8.3) | 11 (91.7) | 0.001* |
| Diffuse astrocytoma | 0 (0) | 9 (100) | 1 (11) | 8 (89) | |||
| Oligodendroglioma | 1 (16.7) | 5 (83.3) | 0 (0) | 6 (100) | |||
| Anaplastic glioma | 5 (71.4) | 2 (28.6) | 4 (57.2) | 3 (42.8) | |||
| Glioblastoma | 18 (69.2) | 8 (30.8) | 21 (80.8) | 5 (19.2) | |||
| Tumor Grade | High | 23 (69.7) | 10 (30.3) | 0.000* | 25 (75.8) | 8 (24.2) | 0.001* |
| Low | 1 (3.7) | 26 (96.3) | 2 (7.4) | 25 (92.6) | |||
| Individual Tumor Grade | Grade I | 0 (0 ) | 12 (100) | 0.000* | 1 (8.3) | 11 (91.7) | 0.000* |
| Grade II | 1 (6.7) | 14 (93.3) | 1 (6.7) | 14 (93.3) | |||
| Grade III | 5 (71.4) | 2 (28.6) | 4 (57) | 3 (43) | |||
| Grade IV | 18 (69.2) | 8 (30.8) | 21 (80.8) | 5 (19.2) | |||
Table 3.
The Relationship between LC3B and CD3 Expression in Studied Glioma Cases
| CD3/LC3B | Positive Expression (%) | Negative expression (%) | Total (%) | P value |
|---|---|---|---|---|
| Positive expression | 19 (70.4) | 8 (29.6) | 27 (45) | 0.000* |
| Negative expression | 5 (15.2) | 28 (84.8) | 33 (55) | |
| Total (%) | 24 (40) | 36 (60) | 60 (100) |
Figure 3.
Correlation between Number of CD3+ve T lymphocytes & LC3B Score
Discussion
Even with the combined aggressive treatment modalities, many of the patients with high-grade glioma experience an early recurrence. The addition of immunotherapy in cancer treatment has shown promising results in many tumors including glioma. However, the standardization of immunotherapy in all cases is not recommended due to autoimmune-like adverse reactions (Anderson, 2014).
The tumor-infiltrating lymphocytes have proved to be a major player in tumor development and progression, opening the way for introducing the immerging immunotherapies (Domingues et al., 2016). However, it is not yet established whether tumor-infiltrating immune lymphocytes exist in glioma and their role in tumor progression (Fan et al., 2020).
Autophagy is regarded as a basic physiological process that maintains homeostasis and survival during cellular adverse conditions. Autophagy usually starts by encapsulation of impaired organelles into an autophagosome, then followed by enzymatic destruction (Jiang et al., 2019).
In general terms, this study aims to detect a correlation between T lymphocyte infiltrate as evidence for immunosurveillance in different grades of gliomas and the phagocytic activity in malignant cells demonstrated by the presence of LC3B.
Regarding LC3B immunohistochemical expression in the present work; 24/60 (40%) of high-grade gliomas showed positive expression, while only 3.7% of low-grade cases showed this positivity with an achieved statistical significance (P value: 0.000). These figures support that high levels of LC3B expression, or promoted autophagy, correlated with tumor advanced grade and aggressiveness; consistent with the potential role of autophagy as tumor enhancer.
A study conducted by Li et al., (2017) concluded that during tumor progression, autophagy can help the cell to withstand the alterations in the tumor microenvironment, allowing it to withstand hypoxic injury, nutrient reduction, and other adverse conditions that normally lead to apoptosis.
In support of our results, Tamrakar et al., (2019) reported a statistically significant relationship between glioma WHO grade and LC3B expression (P-value: 0.004); most high-grade gliomas (74%) showed positive LC3B expression. Also, Jiang and Wu (2018) and Cj et al., (2018) were in concordance with us by showing a statistical difference with glioma grade and LC3B expression; (P values: 0.015 and 0.001 respectively), where LC3B expression was more in higher grade gliomas.
In contrast to our findings, Jennewein et al., (2016) reported that LC3B-positive staining did not significantly differ amongst gliomas of different WHO grades. Different samples’ size, the different LC3B antibody clones and the mechanism of immunostaining used in each study may play a role in these differences.
The expression of LC3B did not statistically differ with the clinic-pathological variables sex, tumor size, tumor site, and recurrence (P values: 0.172, 0.319, 0.158, and 0.480 respectively). However, the expression of LC3B showed a statistical difference with age (P value: 0.047).
Other studies were in harmony with our result that no significant relation was found between LC3B expression, sex of glioma patients, and site of the tumor. Our significant relation with age was not reported by them. Our unique result may be due to different age groups included in the study, we have a wide range of ages, ranging from 6 to 68 years. While most of the other studies showed minimum age of 20 years. (Huang et al., 2010; Winardi et al., 2014; Cj et al., 2018; Jiang and Wu 2018; Tamrakar et al., 2019).
Regarding LC3B expression and tumor recurrence, we did not find a statistical significance in this relation (P value: 0.480), Tamrakar et al., (2019) reached the same results (P value: 0.532).
Regarding glioblastomas in this study; 69.2% of cases showed positive LC3B expression, and this was in harmony with Giatromanolaki et al., (2014) who reported overexpression in most glioblastoma cases. Also, Aoki et al., (2008) studied 65 glioblastoma cases, all of them were LC3B positive with different intensity; 34 cases were weakly positive, and the other 31 cases were strongly positive.
Contrary to our results, Huang et al., (2010) reported that the expressions of LC3B in glioblastoma (GBM) was lower than in other grades of astrocytic tumors (P value: 0.030) due to a downregulated autophagic capacity. Consistent with these results, Winardi et al., (2014) also found that high-level expression of LC3B was associated with lower-grade gliomas; although their correlation did not reach statistical significance (P value: 0.563). These findings support the hypotheses that the autophagy process might have a suppressive role in the progression of gliomas.
Lymphocytes are evident in the stroma of different tumors. The prevalence of Tumor-infiltrating lymphocytes (TILs) is often linked with prolonged overall survival. Tumors harboring significant TILs may have special clinicopathologic features or associated genetic alterations, which could serve as a target for future therapies (Rutledge et al., 2013).
Although the brain has been classically defined as an “immune privileged” organ, recent studies suggest that the CNS actively interplay with the immune system and is regarded as a target for potential immunotherapy. Moreover, it can be considered as a secondary lymphoid organ due to the emerging discovery of lymphatic channels surrounding the brain (Huang et al., 2017).
In the current study, we assessed tumor-infiltrating T lymphocytes by immunohistochemistry, using CD3 antibody, and found that 27/60 cases showed positive CD3 expression (45%) and the rest of cases were negative (55%). These results showed a significant relationship with the patient’s sex, age, tumor histological variants and tumor grade; (P values: 0.035, 0.003, 0.001, and 0.001. respectively). While no significant relation was found with tumor recurrence, tumor size, and tumor site; (P values: 0.728, 0.544, and 0.109 respectively).
Many studies showed conformity with our results; Lohr et al., (2011) reported that infiltration of T lymphocytes was more in high-grade gliomas and mostly in glioblastoma. They also described that T lymphocytes were up to 10 times more in Glioblastoma world health organization (WHO) Grade IV, compared to gliomas WHO grade II and grade III with a significant difference. Also, a statistically significant difference between T lymphocytes and different grades of gliomas was found in a study done by Ben-Horin et al., (2017) that showed T lymphocytes in glioblastoma having a 15- fold higher level than grade II and grade III gliomas.
In agreement with these researches, Kmiecik et al., (2013) wrote a similar conclusion and Cejalvo et al., (2020) reported that T lymphocytes were concentrated more in glioblastomas than grade II and grade III gliomas (P value: <0.001).
Although glioblastoma ranked first in populating more T lymphocytes among gliomas in several studies, it showed the least population compared with lung, cutaneous, cervical, and gastric carcinomas (Castaneda, et al., 2019). They justified this by mentioning; the mutational alterations, and the existence of the brain-blood barrier (Castaneda, et al., 2019).
On the other hand, Robinson et al., (2020) declared different results; they found that low-grade gliomas were characterized by greater CD3 positive T-cell infiltration as well as CD3 positive T-cell density compared with high-grade gliomas. It’s worth mentioning that this study included pediatric patients only, showing different histological variants not included in our study as xanthoastrocytoma and ganglioglioma, hence we expected this discrepancy in results.
Not only tumor grade but also patient’s age and sex showed a significant relation with T lymphocyte infiltration in our study, and this was against the results of other studies where no significant relation with age and sex was reported (Han et al., 2014; Orrego et al., 2018). This discrepancy among studies may be due to the difference in sample size, age group or statistical methods to calculate the positivity and negativity of CD3 expression.
Regarding glioblastoma cases in this study, CD3 positive T cells were shown in 80.8% of cases, that result was not in harmony with Rutledge et al., (2013) findings; that was only 46% of cases showed tumor-infiltrating lymphocytes with no significant associations. Closer to our results, that was found by Berghoff and his colleagues in 2015, as CD3 positive T lymphocytes were found in 66.7% (78/117) of their glioblastoma cases. This incompatibility in results may be explained by the difference in samples size (Berghoff et al., 2015).
Autophagy may have an essential role in tumor immune response. It can enhance the recognition, release, and degradation of tumor surface antigens, by immune cells (Van Den Boorn et al., 2011; Bustos et al., 2020; Gerada et al., 2020; Poillet-Perez et al., 2020) Stimulation of autophagy can ultimately increase the antigen identification and activation of T lymphocytes (Wang et al., 2005).
At present, there are few studies on the correlations between LC3, T lymphocytes, and clinicopathological factors in patients with glioma. In our study, by analyzing the correlation between autophagic activity and the numbers of T lymphocytes in glioma tissues, we found that the expression of LC3B was positively correlated with the numbers of T lymphocytes, suggesting that changes in autophagy levels may be one of the factors that affect the cellular immune response in glioma.
By analyzing our results, there was a significant relation between CD3 & LC3B expression in all 60 cases (P value: 0.000). Also, the number of CD3 positive cells was directly proportionate to the score of LC3B positive cases (the more the score of LC3B, the more the number of CD3 positive T lymphocytes) with a significant relation (P value: 0.001).
In comparison, Zhang et al., (2018) agreed with us that the expression of LC3 in glioma tissues was positively correlated with T lymphocytes. The expression of LC3 and the numbers of T lymphocytes were also positively correlated with tumor grade. Moreover, they explained these findings by suggesting that the grade of glioma affects the local blood supply. The more advanced glioma grade; the higher the metabolic activity of its cells with relatively diminished tumor vascular supply, and the higher the autophagic activity. Autophagy, in turn, promotes the recognition of tumor antigen and enhances the cellular immune response.
LC3B expression has been studied in different malignancies to assess the relation of the autophagy process with the tumor microenvironment, where its activation has been linked with more populating tumor lymphocytic infiltrates as in breast carcinoma and squamous cell carcinoma of the hypopharynx (Ladoire et al., 2016; Gao et al., 2020).
In contrast to these findings, Noman et al., (2011), reported that the autophagy process can suppress immune-mediated mechanisms fighting against tumors, As the hypoxic injury stimulates autophagy in their studied lung cancer cells, which negatively affects T cell infiltrating lymphocytes. Also, Lazova et al., (2012) declared that no specific correlation could be detected betweenLC3B expression and TILs in his studied melanoma cases. May be different results due to different studied malignancies, as each tumor has its own, specific tumor microenvironment.
So autophagy can enhance the development of resistant tumor cells or in contrast cell death; promoting the introduction of both autophagy inhibitors and stimulators (Trejo-Solís et al., 2012).
To sum up, in the current study, LC3B expression in tumor cells and CD3 positive T lymphocytes were significantly higher in high-grade gliomas, supporting the role of LC3B as a potential biomarker in the development and progression of glioma and a marker of tumor aggressiveness. Moreover, it has an important role in modulating tumor microenvironment and immune response. So we think that there is a great chance for immunotherapy and autophagy-targeted therapy in gliomas, especially high-grade ones.
Author Contribution Statement
All authors contributed efficiently to the research and approved the manuscript. SMM and YFE shared in study design and sample collection. RAK and SMM shared in writing the manuscript. All authors shared in results analysis, interpretation, revising, and approving the final version of the manuscript.
Approval
This study was approved by the research committee of pathology department, faculty of medicine, Cairo University and REC committee.
Ethical Declaration
This study obtained the approval of the Kasr alainy research ethics committee (REC) that conducts according to ICH GCP standards and appropriate local and institutional regulations and guiding principles that govern REC operation.
Availability of Data
Data is available upon request according to the institutional regulations and with official permission
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Anderson AC. Tim-3: an emerging target in the cancer immunotherapy landscape. Cancer Immunol Res. 2014;2:393–8. doi: 10.1158/2326-6066.CIR-14-0039. [DOI] [PubMed] [Google Scholar]
- 2.Aoki H, Kondo Y, Aldape K, et al. Monitoring autophagy in glioblastoma with antibody against isoform B of human microtubule-associated protein 1 light chain 3. Autophagy. 2008;4:467–75. doi: 10.4161/auto.5668. [DOI] [PubMed] [Google Scholar]
- 3.Arani R, Mohammadpour H, Moosavi M, et al. The Role of Autophagy-related Proteins of Beclin-1/BECN1, LC3II, and p62/SQSTM1 in Melanoma Tumors. Asian Pac J Can Biol. 2021;4:263–72. [Google Scholar]
- 4.Barth , S , Glick D, Macleod KF. Autophagy: assays and artifacts. J Pathol. 2010;221:117–24. doi: 10.1002/path.2694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ben-Horin I, Shenkar A, Alter-Regev T, et al. Identification of subsets of tumor infiltrating lymphocytes in primary brain tumors using multi-color panel flow cytometry. Ann Oncol. 2017;28:xi25. [Google Scholar]
- 6.Berghoff AS, Kiesel B, Widhalm G, et al. Programmed death ligand 1 expression and tumor-infiltrating lymphocytes in glioblastoma. Neuro Oncol. 2015;17:1064–75. doi: 10.1093/neuonc/nou307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bustos SO, Antunes F, Rangel MC, Chammas R. Emerging Autophagy Functions Shape the Tumor Microenvironment and Play a Role in Cancer Progression-Implications for Cancer Therapy. Front Oncol. 2020;10:2549. doi: 10.3389/fonc.2020.606436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Castaneda CA, Castillo M, Aliaga K, et al. Level of tumor-infiltrating lymphocytes and density of infiltrating immune cells in different malignancies. Biomark Med. 2019;17:1481–91. doi: 10.2217/bmm-2019-0178. [DOI] [PubMed] [Google Scholar]
- 9.Cejalvo T, Gargini R, Segura-Collar B, et al. Immune Profiling of Gliomas Reveals a Connection with IDH1/2 Mutations, Tau Function and the Vascular Phenotype. Cancers. 2020;12:3230. doi: 10.3390/cancers12113230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cj P, Hv E, Vijayakurup V, et al. High LC3/Beclin Expression Correlates with Poor Survival in Glioma: a Definitive Role for Autophagy as Evidenced by In Vitro Autophagic Flux. Pathol Oncol Res. 2019;25:137–48. doi: 10.1007/s12253-017-0310-7. [DOI] [PubMed] [Google Scholar]
- 11.Domingues P, González-Tablas M, Otero Á, et al. Tumor infiltrating immune cells in gliomas and meningiomas. Brain Behav Immun. 2016;53:1–15. doi: 10.1016/j.bbi.2015.07.019. [DOI] [PubMed] [Google Scholar]
- 12.Fan W, Song Y, Ren Z, et al. Glioma cells are resistant to inflammationinduced alterations of mitochondrial dynamics. Int J Oncol. 2020;57:1293–306. doi: 10.3892/ijo.2020.5134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ferlay J, Colombet M, Soerjomataram I, et al. Global and Regional Estimates of the Incidence and Mortality for 38 Cancers: GLOBOCAN 2018. Lyon: International Agency for Research on Cancer/World Health Organization; 2018. [Google Scholar]
- 14.Gao S, Chen J, Han X, et al. LC3B in Malignant Cells Correlates With Immune Infiltrate in Hypopharyngeal Squamous Cell Carcinoma. Technol Cancer Res Treat. 2020;19:1533033820970664. doi: 10.1177/1533033820970664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gerada C, Ryan KM. Autophagy, the innate immune response and cancer. Mol Oncol. 2020;14:1913–29. doi: 10.1002/1878-0261.12774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Giatromanolaki A, Sivridis E, Mitrakas A, et al. Autophagy and lysosomal related protein expression patterns in human glioblastoma. Cancer Biol Ther. 2014;15:1468–78. doi: 10.4161/15384047.2014.955719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Han S, Zhang C, Li Q, et al. Tumour-infiltrating CD4+ and CD8+ lymphocytes as predictors of clinical outcome in glioma. Br J Cancer. 2014;110:2560–8. doi: 10.1038/bjc.2014.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.He H, Dang Y, Dai F, et al. Post-translational modifications of three members of the human MAP1LC3 family and detection of a novel type of modification for MAP1LC3B. J Biol Chem. 2003;278:29278–87. doi: 10.1074/jbc.M303800200. [DOI] [PubMed] [Google Scholar]
- 19.Hewedi I, Ibrahim R, Elserry T, et al. Frequency of primary central nervous system tumors in a tertiary hospital, Cairo, Egypt. J Community Health Manag. 2018;5:140–6. [Google Scholar]
- 20.Huang X, Bai HM, Chen L, Li B, Lu YC. Reduced expression of LC3B-II and Beclin 1 in glioblastoma multiforme indicates a down-regulated autophagic capacity that relates to the progression of astrocytic tumors. J Clin Neurosci. 2010;17:1515–9. doi: 10.1016/j.jocn.2010.03.051. [DOI] [PubMed] [Google Scholar]
- 21.Huang J, Liu F, Liu Z, et al. Immune checkpoint in glioblastoma: promising and challenging. Front Pharmacol. 2017;8:242. doi: 10.3389/fphar.2017.00242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jennewein L, Ronellenfitsch MW, Antonietti P, et al. Diagnostic and clinical relevance of the autophago-lysosomal network in human gliomas. Oncotarget. 2016;7:20016–32. doi: 10.18632/oncotarget.7910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jiang GM, Tan Y, Wang H, et al. The relationship between autophagy and the immune system and its applications for tumor immunotherapy. Mol Cancer. 2019;18:1–22. doi: 10.1186/s12943-019-0944-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jiang T, Wu Z. Immunohistochemical assessment of autophagic protein LC3B and p62 levels in glioma patients. Int J Clin Exp Pathol. 2018;11:862–8. [PMC free article] [PubMed] [Google Scholar]
- 25.Kmiecik J, Poli A, Brons NH, et al. Elevated CD3+ and CD8+ tumor-infiltrating immune cells correlate with prolonged survival in glioblastoma patients despite integrated immunosuppressive mechanisms in the tumor microenvironment and at the systemic level. J Neuroimmunol. 2013;264:71–83. doi: 10.1016/j.jneuroim.2013.08.013. [DOI] [PubMed] [Google Scholar]
- 26.Kohrt HE, Tumeh PC, Benson D, et al. Cancer Immunotherapy Trials Network (CITN) Immunodynamics: a cancer immunotherapy trials network review of immune monitoring in immuno-oncology clinical trials. J Immunother Cancer. 2016;4:1–15. doi: 10.1186/s40425-016-0118-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ladoire S, Enot D, Senovilla L, et al. The presence of LC3B puncta and HMGB1 expression in malignant cells correlate with the immune infiltrate in breast cancer. Autophagy. 2016;12:864–75. doi: 10.1080/15548627.2016.1154244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lazova R, Camp RL, Klump V, et al. Punctate LC3B expression is a common feature of solid tumors and associated with proliferation, metastasis, and poor outcome. Clin Cancer Res. 2012;18:370–9. doi: 10.1158/1078-0432.CCR-11-1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li CJ, Liao WT, Wu MY, Chu PY. New Insights into the Role of Autophagy in Tumor Immune Microenvironment. Int J Mol Sci. 2017;18:1566. doi: 10.3390/ijms18071566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lohr J, Ratliff T, Huppertz A, et al. Effector T-cell infiltration positively impacts survival of glioblastoma patients and is impaired by tumor-derived TGF-β. Clin Cancer Res. 2011;17:4296–308. doi: 10.1158/1078-0432.CCR-10-2557. [DOI] [PubMed] [Google Scholar]
- 31.Noman MZ, Janji B, Kaminska B, et al. Blocking hypoxia-induced autophagy in tumors restores cytotoxic T-cell activity and promotes regression. Cancer Res. 2011;71:5976–86. doi: 10.1158/0008-5472.CAN-11-1094. [DOI] [PubMed] [Google Scholar]
- 32.Orrego E, Castaneda CA, Castillo M, et al. Distribution of tumor-infiltrating immune cells in glioblastoma. CNS Oncol. 2018;7:CNS21. doi: 10.2217/cns-2017-0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Poillet-Perez L, Sharp DW, Yang Y, et al. Autophagy promotes growth of tumors with high mutational burden by inhibiting a T-cell immune response. Nat Cancer. 2020;1:923–34. doi: 10.1038/s43018-020-00110-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Robinson MH, Vasquez J, Kaushal A, et al. Subtype and grade-dependent spatial heterogeneity of T-cell infiltration in pediatric glioma. J Immunother Cancer. 2020;8:e001066. doi: 10.1136/jitc-2020-001066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rutledge WC, Kong J, Gao J, et al. Tumor-infiltrating lymphocytes in glioblastoma are associated with specific genomic alterations and related to transcriptional class. Clin Cancer Res. 2013;19:4951–60. doi: 10.1158/1078-0432.CCR-13-0551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schulte J, Yusifli K, Moosmann B, et al. Novel Insights into the Cellular Localization and Regulation of the Autophagosomal Proteins LC3A, LC3B and LC3C. Cells. 2020;10:2315. doi: 10.3390/cells9102315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tamrakar S, Yashiro M, Kawashima T, et al. Clinicopathological Significance of Autophagy-related Proteins and its Association With Genetic Alterations in Gliomas. Anticancer Res. 2019;39:1233–42. doi: 10.21873/anticanres.13233. [DOI] [PubMed] [Google Scholar]
- 38.Tanida I, Ueno T, Kominami E. LC3 and Autophagy. Methods Mol Biol. 2008;445:77–88. doi: 10.1007/978-1-59745-157-4_4. [DOI] [PubMed] [Google Scholar]
- 39.Trejo-Solís C, Jimenez-Farfan D, Rodriguez-Enriquez S, et al. Copper compound induces autophagy and apoptosis of glioma cells by reactive oxygen species and JNK activation. BMC Cancer. 2012;12:1–15. doi: 10.1186/1471-2407-12-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Van Den Boorn JG, Picavet DI, Van Swieten PF, et al. Skin-depigmenting agent monobenzone induces potent T-cell autoimmunity toward pigmented cells by tyrosinase haptenation and melanosome autophagy. J Invest. Dermatol. 2011;131:1240–51. doi: 10.1038/jid.2011.16. [DOI] [PubMed] [Google Scholar]
- 41.Vessoni AT, Filippi-Chiela EC, Menck CF, Lenz G. Autophagy and genomic integrity. Cell Death Differ. 2013;20:1444–54. doi: 10.1038/cdd.2013.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang LX, Li R, Yang G, et al. Interleukin-7-dependent expansion and persistence of melanoma-specific T cells in lymphodepleted mice lead to tumor regression and editing. Cancer Res. 2005;65:10569–77. doi: 10.1158/0008-5472.CAN-05-2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Winardi D, Tsai HP, Chai CY, et al. Correlation of altered expression of the autophagy marker LC3B with poor prognosis in astrocytoma. Biomed Res Int. 2014;2014:723176. doi: 10.1155/2014/723176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang W, Wu S, Guo K, et al. Correlation and clinical significance of LC3, CD68+ microglia, CD4+ T lymphocytes, and CD8+ T lymphocytes in gliomas. Clin Neurol Neurosurg. 2018;168:167–74. doi: 10.1016/j.clineuro.2018.02.044. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data is available upon request according to the institutional regulations and with official permission