Abstract
Objective:
The study was aimed at understanding the survival of metastatic ovarian cancer spheroids in the malignant ascites microenvironment.
Methods:
All the assays were performed using aseptically collected patient samples. The cells were characterized for the expression of ovarian and cancer stem cell markers using immunocytochemistry. The presence of lipid in the primary metastatic cancer spheroids were confirmed by neutral fat staining using Oil Red-O and transmission electron microscopy. The mRNA expression of autophagy and lipid metabolism genes was analyzed using RT-PCR. The lipid content was analyzed using lipidomics analysis. Etomoxir and chloroquine were used to study the effect of inhibition of autophagy in the metastatic cells. The data were analyzed using appropriate statistical tools and a p-value <0.05 was considered to be statistically significant.
Results:
Metastatic ovarian cancer spheroids exhibit cancer stem like properties and undergo a metabolic reprogramming when they disseminate from the primary tumor. We report here the accumulation of numerous cytoplasmic lipid droplets and lipophagic vesicles in the metastatic cells in contrast to their primary tumors. In addition we also report that these cells depend on lipophagy for the utilization of lipids rather than the conventional lipolytic pathway. The lipidomics analysis data reveals that the metastatic cells possess high levels of unsaturated fatty acids. We have also reported the occurrence of distinct accumulation of multiple nuclei in the patient derived metastatic cells. Inhibition of beta-oxidation and autophagic machinery using etomoxir and chloroquine resulted in cell death suggesting a potential mode to suppress metastatic cancer cells.
Conclusion:
Metabolic reprogramming is a characteristic feature of the metastatic ovarian cancer cells that are persisting in the malignant ascites. Targeting of the metastatic cells by gaining an insight into the various metabolic and molecular changes that occur in the metastatic niche provides a promising therapeutic approach in management of the disease.
Key Words: Metabolic reprogramming, lipid droplets- autophagy- lipophagy- CPT-1a- multinucleated cancer cells
Introduction
Ovarian carcinoma (OC) has the highest rate of mortality among all the gynecological malignancies and this is attributed to late stage diagnosis of the disease. Ovarian cancer metastasis occurs primarily through direct dissemination into the peritoneal cavity (Lengyel, 2010). About 89% of patients diagnosed at III/IV stage are known to present with malignant ascites. Malignant Ascites accumulation is one of the characteristic features during recurrence (Shen-Gunther et al., 2002). Ovarian cancer cells disseminate either as single cell or as multicellular aggregates called spheroids that are known to have a high tumorigenic and chemo resistant property (Latifi et al., 2012; Ahemd et al., 2013). The high tumorigenic potential is mainly because of a sub population of cancer stem-like cells (Zeimet et al., 2012). Several reports suggest that the spheroids are enriched with CSC characteristics and are known to contribute to tumorigenesis, metastasis and chemoresistance (Bozza et al., 2010; Nieman et al., 2011; Ahemd et al., 2013; Beloribi-Djefaflia et al., 2016).
Previous studies have reported that Omental adipocytes provide energy source for the proliferation and metastasis of ovarian cancer cell (Olzmann et al., 2018). However, during primary surgery the clinicians most often perform omentectomy along with total hysterectomy and salphingo-oopherctomy. This led us to investigate how these spheroids would survive in the peritoneal cavity to cause recurrence.
Metabolic reprogramming is emerging as a hallmark of cancer. Cancer cells are shown to switch to lipid metabolism by accumulating neutral lipids and cholesterol esters within lipid droplets (LD). High LD content is known to render cancer cells more aggressive (Olzmann et al., 2018). LDs are dynamic structures that not only function as storage organelle, but also mediate key cellular processes such as inflammation, membrane biogenesis and protection against oxidative stress during cancer progression (Bozza et al., 2010; Olzmann et al., 2018; Petan et al., 2018).
In most tissues, release of free fatty acids from LDs are achieved by the action of cytosolic lipases, adipose triglyceride lipase (ATGL) and hormone sensitive lipase (HSL) (Zechner et al., 2012; Zhang et al., 2017). As opposed to these conventional modes of lipolysis, we report the ATGL-HSL independent lipolysis in the patient derived metastatic ovarian spheroids. The spheroid cells rely on the autophagic pathway for utilizing these stored lipids by a process of lipophagy. Involvement of autopgahy in tumorigenesis of cancers has been extensively studied and autophagy. Studies have also reported the role of autophagy in the metastatic niche (Ferraresi et al., 2020; Lim et al., 2021; Arani et al., 2021).
In this study, we report the occurrence of a metabolic reprogramming that occurs in the metastatic cancer cells when they detach from their primary site. We show that LDs are present only in the patient derived metastatic spheroids and not in their primary tissue counterpart and that the spheroids resort to lipophagy for LD breakdown. Additionally we also report the presence of distinct multinucleation in the metastatic spheroids which could contribute to tumor heterogeneity and repopulating the tumor post surgery and chemotherapy.
Materials and Methods
Reagents
DMEM medium for cell culture was obtained from Gibco (11965092). Oil red-O (O1391), Etomoxir sodium salt (E1905), Chloroquine Diphosphate salt (C6628) were obtained from Sigma Aldrich. CA-125 (CM 101 AK) and Pax8 (ACI 438 A) were obtained from Biocare Medical, CD133 (MA1-219) and CD44 (MA5-15462), E-Cadherin (33-4000), Bodipy493/503, DAPI, and conjugated Alexafluor secondary antibodies were obtained from Invitrogen, USA. Map LC3β (SC-376404) antibody was obtained from Santa Cruz Biotechnologies.
Patient samples
Primary ovarian tumor tissue and malignant ascites were obtained after the approval from the institutional scientific review board and medical ethics committee of Kidwai Memorial Institute of Oncology, Bangalore, India. Informed consent was obtained from all patients prior to sample collection.
Tumor tissues were obtained from patients undergoing primary cytoreductive surgery. The specimens were examined and confirmed histopathologically before including in the study.
Malignant ascites was collected aseptically from OC patients via peritoneal paracentesis. OC spheroids were separated using a 40μm cell strainer (Davidowitz et al., 2014). The spheroids were aliquoted and used for the experiments described below. The cell free ascites was preserved for culturing of the primary OC spheroids.
Establishment of primary cultures from patient spheroids
OC spheroids isolated from ascites were cultured in cell free malignant ascites of the respective patient and/ or in DMEM medium in a 1:1 ratio in normal tissue culture plates and allowed to adhere. The medium was replenished every alternate day. Once confluent the cells were sub cultured and seeded into fresh culture plates.
Immunocytochemistry
The metastatic patient spheroids were fixed in 4% paraformaldehyde in PBS (pH 7.4) and permeabilized using 0.1% Triton-X100 for 15 min at room temperature. The cells were then washed 3 times with PBS and incubated with the blocking solution (1% BSA in PBS) for 1 h at room temperature. The cells were then incubated with the primary antibodies (CA125, PAX8, CD133, LC3-1:100,CD44,E-cadherin-1:200) overnight at 4OC. Post incubation, the cells were washed thrice in PBS and incubated with fluorescent conjugated secondary antibody (1:500 dilutions) for 1 hour in dark. The cells are washed with PBS and stained with fluorophore conjugated phalloidin for 15 mins and washed with PBS. The cells were further counterstained with DAPI (1μg/ml) for 10 mins and rinsed with PBS. The cover slips were mounted with a drop of mounting media and imaged under a confocal microscope.
Staining for neutral fats
The native spheroids were fixed in 4% paraformaldehyde and stained with Oil red-O and Bodipy as described previously (Andersson et al., 2006; Singh et al., 2009).
Transmission electron microscopy:
The isolated spheroids and tumor tissues were fixed in 3% glutaraldehyde in phosphate buffer (pH 7.2). The samples were subsequently washed in phosphate buffer and post-fixed in 1% osmium tetroxide for 1 hr at room temperature followed by dehydration in 70%, 80%, 90% ethyl alcohol for 1 hr and 100% ethanol for 30 min twice, cleared in propylene oxide and embedded in Araldite CY212 resin followed by polymerization at 60O C for 48 h. The blocks were cut on Leica EM UC6 ultra microtome (M/ S Leica Mikrosysteme, Austria). Initially 1 μm thick sections collected on plane glass slide were stained using 1% toluidine blue and viewed under light microscope. Subsequently 400-500 A0 thick ultrathin sections collected on copper grids were stained using uranyl acetate and lead citrate as described by Frasca and Parks (1965). After staining, the ultrathin sections were scanned under JEM-1400 plus Transmission Electron Microscope at 80 KV and representative areas were photographed using Gatan CCD camera.
Gene expression analysis
RNA was isolated from primary tumor tissues and OC spheroids using TRIzol (Invitrogen). 1μg of RNA was reverse transcribed into cDNA using High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems) and gene expression levels were quantified using Power Up SYBR green master mix (Applied Biosystems) on the Stepone plus Real Time - PCR. Primer sequences are provided in the table-S3. Amplification for each sample was performed in triplicates and mRNA levels were normalized to18s rRNA.
Lipidomic analysis
Spheroids were isolated from malignant ascites as described above and used for lipidomic analysis. Lipids were extracted following the method of Bligh and Dyer (1959). The samples were analyzed on UPLC-MS/MS (Thermo Scientific LTQ Orbitrap XL/ Q-Exactive) using a C30 column.
Inhibition of FAO and autophagy
Spheroids were seeded into a 48 well plate in cell free malignant ascites to mimic the in vivo conditions during drug treatment. The concentration of drug doses used were higher than the doses that are reported for monolayer cultures since the IC50 value of drugs for multicellular spheroids are higher compared to monolayer (Nicholson et al., 1997. Jia et al., 2018). The spheroids were treated with varying concentrations of Etomoxir (100uM to 750uM) and chloroquine (50uM to 200uM) individually and in combination for 48hours to inhibit FAO and autophagy respectively. Post incubation with drug the cells were observed and imaged in Motic inverted microscope. To assess for the loss of viability after drug treatment these cells were re-plated into drug free medium and observed for attachment and proliferation.
Statistical Analysis
The data was analyzed using a two-tailed t-test and a p-value <0.05 was considered statistically significant.
Results
Metastatic spheroids possess cancer stem cell-like phenotype
All the experiments were performed using primary metastatic ovarian cancer spheroids isolated from malignant ascites.
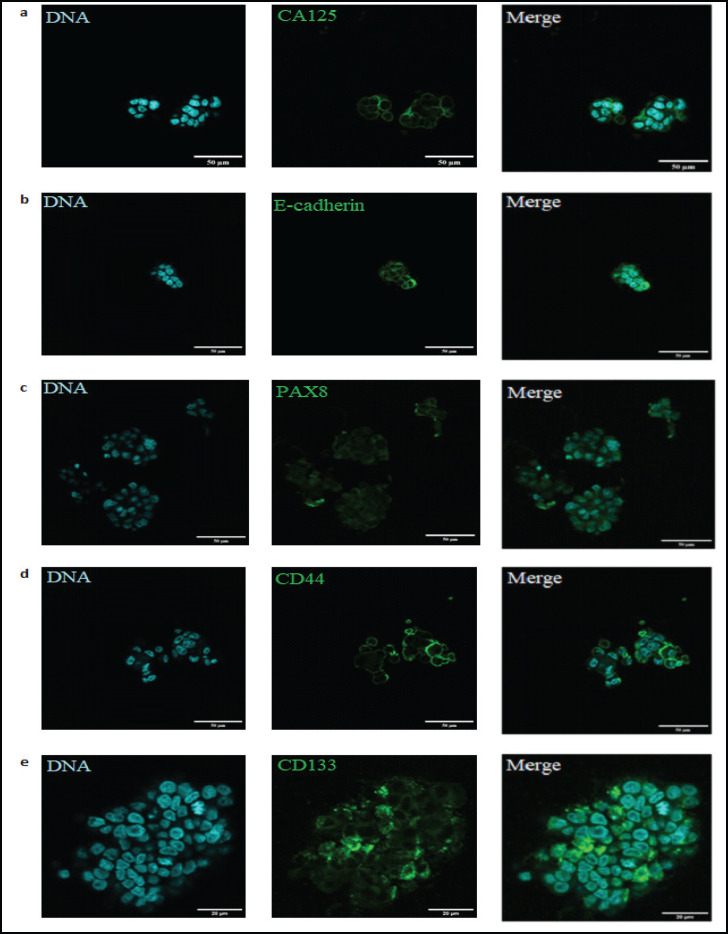
The isolated metastatic spheroids were immediately fixed in paraformaldehyde and assessed for ovarian cancer and stem cell markers such as CA125, PAX8, E-cadherin, CD133 and CD44 by immunocytochemistry. The spheroids showed positive expression of the markers (Figure 1a-e), in all the samples analyzed, confirming their origin and indicating the properties of cancer stem-like phenotype.
Figure 1.
Expression and Immunolocalization of Cancer and Stem Cell Markers in Patient Derived Metastatic OC Spheroids. Metastatic OC Spheroids showing positive expression of CA125 (a); E-cadherin (b); Pax8(c); CD44 (d) and CD133 (e)
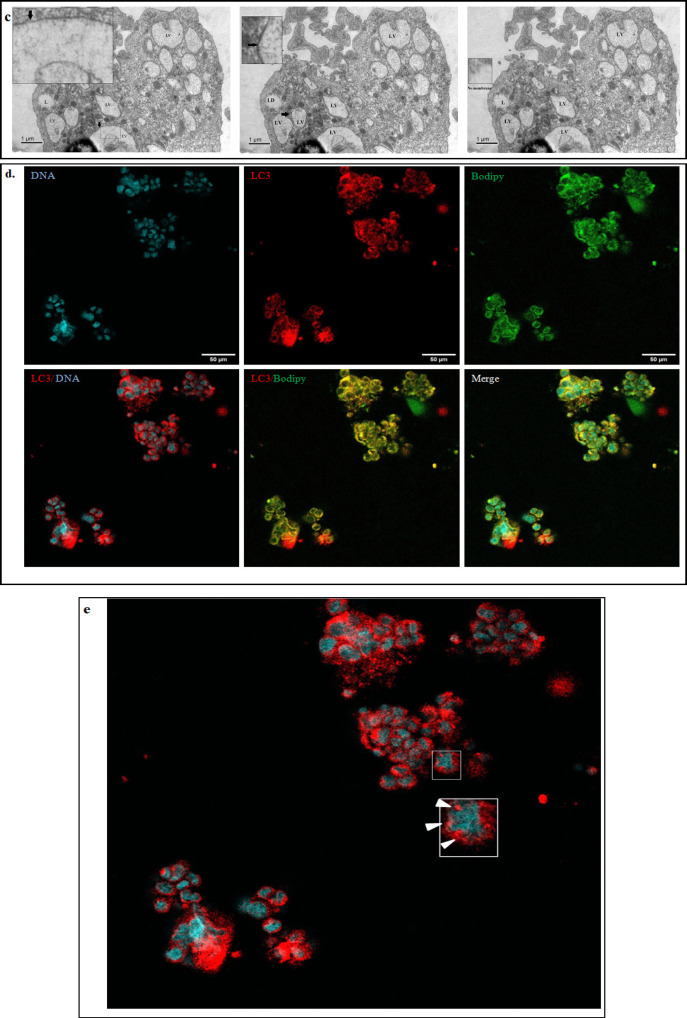
Distinct multinucleation in primary spheroids
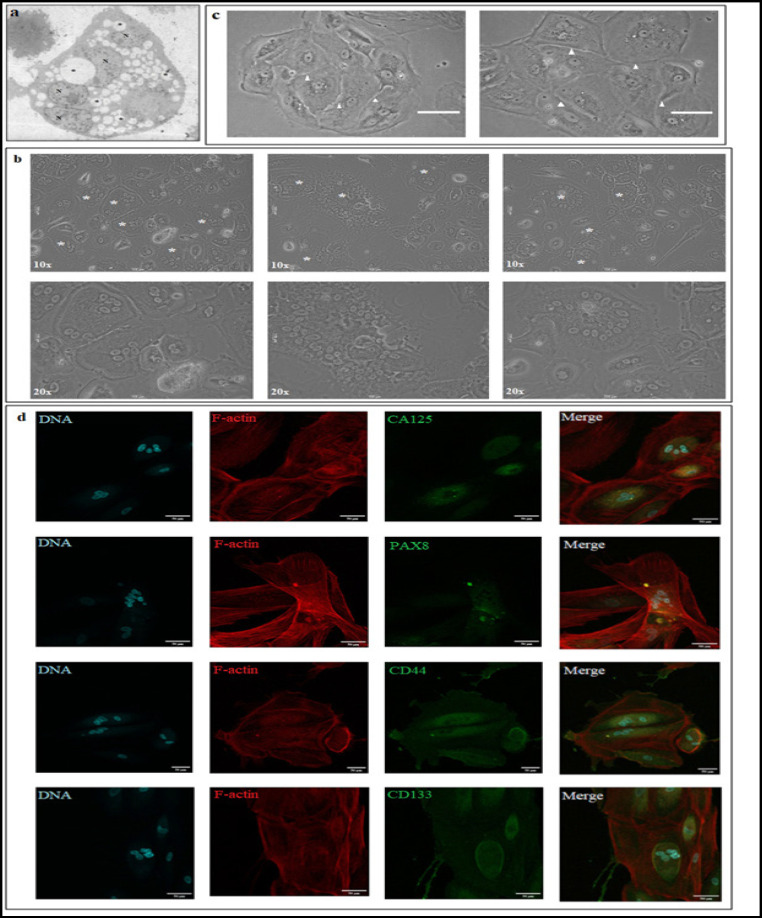
Electron micrographs of primary patient derived metastatic spheroid isolated from malignant ascites showed the presence of multiple nuclei within them (Figure2 a). To ascertain whether these multinucleation was confined to these anchorage independent spheroids or if they continued to exist after the cells attach we attempted to culture them on standard tissue culture flask using two conditions- (i) cultured in patient matched ascites and (ii) in DMEM medium supplemented with 10% FBS. During the course of culturing these cells, we observed that the spheroids remain non adherent initially but eventually begin to attach to the culture dish. Once attached to the culture dish a few Multinucleated cells (MNC) were observed and the number of these cells got enriched overtime. The phase contrast images observed show that the number of nuclei within the cell varied from 3 to over 40 per cell (Figure 2b).
Figure 2.
Multinucleation in Primary OC Spheroids. (a)Electron micrograph of showing multinucleated cell in patient derived spheroid. (*: LD,N: NUCLEUS) Scale: 10μm. (b) Phase contrast micrograph of multinucleated cells derived from metastatic OC spheroids. [* represent multinucleated cells; upper panel represents images acquired in 10x. magnification and lower panel images acquired in 20x magnification] Scale:100μm. (c) Asymmetric cell division with septum –like formation in multinucleated cells (white arrow heads show the septum-like structure that forms during asymmetric division). (d) Expression and immunolocalization of cancer and stem cell markers in MNCs. Scale: 50μm
We also noticed that the division of these cells were slow in both patient matched ascites and in DMEM medium supplemented with 10%FBS. In addition we also noticed that these cells do not divide in the normal symmetrical fashion but follow an asymmetrical cell division. These cells tend to accumulate multiple nuclei within them and divide at once asymmetrically forming a septum like pattern (Figure 2c). This is indicative that the cells might undergo a successful karyokinesis but with delayed cytokinesis, the mechanism of which still remains elusive. Interestingly these MNCs were found to be present in both the primary tumor tissue culture and circulating metastatic spheroids.
Immunocytochemistry data of MNCs revealed an altered localization of cancer and stem cell markers CA125, PAX8, CD133 and CD44. The cancer marker CA125 was found to be localized in the cytoplasm instead of the membrane, PAX 8 was also localized to the cytoplasm instead of the nucleus. The MNCs showed a positive staining for stem cell markers- CD133 and CD44 but with a cytoplasmic localization. (Figure 2d). This data suggests that these cells could be the source for tumor heterogeneity and contribute to stemness properties of the tumor which are responsible for repopulating the tumor during recurrence.
Metabolic reprogramming in circulating OC spheroids
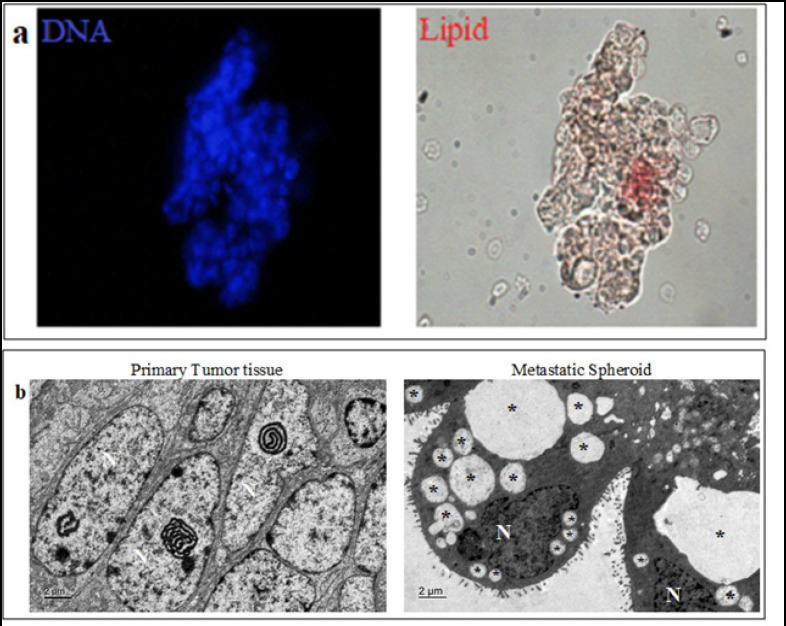
Metastatic spheroids present in the malignant ascites survive post surgery and chemotherapy. We wanted to decipher what drives the survival of these cells within the peritoneal cavity. Since the cancer spheroids showed stem-like phenotype, we speculated that these cells could posses an increased lipid content (a property of stem cells) within them which could aid in their survival. In order to check for the presence of lipids, we stained the spheroids with Oil Red-O. The spheroids stained positive for Oil Red-O confirming the presence of neutral fats (Figure 3a).
Figure 3.
Metabolic Reprogramming and LD Accumulation in Metastatic OC Spheroids. Presence of neutral lipids shown by staining with Oil red-O. (b) Transmission electron micrograph of primary tumor tissue and metastatic spheroid shows that LDs are accumulated only in the metastatic spheroids. (*LDs; N: nucleus)
Ultra structure analysis of patient derived circulating spheroids and corresponding primary tumor tissues were performed using electron microscopy. Interestingly, primary tumor tissues did not show the presence of LDs, but circulating cancer spheroids revealed the presence of numerous LDs within them (Figure 3b). This clearly indicates that a metabolic reprogramming occurs in the cancer cells that disseminate from the primary tumor site during metastasis. This reprogramming could confer survival advantages to the circulating cancer spheroids in their metastatic journey.
Lipidomic analysis reveals accumulation of UFAs
The Lipidomics data reveals that the circulating spheroids have high TG levels compared to MG and DG in all the samples analyzed. A total of 9 MG, 19 DG, 117 TG and 7 cholesterol ester species were identified in the samples.(Supplementary Table S1and S2)
Eicosadienoic acid (MG 20:2), 1-stearoyl-2-linoleoyl-sn-glycerol (DG 18:0/18:2), 1-stearoyl-2-arachidonoyl-sn-glycerol (DG 18:0/20:4) was found to be high in 4 out of five samples analyzed. Out of the 117 species of triglycerides, 1-hexadecanoyl-2,3-di-(9Z-octadecenoyl)-sn-glycerol (TG 16:0/18:1/18:1), 1-hexadecanoyl-2-(9Z-octadecenoyl)-3-(9Z,12Z-octadecadienoyl)-sn-glycerol (TG 16:0/18:1/18:2), 1,2-di-(9Z-octadecenoyl)-3-(9Z,12Z-octadecadienoyl)-sn-glycerol (TG 18:1/18:1/18:1) was found to be present in highest amounts in all the five samples. The functions of many of these fatty acid species in promoting tumorigenesis still remain unclear.
The data analysis also showed the presence of as many as 125 unsaturated fatty acid (UFA) species in a total of 145 neutral lipid species analyzed, a majority of them being PUFAs. This data suggests that these UFAs might be playing a crucial role in aiding survival and in contributing to the stemness of the cancer spheroids.
The purpose of storing UFAs within the cancer spheroids is not fully understood. However, these stored lipids could aid the metastatic cells in a variety of functional roles such as; energy demands, inflammation and membrane biogenesis during invasion into secondary site.
Increased fatty acid oxidation in spheroids
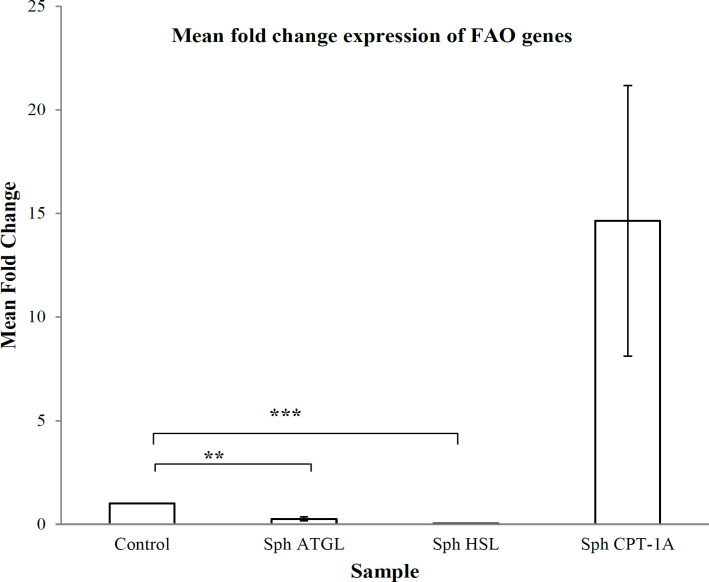
ATGL and HSL are two important cytosolic lipases that are involved in the metabolism and release of free fatty acids from lipid reserves. The free fatty acids that are released, feed into the beta oxidation pathway for energy. We therefore assessed the mRNA expression of these cytosolic lipases and the rate limiting key enzyme of the beta oxidation pathway, CPT-1a in the patient derived spheroids. Contrary to the existing understanding, ATGL and HSL were down regulated but, CPT-1a was up regulated (Figure 4) in the spheroids in comparison to the corresponding tumor tissues used as control. This suggests that the FAO pathway is active in the metastatic spheroids but the release of free fatty acids from the LDs happen in an ATGL-HSL independent mechanism in the spheroids.
Figure 4.
mRNA Expression Profile of Lipolysis and FAO Genes in Metastatic Spheroids. mRNA expression of Lipolysis genes ATGL, HSL and FAO gene CPT1a. mRNA fold change from three individual samples were performed, error bar indicates the SEM of the three spheroid samples. p-value <0.05 is considered statistically significant. (*p<0.05;**p<0.01)
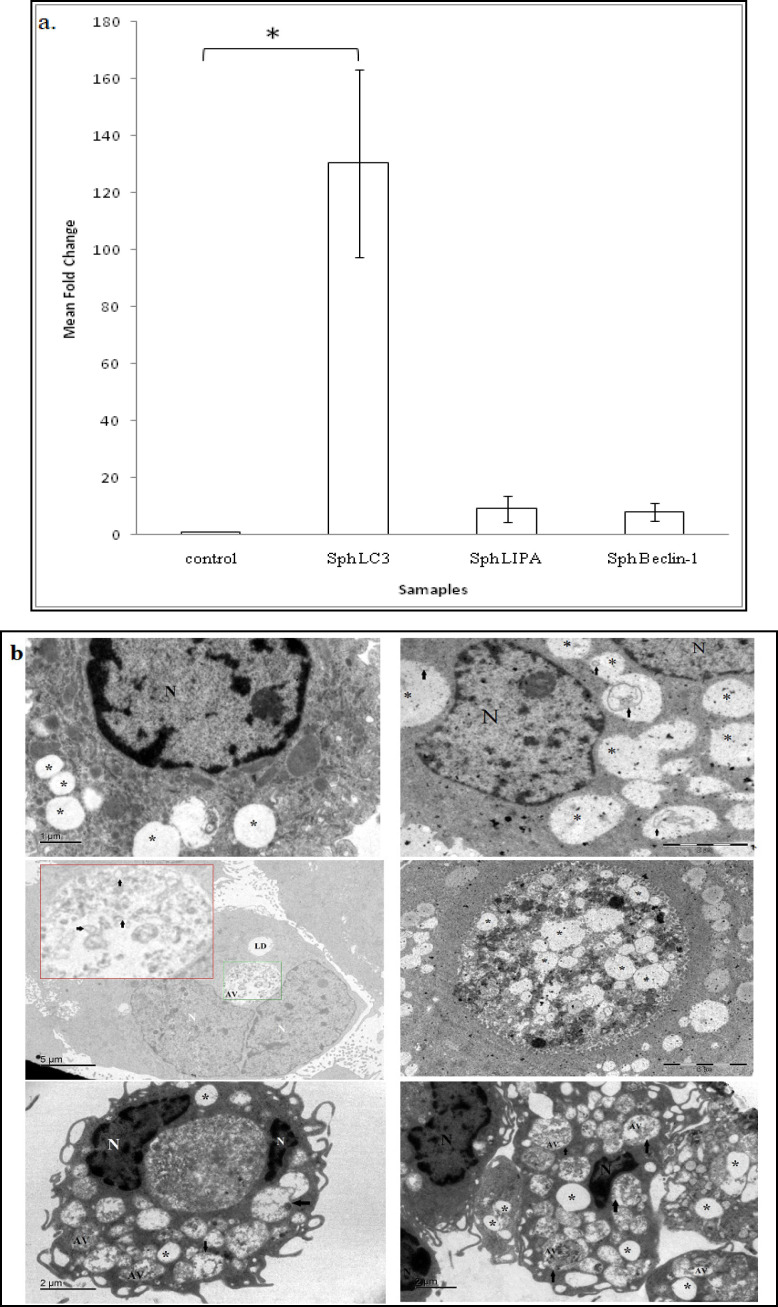
Lipophagy mediates the breakdown of LDs
During non-adherent/ anchorage independent conditions, the cancer cells are known to utilize the autophagic machinery for survival. Therefore, we assessed the mRNA expression of autophagic genes- LC3 and Beclin-1 and in addition to lysosomal acid lipase (LIPA) which mediates the lipolytic process within the phagolysosome. We observed an increased expression of these genes in the metastatic spheroids (Figure 5a). This data was further supported and confirmed by the presence of autophagic and lipophagic vesicles in the electron micrographs of the patient derived metastatic spheroids. The lipophagic vesicle can be clearly distinguished from lipid droplets by the presence of a distinct membrane in the former. The presence of lipophagic vesicles are shown in the Figure 5b and c.
Figure 5.
Involvement of Autophagy in Mediating Lipid Breakdown in Metastatic Spheroids. (c) Lipophagic vesicles can be identified and distinguished by a membrane that surrounds the lipid droplet. LD contains no membrane (Arrow indicates the membrane). (d) Co localization of LC3 and Bodipy was observed in ovarian cancer spheroids. (e) Image depicting expression of LC3 puncta in the metastatic spheroids (white arrow head indicates the puncta).LD, Lipid droplet; AV, autophagic vesicle; LV, lipophagic vesicle; N, nucleus
In order to further confirm that autophagy is involved in lipolysis of LDs, co-localization studies were performed using confocal microscopy. The spheroids were stained for neutral lipids and autophagic marker using Bodipy and LC3 respectively. All the samples analyzed showed positive for both Bodipy and LC3 and revealed a clear co-localization (Figure 5d and e), suggesting the role of lipophagy in mediating the release of free fatty acids from the LDs.
Targeting circulating spheroids via inhibition of FAO and Autophagy
To understand the effect of inhibitions of FAO and autophagy in circulating spheroids, the spheroids were treated with Etomoxir (irreversible inhibitor of CPT-1a) and Chloroquine respectively.
The spheroids were treated with these drugs both individually and in combination for 48 hours and morphological changes were observed and captured using phase contrast microscope.
At higher concentration of Etomoxir and chloroquine (individually and in combination), there was a disintegration in the spheroid structure in two patient samples and a complete dark appearance of spheroids in one patient sample (Figure 6 and S1). To confirm that these cells were no longer viable, the cells were re-plated into culture dish and allowed to recover in drug free medium and observed. These cells failed to adhere and proliferate confirming the loss of viability. This indicates that inhibition of FAO and autophagy had a detrimental effect on the circulating metastatic spheroid.
Figure 6.
Inhibition of FAO and Autophagy Leads to Loss of Viability and Survival of Metastatic Spheroids. Representative image showing the effect of inhibition of FAO and autophagy using etomoxir and chloroquine leading to loss of viability of the metastatic spheroids
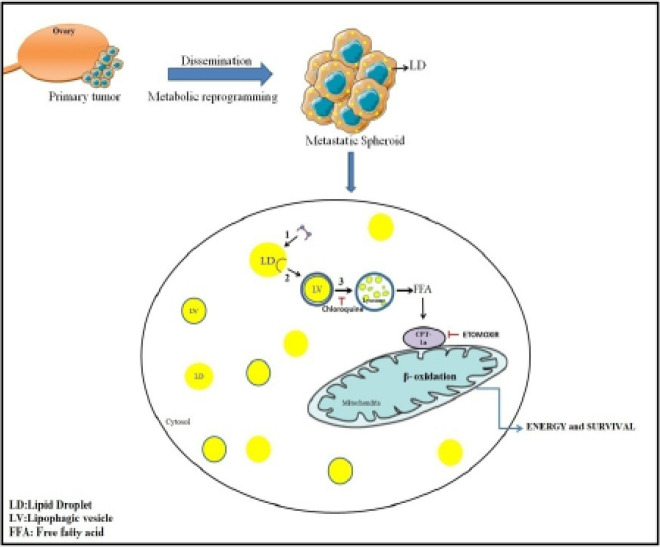
Graphical Abstract
Discussion
The major challenge in the treatment and management of ovarian cancer is the recurrence of the disease. The five- year survival rate of ovarian cancer patients diagnosed at stage III/IV is less that 30% (Kumar et al., 2019).
During primary cytoreductive surgery, clinicians are able to remove only bulky tumors not the micro metastatic cells as they are invisible to clinician’s eyes. Micro metastatic spheroids are known to be chemo resistant, dormant, and non-adherent and have stem-cell properties. The cells within the spheroids have a reduced proliferation rate and thereby escape from the effect of chemotherapeutic drugs (Peart et al., 2015).
Extensive research is being done to understand why >80% of OC patients recur back with the disease. Current therapies kills the cells in the bulk of the tumor, but the CSC remain unaffected. Previous studies have reported that a population of CSC which exists within the OC population that are potent enough to resist chemotherapy and continue to cause disease progression (Bapat et al., 2005; Zhang et al., 2008; Mor et al., 2011; Latifi et al., 2012). We have reported a novel occurrence of multinucleation and unique cell division patterns that arise from metastatic patient derived OC spheroids. These cells were CD44+, CD133+ confirming the presence of stem like cells. Weihua et al., (2011) have reported that these cells are highly tumorigenic, divide asymmetrically and also possess the ability to self renew. It has also been reported that a single MNC has the potential of initiating tumors in vivo (Weihua et al., 2011). Studies have reported that the expression of CD44 and CD133 has been implicated in the regulation of spheroid formation and organ specific metastasis by ovarian cancer cells (Suarez et al., 2019; Peickert et al., 2012). We have observed a cytoplasmic localization of PAX8 and CD133 in the MNCs. Several studies have reported that the cytoplasmic expression of PAX8 and CD133 is associated with greater risk of recurrence and is implicated with poor prognosis and overall survival (Scouten, et al., 2004; Sasaki et al., 2010; Huang et al., 2015; Chen et al., 2017).
Though the occurrence of MNC has been reported in several cancers, there are not many reports which have established an understanding of their phenotype and cell division patterns (Ariizumi et al., 2009; Díaz-Carballo et al., 2018; Mirzayans et al., 2018). Ariizumi et al., 2009) have reported that the myxofibrosarcoma MNCs take about 44-455 hours to double, which is similar to our observations in OC MNCs. This slow division pattern confers a survival advantage to these cells during chemotherapy.
Díaz-Carballo et al., (2018) have reported that MNCs occur within chemotherapy refractory OC, but we have observed this phenomenon within both the chemo naïve and resistant samples. The group also reported that the cells divide by budding, but such a pattern was not observed in our data, instead we have observed an asymmetric cell division pattern.
We speculate that, since these cells have multiple nuclei even if chemotherapy damages DNA within few nuclei, the other nuclei that are present could rescue and continue to repopulate within the cancer site. Also, since these cells carry multiple nuclei, they could have the ability to colonize more aggressively when they reach the secondary site and could contribute to tumor heterogeneity.
Neiman et al., (2011) have reported that omental adipocytes fuel OC cells during metastasis. But our data suggest that OC Spheroids might be capable of making their own lipid bodies even in the absence of omentum. We report here for the first time that the primary tumors lack LDs whereas the metastatic OC spheroids that have detached from the primary site have increased LD content within them. This clearly indicates that there is a metabolic reprogramming that occurs when the cells begins to metastasize.
Recent studies have revealed that LDs are known to have diverse functions within cancer cells. Tirinato et al., (2015), have observed that CR-CSC with high LD content are more tumorigenic. Li et.al.,(2017), have reported that lipid desaturation could serve as a metabolic marker of OC-CSC. We have also seen the presence of UFA, specifically, more PUFAs within the patient derived spheroids. The purpose of storing UFA within the cancer spheroids is not fully understood. However, these stored lipids could aid the metastatic cells in a variety of functional roles such as; energy demands, inflammation and membrane biogenesis during invasion into secondary site. We have observed a higher content of Eicosadienoic acid which is known to aid the production of pro-inflammatory modulators and these inflammatory signatures are attributed to contribute to cancer cell aggregation, adhesion and metastasis (Geng et al., 2013; Geck et al., 2018). SAG (1-stearoyl-2-arachidonoyl-sn glycerol) in addition to efficiently activating Protein kinase- C is also known to bind to Ras-GRP and modulate MAPKinase activation (Madani et al., 2001; Madani et al 2004).
Recent studies have reported that CPT-1a, the rate limiting enzyme of FAO is upregulated in cancers and contributes to anoiksis resistance, cell cycle deregulation and poor survival in ovarian cancer (Shao et al., 2016; Sawyer et al., 2020).
Our report is also in concordance with these finding that CPT1a is upregulated in cells in matrix deprived condition (Wang et al., 2018).
Contrary to the conventional mechanism of lipolysis that occurs with the aid of ATGL and HSL, LDs within OC spheroids are metabolized via lipophagy. Autophagy is known to have a role in both cancer cell survival and destruction. As a survival mechanism, autophagy aids cancer cells to withstand and survive in stressful conditions (White et al., 2015; Bingel et al., 2017). Studies have shown that autophagic proteins are known to play important role in OC dissemination and areindispensible for self renewal and quiescence of OC stem like cells. Recent reports have shown that autophagy also has a role in metabolizing lipids stored in cancer cells (Singh et al., 2009; Roy et al., 2017; Mondal et al., 2019). Similar to these finding we show here that autophagy and FAO might be aiding ovarian cancer spheroids to survive in non adherent condition and could also aid in metabolizing the lipids stored in LDs via lipophagy. Inhibition of autophagy and FAO with Chloroquine and etomoxir caused a disruption of spheroid structure and cell death.
Metabolic reprogramming is a quintessential property for metastasis of OC spheroids. Therefore, targeting lipid metabolism and autophagy could be used as a potential therapeutic strategy to suppress metastasis of OC.
Author Contribution Statement
SKS conceptualized the work. SKS, SNS, RG designed the methodology, SKS and SNS performed the data curation, formal analysis and investigation. SKS, SNS, RG, VRD performed the validation. PCS, PVR, BKCS, performed clinical and pathological examination. SKS, DDS performed lipidomic analysis. SKS, SNS, VRD, RG wrote the manuscript.
Acknowledgements
The authors thank Dr.Ramray Bhat, Molecular Reproduction, Development and Genetics, IISc, Bangalore, India, for extending valuable suggestions.
Funding statement
SKS is the recipient of senior research fellowship from Council for to Of (Council of scientific and Industrial research) and Industrial Research [award no. 09/999/0002/2016-EMR-I (2017-2019)]. RG is the recipient of research grant from Rajiv Gandhi University of Health Sciences, Bangalore for the Research grant (Grant Number: RI004, 2016-2018).
Ethics approval
The study was approved by the institutional scientific review board and medical ethics committee of Kidwai Memorial Institute of Oncology, Bangalore (No:KMIO/MEC/003/30.April.2016).
Conflict of Interest
The authors declare no conflict of interest.
References
- 1.Ahmed N, Stenvers K. Getting to know ovarian cancer ascites: opportunities for targeted therapy-based translational research. Front Oncol. 2013;3:256. doi: 10.3389/fonc.2013.00256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andersson L, Boström P, Ericson J, et al. PLD1 and ERK2 regulate cytosolic lipid droplet formation. J Cell Sci. 2006;119:2246–57. doi: 10.1242/jcs.02941. [DOI] [PubMed] [Google Scholar]
- 3.Arani RH, Mohammadpour H, Moosavi MA, et al. The Role of Autophagy-related Proteins of Beclin-1/BECN1, LC3II, and p62/SQSTM1 in Melanoma Tumors. Asian Pac J Cancer Biol. 2021;6:263–72. [Google Scholar]
- 4.Ariizumi T, Ogose A, Kawashima H, et al. Multinucleation followed by an acytokinetic cell division in myxofibrosarcoma with giant cell proliferation. J Exp Clin Cancer Res. 2009;28:44. doi: 10.1186/1756-9966-28-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bapat SA, Mali AM, Koppikar CB, Kurrey NK. Stem and progenitor-like cells contribute to the aggressive behavior of human epithelial ovarian cancer. Cancer Res. 2005;65:3025–9. doi: 10.1158/0008-5472.CAN-04-3931. [DOI] [PubMed] [Google Scholar]
- 6.Bingel C, Koeneke E, Ridinger J, et al. Three-dimensional tumor cell growth stimulates autophagic flux and recapitulates chemotherapy resistance. Cell Death Dis. 2017;8:e3013. doi: 10.1038/cddis.2017.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beloribi-Djefaflia S, Vasseur S, Guillaumond F. Lipid metabolic reprogramming in cancer cells. Oncogenesis. 2016;5:e189. doi: 10.1038/oncsis.2015.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–7. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 9.Bozza PT, Viola JP. Lipid droplets in inflammation and cancer. Prostaglandins Leukot Essent Fatty Acids. 2010;82:243–50. doi: 10.1016/j.plefa.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 10.Chen YL, Lin PY, Ming YZ, et al. The effects of the location of cancer stem cell marker CD133 on the prognosis of hepatocellular carcinoma patients. BMC Cancer. 2017;17:474. doi: 10.1186/s12885-017-3460-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davidowitz RA, SelforsLM , Iwanicki MP, et al. Mesenchymal gene program–expressing ovarian cancer spheroids exhibit enhanced mesothelial clearance. J Clin Invest. 2014;124:2611–25. doi: 10.1172/JCI69815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Díaz-Carballo D, Saka S, Klein J, et al. A distinct oncogenerative multinucleated cancer cell serves as a source of stemness and tumor heterogeneity. Cancer Res. 2018;78:2318–31. doi: 10.1158/0008-5472.CAN-17-1861. [DOI] [PubMed] [Google Scholar]
- 13.Ferraresi A, Girone C, Esposito A, et al. How Autophagy Shapes the Tumor Microenvironment in Ovarian Cancer. Front Oncol. 2020;10:599915. doi: 10.3389/fonc.2020.599915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Frasca JM, Parks VR. A routine technique for double-staining ultrathin sections using uranyl and lead salts. J Cell Biol. 1965;25:157. doi: 10.1083/jcb.25.1.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Geck P, Viktoria D, Balint T, et al. Analysis of inflammatory signatures of cancer spheroids in blood and their role in metastasis. J Clin Oncol. 2018;36:3090. [Google Scholar]
- 16.Geng Y, Chandrasekaran S, Hsu JW, et al. Phenotypic switch in blood: effects of pro-inflammatory cytokines on breast cancer cell aggregation and adhesion. PLoS One. 2013;8:e54959. doi: 10.1371/journal.pone.0054959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang M, Zhu H, Feng J, Ni S, Huang J. High CD133 expression in the nucleus and cytoplasm predicts poor prognosis in non-small cell lung cancer. Dis Markers. 2015;2015:986095. doi: 10.1155/2015/986095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jia B, Xue Y, Yan X, et al. Autophagy inhibitor chloroquine induces apoptosis of cholangiocarcinoma cells via endoplasmic reticulum stress. Oncol Lett. 2018;16:3509–16. doi: 10.3892/ol.2018.9131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Latifi A, Luwor RB, Bilandzic M, et al. Isolation and characterization of tumor cells from the ascites of ovarian cancer patients: molecular phenotype of chemoresistant ovarian tumors. PLoS One. 2012;7:e46858. doi: 10.1371/journal.pone.0046858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lengyel E. Ovarian cancer development and metastasis. Am J Pathol. 2010;177:1053–64. doi: 10.2353/ajpath.2010.100105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li J, Condello S, Thomes-Pepin J, et al. Lipid desaturation is a metabolic marker and therapeutic target of ovarian cancer stem cells. Cell Stem Cell. 2017;20:303–14. doi: 10.1016/j.stem.2016.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lim SM, Mohamad Hanif EA, Chin SF. Is targeting autophagy mechanism in cancer a good approach? The possible double-edge sword effect. Cell Biosci. 2021;11:56. doi: 10.1186/s13578-021-00570-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Madani S, Hichami A, Charkaoui-Malki M, Khan NA. Diacylglycerols containing Omega 3 and Omega 6 fatty acids bind to RasGRP and modulate MAP kinase activation. J Biol Chem. 2004;279:1176–83. doi: 10.1074/jbc.M306252200. [DOI] [PubMed] [Google Scholar]
- 24.Madani S, Hichami A, Legrand A, Belleville J, Khan NA. Implication of acyl chain of diacylglycerols in activation of different isoforms of protein kinase C. FASEB J. 2001;15:2595–601. doi: 10.1096/fj.01-0753int. [DOI] [PubMed] [Google Scholar]
- 25.Mirzayans R, Andrais B, Murray D. Roles of polyploid/multinucleated giant cancer cells in metastasis and disease relapse following anticancer treatment. Cancers. 2018;10:118. doi: 10.3390/cancers10040118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mondal S, Roy D, Sarkar Bhattacharya S, et al. Therapeutic targeting of PFKFB3 with a novel glycolytic inhibitor PFK158 promotes lipophagy and chemosensitivity in gynecologic cancers. Int J Cancer. 2019;144:178–89. doi: 10.1002/ijc.31868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mor G, Yin G, Chefetz I, Yang Y, Alvero A. Ovarian cancer stem cells and inflammation. Cancer Biol Ther. 2011;11:708–13. doi: 10.4161/cbt.11.8.14967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nicholson KM, Bibby MC, Phillips RM. Influence of drug exposure parameters on the activity of paclitaxel in multicellular spheroids. Eur J Cancer. 1997;33:1291–8. doi: 10.1016/s0959-8049(97)00114-7. [DOI] [PubMed] [Google Scholar]
- 29.Nieman KM, Kenny HA, Penicka CV, et al. Adipocytes promote ovarian cancer metastasis and provide energy for rapid tumor growth. Nat Med. 2011;17:1498–503. doi: 10.1038/nm.2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Olzmann JA, Carvalho P. Dynamics and functions of lipid droplets. Nat Rev Mol Cell Biol. 2018;20:137–55. doi: 10.1038/s41580-018-0085-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Peart T, Ramos Valdes Y, Correa RJ, et al. Intact LKB1 activity is required for survival of dormant ovarian cancer spheroids. Oncotarget. 2015;6:22424–38. doi: 10.18632/oncotarget.4211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Peickert S, Waurig J, Dittfeld C, et al. Rapid re-expression of CD133 protein in colorectal cancer cell lines in vitro and in vivo. Lab Invest. 2012;92:1607–22. doi: 10.1038/labinvest.2012.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Petan T, Jarc E, Jusović M. Lipid droplets in cancer: guardians of fat in a stressful world. Molecules. 2018;23:1941. doi: 10.3390/molecules23081941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roy D, Mondal S, Khurana A, et al. Loss of HSulf-1: The missing link between autophagy and lipid droplets in ovarian cancer. Sci Rep. 2017;7:1–3. doi: 10.1038/srep41977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kumar S, Swamy SN, Premalatha CS, Pallavi VR, Gawari R. Aberrant promoter hypermethylation of RASSF1a and BRCA1 in circulating cell-free tumor DNA serves as a biomarker of ovarian carcinoma. Asian Pac J Cancer Prev. 2019;20:3001–5. doi: 10.31557/APJCP.2019.20.10.3001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sasaki A, Kamiyama T, Yokoo H, et al. Cytoplasmic expression of CD133 is an important risk factor for overall survival in hepatocellular carcinoma. Oncol Rep. 2010;24:537–46. doi: 10.3892/or_00000890. [DOI] [PubMed] [Google Scholar]
- 37.Sawyer BT, Qamar L, Yamamoto TM, et al. Targeting fatty acid oxidation to promote anoikis and inhibit ovarian cancer progression. Mol Cancer Res. 2020;18:1088–98. doi: 10.1158/1541-7786.MCR-19-1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Scouten WT, Patel A, Terrell R, et al. Cytoplasmic localization of the paired box gene, Pax-8, is found in pediatric thyroid cancer and may be associated with a greater risk of recurrence. Thyroid. 2004;14:1037–46. doi: 10.1089/thy.2004.14.1037. [DOI] [PubMed] [Google Scholar]
- 39.Shao H, Mohamed EM, Xu GG, et al. Carnitine palmitoyltransferase 1A functions to repress FoxO transcription factors to allow cell cycle progression in ovarian cancer. Oncotarget. 2016;7:3832. doi: 10.18632/oncotarget.6757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shen-Gunther J, Mannel RS. Ascites as a predictor of ovarian malignancy. Gynecol Oncol. 2002;87:77–83. doi: 10.1006/gyno.2002.6800. [DOI] [PubMed] [Google Scholar]
- 41.Singh R, Kaushik S, Wang Y, et al. Autophagy regulates lipid metabolism. Nature. 2009;458:1131. doi: 10.1038/nature07976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Suarez JS, Main HG, Muralidhar GG, et al. CD44 regulates formation of spheroids and controls organ-specific metastatic colonization in epithelial ovarian carcinoma. Mol Cancer Res. 2019;17:1801–14. doi: 10.1158/1541-7786.MCR-18-1205. [DOI] [PubMed] [Google Scholar]
- 43.Tirinato L, Liberale C, Di Franco S, et al. Lipid droplets: a new player in colorectal cancer stem cells unveiled by spectroscopic imaging. Stem Cells. 2015;33:35–44. doi: 10.1002/stem.1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang YN, Zeng ZL, Lu J, et al. CPT1A-mediated fatty acid oxidation promotes colorectal cancer cell metastasis by inhibiting anoikis. Oncogene. 2018;37:6025–40. doi: 10.1038/s41388-018-0384-z. [DOI] [PubMed] [Google Scholar]
- 45.Weihua Z, Lin Q, Ramoth AJ, Fan D, Fidler IJ. Formation of solid tumors by a single multinucleated cancer cell. Cancer. 2011;117:4092–9. doi: 10.1002/cncr.26021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.White E. The role for autophagy in cancer. J Clin Invest. 2015;125:42–6. doi: 10.1172/JCI73941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zechner R, Zimmermann R, Eichmann TO, et al. FAT SIGNALS-lipases and lipolysis in lipid metabolism and signaling. Cell Metab. 2012;15:279–91. doi: 10.1016/j.cmet.2011.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zeimet AG, Reimer D, Sopper S, et al. Ovarian cancer stem cells. Neoplasma. 2012;59:747–55. doi: 10.4149/neo_2012_094. [DOI] [PubMed] [Google Scholar]
- 49.Zhang S, Balch C, Chan MW, et al. Identification and characterization of ovarian cancer-initiating cells from primary human tumors. Cancer Res. 2008;68:4311–20. doi: 10.1158/0008-5472.CAN-08-0364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang X, Saarinen AM, Hitosugi T, et al. Inhibition of intracellular lipolysis promotes human cancer cell adaptation to hypoxia. Elife. 2017;6:e31132. doi: 10.7554/eLife.31132. [DOI] [PMC free article] [PubMed] [Google Scholar]