Abstract
Internal mammary lymph node (IMLN) metastasis forms part of the clinical node classification for primary breast cancer, which influences the treatment strategy. However, because of the IMLNs’ complicated anatomical structures and relationships with adjacent structures, IMLN biopsy or resection is associated with a limited improvement in prognosis and a high complication rate. The positivity rate also varies broadly according to imaging modality, and there is a low rate of agreement between the imaging and pathological diagnoses, which creates imprecision in the preoperative staging. The IMLN positivity rate also varies remarkably, and there are no clear, accurate, and non-invasive modalities for diagnosing the pre-mastectomy IMLN status. Nevertheless, medical imaging modalities continue to evolve, with functional imaging and image-guided thoracoscopic biopsy of sentinel IMLNs being well established. Thus, personalized decision-making and treatment selection should be based on the modality-specific differences in the diagnosis of IMLN metastasis/recurrence and the patient’s specific risk factors.
Keyword: Internal mammary lymph node, Metastasis/recurrence, Preoperative anatomical imaging, Preoperative functional imaging, Postoperative pathological findings
Background
Early clinical trials have shown that the internal mammary lymph node (IMLN) status is an important prognostic factor for breast cancer, and knowledge of the IMLN status is essential to guide the treatment strategy [1–4]. However, recently clinical trials have indicated that IMLN metastasis does not independently predict overall survival (OS) and progression-free survival (PFS) for patients who receive personalized treatment (e.g., chemotherapy, endocrine treatment, targeted treatment, and radiotherapy) [5–9]. Thus, accurate staging plays a significant role in guiding effective, multidisciplinary, and personalized treatment for newly diagnosed breast cancer patients. The American Joint Committee on Cancer (AJCC) staging guidelines include detailed clinical stages for patients with breast cancer [10], although the IMLNs’ location is relatively deep within the chest wall and in the parasternal intercostal spaces, which precludes palpation during a clinical examination. Moreover, the complex anatomical structures in this region create differences in the rates of preoperative imaging-based IMLN diagnosis and histopathologically confirmed IMLN metastasis. For patients with breast cancer (stage I, II, or III), observed IMLN recurrence rate was < 1.5% after primary breast cancer treatment, even when the internal mammary chain (IMC) not excised or irradiated [11–13]. Patients most likely benefit from systemic therapies and incidental regional node irradiation [14–18]. But according to extended radical mastectomy data, 9.2% of patients with no positive axillary nodes (ALN) present IMLN metastasis [19]. The current indications for IMLN irradiation might result in over-/under-treatment according to the National Comprehensive Cancer Network (NCCN) clinical practice guidelines (Version1. 2016) and the Royal College of Radiologists (RCR) consensus statement [20, 21]. These issues have generated controversy regarding the clinical diagnosis of IMLN metastasis in breast cancer patients, which is required to guide the use of local treatment such as radiation therapy and surgery. Therefore, we have reviewed the detection of IMLN metastases/recurrence based on various imaging modalities, as well as its risk factors and prognostic characteristics.
IMLN metastasis in newly diagnosed patients
Preoperative anatomical imaging
Preoperative anatomical imaging is a non-invasive technique that can be used to identify positive IMLNs, which can be performed using ultrasonography (US), computed tomography (CT), and magnetic resonance image (MRI) (Table 1). Swollen lymph nodes (LNs) are a characteristic sign of IMLN metastasis on CT images (Fig. 1), with contrast-enhanced CT identifying positive IMLNs in nearly 42% of patients with breast cancer, and > 50% of the identifiable IMLNs were > 5 mm in size [22, 23]. However, partial IMLN metastasis with sternal erosion or osteolytic sternal metastasis with a local soft tissue lump have some resemblance to swollen IMLNs with sternal erosion [24], which can make it difficult to diagnose IMLN metastasis based on CT images (Fig. 2). The rate for pathological confirmation of IMLN metastasis rate was 57% among all imaging-positive patients who received neo-adjuvant chemotherapy (NAC), with rates of 74% among US-positive patients and 70% among MRI-positive patients [6], which provided superior sensitivity and specificity, relative to CT.
Table 1.
Summary of IMLN metastasis accuracy of the various imaging techniques
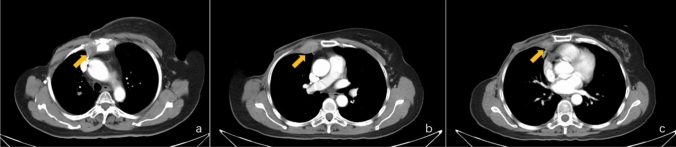
Fig. 1.
Metastatic internal mammary lymph nodes (IMLNs, yellow arrows) detected during computed tomography. A metastatic IMLN in the first intercostal space (a), a metastatic IMLN in the second intercostal space (b), and a metastatic IMLN in the third intercostal space (c)
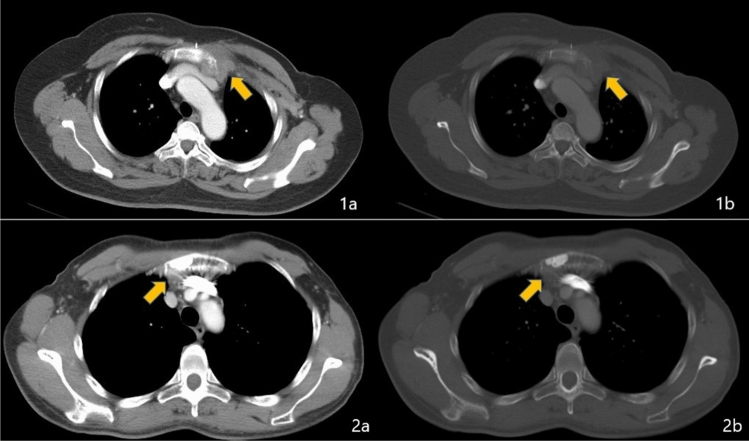
Fig. 2.
Computed tomography reveals internal mammary lymph node metastasis (yellow arrow) with sternal erosion, sternal metastasis (yellow arrow), and a local soft tissue lump. Views are shown in the mediastinal window (a) and bone window (b)
Jeong et al. [25] explored the shape and depth of metastatic IMLNs using US, which revealed that 85% of the metastatic IMLNs had an oval shape and the other 15% had an irregular shape, with positive IMLNs typically being found in the posterior aspect of the intercostal spaces. Among newly diagnosed breast cancer patients, the prevalence of positive IMLNs based on US was 10%, and US-guided needle biopsy confirmed malignancy in 90% of these cases, with 1.3% of the patients having isolated IMLN metastasis. Younger patients had an increased risk of IMLN metastasis. Internal mammary (IM) US has been used to change the N classification for 8% of patients and to change the overall clinical stage for 6.4% of patients, which ultimately necessitated a change in the treatment strategy for some patients [26]. However, approximately 40% of those patients underwent NAC before surgery, which may have prevented micrometastatic IMLNs from being identified at the LN biopsy, and subsequently reduced the positivity rate. Furthermore, the IMLNs cannot be easily identified using US in patients with a high body mass index (BMI) or thick muscle and intermuscular fat. Moreover, perforating branches of the internal mammary veins (IMVs) can easily be confused with IMLNs during US, although color Doppler US can distinguish an IMLN from a perforating branch of the IMVs based on the blood flow ultrasonic signal [27].
Preoperative anatomical imaging can support a diagnosis based on the changing shape and size of LNs, as well as other imaging features, although both physiologic and metastatic IMLNs enhance during dynamic contrast-enhanced breast MRI. Sachdev et al. [28] reported that only 0.3% of breast cancer patients had positive IMLNs that were identified at the pre-treatment MRI, with 96% of positive nodes being located in the first two intercostal spaces and the remaining 4% located in the third intercostal space. For high-risk patients and using a size threshold of 4.5–5 mm, MRI provides good sensitivity (93.3%) and specificity (89.3%) for diagnosing metastatic IMLN [29, 30]. However, clear and universally recognized standards for identifying IMLN metastasis are lacking. Patel et al. [31] analyzed the before and after MRI findings of suspicious IMLNs in breast cancer patients who were receiving neo-adjuvant therapy, and suggested that metastatic IMLNs should be suspected when the diagnostic MRI reveals ≥ 3 ipsilateral IMLNs that are ≥ 6 mm. However, this approach will have an increasing false-negative rate at smaller tumor sizes. The IMV and metastatic IMLN both exhibit high signal intensities during diffusion-weighted MRI, which suggests that using multiple MRI parameters and sequences may help improve the diagnosis rate (Fig. 3).
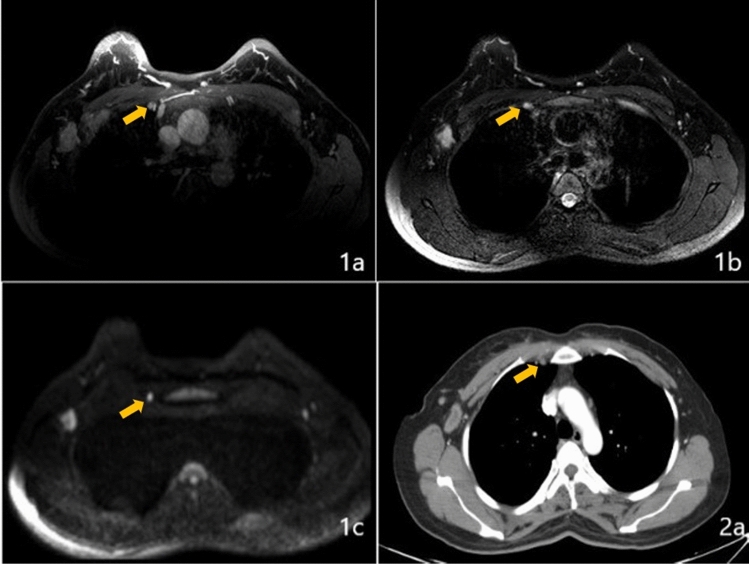
Fig. 3.
A metastatic internal mammary lymph node (yellow arrow) detected during using dynamic contrast-enhanced T1-weighted magnetic resonance imaging (1a), T2-weighted imaging (1b), diffusion-weighted imaging (1c), and computed tomography (2a)
Preoperative functional imaging
Relative to standard anatomical imaging, positron emission tomography (PET) combined with CT or MRI (PET/CT or PET/MRI) improves the ability to detect positive IMLNs in breast cancer patients [32–39]. Furthermore, use of PET provides a positive predictive value of > 80% [40, 41] (Fig. 4), even for patients who received NAC, and the pathological confirmation rate was up to 55% in PET-positive patients [6]. However, some trials have indicated that ≤ 10% of breast cancer patients have positive IMLNs based on PET-CT [40, 42]. In addition, subgroup analysis revealed that disease stage significantly influenced the likelihood of IMLN metastasis, with positive IMLNs relative proportion within all LN region metastases (level I–III, the supraclavicular region, and the internal mammary region) were 3.7% in primary M0 patients and 9.5% in M1 patients [42]. Inflammation, infection, and reactive hyperplasia may create a false-positive result for FDG uptake at the IMLNs during PET/CT.
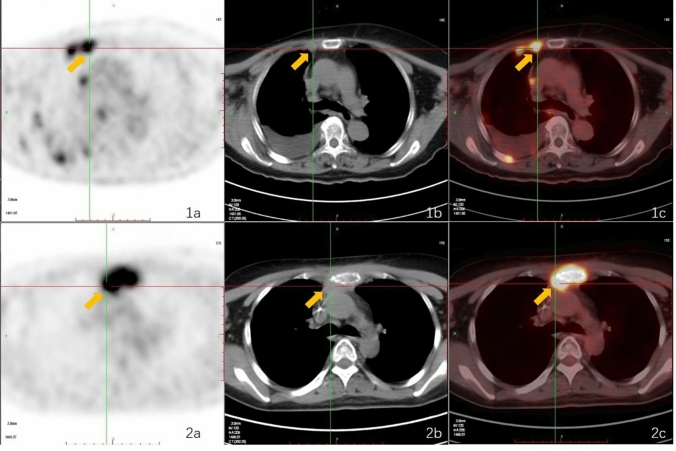
Fig. 4.
Internal mammary lymph node metastasis (yellow arrow) with sternal erosion detected based on FDG uptake (1a), computed tomography (1b), and a fused FDG PET/CT image (1c), as well as sternal metastasis (yellow arrow) with a local soft tissue lump detected based on FDG uptake (2a), computed tomography (2b) and a fused FDG PET/CT image (2c)
Based on better soft tissue contrast and motion correction possibilities [43], compared to PET/CT, statistics display a definite trend toward lower specificity and higher sensitivity of PET/MRI in the lesion-by-lesion analysis [44, 45]. Moreover, PET/CT or PET/MRI are currently available only at select hospitals, and these modalities are more expensive than more traditional modalities.
Histopathologically confirmed IMLN metastasis
Anatomical imaging (US, CT, and MRI) and functional imaging (PET/CT, PET/MRI, and lymphoscintigraphy) reportedly provide high sensitivity and accuracy for diagnosis of IMLN metastasis [6, 29, 30, 40–42]. However, a pathological diagnosis (are intercostal space IMN biopsy or endoscopic lymphatic chain resection) is still important for determining IMLN metastasis, as the pathological LN stage may change in a subset of patients, who may then require a revised treatment strategy [9, 46]. Although the pathological determination of IMLN status remains important, the performance of intercostal space IMN biopsy and endoscopic lymphatic chain resection remains controversial because these techniques are not associated with improvements in survival or in intraoperative/postoperative complications [13, 47, 48]. Thus, IMLN dissection is not routinely recommended.
When using a free flap for immediate autologous reconstruction after mastectomy, the internal mammary artery (IMA) and IMV are the preferred recipient vessels because of their large caliber and high flow rate, which provides a reasonable opportunity to study IMLN metastasis. Recent studies have identified that 3.6–6% of breast cancer patients have positive IMLNs based on biopsy performed during immediate reconstruction after mastectomy [48–50]. However, among 2057 patients who underwent free-flap breast reconstruction, Ochoa et al. [51] found that only 28 patients (1.3%) had positive IMLNs, and the preoperative breast MRI provided 11% sensitivity for detecting the IMLN. Ten of the 28 patients with IMLN metastasis (36%) had nodal metastases that were isolated to the IMLNs, and isolated IMLN involvement was associated with a lower T classification.
While difficult relative to axillary sentinel LN biopsy, the success rate for IMLN biopsy currently averages 90% for experienced teams [52–55]. Thoracoscopic IMLN dissection in breast cancer has also been reported with the same positive IMLN rate and less morbidity than the conventional intercostal space incision [9, 56]. In addition, image-guided (especially preoperative lymphoscintigraphy single-photon emission computed tomography (SPECT) with CT (SPECT/CT)-guided) thoracoscopic biopsy of the sentinel IMLNs can further improve the detection rate [5, 13, 57–59]. Piato et al. [60] found that 15% of patients had a change in the pathological LN staging when lymphoscintigraphy was performed after the intra-tumor technetium-99 m injection. Results from a prospective study also showed that 21% of early-stage breast cancer patients had IMC drainage identified based on lymphoscintigraphy, although only 13% of these patients had pathologically confirmed IMLN metastasis after IM-SLN biopsy. Moreover, there was no difference in recurrence-free survival between the patients with a positive IMLN and the patients without an IMC-sensitive node biopsy [13]. Using the same techniques, a multicenter cohort study revealed that 20.5% of ipsilateral IMLNs were visualized during preoperative lymphoscintigraphy, while only 3.53% of patients were found to have IMLN metastasis [5]. The presence of IMLN metastasis was also associated with ALN metastasis [5, 61], and the same relationship was observed among patients who had received NAC [59].
Minimally invasive diagnosis of IM-SLNB
Compared to extended surgical resection, IM sentinel lymph node biopsy (IM-SLNB) provides a less invasive method of assessing the IMLN. However, low visualization rate of IM-SLN has been a restriction of IM-SLNB. Routine SPECT can help identify lymphatic drainage and the form, size, amount, and distribution of the sentinel lymph nodes (SLNs) based on tracer drug uptake. However, precise identification of the IMLNs and clarification of their relationships with the surrounding structures are also important. Hybrid SPECT/CT can provide complementary functional and anatomical information (Fig. 5), as well as mapping of lymphatic drainage via three-dimensional (3D) reconstruction. Relative to lymphoscintigraphy alone, SPECT/CT provides advantages in terms of accurate anatomical localization, identification of false-positive results, fewer false-negative results, and better guidance regarding the surgical approach. Using lymphoscintigraphy and SPECT/CT after an intra-lesion injection of 99mTc-nanocolloid, the sentinel IMLN was found to contain metastasis in only 24% of patients from a pilot study [60] and in 34% of patients from a Dutch multicenter study [55]. The Breast Cancer Center of Shandong Cancer Hospital optimized the use of 99mTc-labeled sulfur colloid (99mTc-SC) in sentinel IMLN mapping and detection, evaluated the SPECT-CT image acquisition time, and modified the injection technique (peri-areolar intraparenchymal, high volume, and ultrasound guidance), which increased the IM-SLN visualization rate to 71.9% for breast cancer patients who were receiving initial surgery [52, 57, 58, 62, 63]. Furthermore, among patients who underwent NAC, the IM-SLN visualization rate was 33.1% [52, 62]. An acoustic probe commonly captures diffuse activity along the rib cage and care is needed when radioactive IMLNs are present in multiple intercostal spaces, as the sentinel and non-sentinel IMLNs can easily be confused [60]. The sentinel IMLNs near the radiotracer injection site are also easily ignored, which can lead to an increase in the false-negative rate. Finally, the use of NAC can influence the basal metabolic rate, radiotracer injection concentration, and tumor staging, which can lead to inter-patient differences in the radiotracer’s uptake and metabolism [64]. These differences would presumably translate into variability in the detection rate.
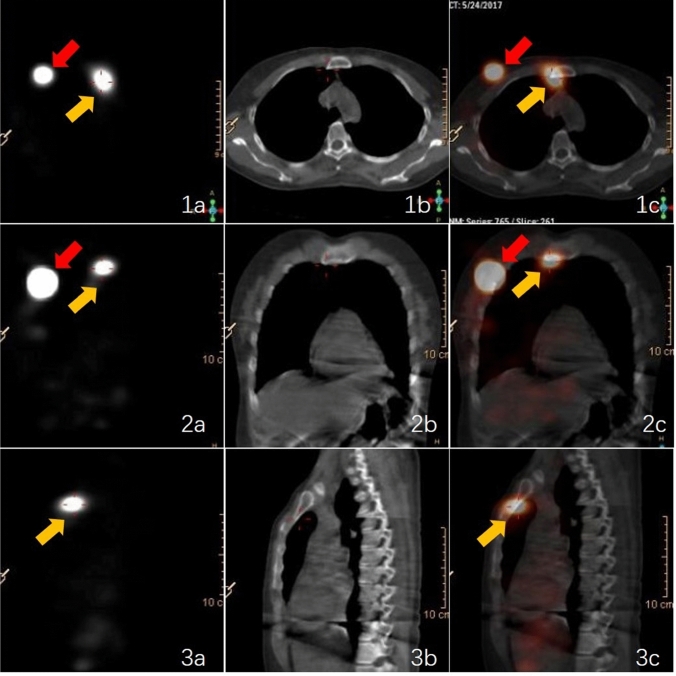
Fig. 5.
A positive internal mammary lymph node (yellow arrow) detected using single-photon emission computed tomography (a), computed tomography (b), and SPECT/CT (c) viewed in the axial plane (1), coronal plane (2), and sagittal plane (3). The red arrow indicates the injection point
IMLN recurrence after treatment
IMLN recurrence after systematic breast cancer treatment is poorly characterized. The 5-year OS in patients with IM and/or supraclavicular (IM–SC) LN recurrence without distant metastasis (DM) was 51% compared with 27% in patients with DM recurrence. The rate of IM–SC LN recurrence without DM was 0.3%, and with DM was 6.5% at the first recurrence [65]. Median OS for patients with IMN recurrences as a first event was 2.5 years, with 5 year OS 28% [66]. Sachdev et al. [28] reported that, after a median follow-up of 38 months for 7070 breast cancer patients who did not undergo IMC resection, loco-regional control could be achieved using IMC irradiation combined with modern systemic therapy. Based on FDG-PET/CT-positive loco-regional LN metastases (defined as the ALNs, supraclavicular LNs, and IMLNs), the rates of LNs in the IMC region were 3.7% for patients with primary M0 cancer and 9.5% for patients with primary M1 cancer, while the rates increased to 9.4% for patients with recurrent M0 cancer and 11.8% for recurrent M1 cancer [42]. Regardless of the imaging modality, follow-up results have indicated that the overall rate of clinically detectable IMLN recurrences remains < 1.5% after primary systemic breast cancer treatment, even when the regional IMLNs were not irradiated [12, 67–71] (Table 2). In addition, the IMLN recurrence rate was reduced to 0.2% for patient who had undergone IMLN radiotherapy (IMLN RT) [12]. Similar to in primary breast cancer patients, the recurrent IMLNs were still concentrated in the second intercostal space (67.7%) and the third intercostal space (19.5%) [68]. Loganadane et al. [71] evaluated the loco-regional failure patterns in 796 women with breast cancer who underwent irradiation of the chest wall ± the supraclavicular region (level IV, 88.3%), ± the infraclavicular region (levels II–III, 77.9%), ± the IMC region (85.6%), ± the ALN region (level I, 14.9%). During a median follow-up of 64 months (range: 6–102 months), only one patient developed IMLN recurrence, which occurred within the irradiated volumes. LN metastasis was observed in 17.5 and 31.5% of cases when irradiation tagret delineation was based on the RTOG and ESTRO guidelines, respectively. As geographic misses outside the ESTRO-clinical target volume (CTV) while within the RTOG-CTVs occurred in 14% of the cases. This may be because the ESTRO-recommended volumes were designed for early-stage breast cancer, while the RTOG guidelines are probably more suitable for advanced tumors [71–73]. Therefore, part of the loco-regional failure after radiotherapy may be related to poor tumor targeting. So, tailored target delineation guideline should be selected.
Table 2.
The overall rate of IMLN recurrences after primary systemic treatment
| Author | Year | No. of patients | IMLN recurrence (%) |
|---|---|---|---|
| Cranenbroek et al. [67] | 2005 | 5912 | 0.1 |
| Chen et al. [68] | 2010 | 8867 | 1.5 |
| Ohsumi et al. [69] | 2011 | 1907 | 0.26 |
| Oh et al. [70] | 2014 | 1906 | 1.47 |
| Poortmans et al. [12] | 2015 | 4004 (all) | 0.5 |
| 2002 (nodal-irradiation group) | 0.2 | ||
| 2002 (thoracic wall irradiation only group) | 0.8 | ||
| Loganadane et al. [71] | 2017 | 796 | 0.13 |
Follow-up results have shown that benign or malignant IMLNs can be detected using MRI in 37.6% of breast cancer patients who underwent silicone implant-based oncoplastic surgery, with median short-axis and long-axis measurements of 0.40 and 0.70 cm. However, the surgical and percutaneous biopsy results revealed that only 0.48% of the IMLNs (1/207) were malignant, which corresponded to a positive predictive value of 0.5% for predicting malignancy based on MRI-detected enlarged IMLNs [74]. The high rate of false-positive IMLN metastasis, which were incorrectly identified during preoperative imaging and reviewed after breast cancer surgery, may be related to several factors. First, inflammation or LN reactive hyperplasia may be a confounding factor. Second, patients who receive silicone implants have increasing risks over time of silicone migration and possibly silicone granulomatous lymphadenitis [75, 76]. Third, some studies have shown that lymphatic drainage patterns might be altered by lymph vessel shrinkage, fibrosis, and obstruction caused by cellular material or tumor emboli, especially for patients who have received chemotherapy [77, 78]. Fourth, various tissues can mimic IMLNs during parasternal imaging, such as mature adipose tissue, costal cartilage, part of a vessel, skeletal muscle, or fibroblasts [25]. Finally, granulomatosis or acute infective pleurisy are also potential confounding factors [79]. Therefore, for patients with large IMLNs or functional imaging-positive IMLNs after surgery, it may be more appropriate to use a short-interval follow-up with enhanced CT, MRI, US, or biopsy, rather than an immediate full oncologic work-up, depending on the size and location of the IMLNs.
Predictive and prognostic factors of IMLN metastasis
Many studies have investigated the relationships between IMLN metastasis and various clinical, pathological, and immunohistochemical parameters. Among newly diagnosed breast cancer patients, IMLN metastasis is associated with age, tumor location/depth, and the tumor’s expression of estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor-2 (HER-2) [2, 9, 40, 50, 80–85]. For example, Heuts et al. [2] reported that the proportions of IMLN metastasis were 32% for < 50-year-old patients, 15% for 51- to 70-year-old patients, and 11% for > 70-year-old patients in their cohort. Huang et al. [81] also reported that the proportions of IMLN metastasis were 21.8% for < 35-year-old patients, 15.7% for 35- to 50-year-old patients, and 12.9% for > 50-year-old patients in their cohort. Thus, younger patients appear to have a higher risk of IMLN metastasis. The tumor was located in the central or medial breast in 60% of patients with proven positive IMLNs [2], while tumors located in the upper outer quadrant had a smaller likelihood of having positive IMLNs (approximately 10%) [83]. Patients with negative ER expression were 2.8 times more likely to have positive IMLNs, relative to patients with positive ER expression, based on findings from FDG-PET/CT [40], and other multivariate analyses have revealed that IMLN metastasis was independently associated with positive HER-2 expression [9, 50, 85]. Furthermore, the risk of IMLN metastasis increased at higher numbers of involved ALNs [9, 13, 81, 84], and the negative predictive value (NPV) of ALN metastasis was 92.3% for tumor-positive internal mammary chain sentinel nodes [59]. Therefore, based on the current evidence, an elevated risk of IMLN metastasis appears to be associated with a medial or central location, younger age (< 50 years old), triple-negative hormone receptor status or positive HER-2 expression, and involvement of ≥ 4 ALNs metastases, and high nuclear grade.
A recent study also indicated that nipple inversion and mammographic calcification were strongly associated with a higher rate of IMLN metastasis [85]. Other results have suggested that IMLN metastasis/recurrence was independently influenced by tumor size, especially for invasive tumors with a size of > 2 cm [50, 80, 85]. However, in a large series of 2269 Chinese breast cancer patients who underwent extended radical mastectomy, IMLN metastasis was independently associated with number of ALNs and age, but not tumor size [81]. These conflicting findings may be related to racial or regional differences. During the follow-up evaluations, 66.2% of the patients had other concurrent metastasis sites with the IMLN recurrence, and these patients had a median survival time of only 42 months, relative to the 63 months for patients with isolated IMLN recurrence. Other independent factors that might delay IMLN recurrence were a small tumor size and positive ER/PR expression [68].
Based on clinicopathological risk factors, Sun Yat-sen University Cancer Center found that IMLN metastasis was significantly correlated with tumor location, lymphovascular invasion (LVI) and pathological ALN (pALN) stage in multivariable analysis [86]. Based on the multivariable logistic regression, they developed a user-friendly and effective nomogram to a prediction model constructed for IMLN status in breast cancer, but compared to IM-SLNB, the false-negative (FN) rate of nomogram for the detection of IMLN disease was still higher than that of IM-SLNB (13.9% vs. 3.3%) [86, 87]. On further expansion of the sample size (444 patients in the training cohort and 180 patients in the validation cohort) and independent external validation cohorts from other hospitals, non-invasive nomogram still had a 34 and 7% FN rate in the training and validation cohorts, respectively [88]. Non-invasive prediction tool must be based on prospective, large-scale and multicenter clinical trials, that to select patients with high risk of IMLN metastasis to undergo tailored treatment strategies, while for low risk patients can omit IMLN surgery or irradiation.
Conclusions
The results of various population-based studies (22,922/10925, MA.20, and DBCG-IMN) have supported the use of IMLN RT after surgery, based on the therapeutic effect and long-term cardiotoxicity of IMLN irradiation [12, 89, 90]. Furthermore, the National Comprehensive Cancer Network Guidelines (version 1.2016) updated the level of evidence and strength of the recommendation for IMLN RT [20]. However, 56% of cardiac events occur after 10 years [91], and the Early Breast Cancer Trialists’ Collaborative Group proved that non-cancer deaths increased for breast cancer patients with N1 disease who underwent regional LN irradiation [92]. Therefore, clinicians must still comprehensively and conservatively estimate the potential benefits of IMLN RT and the risks of any toxic reactions. This assessment should include tumor position, volume, and histopathological grading, heart and lung functions, any use of anthracycline/trastuzumab, and reasonable life expectations [2, 9, 40, 50, 80–85, 93, 94]. Patients with increased long-term radiation-induced cardiovascular risks in coronary heart disease patients is greater than that in the general population [95]. Breast cancer chemotherapy with anthracycline and targeted therapy with trastuzumab increases the risk of cardiovascular risk. Drug-induced myocardial injury along with radiation-induced endothelial injury and inflammatory cell infiltration may further contribute to the increased risk of ischemic heart disease. Therefore, superior diagnostic and/ or predicted tools should be developed. Further studies are needed to examine the genomic and radiomic characteristics of breast cancer patients who have an elevated risk of IMLN metastasis, which will allow physicians to develop personalized and reasonable IMLN surgery or irradiation strategies to avoid over-/under-treatment.
Acknowledgements
This manuscript was edited by Elsevier Language Editing Services.
Author contributions
WW and LJ participated in the study design, contributed to the data collection, and draft the manuscript. QP made important contributions in collecting image collection. All authors read and approved the final manuscript.
Funding
This study has received funding from the Natural Science Foundation of Shandong Provence [grant number ZR2020QH260; ZR20210219068]; Taishan Scholars Program of Shandong Province [NO. ts 20190982]; National Natural Science Foundation of China [grant number 82172873]; China Postdoctoral Science Foundation (2021M691334); Bethune Charitable Foundation "Shared Sunshine—Major Disease Clinical Research Project" [grant number G-X-2019-0101-12]; Breast Disease Science Foundation of Shandong Province Medical Association (No. YXH2020ZX062).
Availability of data and materials
The datasets/or images are available from the corresponding author on reasonable request.
Declarations
Competing interests
The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article.
Ethics approval and consent to participate
These issues are not applicable for this review.
Consent for publication
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Lê MG, Arriagada R, de Vathaire F, Dewar J, Fontaine F, Lacour J, et al. Can internal mammary chain treatment decrease the risk of death for patients with medial breast cancers and positive axillary lymph nodes? Cancer. 1990;66:2313–2318. doi: 10.1002/1097-0142(19901201)66:11<2313::aid-cncr2820661110>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 2.Heuts EM, van der Ent FW, Hulsewé KW, von Meyenfeldt MF, Voogd AC. Results of tailored treatment for breast cancer patients with internal mammary lymph node metastases. Breast. 2009;18:254–258. doi: 10.1016/j.breast.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 3.Sugg SL, Ferguson DJ, Posner MC, Heimann R. Should internal mammary nodes be sampled in the sentinel lymph node era? Ann Surg Oncol. 2000;7:188–192. doi: 10.1007/bf02523652. [DOI] [PubMed] [Google Scholar]
- 4.Dellapasqua S, Bagnardi V, Balduzzi A, Iorfida M, Rotmensz N, Santillo B, et al. Outcomes of patients with breast cancer who present with ipsilateral supraclavicular or internal mammary lymph node metastases. Clin Breast Cancer. 2014;14:53–60. doi: 10.1016/j.clbc.2013.09.008. [DOI] [PubMed] [Google Scholar]
- 5.Madsen EV, Aalders KC, van der Heiden-van der LM, Gobardhan PD, van Oort PM, van der Ent FW, et al. Prognostic significance of tumor-positive internal mammary sentinel lymph nodes in breast cancer: a multicenter cohort study. Ann Surg Oncol. 2015;22:4254–4262. doi: 10.1245/s10434-015-4535-y. [DOI] [PubMed] [Google Scholar]
- 6.Joo JH, Kim SS, Ahn SD, Choi EK, Jung JH, Jeong Y, et al. Impact of pathologic diagnosis of internal mammary lymph node metastasis in clinical N2b and N3b breast cancer patients. Breast Cancer Res Treat. 2017;166:511–518. doi: 10.1007/s10549-017-4422-2. [DOI] [PubMed] [Google Scholar]
- 7.Tan C, Caragata R, Bennett I. Is sentinel node biopsy of the internal mammary lymph nodes relevant in the management of breast cancer? Breast J. 2017;23:410–414. doi: 10.1111/tbj.12754. [DOI] [PubMed] [Google Scholar]
- 8.Abdel-Rahman O. Impact of regional nodal irradiation on the outcomes of N1 breast cancer patients referred for adjuvant treatment: a patient-level pooled analysis of 2 clinical trials. Clin Breast Cancer. 2018;18:504–510. doi: 10.1016/j.clbc.2018.07.016. [DOI] [PubMed] [Google Scholar]
- 9.Qi XW, Du JZ, Tang P, Liu X, He QQ, Zhong L, et al. Clinical significance of internal mammary lymph node metastasis for breast cancer: analysis of 337 breast cancer patients. Surg Oncol. 2018;27:185–191. doi: 10.1016/j.suronc.2018.03.006. [DOI] [PubMed] [Google Scholar]
- 10.Edge S, Byrd D, Compton C, Fritz AG, Greene F, Trotti A, editors. AJCC cancer staging manual. 7. New York: Springer; 2010. [Google Scholar]
- 11.Xie L, Higginson DS, Marks LB. Elective regional nodal irradiation in patients with early-stage breast cancer. Semin Radiat Oncol. 2011;21:66–78. doi: 10.1016/j.semradonc.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 12.Poortmans PM, Collette S, Kirkove C, Van Limbergen E, Budach V, Struikmans H, et al. Internal mammary and medial supraclavicular irradiation in breast cancer. N Engl J Med. 2015;373:317–327. doi: 10.1056/NEJMoa1415369. [DOI] [PubMed] [Google Scholar]
- 13.van Loevezijn AA, Bartels SAL, van Duijnhoven FH, Heemsbergen WD, Bosma SCJ, Elkhuizen PHM, et al. Internal mammary chain sentinel nodes in early-stage breast cancer patients: toward selective removal. Ann Surg Oncol. 2019;26:945–953. doi: 10.1245/s10434-018-7058-5. [DOI] [PubMed] [Google Scholar]
- 14.Giuliano AE, Ballman K, McCall L, et al. Locoregional recurrence after sentinel lymph node dissection with or without axillary dissection in patients with sentinel lymph node metastases: long-term follow-up from the American college of surgeons oncology group (Alliance) ACOSOG Z0011 randomized trial. Ann Surg. 2016;264(3):413–420. doi: 10.1097/SLA.0000000000001863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Krag DN, Anderson SJ, Julian TB, Brown AM, Harlow SP, Costantino JP, et al. Sentinel-lymph-node resection compared with conventional axillary-lymph-node dissection in clinically node-negative patients with breast cancer: overall survival findings from the NSABP B-32 randomised phase 3 trial. Lancet Oncol. 2010;11:927–933. doi: 10.1016/S1470-2045(10)70207-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang W, Wang J, Qiu P, et al. Factors influencing the incidental dose distribution in internal mammary nodes: a comparative study. Front Oncol. 2020;10:456. doi: 10.3389/fonc.2020.00456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Song Y, Yu T, Wang W, et al. Dosimetric comparison of incidental radiation to the internal mammary nodes after breast-conserving surgery using 3 techniques-inverse intensity-modulated radiotherapy, field-in-field intensity-modulated radiotherapy, and 3-dimensional conformal radiotherapy: a retrospective clinical study. Medicine (Baltimore) 2019;98(41):e17549. doi: 10.1097/MD.0000000000017549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang W, Zhang Y, Xu M, et al. Postmastectomy radiotherapy using three different techniques: a retrospective evaluation of the incidental dose distribution in the internal mammary nodes. Cancer Manag Res. 2019;11:1097–1106. doi: 10.2147/CMAR.S191047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cong BB, Cao XS, Cao L, et al. Internal mammary lymph nodes radiotherapy of breast cancer in the era of individualized medicine. Oncotarget. 2017;8:81583–81590. doi: 10.18632/oncotarget.20186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.National Comprehensive Cancer Network. Clinical practice guidelines in oncology: breast cancer (Version 1.2016)—January 20, 2016. http://www.nccn.org.
- 21.Bloomfield DJ, Core Group facilitated by The Royal College of Radiologists Development of postoperative radiotherapy for breast cancer: UK consensus statements—a model of patient, clinical and commissioner engagement? Clin Oncol (R Coll Radiol) 2017;29(10):639–641. doi: 10.1016/j.clon.2017.06.011. [DOI] [PubMed] [Google Scholar]
- 22.Singh S, Ramani SK, Rastogi A, Thakur MH. Incidence of internal mammary node in locally advanced breast cancer and its correlation with metastatic disease: a retrospective observational study. Br J Radiol. 2019;92:20190098. doi: 10.1259/bjr.20190098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Savaridas SL, Spratt JD, Cox J. Incidence and potential significance of internal mammary lymphadenopathy on computed tomography in patients with a diagnosis of primary breast cancer. Breast Cancer (Auckl) 2015;9:59–65. doi: 10.4137/BCBCR.S25782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kwai AH, Stomper PC, Kaplan WD. Clinical significance of isolated scintigraphic sternal lesions in patients with breast cancer. J Nucl Med. 1988;29:324–328. [PubMed] [Google Scholar]
- 25.Jeong CJ, Bae JM, Park SY, Han BK, Ko ES, Ko EY. Metastatic internal mammary lymph nodes or mimickers on parasternal ultrasonography: focusing on their distribution and depth. Acta Radiol. 2018;59:34–40. doi: 10.1177/0284185117702180. [DOI] [PubMed] [Google Scholar]
- 26.Dogan BE, Dryden MJ, Wei W, Fornage BD, Buchholz TA, Smith B, et al. Sonography and sonographically guided needle biopsy of internal mammary nodes in staging of patients with breast cancer. AJR Am J Roentgenol. 2015;205:905–911. doi: 10.2214/AJR.15.14307. [DOI] [PubMed] [Google Scholar]
- 27.Urano M, Denewar FA, Murai T, Mizutani M, Kitase M, Ohashi K, et al. Internal mammary lymph node metastases in breast cancer: what should radiologists know? Jpn J Radiol. 2018;36:629–640. doi: 10.1007/s11604-018-0773-9. [DOI] [PubMed] [Google Scholar]
- 28.Sachdev S, Goodman CR, Neuschler E, Kalakota K, Cutright D, Donnelly ED, et al. Radiotherapy of MRI-detected involved internal mammary lymph nodes in breast cancer. Radiat Oncol. 2017;12:199. doi: 10.1186/s13014-017-0934-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kinoshita T, Odagiri K, Andoh K, Doiuchi T, Sugimura K, Shiotani S, et al. Evaluation of small internal mammary lymph metastases in breast cancer by MRI. Radiat Med. 1999;17:189–193. [PubMed] [Google Scholar]
- 30.Mack M, Chetlen A, Liao J. Incidental internal mammary lymph nodes visualized on screening breast MRI. AJR Am J Roentgenol. 2015;205:209–214. doi: 10.2214/AJR.14.13586. [DOI] [PubMed] [Google Scholar]
- 31.Patel S, Delikat A, Liao J, Chetlen AL. Pre- and post-magnetic resonance imaging features of suspicious internal mammary lymph nodes in breast cancer patients receiving neo-adjuvant therapy: Are any imaging features predictive of malignancy? Breast J. 2018;24:997–1000. doi: 10.1111/tbj.13102. [DOI] [PubMed] [Google Scholar]
- 32.Cermik TF, Mavi A, Basu S, Alavi A. Impact of FDG PET on the preoperative staging of newly diagnosed breast cancer. Eur J Nucl Med Mol Imaging. 2008;35:475–483. doi: 10.1007/s00259-007-0580-5. [DOI] [PubMed] [Google Scholar]
- 33.Heusner TA, Kuemmel S, Hahn S, Koeninger A, Otterbach F, Hamami ME, et al. Diagnostic value of full-dose FDG PET/CT for axillary lymph node staging in breast cancer patients. Eur J Nucl Med Mol Imaging. 2009;36:1543–1550. doi: 10.1007/s00259-009-1145-6. [DOI] [PubMed] [Google Scholar]
- 34.Riegger C, Koeninger A, Hartung V, Otterbach F, Kimmig R, Forsting M, et al. Comparison of the diagnostic value of FDG-PET/CT and axillary ultrasound for the detection of lymph node metastases in breast cancer patients. Acta Radiol. 2012;53:1092–1098. doi: 10.1258/ar.2012.110635. [DOI] [PubMed] [Google Scholar]
- 35.Ergul N, Kadioglu H, Yildiz S, Yucel SB, Gucin Z, Erdogan EB, et al. Assessment of multifocality and axillary nodal involvement in early-stage breast cancer patients using 18F-FDG PET/CT compared to contrast-enhanced and diffusion-weighted magnetic resonance imaging and sentinel node biopsy. Acta Radiol. 2015;56:917–923. doi: 10.1177/0284185114539786. [DOI] [PubMed] [Google Scholar]
- 36.Boellaard R, Delgado-Bolton R, Oyen WJ, Giammarile F, Tatsch K, Eschner W, et al. FDGPET/CT: EANM procedure guidelines for tumor imaging: version 2.0. Eur J Nucl Med Mol Imaging. 2015;42:328–354. doi: 10.1007/s00259-014-2961-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Melsaether AN, Raad RA, Pujara AC, Ponzo FD, Pysarenko KM, Jhaveri K, et al. Comparison of whole-body (18)F FDG PET/MR imaging and whole-body (18)F FDG PET/CT in terms of lesion detection and radiation dose in patients with breast cancer. Radiology. 2016;281:193–202. doi: 10.1148/radiol.2016151155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Orsaria P, Chiaravalloti A, Caredda E, Marchese PV, Titka B, Anemona L, et al. Evaluation of the usefulness of FDG-PET/CT for nodal staging of breast cancer. Anticancer Res. 2018;38:6639–6652. doi: 10.21873/anticanres.13031. [DOI] [PubMed] [Google Scholar]
- 39.van Nijnatten TJA, Goorts B, Vöö S, de Boer M, Kooreman LFS, Heuts EM, et al. Added value of dedicated axillary hybrid 18F-FDG PET/MRI for improved axillary nodal staging in clinically node-positive breast cancer patients: a feasibility study. Eur J Nucl Med Mol Imaging. 2018;45:179–186. doi: 10.1007/s00259-017-3823-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang CL, Eissa MJ, Rogers JV, Aravkin AY, Porter BA, Beatty JD. (18)F-FDG PET/CT-positive internal mammary lymph nodes: pathologic correlation by ultrasound-guided fine-needle aspiration and assessment of associated risk factors. AJR Am J Roentgenol. 2013;200:1138–1144. doi: 10.2214/AJR.12.8754. [DOI] [PubMed] [Google Scholar]
- 41.Seo MJ, Lee JJ, Kim HO, Chae SY, Park SH, Ryu JS, et al. Detection of internal mammary lymph node metastasis with 18F-fluorodeoxyglucose positron emission tomography/computed tomography in patients with stage III breast cancer. Eur J Nucl Med Mol Imaging. 2014;41:438–445. doi: 10.1007/s00259-013-2600-y. [DOI] [PubMed] [Google Scholar]
- 42.Borm KJ, Voppichler J, Düsberg M, Oechsner M, Vag T, Weber W, et al. FDG/PET-CT-based lymph node atlas in breast cancer patients. Int J Radiat Oncol Biol Phys. 2019;103:574–582. doi: 10.1016/j.ijrobp.2018.07.2025. [DOI] [PubMed] [Google Scholar]
- 43.Ehman EC, Johnson GB, Villanueva-Meyer JE, et al. PET/MRI: Where might it replace PET/CT? J Magn Reson Imaging. 2017;46(5):1247–1262. doi: 10.1002/jmri.25711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Botsikas D, Bagetakos I, Picarra M, et al. What is the diagnostic performance of 18-FDG-PET/MR compared to PET/CT for the N- and M- staging of breast cancer? Eur Radiol. 2019;29(4):1787–1798. doi: 10.1007/s00330-018-5720-8. [DOI] [PubMed] [Google Scholar]
- 45.de Mooij CM, Sunen I, Mitea C, et al. Diagnostic performance of PET/computed tomography versus PET/MRI and diffusion-weighted imaging in the N- and M-staging of breast cancer patients. Nucl Med Commun. 2020;41(10):995–1004. doi: 10.1097/MNM.0000000000001254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Coombs NJ, Boyages J, French JR, Ung OA. Internal mammary sentinel nodes: ignore, irradiate or operate? Eur J Cancer. 2009;45:789–794. doi: 10.1016/j.ejca.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 47.Postma EL, van Wieringen S, Hobbelink MG, Verkooijen HM, van den Bongard HJ, Borel Rinkes IH, et al. Sentinel lymph node biopsy of the internal mammary chain in breast cancer. Breast Cancer Res Treat. 2012;134:735–741. doi: 10.1007/s10549-012-2086-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang X, Jaffer S, Bleiweiss IJ, Nayak A. The clinical significance of internal mammary lymph node (IMLN) biopsy during autologous reconstruction in breast cancer patients. Breast Cancer Res Treat. 2015;153:565–572. doi: 10.1007/s10549-015-3569-y. [DOI] [PubMed] [Google Scholar]
- 49.Rose JF, Zavlin D, Menn ZK, Eldor L, Chegireddy V, Baker TP, et al. Implications of internal mammary lymph node sampling during microsurgical breast reconstruction. Ann Surg Oncol. 2018;25:3134–3140. doi: 10.1245/s10434-018-6679-z. [DOI] [PubMed] [Google Scholar]
- 50.Hong KY, Lee HB, Yim S, Lee J, Kim TY, Han W, et al. Opportunistic biopsy of internal mammary lymph nodes during immediate breast reconstruction after mastectomy for breast malignancies. Ann Surg Oncol. 2017;24:1881–1888. doi: 10.1245/s10434-017-5837-z. [DOI] [PubMed] [Google Scholar]
- 51.Ochoa O, Azouz V, Santillan A, Pisano S, Chrysopoulo M, Ledoux P, et al. Internal mammary lymph node biopsy during free-flap breast reconstruction: optimizing adjuvant breast cancer treatment through comprehensive staging. Ann Surg Oncol. 2018;25:1322–1328. doi: 10.1245/s10434-018-6352-6. [DOI] [PubMed] [Google Scholar]
- 52.Qiu PF, Zhao RR, Wang W, Sun X, Chen P, Liu YB, et al. Internal mammary sentinel lymph node biopsy in clinically axillary lymph node-positive breast cancer: diagnosis and implications for patient management. Ann Surg Oncol. 2019 doi: 10.1245/s10434-019-07705-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Madsen E, Gobardhan P, Bongers V, Albregts M, Burgmans J, De Hooge P, et al. The impact on post-surgical treatment of sentinel lymph node biopsy of internal mammary lymph nodes in patients with breast cancer. Ann Surg Oncol. 2007;14:e1486–e1492. doi: 10.1245/s10434-006-9230-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hindié E, Groheux D, Hennequin C, Zanotti-Fregonara P, Vercellino L, Berenger N, et al. Lymphoscintigraphy can select breast cancer patients for internal mammary chain radiotherapy. Int J Radiat Oncol Biol Phys. 2012;83:1081–1088. doi: 10.1016/j.ijrobp.2011.09.016. [DOI] [PubMed] [Google Scholar]
- 55.Gong J, Yu Y, Wu G, Lin C, Tu X. Should internal mammary lymph node sentinel biopsy be performed in breast cancer: a systematic review and meta-analysis. World J Surg Oncol. 2019;17:135. doi: 10.1186/s12957-019-1683-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Long H, Lin Z, Situ D, Ma G, Zheng Y, Rong T. Stage migration and therapy modification after thoracoscopic internal mammary lymph node dissection in breast cancer patients. Breast. 2010;20:129–133. doi: 10.1016/j.breast.2010.10.005. [DOI] [PubMed] [Google Scholar]
- 57.Cong BB, Qiu PF, Wang YS. Internal mammary sentinel lymph node biopsy: minimally invasive staging and tailored internal mammary radiotherapy. Ann Surg Oncol. 2014;21:2119–2121. doi: 10.1245/s10434-014-3650-5. [DOI] [PubMed] [Google Scholar]
- 58.Qiu PF, Cong BB, Zhao RR, Yang GR, Liu YB, Chen P, et al. Internal mammary sentinel lymph node biopsy with modified injection technique: high visualization rate and accurate staging. Medicine. 2015;94:e1790. doi: 10.1097/MD.0000000000001790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bi Z, Chen P, Liu J, Liu Y, Qiu P, Yang Q, et al. Internal mammary sentinel lymph node biopsy after neoadjuvant chemotherapy in breast cancer. J Breast Cancer. 2018;21:442–446. doi: 10.4048/jbc.2018.21.e49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Piato JR, Filassi JR, Dela Vega AJ, Coura-Filho GB, Aguiar FN, Porciuncula LM, et al. SPECT-CT-guided thoracoscopic biopsy of sentinel lymph nodes in the internal mammary chain in patients with breast cancer: a pilot study. Innovations (Phila) 2016;11:94–98. doi: 10.1097/IMI.0000000000000257. [DOI] [PubMed] [Google Scholar]
- 61.Brouwer OR, Vermeeren L, van der Ploeg IM, Valdés Olmos RA, Loo CE, Pereira-Bouda LM, et al. Lymphoscintigraphy and SPECT/CT in multicentric and multifocal breast cancer: does each tumour have a separate drainage pattern? Results of a Dutch multicentre study (MULTISENT) Eur J Nucl Med Mol Imaging. 2012;39:1137–1143. doi: 10.1007/s00259-012-2131-y. [DOI] [PubMed] [Google Scholar]
- 62.Cao XS, Yang GR, Cong BB, Qiu PF, Wang YS. The lymphatic drainage pattern of internal mammary sentinel lymph node identified by small particle radiotracer (99mTc-Dextran 40) in breast. Cancer Res Treat. 2019;51:483–492. doi: 10.4143/crt.2018.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cong BB, Cao XS, Qiu PF, Liu YB, Zhao T, Chen P, et al. Validation study of the modified injection technique for internal mammary sentinel lymph node biopsy in breast cancer. Onco Targets Ther. 2015;8:2705–2708. doi: 10.2147/OTT.S91185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ferreres García K, Siegrist Ridruejo J, Rincón Olbés P, Luque Molina MS, Almoguera Arias MI. Therapeutic attitude towards internal mammary chain drainage in patients with breast cancer. Breast. 2016;30:1–4. doi: 10.1016/j.breast.2016.08.001. [DOI] [PubMed] [Google Scholar]
- 65.Inari H, Teruya N, Kishi M, et al. Clinicopathological features of breast cancer patients with internal mammary and/or supraclavicular lymph node recurrence without distant metastasis. BMC Cancer. 2020;20:932. doi: 10.1186/s12885-020-07442-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Xu AJ, DeSelm CJ, Ho AY, et al. Overall survival of breast cancer patients with loco regional failures involving internal mammary nodes. Adv Radiat Oncol. 2019;4(3):447–452. doi: 10.1016/j.adro.2019.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cranenbroek S, van der Sangen MJ, Kuijt GP, Voogd AC. Diagnosis, treatment and prognosis of internal mammary lymph node recurrence in breast cancer patients. Breast Cancer Res Treat. 2005;89:271–275. doi: 10.1007/s10549-005-2469-y. [DOI] [PubMed] [Google Scholar]
- 68.Chen L, Gu Y, Leaw S, Wang Z, Wang P, Hu X, et al. Internal mammary lymph node recurrence: rare but characteristic metastasis site in breast cancer. BMC Cancer. 2010;10:479. doi: 10.1186/1471-2407-10-479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ohsumi S, Inoue T, Kiyoto S, Hara F, Takahashi M, Takabatake D, et al. Detection of isolated ipsilateral regional lymph node recurrences by F18-fluorodeoxyglucose positron emission tomography-CT in follow-up of postoperative breast cancer patients. Breast Cancer Res Treat. 2011;130:267–272. doi: 10.1007/s10549-011-1561-8. [DOI] [PubMed] [Google Scholar]
- 70.Oh JK, Chung YA, Kim YS, Jeon HM, Kim SH, Park YH, et al. Value of F-18 FDG PET/CT in detection and prognostication of isolated extra-axillary lymph node recurrences in postoperative breast cancer. Biomed Mater Eng. 2014;24:1173–1184. doi: 10.3233/BME-130918. [DOI] [PubMed] [Google Scholar]
- 71.Loganadane G, Xi Z, Xu HP, Grellier Adedjouma N, Bazire L, Fourquet A, et al. Patterns of loco regional failure in women with breast cancer treated by post mastectomy conformal electron beam radiation therapy (PMERT): large scale single center experience. Clin Transl Radiat Oncol. 2017;4:46–50. doi: 10.1016/j.ctro.2017.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Offersen BV, Boersma LJ, Kirkove C, Hol S, Aznar MC, Biete Sola A, et al. ESTRO consensus guideline on target volume delineation for elective radiation therapy of early stage breast cancer. Radiother Oncol. 2015;114:3–10. doi: 10.1016/j.radonc.2014.11.030. [DOI] [PubMed] [Google Scholar]
- 73.Chang JS, Byun HK, Kim JW, Kim KH, Lee J, Cho Y, et al. Three-dimensional analysis of patterns of loco regional recurrence after treatment in breast cancer patients: validation of the ESTRO consensus guideline on target volume. Radiother Oncol. 2017;122:24–29. doi: 10.1016/j.radonc.2016.10.016. [DOI] [PubMed] [Google Scholar]
- 74.Sutton EJ, Watson EJ, Gibbons G, Goldman DA, Moskowitz CS, Jochelson MS, et al. Incidence of internal mammary lymph nodes with silicone breast implants at MR imaging after oncoplastic surgery. Radiology. 2015;277:381–387. doi: 10.1148/radiol.2015142717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Soudack M, Yelin A, Simansky D, Ben-Nun A. Fluorodeoxyglucose-positive internal mammary lymph node in breast cancer patients with silicone implants: is it always metastatic cancer? Eur J Cardiothorac Surg. 2013;44:79–82. doi: 10.1093/ejcts/ezs625. [DOI] [PubMed] [Google Scholar]
- 76.Pardolesi A, Bertolaccini L, Brandolini J, Solli P. Robotic internal mammary lymphadenectomy: another possible minimally invasive approach to sampling lymph nodes in breast cancer patients. J Vis Surg. 2018;12(4):71. doi: 10.21037/jovs.2018.03.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kuerer HM, Hunt KK. The rationale for integration of lymphatic mapping and sentinel node biopsy in the management of breast cancer after neoadjuvant chemotherapy. Semin Breast Dis. 2002;5:80–87. [Google Scholar]
- 78.Sharkey FE, Addington SL, Fowler LJ, Page CP, Cruz AB. Effects of preoperative chemotherapy on the morphology of resectable breast carcinoma. Mod Pathol. 1996;9:893–900. [PubMed] [Google Scholar]
- 79.Messineo L, Quadri F, Valsecchi A, Lonni S, Palmiotti A, Giampietro M. Internal mammary lymph node visualization as a sentinel sonographic sign of tuberculous pleurisy. Ultraschall Med. 2019;40:488–494. doi: 10.1055/a-0879-1758. [DOI] [PubMed] [Google Scholar]
- 80.Lukesova L, Vrana D, Gatek J, Koranda P, Cwiertka K, Radova L, et al. Predictive parameters for internal mammary node drainage in patients with early breast cancer. Tumori. 2014;100:254–258. doi: 10.1700/1578.17194. [DOI] [PubMed] [Google Scholar]
- 81.Huang O, Wang L, Shen K, Lin H, Hu Z, Liu G, et al. Breast cancer subpopulation with high risk of internal mammary lymph nodes metastasis: analysis of 2,269 Chinese breast cancer patients treated with extended radical mastectomy. Breast Cancer Res Treat. 2008;107:379–387. doi: 10.1007/s10549-007-9561-4. [DOI] [PubMed] [Google Scholar]
- 82.Ray KM, Munir R, Wisner DJ, Azziz A, Holland BC, Kornak J, et al. Internal mammary lymph nodes as incidental findings at screening breast MRI. Clin Imaging. 2015;39:791–793. doi: 10.1016/j.clinimag.2015.05.011. [DOI] [PubMed] [Google Scholar]
- 83.Byrd DR, Dunnwald LK, Mankoff DA, Anderson BO, Moe RE, Yeung RS, et al. Internal mammary lymph node drainage patterns in patients with breast cancer documented by breast lymphoscintigraphy. Ann Surg Oncol. 2001;8:234–240. doi: 10.1007/s10434-001-0234-y. [DOI] [PubMed] [Google Scholar]
- 84.Nikolaevich NS, Vasilevich KS. Why do we need irradiation of internal mammary lymph nodes in patients with breast cancer: analysis of lymph flow and radiotherapy studies. Rep Pract Oncol Radiother. 2017;22:37–41. doi: 10.1016/j.rpor.2016.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wang K, Zhang X, Zheng K, Yin XD, Xing L, Zhang AJ, et al. Predictors of internal mammary lymph nodes (IMLN) metastasis and disease-free survival comparison between IMLN-positive and IMLN-negative breast cancer patients: results from Western China Clinical Cooperation Group (WCCCG) database (CONSORT) Medicine (Baltimore) 2018;97:e11296. doi: 10.1097/MD.0000000000011296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wang YS. The multi-center validation study of internal mammary lymph biopsy with modified injection technique in breast cancer patients. ClinicalTrials.gov Identifier: NCT03024463.
- 87.Xie X, Xiong Z, Xing Li X, et al. Nomogram to predict internal mammary lymph nodes metastasis in patients with breast cancer. Front Oncol. 2019;9:1193. doi: 10.3389/fonc.2019.01193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hu XE, Xue J, Peng S, et al. Preoperative nomogram for predicting sentinel lymph node metastasis risk in breast cancer: a potential application on omitting sentinel lymph node biopsy. Front Oncol. 2021;11:665240. doi: 10.3389/fonc.2021.665240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Whelan TJ, Olivotto IA, Parulekar WR, Ackerman I, Chua BH, Nabid A, et al. Regional nodal irradiation in early-stage breast cancer. N Engl J Med. 2015;373:307–316. doi: 10.1056/NEJMoa1415340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Thorsen LB, Offersen BV, Danø H, Berg M, Jensen I, Pedersen AN, et al. DBCG-IMN: a population-based cohort study on the effect of internal mammary node irradiation in early node-positive breast cancer. J Clin Oncol. 2016;34:314–320. doi: 10.1200/JCO.2015.63.6456. [DOI] [PubMed] [Google Scholar]
- 91.Darby SC, Ewertz M, McGale P, Bennet AM, Blom-Goldman U, Brønnum D, et al. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med. 2013;368:987–998. doi: 10.1056/NEJMoa1209825. [DOI] [PubMed] [Google Scholar]
- 92.EBCTCG (Early Breast Cancer Trialists’ Collaborative Group) McGale P, Taylor C, Correa C, Cutter D, Duane F, et al. Effect of radiotherapy after mastectomy and axillary surgery on 10-year recurrence and 20-year breast cancer mortality: meta-analysis of individual patient data for 8135 women in 22 randomised trials. Lancet. 2014;383:2127–2135. doi: 10.1016/S0140-6736(14)60488-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Verma V, Vicini F, Tendulkar RD, Khan AJ, Wobb J, Edwards-Bennett S, et al. Role of internal mammary node radiation as a part of modern breast cancer radiation therapy: a systematic review. Int J Radiat Oncol Biol Phys. 2016;95:617–631. doi: 10.1016/j.ijrobp.2016.01.058. [DOI] [PubMed] [Google Scholar]
- 94.Green M, Tafazal H, Swati B, Vidya R. Time to redefine the intramammary lymph node as a separate entity? Clin Anat. 2018;31:684–687. doi: 10.1002/ca.23186. [DOI] [PubMed] [Google Scholar]
- 95.Cheng YJ, Nie XY, Ji CC, Lin XX, Liu LJ, Chen XM, et al. Long-term cardiovascular risk after radiotherapy in women with breast cancer. J Am Heart Assoc. 2017;6(5):e005633. doi: 10.1161/JAHA.117.005633. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets/or images are available from the corresponding author on reasonable request.