Abstract
Although most published epidemiological studies have found little evidence of systemic autoimmune disease associated with silicone breast implants, there still remains a question of whether silicones can cause local and/or systemic immune dysfunction. This study further investigates the effects of silicones on autoantibody and immunoglobulin production and macrophage activation in female A.SW mice. Sixty mice were divided among four treatment groups receiving a 0.5-ml intraperitoneal injection of either phosphate-buffered saline (PBS), pristane, silicone gel, or silicone oil. Test bleeds were taken periodically for 6 months. In contrast to pristane, neither silicone gel nor silicone oil induced lupus-associated antinuclear autoantibodies (immunoglobulin G [IgG] anti-nRNP/Sm, Su, and ribosomal P) or lupus nephritis. However, serum IgM became elevated persistently within 1 month of silicone gel or silicone oil administration. Also, the level of IgG3 was clearly elevated in silicone oil-treated mice. In contrast, IgG1, IgG2a, and IgG2b levels were not affected greatly by either silicone gel or oil. Furthermore, peritoneal macrophages from silicone- and pristane-treated mice produced higher levels of interleukin-1β (IL-1β) and IL-6 than those from PBS-treated mice after lipopolysaccharide stimulation. These results suggest that silicone gels and oils are capable of inducing hypergammaglobulinemia and activating macrophages in female A.SW mice.
The relative safety of silicone breast implants (SBI) has been and continues to be controversial. Most published epidemiological studies have found little, if any, evidence of systemic autoimmune disease associated with SBI (2, 3, 6, 14, 17). However, many of these retrospective studies were limited to detecting an increased frequency of classic autoimmune and/or clinically defined diseases. The question remains whether susceptible individuals may develop an ill-defined immunological syndrome as a result of silicone exposure. The present study represents an effort to further examine the effects of silicones on immune function under defined experimental conditions.
Silicone is the generic description for synthetic polymers containing a repeating Si-O backbone and organic groups attached to the silicon atom (7). Medical-grade silicones consist primarily of polydimethylsiloxane (PDMS), which is a synthetic polymer having (CH3)2SiO as its repeating unit. Depending upon the number of repeat units in the polymer chain and the degree of cross-linking, medical-grade silicones can be found as a fluids (oils), gels, or elastomers. Silicone oils are straight chains of PDMS, which are chain terminated with trimethylsilyl groups and come in a variety of viscosities (7). Silicone gels are lightly cross-linked PDMS matrixes which produce three-dimensional networks that are swollen with PDMS fluids. Silicone elastomers are highly cross-linked polymers to which amorphous silica has been added to give greater tensile strength. All SBI are made up of a silicone elastomer envelope which contains either silicone gel filler or saline.
Over the past several years our laboratory has shown that certain forms of silicone are immunologically active. Silicone gel is a potent humoral adjuvant and a weak adjuvant for cell-mediated immunity (CMI) (9). Low-molecular-weight (low-MW) silicone oils are weak adjuvants, whereas the highest-MW and most viscous oil tested showed significant humoral adjuvant properties (10). Silicone gel when mixed with bovine type II collagen is arithritogenic in female DA rats (11). Silicone elastomer, preadsorbed with plasma proteins, activates human monocytes/macrophages in vitro to secrete interleukin-1β (IL-1β), IL-6, and tumor necrosis factor alpha (TNF-α) (13). Also, recent work by McDonald et al. has demonstrated that silicone gel enhances the development of autoimmune disease in NZB mice and has little effect on BALB/c mice (8). Thus, there is substantial evidence that silicones have an effect on immune cells.
Like BALB/c (H-2b) mice, the A.SW (H-2s) mice do not develop spontaneous autoimmunity, making this strain an appropriate model for investigating the immune effects of silicones. In view of the higher prevalence of autoimmunity in women (22) and the preponderance of women receiving SBI, we studied female mice only. We report here on the induction of hypergammaglobulinemia and macrophage activation in female A.SW mice by silicone gels and oils. Also, since silicone gels and oils have been shown to be weak CMI adjuvants (9, 10), only humoral immunity was examined here.
MATERIALS AND METHODS
Sixty female (6- to 8-week-old) A.SW (H-2s-T18b-/SnJ) mice were obtained from The Jackson Laboratory (Bar Harbor, Maine) and were divided equally among four treatment groups as follows: phosphate-buffered saline (PBS) (negative control), pristane (2,6,10,14-tetramethylpentadecane; Sigma, St. Louis, Mo.) (positive control), silicone gel (Silastic II Mammary Implant, lot HH019581; Dow Corning, Midland, Mich.), and silicone oil (360 Medical Fluid 1000cs; Dow Corning). The silicone gel was aseptically removed from an unused mammary implant and was churned with a Brinkman tissue homogenizer to help reduce the gel's viscosity to facilitate injection. Mice received a single 0.5-ml intraperitoneal injection of one of the three materials or an equal volume of PBS. Prior to injection, a pretest bleed (time zero) was taken via orbital puncture, after which blood samples were obtained monthly for 6 months. Serum from each mouse was stored at −70°C until analyzed. All mice were housed under conventional conditions. At 6 months after injection mice were anesthetized and euthanized by cervical dislocation, after which peritoneal macrophages were collected by lavage from some mice. The kidneys were excised, and one was fixed in formalin, while the other was snap frozen in liquid nitrogen and stored at −70°C.
Immunoprecipitation.
Six-month sera were screened for autoantibodies to the Su and nRNP/Sm antigens by immunoprecipitation by using [35S]methionine-[35S]cysteine-labeled K562 cell extracts and 3.0 μl of mouse serum as described previously (19).
Fluorescent antinuclear antibody (FANA).
Indirect immunofluorescence of autoantibodies to nuclear and cytoplasmic antigens was determined by using methanol-fixed HeLa cells as described previously with some modifications (18). Briefly, cells were incubated for 30 min with a 1:40 dilution of mouse serum, washed three times with PBS, and then incubated for 30 min with a 1:40 dilution of fluorescein isothiocyanate (FITC)-conjugated goat anti-mouse IgG (gamma-chain specific; Southern Biotechnology Associates). After being washed, the cells were viewed with an epifluorescence microscope.
ELISAs for IgG anti-chromatin, IgG anti-ssDNA antibodies, and antiribosomal P antibodies.
Immunoglobulin G (IgG) anti-chromatin antibodies were measured as previously described (1). In brief, wells of microtiter plates (Maxisorp; Nunc, Naperville, Ill.) were coated with 100 μl of a 1-μg/ml concentration of chicken chromatin antigens in borate-buffered saline. Sera were tested at a 1:500 dilution, followed by alkaline phosphatase-labeled goat anti-mouse IgG (1:1,000) and p-nitrophenyl phosphate substrate (Sigma). The optical density (OD) was measured at 405 nm by using a Molecular Devices (Menlo Park, Calif.) enzyme-linked immunosorbent assay (ELISA) plate reader. Antibodies to single-stranded DNA (ssDNA) were measured as described previously (20). Briefly, antibodies to heat-denatured calf thymus DNA (Sigma) were detected with a 1:500 dilution of mouse sera and alkaline phosphatase-conjugated goat anti-mouse IgG or IgM antibodies. The absorbance at 405 nm of each sample for anti-ssDNA and anti-chromatin antibodies was converted into units based on a standard curve produced by pooled sera from MRL/lpr mice. The units were assigned as follows: 1:500 dilution, 1,000 U; 1:5,000 dilution, 100 U; 1:50,000 dilution, 10 U; 1:500,000 dilution, 1 U; 1:5,000,000 dilution, 0.1 U; and 1:50,000,000 dilution, 0.01 U. Antiribosomal P antibodies were detected by ELISA using a 22-amino-acid C-terminal peptide as the antigen (18). The results were expressed as the number of mice producing a positive antibody response that was defined as an antibody unit value exceeding the mean plus three standard deviations (SD) of the PBS-treated mice.
Measurement of IgM and IgG.
Total levels of each immunoglobulin isotype were determined by ELISA (5). Microtiter plate wells were coated for 12 h at 4°C with 50 μl of a 3-μg/ml concentration of goat anti-mouse κ- or λ-light-chain antibodies per well (9:1 ratio; Southern Biotechnology) in 20 mM Tris (pH 8.0). Wells were washed with 0.15 M NaCl–2 mM EDTA–50 mM Tris (NET) containing 0.3% Nonidet P-40 (NET–NP-40) and then blocked with 0.5% bovine serum albumin (BSA) in NET–NP-40. Mouse sera were diluted 1:200,000 with NET–NP-40 containing 0.5% BSA and added to the wells for 12 h at 4°C. Immunoglobulin isotype standards (IgG1, IgG2a, IgG2b, IgG3, and IgM [Southern Biotechnology], each at 100 μg/ml, with a 12-h incubation at 4°C) were used for standard curve fitting at concentrations of 100, 50, 25, 10, 5, 2.5, 1, and 0.5 ng/ml. After being washed, the wells were incubated with a 1:1,000 dilution of alkaline phosphatase-labeled goat anti-mouse antibodies specific for IgG1, IgG2a, IgG2b, IgG3, or IgM (Southern Biotechnology); p-nitrophenyl phosphate substrate was then added, and the OD was determined at 405 nm. Statistical analysis was performed by using SigmaStat statistical software (SPSS, Inc., Chicago, Ill.) employing the one-way analysis-of-variance technique applying multiple comparison corrections.
Peritoneal macrophage culture and cytokine measurements.
To determine the effect of silicone gels and oils on macrophage cytokine priming in vivo, peritoneal macrophages from four randomly selected mice were pooled at the time of euthanasia. The cells were washed with PBS and placed in a 24-well culture plate (2 × 105 cells per well in PBS). Lipopolysaccharide (LPS; Escherichia coli, serotype O111:B4; Sigma) was added at 0.5, 5.0, or 25 ng/ml. PBS was added to a fourth set of wells and served as an unstimulated control. The cells were incubated for 16 h at 37°C, the plates were centrifuged, and the supernatants were collected and stored at −70°C until used. Mouse IL-6 and IL-1β were measured by employing commercial ELISA kits from R&D Systems (Minneapolis, Minn.).
Kidney histology and immunofluorescence.
Kidney pathology was assessed as described previously (20). For light microscopy, kidneys were fixed in formalin, dehydrated, and embedded in paraffin, and 6-μm sections were cut and stained with hematoxylin and eosin. For direct immunofluorescence, kidneys were snap frozen, and 5-μm cryostat sections were cut and stained with either FITC-conjugated rabbit anti-mouse IgG (1:100 dilution), goat anti-mouse IgM (1:100 dilution) (Sigma), or rabbit anti-mouse complement 3 (C3) (1:300 dilution) (ICN, Auora, Ohio) antibodies. Sections were examined with a epifluorescence microscope equipped with an FITC filter.
RESULTS
Anticytoplasmic and antinuclear autoantibodies.
Table 1 summarizes the frequency of anticytoplasmic and antinuclear antibody production in A.SW mice 6 months after injection with either PBS, pristane, silicone gel, or silicone oil. Antibodies against ribosomal P, nRNP/Sm, and Su antigens are lupus-associated autoantibodies produced by BALB/c and other strains of mice treated with pristane (5, 18–21). As expected, pristane induced all of these specificities in A.SW mice, as well as anti-ssDNA antibodies. In contrast, mice treated with PBS, silicone gel, or silicone oil did not produce anti-nRNP/Sm, -Su or -ribosomal P antibodies. However, three of the silicone oil-treated mice produced relatively low levels IgG anti-ssDNA. Five silicone gel- and nine silicone oil-treated mice showed a positive FANA reaction. However, this result was not statistically significant because four of the PBS-treated mice also were FANA positive. Table 2 shows the frequency of IgG antichromatin antibodies production over time. At 6 months, one silicone gel and three silicone oil-treated mice produced IgG antichromatin antibodies at levels three to eight times greater than the highest value from the PBS-treated mice. However, on average, only pristane-treated mice produced significant amounts of IgG antichromatin antibodies at between 2 and 5 months.
TABLE 1.
Frequency of positive antinuclear and anticytoplasmic antibody production at 6 months
| Treatment | n | rib-Pa | IgG ssDNAa | FANA grade
|
No. of antibodies
|
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| − | + | 2+ | 3+ | Cyto.b | nRNP/Smc | Suc | ||||
| PBS | 14 | 0 (0) | 0 (0.11 ± 0.11) | 10 | 0 | 3 | 1 | 0 | 0 | 0 |
| Pristane | 14 | 9*d (5.8 ± 13.2) | 10* (8.4 ± 10.5) | 5 | 3 | 1 | 5 | 8**d | 7†d | 9* |
| Silicone gel | 14 | 0 (0) | 0 (0.1 ± 0.07) | 9 | 3 | 0 | 2 | 0 | 0 | 0 |
| Silicone oil | 15 | 0 (0) | 3e (0.23 ± 0.22) | 6 | 5 | 2 | 2 | 0 | 0 | 0 |
Positive response defined as an antibody unit value exceeding the mean plus three SD of the PBS-treated mice (rib-P, antiribosomal antibodies). Values in parentheses represent the mean antibody units ± the SD as determined by ELISA.
Cyto., anticytoplasmic antibodies as detected by fluorescence.
As determined by immunoprecipitation.
∗, P < 0.001; ∗∗, P = 0.002; and †, P = 0.006 (versus PBS as determined by the Fisher exact test [4]).
Not statistically significant versus the PBS group.
TABLE 2.
Frequency of positive IgG antichromatin antibodies
| Treatment | No. positive/total no. (mean antibody units ± SD)a at:
|
||||||
|---|---|---|---|---|---|---|---|
| 0 mo | 1 mo | 2 mo | 3 mo | 4 mo | 5 mo | 6 mo | |
| PBS | 0/15 (0.1 ± 0) | 0/15 (0.11 ± 0.01) | 1/14 (0.11 ± 0.02) | 1/14 (0.11 ± 0.02) | 0/14 (0.25 ± 0.13) | 0/14 (0.23 ± 0.22) | 0/14 (1.7 ± 2.8) |
| Pristane | 0/15 (0.1 ± 0) | 2/15 (0.11 ± 0.03) | 11/15b (1.1 ± 1.5) | 11/14b (1.2 ± 1.5) | 9/14b (6.8 ± 13.2) | 9/14b (11.2 ± 23.4) | 2/14 (6.0 ± 11.6) |
| Silicone gel | 0/15 (0.1 ± 0) | 0/15 (0.1 ± 0.01) | 1/15 (0.11 ± 0.02) | 1/14 (0.11 ± 0.02) | 0/14 (0.22 ± 0.15) | 1/14 (1.0 ± 3.0) | 2/14 (3.3 ± 8.0) |
| Silicone oil | 0/15 (0.1 ± 0) | 0/15 (0.1 ± 0.04) | 1/15 (0.11 ± 0.02) | 1/15 (0.11 ± 0.02) | 2/15 (0.49 ± 0.71) | 2/15 (0.81 ± 1.8) | 3/15c (12.6 ± 26.6) |
Positive response was defined as an antibody unit value exceeding the mean value plus three SD of the PBS-treated mice. Values in parentheses represent the mean antibody units ± the SD as determined by ELISA.
P < 0.001 versus PBS as determined by the Fisher exact test (4).
Not statistically significant versus the PBS group.
Immunoglobulin levels.
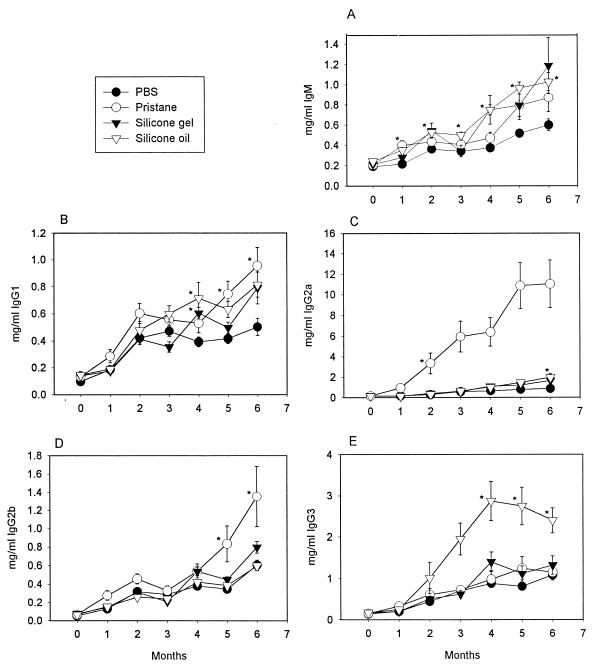
To determine whether silicones affected immunoglobulin production in a nonspecific manner, we examined the major immunoglobulin isotypes. Figure 1A through E shows the mean serum levels and time courses of IgM, IgG1, IgG2a, IgG2b, and IgG3, respectively, from mice treated with PBS, pristane, silicone gel, and silicone oil. As expected, there was a general increase in all immunoglobulins measured over time in mice treated with PBS, a result probably reflecting the normal response to microbial stimulation (5). Pristane-treated mice showed a significant rise in IgM levels (Fig. 1A) within 1 month of injection; these levels began to decline and then followed a course parallel to those of the PBS-treated mice. Silicone gel- and silicone oil-treated mice showed a significant rise in IgM levels within 2 months of injection, and these levels remained elevated for a prolonged period in contrast to the pristane-induced IgM levels. IgG1 (Fig. 1B) serum levels tended to become significantly elevated at 4 months for silicone gel- and silicone oil-treated mice, while the pristane-treated mice showed significant elevations at 5 and 6 months. IgG2a levels increased dramatically in pristane-treated mice (Fig. 1C) compared to all other mice. However, lower but still significant elevations were seen in the silicone oil-treated mice (Fig. 1C) at 6 months. IgG2b (Fig. 1D) levels were only elevated in pristane-treated mice at 5 and 6 months. IgG3 (Fig. 1E) levels became significantly elevated only in silicone oil-treated mice between 4 and 6 months.
FIG. 1.
Mean serum IgM (A), IgG1 (B), IgG2a (C), IgG2b (D), and IgG3 (E) levels (± the standard error of the mean) from mice treated with either PBS, pristane, silicone gel, or silicone oil over time. (A) ∗, P < 0.05 for pristane versus PBS at 1 month, silicone oil versus PBS at 1 through 6 months, and silicone gel versus PBS at 4 months. (B) ∗, P < 0.05 for silicone oil and silicone gel versus PBS at 4 months and pristane versus PBS at 5 and 6 months. (C) ∗, P < 0.05 for pristane versus PBS at 2 through 6 months and silicone oil versus PBS at 6 months. (D) ∗, P < 0.05 for pristane versus PBS at 5 and 6 months. (E) ∗, P < 0.05 for silicone oil versus PBS at 4 through 6 months.
IL-6 and IL-1β secreted from mouse peritoneal macrophages.
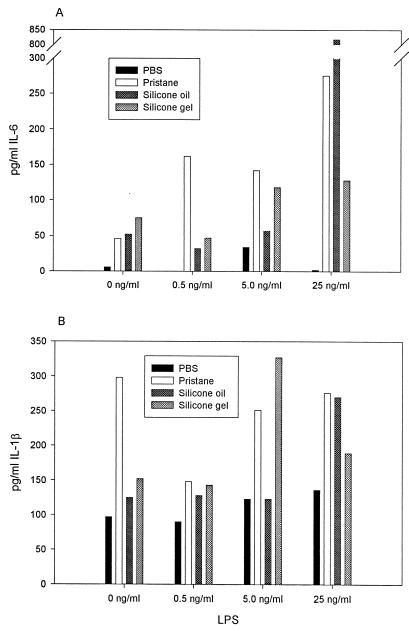
Since we had previously observed that silicones activate monocytes/macrophages in vitro (13), this experiment afforded us an opportunity to study the effects of silicone on macrophages in vivo. The production of IL-6 and IL-1β by pooled peritoneal macrophages from mice treated with silicone oil, silicone gel, pristane, or PBS are presented in Fig. 2A and B, respectively. Since the data represent a single measurement for each bar, only qualitative comparisons can be made. Under nonstimulating conditions, macrophages from PBS-treated mice secreted little IL-6 (Fig. 2A), while pristane-, silicone oil-, and silicone gel-treated mice spontaneously secreted IL-6. Macrophages from PBS-treated mice, cultured with suboptimal amounts of LPS, produced low amounts of IL-6. In contrast, macrophages from pristane-, silicone gel-, and silicone oil-treated mice produced much greater amounts of IL-6 when cultured with LPS in a dose-dependent manner. IL-1β (Fig. 2B) was produced spontaneously from all sampled mouse macrophages, with pristane-treated mice producing the greatest amount. There was generally increased production of IL-1β from macrophages collected from pristane-, silicone gel-, and silicone oil-treated mice when cultured with increasing amounts of LPS. However, this trend was not as pronounced as that seen with IL-6. Although each datum point represents a single cytokine measurement (Fig. 2), there appears nonetheless to be a dose response to LPS, especially for IL-6 (Fig. 2A).
FIG. 2.
IL-6 (A) and IL-1β (B) secreted from peritoneal macrophages pooled from four mice from each treatment group and cultured overnight in PBS with 0, 0.5, 5.0, or 25 ng of LPS per ml.
Kidney pathology.
No significant immune-complex glomerulonephritis was observed by light microscopy in mice treated with either pristane, silicone gel, or silicone oil compared to the PBS control mice. Immunofluorescence revealed no significant deposition of IgM, IgG, or C3 in glomeruli from the experimental mice compared with the PBS control mice. We noted that a few kidney sections from mice treated with either silicone gel or oil had large vacuoles within some of their glomeruli. Although we do not have definitive analytical evidence, we speculate that these vacuoles once contained silicone that was washed out during histological processing. Curiously, these vacuoles were not observed in kidney sections obtained from pristane-treated mice.
DISCUSSION
The immunological response to silicones is not fully understood. When mixed with an antigen, silicone gel behaves as a potent humoral adjuvant but does not enhance CMI (9). In comparison, low-MW silicone oils are generally weak adjuvants. The lack of adjuvancy by silicone oils has been ascribed to their inability to form an optimal emulsion with an antigen, in contrast to the more efficient formation of an emulsion with silicone gels (12). Because low-MW silicone oils are poor adjuvants, we had assumed that only ruptured silicone gel-filled implants may pose a health risk. More recently, however, we demonstrated that both silicone gel and silicone oil (1,000 cs) can activate monocytes in vitro to overproduce proinflammatory cytokines (IL-6, IL-1β, and TNF-α) (13). Thus, our revised working hypothesis is that both silicone gels and silicone oils incite a chronic inflammation both locally and systemically. This chronic inflammatory response may perturb immune regulatory pathways, but it is unlikely to lead to classic autoimmunity, e.g., rheumatoid arthritis, systemic lupus erythematosus, and scleroderma, at least in individuals without genetic susceptibility. This hypothesis is supported by the present investigations. However, the question of whether or not these materials can exacerbate autoimmunity in mice predisposed to lupus-like disease (8) was not examined here.
Induction of hypergammaglobulinemia by silicone oil or gel.
Polyclonal hypergammaglobulinemia was perhaps the most striking abnormality induced by the intraperitoneal administration of silicone oil and silicone gel. The increased level of IgG3 induced by silicone oil and the increased total IgM level induced by both gel and oil was even higher than that seen in pristane-treated mice, whereas the increased levels of IgG1 were comparable. In contrast, pristane induced far higher levels of IgG2a and IgG2b than silicone oil or gel. The preferential increase in IgM and IgG3 is consistent with the first phase of immunoglobulin production induced by pristane (20). In pristane-induced lupus, this early phase has been hypothesized to reflect polyclonal B-1 cell activation and expansion (15). However, the comparable levels of IgG1, a T-cell-dependent isotype, induced by silicone gels and oils and pristane suggest that there may be effects on conventional B cells as well. This may be mediated by IL-4 (23). In contrast to silicone gels and oils, pristane appears to be far more potent at inducing the production of IgG2a autoantibodies, suggesting that pristane may stimulate gamma interferon (IFN-γ) production more efficiently (23). Recent data are consistent with the importance of IFN-γ in the pathogenesis of pristane-induced lupus (H. B. Richards et al., manuscript in preparation).
Enhancement of IL-6 and IL-1β production.
A second immune effect of intraperitoneal silicone oil or gel administration is the enhancement of IL-6 and IL-1β production by peritoneal macrophages. Silicone oil, silicone gel, and pristane all appear to prime these cells to overproduce IL-6 and IL-1β in response to LPS. This result is consistent with previous observations that silicones preabsorbed with protein activate monocytes in vitro (13). We ascribed this activation to the very hydrophobic nature of silicones, since polystyrene, preadsorbed with protein, also can activate monocytes in vitro (13). Thus, IL-6 and IL-1β may play a role in mediating the inflammatory response to silicones. Moreover, IL-6 may be involved in the pathogenesis of the hypergammaglobulinemia. This cytokine has well-known effects on immunoglobulin production and plasma cell differentiation (1) and is critical for the production of anti-DNA and antichromatin autoantibodies by pristane-treated BALB/c mice (16). In contrast, the production of anti-nRNP/Sm and Su autoantibodies is less IL-6 dependent. It is of interest, therefore, that silicone gels and oils augment spontaneous, as well as LPS-inducible, IL-6 overproduction by peritoneal macrophages along with the production of high levels of anti-DNA and antichromatin autoantibodies in at least some mice (Table 2).
Induction of autoantibodies by silicone oil or gel.
In contrast, none of the silicone gel- or oil-treated mice produced anti-nRNP/Sm, Su, or ribosomal P autoantibodies, whereas all of these specificities were produced by pristane-treated A.SW mice. We speculate that this may be related to the inefficient stimulation of IFN-γ production by silicones, as reflected in the markedly lower total IgG2a levels compared to pristane-treated mice (Fig. 1C). In support of this interpretation is evidence that the frequency of anti-nRNP/Sm autoantibodies is greatly reduced in IFN-γ-deficient compared with wild-type or IL-4-deficient BALB/c mice (Richards et al., in preparation).
The presence of suspected silicone droplets in the glomeruli of some mice 6 months after treatment is an indicator of silicone's durability and capacity for systemic dissemination throughout the host. These suspected droplets of silicone would likely present a large effective surface area, allowing for persistent macrophage activation and the expression of proinflammatory cytokines.
In conclusion, the present studies suggest that the intraperitoneal administration of silicone oils or silicone gels induces some of the abnormalities typical of pristane-induced lupus in mice. Polyclonal IgM, IgG3, and IgG1 hypergammaglobulinemia, as well as overproduction of IL-6 and IL-1β, are especially prominent. However, in contrast to pristane-induced lupus, there is a quantitative difference in the stimulation of IgG2a production, a difference presumably related to lower levels of IFN-γ. This may be responsible for the relatively poor efficacy of silicone gels and oils in stimulating autoantibody production. Nevertheless, the high levels of antichromatin autoantibodies produced by some of the mice suggest that in some cases chronic immune stimulation by silicone gels and oils may lead to the production of a subset of autoantibodies, although not the full spectrum seen in pristane-treated mice. These observations may be relevant to understanding the vague and often poorly defined autoimmune-like phenomena occurring in certain individuals with silicone implants. However, further experimental and clinical studies will be necessary to address this question.
ACKNOWLEDGMENTS
This work was supported by U.S. Public Health Service grants AI34597, AR44731, AI44074, P60-AR30701, and T32-AR7416.
REFERENCES
- 1.Akira S, Taga T, Kishimoto T. Interleukin-6 in biology and medicine. Adv Immunol. 1993;54:1–78. doi: 10.1016/s0065-2776(08)60532-5. [DOI] [PubMed] [Google Scholar]
- 2.Edworthy S M, Martin L, Barr S G, Birdsell D C, Brant R F, Fritzler M J. A clinical study of the relationship between silicone breast implants and connective tissue disease. J Rheumatol. 1998;25:254–260. [PubMed] [Google Scholar]
- 3.Gabriel S E, O'Fallon W M, Kurland L T, Beard C M, Woodsand J E, Melton L J. Risk of connective-tissue diseases and other disorders after breast implantation. N Engl J Med. 1994;330:1697–1702. doi: 10.1056/NEJM199406163302401. [DOI] [PubMed] [Google Scholar]
- 4.Glantz S A, editor. Primer of biostatistics. 3rd ed. New York, N.Y: McGraw-Hill; 1992. pp. 110–154. [Google Scholar]
- 5.Hamilton K J, Satoh M, Swartz J, Richards H B, Reeves W H. Influence of microbial stimulation on hypergammaglobulinemia and autoantibody production in pristane-induced lupus. Clin Immunol Immunopathol. 1998;86:271–279. doi: 10.1006/clin.1997.4481. [DOI] [PubMed] [Google Scholar]
- 6.Hennekens C H, Lee I, Cook N R, Hebert P R, Karlson E W, LaMotte F, Manson J E, Buring J E. Self-reported breast implants and connective-tissue diseases in female health professionals. JAMA. 1996;275:616–621. [PubMed] [Google Scholar]
- 7.Lane T H, Burns S A. Silica, silicon, and silicone … unraveling the mystery. Curr Top Microbiol Immunol. 1996;210:3–12. doi: 10.1007/978-3-642-85226-8_1. [DOI] [PubMed] [Google Scholar]
- 8.McDonald A H, Weir K, Schneider M, Gudenkuaf L, Sanger J R. Silicone gel enhances the development of autoimmune disease in New Zealand black mice but fails to induce it in BALB/cAnPt mice. Clin Immunol Immunopathol. 1998;87:248–255. doi: 10.1006/clin.1998.4532. [DOI] [PubMed] [Google Scholar]
- 9.Naim J O, Lanzafame R J, van Oss C J. The effect of silicone-gel on antibody formation in rats. Immunol Investig. 1993;22:151–161. doi: 10.3109/08820139309063397. [DOI] [PubMed] [Google Scholar]
- 10.Naim J O, Ippolito K M, Lanzafame R J, van Oss C J. The effect of molecular weight and gel preparation on humoral adjuvancy of silicone oils and silicone gels. Immunol Investig. 1995;24:537–547. doi: 10.3109/08820139509066849. [DOI] [PubMed] [Google Scholar]
- 11.Naim J O, Ippolito K M, Lanzafame R J, van Oss C J. Induction of type II collagen arthritis in the DA rat using silicone gels and oils as adjuvant. J Autoimmunity. 1995;8:751–761. doi: 10.1006/jaut.1995.0056. [DOI] [PubMed] [Google Scholar]
- 12.Naim J O, Ippolito K M L, van Oss C J. Adjuvancy effect of different types of silicone gel. J Biomed Materials Res. 1997;37:534–538. doi: 10.1002/(sici)1097-4636(19971215)37:4<534::aid-jbm12>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 13.Naim J O, van Oss C J, Ippolito K M L, Zhang J-W, Jin L-P, Fortuna R, Buehner N A. In vitro activation of human monocytes by silicones. Colloids Surfaces B Biointerfaces. 1998;11:79–86. [Google Scholar]
- 14.Park A J, Black R J, Sarhadi N S, Chetty U, Watson A C. Silicone gel filled breast implants and connective tissue diseases. Plastic Reconstruct Surg. 1998;101:261–268. doi: 10.1097/00006534-199802000-00001. [DOI] [PubMed] [Google Scholar]
- 15.Richards H B, Satoh M, Jennette J C, Okano T, Kanwar Y S, Reeves W H. Disparate T cell requirements of two subsets of lupus-specific autoantibodies in pristane-treated mice. Clin Exp Immunol. 1999;115:547–553. doi: 10.1046/j.1365-2249.1999.00825.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Richards H B, Satoh M, Shaw M, Libert C, Poli V, Reeves W H. Interleukin-6 dependence of anti-DNA antibody production: evidence for two pathways of autoantibody formation in pristane-induced lupus. J Exp Med. 1998;188:985–990. doi: 10.1084/jem.188.5.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sanchez-Guerrero J, Colditz G A, Karlson E W, Hunter D J, Speizer F E, Liang M H. Silicone breast implants and the risk of connective-tissue diseases and symptoms. N Engl J Med. 1995;332:1666–1670. doi: 10.1056/NEJM199506223322502. [DOI] [PubMed] [Google Scholar]
- 18.Satoh M, Hamilton K J, Ajmani A K, Dong X, Wang J, Kanwar Y S, Reeves W H. Autoantibodies to ribosomal P antigens with immune complex glomerulonephritis in SJL mice treated with pristane. J Immunol. 1996;157:3200–3206. [PubMed] [Google Scholar]
- 19.Satoh M, Reeves W H. Induction of lupus-associated autoantibodies in BALB/c mice by intraperitoneal injection of pristane. J Exp Med. 1994;180:2341. doi: 10.1084/jem.180.6.2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Satoh M, Kumar A, Kanwar Y S, Reeves W H. Anti-nuclear antibody production and immune-complex glomerulonephritis in BALB/c mice treated with pristane. Proc Natl Acad Sci USA. 1995;92:10934–10938. doi: 10.1073/pnas.92.24.10934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Satoh M, Treadwell E L, Reeves W H. Pristane induces high titers of anti-Su and anti-nRNP/Sm autoantibodies in BALB/c mice: quantitation by antigen capture ELISAs based on monospecific human autoimmune sera. J Immunol Methods. 1995;182:51–62. doi: 10.1016/0022-1759(95)00022-3. [DOI] [PubMed] [Google Scholar]
- 22.Schwartz R S. Autoimmunity and autoimmune diseases. In: Paul W E, editor. Fundamental immunology. 3rd ed. New York, N.Y: Raven Press; 1993. pp. 1064–1065. [Google Scholar]
- 23.Snapper C M, Paul W E. Interferon-gamma and B cell stimulatory factor-1 reciprocally regulate Ig isotype production. Science. 1987;236:944–947. doi: 10.1126/science.3107127. [DOI] [PubMed] [Google Scholar]