Abstract
Substance P (SP) has been implicated in peripheral and mucosal neuroimmunoregulation. However, confusion remains regarding immunocyte expression of the receptor for SP, neurokinin-1 receptor (NK-1R), and whether there is differential NK-1R expression in the mucosal versus the peripheral immune system. In the same assay systems, we examined the expression of NK-1R in human lamina propria mononuclear cells (LPMC), peripheral blood mononuclear cells (PBMC), peripheral blood lymphocytes (PBL), monocytes, and monocyte-derived macrophages (MDM). Using standard reverse transcription (RT)-PCR, mRNA expression of both the long and the short isoforms of the NK-1R was evident in LPMC but not in PBMC, PBL, monocytes, or MDM. However, by using nested RT-PCR NK-1R mRNA expression was detected in PBMC, PBL, monocytes, and MDM. This level of expression was found to represent one NK-1R mRNA transcript in >1,000 cells. In contrast, by using competitive RT-PCR we demonstrate that LPMC express a more biologically significant level of eight NK-1R mRNA transcripts per cell. Flow cytometric detection of NK-1R expression at the protein level was evident in LPMC but not in PBMC. These findings illustrate the extreme sensitivity of nested RT-PCR and the advantages of competitive RT-PCR in comparative studies of receptor expression in different cell populations. This study suggests that, under normal conditions, readily detectable expression of NK-1R in human mononuclear cells occurs at the mucosal level rather than in the peripheral circulation.
Evidence for a reciprocal or bidirectional communication between the immune system and the neuroendocrine system is well established (4). Intersystem cross-talk is mediated via a common biochemical language consisting of cytokines, neuropeptides, and their receptors (4). The gastrointestinal tract, the largest lymphoid organ of the body, is rich in peptidergic innervation and neuropeptide content and provides an ideal milieu for neuroimmune interactions (43). The mucosal neuroimmune axis is now recognized as an important modulator of gut homeostasis and pathophysiology (10, 42).
The neuropeptide substance P (SP) has been extensively characterized as an immunomodulator (31). SP influences lymphocyte traffic in vivo (33), stimulates the proliferation of peripheral and mucosal lymphocytes (35, 45), enhances immunoglobulin production by Peyer's patches', splenic and mesenteric lymph node lymphocytes (45), and stimulates the differentiation of B lymphocytes (6). SP induces the chemotaxis of monocytes (40), stimulates monocyte production of interleukin-1 (IL-1), IL-6, IL-10, and tumor necrosis factor alpha (20, 26), activates macrophages (17), and enhances macrophage phagocytosis (2). Peritoneal and mucosal mast cells release histamine upon stimulation with SP (44). The primary source of SP is neuronal. However, lymphocytes (25), monocytes (21), macrophages (21), and eosinophils (32) have been identified as alternative sources of SP.
SP mediates its biologic effects by preferentially binding to the neurokinin-1 receptor (NK-1R) (38). Binding sites for SP have been described on a subset of human T lymphocytes (36). Mantyh and coworkers have autoradiographically demonstrated SP binding sites in the germinal centers of colonic lymph nodules (12, 28). In contrast to these findings, radioligand binding studies by Roberts et al. reported the absence of SP binding sites on human peripheral blood lymphocytes (PBL), polymorphonuclear leukocytes, splenocytes, intraepithelial lymphocytes, or jejunal lamina propria lymphocytes (39). A non-neurokinin receptor for SP has been demonstrated on human monocytes (23). It has also been reported that SP can activate T lymphocytes independent of the receptor (24). Using the techniques of RT-PCR, in situ hybridization, immunohistochemistry, and flow cytometry, we previously demonstrated that NK-1R is expressed by human mucosal mononuclear cells but not by peripheral blood mononuclear cells (PBMC) (15). In contrast, using nested reverse transcription (RT)-PCR, it has recently been reported that human lymphocytes, monocytes, and monocyte-derived macrophages (MDM) express NK-1R mRNA (21, 25).
Despite the substantial evidence implicating SP in peripheral and mucosal neuroimmunoregulation, considerable confusion remains regarding immunocyte expression of the NK-1 receptor. This may possibly be explained by differences in the sensitivity of techniques for demonstration of receptor expression. In this study we compared the techniques of nested RT-PCR, competitive RT-PCR, and flow cytometry in our analysis of NK-1R expression by human PBMC, monocytes, MDM, and lamina propria mononuclear cells (LPMC).
MATERIALS AND METHODS
Specimens.
Peripheral blood specimens were obtained from healthy volunteers. Colonic tissue was obtained from patients undergoing surgical resection for colorectal adenocarcinoma. Only histologically normal tissue from an uninvolved area of the colon was used. All protocols were approved by the University Teaching Hospitals Ethics Committee (Cork, Ireland).
Cell isolation.
PBMC were isolated from heparinized blood by centrifugation over Ficoll-Hypaque gradients (Sigma Chemical Co., St. Louis, Mo.) (7). Cells were recovered and washed twice in Dulbecco modified Eagle medium (DMEM). Monocytes were isolated according to previously described techniques (18, 19). Briefly, mononuclear cells were incubated with DMEM in a 2% gelatin-coated flask for 45 min at 37°C, followed by the removal of nonadherent cells with DMEM. Monocytes were detached by EDTA. Following the initial purification, at least 97% of the cells were monocytes, as determined by nonspecific esterase staining and fluorescence-activated cell sorter (FACS) analysis using a monoclonal antibody against CD14 (Leu-M3) and low-density lipoprotein specific for monocytes and macrophages. Nonadherent PBL were collected from gelatin-coated flasks and washed three times with phosphate-buffered saline (PBS).
LPMC were isolated by a technique originally described by Bull and Bookman (8). The entire procedure was performed in 6 h without interruption. The colonic specimen was washed in saline, and the adherent debris was removed. The mucosa was stripped free from underlying musculature and was cut into small pieces (1 by 3 cm). To remove the epithelium, tissue was incubated in a shaking water bath at 37°C for 30 min in calcium-magnesium-free Hanks balanced salt solution containing 1 mM EDTA and 50 μg of gentamicin per ml. Then, 100 U of penicillin, 100 μg of streptomycin, and 0.25 μg of fungizone per ml were also added as a 1% anti-fungal-bacterial solution. The incubations were repeated every 30 min with fresh medium until the supernatant was free of epithelial cells.
The remaining tissue was then minced into smaller pieces and digested in a shaking water bath at 37°C for 1 h with 0.5 mg of collagenase and 1 mg of hyaluronidase (Sigma Chemical Co.) per ml in RPMI 1640 containing 10% fetal calf serum (FCS), 2 mM l-glutamine, 1% anti-fungal-bacterial solution, and 50 μg of gentamicin per ml. The supernatant was collected, and the cells were pelleted by centrifugation (500 × g for 5 min), resuspended in the same medium without the enzyme solution, and then filtered through a 60-μm (pore-size) nylon mesh to remove the particulate material. The filtrate was then centrifuged over a Ficoll-Hypaque density gradient. LPMC were recovered, washed twice in DMEM, and resuspended in DMEM supplemented with 10% FCS and antibiotics as detailed above. LPMC isolations were routinely examined by FACS analysis and were found to show >95% CD45 positivity.
Cell culture.
The human IM-9 B lymphoblastoid cell line was obtained from American Type Culture Collection (Rockville, Md.). IM-9 cells were cultured in DMEM supplemented with 10% FCS, 2 mM glutamine, and antibiotics. Cells were maintained at 37°C in a humidified atmosphere containing 5% CO2.
PBL were cultured in RPMI 1640 medium containing 10% FCS, with or without 1 μg of phytohemagglutinin (PHA) per ml for 72 h. Two different activation procedures were employed to activate PBMC. PBMC (106/ml) were cultured either with PHA (3 μg/ml) or with phorbol myristate acetate (PMA) (10 ng/ml), plus ionomycin (500 ng/ml), in DMEM with 10% FCS at 37°C in a humidified 5% CO2 atmosphere for 48 and 24 h, respectively.
Freshly isolated monocytes were plated in 48-well culture plates at a density of 2.5 × 105 cells/well in DMEM containing 20% FCS. The total length of time in culture for fresh monocytes was no more than 48 h, whereas MDM refers to 7- to 10-day-cultured monocytes in vitro. Monocyte and MDM viability was monitored by trypan blue exclusion and maintenance of cell adherence.
Primer design.
PCR primers were designed using the DNASTAR Lasergene Primerselect program (DNASTAR, Inc., Madison, Wis.). Primers were selected that showed insignificant homology to any other genes in the EMBL DNA sequence database. Primer pairs were chosen to span introns in their genomic sequences, thus ensuring mRNA-specific amplification.
NK-1R PCR was performed using the primers TGACCGCTACCACGAGCAAGTCTC (sense) and ATAGTCGCCGGCGCTGATGAAG (antisense), which correspond to nucleotides 699 to 722 and 993 to 972, respectively, of human NK-1R cDNA and yield an amplification product size of 295 bp. Nested PCR for NK-1R utilized the primers TCTCTGCCAAGCGCAAGGTGGTCA (sense) and GAAGGCATGCTTGAAGCCCAGACG (antisense), which correspond to nucleotides 719 to 742 and 960 to 937, respectively, of human NK-1R cDNA and yield an amplification product size of 242 bp.
PCR specific for the short isoform of the NK-1R was performed using the primers CCCCGGGGACTCCTCTGACC (sense) and CTACTCCGGGC-TCCCATTCCTG (antisense) corresponding to nucleotides 684 to 703 and 1121 to 1100, respectively, of human NK-1R (short isoform) cDNA (11). β-Actin control PCR utilized the primers CCTTCCTGGGCATGGAGTCCTG (sense) and GGAGCAATGATCTTGATCTTC (antisense) corresponding to nucleotides 794 to 815 and 995 to 975, respectively, of human β-actin cDNA.
Detection of NK-1R mRNA expression by RT-PCR.
Total RNA was isolated by phenol-chloroform extraction of guanidinium isothiocyanate lysates (9). cDNA was synthesized by using approximately 1 μg of total RNA, 9 U of avian myeloblastosis virus reverse transcriptase (Promega Corp., Madison, Wis.), 40 U of RNasin (Promega Corp.), 500 μM deoxynucleoside triphosphates (dNTPs), and either 500 nM NK-1R-specific antisense primer GGATTTCATTTCCAGCCCCT or 125 nM random hexanucleotide primers (Boehringer Mannheim GmbH, Mannheim, Germany) per 30-μl reaction for 90 min at 42°C.
PCR was performed on 1% of the cDNA using a final concentration of 1.5 μM MgCl2, 50 μM dNTPs, 0.1 μM concentrations of each primer, and 1 U of Taq DNA polymerase (Promega Corp.) per 50-μl reaction. Negative controls were performed either by omitting reverse transcriptase from cDNA synthesis or by omitting cDNA from the PCR amplifications. As a positive control, RNA from cells known to abundantly express NK-1R mRNA was used, i.e., the IM-9 B lymphoblastoid cell line. Hot start PCR was employed to increase the specificity of the amplification.
Thermal-cycling programs were as follows. For NK-1R PCR, denaturation was at 96°C for 15 s, annealing was at 60°C for 30 s, and extension was at 72°C for 1 min 30 s for 45 cycles. For nested NK-1R PCR, denaturation was at 96°C for 15 s, annealing was at 60°C for 30 s, and extension was at 72°C for 1 min 30 s for 35 cycles. For NK-1R (short isoform) PCR, denaturation was at 96°C for 15 s, annealing was at 62°C for 30 s, and extension was at 72°C for 3 min for 45 cycles. For β-actin PCR, denaturation was at 96°C for 15 s, annealing was at 55°C for 30 s, and extension was at 72°C for 3 min for 35 cycles.
For nested-PCR purposes, the primary and secondary PCR amplifications were performed using identical reagent concentrations, and the thermal cycles were as detailed above, except that the secondary PCR was performed on 1% of the amplification products obtained from the primary PCR.
PCR products were analyzed by electrophoresis through 2% agarose gels and viewed under UV light after ethidium bromide staining. Product specificities were confirmed by DNA sequence analysis using an ABI Prism 310 Genetic Analyzer (Perkin-Elmer, Norwalk, Conn.). The sensitivities of the primary and secondary (nested) RT-PCR assays for NK-1R were calculated by using a dilution series of a known quantity of in vitro-transcribed NK-1R transcript. The primary RT-PCR assay is capable of detecting fewer than 50 NK-1R transcripts, while the nested RT-PCR assay can detect fewer than 10 NK-1R transcripts in the starting RNA template.
Immunofluorescence flow cytometric measurement of NK-1R.
The NK-1R antibody was raised in rabbits against a synthetic peptide (MDNVLPVDSDLSP) corresponding to the extracellular N-terminal amino acids 1 to 13 of human NK-1R. The immunoglobulin G (IgG) fraction was affinity purified on an AH-Sepharose column to which the peptide had been coupled. The antibody specificity was confirmed by extensive radioimmunoassays and Western blotting.
PBMC and LPMC isolations were fixed with 2% paraformaldehyde for 10 min on ice, permeabilized with 70% methanol for 2 min on ice, and then washed twice in PBS containing 2% FCS (used for all subsequent wash steps). A total of 5 × 105 cells were incubated with rabbit polyclonal anti-human NK-1R-specific IgG at a dilution of 1:50 for 30 min on ice. Cells were washed twice and fluorescein isothiocyanate-conjugated anti-rabbit IgG (Dako Corp., Carpinteria, Calif.) was added for 30 min on ice. Cells were washed twice again and analyzed using an Epics Elite flow cytometer (Coulter Corp., Hialeah, Fla.). Isotype-matched control antibodies were used in negative control staining. A total of 10,000 cells were analyzed for each determination. Events contained within the forward-light scatter window corresponding to lymphocytes were selected and analyzed.
Quantitation of NK-1R mRNA expression by quantitative competitive RT-PCR (qcRT-PCR).
To facilitate quantitation of NK-1R mRNA, a competitive internal RNA standard was constructed (34). This standard is identical to the target NK-1R sequence except for an internal deletion of 71 bp. Construction of the competitive standard involved cloning a NK-1R PCR product (324 bp) into pBluescript (Stratagene, La Jolla, Calif.) at the EcoRV site. This NK-1R PCR product was obtained by using the following primers: GACTCCTCTGACCGCTACCA (sense) and GGATTTCATTTCCAGCCCCT (antisense) corresponding to nucleotides 691 to 710 and 1014 to 995, respectively, of the human NK-1R cDNA. Orientation of the PCR product insert within the plasmid was determined by restriction mapping with HinfI. Digestion at the BsrGI and BglII unique restriction sites within the cloned insert resulted in the deletion of a 71-bp fragment. Sticky ends of the plasmid were then filled in by Klenow DNA polymerase (Promega), and blunt-ended recircularization of the plasmid was performed using T4 DNA ligase (Promega). After propagation in Escherichia coli, the deleted recombinant plasmid was subjected to in vitro transcription with T3 RNA polymerase (Promega) to synthesize deleted sense RNA transcripts (257 bp) for use as a competitive standard in qcRT-PCR.
For qcRT-PCR, various amounts of RNA standard transcripts of known concentration were spiked into aliquoted target RNA samples, and the mixtures were then subjected to RT-PCR as described above. As the internal standard is spiked in at the cDNA synthesis step, competition for both RT and PCR amplification occurs. Equivalence of PCR products occurred when target and standard templates were present at equal initial concentrations, permitting quantitation of the target template. Equivalence was determined as the point at which target and competitive standard PCR products were of equal band intensity. Results for NK-1R mRNA quantitation are expressed as the number of NK-1R mRNA molecules per microgram of total RNA isolated. Total RNA was quantified using a nucleic acid quantitation kit (Invitrogen, Leek, The Netherlands). The assay is sufficiently sensitive to quantitate 100 NK-1R mRNA copies. This is equivalent to 103 NK-1R mRNA transcripts/μg of RNA isolated.
RESULTS
NK-1R mRNA is differentially expressed by mucosal and peripheral lymphoid cells.
We examined the expression of NK-1R mRNA in human LPMC, PBMC, PBL, monocytes, and MDM by RT-PCR. Using standard single-round RT-PCR, NK-1R mRNA expression was detected in the IM-9 positive control cells and in the LPMC but not in PBMC, PBL, monocytes, or MDM (Fig. 1C). When the input cDNA concentration in the PCR amplifications was increased from 1 to 10%, identical results arose, namely, IM-9 cells and LPMC were found to express NK-1R mRNA, whereas PBMC, PBL, monocytes, and MDM were negative for NK-1R mRNA expression (Fig. 1E).
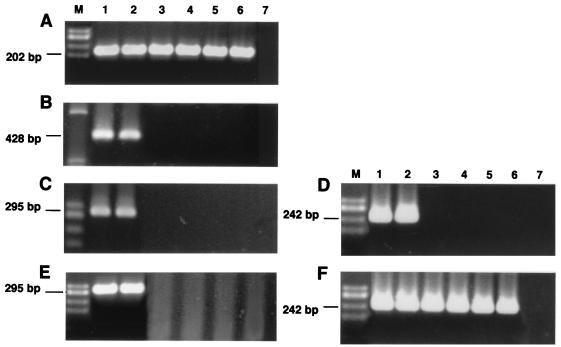
FIG. 1.
NK-1R mRNA expression was analyzed by RT-PCR of equalized input RNA from IM-9 cells, LPMC, resting PBMC, PBMC activated with PHA, monocytes, MDM, and negative controls (lanes 1 to 7, respectively). HaeIII-digested φX174 DNA size markers were used (lane M). Gel A shows mRNA-specific amplification products (202 bp) for β-actin to control for amplification efficiency. Gel B shows mRNA-specific amplification products for the short isoform of NK-1R (438 bp). Gels C and E show mRNA-specific amplification products (295 bp) from primary-round RT-PCR for NK-1R using 1% (C) and 10% (E) cDNA. Gels D and F show mRNA-specific amplification products for NK-1R (242 bp) from secondary-round nested RT-PCR using 1% (D) and 10% (F) cDNA.
In order to increase the sensitivity of mRNA detection, we performed nested RT-PCR on the primary PCR amplification products (as discussed above), using primers which spanned a region internal to that examined previously. Results obtained from nested RT-PCR analysis of NK-1R mRNA expression are shown in Fig. 1D and F. When the input cDNA concentration in the primary PCR amplification was 1%, nested-RT-PCR results were identical to those achieved by standard RT-PCR, namely, IM-9 cells and LPMC were shown to be positive for NK-1R mRNA expression, while PBMC, PBL, monocytes, and MDM were shown to be negative for NK-1R mRNA expression (Fig. 1D). However, when the input cDNA concentration in the primary PCR amplification was increased to 10%, nested-RT-PCR analysis demonstrated an NK-1R-specific mRNA amplification product in IM-9 cells, LPMC, PBMC, PBL, monocytes, and MDM (Fig. 1F).
We have previously reported that activation of IM-9 lymphoblastoid cells with PMA plus ionomycin caused a sevenfold upregulation in NK-1R mRNA expression levels (14). Since freshly isolated PBMC were found to express a very low level of NK-1 mRNA de novo, detectable only by extremely sensitive nested RT-PCR, we decided to investigate whether this basal level of expression could be upregulated by activation. We observed that activation of PBMC with either PHA (Fig. 1, lane 4) or PMA-ionomycin (data not shown) did not significantly upregulate NK-1R mRNA expression levels, since expression was not detected in activated PBMC when an input cDNA concentration of 1% was used but was detected when a starting concentration of 10% cDNA was used for nested RT-PCR. This suggests that activated PBMC express a similar level of NK-1R mRNA as resting PBMC (Fig. 1, lane 3).
A short isoform of the NK-1R has been characterized in human brain and in rat submaxillary gland (11, 22). The short isoform differs from the long isoform of NK-1R in the length of its carboxyl-terminal tail (11, 22). Since previous reports of NK-1R expression by human PBMC (21, 25) could perhaps be attributed to expression of the short isoform of NK-1R, we decided to examine our lymphoid cell isolations for mRNA expression of the short isoform of NK-1R. Using standard RT-PCR, mRNA expression of the short isoform of NK-1R was detected in IM-9 cells and LPMC but not in PBMC, PBL, monocytes, and MDM (Fig. 1B). Since IM-9 cells and LPMC were also found to express mRNA for the long isoform of NK-1R by standard RT-PCR, this finding suggests that the short isoform of the NK-1R is derived from alternative splicing of NK-1R pre-mRNA.
All RT-PCR assays were controlled by equalization of input RNA for each cell isolation. Comparable amplification efficiencies were achieved in all RNA samples, as evidenced by the uniformity of control β-actin RT-PCR product yields (Fig. 1A). Negative control RT-PCRs showed no amplification product (Fig. 1, lane 7).
Quantitative assessment of NK-1R mRNA expression levels in peripheral versus mucosal mononuclear cells.
Using a dilution series of a known quantity of in vitro-transcribed NK-1R RNA, we have defined the sensitivity of our primary and secondary (nested) NK-1R-specific RT-PCR assays. The primary RT-PCR assay can detect fewer than 50 NK-1R transcripts, while the nested-RT-PCR assay can detect fewer than 10 NK-1R transcripts in the starting RNA template. These defined sensitivities allowed us to estimate the number of NK-1R mRNA transcripts expressed per cell in the different lymphoid cell isolations.
The RNA preparations used in the above experiments were each obtained from approximately 5 × 106 cells. We used one-fifth of the RNA preparations for cDNA synthesis (equivalent to 106 cells) and either 1% (equivalent to 104 cells) or 10% (equivalent to 105 cells) of the cDNA for nested PCR. When the starting cDNA concentration was 1%, nested PCR did not yield an NK-1R-specific PCR product in PBMC, PBL, monocytes, or MDM. However, an NK-1R PCR product was evident in these samples when 10% cDNA was used. Since our nested-RT-PCR assay can detect fewer than 10 NK-1R RNA transcripts in the template RNA, what we detected therefore was fewer than 10 NK-1R mRNA transcripts per 104 to 105 cells in the PBMC, PBL, monocyte, and MDM samples. The only variable we haven't accounted for is the percent yield of RNA from the cells (it is difficult to estimate how much RNA a given cell could contain). Even if the recovery of RNA was only 50%, this detection would still reflect single NK-1R mRNA transcripts in 500 to 5,000 cells.
Mucosal but not peripheral lymphoid cells express detectable NK-1R protein.
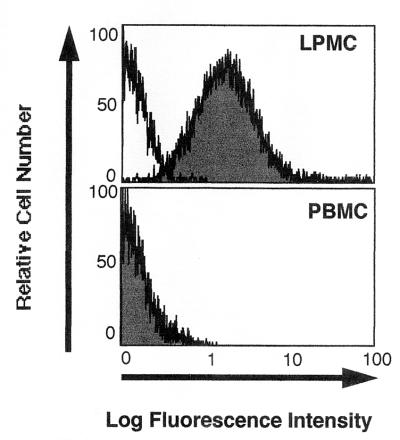
A polyclonal antibody specific for the extracellular N terminus of the human NK-1R enabled flow cytometric analysis of NK-1R protein expression levels in PBMC and LPMC. The fluorescence profiles of NK-1R expression in PBMC and LPMC are shown in Fig. 2. NK-1R immunofluorescence was not detected in PBMC but was detected in LPMC preparations.
FIG. 2.
Fluorescence profiles of NK-1R expression in PBMC and LPMC (as indicated). NK-1R antibody staining (shaded profile) relative to control antibody staining (open profile) is shown for PBMC and LPMC. Data are representative of three experiments.
Quantitation of NK-1R mRNA expression in mucosal lymphoid cells.
We had previously developed a qcRT-PCR assay that allows specific quantitation of human NK-1R mRNA expression (34). The accuracy, sensitivity, and reproducibility of the assay have all been confirmed in control experiments (34). The assay is sufficiently sensitive to quantitate 100 NK-1R mRNA molecules, which is equivalent to 103 NK-1R mRNA transcripts/μg of total RNA isolated. The sensitivity of the qcRT-PCR assay is less than that of the qualitative (standard) RT-PCR assay, since two amplicons compete for reagents in qcRT-PCR.
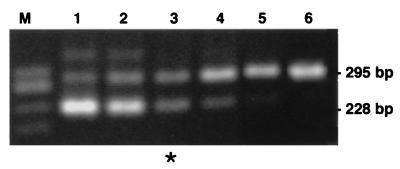
Figure 3 illustrates quantitation of NK-1R mRNA expression in a representative LPMC isolation. Quantitation of NK-1R mRNA expression levels in several LPMC isolations revealed a mean level of expression of 7.5 × 105 ± 2.2 × 105 NK-1R mRNA transcripts/μg of total RNA (n = 7). Since 1 μg of cellular RNA was the average RNA yield from approximately 105 cells, this level of expression corresponds to approximately 7.5 ± 2.2 NK-1R mRNA transcripts per LPMC. Since PBMC were found to be negative for NK-1R mRNA expression by quantitative RT-PCR and since the sensitivity of the qcRT-PCR assay was calculated to be 103 NK-1R transcripts/μg of RNA or 1 transcript/1,000 cells, we can further estimate that the level of NK-1R mRNA expression detected in PBMC by nested RT-PCR (10% cDNA) corresponds to one NK-1R transcript in >1,000 cells.
FIG. 3.
Quantitation of NK-1R mRNA expression in a representative LPMC isolation. Competitive standard transcripts were spiked into the aliquoted LPMC RNA sample at concentrations ranging from 4.0 × 105 to 4.1 × 103 (2.5-fold dilution series), followed by NK-1R mRNA-specific RT-PCR (lanes 1 to 6). HaeIII-digested φX174 DNA size markers were used (lane M). Equivalence (∗) is seen at 6.4 × 104 NK-1R mRNA molecules in which target (295 bp) and competitive-standard (228 bp) RT-PCR products are of equal band intensity. When adjusted for the amount of total RNA in the assay, this represents a level of expression of 7.5 × 105 NK-1R mRNA transcripts/μg of total RNA isolated.
DISCUSSION
In this study we examined NK-1R expression by human peripheral and mucosal lymphoid cells by using various molecular techniques of differing sensitivities. Our results confirm that human NK-1R is differentially expressed by lymphoid cells of the periphery and the mucosa and is more likely to have biological significance at the mucosal level.
Our RT-PCR results consistently demonstrated the expression of NK-1R mRNA by LPMC isolated from human colonic resections. Receptor mRNA levels were quantitated by qcRT-PCR, and LPMC were found to express a mean of eight NK-1R mRNA transcripts/cell. This level of expression correlates with that observed for other G protein-coupled receptors, which are generally associated with a low abundance of mRNA. For example, only 15 mRNA transcripts per cell have been reported for the β2-adrenergic receptor (16). We also demonstrated the presence of NK-1R protein in LPMC by immunofluorescence flow cytometry, thereby confirming LPMC expression of NK-1R at the protein level. Indeed, we have previously reported NK-1R expression in CD4+, CD8+, CD45RO+, CD45RA+, CD19+, and CD14+ LPMC (15). NK-1R protein, however, was undetectable in PBMC by the same technique. Hence, while NK-1R protein is undetectable in peripheral mononuclear cells, in the mucosa expression occurs in helper and cytotoxic T cells of both the memory and naive phenotypes, in B cells, and in monocytes/macrophages. Our findings complement the autoradiographic studies by Mantyh et al., which described SP binding sites in the lymphoid follicles of human and canine colon (12, 28, 30). SP binding sites have also been reported in murine Peyer's patch T and B cells (46). Our observation that LPMC also express the short isoform of NK-1R suggests differential splicing of NK-1R pre-mRNA in these cells. While NK-1R is expressed in lymphoid cells in the colonic mucosa, which is the largest mucosal site in the body, there is limited data on NK-1R expression in other mucosal sites. A recent report showed that macrophages from human sputum were positive for NK-1R, indicating that macrophages in the respiratory mucosa express NK-1R (13). We are currently investigating NK-1R expression in lymphoid cells within the bronchial mucosa.
Previous studies of NK-1R expression by peripheral lymphoid cells have used different techniques yielding conflicting results. SP binding sites have been identified on a subset of human T lymphocytes (36) and on human monocytes/macrophages (27). Recent nested-RT-PCR studies have demonstrated NK-1R mRNA expression by human PBL, monocytes, and MDM (21, 25). Lipopolysaccharide-activated murine macrophages have also been shown to express NK-1R mRNA (5). However, others have reported the absence of SP binding sites on human PBL (39). A non-neurokinin receptor for SP has been identified on human monocytes (23). SP has also been shown to activate human T cells receptor independently (24). When we analyzed peripheral lymphoid cells for NK-1R mRNA expression by RT-PCR, an apparent disparity of results also arose. NK-1R mRNA expression was not detected in PBMC, PBL, monocytes, and MDM by standard single-round RT-PCR or by nested RT-PCR using a starting cDNA concentration of 1%. However, NK-1R expression was detected when a starting cDNA concentration of 10% was used in nested RT-PCR. When we defined the sensitivities of our qualitative and quantitative RT-PCR assays, we could estimate that the expression detected in resting and activated peripheral lymphoid cells represented one NK-1R transcript in >1,000 cells. This contrasts sharply with a level of expression of eight NK-1R transcripts per cell in LPMC. Further evidence from flow cytometry confirmed the absence of NK-1R protein expression on PBMC. We conclude, therefore, that detection of NK-1R mRNA in the periphery may reflect intermucosal traffic involving the transient presence of circulating mucosal homing cells (41).
Our results illustrate the caution that is needed when interpreting results from sensitive, qualitative nested-RT-PCR assays, especially in the absence of confirmatory data such as from immunofluorescence flow cytometry. This study also illustrates the importance of establishing technique sensitivity and highlights the distinct advantages of competitive RT-PCR, a technique which retains the remarkable sensitivity of detection of PCR but also allows accurate quantitation of the mRNA transcript number.
In conclusion, our findings demonstrate significant expression of NK-1R by human mucosal, but not peripheral, mononuclear cells, and thereby provide further evidence to support the hypothesis that NK-1R expression is a marker of human mucosal mononuclear cells. Our finding that mucosal but not peripheral lymphoid cells express NK-1R supports the notion that SP plays a role specifically in mucosal immunoregulation. SP therefore probably contributes to mucosal inflammation in pathological conditions such as inflammatory bowel disease. We have recently found increased levels of NK-1R mRNA in colonic biopsies from sites of active inflammation in inflammatory bowel disease (14). This appears to be due mainly to the presence of increased numbers of NK-1R-expressing inflammatory cells, although neural expression of NK-1R in the myenteric plexus is also upregulated in Crohn's disease (14, 29). We found that there was a statistically significant higher level of NK-1R mRNA in colonic biopsies from Crohn's disease compared with ulcerative colitis, suggesting NK-1R mRNA quantitation as a potential diagnostic aid for differentiating between both diseases in cases of uncertain diagnosis. There is evidence that colonic levels of SP are increased in ulcerative colitis, and the level appears to correlate with inflammatory activity (3). Specific NK-1R antagonists have been shown to have anti-inflammatory effects in animal models of colonic inflammation (37). There is also evidence that NK-1R upregulation is involved in the pathogenesis of airway inflammation in patients with asthma and chronic obstructive pulmonary disease (1). Our finding that NK-1R expression is specific for mucosal rather than peripheral immunocytes will help to elucidate the role of SP in mucosal neuroimmunoregulation in both health and disease.
ACKNOWLEDGMENTS
This work was supported by the Health Research Board of Ireland, The Wellcome Trust (to J. O'Connell), U.S. National Institutes of Health (NIH) grant MH49981 (to S. D. Douglas), and U.S. NIH grant DA11887 (to W.-Z. Ho).
REFERENCES
- 1.Bai T R, Zhou D, Weir T, Walker B, Hegele R, Hayashi S, McKay K, Bondy G P, Fong T. Substance P (NK1)- and neurokinin A (NK2)-receptor gene expression in inflammatory airway diseases. Am J Physiol. 1995;269:L309–L317. doi: 10.1152/ajplung.1995.269.3.L309. [DOI] [PubMed] [Google Scholar]
- 2.Bar-Shavit Z, Goldman R, Stabinsky Y, Gottlieb P, Fridkin M, Teichberg V I, Blumberg S. Enhancement of phagocytosis—a newly found activity of substance P residing in its N-terminal tetrapeptide sequence. Biochem Biophys Res Commun. 1980;94:1445–1451. doi: 10.1016/0006-291x(80)90581-1. [DOI] [PubMed] [Google Scholar]
- 3.Bernstein C N, Robert M E, Eysselein V E. Rectal substance P concentrations are increased in ulcerative colitis but not in Crohn's disease. Am J Gastroenterol. 1993;88:908–913. [PubMed] [Google Scholar]
- 4.Blalock J E. The syntax of immune-neuroendocrine communication. Immunol Today. 1994;15:504–510. doi: 10.1016/0167-5699(94)90205-4. [DOI] [PubMed] [Google Scholar]
- 5.Bost K L. Quantification of macrophage-derived substance P receptor mRNA using competitive polymerase chain reaction. In: Sharp B, editor. The brain: immune axis and substance abuse. New York, N.Y: Plenum Press; 1995. pp. 219–223. [DOI] [PubMed] [Google Scholar]
- 6.Bost K L, Pascual D W. Substance P: a late-acting B lymphocyte differentiation cofactor. Am J Physiol. 1992;262:C537–C545. doi: 10.1152/ajpcell.1992.262.3.C537. [DOI] [PubMed] [Google Scholar]
- 7.Boyum A. Isolation of mononuclear cells and granulocytes from human blood. Scand J Clin Lab Investig. 1968;21:77–89. [PubMed] [Google Scholar]
- 8.Bull D M, Bookman M A. Isolation and functional characterization of human intestinal mucosal lymphoid cells. J Clin Investig. 1977;59:966–974. doi: 10.1172/JCI108719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chomczynski P, Saachi N. Single-step method of RNA isolation by guanidium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 10.Cooke H J. Neuroimmune signaling in regulation of intestinal ion transport. Am J Physiol. 1994;266:G167–G178. doi: 10.1152/ajpgi.1994.266.2.G167. [DOI] [PubMed] [Google Scholar]
- 11.Fong T M, Anderson S A, Yu H, Huang R C, Strader C D. Differential activation of intracellular effector by two isoforms of human neurokinin-1 receptor. Mol Pharmacol. 1991;41:24–30. [PubMed] [Google Scholar]
- 12.Gates T S, Zimmerman R P, Mantyh C R, Vigna S R, Maggio J E, Welton M L, Passaro E P, Mantyh P W. Substance P and substance K receptor binding sites in the human gastrointestinal tract: localization by autoradiography. Peptides. 1988;9:1207–1219. doi: 10.1016/0196-9781(88)90184-2. [DOI] [PubMed] [Google Scholar]
- 13.Germonpre P R, Bullock G R, Lambrecht B N, Van De Velde V, Luyten W H M L, Joos G F, Pauwels R A. Presence of substance P and neurokinin-1 receptors in human sputum macrophages and U-937 cells. Eur Respir J. 1999;14:776–782. doi: 10.1034/j.1399-3003.1999.14d08.x. [DOI] [PubMed] [Google Scholar]
- 14.Goode C, O'Connell J, O'Sullivan G C, Collins J K, Shanahan F. Cellular localization and quantitation of substance P (NK-1) receptor mRNA in normal and inflamed human colonic mucosa. Gastroenterology. 1997;112:A982. [Google Scholar]
- 15.Goode T, O'Connell J, Sternini C, Anton P, Wong H, O'Sullivan G C, Collins J K, Shanahan F. Substance P (neurokinin-1) receptor is a marker of human mucosal but not peripheral mononuclear cells: molecular quantitation and localization. J Immunol. 1998;161:2232–2240. [PubMed] [Google Scholar]
- 16.Hadcock J R, Williams D L, Malbon C C. Physiological regulation at the level of mRNA: analysis of steady state levels of specific mRNAs by DNA-excess solution hybridization. Am J Physiol. 1989;256:C457–C465. doi: 10.1152/ajpcell.1989.256.3.C457. [DOI] [PubMed] [Google Scholar]
- 17.Hartung H P, Toyka K V. Activation of macrophages by substance P: induction of oxidative burst and thromboxane release. Eur J Pharmacol. 1983;89:301–305. doi: 10.1016/0014-2999(83)90511-3. [DOI] [PubMed] [Google Scholar]
- 18.Hassan N F, Campbell D E, Douglas S D. Purification of human monocytes on gelatin-coated surfaces. J Immunol Methods. 1986;95:273–276. doi: 10.1016/0022-1759(86)90415-1. [DOI] [PubMed] [Google Scholar]
- 19.Hassan N F, Cutilli J R, Douglas S D. Isolation of highly purified human blood monocytes for in vitro HIV-1 infection studies of monocytes/macrophages. J Immunol Methods. 1990;130:283–285. doi: 10.1016/0022-1759(90)90058-4. [DOI] [PubMed] [Google Scholar]
- 20.Ho W-Z, Kaufman D, Uvaydova M, Douglas S D. Substance P augments interleukin-10 and tumor necrosis factor-alpha release by human cord monocytes and macrophages. J Neuroimmunol. 1996;71:73–80. doi: 10.1016/s0165-5728(96)00132-4. [DOI] [PubMed] [Google Scholar]
- 21.Ho W-Z, Lai J-P, Zhu X-H, Uvaydova M, Douglas S D. Human monocytes and macrophages express substance P and neurokinin-1 receptor. J Immunol. 1997;159:5654–5660. [PubMed] [Google Scholar]
- 22.Kage R, Leeman S E, Boyd N D. Biochemical characterization of two different forms of the substance P receptor in rat submaxillary gland. J Neurochem. 1993;60:347–351. doi: 10.1111/j.1471-4159.1993.tb05857.x. [DOI] [PubMed] [Google Scholar]
- 23.Kavelaars A, Broeke D, Jeurissen F, Kardux J, Meijer A, Franklin R, Gelfand E W, Heijnen C J. Activation of human monocytes via a non-neurokinin substance P receptor that is coupled to Gi protein, calcium, phospholipase D, MAP kinase, and IL-6 production. J Immunol. 1994;153:3691–3699. [PubMed] [Google Scholar]
- 24.Kavelaars A, Jeurissen F, von Frijtag Drabbe Kunzel J, van Roijen J H, Rijkers G T, Heijnen C J. Substance P induces a rise in intracellular calcium concentration in human T lymphocytes in vitro: evidence of a receptor-independent mechanism. J Neuroimmunol. 1993;42:61–70. doi: 10.1016/0165-5728(93)90213-i. [DOI] [PubMed] [Google Scholar]
- 25.Lai J-P, Douglas S D, Ho W-Z. Human lymphocytes express substance P and its receptor. J Neuroimmunol. 1998;86:80–86. doi: 10.1016/s0165-5728(98)00025-3. [DOI] [PubMed] [Google Scholar]
- 26.Lotz M, Vaughan J H, Carson D A. Effect of neuropeptides on production of inflammatory cytokines by human monocytes. Science. 1988;241:1218–1221. doi: 10.1126/science.2457950. [DOI] [PubMed] [Google Scholar]
- 27.Lucey D R, Novak J M, Polonis V R, Liu Y, Gartner S. Characterization of substance P binding to human monocytes/macrophages. Clin Diagn Lab Immunol. 1994;1:330–335. doi: 10.1128/cdli.1.3.330-335.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mantyh C R, Gtes T S, Zimmermann R P, Welton M L, Passaro E P, Vigna S R, Maggio J E, Kruger L, Mantyh P W. Receptor binding sites for substance P, but not substance K or neuromedin K, are expressed in high concentrations by arterioles, venules, and lymph nodules in surgical specimens obtained from patients with ulcerative colitis and Crohn's disease. Proc Natl Acad Sci USA. 1988;85:3235–3239. doi: 10.1073/pnas.85.9.3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mantyh C R, Vigna S R, Bollinger R R, Mantyh P W, Maggio J E, Pappas T N. Differential expression of substance P receptors in patients with Crohn's disease and ulcerative colitis. Gastroenterology. 1995;109:850–860. doi: 10.1016/0016-5085(95)90394-1. [DOI] [PubMed] [Google Scholar]
- 30.Mantyh P W, Mantyh C R, Gates T, Vigna S R, Maggio J E. Receptor binding sites for substance P and substance K in the canine gastrointestinal tract and their possible role in inflammatory bowel disease. Neuroscience. 1988;25:817–837. doi: 10.1016/0306-4522(88)90038-3. [DOI] [PubMed] [Google Scholar]
- 31.McGillis J P, Mitsuhashi M, Payan D G. Immunologic properties of substance P. In: Ader R, editor. Psychoneuroimmunology. London, England: Academic Press; 1991. pp. 209–223. [Google Scholar]
- 32.Metwali A, Blum A M, Ferraris L, Klein J S, Fiocchi C, Weinstock J V. Eosinophils within healthy or inflamed human intestine produce substance P and vasoactive intestinal peptide. J Neuroimmunol. 1994;52:69–78. doi: 10.1016/0165-5728(94)90164-3. [DOI] [PubMed] [Google Scholar]
- 33.Moore T C, Lami J L, Spruck C H. Substance P increases lymphocyte traffic and lymph flow through peripheral lymph nodes of sheep. Immunology. 1989;67:109–114. [PMC free article] [PubMed] [Google Scholar]
- 34.O'Connell J, Goode T, Shanahan F. Quantitative measurement of mRNA expression by competitive RT-PCR. Methods Mol Biol. 1998;92:183–193. doi: 10.1385/0-89603-497-6:183. [DOI] [PubMed] [Google Scholar]
- 35.Payan D G, Brewster D R, Goetzl E J. Specific stimulation of human T lymphocytes by substance P. J Immunol. 1983;131:1613–1615. [PubMed] [Google Scholar]
- 36.Payan D G, Brewster D R, Missirian-Bastian A, Goetzl E J. Substance P recognition by a subset of human T lymphocytes. J Clin Investig. 1984;74:1532–1539. doi: 10.1172/JCI111567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pothoulakis C, Castagliuolo I, LaMont T J, Jaffer A, O'Keane J C, Snider R M, Leeman S E. CP-96,345, a substance P antagonist, inhibits rat intestinal responses to Clostridium difficile toxin A but not cholera toxin. Proc Natl Acad Sci USA. 1994;91:947–951. doi: 10.1073/pnas.91.3.947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Regoli D, Drapeau G, Dion S, D'Orleans-Juste P. Pharmacological receptors for substance P and neurokinins. Life Sci. 1987;40:109–117. doi: 10.1016/0024-3205(87)90349-3. [DOI] [PubMed] [Google Scholar]
- 39.Roberts A I, Taunk J, Ebert C. Human lymphocytes lack substance P receptors. Cell Immunol. 1992;141:457–465. doi: 10.1016/0008-8749(92)90163-j. [DOI] [PubMed] [Google Scholar]
- 40.Ruff M R, Wahl S M, Pert C B. Substance P receptor-mediated chemotaxis of human monocytes. Peptides. 1985;6:107–111. doi: 10.1016/0196-9781(85)90142-1. [DOI] [PubMed] [Google Scholar]
- 41.Shanahan F. The intestinal immune system. In: Johnson L R, editor. Physiology of the gastrointestinal tract. New York, N.Y: Raven Press; 1994. pp. 643–684. [Google Scholar]
- 42.Shanahan F, Anton P A. Neuroendocrine modulation of the immune system. Possible implications for inflammatory bowel disease. Dig Dis Sci. 1988;33:41S–49S. doi: 10.1007/BF01538130. [DOI] [PubMed] [Google Scholar]
- 43.Shanahan F, Anton P A. Role of peptides in the regulation of the mucosal immune and inflammatory response. In: Walsh J H, Dockray G J, editors. Gut peptides: biochemistry and physiology. New York, N.Y: Raven Press; 1994. pp. 851–867. [Google Scholar]
- 44.Shanahan F, Denburg J A, Fox J, Bienenstock J, Befus D. Mast cell heterogeneity: effects of neuroenteric peptides on histamine release. J Immunol. 1985;135:1331–1337. [PubMed] [Google Scholar]
- 45.Stanisz A M, Befus D, Bienenstock J. Differential effects of vasoactive intestinal peptide, substance P, and somatostatin on immunoglobulin synthesis and proliferations by lymphocytes from Peyer's patches, mesenteric lymph nodes, and spleen. J Immunol. 1986;136:152–156. [PubMed] [Google Scholar]
- 46.Stanisz A M, Scicchitano R, Dazin P, Bienenstock J, Payan D G. Distribution of substance P receptors on murine spleen and Peyer's patch T and B cells. J Immunol. 1987;139:749–754. [PubMed] [Google Scholar]