Key Points
Question
What are the rates of viral shedding after first-episode genital herpes simplex virus type 1 (HSV-1) infection?
Findings
In this prospective cohort study, 82 participants with first-episode genital HSV-1 infection, of whom 42 had primary HSV-1 infection, self-collected oral and genital swabs daily for HSV polymerase chain reaction testing for two 30-day periods (2 months and 11 months after initial symptoms). Genital HSV-1 shedding was detected on 12.1% of days at 2 months and declined significantly to 7.1% of days at 11 months. Most genital shedding was asymptomatic; genital and oral lesions and oral shedding were rare.
Meaning
Genital HSV-1 shedding was frequent after first-episode genital HSV-1 and declined rapidly during the first year of infection.
Abstract
Importance
Herpes simplex virus type 1 (HSV-1) is the leading cause of first-episode genital herpes in many countries.
Objective
To inform counseling messages regarding genital HSV-1 transmission, oral and genital viral shedding patterns among persons with first-episode genital HSV-1 infection were assessed. The trajectory of the development of HSV-specific antibody and T-cell responses was also characterized.
Design, Setting, and Participants
Prospective cohort followed up for up to 2 years, with 82 participants followed up between 2013 and 2018. Participants were recruited from sexual health and primary care clinics in Seattle, Washington. Persons with laboratory-documented first-episode genital HSV-1 infection, without HIV infection or current pregnancy, were referred for enrollment.
Exposures
First-episode genital HSV-1 infection.
Main Outcomes and Measures
Genital and oral HSV-1 shedding and lesion rates at 2 months, 11 months, and up to 2 years after initial genital HSV-1 infection. Participants self-collected oral and genital swabs for HSV polymerase chain reaction testing for 30 days at 2 and 11 months and up to 2 years after diagnosis of genital HSV-1. Blood samples were collected at serial time points to assess immune responses to HSV-1. Primary HSV-1 infection was defined as absent HSV antibody at baseline or evolving antibody profile using the University of Washington HSV Western Blot. HSV-specific T-cell responses were detected using interferon γ enzyme-linked immunospot.
Results
Among the 82 participants, the median (range) age was 26 (16-64) years, 54 (65.9%) were women, and 42 (51.2%) had primary HSV-1 infection. At 2 months, HSV-1 was detected from the genital tract in 53 participants (64.6%) and in the mouth in 24 participants (29.3%). Genital HSV-1 shedding was detected on 275 of 2264 days (12.1%) at 2 months and declined significantly to 122 of 1719 days (7.1%) at 11 months (model-predicted rate, 6.2% [95% CI, 4.3%-8.9%] at 2 months vs 3.2% [95% CI, 1.8%-5.7%] at 11 months; relative risk, 0.52 [95% CI, 0.29-0.93]). Genital lesions were rare, reported on 65 of 2497 days (2.6%) at 2 months and 72 of 1872 days (3.8%) at 11 months. Oral HSV-1 shedding was detected on 88 of 2247 days (3.9%) at 2 months. Persons with primary HSV-1 infection had a higher risk of genital shedding compared with those with nonprimary infection (model-predicted rate, 7.9% [95% CI, 5.4%-11.7%] vs 2.9% [95% CI, 1.7%-5.0%]; relative risk, 2.75 [95% CI, 1.40-5.44]). Polyfunctional HSV-specific CD4+ and CD8+ T-cell responses were maintained during the follow-up period.
Conclusions and Relevance
Genital HSV-1 shedding was frequent after first-episode genital HSV-1, particularly among those with primary infection, and declined rapidly during the first year after infection.
This study examines genital HSV-1 transmission and oral and genital viral shedding patterns among persons with first-episode genital HSV-1 infection.
Introduction
Both herpes simplex virus type 1 and 2 (HSV-1 and HSV-2) cause genital herpes, a chronic infection characterized by recurrent, self-limited genital ulcers. Genital herpes is associated with large medical, social, and financial burdens. In 2018, there were an estimated 18.6 million prevalent and 572 000 incident HSV-2 infections among those aged 18 to 49 years in the US,1 accounting for 27% of all prevalent sexually transmitted infections (STIs)2; millions of infections would be added if genital HSV-1 was included.1 The lifetime medical cost of genital herpes due to HSV-2 acquired in 2018 alone in the US was estimated to be $90.7 million dollars.3 These costs do not account for effects on sexual health, including stress associated with potential for transmission to sexual partners or neonates and social stigma.
HSV-1 is an increasing cause of genital herpes, especially in high-income countries that have a declining rate of oral HSV-1 acquisition during childhood,4,5 leaving individuals susceptible at initiation of sexual activity. An “epidemiologic transition” of HSV-1 from oral to genital infection is predicted, with up to 25% of all HSV-1 acquisitions in the genital tract by 2050 in the US.6 Given the changing epidemiology of HSV-1 infection, it is important for clinicians to understand outcomes of genital HSV-1 viral shedding and risk of recurrence over time.
Although HSV-1 is adapted to the trigeminal, superior cervical, and ciliary ganglia, where it establishes latency and reactivates causing orolabial ulcerations and asymptomatic shedding, the frequency of HSV-1 reactivation in the genital tract, the prevalence of concurrent oral and genital HSV-1 infection, and development of cellular and humoral responses to HSV-1 have not been determined. To answer these questions, a cohort of persons with first-episode genital HSV-1 infections was prospectively followed up.
Methods
Participants and Study Design
All procedures were approved by the University of Washington Human Subjects Division. Participants provided written informed consent in English. Eligible participants had first-episode genital HSV-1 within the past 8 weeks, were seronegative for HSV-2 and HIV, and were not pregnant. First-episode genital HSV-1 was defined as detection of HSV-1 from genital ulcers among persons with no prior history of genital herpes. Participants were followed up between 2013 and 2018 and recruited from the Public Health-Seattle and King County Sexual Health Clinic, the University of Washington Healthcare System, and the surrounding community.
Antiviral therapy active against HSV was used for the initial episode and participants consented to avoid suppressive antiviral therapy during swabbing sessions. Participants collected daily oral and anogenital swabs and completed a diary of genital symptoms between 8 and 12 weeks (session 1) and between 48 and 52 weeks (session 2) after first-episode genital HSV-1.7 Participants with detectable genital HSV-1 shedding during session 2 were invited to complete a third session 24 months after infection. Oral swabs were collected by rubbing a Dacron swab on the tonsils, tongue, hard palate, and gums. Anogenital swabs were collected by rotating a Dacron swab into the vagina, followed by swabbing the labia majora/minora, perianal area, and rectal area or swabbing the entire penile shaft, including underneath the foreskin if present, perianal area, and rectal area. Swabs were placed into 1-mL 1X polymerase chain reaction (PCR) buffer and stored at room temperature or 4 °C. Blood was drawn every 2 weeks during shedding sessions and at week 24, with additional blood draws at 2, 4, and 6 weeks among participants with primary genital HSV-1.
Participants were contacted monthly to assess for genital herpes recurrences and encouraged to come to the clinic during recurrences for laboratory confirmation.
Laboratory Assays
Immunology
Serology and Definition of Acquisition Type
Serum was tested for HSV-specific IgG antibodies at enrollment and at 12, 24, and 52 weeks using the University of Washington Western Blot, which differentiates between HSV-1 and HSV-2 infection.8 Samples were considered HSV-1 or HSV-2 positive (4 bands that comigrated with HSV-1 or HSV-2 antigens), HSV-1 negative (no bands), or HSV-1 indeterminate (1-3 bands).8 Participants were classified as having primary infection if they were HSV-1 seronegative or HSV-1 indeterminate at baseline and seroconverted or had maturation of HSV-1 antibody response over time. Persons who were HSV-1 seropositive at baseline and had blood drawn less than 42 days after the first episode had nonprimary infection and participants were classified as unknown if they did not demonstrate seroconversion or the first blood draw was 42 days or more after the first episode. A 3-person panel blinded to HSV-1 shedding rates adjudicated the HSV seroconversion of each participant.
T-Cell Responses
Peripheral blood mononuclear cells (PBMCs) were isolated by density gradient centrifugation and cryopreserved.9 CD4+ T-cell responses were measured by interferon γ enzyme-linked immunospot and stimulation with ultraviolet-irradiated HSV-1 strain E115 grown in Vero cells (ATCC) or ultraviolet-treated Vero cells as a negative control.10 Intracellular cytokine staining (106 PBMC/well) was used to characterize the expression of interferon γ, interleukin 2, tumor necrosis factor α, and CD40L in single live CD3+CD4+CD8 T cells.11 Samples that did not meet PBMC functional viability standards were censored.11 CD8+ T-cell responses were measured in duplicate by interferon γ enzyme-linked immunospot by stimulating PBMCs (2.5 × 105 cells/well) from each time point with 117 HSV-1 peptide epitopes known to be recognized by CD8+ T cells in 3 pools.10 For select samples responding to peptide pools, responses were deconvoluted to the single peptide level using methods analogous to those described for SARS-CoV-2.12 Single peptides recognized by CD8+ T-cells and reactive in this study have been previously reported13,14,15,16 with details documented in Immune Epitope Database and Analysis Resource.17
HSV-1 Detection
DNA was extracted and PCR testing was performed using type-common primers to the HSV glycoprotein B gene, with the first positive swab from each participant confirmed to be HSV-1 using a type-specific probe.18 Swabs with at least 2.3 log10 HSV-1 copies/mL (3 copies/reaction) were considered positive. Swabs from genital and oral lesions were placed into a virus-transport medium and grown in cell culture as previously described.19
Full-genome HSV-1 DNA was sequenced from cultures on the Illumina platform20 with assembly.21 GenBank accession numbers are provided for newly and previously sequenced HSV-1 genomes (eTables 1-2 in the Supplement).
Statistical Analysis
Genital and oral shedding rates were compared in session 2 and session 1 and among participants with primary compared with nonprimary HSV-1 infection using a Poisson mixed model regression to calculate the relative risk (RR) of viral shedding. Mixed models accounted for association in outcomes between repeated sessions on the same individuals. The model included the number of days positive for HSV-1 shedding as the outcome, with log number of days with swabs as the offset. No assumption of linearity was included because all covariates were binary. The Poisson distribution was not assumed to determine the variance structure because the error variance was estimated empirically. Initial multivariable analyses included 5 terms: sex, age (<26 y vs ≥26 y), session number (1 vs 2), infection type (primary vs nonprimary), and the interaction between session and infection type. Backward elimination was used to select a final model. Similar analyses were performed for lesion frequency, using days with reported lesions as the outcome and log number of diary days recorded as the offset. The association between HSV-1 shedding rates and polyfunctional T-cell function was assessed using Spearmen correlation. Two-sided P values <.05 were considered statistically significant. Analyses were conducted using SAS, version 9.4, for Windows.
Results
Participants
Ninety-two individuals were screened and 82 with first-episode genital HSV-1 were enrolled (Table 1; eFigure 1 in the Supplement). The median (range) age was 26 (16-64) years, 54 participants (65.9%) were women, and 66 (80.5%) self-identified as White. Ten participants (12.2%) reported a prior history of oral HSV infection. Eighteen participants (22.0%) were lost to follow-up or withdrew (eFigure 1 in Supplement); their baseline characteristics were similar to those who completed the study (Table 1; eTable 3 in the Supplement). Two participants had concurrent syphilis and 1 had chlamydia.
Table 1. Demographic and Clinical Characteristics of Participants in a Study of Viral Shedding 1 Year Following First-Episode Genital Herpes Simplex Virus Type 1 (HSV-1) Infection.
| Baseline characteristic | No. (%) | |
|---|---|---|
| All enrolled (n = 82) | Completed 12-mo follow-up (n = 64) | |
| Age, median (range), y | 26 (16-64) | 26 (16-64) |
| Age ≥26 y | 42 (51.2) | 33 (51.6) |
| Sex | ||
| Women | 54 (65.9) | 43 (67.2) |
| Men | 28 (34.2) | 21 (32.8) |
| Racea | ||
| American Indian/Alaska Native | 0 | 0 |
| Asian | 2 (2.4) | 1 (1.6) |
| Black | 1 (1.2) | 0 |
| Native Hawaiian/Pacific Islander | 0 | 0 |
| White | 66 (80.5) | 53 (82.8) |
| More than 1 option | 10 (12.2) | 8 (12.5) |
| Other | 3 (3.7) | 2 (3.1) |
| Acquisition typeb | ||
| Primaryc | 42 (51.2) | 35 (54.7) |
| Nonprimaryd | 23 (28.1) | 17 (26.7) |
| Unknowne | 17 (20.7) | 12 (18.8) |
| Time since genital HSV acquisition if known, median (IQR), d | 25 (16-42) [n = 82] | 24 (16-59) [n = 64] |
| History of oral HSVf | 10 (12.2)g | 5 (8.3) |
| Days since oral HSV acquisition if known, median (range) | 765 (54-7538) [n = 11] | 65 (54-6059) [n = 6] |
| Method for genital HSV-1 diagnosis | ||
| Only polymerase chain reaction | 28 (34.2) | |
| Only culture | 40 (48.8) | |
| Polymerase chain reaction and culture | 14 (17.1) | |
| No. of sexual partners in past 4 wk, median (range) | 1 (0-6) [n = 75] | 1 (0-6) [n = 61] |
Selected by the participant from a closed list of options that included “other.”
Based on HSV serostatus using the HSV Western Blot at screening visit.
Primary defined as HSV-1 seronegative or HSV-1 indeterminate at first blood draw or increasing antibody over time.
Nonprimary defined as HSV-1 seropositive with first blood sample drawn <42 days after symptom onset.
Unknown defined as HSV-1 seropositive with first blood sample drawn ≥42 days after symptom onset or seroconversion not observed.
Oral HSV was defined as self-reported history of symptoms consistent with oral HSV infection.
Three participants with primary HSV-1 reported history of oral HSV. Two participants had oral HSV symptoms with first-episode genital HSV-1. One participant reported a distant prior history of oral HSV symptoms; this participant had clear evidence of HSV-1 acquisition at the time of first-episode genital HSV-1 based on HSV serologic response, and thus was classified as having primary HSV-1.
HSV Serostatus and Seroconversion
Overall, 42 participants (51.2%) had primary HSV-1 infection, 23 (28.1%) had nonprimary infection, and 17 (20.7%) were classified as unknown. Of 80 participants with sera available 12 weeks after the first symptoms of genital infection, 69 (86.3%) were HSV-1 seropositive, 2 (2.5%) were HSV-1 seronegative, and 9 (11.3%) were indeterminate. By 52 weeks, 60 of 63 participants (95.2%) were HSV-1 seropositive, 1 (1.6%) remained HSV-1 negative, and 2 (3.2%) remained indeterminate. All participants remained HSV-2 seronegative throughout the study.
Genital Shedding and Lesions
All participants completed session 1 and 64 (78.1%) completed session 2 (Table 2 and Figure 1). Overall, 2170 of 2460 (88.2%) expected swabs were collected and 2350 of 2460 (95.5%) diary days were recorded. No participants had fewer than 13 swabs or 17 diary days and there was no antiviral use during either session.
Table 2. Shedding Rates, Lesion Rates, and Quantity of Herpes Simplex Virus Type 1 (HSV-1) Detected .
| Outcome | No. (%) | |||||
|---|---|---|---|---|---|---|
| 8-12 wk after onset (session 1) | 48-52 wk after onset (session 2) | |||||
| All (n = 82) | Primary HSV-1 infection (n = 42)a | Prior or unknown HSV-1 infection (n = 40)b,c | All (n = 64) | Primary HSV-1 infection (n = 35)a | Prior or unknown HSV-1 infection (n = 29)b,c | |
| HSV-1 DNA detected by polymerase chain reaction testing | ||||||
| Persons with any genital shedding | 53 (64.6) | 32 (76.2) | 21 (52.5) | 21 (32.8) | 14 (40.0) | 7 (24.1) |
| Overall genital shedding rated | 275/2264 (12.1) | 199/1156 (17.2) | 76/1108 (6.9) | 122/1719 (7.1) | 79/933 (8.5) | 43/786 (5.5) |
| Asymptomatic genital sheddinge | 239/2189 (10.9) | 167/1099 (15.2) | 72/1090 (6.6) | 89/1637 (5.4) | 48/871 (5.5) | 41/766 (5.4) |
| Persons with any genital lesions | 12 (14.6) | 8 (19.1) | 4 (10.0) | 15 (23.4) | 12 (34.3) | 3 (10.4) |
| Days with genital lesionsf | 65/2497 (2.6) | 60/1271 (4.7) | 5/1226 (0.4) | 72/1872 (3.8) | 66/1042 (6.3) | 6/830 (0.7) |
| Genital lesion sheddingg | 34/62 (54.8) | 32/57 (56.1) | 2/5 (40.0) | 33/68 (48.5) | 31/62 (50.0) | 2/6 (33.3) |
| Persons with any oral shedding | 24 (29.3) | 16 (38.1) | 8 (20.0) | 17 (26.6) | 11 (31.4) | 6 (20.7) |
| Overall oral shedding rated | 88/2247 (3.9) | 63/1140 (5.5) | 25/1107 (2.3) | 87/1714 (5.1) | 64/925 (6.9) | 23/789 (2.9) |
| Asymptomatic oral sheddinge | 87/2223 (3.9) | 63/1140 (5.5) | 24/1083 (2.2) | 86/1690 (5.1) | 64/919 (7.0) | 22/771 (2.9) |
| Persons with any oral lesions | 2 (2.4) | 0 | 2 (5.0) | 3 (4.7) | 1 (2.9) | 2 (6.9) |
| Days with oral lesionsf | 11/2497 (0.4) | 0/1271 | 11/1226 (0.9) | 10/1872 (0.5) | 6/1042 (0.6) | 4/830 (0.5) |
| Oral lesion sheddingg | 1/11 (9.1) | 1/11 (9.1) | 1/10 (10.0) | 0/6 | 1/4 (25.0) | |
| Log10 copies/mL HSV-1, median (IQR) | ||||||
| Genital | 2.9 (2.5-3.6) [n = 275] | 2.9 (2.5-3.7) [n = 199] | 2.8 (2.5-3.4) [n = 76] | 4.4 (3.2-5.8) [n = 122] | 4.5 (3.1-6.5) [n = 79] | 4.4 (3.2-4.7) [n = 43] |
| When lesions present | 5.1 (2.5-7.1) [n = 34] | 5.2 (2.6-7.3) [n = 32] | 2.6 (2.3-2.8) [n = 2] | 6.1 (4.3-6.9) [n = 33] | 6.1 (4.3-7.4) [n = 31] | 5.0 (3.2-6.9) [n = 2] |
| When asymptomatic | 2.8 (2.5-3.4) [n = 239] | 2.8 (2.5-3.4) [n = 167] | 2.8 (2.5-3.4) [n = 72] | 4.2 (3.0-4.8) [n = 89] | 3.9 (2.9-5.4) [n = 48] | 4.4 (3.2-4.7) [n = 41] |
| Oral | 3.5 (2.5-4.3) [n = 88] | 3.4 (2.5-4.4) [n = 63] | 3.5 (2.6-4.2) [n = 25] | 3.6 (2.7-4.7) [n = 87] | 3.5 (2.7-4.8) [n = 64] | 3.8 (2.7-4.7) [n = 23] |
| When lesions present | 3.0h | 3.0h | 2.2h | 2.2h | ||
| When asymptomatic | 3.5 (2.5-4.3) [n = 87] | 3.4 (2.5-4.4) [n = 63] | 3.6 (2.5-4.2) [n = 24] | 3.6 (2.8-4.7) [n = 86] | 3.5 (2.7-4.8) [n = 64] | 3.9 (2.9-4.7) [n = 22] |
Primary defined as HSV-1 seronegative or HSV-1 indeterminate at first blood draw or increasing antibody over time.
Nonprimary defined as HSV-1 seropositive with first blood sample drawn <42 days after symptom onset.
Unknown defined as HSV-1 seropositive with first blood sample drawn ≥42 days after symptom onset or seroconversion not observed.
Overall shedding includes all positive swabs over all swabs taken and includes all persons (days with shedding/total daily swabs).
Asymptomatic shedding is defined as the proportion of days with shedding in the absence of genital lesions/total daily swabs collected in absence of lesions.
The denominator for “days with [genital or oral] lesions” is obtained from diary data. There are slight discrepancies between days in which the diary was completed and days with swabs obtained.
Lesion shedding is defined as the proportion of days that with shedding in the presence of genital lesions/total days with genital lesions.
The CI was omitted because there was only 1 swab collected.
Figure 1. Genital and Oral Herpes Simplex Virus Type 1 (HSV-1) Shedding Rates.
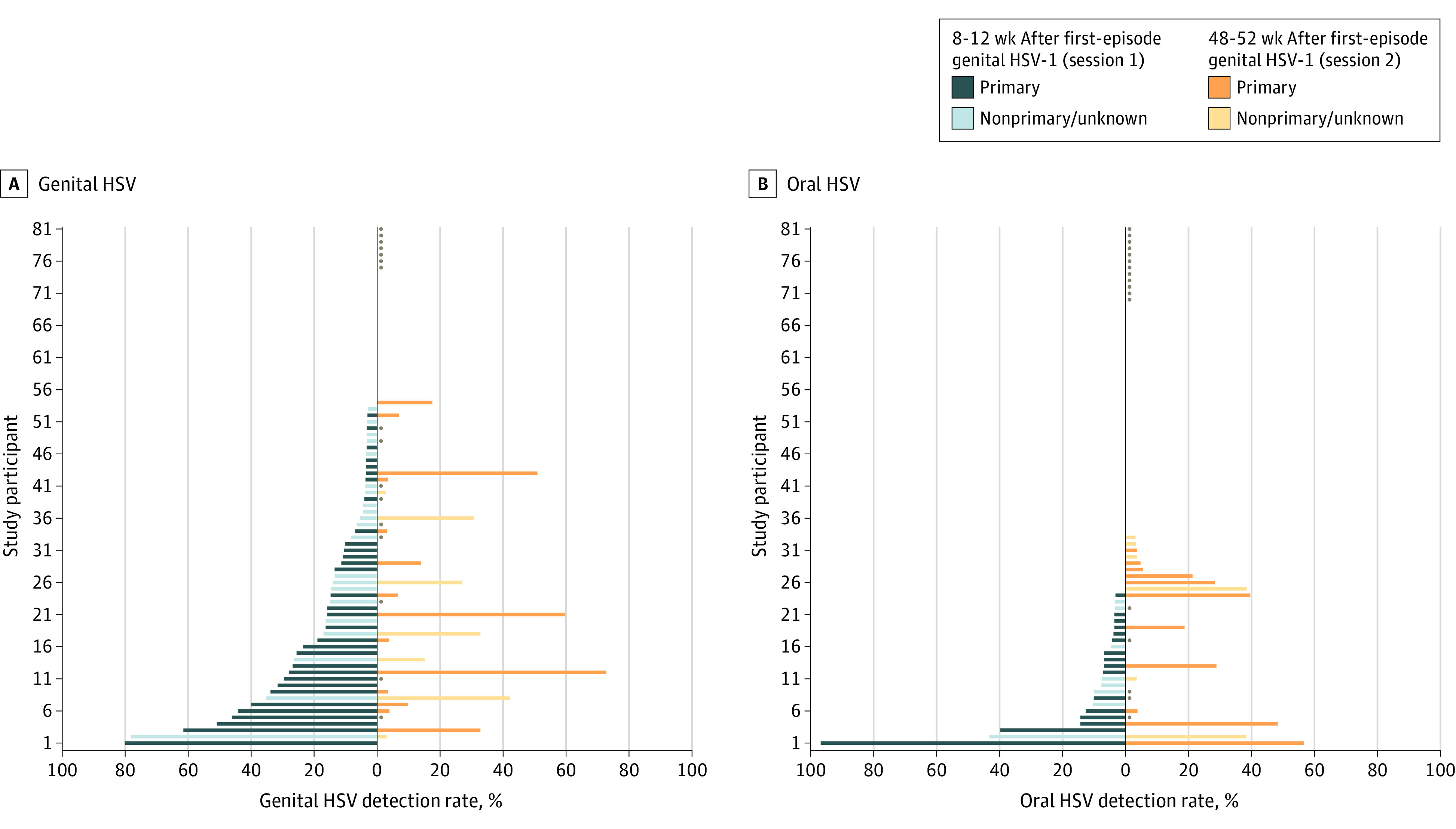
All participants are included; those who completed only the first session are indicated by a dot for the second session. Twenty-eight participants did not have any genital shedding detected and 49 participants did not have any oral shedding detected. Shedding rates are calculated by dividing the number of days with HSV detected by the total number of days with swabs collected. Study participants are plotted in reverse rank order from least to greatest shedding rate. The order of study participants is not equivalent in panels A and B, and the participant numbers do not relate directly to viral genome isolate numbers in eFigure 3 in the Supplement.
During session 1, a total of 53 participants (64.6%) had genital HSV-1 detected on 275 of 2264 days (12.1%) (Table 2). Asymptomatic genital HSV-1 shedding occurred on 239 of 2189 days (10.9%). Twelve participants (14.6%) had genital lesions reported on 65 of 2497 days (2.6%) during session 1. Among 42 persons with primary HSV-1 infection, 32 (76.2%) had genital shedding, with an overall shedding rate of 17.2%, mostly due to asymptomatic shedding (15.2%) (Table 2). Genital lesions were present on 60 of 1271 days (4.7%). Of 40 participants with nonprimary infection, 21 (52.5%) had HSV-1 detected. The overall genital shedding rate was 6.9%, with an asymptomatic genital shedding rate of 6.6%. Lesions were reported on 5 of 1266 days (0.4%).
During session 2, HSV-1 was detected in 21 of 64 people (32.8%) on 122 of 1719 days (7.1%), with asymptomatic shedding detected on 89 of 1637 days (5.4%) (Table 2). Genital lesions were reported on 72 of 1872 days (3.8%). Among 35 persons with primary genital infection, 14 (40.0%) had genital shedding during session 2, with an overall genital shedding rate of 8.5%. Genital lesions were reported on 66 of 1042 days (6.3%). Among 29 persons with nonprimary infection, 7 participants (24.1%) had genital shedding and the overall shedding rate was 5.5%, with genital lesions reported on 6 of 830 days (0.7%). The median (IQR) quantity of genital HSV-1 detected was 2.9 (2.5-3.6) log10 copies/mL during session 1 and 4.4 ( 3.2-5.8) log10 copies/mL during session 2, a difference of 0.95 log10 copies/mL more during session 2 (95% CI, 0.60-1.31; P < .0001).
Oral Shedding and Lesions
Oral shedding and lesions were detected infrequently throughout the study. During session 1, a total of 24 participants (29.3%) had oral HSV-1 shedding. The oral shedding rate was 3.9% (88 of 2247 days). Oral lesions were reported on 11 of 2497 days (0.4%). Shedding rates were similar between the 2 sessions and in participants with primary and nonprimary infection. The median quantity of HSV-1 detected from the oral cavity was similar during both sessions (Table 2).
Genital and Oral Recurrences
Among 42 people with primary infection, 30 (71.4%) had recurrences over the first year, with a median (range) of 1 (0-7) genital recurrence in the first year. Of those with nonprimary infection, 13 (32.5%) had a recurrence, with a median (range) of 0 (range, 0-7) recurrences. Overall, 98 recurrences were reported among 43 study participants, with 82 recurrences (83.7%) in the genital area. Four people (9.5%) with primary infection and 6 (15.0%) with nonprimary infection reported oral recurrences. Twelve participants (14.6%) used suppressive antiviral therapy between session 1 and session 2.
Long-term HSV-1 Shedding
Eleven participants had shedding on at least 10.0% of days during session 2. Of these participants, 6 (54.5%) completed an additional 30 days of daily oral and genital swabbing at least 2 years after infection. Participants had genital shedding on 1.3% of days, while oral shedding rates were similar to prior sessions (3.2%). One participant (1.6%) had genital lesions; no oral lesions were reported.
Predictors of Genital and Oral HSV-1 Shedding
In a bivariable model, there was a significant decrease in genital shedding between session 1 (predicted shedding rate, 6.2% [95% CI, 4.3%-8.9%]) and session 2 (predicted shedding rate, 3.2% [95% CI, 1.8%-5.7%]) (RR, 0.52 [95% CI, 0.29-0.93]; P = .03), with a negative but nonsignificant association for persons with primary and nonprimary infection (Figure 2). Participants with primary genital HSV-1 had a higher risk of genital HSV-1 shedding (model-predicted shedding rate, 7.9% [95%, 5.4%-11.7%]) compared with persons with nonprimary/unknown infections (model-predicted shedding rate, 2.9% [95% CI, 1.7%-5.0%]) (RR, 2.75 [95% CI, 1.40-5.44]; P = .004) (Figure 2) and an increased risk of genital lesions (model-predicted lesion rate of 2.1% [95% CI, 1.1%-3.8%] vs 0.3% [95% CI, 0.1%-0.7%]; RR, 6.50 [95% CI, 2.37-17.8]; P < .001) (eTable 4 in the Supplement). Sex and age were not significantly associated with genital shedding rates. In a multivariable model containing an interaction term between session and acquisition type, persons with primary infection had a higher risk of shedding during the first session compared with those with nonprimary infection (model-predicted shedding rate of 10.2% [95% CI, 6.7%-15.6%]vs 3.4% [95% CI, 1.9%-6.1%]; RR, 3.02 [95% CI, −1.45 to 6.29]; P = .004) (Figure 2).
Figure 2. Bivariable and Multivariable Risk Factors Associated With Genital and Oral Herpes Simplex Virus Type 1 (HSV-1) Shedding.
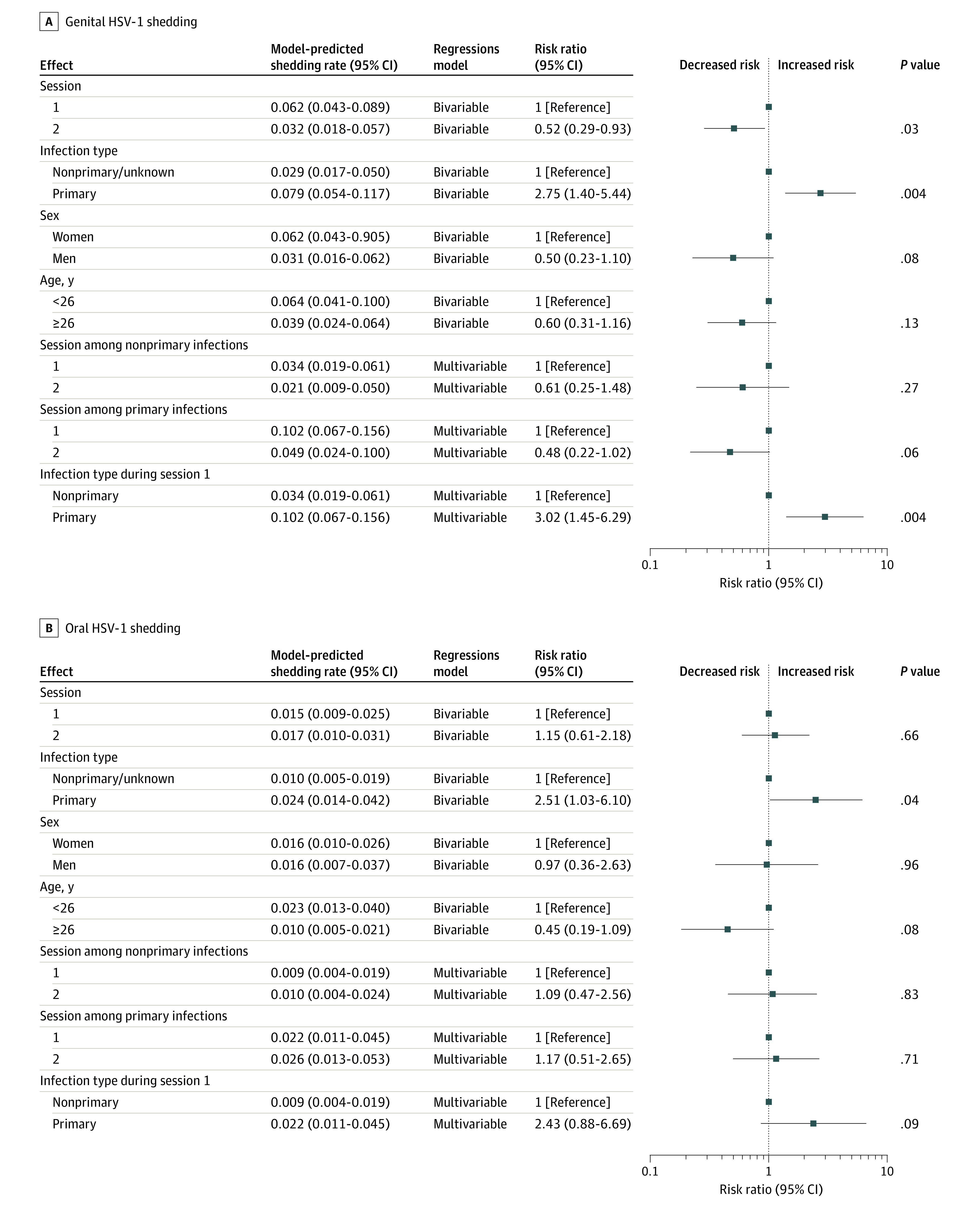
For this analysis, those with unknown acquisition type are grouped with those with nonprimary infection. For the comparison between first and second session, the model does not distinguish primary from nonprimary or unknown acquisition type. For the comparison between nonprimary unknown and primary the model does not distinguish the first from the second session. The multivariable model included an interaction term between session and acquisition type. In multivariable models including both age and sex, neither age nor sex contributed to the model in estimating shedding frequencies, so those measures were removed in backward elimination. Forest plots indicate the point estimate and 95% CI for each comparison. The reference is indicated by a box over the midline without a CI.
In a multivariable model, oral shedding was not significantly different among persons with primary compared with nonprimary infection or among those with primary infection over time (Figure 2). A sensitivity analysis excluding 12 people who received suppressive antiviral therapy between session 1 and session 2 yielded similar results for all models (eTable 5 in the Supplement).
HSV-1 Sequencing
To determine whether HSV-1 strains found in the genital tract had unique genomic properties compared with HSV-1 sequences available in GenBank, all isolates from unique participants grown in culture were sequenced (n = 27) (eFigure 2 and eTable 1 in the Supplement). The genital HSV-1 genomes were dispersed throughout a network graph of a globally distributed collection of previously sequenced HSV-1 isolates.
T-cell Responses to HSV-1
Among a subset of participants with primary genital HSV-1, PBMC CD4+ and CD8+ T-cell responses to HSV-1 were performed at specified points during follow-up to determine the kinetics of the development of the cellular immune response (Figure 3). CD4+ T-cell responses to ultraviolet-irradiated HSV-1 were detected at the earliest time point available (2 weeks) after HSV-1 acquisition. All participants had detectable CD4+ T-cell responses, and the responses remained stable over the first year of infection for most participants. Regardless of quantity of HSV-1–specific CD4+ T cells, a substantial proportion were polyfunctional, as measured by expression of 3 or 4 of the functional markers interferon γ, interleukin 2, tumor necrosis factor-α, and CD40L. The proportion of cells expressing polyfunctionality did not change qualitatively over time (eFigure 3 in the Supplement). There was no association between genital or oral shedding rates and the proportion of cells that expressed 2, 3, or 4 cytokines in either session (eTable 6 in the Supplement). HSV-1–specific CD8+ T-cell responses were detected using peptide pools and were sustained, validated in select cases to single HSV-1 CD8+ T-cell peptide epitopes (eFigure 4 and eTable 7 in the Supplement).
Figure 3. Cellular Immune Responses Measured Over Time for the First 20 Participants With Primary Genital Herpes Simplex Virus Type 1 (HSV-1).
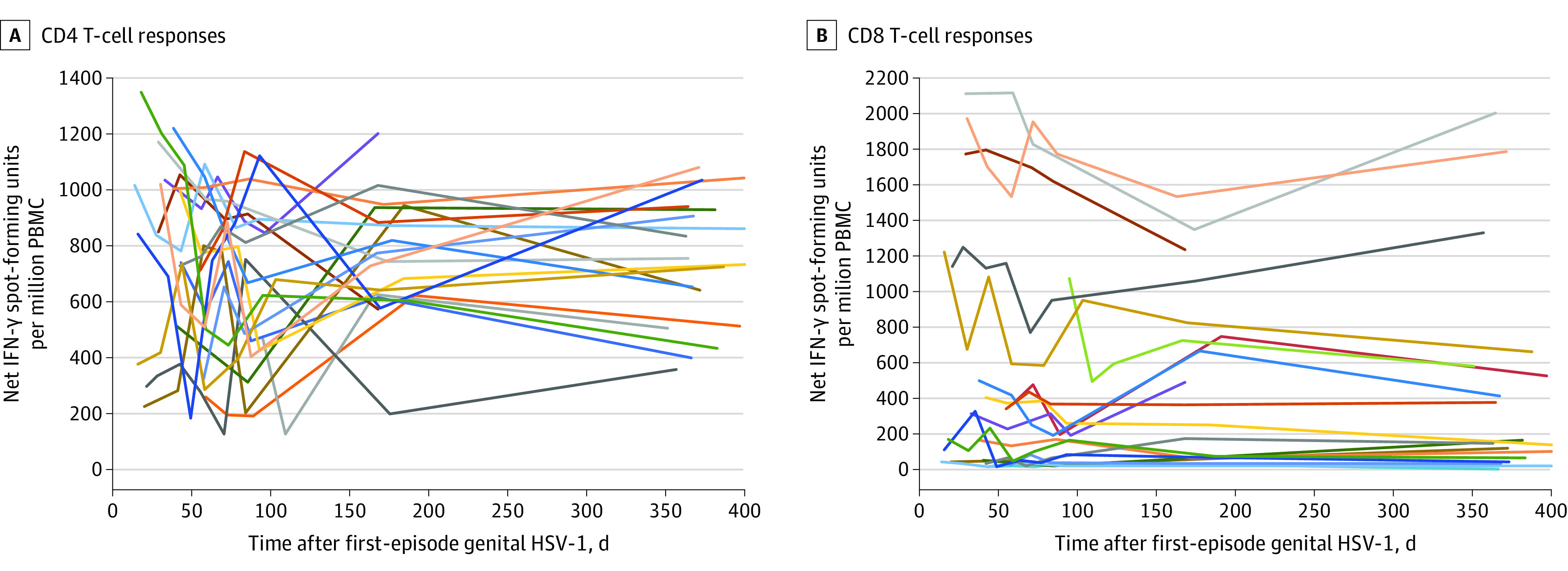
Each line represents the time course for a single participant, and participants are represented by the same line color for both graphs. IFN indicates interferon; PBMC, peripheral blood mononuclear cells.
Discussion
In this study, genital HSV-1 shedding was frequent after first-episode genital HSV-1, particularly among individuals with primary infection, and declined rapidly in the first year after infection. In contrast, symptomatic genital HSV-1 lesions and oral HSV-1 shedding and lesions were uncommon.
The prevalence of genital HSV-1 infection is difficult to determine because diagnosis often relies on HSV-1 antibody tests, which cannot differentiate between oral and genital infection, and there is no surveillance or reporting for genital herpes infections as is the case for other STIs. There were an estimated 192 million prevalent cases of genital HSV-1 infection in 2016 among people aged 15 to 49 years, mostly in the World Health Organization regions of the Americas and Europe.22 HSV-1 has surpassed HSV-2 to become the leading cause of first-episode genital herpes in some populations, and this is expected to increase over time. Although it is known that HSV-1 causes less-frequent genital recurrences than HSV-2,23 this is the first study to our knowledge to comprehensively assess genital and oral HSV-1 viral shedding using PCR. Characterizing shedding rates is clinically important because patients with genital herpes are often concerned about transmission to sexual partners, which usually occurs in the absence of lesions.24 A previous study using viral culture to assess genital HSV-1 shedding was small, involving only 14 women, and detected a shedding rate of 1.8%.25 The current study showed that shedding rates detected by PCR, the current standard for viral diagnosis, were higher, particularly in the first months of infection, but declined substantially over the first year. However, these rates are lower than genital HSV-2 shedding rates, which is found on 33.6% of days in persons in the first year after the first clinical episode and persists at 16.7% of days even 10 years after genital HSV-2 infection.26 Although the threshold quantity of virus for HSV-1 transmission is not known, quantities of HSV-2 greater than 4 log10 copies/mL have been modeled as leading to genital HSV-2 transmission.27 The higher quantity of HSV-1 DNA detected from swabs in session 2 compared with session 1 likely reflects a larger proportion of swabs collected from lesions in session 2. Persons who lack HSV antibodies at presentation may be counseled to expect more frequent shedding and recurrences and may be candidates for suppressive antiviral therapy for the initial year of infection.
Whether the decline in genital HSV-1 shedding over time is due to virologic or immunologic factors is unclear. HSV reactivation patterns depend on anatomic site and viral type, with HSV-1 recurring more frequently in the oral compared with the genital niche.28 Antibody responses to HSV-1 developed quickly over time, with most people considered HSV-1 seropositive or indeterminate by 12 weeks after first-episode genital HSV-1. A small rate of nonseroconversion at 1 year was found. This is in contrast with HSV-2, in which 100% seroconversion by 6 months has been previously reported.29
Cellular immunity is essential to contain HSV recurrences,30 and this study demonstrated that polyfunctional HSV-1–specific CD4+ and CD8+ T cells developed early and were sustained after primary HSV-1. Tissue resident memory T cells may also be critical for containment of HSV-1, as has been shown for HSV-2 infection.31 There was considerable heterogeneity between participants for levels of HSV-1–specific CD8+ T cells. The basis for this observation is unknown, but may be linked to the very large genome size of HSV and the set of 117 proven CD8+ T cell epitopes chosen. Understanding effective immune responses to contain genital HSV-1 infection may inform future development of HSV vaccines.
Although neonatal herpes is rare, with an estimated 10 cases per 100 000 livebirths globally, mortality, morbidity, and cost remain high.32,33 Genital HSV-1 is estimated to contribute more cases to neonatal herpes than HSV-2 in the World Health Organization regions of the Americas, Europe, and Western Pacific.32 The finding that genital HSV-1 shedding occurred at a high rate 2 to 3 months after first-episode genital herpes, particularly among those with primary infection, is consistent with the increased risk of neonatal herpes observed after first-episode genital HSV in pregnancy.34 Identifying pregnant people at risk of genital HSV-1 acquisition or who acquire HSV-1 during pregnancy is a high priority for prevention of neonatal herpes.
This study provides a large contribution of genital HSV-1 specimens to the HSV-1 genomic sequence database. There was no clustering of sequences on the whole genome level to suggest that HSV-1 strains inhabiting the genital niche have unique genetic features. In addition, there was no evidence of transmission clusters in the Seattle area over a 4-year period, suggesting that transmitted HSV-1 infections are from latently infected hosts. Whether people with both oral and genital HSV-1 infection are infected with the same strain simultaneously or can be infected with 2 different strains at the different sites requires additional analysis of the genomic data. Given that some people presented with newly symptomatic genital HSV-1 infection in the setting of fully developed HSV-1 antibody, new genital HSV-1 infection appears possible in persons with prior oral HSV-1.
Limitations
This study has several limitations. First, there was a 22% loss to follow-up at the end of year 1. Second, the study was conducted in a single geographic location in the US and the population comprised predominantly White individuals. This may reflect higher HSV-1 prevalence in childhood among racial and ethnic minority populations, likely due to social determinants of health.35,36 Third, although no participants used antiviral medication during the swabbing periods, the use of antiviral medications was incompletely captured between the swabbing periods, and recurrences were captured by monthly self-report. Therefore, the number of oral and genital recurrences may be underestimated. Fourth, there were a limited number of participants who were followed up beyond 1 year.
Conclusions
Genital shedding was frequent after first-episode genital HSV-1, particularly among those with primary infection, and declined rapidly in the first year of infection.
eFigures
eTables
eMethods
References
- 1.Spicknall IH, Flagg EW, Torrone EA. Estimates of the prevalence and incidence of genital herpes, United States, 2018. Sex Transm Dis. 2021;48(4):260-265. doi: 10.1097/OLQ.0000000000001375 [DOI] [PubMed] [Google Scholar]
- 2.Kreisel KM, Spicknall IH, Gargano JW, et al. Sexually transmitted infections among US women and men: prevalence and incidence estimates, 2018. Sex Transm Dis. 2021;48(4):208-214. doi: 10.1097/OLQ.0000000000001355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chesson HW, Spicknall IH, Bingham A, et al. The estimated direct lifetime medical costs of sexually transmitted infections acquired in the United States in 2018. Sex Transm Dis. 2021;48(4):215-221. doi: 10.1097/OLQ.0000000000001380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ryder N, Jin F, McNulty AM, Grulich AE, Donovan B. Increasing role of herpes simplex virus type 1 in first-episode anogenital herpes in heterosexual women and younger men who have sex with men, 1992-2006. Sex Transm Infect. 2009;85(6):416-419. doi: 10.1136/sti.2008.033902 [DOI] [PubMed] [Google Scholar]
- 5.Bradley H, Markowitz LE, Gibson T, McQuillan GM. Seroprevalence of herpes simplex virus types 1 and 2–United States, 1999-2010. J Infect Dis. 2014;209(3):325-333. doi: 10.1093/infdis/jit458 [DOI] [PubMed] [Google Scholar]
- 6.Ayoub HH, Chemaitelly H, Abu-Raddad LJ. Characterizing the transitioning epidemiology of herpes simplex virus type 1 in the USA: model-based predictions. BMC Med. 2019;17(1):57. doi: 10.1186/s12916-019-1285-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wald A, Zeh J, Selke S, et al. Reactivation of genital herpes simplex virus type 2 infection in asymptomatic seropositive persons. N Engl J Med. 2000;342(12):844-850. doi: 10.1056/NEJM200003233421203 [DOI] [PubMed] [Google Scholar]
- 8.Ashley RL, Militoni J, Lee F, Nahmias A, Corey L. Comparison of Western blot (immunoblot) and glycoprotein G-specific immunodot enzyme assay for detecting antibodies to herpes simplex virus types 1 and 2 in human sera. J Clin Microbiol. 1988;26(4):662-667. doi: 10.1128/jcm.26.4.662-667.1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koelle DM, Corey L, Burke RL, et al. Antigenic specificities of human CD4+ T-cell clones recovered from recurrent genital herpes simplex virus type 2 lesions. J Virol. 1994;68(5):2803-2810. doi: 10.1128/jvi.68.5.2803-2810.1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ott M, Jing L, Lorenzo L, Casanova J-L, Zhang S-Y, Koelle DM. T-cell responses to HSV-1 in persons who have survived childhood herpes simplex encephalitis. Pediatr Infect Dis J. 2017;36(8):741-744. doi: 10.1097/INF.0000000000001631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dropulic LK, Oestreich MC, Pietz HL, et al. A randomized, double-blinded, placebo-controlled, phase 1 study of a replication-defective herpes simplex virus (HSV) type 2 vaccine, hsv529, in adults with or without HSV infection. J Infect Dis. 2019;220(6):990-1000. doi: 10.1093/infdis/jiz225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jing L, Wu X, Krist MP, et al. T cell response to intact SARS-CoV-2 includes coronavirus cross-reactive and variant-specific components. JCI Insight. 2022;7(6):e158126. doi: 10.1172/jci.insight.158126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jing L, Haas J, Chong TM, et al. Cross-presentation and genome-wide screening reveal candidate T cells antigens for a herpes simplex virus type 1 vaccine. J Clin Invest. 2012;122(2):654-673. doi: 10.1172/JCI60556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koelle DM, Liu Z, McClurkan CL, et al. Immunodominance among herpes simplex virus-specific CD8 T cells expressing a tissue-specific homing receptor. Proc Natl Acad Sci U S A. 2003;100(22):12899-12904. doi: 10.1073/pnas.2131705100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Srivastava R, Khan AA, Spencer D, et al. HLA-A02:01-restricted epitopes identified from the herpes simplex virus tegument protein VP11/12 preferentially recall polyfunctional effector memory CD8+ T cells from seropositive asymptomatic individuals and protect humanized HLA-A*02:01 transgenic mice against ocular herpes. J Immunol. 2015;194(5):2232-2248. doi: 10.4049/jimmunol.1402606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dervillez X, Qureshi H, Chentoufi AA, et al. Asymptomatic HLA-A*02:01-restricted epitopes from herpes simplex virus glycoprotein B preferentially recall polyfunctional CD8+ T cells from seropositive asymptomatic individuals and protect HLA transgenic mice against ocular herpes. J Immunol. 2013;191(10):5124-5138. doi: 10.4049/jimmunol.1301415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martini S, Nielsen M, Peters B, Sette A. The Immune Epitope Database and Analysis Resource Program 2003-2018: reflections and outlook. Immunogenetics. 2020;72(1-2):57-76. doi: 10.1007/s00251-019-01137-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ryncarz AJ, Goddard J, Wald A, Huang ML, Roizman B, Corey L. Development of a high-throughput quantitative assay for detecting herpes simplex virus DNA in clinical samples. J Clin Microbiol. 1999;37(6):1941-1947. doi: 10.1128/JCM.37.6.1941-1947.1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wald A, Huang ML, Carrell D, Selke S, Corey L. Polymerase chain reaction for detection of herpes simplex virus (HSV) DNA on mucosal surfaces: comparison with HSV isolation in cell culture. J Infect Dis. 2003;188(9):1345-1351. doi: 10.1086/379043 [DOI] [PubMed] [Google Scholar]
- 20.Shipley MM, Renner DW, Ott M, et al. Genome-wide surveillance of genital herpes simplex virus type 1 from multiple anatomic sites over time. J Infect Dis. 2018;218(4):595-605. doi: 10.1093/infdis/jiy216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rathbun MM, Shipley MM, Bowen CD, et al. Comparison of herpes simplex virus 1 genomic diversity between adult sexual transmission partners with genital infection. PLoS Pathog. 2022;18(5):e1010437. doi: 10.1371/journal.ppat.1010437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.James C, Harfouche M, Welton NJ, et al. Herpes simplex virus: global infection prevalence and incidence estimates, 2016. Bull World Health Organ. 2020;98(5):315-329. doi: 10.2471/BLT.19.237149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Benedetti JK, Zeh J, Corey L. Clinical reactivation of genital herpes simplex virus infection decreases in frequency over time. Ann Intern Med. 1999;131(1):14-20. doi: 10.7326/0003-4819-131-1-199907060-00004 [DOI] [PubMed] [Google Scholar]
- 24.Mertz GJ, Schmidt O, Jourden JL, et al. Frequency of acquisition of first-episode genital infection with herpes simplex virus from symptomatic and asymptomatic source contacts. Sex Transm Dis. 1985;12(1):33-39. doi: 10.1097/00007435-198501000-00007 [DOI] [PubMed] [Google Scholar]
- 25.Wald A, Zeh J, Selke S, Ashley RL, Corey L. Virologic characteristics of subclinical and symptomatic genital herpes infections. N Engl J Med. 1995;333(12):770-775. doi: 10.1056/NEJM199509213331205 [DOI] [PubMed] [Google Scholar]
- 26.Phipps W, Saracino M, Magaret A, et al. Persistent genital herpes simplex virus-2 shedding years following the first clinical episode. J Infect Dis. 2011;203(2):180-187. doi: 10.1093/infdis/jiq035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schiffer JT, Mayer BT, Fong Y, Swan DA, Wald A. Herpes simplex virus-2 transmission probability estimates based on quantity of viral shedding. J R Soc Interface. 2014;11(95):20140160. doi: 10.1098/rsif.2014.0160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lafferty WE, Coombs RW, Benedetti J, Critchlow C, Corey L. Recurrences after oral and genital herpes simplex virus infection. Influence of site of infection and viral type. N Engl J Med. 1987;316(23):1444-1449. doi: 10.1056/NEJM198706043162304 [DOI] [PubMed] [Google Scholar]
- 29.Ashley-Morrow R, Krantz E, Wald A. Time course of seroconversion by HerpeSelect ELISA after acquisition of genital herpes simplex virus type 1 (HSV-1) or HSV-2. Sex Transm Dis. 2003;30(4):310-314. doi: 10.1097/00007435-200304000-00007 [DOI] [PubMed] [Google Scholar]
- 30.Koelle DM, Posavad CM, Barnum GR, Johnson ML, Frank JM, Corey L. Clearance of HSV-2 from recurrent genital lesions correlates with infiltration of HSV-specific cytotoxic T lymphocytes. J Clin Invest. 1998;101(7):1500-1508. doi: 10.1172/JCI1758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhu J, Peng T, Johnston C, et al. Immune surveillance by CD8αα+ skin-resident T cells in human herpes virus infection. Nature. 2013;497(7450):494-497. doi: 10.1038/nature12110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Looker KJ, Magaret AS, May MT, et al. First estimates of the global and regional incidence of neonatal herpes infection. Lancet Glob Health. 2017;5(3):e300-e309. doi: 10.1016/S2214-109X(16)30362-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee SWH, Gottlieb SL, Chaiyakunapruk N. Healthcare resource utilisation pattern and costs associated with herpes simplex virus diagnosis and management: a systematic review. BMJ Open. 2022;12(1):e049618. doi: 10.1136/bmjopen-2021-049618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brown ZA, Wald A, Morrow RA, Selke S, Zeh J, Corey L. Effect of serologic status and cesarean delivery on transmission rates of herpes simplex virus from mother to infant. JAMA. 2003;289(2):203-209. doi: 10.1001/jama.289.2.203 [DOI] [PubMed] [Google Scholar]
- 35.Xu F, Lee FK, Morrow RA, et al. Seroprevalence of herpes simplex virus type 1 in children in the United States. J Pediatr. 2007;151(4):374-377. doi: 10.1016/j.jpeds.2007.04.065 [DOI] [PubMed] [Google Scholar]
- 36.Delaney AS, Thomas W, Balfour HH Jr. Coprevalence of Epstein-Barr virus, cytomegalovirus, and herpes simplex virus type-1 antibodies among United States children and factors associated with their acquisition. J Pediatric Infect Dis Soc. 2015;4(4):323-329. doi: 10.1093/jpids/piu076 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigures
eTables
eMethods


