Abstract
Background
Growth differentiation factor 15 (GDF15) was newly discovered to be a promising target of metformin. The study was aimed to investigate the relationship between GDF15 and glycemic control after metformin treatment in patients with type 2 diabetes mellitus.
Methods
The study was a post-hoc analysis of AIM (the effect of Acarbose on glycemic variability in patients with type 2 diabetes mellitus using premixed Insulin compared to Metformin) study. The participants were randomly assigned to 12 weeks of metformin (MET) or acarbose (ACA) treatment combined with insulin. Serum GDF15 levels of 51 subjects from MET group and 53 subjects from ACA group were measured at baseline and after a 12-week treatment. Fasting plasma glucose (FPG), 2-h postprandial plasma glucose (2-h PG) and glycated hemoglobin A1c (HbA1c) were measured at baseline and endpoint.
Results
After a 12-week treatment, serum GDF15 levels significantly increased in MET group [baseline vs. endpoint, 936.70 (741.00, 1205.40) pg/mL vs. 1265.20 (1027.90, 1634.00) pg/mL, P < 0.001], but not in ACA group [baseline vs. endpoint, 920.60 (701.45, 1332.55) pg/mL vs. 893.80 (663.25, 1284.05) pg/mL, P = 0.944]. However, there were no significant differences of glycemic control parameters (ΔFPG, Δ2-h PG and ΔHbA1c) between subgroups of MET group divided by median of ΔGDF15 (all P > 0.05). Spearman correlation coefficient and analysis of covariance after adjustment for baseline HbA1c levels showed that ΔGDF15 was not correlated with ΔFPG, Δ2-h PG and ΔHbA1c (all P > 0.05).
Conclusion
Serum GDF15 levels were significantly elevated after metformin treatment in patients with type 2 diabetes mellitus. However, the increase was not an indicator of the glucose-lowering effect of metformin.
Trial registration
Clinicaltrials.gov, NCT02438397. Registered 8 May 2015.
Keywords: Growth differentiation factor 15, Metformin, Glycemic control, Type 2 diabetes mellitus
Background
Metformin is the most commonly used oral antidiabetic drug [1–3], which can reduce hepatic glucose production and peripheral insulin resistance and thereby lower blood glucose levels [4–6]. Growth differentiation factor 15 (GDF15), also known as macrophage inhibitory cytokine 1, is a stress responsive cytokine [7–9]. Previous studies found that GDF15 was closely related to diabetes mellitus [7, 10, 11], while GDF15 was newly discovered to be a promising target of metformin [12, 13]. Coll et al. [13] revealed that metformin treatment was related to increased levels of circulation GDF15 in people without diabetes mellitus, while the change of GDF15 levels in metformin group was significantly corelated with weight loss. Our previous study also revealed that increased serum GDF15 was related to metabolic improvement by lifestyle intervention among young overweight and obese adults [14]. However, in addition to its association with weight control, the relationship between GDF15 and glycemic control during metformin therapy still remains unknown.
Therefore, in the present study, we measured the serum GDF15 concentrations from the AIM (the effect of Acarbose on glycemic variability in patients with type 2 diabetes mellitus using premixed Insulin compared to Metformin) study [15] to explore the association between metformin treatment, glycemic control and the change of serum GDF15 levels.
Materials and methods
Study design and participants
The present study is a post-hoc analysis of the AIM study. The AIM study was an open-labeled randomized clinical trial designed to investigate the effect of acarbose on glycemic variability in patients with type 2 diabetes mellitus using premixed insulin compared to metformin. The study protocol and main results of the trial have been published [15]. The AIM study was a prospective trail registered at www.ClinicalTrials.gov with clinical trial registration number NCT02438397.
In brief, the AIM study enrolled patients with type 2 diabetes using premixed insulin and the glycated hemoglobin A1c (HbA1c) levels were between 7 and 10% before randomization. Patients taking more than two oral antidiabetic drugs or taking one oral antidiabetic drug at the maximum therapeutic dose were excluded. The main inclusion and exclusion criteria were described in detail in the previously published article [15].
The eligible patients were randomly assigned to 12 weeks of metformin (MET group, n = 62) or acarbose (ACA group, n = 62) treatment combined with insulin according to the random encoder [15]. The initial dose of ACA was 50 mg three times a day at three meals and the dose was raised to 100 mg three times a day at three meals 1 week later. The dose of metformin was 500 mg three times a day throughout the study. The patients have already made adjustment to their lifestyle before the enrollment and did not change their previous lifestyle during the 12-week hypoglycemic intervention. Finally, 54 subjects from MET group and 61 from ACA group completed the whole study.
The study was approved by the Ethics Committee of Shanghai Jiao Tong University Affiliated Sixth People’s Hospital and was in accordance with the 1964 Declaration of Helsinki. Informed consent was obtained from each subject at the beginning of the study.
Anthropometric and biochemical measurements
Each subject had standardized meal tests at baseline and at the 12-week follow-up visit. Fasting blood samples were collected after an 8-hour overnight fast and postprandial blood was collected 2-hour later. The standard meal test was standardized instant noodles containing 69.3 g of carbohydrates, 9.3 g of protein, and 1.5 g of fat [15]. Blood pressure, height and waist were measured. BMI = weight (kg)/height(m)2.
Plasma glucose including fasting plasma glucose (FPG) and 2-h postprandial plasma glucose (2-h PG) levels were measured by glucose oxidase method. HbA1c was measured by using high-performance liquid chromatography with a VARIANT II Hemoglobin A1c analyzer (Bio-Rad Laboratories, Hercules, CA). Triglycerides (TG), total cholesterol (TC), high-density lipoprotein cholesterol (HDL-c), and low-density lipoprotein cholesterol (LDL-c) were determined by applying standard enzymatic methods using a biochemical analyzer (7600–120; Hitachi, Tokyo, Japan). The criteria for the serum sample included: 1) the volume of the serum sample was enough for the assay; 2) serum sample was stored properly. For fasting serum GDF15 levels, 51 of subjects from MET group and 53 subjects from ACA group were measured at the baseline and endpoint by quantitative sandwich enzyme-linked immunosorbent assay (R&D Systems, Minneapolis, USA).
Statistical analyses
Statistical analyses were performed in SPSS version 24.0 (SPSS, Inc., Chicago, IL). The Kolmogorov-Smirnov test was performed to determine normality of the data distribution. Variables with a normal distribution were presented as means ± standard deviation (SD), and variables with a skewed distribution were presented as median (interquartile range). Differences of the parameters before and after treatment were compared by the paired t-test or Wilcoxon signed rank test. Differences between groups were compared using the student t-test or the Mann-Whitney test. The correlation between clinical parameters and the change of serum GDF15 levels were assessed by Spearman correlation coefficient and analysis of covariance (ANCOVA). A P value of < 0.05 (two-tailed) was considered statistically significant.
Results
Basic characteristics of the subjects
We finally enrolled a total of 51 subjects from MET group and 53 subjects from ACA group in the present analysis. The characteristics of the participants at baseline and at the endpoint are presented in Table 1. Compared with baseline levels, glycemic control parameters (FPG, 2-h PG, HbA1c) all significantly improved in both MET and ACA group after a 12-week therapy (all P < 0.01, Table 1). Body weight, BMI and waist circumference did not change in MET group (all P > 0.05, Table 1).
Table 1.
Clinical characteristics of the participants at the baseline and endpoint
| Metformin (N = 51) | Acarbose (N = 53) | |||||
|---|---|---|---|---|---|---|
| Baseline | Endpoint | P | Baseline | Endpoint | P | |
| Sex (male/female) | 28/23 | – | – | 32/21 | – | – |
| Age (year) | 60.00 (53.00, 64.00) | – | – | 63.00 (57.00, 67.00) | – | – |
| Diabetes duration (year) | 14.00 (10.00, 17.00) | – | – | 16.00 (11.00, 20.00) | – | – |
| Body weight (kg) | 74.13 ± 10.22 | 73.65 ± 10.14 | 0.181 | 69.66 ± 9.23 | 68.63 ± 9.34 | < 0.001 |
| BMI (kg/m2) | 26.37 ± 2.80 | 26.21 ± 2.83 | 0.197 | 25.31 ± 2.42 | 24.94 ± 2.43 | < 0.001 |
| Waist circumference (cm) | 91.11 ± 10.66 | 92.35 ± 9.61 | 0.186 | 88.68 ± 8.33 | 88.49 ± 8.10 | 0.736 |
| SBP (mmHg) | 137.00 ± 13.00 | 133.00 ± 16.00 | 0.107 | 136.00 ± 16.00 | 130.00 ± 14.00 | 0.005 |
| DBP (mmHg) | 81.00 (76.00, 86.00) | 80.00 (76.00, 85.00) | 0.587 | 81.00 (73.00, 85.00) | 77.00 (71.00, 82.00) | 0.089 |
| TC (mmol/L) | 4.97 ± 1.18 | 4.82 ± 1.06 | 0.386 | 4.93 ± 0.90 | 4.97 ± 1.08 | 0.781 |
| TG (mmol/L) | 1.63 (1.10, 2.25) | 1.55 (1.02, 2.18) | 0.636 | 1.33 (0.95, 2.10) | 1.19 (0.89, 1.53) | 0.025 |
| HDL-c (mmol/L) | 1.09 (0.96, 1.35) | 1.14 (0.95, 1.30) | 0.881 | 1.17 (1.03, 1.46) | 1.15 (0.97, 1.33) | 0.089 |
| LDL-c (mmol/L) | 2.89 ± 0.99 | 2.76 ± 0.83 | 0.345 | 2.94 ± 0.76 | 3.00 ± 0.85 | 0.552 |
| FPG (mmol/L) | 9.91 (8.79, 12.16) | 8.28 (7.29, 9.76) | < 0.001 | 10.29 (8.88, 12.14) | 8.71 (7.49, 10.42) | 0.003 |
| 2-h PG (mmol/L) | 20.03 ± 3.31 | 15.48 ± 3.53 | < 0.001 | 20.37 ± 3.72 | 11.60 ± 3.75 | < 0.001 |
| HbA1c (%) | 8.40 (7.70, 9.00) | 7.50 (7.00, 8.10) | < 0.001 | 8.50 (7.80, 9.10) | 7.50 (7.00, 8.10) | < 0.001 |
Data are expressed as mean ± SD or median (interquartile range)
Abbreviations: BMI Body mass index, SBP Systolic blood pressure, DBP Diastolic blood pressure, TC Total cholesterol, TG Triglycerides, HDL-c High-density lipoprotein cholesterol, LDL-c Low-density lipoprotein cholesterol, FPG Fasting plasma glucose, 2-h PG 2-h postprandial plasma glucose, HbA1c Glycated hemoglobin A1c
Change of serum GDF15 levels before and after treatment
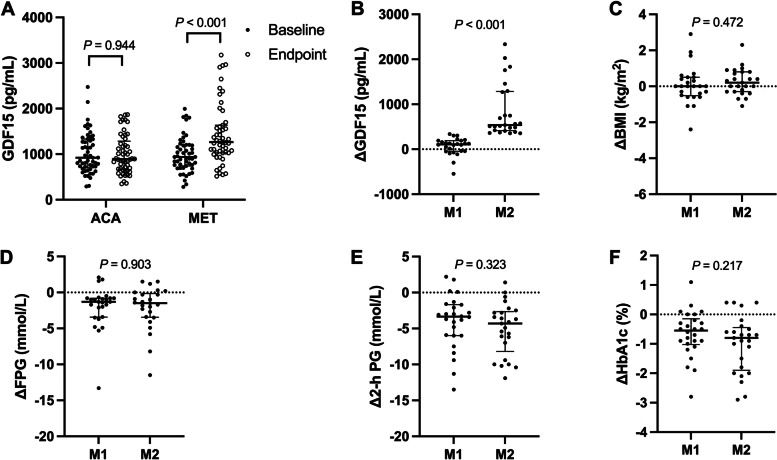
After a 12-week treatment, serum GDF15 levels significantly increased in MET group [baseline vs. endpoint, 936.70 (741.00, 1205.40) pg/mL vs. 1265.20 (1027.90, 1634.00) pg/mL, P < 0.001, Fig. 1A], but not in ACA group [baseline vs. endpoint, 920.60 (701.45, 1332.55) pg/mL vs. 893.80 (663.25, 1284.05) pg/mL, P = 0.944, Fig. 1A]. There were no differences of serum GDF15 levels between the MET group and ACA group at baseline (P = 0.946), while serum GDF15 levels at the endpoint were much higher in MET group than those in ACA group (P < 0.001).
Fig. 1.
A The comparison of serum GDF15 levels between baseline and endpoint of ACA group and MET group; B-F The comparison of ΔGDF15, ΔBMI, ΔFPG, Δ2-h PG and ΔHbA1c levels between M1 and M2 subgroup (divided by the median of ΔGDF15 in MET group; M1, ΔGDF15 ≤ 338.50 pg/mL; M2, ΔGDF15 > 338.50 pg/mL). GDF15, growth differentiation factor 15; BMI, body mass index; FPG, fasting plasma glucose; 2-h PG, 2-h postprandial plasma glucose; HbA1c, glycated hemoglobin A1c
Relationship between serum GDF15 levels and glycemic parameters in the MET group
Since the differences of serum GDF15 levels after treatment were only discovered in the MET group, we divided the MET group into two subgroups (M1 group, n = 26; M2 group, n = 25) according to the median of ΔGDF15 levels [338.50 (101.10, 538.00) pg/mL], to further explore the relationship between ΔGDF15 and glycemic control parameters. The clinical characteristics of the participants of M1 and M2 subgroups are presented in Table 2. Body weight, BMI and waist circumference showed no significant differences in M1 and M2 group (all P > 0.05, Table 2). TG levels were lower while HDL-c levels were higher in M2 group at baseline (all P < 0.05, Table 2). There were no significant differences between M1 and M2 subgroup in glycemic control parameters (FPG, 2-h PG and HbA1c) at both baseline and endpoint (all P > 0.05, Table 2).
Table 2.
Clinical characteristics of the participants of M1 and M2 subgroups
| M1 (N = 26) | M2 (N = 25) | |||||
|---|---|---|---|---|---|---|
| Baseline | Endpoint | P | Baseline | Endpoint | P | |
| Sex (male/female) | 16/10 | – | – | 12/13 | – | – |
| Age (year) | 60.00 (55.00, 62.00) | – | – | 62.00 (49.00, 67.00) | – | – |
| Diabetes duration (year) | 14.00 (10.00, 17.00) | – | – | 13.00 (10.00, 16.00) | – | – |
| Body weight (kg) | 75.34 ± 10.15 | 75.10 ± 10.20 | 0.685 | 72.88 ± 10.35 | 72.14 ± 10.06 | 0.072 |
| BMI (kg/m2) | 26.63 ± 3.10 | 26.55 ± 3.24 | 0.716 | 26.11 ± 2.50 | 25.85 ± 2.35 | 0.094 |
| Waist circumference (cm) | 91.44 ± 9.55 | 92.46 ± 7.65 | 0.473 | 90.76 ± 11.90 | 92.24 ± 11.46 | 0.243 |
| SBP (mmHg) | 137.00 ± 12.00 | 131.00 ± 15.00 | 0.092 | 138.00 ± 15.00 | 136.00 ± 16.00 | 0.608 |
| DBP (mmHg) | 80.00 ± 6.00 | 80.00 ± 7.00 | 0.980 | 82.00 ± 7.00 | 81.00 ± 8.00 | 0.305 |
| TC (mmol/L) | 4.80 ± 1.00 | 4.68 ± 1.10 | 0.587 | 5.14 ± 1.33 | 4.97 ± 1.02 | 0.510 |
| TG (mmol/L) | 1.90 (1.28, 3.03) | 1.58 (1.07, 2.50) | 0.041 | 1.26 (0.79, 1.82) * | 1.55 (1.01, 1.94) | 0.087 |
| HDL-c (mmol/L) | 1.04 (0.92, 1.21) | 1.13 (0.89, 1.25) | 0.096 | 1.14 (1.00, 1.54) * | 1.22 (0.99, 1.35) | 0.277 |
| LDL-c (mmol/L) | 2.62 ± 0.78 | 2.69 ± 0.93 | 0.683 | 3.17 ± 1.12 | 2.83 ± 0.72 | 0.121 |
| FPG (mmol/L) | 9.91 (9.08, 12.22) | 8.18 (6.82, 10.36) | 0.001 | 10.25 (8.49, 11.96) | 8.28 (7.46, 9.61) | 0.001 |
| 2-h PG (mmol/L) | 18.74 (17.55, 21.06) | 14.52 (12.67, 18.18) | < 0.001 | 20.36 (18.06, 22.72) | 16.09 (14.64, 17.57) | < 0.001 |
| HbA1c (%) | 8.20 ± 0.70 | 7.50 ± 0.60 | < 0.001 | 8.60 ± 0.90 | 7.60 ± 0.80 | < 0.001 |
| GDF15 (pg/mL) | 983.05 (694.70, 1324.85) | 1040.65 (853.75, 1333.93) | 0.016 | 897.40 (769.90, 1171.30) | 1559.70 (1247.35, 2416.00) ** | < 0.001 |
Data are expressed as mean ± SD or median (interquartile range)
M1 subgroup, ΔGDF15 ≤ 338.50 pg/mL; M2 subgroup, ΔGDF15 > 338.50 pg/mL
Compared to M1 group: *, P < 0.05; **, P < 0.01
Abbreviations: BMI Body mass index, SBP Systolic blood pressure, DBP Diastolic blood pressure; TC Total cholesterol, TG Triglycerides, HDL-c High-density lipoprotein cholesterol, LDL-c Low-density lipoprotein cholesterol; FPG Fasting plasma glucose, 2-h PG 2-h postprandial plasma glucose, HbA1c Glycated hemoglobin A1c, GDF15 Growth differentiation factor 15
Serum GDF15 levels at baseline were 983.05 (694.70, 1324.85) and 897.40 (769.90, 1171.30) in M1 and M2 group (P = 0.692), respectively. Serum GDF15 levels at endpoint were 1040.65 (853.75, 1333.93) and 1559.70 (1247.35, 2416.00) in M1 and M2 group (P < 0.001), respectively. The median (interquartile range) of ΔGDF15 of M1 and M2 subgroup were 108.15 (− 44.23, 191.68) and 538.00 (415.00, 1283.55), respectively (Fig. 1B, P < 0.001). There were no significant differences of ΔBMI, ΔFPG, Δ2-h PG and ΔHbA1c between the M1 and M2 subgroup [ΔBMI, 0.00 (− 0.49, 0.50) vs. 0.17 (− 0.31, 0.76); ΔFPG, − 1.3 (− 3.40, − 0.76) vs. -1.49 (− 3.47, − 0.13); Δ2-h PG, − 3.32 (− 6.02, − 1.68) vs. -4.26 (− 8.21, − 2.63); ΔHbA1c, − 0.55 (− 1.03, − 0.15) vs. -0.80 (− 1.90, − 0.45), all P > 0.05, Fig. 1C-F).
Spearman correlation coefficient was used to analyze the possible relationship between glycemic control parameters and results showed that ΔGDF15 was not correlated to ΔFPG, Δ2-h PG and ΔHbA1c (all P > 0.05, Table 3). To further verify the relationship, analysis of covariance after adjustment for baseline HbA1c levels showed that ΔGDF15 was also not correlated to ΔFPG, Δ2-h PG and ΔHbA1c (ΔFPG, P = 0.682; Δ2-h PG, P = 0.704; ΔHbA1c, P = 0.725).
Table 3.
Spearman correlation analysis of ΔGDF15 and the change of clinical parameters in MET group
| ΔGDF15 | ||
|---|---|---|
| r | P | |
| ΔFPG (mmol/L) | 0.075 | 0.602 |
| Δ2-h PG (mmol/L) | −0.131 | 0.361 |
| ΔHbA1c (%) | −0.166 | 0.245 |
Abbreviations: GDF15 Growth differentiation factor 15, FPG Fasting plasma glucose, 2-h PG 2-h postprandial plasma glucose, HbA1c Glycated hemoglobin A1c
Discussion
It has been reported that GDF15 was a promising biomarker for metformin treatment and could reflect the dosage of metformin treatment at the same time [10, 16]. In a nested case-control study, Natali et al. [17] found that in patients with diabetes mellitus, metformin treatment was associated with a 40% rise in serum GDF15 levels. Although these cross-sectional studies have focused on GDF15 and metformin, our study first investigated the change of serum GDF15 levels before and after metformin treatment in patients with type 2 diabetes mellitus. We found that compared with baseline levels, serum GDF15 levels increased about 35% after a 12-week metformin treatment in patients with type 2 diabetes mellitus. Moreover, our results showed that after acarbose treatment, serum GDF15 levels did not change. It is similar with previous studies that serum GDF15 levels were not associated with other hypoglycemia therapy [10, 17].
Although the increase of GDF15 levels was a unique characteristic of metformin treatment, the change of GDF15 in previous studies was discovered to be mainly associated with weight loss so far [18]. A previous study focusing on people without diabetes mellitus revealed that metformin treatment was associated with significantly increased levels of circulation GDF15 with lost about 3.5% of body weight [12]. There still remains a paucity of information on the relationship between GDF15 and the glucose-lowering effect of metformin in patients with type 2 diabetes mellitus. The present study first found that the increase of serum GDF15 levels after metformin intervention was not related to the improvement of glycemic control parameters (ΔFPG, Δ2-h PG or ΔHbA1c) in patients with type 2 diabetes mellitus. Our results also showed that serum GDF15 levels did not increase in nearly half of the subjects after a 12-week metformin treatment, while serum GDF15 levels of the other half increased approximately 70%. However, the changes of glycemic control parameters were similar in the two subgroups (divided according to the median of ΔGDF15), which further proved that the elevation of serum GDF15 levels was not directly associated with glucose metabolism improvement in patients with type 2 diabetes mellitus. Still, reasons for the differences of ΔGDF15 between these two subgroups after metformin intervention need further detailed investigation.
To be noted, body weight of the MET group did not change after the intervention. Possible reasons may be the features of participants in our study. Firstly, the participants included were mostly overweight, not obese. Moreover, the improvement of the glycemic control in MET group may have prevented the loss of nutrition and partly neutralized the weight-loss effect of metformin. Without weight changes, this study population was definitely a proper model to explore the underlying relationship between GDF15 and the glucose-lowering effect of metformin. Previous animal studies showed that overexpression of GDF15 levels in mice led to decreased food intake, body weight and improved glucose metabolism [19, 20]. Taking the results from our study into consideration, the improved glucose metabolism in animal studies may be secondary to the decreased food intake and body weight.
There are some limitations of the present study. First, this was a pilot study, so the sample size was relatively small. In addition, we did not evaluate GDF15 levels after the standard meal. Therefore, further large-scale studies with more intense investigation on the change of serum GDF15 levels are needed to precisely elucidate the relationship among GDF15, glycemic control and metformin treatment.
In conclusion, the increase of serum GDF15 levels was an indicator of metformin treatment. However, the increase was not associated with the improvement of glycemic control in patients with type 2 diabetes mellitus.
Acknowledgements
We would like to thank all the involved clinicians, nurses, and technicians for their contribution to the study. We are grateful to all the participants for their dedication in data collection and laboratory measurements.
Abbreviations
- GDF15
Growth differentiation factor 15
- BMI
Body mass index
- HbA1c
Glycated hemoglobin A1c
- FPG
Fasting plasma glucose
- 2-h PG
2-h postprandial plasma glucose
- SBP
Systolic blood pressure
- DBP
Diastolic blood pressure
- TC
Total cholesterol
- TG
Triglycerides
- HDL-c
High-density lipoprotein cholesterol
- LDL-c
Low-density lipoprotein cholesterol
Authors’ contributions
J.Z., X.M. and J.Y. designed the study. F.G. and C.L. contributed to data analysis and writing the paper. Y.W. helped with the measurement of growth differentiation factor 15. F.G., W.L. and X.M. contributed to data collection. J. L, J. Z, J.Y. and X.M. contributed to interpretation of data and revision of the manuscript. All authors have read and approved the final manuscript. J.Y. and X.M. are the guarantors of this work and had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. All authors have read and approved the final manuscript.
Funding
This work was funded by the Shanghai Municipal Commission of Health and Family planning General Program (201840232), Shanghai Municipal Education Commission—Gaofeng Clinical Medicine Grant Support (20172025, 20161430), National Natural Science Foundation of China (82070885), and Shanghai Municipal Key Clinical Specialty. The funding body provided financial support for the present study and supervised the study design, conduct, and data collection and analysis.
Availability of data and materials
The datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The study was approved by the Ethics Committee of Shanghai Jiao Tong University Affiliated Sixth People’s Hospital and was in accordance with the 1964 Declaration of Helsinki. Informed consent was obtained from each subject at the beginning of the study.
Consent for publication
Not applicable.
Competing interests
No potential conflicts of interest relevant to this article were reported.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Fei Gao and Cheng Li contributed equally to this work.
Contributor Information
Jun Yin, Email: yinjun@sjtu.edu.cn.
Xiaojing Ma, Email: maxiaojing@sjtu.edu.cn.
References
- 1.Jia W, Weng J, Zhu D, et al. Standards of medical care for type 2 diabetes in China 2019. Diabetes Metab Res Rev. 2019;35(6):e3158. doi: 10.1002/dmrr.3158. [DOI] [PubMed] [Google Scholar]
- 2.Marx N, Davies MJ, Grant PJ, et al. Guideline recommendations and the positioning of newer drugs in type 2 diabetes care. Lancet Diabetes Endocrinol. 2021;9(1):46–52. doi: 10.1016/S2213-8587(20)30343-0. [DOI] [PubMed] [Google Scholar]
- 3.Doyle-Delgado K, Chamberlain JJ, Shubrook JH, et al. Pharmacologic approaches to glycemic treatment of type 2 diabetes: synopsis of the 2020 American Diabetes Association’s Standards of Medical Care in Diabetes Clinical Guideline. Ann Intern Med. 2020;173(10):813–821. doi: 10.7326/M20-2470. [DOI] [PubMed] [Google Scholar]
- 4.Rena G, Hardie DG, Pearson ER. The mechanisms of action of metformin. Diabetologia. 2017;60(9):1577–1585. doi: 10.1007/s00125-017-4342-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yerevanian A, Soukas AA. Metformin: mechanisms in human obesity and weight loss. Curr Obes Rep. 2019;8(2):156–164. doi: 10.1007/s13679-019-00335-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cree-Green M, Bergman BC, Cengiz E, et al. Metformin improves peripheral insulin sensitivity in youth with type 1 diabetes. J Clin Endocrinol Metab. 2019;104(8):3265–3278. doi: 10.1210/jc.2019-00129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Adela R, Banerjee SK. GDF-15 as a target and biomarker for diabetes and cardiovascular diseases: a translational prospective. J Diabetes Res. 2015;2015:490842. doi: 10.1155/2015/490842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Patel S, Alvarez-Guaita A, Melvin A, et al. GDF15 provides an endocrine signal of nutritional stress in mice and humans. Cell Metab. 2019;29(3):707–18.e8. doi: 10.1016/j.cmet.2018.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Desmedt S, Desmedt V, De Vos L, et al. Growth differentiation factor 15: a novel biomarker with high clinical potential. Crit Rev Clin Lab Sci. 2019;56(5):333–350. doi: 10.1080/10408363.2019.1615034. [DOI] [PubMed] [Google Scholar]
- 10.Breit SN, Brown DA, Tsai VW. The GDF15-GFRAL pathway in health and metabolic disease: friend or foe? Annu Rev Physiol. 2021;83:127–151. doi: 10.1146/annurev-physiol-022020-045449. [DOI] [PubMed] [Google Scholar]
- 11.Berezin AE. Diabetes mellitus related biomarker: the predictive role of growth-differentiation factor-15. Diabetes Metab Syndr. 2016;10(1 Suppl 1):S154–S157. doi: 10.1016/j.dsx.2015.09.016. [DOI] [PubMed] [Google Scholar]
- 12.Gerstein HC, Pare G, Hess S, et al. Growth differentiation factor 15 as a novel biomarker for metformin. Diabetes Care. 2017;40(2):280–283. doi: 10.2337/dc16-1682. [DOI] [PubMed] [Google Scholar]
- 13.Coll AP, Chen M, Taskar P, et al. GDF15 mediates the effects of metformin on body weight and energy balance. Nature. 2020;578(7795):444–448. doi: 10.1038/s41586-019-1911-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cai L, Li C, Wang Y, et al. Increased serum GDF15 related to improvement in metabolism by lifestyle intervention among young overweight and obese adults. Diabetes Metab Syndr Obes. 2021;14:1195–1202. doi: 10.2147/DMSO.S302033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gao F, Ma X, Peng J, et al. The effect of Acarbose on glycemic variability in patients with type 2 diabetes mellitus using premixed insulin compared to metformin (AIM): an open-label randomized trial. Diabetes Technol Ther. 2020;22(4):256–264. doi: 10.1089/dia.2019.0290. [DOI] [PubMed] [Google Scholar]
- 16.Day EA, Ford RJ, Smith BK, et al. Metformin-induced increases in GDF15 are important for suppressing appetite and promoting weight loss. Nat Metab. 2019;1(12):1202–1208. doi: 10.1038/s42255-019-0146-4. [DOI] [PubMed] [Google Scholar]
- 17.Natali A, Nesti L, Venturi E, et al. Metformin is the key factor in elevated plasma growth differentiation factor-15 levels in type 2 diabetes: a nested, case-control study. Diabetes Obes Metab. 2019;21(2):412–416. doi: 10.1111/dom.13519. [DOI] [PubMed] [Google Scholar]
- 18.Wang D, Day EA, Townsend LK, et al. GDF15: emerging biology and therapeutic applications for obesity and cardiometabolic disease. Nat Rev Endocrinol. 2021;17(10):592–607. doi: 10.1038/s41574-021-00529-7. [DOI] [PubMed] [Google Scholar]
- 19.Xiong Y, Walker K, Min X, et al. Long-acting MIC-1/GDF15 molecules to treat obesity: evidence from mice to monkeys. Sci Transl Med. 2017;9(412):eaan8732. doi: 10.1126/scitranslmed.aan8732. [DOI] [PubMed] [Google Scholar]
- 20.Macia L, Tsai VW, Nguyen AD, et al. Macrophage inhibitory cytokine 1 (MIC-1/GDF15) decreases food intake, body weight and improves glucose tolerance in mice on normal & obesogenic diets. Plos One. 2012;7(4):e34868. doi: 10.1371/journal.pone.0034868. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.