Abstract
Background
Urban malaria has received insufficient attention in the literature. The prevalence and clinical characteristics of Plasmodium falciparum infection amongst patients presenting with suspected malaria were investigated at a major urban hospital in Douala, Cameroon with a particular focus on anaemia.
Methods
A cross-sectional, 18-week demographic and clinical survey was conducted of patients presenting to the Emergency Department of Douala Military Hospital with suspected malaria, largely defined by the presence or recent history of fever. Venous samples were tested for P. falciparum using rapid diagnostic tests and PCR, and anaemia was defined by haemoglobin level according to WHO definitions. Likelihood ratios (LR), odds ratios (OR), and population attributable risk percent (PARP) were calculated.
Results
Participants were ages 8 months to 86 years, 51% were women (257/503), and all districts of Douala were represented. Overall, 38.0% (n = 189/497) were anaemic, including 5.2% (n = 26/497) with severe anaemia. Anaemia prevalence was significantly higher (OR: 2.20, 95% CI 1.41–3.45) among children < 15 years (53.1%, n = 52/98) compared to adults (34%, n = 133/392). Plasmodium falciparum was detected in 37.2% by nested PCR. Among all participants, several factors were associated with clinically significant LR for P. falciparum infection, including age 10–14 years (positive LR: 3.73), living in the island district of Douala VI (positive LR: 3.41), travel to any of three northern regions (positive LR: 5.11), and high fever > 40 °C at presentation (positive LR: 4.83). Among all participants, 8.7% of anaemia was associated with P. falciparum infection, while the PARP was 33.2% among those < 15 years of age and 81.0% among 10–14-year-olds.
Conclusions
The prevalence of P. falciparum infection in the urban hospital was high. Mirroring trends in many rural African settings, older children had the highest positivity rate for P. falciparum infection. Anaemia was also common in all age groups, and for those 10–14 years of age, 80% of the risk for anaemia was associated with P. falciparum infection. Malaria rates in major urban population centres can be high, and more research into the multifactorial causes of anaemia across the age spectrum are needed.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12936-022-04315-2.
Keywords: Malaria, Plasmodium falciparum, Anaemia, Cameroon, Douala, Febrile illness, Urban
Background
Malaria remains one of the most important global infectious diseases, with an estimated 241 million incident cases and 627,000 estimated malaria deaths in 2020 [1]. Malaria morbidity and mortality fall disproportionally on the African continent, with 95% of malaria cases and 96% of malaria deaths occurring in the World Health Organization’s (WHO) African Region in 2020 [1]. In sub-Saharan Africa, the risk of malaria has been traditionally considered to be significantly higher in rural than urban settings [2]. This increased risk has been attributed to multiple factors, including a lack of vectors ideally suited to urban environments, better housing, and improved access to care. However, many epidemiologic and demographic shifts have been occurring on the continent, including reports of shifting age burdens for malaria, emergence of unique risk factors for urban malaria, and recent reports of expanding ranges for the urban dwelling Anopheles stephensi vector [3–7].
Located in the WHO Central Africa region, Cameroon is one of the WHO’s designated high burden to high impact malaria countries with > 6.9 million malaria cases and 14,841 malaria deaths estimated for 2020 [1, 8, 9]. The health system includes 10 health regions, 189 health districts, about 1700 health areas, and a community health component in 90 districts, with only 1.07 doctors and nurses per 1000 persons [8, 10]. While the entire country remains at risk for malaria, Cameroon can be divided into multiple epidemiological zones each with varying levels of malaria transmission. For example, the Mapping Malaria Risk in Africa epidemiological stratification divides the country from South to North into three major epidemiological facets: (i) the Equatorial facet, (ii) the Tropical/Sudanian facet that include the North Region, and (iii) the Sahelian facet in the Far North zone [11]. Recent studies have also divided the country into five climate zones: the Sahelian zone (including the Extreme North region), the Soudanian zone (North region), the Sahelo-Guinean zone (Adamaoua region), the Humid Savannah zone (North–West and West regions), and the more southern Forest zone (Central, East, South, Littoral, and South–West regions) [8, 12, 13]. Overall, Plasmodium falciparum is estimated to account for > 95% of malaria cases in Cameroon, though Plasmodium malariae, Plasmodium ovale, and even Plasmodium vivax have been detected [13–19].
The economic hub and most populous city of Douala has a smaller representation in the malaria literature than the capital, Yaoundé. Lying within the Littoral Region of the Forest zone, Douala is home to a population of approximately 3 million people [13]. This zone experiences two rainy seasons with annual rainfall between 1500 and 4000 mm and the entomological inoculation rate (EIR) ranges from 0 to 90 infective bites per person per year in Douala [13]. Studies conducted in Douala in 2009–2010 and 2014 estimated the EIR to be 31 and 110, respectively [20, 21]. The Cameroon National Malaria Control Programme reported a country-wide incidence of 103.1 cases per 1000 inhabitants in 2019, with 96.0 cases per 1000 inhabitants in the Littoral Region [22]. The 2018 Demographic and Health Survey (DHS), which focuses on community-based sampling, found 7.8% of children 6–59 months to be RDT-positive for malaria in Douala [23]. Thus, while EIR surveys have suggested high rates of transmission potential in Doula, literature on the prevalence and characteristics of malaria in this populous urban environment are relatively scarce.
Uncomplicated malaria often presents with nonspecific symptoms including malaise, headache, fatigue, abdominal discomfort, and myalgias/arthralgias followed by fever, chills, diaphoresis, anorexia, nausea, and emesis [24]. In infants, malaria may present like any other cause of sepsis with lethargy and poor feeding, and young children may also develop cough or gastrointestinal symptoms [24]. Signs and symptoms of severe malaria include coma, convulsions, impaired consciousness, acute pulmonary edema, renal failure, metabolic acidosis, severe anaemia, or severe hypoglycemia [24], and severe anaemia may be the most common severe manifestation [1]. In settings such as urban Cameroon, anaemia may be caused by several overlapping factors including non-malarial infections, haemoglobinopathies, and poor nutritional status. In a community sample of 6 to 59-month-old children from Douala, the 2018 DHS found a haemoglobin < 11.0 g/dL (anaemia of any severity), 7.0–9.9 g/dL (moderate anaemia), < 8.0 g/dL (regarded as malaria-related anaemia), and < 7.0 g/dL (severe anaemia) in 56.5%, 25.1%, 5.8%, and 1.7% of children, respectively [23]. Individual studies in Douala have examined haemoglobin levels in clinical settings, but comorbidities varied, and different studies used different haemoglobin cutoffs [25–28].
Urban studies looking at rates of malaria and anaemia in those presenting to high level care centers with fever are also less prevalent in the literature. In Cameroon, the few existing studies in Douala over the past 20+ years have been limited by reliance on microscopy for diagnosis, recruitment of a narrow age range, inconsistent haemoglobin thresholds used to define anaemia, and minimal assessment of additional symptomatology. To characterize the burden and presentation of malaria and anaemia in the most populated urban centre in Cameroon, a descriptive survey of symptomatic patients presenting to the Emergency Department (ED) of Douala Military Hospital (DMH) was conducted. This manuscript reports on the prevalence of P. falciparum infection and the clinical characteristics of these infections, with a specific emphasis on anaemia.
Methods
Study design and study population
A cross-sectional epidemiological and clinical survey of symptomatic patients presenting to the ED of DMH was conducted. The hospital sits in the Douala I district and treats both military and civilian patients. Its ED consists of 2–3 physicians, a triage station staffed by 5–7 nurses, and approximately 18 beds. The study planned to recruit a convenience sample of 450 patients presenting to the ED for whom there was a suspicion of malaria. Recruitment was planned for consecutive, weekday enrollment due to the availability of the laboratory staff and equipment. ED physicians and nurses were asked to refer patients meeting the following eligibility criteria to the study team:
1) Age of 6 months and older.
2) At least one of the following: (a) temperature ≥ 37.5 °C on presentation to the triage nurse station; (b) subjective history of fever in the previous three days; or (c) suspicion of malaria infection by the ED physician.
Clinical evaluation and point-of-care testing for malaria and anaemia
The recruitment team administered a questionnaire including demographics, travel history, prior medications and traditional remedies used, and symptomology. The team also assessed for clinical signs of anaemia (palmar pallor, conjunctival pallor, and koilonychia). Symptoms and signs were rated on a four-point scale from 0 to 3 with 0 indicating the absence of the symptom/sign, 1 indicating borderline or mild (or once per day in the case of emesis and diarrhoea), 2 indicating moderate (or twice per day for emesis and diarrhoea), and 3 indicating severe symptoms (or ≥ 3 times per day for emesis and diarrhoea). Temperature from the triage station was recorded from the patient’s medical card if available, and an oral temperature (or rectal in infants) was taken by study personnel for all participants.
In the ED, a 2 mL venous blood sample was collected, used to measure haemoglobin level using the HemoCue® 201 + system (HemoCue America, Brea, CA, USA), and tested with a WHO pre-qualified histidine-rich protein 2 (HRP-2)/Plasmodium lactate dehydrogenase (pLDH) malaria rapid diagnostic test (RDT) commonly used throughout Cameroon (Standard Q Malaria Pf/Pan Ag RDT, SD Biosensor, Suwon, South Korea) [29].
Molecular-based speciation from red blood cell pellets
Whole blood samples were centrifuged at 3000 rpm for 5–10 min and then pipetted into plasma and red blood cell (RBC) pellet samples. All samples were stored at -20° C in the DMH lab and later transported on ice to the Malaria Unit of the Centre Pasteur of Cameroon (CPC) in Yaoundé, Cameroon. DNA was extracted from the RBC pellets at the CPC using DNeasy® Blood and Tissue DNA extraction kits (QIAGEN, Germantown, MD, USA) then shipped to the Yale School of Public Health in New Haven, USA on dry ice. Extracted DNA was amplified by nested polymerase chain reaction (PCR) (Additional file 1: Tables S1–2) using GoTaq® Flexi DNA Polymerase kit (Promega Corporation, Madison, WI, USA), according to established protocols with the expected sizes for the species-specific Nest 2 products of 205 bp for P. falciparum, 144 bp for P. malariae, 120 bp for P. vivax, and approximately 800 bp for P. ovale [30, 31].
Data management and statistical analysis
All recruitment data were recorded onto paper standardized case record forms by study staff and then double entered into the database. Participants were grouped into ages < 15 years-old (children) and ≥ 15 years old (adults). Children were further divided into those < 5 years old, 5–9 years old, and 10–14 years old. Detection by PCR constituted the reference standard for the presence of malaria infection. Anaemia was defined by WHO criteria (see Additional file 1: Table S3) based on haemoglobin level (g/dL) assessed with the HemoCue® system [32]. Continuous variables were reported as means ± standard deviation (SD), while categorical variables were reported as number and percent. Means were compared between P. falciparum-positive and negative groups using the student t-test, and association between categorical variables and malaria status was assessed using the Pearson Chi-square. Prevalence odds ratios (OR) with 95% confidence intervals (CI) were calculated using binomial logistic regression in which a single predictor variable (e.g. sex) with two categories was entered [33]. Population attributable risk percent (PARP) was defined as (incidence among all participants within the population − incidence among non-exposed)/incidence among all participants within the population. Likelihood ratios (LR) were defined and interpreted according to McGee: the positive LR refers to the LR when the symptom/sign is present and was defined as sensitivity/(1 − specificity), while the negative LR refers to the LR when the symptom/sign is absent and was defined as (1-sensitivity)/specificity [34]. A significant LR was defined as one which changes the probability by ≥ 20%; therefore, a clinically significant positive LR was defined as ≥ 3, while a clinically significant negative LR was defined as ≤ 0.4 (Additional file 1: Table S4) [34]. Statistical significance was defined as 2-tailed p-value < 0.05 for all analyses. Data were analysed in Microsoft Excel and IBM SPSS Version 28 (IBM Corporation, Armonk, NY, USA).
Results
Study participant profile
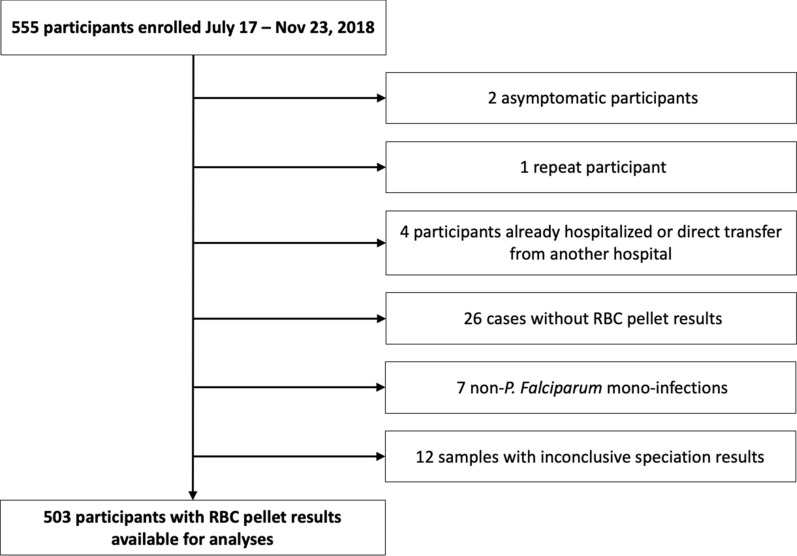
Between July 17 and November 23, 2018, a total of 555 participants were enrolled in the study, and 503 results from RBC pellet samples were available for molecular analyses (Fig. 1). Characteristics for these 503 participants are provided in Table 1, and these were similar for the full cohort of recruited participants. Participant sex was balanced, and participant’s mean age was 32 years old (range 8 months to 86 years). There were 7 pregnant women. The cohort represented all districts within Douala: 26.2% (n = 132/503) from Douala I (Bonanjo; site of DMH), 10.5% (n = 53/503) from Douala II (New Bell), 29.4% (n = 148/503) from Douala III (Bonassama), 5.6% (n = 28/503) from Douala IV (Logbaba), 18.9% (n = 95/503) from Douala V (Kotto), and 1.8% (n = 9/503) from the island district of Douala VI (Manoka). Only 3.8% (n = 19/503) of participants were from outside Douala. Over one quarter had traveled outside of Douala during the previous 30 days; the most common destinations included the Central region which includes Yaoundé (9.0%, n = 45/500), the West region (5.8%, n = 29/500) and the greater Littoral region (4.2%, n = 21/500). More participants had already taken an antipyretic (40.3%) than an anti-malarial (17.3%) or a traditional remedy (10.1%).
Fig. 1.
Participant recruitment and inclusion. Diagram describes recruited patients excluded from the analyses for this manuscript. Demographics for the full cohort of recruited patients were similar to the demographics reported here for the 503 participants included in these analyses
Table 1.
Participant characteristics by PCR-determined malaria status
| All n = 503 |
P. falciparum* positive n = 187 |
P. falciparum negative n = 316 |
p** | |
|---|---|---|---|---|
| Age |
n = 498 32 ± 20 years |
n = 186 30 ± 20 years |
n = 312 34 ± 20 years |
.038 |
| Female*** |
n = 503 257 (51.1) |
n = 187 84 (44.9) |
n = 316 173 (54.7) |
.033 |
| Anti-malarial |
n = 501 87 (17.4) |
n = 186 40 (21.5) |
n = 315 47 (14.9) |
.060 |
| Antipyretic |
n = 501 202 (40.3) |
n = 186 86 (46.2) |
n = 315 116 (36.8) |
.038 |
| Traditional remedy |
n = 495 50 (10.1) |
n = 185 25 (13.5) |
n = 310 25 (8.1) |
.052 |
| Travel |
n = 500 143 (28.6) |
n = 185 55 (29.7) |
n = 315 88 (27.9) |
.668 |
| Fever by history |
n = 502 488 (97.2) |
n = 186 183 (97.8) |
n = 316 306 (96.8) |
.505 |
| Fever on presentation |
n = 488 322 (66.0) |
n = 179 121 (67.6) |
n = 309 201 (65.0) |
.567 |
| Temperature on presentation |
n = 488 37.8 ± 1.0 |
n = 179 38.1 ± 1.1 |
n = 309 37.7 ± 0.9 |
< .001 |
Values reported as mean ± SD for age and number (valid percent) for other variables
Anti-malarial, antipyretic, and traditional remedy refers to participant reported medication use for the current illness episode
Travel refers to participant reported travel within the previous 30 days
Fever by history refers to participant-reported fever within the preceding 72 h. Note that only 2.8% of participants did not have history of fever, and the percentage of participants with history of fever was equivalent between the two groups
Fever on presentation was defined as temperature ≥ 37.5 °C on measurement by either emergency department staff or oral measurement by study personnel
*Includes 9 cases of P. falciparum/P. malariae and 5 cases of P. falciparum/P. ovale mixed infections
**Means of continuous variables compared using independent samples t-test and categorical variables compared using Pearson Chi square. Significant p-values < 0.05 bolded
***Includes 7 pregnant women
Clinical presentation of participants
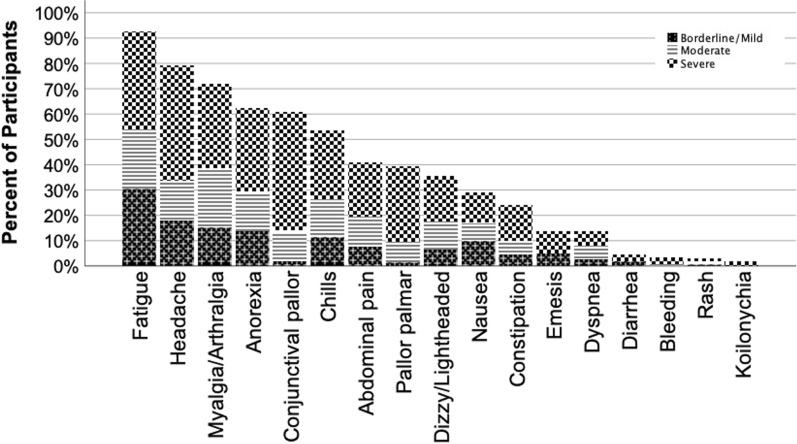
Almost all patients (97.2%, n = 488 / 502) had a history of fever (Table 1), as expected given eligibility criteria. Figure 2 presents the proportion of all participants reporting additional symptoms or with detected signs, as well as the severity of these symptoms and signs. Nonspecific symptoms of fatigue (92.4%, n = 465/503), headache (79.4%, n = 392/494), myalgias/arthralgias (71.8%, n = 359/500), anorexia (62.5%, n = 313/501), and chills (53.5%, n = 268/501) were more frequently reported. Other symptoms were reported by less than half of participants. Among signs of anaemia, conjunctival pallor was observed in 60.7% (n = 304/501), while pallor earning a score of moderate (conjunctival: 11.4%, n = 57/501; palmar: 8.0%, n = 40/501) or severe (conjunctival: 2.0%, n = 10/501; palmar: 1.4%, n = 7/501) was infrequent. Koilonychia was rare and rated as mild/borderline in all cases.
Fig. 2.
Presenting symptoms and signs. Presenting symptoms and signs of participants. Pattern of shading indicates severity of reported symptom. In addition to the symptoms reported in this figure, 97.2% of participants reported a history of fever in the preceding 72 h
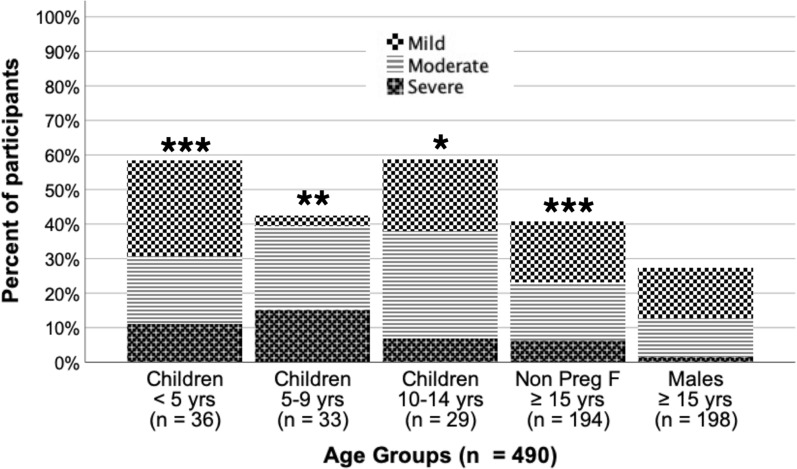
Among all participants, the prevalence of anaemia was 38.0% (n = 189/497), including 16.3% (n = 81/497) with moderate and 5.2% (n = 26/497) with severe anemia. Clinical findings associated with anaemia can be found in Additional file 1: Table S5. Four participants under the age of 12 had a haemoglobin < 5 g/dL, and 8 participants ≥ 12 years old had a haemoglobin < 7 g/dL, which are part of the criteria for severe malaria based on the presence of low haemoglobin. The prevalence of anaemia (any severity) was significantly higher (OR: 2.20, 95% CI 1.41–3.45) among children < 15 years (53.1%, n = 52/98) compared to adults ≥ 15 years old (34%, n = 133/392). The prevalence of severe anaemia was also significantly higher (OR: 3.18, 95% CI 1.41–7.16) among children (11.2%, n = 11/98) compared to adults (3.8%, n = 15/392). Considering different age groups, anaemia (any severity) was significantly higher among children < 5 years old (OR: 3.73, 95% CI 1.79–7.77), children 10–14 years (OR: 3.78, 95% CI 1.69–8.43), and nonpregnant women ≥ 15 years old (OR: 1.83, 95% CI 1.20–2.80) compared to men ≥ 15 years old (Fig. 3). Furthermore, severe anaemia was significantly higher among children < 5 years old (OR: 8.13, 95% CI 1.74–38.01), children 5–9 years (OR: 11.61, 95% CI 2.63–51.25), and nonpregnant women ≥ 15 years old (OR: 4.29, 95% CI 1.19–15.43) compared to men ≥ 15 years old (Fig. 3).
Fig. 3.
Anaemia by age groups. Percent of participants with anaemia by age group. For participants 15 years old, the group is split between males and females with pregnant women excluded. Shading pattern indicates anaemia severity. See text for complete details. N Preg F: non pregnant females. *Indicates anaemia (any severity) was significant higher compared to males ≥ 15 years old. **Indicates severe anaemia was significantly higher compared to males ≥ 15 years old. *** Indicates both anaemia (any severity) and severe anaemia were higher compared to males ≥ 15 years old
Diagnosis of P. falciparum infection
One quarter (25.2%, n = 126/500) of participants were positive by RDT, with 12.2% (n = 61/500) positive for HRP-2 only (P. falciparum infection only), 12.6% (n = 63/500) positive for both HRP-2 and pLDH (P. falciparum mono- or mixed infection), and only 0.4% (n = 2/500) positive for pLDH alone (either mono- or mixed non-P. falciparum species infection).
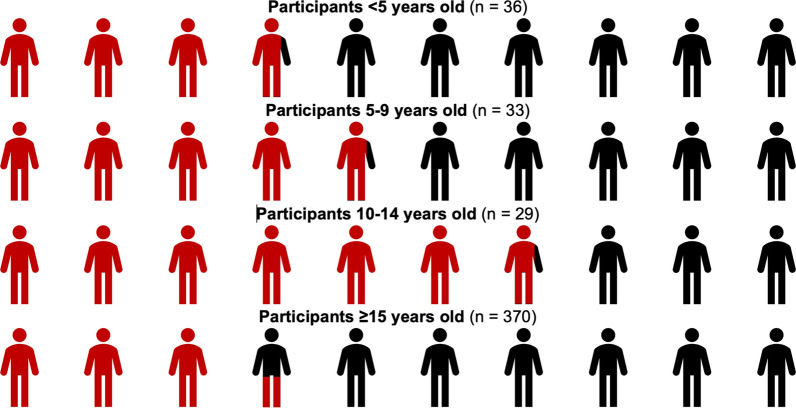
Malaria rates were higher by nested PCR; among all participants, 37.2% (n = 187/503) were positive for P. falciparum, including 34.4% (n = 173/503) P. falciparum mono-infection, 1.8% (n = 9/503) P. falciparum/P. malariae mixed infection, and 1.0% (n = 5/503) P. falciparum/P. ovale mixed infection. P. falciparum infection status varied by age group (Fig. 4), with P. falciparum detected by PCR in 38.9% (n = 14/36) of < 5 year-olds, 48.5% (n = 16/33) of 5–9 year-olds, 69.0% (n = 20/29) of 10–14 year-olds, and 34.0% (n = 136/400) of ≥ 15 years old. There was a significant association between age group and either positive RDT (χ2(3) = 31.31, p < 0.001) or detection of P. falciparum by PCR (χ2(3) = 16.09, p < 0.001) with the highest standardized residuals for the 10–14-year-old age group. The prevalence of P. falciparum as diagnosed by PCR was significantly higher among participants aged 10–14 compared to participants < 5 years old (OR: 3.49, 95% CI 1.24–9.82) and ≥ 15 years old (OR: 4.31, 95% CI 1.91–9.73).
Fig. 4.
Plasmodium falciparum infection by age group. Each stick-figure represents 10% of participants in that age group, and red indicates P. falciparum was detected by PCR. The prevalence of P. falciparum was significantly higher among participants aged 10–14 compared to participants < 5 years old and compared to participants ≥ 15 years old. See text for complete details
Comparison to DHS data
Regarding measurement of haemoglobin level among children 6–59 months old, haemoglobin was < 11.0 g/dL in 58.3% (compared to 56.5% in the DHS), between 7.0–9.9 g/dL in 19.4% (compared to 25.1%), < 8.0 g/dL in 13.9% (compared to 5.8%), and < 7.0 g/dL in 11.1% (compared to 1.7%). Regarding prevalence of malaria infection as diagnosed by RDT, 25.0% of participants 6–59 months were RDT positive, compared to 7.8% in the DHS.
Characteristics of P. falciparum PCR-positive participants
PCR results were used as the reference standard for malaria diagnosis. Compared to PCR-negative participants, P. falciparum PCR-positive participants were younger (t = 2.08, p = 0.038) and more likely to have reported already trying an antipyretic (χ2(1) = 4.31, p = 0.038) (Table 1). Presenting temperature was also higher for P. falciparum-positive versus negative participants (t = − 3.6, p < 0.001). Among epidemiological factors, clinically significant positive LRs for P. falciparum infection by PCR were associated with age 10–14 years (positive LR: 3.73), living in the island district of Douala VI (positive LR: 3.41), and travel to any of three northern regions (positive LR: 5.11). Clinically, high fever > 40 °C at the time of presentation (positive LR: 4.83) and frequent emesis ≥ 2 times per day (19.85) were associated with clinically significant positive LRs. Several other gastrointestinal symptoms weakly increased the probability of P. falciparum infection with positive LR >2.0 (moderate to severe abdominal pain, nausea, or diarrhoea). Considering PCR as the gold standard, RDTs demonstrated high positive and negative predictive value in this urban setting. A positive RDT (any band) increased the probability of malaria dramatically (positive LR: 28.70), and an HRP-2/pLDH positive result had a positive predictive value close to 1.0 (positive predictive value: 0.997, positive LR > 100). The absence of a positive RDT (i.e., a negative RDT) was associated with a clinically significant negative LR (0.37). Table 2 presents likelihood ratios separately for children and adults. Notably, moderate to severe anaemia significantly increased the probability of P. falciparum infection among children (positive LR: 3.24–4.32), but not adults (positive LR: 0.70–0.91).
Table 2.
Diagnostic characteristics of clinical findings for PCR-confirmed P. falciparum infection
| Children < 15 years old (n = 98) | Adults ≥ 15 years old (n = 400) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | Sensitivity | Specificity | Positive LR | Negative LR | n | Sensitivity | Specificity | Positive LR | Negative LR | |
| Epidemiological history | ||||||||||
| Sex | 98 | |||||||||
| Female | .560 | .479 | 1.08 | .92 | 400 | .404 | .443 | .73 | 1.34 | |
| District of residence | 94 | 385 | ||||||||
| Douala I | .213 | .574 | .50 | 1.37 | .168 | .685 | .53 | 1.21 | ||
| Douala II | .191 | .915 | 2.25 | .88 | .130 | .913 | .91 | .95 | ||
| Douala III | .383 | .766 | 1.64 | .81 | .298 | .697 | .98 | 1.01 | ||
| Douala IV | .021 | .957 | .50 | 1.02 | .038 | .925 | .51 | 1.04 | ||
| Douala V | .128 | .809 | .68 | 1.08 | .252 | .815 | 1.36 | .92 | ||
| Douala VI | .043 | 1.000 | 1.00 | .96 | .031 | .988 | 2.59 | .98 | ||
| Outside Douala | .021 | .979 | 1.00 | .084 | .976 | 3.56 | .94 | |||
| Travel | 98 | 397 | ||||||||
| Anywhere | .160 | .792 | .77 | 1.06 | .343 | .707 | 1.17 | .93 | ||
| Northern Regions | .040 | 1.000 | *** | .96 | .030 | .992 | 3.93 | .98 | ||
| Medication use | 398 | |||||||||
| Antipyretic | 98 | .580 | .667 | 1.74 | .63 | .415 | .620 | 1.09 | .94 | |
| Antimalarial | 98 | .280 | .854 | 1.92 | .84 | .193 | .852 | 1.30 | .95 | |
| Traditional remedy | 97 | .061 | .917 | .74 | 1.02 | .156 | .919 | 1.91 | .92 | |
| Clinical history | ||||||||||
| Fever at presentation | 90 | 393 | ||||||||
| ≥ 37.5 °C | .787 | .326 | 1.17 | .65 | .641 | .355 | .99 | 1.01 | ||
| ≥ 40.0 °C | .149 | .953 | 3.20 | .89 | .053 | .989 | 4.67 | .96 | ||
| Fatigue | 98 | 400 | ||||||||
| Any severity | .940 | .125 | 1.07 | .48 | .926 | .072 | 1.00 | 1.02 | ||
| Moderate-Severe | .740 | .479 | 1.42 | .54 | .610 | .538 | 1.32 | .72 | ||
| Headache | 90 | 399 | ||||||||
| Any severity | .812 | .217 | 1.04 | .86 | .837 | .186 | 1.03 | .88 | ||
| Moderate-Severe | .489 | .867 | 3.67 | .59 | .452 | .712 | 1.57 | .77 | ||
| Myalgias/Arthralgias | 96 | 399 | ||||||||
| Any severity | .540 | .652 | 1.55 | .71 | .800 | .231 | 1.04 | .87 | ||
| Moderate-Severe | .240 | .891 | 2.21 | .85 | .526 | .610 | 1.35 | .78 | ||
| Anorexia | 98 | 398 | ||||||||
| Any severity | .840 | .417 | 1.44 | .38 | .662 | .424 | 1.15 | .80 | ||
| Moderate-Severe | .600 | .688 | 1.92 | .58 | .360 | .802 | 1.82 | .80 | ||
| Chills | 98 | 398 | ||||||||
| Any severity | .660 | .525 | 1.76 | .54 | .596 | .496 | 1.18 | .82 | ||
| Moderate-Severe | .400 | .833 | 2.40 | .72 | .375 | .798 | 1.86 | .78 | ||
| Abdominal pain | 95 | 397 | ||||||||
| Any severity | .633 | .478 | 1.21 | .77 | .511 | .706 | 1.74 | .69 | ||
| Moderate-Severe | .408 | .848 | 2.68 | .70 | .259 | .878 | 2.12 | .84 | ||
| Dizzy/Lightheaded | 94 | 399 | ||||||||
| Any severity | .479 | .783 | 2.20 | .67 | .422 | .674 | 1.30 | .86 | ||
| Moderate-Severe | .354 | .870 | 2.72 | .74 | .178 | .848 | 1.17 | .97 | ||
| Nausea | 94 | 396 | ||||||||
| Any severity | .490 | .733 | 1.84 | .70 | .368 | .781 | 1.68 | .81 | ||
| Moderate-Severe | .449 | .911 | 5.05 | .60 | .206 | .889 | 1.86 | .89 | ||
| Constipation | 96 | 398 | ||||||||
| Any severity | .306 | .830 | 1.80 | .84 | .200 | .741 | .774 | 1.08 | ||
| Moderate-Severe | .143 | .894 | 1.34 | .96 | .104 | .909 | 1.14 | .99 | ||
| Emesis | 88 | |||||||||
| Any frequency | .386 | .818 | 2.13 | .75 | 381 | .161 | .922 | 2.07 | .91 | |
| ≥ 2 times/day | .250 | .977 | 11.00 | .77 | .089 | .996 | 22.80 | .91 | ||
| Dyspnea | 95 | 398 | ||||||||
| Any severity | .125 | .936 | 1.96 | .93 | .215 | .886 | 1.88 | .89 | ||
| Moderate-Severe | .104 | .957 | 2.45 | .94 | .111 | .935 | 1.70 | .95 | ||
| Diarrhoea | 96 | 385 | ||||||||
| Any frequency | .104 | .958 | 2.50 | .93 | .055 | .969 | 1.76 | .98 | ||
| ≥ 2 times/day | .042 | 1.000 | *** (2) | .96 | .023 | .098 | 1.51 | .99 | ||
| Bleeding/Haemorrhage | 97 | 393 | ||||||||
| Any severity | .061 | .958 | 1.47 | .98 | .038 | .98 | 1.61 | .99 | ||
| Moderate-Severe | .020 | 1.000 | *** (1) | .98 | .000 | .992 | 1.96 | .99 | ||
| Cutaneous eruptions | 97 | 399 | ||||||||
| Any severity | .000 | .915 | .000 | 1.09 | .044 | .981 | 2.35 | .97 | ||
| Moderate-Severe | .000 | .957 | .000 | 1.04 | .015 | .992 | 1.96 | .99 | ||
| Point of care testing | ||||||||||
| RDT | 98 | 397 | ||||||||
| Any positive | .800 | .958 | 19.20 | 0.21 | .585 | .981 | 30.66 | .42 | ||
| HRP-2 positive | .360 | .979 | 17.28 | 0.65 | .274 | .981 | 14.36 | .74 | ||
| Both HRP-2/LDH positive | .440 | .979 | 21.12 | 0.57 | .296 | 1.00 | *** (40) | .70 | ||
| Anaemia | 98 | 399 | ||||||||
| Any severity | .700 | .646 | 1.98 | .56 | .338 | .654 | .98 | 1.01 | ||
| Moderate-Severe | .540 | .833 | 3.24 | .55 | .169 | .814 | .91 | 1.02 | ||
| Severe | .180 | .958 | 4.32 | .86 | .029 | .958 | .70 | 1.01 | ||
LR: likelihood ratio. Clinically significant likelihood ratios bolded
***Indicated LR could not be calculated as all the patients (number listed in parentheses) with the symptom/sign were found to be P. falciparum positive
Northern regions include Adamaoua, North, and Far-North
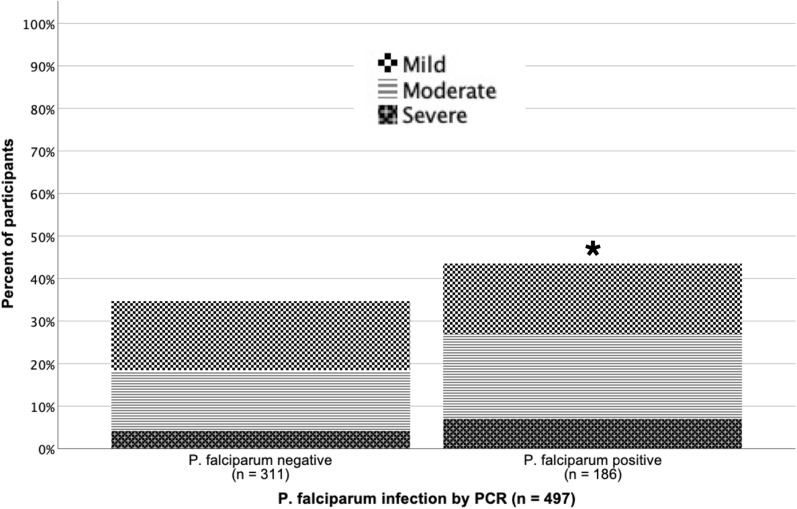
Among all participants, 8.7% (the PARP) of anaemia (any severity) was associated with P. falciparum infection, but this varied dramatically by age as discussed below. The prevalence of moderate to severe anaemia was significantly higher (OR: 1.64, 95% CI 1.06–2.53) among P. falciparum-positive (26.9%, n = 50/186) versus P. falciparum-negative participants (18.3%, n = 57/311), and the association between overall rates of anaemia and P. falciparum infection trended toward statistical significance (OR: 1.45, 95% CI 1.00–2.10) (Fig. 5b). Among children < 15 years old, 33.3% of anaemia was associated with P. falciparum infection, and the prevalence of anaemia (of any severity) was significantly higher (OR: 4.26, 95% CI 1.83–9.92) among participants with P. falciparum (70.0%, n = 35/50) compared to those negative for P. falciparum (35.4%, n = 17/48). For those ≥ 15 years old, anaemia was not associated with malaria (PARP -0.8%) and anaemia rates did not differ statistically by P. falciparum infection status (OR: 0.97, 95% CI 0.62–1.50). The percent of anaemia associated with P. falciparum infection varied by age, as the PARP was 14.3% among children < 5 years old, 30.7% among children 5–9-year-olds, and 81.0% among 10–14-year-olds.
Fig. 5.
Anaemia by P. falciparum infection status. Percent of participants with anaemia by P. falciparum infection status. Pattern of shading indicates anaemia severity. See text for complete details. *Indicates moderate-severe anaemia was significantly higher among P. falciparum positive participants
Discussion
An 18-week cross-sectional study of symptomatic patients across the age spectrum presenting to an urban hospital in the most populous city in Cameroon was conducted and found a high incidence of both P. falciparum infection and anaemia. As DMH is a fee-for-service hospital in a major metropolitan area, a lower prevalence of malaria infection may have been expected, as such centres receive febrile patients with a variety of aetiologies; however, over one third of individuals presenting with fever, history of fever, or other suspicion for malaria were PCR-positive for P. falciparum. This study echoes findings from an urban hospital in Sierra Leone during the same year [35]. In that study, febrile patients presenting to the hospital were found to have 50% positivity for malaria, overwhelmingly P. falciparum, using a highly sensitive multiplex real-time PCR assay. Recent research shows that new vectors may emerge in large, urban environments [3, 36] and that malaria transmission may have actually increased within cities across sub-Saharan Africa in the new millennium [5]. Within the context of these broader trends, these findings emphasize the need for proper surveillance and recognition that incidence of P. falciparum infection may remain high even within the largest population centres traditionally felt to be at lower risk.
Several epidemiologic and clinical characteristics were associated with a higher probability of P. falciparum infection among these participants. Residence on the island district of Manoka (Douala VI) or travel to any of the three northern regions (Adamaoua, North, or Far-North) increased the probability of P. falciparum detection. Travel as an important risk factor among an urban population has been previously described in Malawi [6]. Children 10–14 years of age were more likely than children < 5 years old to be P. falciparum positive, which may reflect broader trends in higher prevalence among older children across sub-Saharan Africa [7, 37]. Possible explanations for these trends in similar settings include the targeting of malaria control measures (e.g. bed nets, seasonal malaria chemoprevention) and the distribution of antimalarials at no charge to children under 5 years of age in many countries. Among all participants, high fever > 40 °C at presentation and frequent emesis increased the probability of P. falciparum infection. Among children, more severe symptoms generally (headache, emesis, diarrhaea) increased the probability of P. falciparum. Importantly, while more severe degrees of anaemia increased the probability of P. falciparum infection among children < 15 years old, this was not seen among participants ≥ 15 years old.
Applying these results to children < 15 years old, the prevalence (pre-test probability) of P. falciparum infection was 51%, and a child presenting with fever > 40 °C and severe anaemia would have over 95% probability of P. falciparum infection. Among adults ≥ 15 years old, the prevalence (pre-test probability) of P. falciparum infection was lower at 34%. However, an individual in this age group presenting with frequent emesis and recent travel to the North would have a near 100% probability of P. falciparum infection. While not a replacement for malaria diagnostic tests, a better understanding of characteristic epidemiological and clinical trends can help health care providers risk stratify patients to streamline testing and treatment. Criteria and calculators to stratify risk for other infections are already employed in urgent care and emergency settings, for example the Centor score for Streptococcal pharyngitis [38] or the Alvarado score for appendicitis [39]. Creating site specific criteria for individual health care centres in high-transmission zones could prove a powerful tool in the clinical management of malaria.
In this cohort, over 17% had already taken an antimalarial, and among these, fewer than 50% were found to have P. falciparum infection by PCR testing. Self-medication prior to presentation to the hospital is quite common. Private pharmacies are proliferating in urban centres, where most drugs are available without a need for clinical consultation. A recent survey of customers at pharmacies in Douala IV found 47% of customers buying antibiotics did so without a prescription [40]. Determining which medication was taken can be challenging, and home treatment can clearly affect both presenting symptomology and the results of diagnostic testing. Self-medication, therefore, complicates the diagnostic picture for clinicians, and it is critical for clinicians to inquire about prior medication usage when evaluating patients.
Almost 40% of participants had anemia, with > 5% meeting criteria for severe anemia. While the burden of anaemia was not confined to younger age groups, the prevalence of anaemia was higher among children and women compared to adult men. In comparison to the community sample of children < 5 years old in the 2018 DHS, there was a higher percentage of more severe anaemia [23]. Such a difference may reflect the different populations from which participants were drawn, as the current study targeted symptomatic patients who were presenting to an ED and much more likely to have clinical illness that might result in anaemia. In comparison to other clinical samples, one older study in Douala assessed anaemia among microscopy-positive children < 15 years old and found 28.7% had a haemoglobin < 8 g/dL [25]. Other studies have investigated haemoglobin status in malaria patients with and without the comorbidities of sickle cell disease and HIV in Douala. One study of patients with malaria and HIV found the overall prevalence of haemoglobin < 11 g/dL was 28.5%, and the prevalence among malaria mono-infected individuals and among malaria/HIV co-infected individuals was 20.4% and 43.3%, respectively [27]. In a different study, 6.7% of the non-sickle cell malaria patients had a haemoglobin < 5 g/dL [28], while in yet another study, no patients with malaria infection without HIV had a haemoglobin < 7 g/dL [27]. While direct comparison across clinical settings is difficult, the results of the current study and others highlight the high level of burden of anaemia among inhabitants of Douala, particularly children.
This study further demonstrates that a considerable risk for anaemia among symptomatic children is associated with malaria infection. Considering 10–14-year-olds for example, this group had a higher prevalence of P. falciparum infection than young children or adults, and over 80% of anaemia was associated with malaria infection. The role of malaria in the burden of anaemia in Cameroon may extend even to asymptomatic malaria infection as a recent longitudinal study across multiple villages in Cameroon’s Central Region found asymptomatic malaria infection (diagnosed by PCR) was associated with 69.2% of anaemia in the study population [41]. Despite this association, the relationship between anaemia and malaria in children remains complex. Iron deficiency anaemia may be associated with decreased risk of malaria infection and clinical illness in children, while universal iron and folate supplementation in children may lead to worse outcomes [42, 43]. Certain anti-malarials can also result in anemia; for example primaquine can result in acute haemolytic anaemia in patients with glucose-6-phosphate dehydrogenase deficiency [44].
These findings have implications for clinical practice. First, effectively detecting and treating malaria infection may reduce the overall burden of anaemia among children. Indeed, chemoprophylaxis against malaria was recently found to reduce death and readmissions among children admitted for severe anaemia [45]. Second, the risk factors for anaemia in the setting of malaria infection need to be better understood. In Cameroon, some of these factors may include parasitemia, duration of clinical illness, presence of stunting, and preexisting iron deficiency [46]. Third, follow-up haemoglobin/haematocrit measurements may be warranted following recovery from malaria to determine the need for additional workup or intervention. In Cameroon, longitudinal follow up of children ≤ 14 years old in whom both P. falciparum and anaemia were detected has shown that over 50% may remain with low haemoglobin even weeks after treatment for malaria [46]. While iron supplementation may not be recommended during acute infections during the high transmission season, iron repletion may be beneficial during the low transmission season [47].
There are important limitations to this study. First, parasitaemia levels by microscopy or quantitative PCR were not available, and the patient’s final diagnosis was not known. Thus, some percentage of P. falciparum infections were either incidental findings or one or multiple comorbid infections responsible for the patient’s febrile illness. This study can therefore only comment on prevalence of P. falciparum infection rather than the clinical entity of P. falciparum malaria, including severe malaria. Second, recruitment took place at a single urban site over less than half the year, specifically during and after the summer rainy season when malaria transmission would be expected to be highest, and only during weekdays when laboratory resources were available. The DMH itself is a private, fee-for-service secondary care facility located proximal to major government facilities, and it serves as the regional medical centre for the Cameroonian Army, therefore serving a unique client base. Thus, participants in this study likely do not reflect the population of Douala as a whole. Third, the study was not powered to test specific hypotheses, and the small number of participants in younger age groups greatly limited the power of the study to detect differences among these age groups. Fourth, additional laboratory data to classify the type of anaemia was not obtained, nor were laboratory tests for haemoglobinopathies or helminth infections performed. As mentioned above, there are a myriad of possible etiologies for anaemia among these participants. Thus, this study cannot identify the etiology of anaemia among the participants and can only comment on possible association, rather than causation, between malaria and anemia. Finally, the assessment of presenting symptoms was subjective.
Conclusions
The current study describes clinical characteristics of P. falciparum infection in Douala, Cameroon. A high burden of both P. falciparum infection and anaemia was found in this populous urban setting. Older children tended to be at higher risk for P. falciparum infection, and several clinical and epidemiologic characteristics significantly increased the likelihood of malaria infection. Anaemia was higher among children and woman compared to adult men, and the presence of anaemia was associated with P. falciparum infection only among children. With recent slowing or even reversal of gains in malaria control efforts, additional epidemiologic studies in urban centres as well as across the full age spectrum in sub-Saharan Africa are recommended, so that appropriate interventions can be developed and deployed for these unique settings and demographics.
Supplementary Information
Additional file 1: Table S1. PCR Mastermix reagents and concentrations for PCR speciation assays. Table S2. PCR amplification parameters for PCR speciation assays. Table S3. WHO haemoglobin classification of anaemia. Table S4. Interpreting likelihood ratios. Table S5. Diagnostic characteristics of history and physical findings for anaemia.
Acknowledgements
The authors would like to thank Oussmane Biri, Cathy Nyemb, and Maritte Tresor Tchouachidjom Noubissie for their efforts as part of the recruitment teams and Abigail Drew Pershing for help compiling the master database.
Abbreviations
- CI
Confidence interval
- CPC
Centre Pasteur of Cameroon
- DHS
Demographic and Health Survey
- DMH
Douala Military Hospital
- ED
Emergency Department
- EIR
Entomological inoculation rate
- HRP-2
Histidine rich-protein 2
- LR
Likelihood ratio
- OR
Odds ratio
- PARP
Population attributable risk percent
- PCR
Polymerase chain reaction
- RBC
Red blood cell
- SD
Standard deviation
- RDT
Rapid diagnostic test
- WHO
World Health Organization
Author contributions
DZH conducted literature reviews, applied for all grants, managed the IRB submission at Yale, managed the database, and performed all statistical analyses. DZH and SP drafted and revised the manuscript. YME coordinated all on-site elements at DMH, managed the IRB submissions to the University of Douala and the DMH, and assisted with data entry and analysis. DZH and YME coordinated and led recruitment efforts at DMH. NMN coordinated all lab operations at DMH. RM and SMD helped create the recruitment materials, recruited participants, and assisted with data entry. JG assisted with PCR protocols and completion of PCR at Yale School of Public Health. GCN and TN performed DNA extraction at Centre Pasteur of Cameroon. JA, JJA, and EZ performed molecular speciation at Yale School of Public Health. MW coordinated all lab operations at Yale School of Public Health. CEEM and YB oversaw recruitment and sample processing at DMH and assisted in revisions to the manuscript. CEEM oversaw all activities at Centre Pasteur of Cameroon. CEEM, YB, and SP oversaw all study activities. All authors read and approved the final manuscript.
Funding
This research was funded in part by a Wilbur G. Downs International Health Travel Fellowship from the Yale School of Public Health (New Haven, USA), a Medical Student Fellowship, Lowe Endowment Funds, and a G.D. Hsiung PhD Student Research Fellowship from the Yale School of Medicine (New Haven, USA), and a Benjamin H. Kean Travel Fellowship in Tropical Medicine from the American Society of Tropical Medicine and Hygiene (Oakbrook Terrance, USA). Material support was also provided by the Yale School of Public Health (New Haven, USA), Douala Military Hospital (Douala, Cameroon), and the Centre Pasteur of Cameroon (Yaoundé, Cameroon).
Availability of data and materials
The datasets generated and analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
This study was conducted in accordance with ethics directives related to research on humans in Cameroon. The study was approved by the Institutional Committee of Ethics for Research for Human Health of the University of Douala (N° 1617 IEC-UD/ 04/2018/T) and the Yale Human Research Protection Program (HIC #2000023509), and administrative authorization (01800776 14 MAI 2018) was obtained from Douala Military Hospital. All those ≥ 18 years old provided consent, parents/guardians provided consent for minors < 18 years old, and minors aged 8–17 years additionally provided assent. Consent forms were available and approved in both English and French, depending on the preference of the participant and/or guardian. Participants with a positive RDT who did not need to be hospitalized were provided first-line artemisinin-based combination therapy free of charge in accordance with the guidelines from the Cameroon National Malaria Control Programme.
Consent for publication
Not applicable.
Competing interests
The authors report that they have no competing interests to report relevant to the current study.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yap Boum II and Sunil Parikh contributed equally as co-senior authors
References
- 1.World Health Organization . World malaria report 2021. Geneva: World Health Organization; 2021. [Google Scholar]
- 2.Hay SI, Guerra CA, Tatem AJ, Atkinson PM, Snow RW. Urbanization, malaria transmission and disease burden in Africa. Nat Rev Microbiol. 2005;3:81–90. doi: 10.1038/nrmicro1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Takken W, Lindsay S. increased threat of urban malaria from Anopheles stephensi mosquitoes, Africa. Emerg Infect Dis. 2019;25:1431–1433. doi: 10.3201/eid2507.190301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nkumama IN, O'Meara WP, Osier FHA. Changes in malaria epidemiology in Africa and new challenges for elimination. Trends Parasitol. 2017;33:128–140. doi: 10.1016/j.pt.2016.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Doumbe-Belisse P, Kopya E, Ngadjeu CS, Sonhafouo-Chiana N, Talipouo A, Djamouko-Djonkam L, et al. Urban malaria in sub-Saharan Africa: dynamic of the vectorial system and the entomological inoculation rate. Malar J. 2021;20:364. doi: 10.1186/s12936-021-03891-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mathanga DP, Tembo AK, Mzilahowa T, Bauleni A, Mtimaukenena K, Taylor TE, et al. Patterns and determinants of malaria risk in urban and peri-urban areas of Blantyre, Malawi. Malar J. 2016;15:590. doi: 10.1186/s12936-016-1623-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Andolina C, Rek JC, Briggs J, Okoth J, Musiime A, Ramjith J, et al. Sources of persistent malaria transmission in a setting with effective malaria control in eastern Uganda: a longitudinal, observational cohort study. Lancet Infect Dis. 2021;21:1568–1578. doi: 10.1016/S1473-3099(21)00072-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.US President's Malaria Initiative. FY 2017 Cameroon Malaria Operational Plan. Yaoundé, Cameroon, 2017. https://d1u4sg1s9ptc4z.cloudfront.net/uploads/2021/03/fy-2017-cameroon-malaria-operational-plan.pdf.
- 9.Mbenda HG, Awasthi G, Singh PK, Gouado I, Das A. Does malaria epidemiology project Cameroon as 'Africa in miniature'? J Biosci. 2014;39:727–738. doi: 10.1007/s12038-014-9451-y. [DOI] [PubMed] [Google Scholar]
- 10.US President's Malaria Initiative. FY 2018/2019 Cameroon Malaria Operational Plan. Yaoundé, Cameroon, 2018. https://d1u4sg1s9ptc4z.cloudfront.net/uploads/2021/03/fy-2018-cameroon-malaria-operational-plan.pdf.
- 11.Snow RW, Noor AM. Malaria risk mapping in Africa: the historical context to the information for malaria (INFORM) project. Nairobi, Kenya. Working Paper in support of the INFORM Project funded by the Department for International Development and the Wellcome Trust 2015.
- 12.Kwenti TE, Kwenti TDB, Njunda LA, Latz A, Tufon KA, Nkuo-Akenji T. Identification of the Plasmodium species in clinical samples from children residing in five epidemiological strata of malaria in Cameroon. Trop Med Health. 2017;45:14. doi: 10.1186/s41182-017-0058-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Antonio-Nkondjio C, Ndo C, Njiokou F, Bigoga JD, Awono-Ambene P, Etang J, et al. Review of malaria situation in Cameroon: technical viewpoint on challenges and prospects for disease elimination. Parasit Vectors. 2019;12:501. doi: 10.1186/s13071-019-3753-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Noubouossie D, Tagny CT, Same-Ekobo A, Mbanya D. Asymptomatic carriage of malaria parasites in blood donors in Yaoundé. Transfus Med. 2012;22:63–67. doi: 10.1111/j.1365-3148.2011.01121.x. [DOI] [PubMed] [Google Scholar]
- 15.Zakeri S, Lindergard G, Davies RM, Boudin C, Louis F, Hommel M. Identification and typing of Cameroonian isolates of P. malariae using monoclonal antibodies against P. brasilianum. Acta Trop. 2006;99:97–101. doi: 10.1016/j.actatropica.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 16.Bonnet S, Paul RE, Gouagna C, Safeukui I, Meunier JY, Gounoue R, et al. Level and dynamics of malaria transmission and morbidity in an equatorial area of South Cameroon. Trop Med Int Health. 2002;7:249–256. doi: 10.1046/j.1365-3156.2002.00861.x. [DOI] [PubMed] [Google Scholar]
- 17.Roman DNR, Rosalie NNA, Kumar A, Luther KMM, Singh V, Albert MS. Asymptomatic Plasmodium malariae infections in children from suburban areas of Yaounde, Cameroon. Parasitol Int. 2018;67:29–33. doi: 10.1016/j.parint.2017.02.009. [DOI] [PubMed] [Google Scholar]
- 18.Kojom Foko LP, Kouemo Motse FD, Kamgain Mawabo L, Pande V, Singh V. First evidence of local circulation of Plasmodium ovale curtisi and reliability of a malaria rapid diagnostic test among symptomatic outpatients in Douala, Cameroon. Infect Genet Evol. 2021;91:104797. doi: 10.1016/j.meegid.2021.104797. [DOI] [PubMed] [Google Scholar]
- 19.Djeunang Dongho GB, Gunalan K, L'Episcopia M, Paganotti GM, Menegon M, Sangong RE, et al. Plasmodium vivax infections detected in a large number of febrile Duffy-negative Africans in Dschang, Cameroon. Am J Trop Med Hyg. 2021;104:987–992. doi: 10.4269/ajtmh.20-1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Antonio-Nkondjio C, Defo-Talom B, Tagne-Fotso R, Tene-Fossog B, Ndo C, Lehman LG, et al. High mosquito burden and malaria transmission in a district of the city of Douala, Cameroon. BMC Infect Dis. 2012;12:275. doi: 10.1186/1471-2334-12-275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Akono Ntonga P, Tonga C, Mbida Mbida JA, Ndo C, Awono Ambene P, Lehman LG, et al. Malaria transmission and the sensitivity of aggressive mosquitoes to insecticides in a poorly urbanized area of the Deido health district in Douala (Cameroon) Med Sante Trop. 2017;27:82–89. doi: 10.1684/mst.2016.0624. [DOI] [PubMed] [Google Scholar]
- 22.PNLP. Rapport d’activités 2019 du Programme National de Lutte contre le Paludisme. 2020.
- 23.Institut National de la Statistique (INS), The DHS Program. République du Cameroun Enquête Démographique et de Santé 2018. 2020.
- 24.WHO . Guidelines for the treatment of malaria–3rd edition. Geneva: World Health Organization; 2015. [PubMed] [Google Scholar]
- 25.Mfonkeu JB, Gouado I, Kuate HF, Zambou O, Grau G, Combes V, et al. Clinical presentation, haematological indices and management of children with severe and uncomplicated malaria in Douala, Cameroon. Pak J Biol Sci. 2008;11:2401–2406. doi: 10.3923/pjbs.2008.2401.2406. [DOI] [PubMed] [Google Scholar]
- 26.Tchinda GG, Atashili J, Achidi EA, Kamga HL, Njunda AL, Ndumbe PM. Impact of malaria on hematological parameters in people living with HIV/AIDS attending the Laquintinie Hospital in Douala, Cameroon. PLoS ONE. 2012;7:e40553. doi: 10.1371/journal.pone.0040553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nkuo-Akenji T, Tevoufouet EE, Nzang F, Ngufor N, Fon E. High prevalence of HIV and malaria co-infection in urban Douala, Cameroon. Afr J AIDS Res. 2008;7:229–235. doi: 10.2989/AJAR.2008.7.2.8.525. [DOI] [PubMed] [Google Scholar]
- 28.Ngo Linwa EE, Cumber SN, Eposse Ekoube C, Esuh EL, Mandeng MLE, Nkfusai CN, et al. Malaria in patients with sickle cell anaemia: burden, risk factors and outcome at the Laquintinie hospital, Cameroon. BMC Infect Dis. 2020;20:40. doi: 10.1186/s12879-019-4757-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.WHO . Malaria rapid diagnostic test performance: results of WHO product testing of malaria RDTs: round 8 (2016–2018) Geneva: World Health Organization; 2018. [Google Scholar]
- 30.Snounou G, Viriyakosol S, Zhu XP, Jarra W, Pinheiro L, do Rosario VE, et al. High sensitivity of detection of human malaria parasites by the use of nested polymerase chain reaction. Mol Biochem Parasitol. 1993;61:315–20. doi: 10.1016/0166-6851(93)90077-B. [DOI] [PubMed] [Google Scholar]
- 31.Snounou G, Pinheiro L, Gonçalves A, Fonseca L, Dias F, Brown KN, et al. The importance of sensitive detection of malaria parasites in the human and insect hosts in epidemiological studies, as shown by the analysis of field samples from Guinea Bissau. Trans R Soc Trop Med Hyg. 1993;87:649–653. doi: 10.1016/0035-9203(93)90274-T. [DOI] [PubMed] [Google Scholar]
- 32.World Health Organization . Haemoglobin concentrations for the diagnosis of anaemia and assessment of severity. Geneva: World Health Organization; 2011. [Google Scholar]
- 33.Field A. Discovering statistics using IBM SPSS statistics. Washington: SAGE Publications Inc.; 2013. [Google Scholar]
- 34.McGee S. Evidence-based physical diagnosis. 4. Philadelphia: Elsevier; 2018. [Google Scholar]
- 35.Leski TA, Taitt CR, Swaray AG, Bangura U, Reynolds ND, Holtz A, et al. Use of real-time multiplex PCR, malaria rapid diagnostic test and microscopy to investigate the prevalence of Plasmodium species among febrile hospital patients in Sierra Leone. Malar J. 2020;19:84. doi: 10.1186/s12936-020-03163-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sinka ME, Pironon S, Massey NC, Longbottom J, Hemingway J, Moyes CL, et al. A new malaria vector in Africa: predicting the expansion range of Anopheles stephensi and identifying the urban populations at risk. Proc Natl Acad Sci USA. 2020;117:24900–24908. doi: 10.1073/pnas.2003976117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.O'Meara WP, Mangeni JN, Steketee R, Greenwood B. Changes in the burden of malaria in sub-Saharan Africa. Lancet Infect Dis. 2010;10:545–55. doi: 10.1016/S1473-3099(10)70096-7. [DOI] [PubMed] [Google Scholar]
- 38.Fine AM, Nizet V, Mandl KD. Large-scale validation of the Centor and McIsaac scores to predict group A streptococcal pharyngitis. Arch Intern Med. 2012;172:847–852. doi: 10.1001/archinternmed.2012.950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Coleman JJ, Carr BW, Rogers T, Field MS, Zarzaur BL, Savage SA, et al. The Alvarado score should be used to reduce emergency department length of stay and radiation exposure in select patients with abdominal pain. J Trauma Acute Care Surg. 2018;84:946–950. doi: 10.1097/TA.0000000000001885. [DOI] [PubMed] [Google Scholar]
- 40.Elong Ekambi GA, Okalla Ebongue C, Penda IC, Nnanga Nga E, Mpondo Mpondo E, Eboumbou Moukoko CE. Knowledge, practices and attitudes on antibiotics use in Cameroon: self-medication and prescription survey among children, adolescents and adults in private pharmacies. PLoS ONE. 2019;14:e0212875. doi: 10.1371/journal.pone.0212875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fogang B, Biabi MF, Megnekou R, Maloba FM, Essangui E, Donkeu C, et al. High Prevalence of asymptomatic malarial anemia and association with early conversion from asymptomatic to symptomatic infection in a Plasmodium falciparum hyperendemic setting in Cameroon. Am J Trop Med Hyg. 2021;106:293–302. doi: 10.4269/ajtmh.21-0316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sazawal S, Black RE, Ramsan M, Chwaya HM, Stoltzfus RJ, Dutta A, et al. Effects of routine prophylactic supplementation with iron and folic acid on admission to hospital and mortality in preschool children in a high malaria transmission setting: community-based, randomised, placebo-controlled trial. Lancet. 2006;367:133–143. doi: 10.1016/S0140-6736(06)67962-2. [DOI] [PubMed] [Google Scholar]
- 43.Clark MA, Goheen MM, Fulford A, Prentice AM, Elnagheeb MA, Patel J, et al. Host iron status and iron supplementation mediate susceptibility to erythrocytic stage Plasmodium falciparum. Nat Commun. 2014;5:4446. doi: 10.1038/ncomms5446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ashley EA, Poespoprodjo JR. Treatment and prevention of malaria in children. Lancet Child Adolesc Health. 2020;4:775–789. doi: 10.1016/S2352-4642(20)30127-9. [DOI] [PubMed] [Google Scholar]
- 45.Kwambai TK, Dhabangi A, Idro R, Opoka R, Watson V, Kariuki S, et al. Malaria chemoprevention in the postdischarge management of severe anemia. N Engl J Med. 2020;383:2242–2254. doi: 10.1056/NEJMoa2002820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sumbele IU, Samje M, Nkuo-Akenji T. A longitudinal study on anaemia in children with Plasmodium falciparum infection in the Mount Cameroon region: prevalence, risk factors and perceptions by caregivers. BMC Infect Dis. 2013;13:123. doi: 10.1186/1471-2334-13-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Atkinson SH, Armitage AE, Khandwala S, Mwangi TW, Uyoga S, Bejon PA, et al. Combinatorial effects of malaria season, iron deficiency, and inflammation determine plasma hepcidin concentration in African children. Blood. 2014;123:3221–3229. doi: 10.1182/blood-2013-10-533000. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. PCR Mastermix reagents and concentrations for PCR speciation assays. Table S2. PCR amplification parameters for PCR speciation assays. Table S3. WHO haemoglobin classification of anaemia. Table S4. Interpreting likelihood ratios. Table S5. Diagnostic characteristics of history and physical findings for anaemia.
Data Availability Statement
The datasets generated and analysed during the current study are available from the corresponding author on reasonable request.