Abstract
We characterized antigenic markers recognized by human serum samples from patients presenting with acute and chronic toxoplasmosis by the determination of immunoglobulin G (IgG) antibody avidity by a Western blot modified technique (avidity immunoblotting) that includes the dissociation of the antigen-antibody interaction with 6 or 8 M urea solutions. Human serum samples from 20 patients presenting with recent infection and from 20 patients with chronic infection were analyzed. It was observed that bands p16, p32, p38, p40, p43, p54, p60, p66, and p97 were more frequently recognized by low-avidity IgG in recent infection and by high-avidity IgG in chronic toxoplasmosis. From these antigenic bands, p38 can be characterized as an optimal antigenic marker of low avidity for recent forms of toxoplasmosis due to a significant decrease of their frequencies (from 80 to 0%) after treatment with 6 M urea solutions. The p30 antigen was not considered a good marker to distinguish acute from chronic infection since corresponding IgG antibodies were determined to have high avidity in both phases of the infection. Thus, the avidity immunoblotting assay proved to be a useful tool for determining antigenic markers of recent and chronic phases of Toxoplasma gondii infection.
Toxoplasmosis is an infection that occurs worldwide; immunocompetent subjects are usually asymptomatic (5), but when the infection occurs during pregnancy, it frequently leads to congenital toxoplasmosis. In such cases, frequent disorders can occur, such as chorioretinitis and neurologic defects. On the other hand, reactivation of latent infection happens frequently in immunosuppressed patients (7). These facts emphasize the importance of making a clear distinction between primary infection and reactivation, especially during pregnancy, to evaluate more accurately the time of primary infection.
The infection is generally diagnosed by demonstration of specific antibodies to Toxoplasma antigens in the serum samples of infected patients (4). The cases of acute toxoplasmosis can be identified by the most valuable serological marker, that is, the presence of Toxoplasma-specific immunoglobulin M (IgM) antibodies. Another marker has been the demonstration of a significant increase in the specific IgG antibody titers in paired samples. However, these markers present conflicting results, since IgM antibodies to Toxoplasma spp. can be detected for a very long time after the acute phase of infection in some patients. On the other hand, high IgG levels can already be present after the onset of symptoms (3). Additional factors that make the diagnosis difficult include (i) the cross-reactivity of IgM antibodies, which are present in several infections with common antigens or are induced by B-lymphocyte polyclonal stimulation, (ii) the presence of IgM rheumatoid factor or antinuclear antibodies, and (iii) the use of heat-inactivated sera (7).
Recently, IgG avidity assays have been proposed in order to distinguish reactivation from primary infections in several diseases such as tuberculosis, periodontitis, and viral infections (herpes simplex virus, cytomegalovirus, Epstein-Barr virus, parainfluenza virus, rubella virus, and hepatitis C virus) (6, 10). In the case of toxoplasmosis, assays were developed to differentiate the low-avidity IgG antibodies produced at an early stage of infection from those with a higher binding strength (high-avidity) that reflect a latent or chronic infection (3, 7–9, 12, 15).
In order to evaluate the avidity of IgG antibodies, a simple technique has been described (7). This assay is based on the dissociation of low-avidity antibodies as a result of a hydrogen bond-disrupting agent, such as urea hypermolar solutions. An enzyme-linked immunosorbent assay (ELISA) was developed to measure IgG avidity that was able to distinguish serum samples from recently infected (low-avidity index) to chronically infected (high-avidity index) patients when using 6 M urea as an elution agent (8). However, there are no reports in the literature about possible antigenic markers of Toxoplasma gondii that could be related to a recent or chronic phase of the infection in avidity assays.
In the present study, we sought to characterize antigenic markers of T. gondii for acute and chronic forms of toxoplasmosis, which are recognized by low- and high-avidity IgG antibodies, respectively, by using a modified immunoblotting assay.
MATERIALS AND METHODS
Samples.
A total of 60 human serum samples were analyzed and divided into three groups based on serological profiles previously characterized by conventional laboratory assays that made it possible to classify the samples as follows.
Group I consisted of 20 human serum samples from patients with an acute phase of toxoplasmic infection, in which the presence of specific IgM antibodies was detected by IgM-ELISA by the Fleury Laboratory, São Paulo, Brazil.
Group II consisted of 20 human serum samples from patients in the chronic phase of toxoplasmic infection, in which the presence of specific IgG antibodies with titers of ≥16 was measured by IgG-ELISA at the Clinical Analysis Laboratory of the Hospital das Clínicas, Uberlândia, Brazil (HC-UFU).
Group III included 20 human serum samples from T. gondii nonreactive subjects; these samples were also provided by HC-UFU.
All human serum samples were collected and preserved at −20°C until tested.
Toxoplasma antigen.
T. gondii (RH strain) was grown intraperitoneally in Swiss mice for 48 to 72 h. The peritoneal exudate was obtained and washed three times by centrifugation at 1,000 × g for 10 min with 0.01 M phosphate-buffered saline (pH 7.2) (PBS). The final parasite pellet was suspended in PBS to adjust it to a concentration of 2 × 106 tachyzoites/ml.
Polystyrene microtiter plates (Interlab, São Paulo, Brazil) were coated with 105 tachyzoites/well in PBS plus 1% gelatin by incubating them for 18 h at 37°C and then stored at −20°C until use in an ELISA.
The antigen used in the immunoblotting assays was prepared from the parasite suspension obtained from peritoneal exudates of previously infected Swiss mice, as described above. This preparation was homogenized in sample buffer solution consisting of 0.1 M Tris-HCl (pH 6.8), 4% sodium dodecyl sulfate (SDS), 20% glycerol, and 2% bromophenol blue. After the mixture was boiled for 3 min, antigen aliquots were used in the immunoblotting assays.
Avidity ELISA.
The avidity of T. gondii-specific IgG antibodies was determined as previously described (7), with some modifications. Microtiter plates previously coated with Toxoplasma tachyzoites were washed three times with PBS plus 0.05% Tween 20 (PBST). The serum samples, in serial twofold dilutions starting from 1:16 to 1:2,048, were added in duplicate on separate plates. After incubation for 45 min at 37°C, the plates were subjected to differential washing as follows: one plate was washed with 6 M urea solution in PBS for 5 min, while the other plate was washed only with PBST for 5 min. Furthermore, both plates were washed twice with PBST for 5 min. The residual antigen-bound IgG was detected with a rabbit anti-human IgG conjugated to horseradish peroxidase (Sigma Chemical Co., St. Louis, Mo.) at 1:3,000 and incubated for 45 min at 37°C. After new washes with PBST, the reaction was revealed with a substrate solution consisting of orthophenylenediamine at 0.5 mg/ml in 0.1 M citrate-phosphate buffer (pH 5.0) and 0.012% H2O2. After incubation for 15 min at room temperature, the reaction was stopped with 2 N H2SO4. The absorbances were measured at 492 nm by using a plate reader system (Titertek Multiskan Plus; Flow Laboratories, Geneva, Switzerland). The avidity index (AI) was calculated as the ratio between the absorbance (Abs) obtained for the plate washed with urea (U+) and the plate without urea (U−) and is expressed as a percentage: AI = Abs(U+)/Abs(U−) × 100.
Avidity immunoblotting.
In order to detect major antigenic proteins from T. gondii (RH strain) which are recognized by human sera, antigenic preparations were separated by SDS–12% polyacrylamide gel electrophoresis as previously described (11). Phosphorylase b (97 kDa), bovine serum albumin (67 kDa), ovalbumin (45 kDa), glyceraldehyde-3-phosphate dehydrogenase (36 kDa), carbonic anhydrase (29 kDa), and α-lactoalbumin (14 kDa) were used as molecular mass standards (Sigma). Proteins separated by gel electrophoresis were further transferred to a 0.45-μm (pore size) nitrocellulose membrane for 2 h by using a semidry transfer system (Multiphor II Electrophoresis Unit; Pharmacia-LKB, Uppsala, Sweden) as described elsewhere (14). After transfer, nitrocellulose strips were blocked with 5% nonfat milk (Molico-Nestlé) in PBST for 2 h at room temperature to saturate protein binding sites. Strips were further incubated with serum samples, in triplicate, diluted in 1% nonfat milk–PBST (PBSTM). The selected dilutions corresponded to the smallest AI obtained from the ELISA or to the adjacent AI, if this had corresponded to the first or last dilution (endpoint). After incubation overnight at 4°C, one strip of the triplicate was washed with PBSTM three times for 5 min. The second and third nitrocellulose strips were washed with 6 and 8 M urea solutions in PBS, respectively, three times for 5 min. Finally, the nitrocellulose strips of each serum sample were then submitted to three washing cycles with PBSTM for 5 min. Conjugate anti-human IgG-peroxidase (Sigma) diluted at 1:200 in PBSTM and incubated for 2 h at room temperature was added. After a new washing step repeated six times for 5 min in PBS, the strips were treated with 0.4% hydrogen peroxide–3,3′-diaminobenzidine tetrahydrochloride (Sigma) in PBS. The reaction was stopped by washing it with distilled water once the stained protein bands had been visualized.
Statistical analysis.
The apparent molecular masses of the T. gondii antigen bands were estimated in comparison with the linear regression curve, which plotted the molecular masses of the markers against relative mobility (Rf).
Frequencies of the antigen bands recognized by serum samples from patients presenting with recent or chronic toxoplasmosis that were obtained following different assay conditions were subjected to comparative analysis between two proportions (Statistic for Windows, release 4.5A; Statesoft, Inc.). Differences were considered significant when P was <0.05.
RESULTS
Avidity ELISA.
AIs were calculated in each dilution of the human serum samples, for both recent and chronic phases of toxoplasmic infection. Thus, in the serum samples from recent infection cases, AIs ranged from 12.3 to 55.9% corresponding to dilutions from 1:64 to 1:2,048. For the serum samples from chronic cases, AIs ranged from 50 to 100%, corresponding to dilutions from 1:16 to 1:1,024.
Avidity immunoblotting.
Serum samples were used at dilutions corresponding to the smallest AI obtained from the avidity ELISA. Thus, for human serum samples from cases of recent infection the most frequently used dilutions were 1:512 and 1:2,048, and for those from the chronic phase the most-used dilution was 1:64.
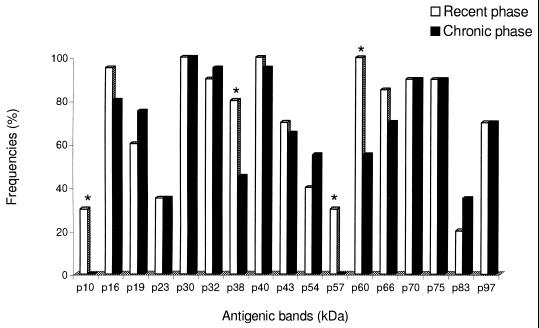
The frequencies of T. gondii antigenic proteins that were recognized by specific IgG antibodies from the sera of group I (recent phase) and group II (chronic phase), without treatment with urea, are show in Fig. 1. Statistically significant differences were observed for the following antigenic bands of apparent molecular masses expressed in kilodaltons: p10, p38, p57, and p60, which were found predominantly in the group I (P < 0.05).
FIG. 1.
Frequencies of T. gondii antigens recognized by IgG antibodies in human sera from recent and chronic forms of toxoplasmosis, as determined by immunoblotting without urea treatment. Antigenic bands with statistically significant differences (P < 0.05) are indicated with an asterisk.
When comparing the frequency of T. gondii antigenic bands which were recognized by specific IgG from the sera of group I after treatment with 6 M urea, statistically significant differences (P < 0.05) were observed for the following bands: p16, p32, p38, p40, p43, p54, p60, p66, and p97 (Table 1). The p38 band presented accentuated reduction of their frequency (80 to 0%) after treatment with 6 M urea. In addition, after treatment with 8 M urea, another five bands (p19, p23, p30, p70, and p75) showed a significant reduction (P < 0.05) in their frequencies compared to the respective untreated serum samples.
TABLE 1.
Frequency of T. gondii antigenic bands recognized by IgG antibodies in human sera from the recent form of toxoplasmosis, as determined by immunoblotting without or with 6 or 8 M urea
| Antigenic band (kDa)a | Frequency (%) of antigenic bands recognized by sera from patients with recent infections
|
||
|---|---|---|---|
| Without urea | With 6 M urea | With 8 M urea | |
| p10 | 30 | 15 | 15 |
| p16* | 95 | 45 | 40 |
| p19** | 60 | 30 | 20 |
| p23** | 35 | 10 | 0 |
| p30** | 100 | 85 | 65 |
| p32* | 90 | 35 | 20 |
| p38* | 80 | 0 | 0 |
| p40* | 100 | 50 | 45 |
| p43* | 70 | 35 | 15 |
| p54* | 40 | 0 | 0 |
| p57 | 30 | 20 | 20 |
| p60* | 100 | 25 | 10 |
| p66 | 85 | 55 | 25 |
| p70** | 90 | 65 | 55 |
| p75** | 90 | 65 | 40 |
| p83 | 20 | 10 | 10 |
| p97* | 70 | 25 | 20 |
∗, Statistically significant differences after both 6 and 8 M urea treatments (P < 0.05); ∗∗, statistically significant differences only after the 8 M urea treatment (P < 0.05).
By analyzing the group II (chronic phase), no statistically significant difference (P > 0.05) was found in the frequency of bands recognized by sera after 6 M urea treatment. However, after treatment with 8 M urea, eight bands (p19, p38, p43, p54, p60, p66, p70, and p75) showed significantly reduced frequencies (Table 2).
TABLE 2.
Frequency of T. gondii antigens in bands recognized by IgG antibodies in human sera from the chronic form of toxoplasmosis, as determined by immunoblotting without or with 6 or 8 M urea
| Antigenic band (kDa)a | Frequency (%) of antigenic bands recognized by sera from patients with chronic infections
|
||
|---|---|---|---|
| Without urea | With 6 M urea | With 8 M urea | |
| p10 | 0 | 0 | 0 |
| p16 | 80 | 75 | 65 |
| p19** | 75 | 70 | 40 |
| p23 | 35 | 15 | 10 |
| p30 | 100 | 95 | 90 |
| p32 | 95 | 95 | 80 |
| p38** | 45 | 35 | 5 |
| p40 | 95 | 90 | 80 |
| p43** | 65 | 45 | 10 |
| p54** | 56 | 45 | 15 |
| p57 | 0 | 0 | 0 |
| p60** | 55 | 55 | 15 |
| p66** | 70 | 65 | 25 |
| p70** | 90 | 90 | 60 |
| p75** | 90 | 70 | 55 |
| p83 | 35 | 30 | 15 |
| p97 | 70 | 70 | 40 |
∗∗, Statistically significant differences only after the 8 M urea treatment (P < 0.05).
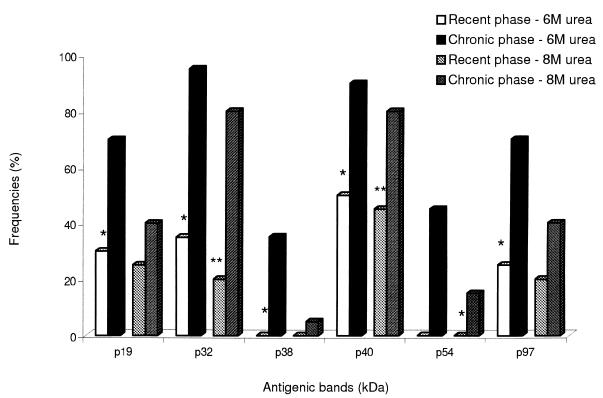
When comparing the recent (I) and chronic (II) groups, the bands that showed significant reduction in their frequencies after treatment with 6 M urea in sera of group I were p19, p32, p38, p40, p54, and p97 (P < 0.05) (Fig. 2). On the other hand, after treatment with 8 M urea, only p32 and p40 presented significantly reduced frequencies in this group. The bands p10 and p57 that were exclusively found in sera from the recent phase did not show a significant reduction in frequency after treatment with 6 or 8 M urea (Fig. 1).
FIG. 2.
Frequencies of T. gondii antigens recognized by IgG antibodies in human sera from recent and chronic forms of toxoplasmosis, as determined by immunoblotting after treatment with 6 or 8 M urea. Statistically significant differences after 6 M urea treatment (P < 0.05) are indicated with a single asterisk. Statistically significant differences only after 8 M urea treatment (P < 0.05) are indicated by double asterisks.
SAG-1 antigen (p30) was not considered a good antigenic marker for distinguishing recent from chronic infections since this molecule was recognized by high-avidity IgG antibodies in both phases of the infection.
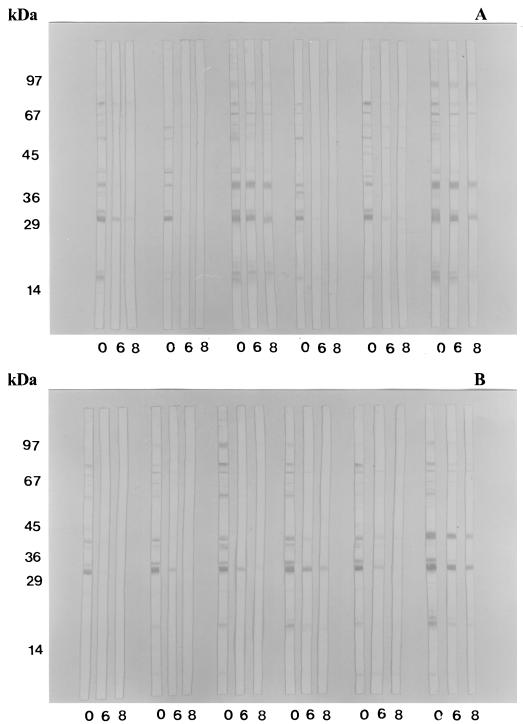
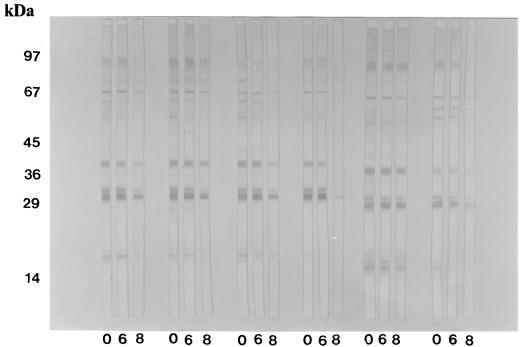
Figures 3 and 4 show the T. gondii antigenic fractions recognized by human serum samples from recent and chronic phases of infection when submitted to the different treatments, i.e., either without urea, with 6 M urea, or with 8 M urea.
FIG. 3.
Antigenic profile of T. gondii recognized by specific IgG antibodies in 12 human serum samples of the recent form of toxoplasmosis (group I), as determined by immunoblotting without or with 6 or 8 M urea treatment. 0, Without urea; 6, 6 M urea; 8, 8 M urea. (A) Sample sets 1 to 6. (B) Sample sets 7 to 12.
FIG. 4.
Antigenic profile of T. gondii recognized by specific IgG antibodies in six human serum samples of the chronic form of toxoplasmosis (group II), as determined by immunoblotting without or with 6 or 8 M urea treatment. 0, Without urea; 6, 6 M urea; 8, 8 M urea.
DISCUSSION
Serological evaluation of the time of acquisition of Toxoplasma infection is of fundamental importance in pregnant women, since infection during pregnancy requires intervention and treatment. In this phase, both eventual clinical manifestations and congenital toxoplasmosis may occur (3).
Various assays have been used to measure IgM antibodies as indicators of the recent phase (2). However, in some patients, IgM antibodies can be detected for a very long time after the acute phase of infection (1); thus, the presence of IgM antibodies is not always an indication of recent infection.
The IgG avidity determination is an important serological marker that can be used to distinguish between recent and chronic infections, allowing such a diagnosis of acute infection to be made from a single serum sample (2, 3, 7–9, 12).
In the present study, the AI of specific IgG antibodies, determined from the avidity ELISA, was shown to be comparable to that found in previous reports (3) due to the presence of low-avidity IgG antibodies. High AIs were characteristic of the chronic phase, as previously described (7, 12), thus suggesting a progressive maturation of the affinity of T. gondii-specific IgG antibodies after the initial antigenic challenge. However, avidity ELISA results can exhibit wide range of AI values for both recent and chronic forms of toxoplasmosis, leading to unreliable results.
An immunoblot assay was described by Rahmah and Anuar for the characterization of T. gondii antigens recognized by serum samples from the acute and chronic phases of infected mice, providing evidence of predominant or exclusive antigenic components at different stages of infection (13).
In the present study, when we analyzed human sera from recent and chronic forms of toxoplasmosis by immunoblotting, exclusive antigenic fractions of the recent phase, i.e., p10 and p57, were detected. Upon analyzing the avidity of IgG antibodies that recognized these antigens, it was verified that these antibodies are of high avidity even during recent infection, since the frequency of these antigenic bands did not present significant reduction after treatment with 6 or 8 M urea solutions. The bands p38 and p60 showed a high incidence in the recent phase, also a characteristic of this infection stage. However, these antigens differ from the previous ones (p10 and p57) because they were recognized by IgG antibodies of low avidity. Therefore, p38 and p60 could be identified as good diagnostic markers for the recent phase of toxoplasmosis.
By analyzing the different treatments (without urea and with 6 or 8 M urea solutions) in sera from recent forms of toxoplasmosis (group I), the antigens p16, p32, p38, p40, p43, p54, p60, p66, and p97 were recognized by low-avidity IgG antibodies after submitting the sera to treatments with 6 and 8 M urea solutions. In addition to these antigens, five other antigenic bands showed significantly reduced frequency after treatment with 8 M urea: p19, p23, p30, p70, and p75.
No significant differences were found in the frequency of antigens in both conditions (without or with 6 M urea) in the sera from the chronic phase of infection, thus characterizing the respective IgG antibodies as being of high avidity. However, after treatment with 8 M urea, the antigens p19, p38, p43, p54, p60, p66, p70, and p75 presented significant differences for the dissociation of the immune complexes compared to untreated samples or samples treated with 6 M urea. These data indicate that the treatment with 8 M urea leads to a greater dissociation of the immune complex with a decrease in IgG antibody avidity in the chronic phase of infection.
The antigens that were recognized by low-avidity IgG antibodies when treated with 6 M urea can be defined as good antigenic markers of the recent phase of infection, since no significant differences were found for the IgG avidity between the treatments with 6 and 8 M urea solutions in the recent infection samples and also between the untreated and the 6 M urea-treated samples in the chronic infection cases.
Therefore, the antigenic bands p10 and p57 can be considered exclusive markers of high avidity for the recent form of toxoplasmosis even though they were present in only 30% of the samples. On the other hand, the bands p16, p32, p38, p40, p43, p54, p60, p66, and p97 that were recognized by low-avidity IgG antibodies from recent infection sera when submitted to treatment with 6 M urea can be considered as antigenic markers of low avidity. In addition, in the chronic infection samples submitted to treatment with 6 M urea, the bands were not dissociated, thus characterizing their recognition by high-avidity IgG antibodies. From these antigenic bands, p38 can be characterized as an optimal antigenic marker of low avidity for the recent form of toxoplasmosis due to a significant decrease of their frequencies (from 80 to 0%) after treatment with 6 M urea solutions. Moreover, after treatment with 8 M urea, the bands p16, p32, p40, and p97 were still detected by high-avidity IgG antibodies in the chronic phase. It is worth noting that the frequencies of the p38 and p54 bands were totally reduced after treatment with 6 or 8 M urea solutions, thus demonstrating their recognition by low-avidity IgG antibodies in the recent phase of infection.
Taken together, these findings show that the avidity immunoblotting technique has a potential complementary role in determining with greater accuracy the diagnosis markers in the different phases of Toxoplasma infection.
ACKNOWLEDGMENTS
We thank Erika de Arruda Chaves and Flávia Andrade Chaves Borges for their critical analysis and suggestions for the manuscript.
This study was financially supported by the FAPEMIG and CNPq Research Agencies, Brazil.
REFERENCES
- 1.Brooks R G, McCabe R E, Remington J S. Role of serology in the diagnosis of toxoplasmic lymphadenophathy. Rev Infect Dis. 1987;9:1055–1062. doi: 10.1093/clinids/9.5.1055. [DOI] [PubMed] [Google Scholar]
- 2.Camargo M E. Alguns aspectos atuais do diagnóstico de laboratório da Toxoplasmose. An Acad Nac Med. 1995;155:236–239. [Google Scholar]
- 3.Camargo M E, Silva S M, Leser P G, Granato C H. Avidez de anticorpos IgG específicos como marcadores de infecção primária recente pelo Toxoplasma gondii. Rev Inst Med Trop Sp. 1991;33:213–218. [PubMed] [Google Scholar]
- 4.Cozon G J N, Ferrandiz J, Nebhi H, Wallon M, Peyron F. Estimation of the avidity of immunoglobulin G for routine diagnosis of chronic Toxoplasma gondii infection in pregnancy women. Eur J Clin Microbiol Infect Dis. 1998;17:32–36. doi: 10.1007/BF01584360. [DOI] [PubMed] [Google Scholar]
- 5.Frenkel J K. Toxoplasmose. In: Veronesi R, editor. Doenças infecciosas e parasitárias. 8th ed. São Paulo, Brazil: Guanabara Koogan; 1995. pp. 734–749. [Google Scholar]
- 6.Gutierrez J, Maroto C. Are IgG antibody avidity assays useful in the diagnosis of infectious diseases? A review. Microbios. 1996;87:113–121. [PubMed] [Google Scholar]
- 7.Hedman K, Lappalainen M, Seppaia I, Makela O. Recent primary toxoplasma infection indicated by a low avidity of specific IgG. J Infect Dis. 1989;159:736–740. doi: 10.1093/infdis/159.4.736. [DOI] [PubMed] [Google Scholar]
- 8.Holliman R E, Raymond R, Renton N, Johnson J D. The diagnosis of toxoplasmosis using IgG avidity. Epidemiol Infect. 1994;112:399–408. doi: 10.1017/s0950268800057812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Joynson D H M, Payne R A, Rawal B K. Potential role of IgG avidity for diagnosing toxoplasmosis. J Clin Pathol. 1990;43:1032–1033. doi: 10.1136/jcp.43.12.1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Korhonen M H, Brunstein J, Haario H, Katnikov A, Rescaldani R, Hedman K. A new method with general diagnostic utility for the calculation of immunoglobulin G avidity. Clin Diagn Lab Immunol. 1999;6:725–728. doi: 10.1128/cdli.6.5.725-728.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 12.Lappalainen M, Koskela P, Koskiniemi M, Ammala P, Hiilesmaa V, Teramo K, Raivio K, Remington J F, Hedman K. Toxoplasmosis acquired during pregnancy: improved serodiagnosis based on avidity of IgG. J Infect Dis. 1993;167:691–697. doi: 10.1093/infdis/167.3.691. [DOI] [PubMed] [Google Scholar]
- 13.Rahmah N, Anuar K. Demonstration of antigenic similarities and variations in excretor/secretory antigens of Toxoplasma gondii. Biochem Biophys Res Commun. 1992;187:294–298. doi: 10.1016/s0006-291x(05)81491-3. [DOI] [PubMed] [Google Scholar]
- 14.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of protein from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1994;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vinhal F A, Pena J D O, Katina J H, Brandão E O, Silva D A O, Orefice F, Mineo J R. Analysis of aqueous humor in ocular toxoplasmosis: detection of low avidity IgG specific to Toxoplasma gondii. Appl Parasitol. 1994;35:1–7. [PubMed] [Google Scholar]