Abstract
Objective(s):
Although various studies have revealed the beneficial effects of crocin (derived from saffron), such as anti-inflammatory, anti-cancer, antioxidant, and immune modulator, however, its exact mechanism is unknown. The present study aimed to investigate the effect of crocin on the expression ratio of T-bet/GATA-3 as an indicator of altered immune responses in the lung tissue of ovalbumin (OVA)-sensitized mice. In addition, the effect of crocin on the expression level of miR-146a and miR-106a in the lung tissue OVA-sensitized mice was investigated.
Materials and Methods:
Mice were randomly divided into five groups (n=6): Control; OVA, OVA + Crocin 25, OVA + Cro 50, and OVA + Cro100 groups. Crocin was administrated intraperitoneally at doses of 25, 50, and 100 mg/kg for five consecutive days. One day after asthma induction, animals were euthanized, and lungs were sampled for pathological and gene expression analysis.
Results:
OVA-sensitization led to increased inflammation and histopathological changes in the lung tissue of mice. In addition, GATA-3 expression increased (P<0.001) and T-bet expression decreased (P<0.001) in OVA-sensitized groups. The T-bet/GATA3 ratio was also reduced markedly in asthma groups (P<0.001). Furthermore, increased expression of miR-146a and miR-106a levels was evident in the lung tissue of OVA-sensitized mice (P<0.001 for both). Intervention with high concentrations of crocin (50 and 100 mg/kg) significantly reduced airway inflammation, GATA-3 expression, miR-146a expression, and miR-106a expression and corrected the T-bet/GATA-3 ratio (P<0.05 to P<0.001).
Conclusion:
Treatment with crocin led to a decrease in the severity of lung inflammation in OVA-sensitized mice, which is probably through the reduction of the T-bet/GATA-3 ratio, and mir-146a and mir-106a expression level.
Key Words: Asthma, GATA-3, microRNA-106a, microRNA-146a, T-box transcription factor
Introduction
Asthma is a chronic inflammatory disease associated with various symptoms, including airway obstruction and hyper-responsiveness (1). Inflammation in asthma is caused by increased and decreased immune responses of Th2 and Th1 cells, respectively (2). Increased Th2 cell activity led to elevated IL-4 levels, and decreased Th1 cell activity led to decreased INF-γ levels, indicating altered immune responses in patients with asthma (2). GATA-3 transcription factor acts as the primary regulator of Th2 differentiation and increases its production of inflammatory cytokines such as IL-4, IL-5, and IL-13 (3, 4). On the other hand, transcription factor T-bet, a member of the T-box family, plays a vital role in the differentiation and effector function of Th1 cells (5, 6). An imbalance between T-bet and GATA-3 transcription factors has recently been reported in bronchial asthma (7). Therefore, one of the treatment goals in patients with asthma is to correct the Th1/Th2 imbalance, so increasing the Th1/Th2 ratio is an essential indicator of improving immune responses in asthmatic patients (8, 9).
Micro-RNAs (miRs) are small non-coding RNAs that regulate gene expression after transcription by inhibiting mRNA or inducing its degradation (10, 11). Recently, miRs have been shown to play a key role in regulating immune and inflammatory responses, lymphocyte activation, and eosinophil evolution (12). However, the role of many of them in patients with asthma is not well understood. Elevated miR-146a levels have been observed in patients with asthma (13). Interestingly, in obese ovalbumin-sensitized rats, increased expression level of miR-146a was more evident in lung tissue (13). Increased expression of miR-106a has also been reported in experimental asthma. By inhibiting miR-106a activity, a reduction in airway inflammation and mucous secretions occurred in the lung tissue of OVA-sensitized mice by increasing IL-10 production and decreasing the infiltration of inflammatory cells into the airways (14, 15). Therefore, another therapeutic goal in patients with asthma can be to focus on the activity of miRs.
The effectiveness of medicinal plants in chronic inflammatory diseases has been demonstrated in various human and animal studies (16-19). One recommended herbal medicine for asthma patients is saffron and its active ingredient crocin (20, 21). Experimental and clinical studies have revealed the effectiveness of crocin in chronic inflammatory diseases such as rheumatoid arthritis, heart disease, central nervous system, kidney disease, and lung disease (21-25). Although various mechanisms have been reported for the effectiveness of crocin in asthma conditions, such as decreased Th2 lymphocyte activity, modulated expression of endoplasmic reticulum stress genes, and improved oxidant/antioxidant imbalance (21, 22), the exact mechanism is not well understood. Therefore, the present study aimed to investigate the effect of crocin on the expression ratio of T-bet/GATA-3 as an indicator of altered immune responses in the lung tissue of ovalbumin-sensitive mice. The current study also evaluated the effects of crocin on miR-146a and miR-106a expression.
Materials and Methods
Chemicals
Quantitative enzyme-linked immunosorbent assay (ELISA) kits for OVA-sensitive IgE were obtained from Crystal Day (Shanghai, China). The crocin standard (>98%) was obtained from Sigma-Aldrich (St. Louis, MO, USA). OVA was obtained from Sigma-Aldrich (St. Louis, MO, USA). Aluminum hydroxide gel was obtained from Thermo Fisher (Waltham, MA, USA). All RT-PCR chemicals were obtained from Yekta Tajhiz Co. (Tehran, Iran). All other chemicals used in the study were of analytical grade.
Experimental design
This study used 30 adult male mice weighing 25 to 30 g. The animals were obtained from the Animal House of Tabriz University of Medical Sciences and adapted to the environment for one week. A temperature of 22±2 °C, a light-dark cycle of 12 hr: 12 hr, and free access to water and food were provided for all animals during the study. All animal-related interventions were performed after approval by the TBZMED ethics committee (IR.TBZMED.VCR.REC.1399.059).
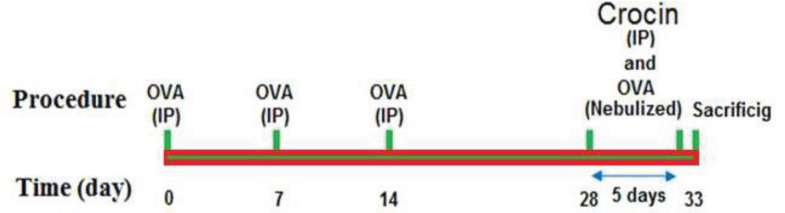
The animals were divided into five groups (n = 6 in each group) according to Figure 1. For sensitization with ovalbumin (OVA), the model of previous studies was used (21). In summary, 10 μg of OVA and 2 mg of aluminum hydroxide (Al (OH)3) were injected intraperitoneally on days 0, 7, and 14. From the 28th to the 32nd day, the animals were then exposed to 1% OVA aerosol through the nose for 30 min. In the control group, the animals received normal saline instead of OVA. In the intervention groups, one hour before the challenge with OVA, the animals were treated with crocin (IP).
Figure 1.
Experimental design flow chart and treatment with saline and crocin (last 5 days of the model). OVA; Ovalbumin, IP; Intraperitoneally
Bronchoalveolar lavage fluid (BALF) collection
In order to collect BALF of animals after anesthesia with ketamine and xylazine (100 mg/kg and 10 mg/kg, IP, respectively), tracheal cannulation was performed. Sample collection was performed by injecting and aspirating 0.5 ml of phosphate buffer saline (PBS) (three times). The supernatant prepared from the BALF sample was used for total white blood cell (WBC) and differential cell count (26).
Total and differential white blood cell (WBC) count
Total WBC count was performed using a hemocytometer and Wright-Giemsa staining. Differential cell count was performed by a light microscope with ×400 magnification and following the standard protocol (26).
Tissue sampling and protein measurement
Right lungs were frozen in liquid nitrogen and stored at -70 °C until OVA-sensitive IgE was measured. To prepare a supernatant, tissue samples were weighed and homogenized in PBS (pH 7.2–7.4) and centrifuged for 20 min at 4 °C at 3000 rpm (21). According to the manufacturer’s instructions, OVA-specific IgE (µg/gram total protein) was measured using mouse ELISA commercial kits (Crystal Day, Shanghai, China).
Real-time polymerase chain reaction
We performed real-time PCR analysis to evaluate GATA-3 and T-bet mRNA expression levels and miR-146a and miR-106a (10, 27). Table 1 shows the locked nucleic acid (LNA) forward and reverses mRNA’s primer sets (Exiqon). The PCR products were normalized with β-actin genes for mRNA samples. Results were expressed as fold change versus controls.
Table 1.
Primer sequence
| Gene | Forward | Reverse |
| β-actin | GGCACCACACCTTCTACAATG | GGGGTGTTGAAGGTCTCAAAC |
| T-bet | GGGTGGACATATAAGCGGTTC | AGCAGCCGCTCACGGAG |
| GATA-3 | GCGGGCTCTATCACAAAATGA | GCCTTCGCTTGGGCTTAAT |
Pathological assessment
Isolated left lung tissue was fixed in 10% neutral buffered formalin (37%, Merck, Germany) and embedded with paraffin blocks. The paraffin blocks were then cut to 4 μm, stained with hematoxylin-eosin, and evaluated under a light microscope. Pathological changes included a detachment of epithelium, bronchioles infiltration of lymphocytes, and interstitial tissue pneumonia. Scoring for each pathological lung change was identified from 0 to 3 as follows: 0= normal; 1= patchy injury, 2= local injuries, and 3= scattered injuries (27, 28).
Statistical analysis
In the current study, results are reported as mean ±SEM. Comparisons among different groups were performed using variance (ANOVA) with Tukey-Kramer post hoc test. In addition, Kruskal-Wallis statistical test was used to analyze the pathological results. P<0.05 was considered the significance level.
Results
Effects of crocin on the BALF cell infiltration
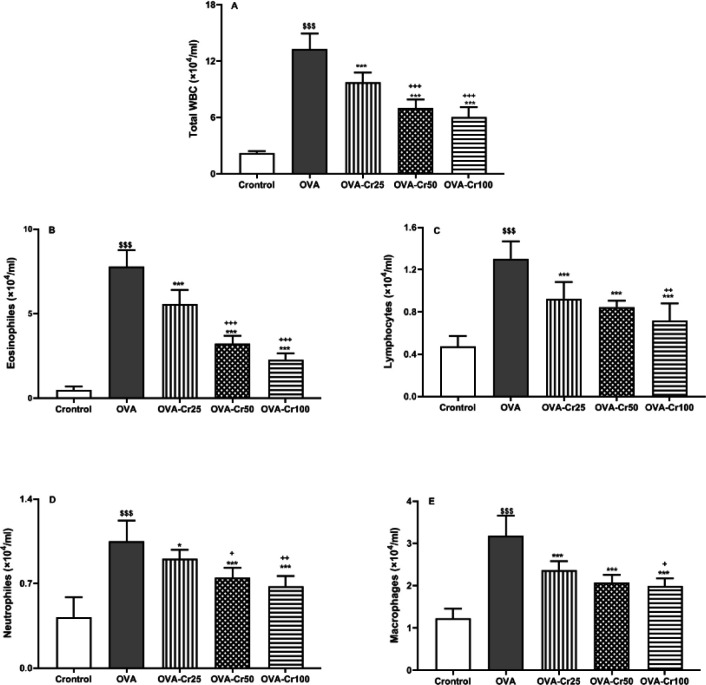
The total number of WBCs in the sensitized group was significantly higher in comparison with the control group (P<0.001). Instead, the number of leukocytes in the crocin-treated groups was significantly lower than in the OVA group (P<0.001 for all) (Figure 2A).
Figure 2.
Total WBC (A), eosinophil (B), lymphocyte (C), neutrophil (D), and macrophage (E) counts in the BALF of the control, asthmatic (OVA), OVA- crocin 25 mg/kg (OVA-Cr25), OVA- crocin 50 mg/kg (OVA-Cr50), and OVA-crocin 100 mg/kg (OVA-Cr100) groups. Data are shown as mean±SEM. $$$: P<0.001 control vs OVA group. *: P<0.05 and ***: P<0.001 OVA group vs crocin treated groups. +: P<0.05, ++: P<0.01, and +++: P<0.001 OVA-Cr25 group vs OVA-Cr50 and OVA-Cr100 groups. &&: P<0.01 OVA-Cr50 group vs OVA-Cr100 group. For each group, n = 6. Comparisons between groups were made using the ANOVA test
WBC: white blood cell; BALF: Bronchoalveolar lavage fluid
Here we reported that in the OVA group, levels of all inflammatory cells, including eosinophils, lymphocytes, neutrophils, and macrophages were significantly higher than in control animals (P<0.001 for all cases, Figure 2B-E). The significant improvement in the levels of all inflammatory cells in the treated groups (OVA-Cr25, OVA-Cr50, and OVA-cr100) was seen in comparison with the OVA group (P<0.001 for all cases, Figures 2B-E). However, total and differential leucocyte count indices in the BAL samples of all treated groups were still higher than in the healthy animals.
Effect of crocin on GATA-3 and T-bet expression levels in the lung tissue
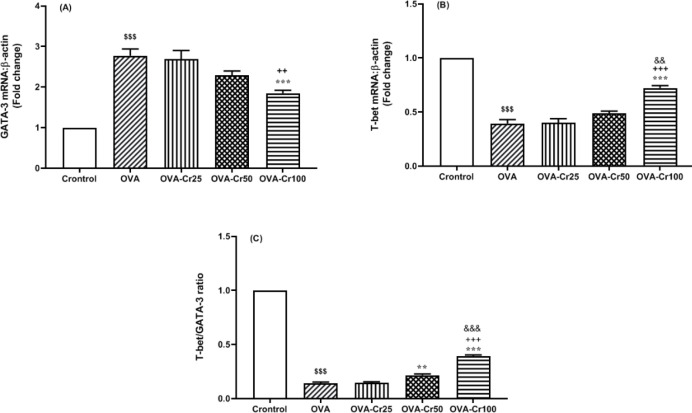
OVA-sensitization increased the expression level of GATA-3 in the lung tissue of mice, which crocin at a concentration of 100 mg/kg significantly prevented (P<0.01, Figure 3A). The inhibitory effect of crocin at 100 mg/kg was significantly higher than that of 50 mg/kg (P<0.05). On the other hand, the expression of T-bet mRNA level in lung tissue of OVA-sensitized mice was significantly reduced compared with the control group (P<0.001 for all) (Figure 3B). Crocin at a 100 mg/kg concentration resulted in a significant increase in T-bet expression levels (P<0.001, Figure 3B). Furthermore, the results showed that the T-bet/GATA-3 ratio was significantly reduced in the OVA-sensitized groups compared with the control group (Figure 3C). Intervention with 50 and 100 mg/kg crocin concentrations prevented T-bet/GATA-3 reduction.
Figure 3.
Lug tissue GATA-3 and T-bet gene expression levels. Mean+SEM of A) GATA-3, B) T-bet, and C) T-bet/GATA-3 ration. Abbreviations are the same as Figure 2. $$$: P<0.001 control vs OVA group. **: P<0.01 and ***: P<0.001 OVA group vs crocin treated groups. ++: P<0.01 and +++: P<0.001 OVA-Cr25 group vs OVA-Cr50 and OVA-Cr100 groups. &&: P<0.01 and &&&: P<0.001 OVA-Cr50 group vs OVA-Cr100 group. For each group, n = 6. Comparisons between groups were made using the ANOVA test
OVA: Ovalbumin
Effect of crocin on miR-146a and miR-106a expression levels in the lung tissue
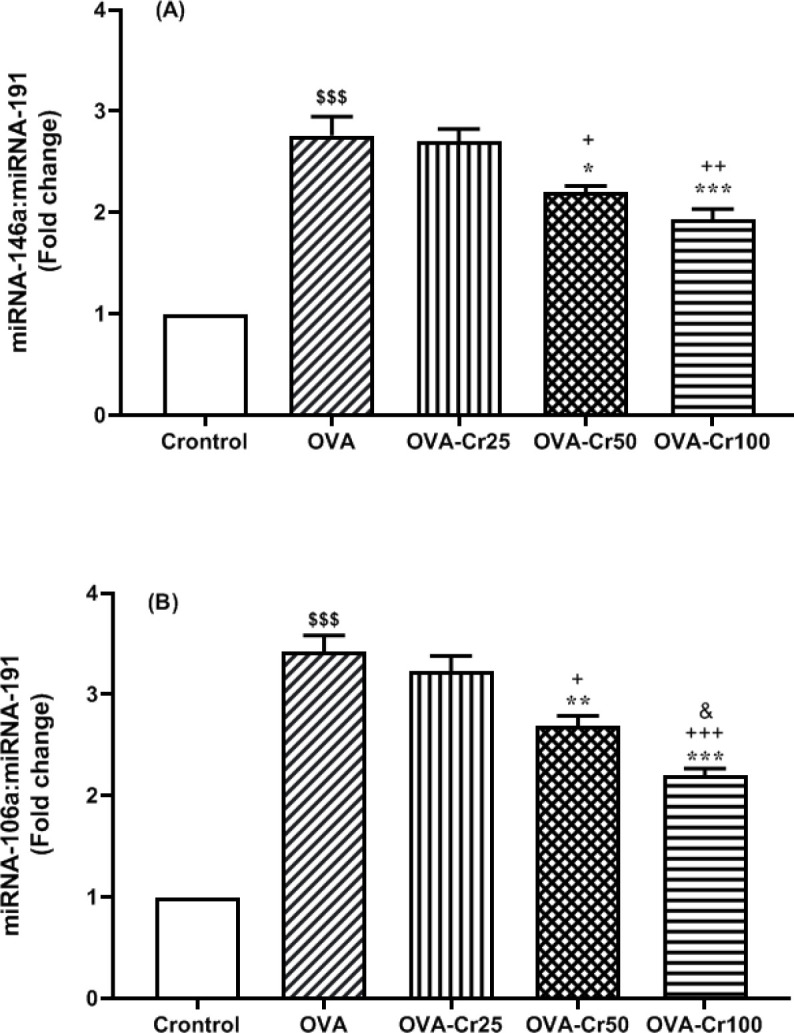
The elevated expression level of miR-146a was significantly observed in the OVA-sensitized groups compared with the control group (P<0.001 for all). Concentration-dependent crocin-treatment (50 and 100 mg/kg) inhibited the increased expression of miR-146a (P<0.05 and P<0.01, respectively), which had a higher effect on crocin 100 than 50 mg/kg (P<0.05) (Figure 4A). The OVA-sensitization effects were also significantly associated with enhanced miR-106a expression levels in mice lung tissue compared with the control group (P<0.001 for all). Crocin 50 and 100 mg/kg significantly inhibited the increase in miR-106a expression compared with the OVA group (P<0.01 and P<0.001, respectively) (Figure 4B).
Figure 4.
Lug tissue miR-146a and miR-106a gene expression levels. Mean+SEM of A) miR-146a and B) miR-106a expression levels. Abbreviations are the same as Figure 2. $$$: P<0.001 control vs OVA group. *: P<0.05, **: P<0.01, and ***: P<0.001 OVA group vs crocin-treated groups. +: P<0.05, ++: P<0.01, and +++: P<0.001 OVA-Cr25 group vs OVA-Cr50 and OVA-Cr100 groups. &: P<0.05 OVA-Cr50 group vs OVA-Cr100 group. For each group, n=6. Comparisons between groups were made using the ANOVA test
OVA: Ovalbumin
Effects of crocin on the OVA-specific IgE protein levels in the lung tissue
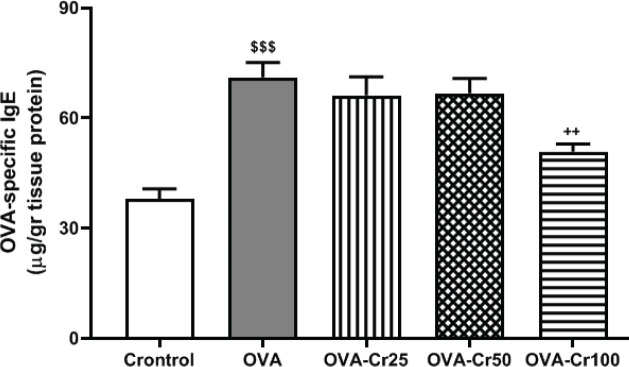
OVA-sensitization resulted in increased OVA-specific IgE protein levels (69.17 ± 9.92 µg) compared with the control group (36.50 ± 6.17 µg) (P<0.001, Figure 5). Crocin at a concentration of 100 mg/kg significantly reduced OVA-specific IgE protein levels (49.83 ± 5.18 µg) compared with the OVA group (P<0.01), while concentrations of 25 and 50 mg/kg had no effect (Figure 5).
Figure 5.
Lug tissue protein levels of OVA-specific IgE. Values are expressed as mean+SEM. Abbreviations are the same as Figure 2. $$$: P<0.001 control vs OVA group. ++: P<0.01 OVA group vs crocin-treated groups. For each group, n=6. Comparisons between groups were made using the ANOVA test
OVA: Ovalbumin
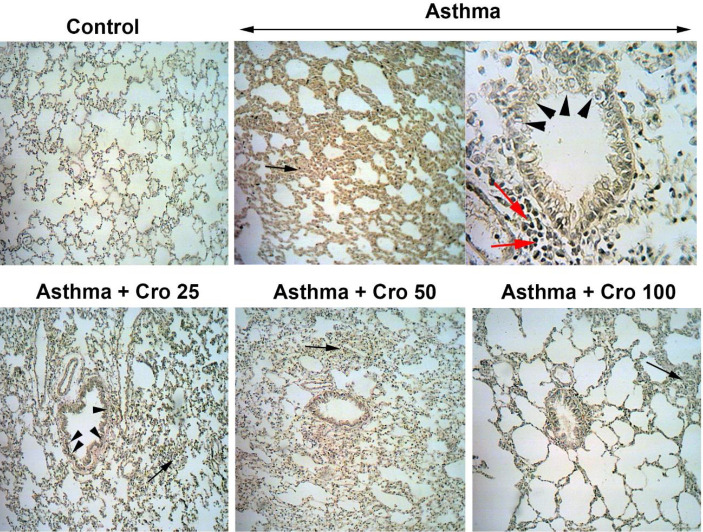
Effects of crocin on lung tissue pathological changes
The pathological findings revealed that the severity of changes such as lymphocyte infiltration, epithelial layer detachment, and interstitial tissue pneumonia (interalveolar septal thickening) was significantly higher in the OVA-sensitized mice than in the control group (P<0.001 for all) (Table 2 and Figure 6). Intervention with 50 and 100 mg/kg of crocin concentrations significantly reduced histopathological changes (Table 2).
Table 2.
Lung pathological scales in different groups
| Pathological results | Scores in groups (for each group, n=6) (Minimum-maximum) |
||||
|---|---|---|---|---|---|
| Control | OVA | OVA-Cr25 | OVA-Cr50 | OVA-Cr100 | |
| Detachment of epithelium | (0-0) | (2-3)$$$ | (1-3)$$$ | (1-3)$$$ | (0-2)++ |
| Lymphocytes bronchioles infiltration | (0-1) | (2-3)$$$ | (2-3)$$$ | (1-3)$$+ | (1-3)$$+ |
| Interstitial pneumonia | (0-1) | (2-3)$$$ | (2-3)$$$ | (1-3)$$$+ | (0-2)$+ |
Figure 6.
Pathological changes of pulmonary tissue (magnification=X40) in the control group, OVA-sensitized group (OVA), and crocin-treated groups (OVA-Cr25, OVA-Cr50, and OVA-Cr100). The asterisk indicates interstitial pneumonia, the headache sign indicates epithelial detachment, and the red arrow sign indicates lymphocyte infiltration
Discussion
This study showed that OVA-sensitization led to an increase in inflammatory cells in the airways of mice and histopathological changes in the lung tissues that were prevented by treatment with crocin, especially at high concentrations. In addition, in OVA-sensitized mice, increased expression of GATA-3 and decreased T-bet occurred in lung tissue, and crocin with high concentrations (50 and 100) significantly prevented their changes. Finally, increased expression of mir-146a and mir-106a in lung tissue of OVA-sensitized mice was suppressed by crocin intervention.
Asthma is an inflammatory disease characterized by AHR, the production of Th2 inflammatory cytokines (IL-4, IL-5, and IL-13), and the increased infiltration of inflammatory cells into the airways that results from the activation of Th2 cells (29, 30). In addition, histopathological changes in asthma patients are evident, including mucus secretion, airway smooth muscle hypertrophy, lymphocyte infiltration, epithelial detachment, interstitial tissue pneumonia, subepithelial fibrosis, and goblet cell hyperplasia (31, 32).
One of the causes of AHR in asthmatic patients is the infiltration of eosinophils in the respiratory airways, which affects the severity of the disease by producing Th2 inflammatory cytokines (5). The present study results identified that increased levels of eosinophils, macrophages, and neutrophils were evident in the BALF samples of OVA-sensitized mice. In addition, OVA-induced histopathological changes such as lymphocyte infiltration, epithelial detachment, and interstitial tissue pneumonia also occurred, indicating induction of the asthma model in this study. Accordingly, one of the therapeutic goals in patients with asthma may be to prevent the eosinophil requirement (5).
The anti-inflammatory, antioxidant, and anti-allergic effects of saffron and its active ingredients (crocin, crocetin, and safranal) have been reported in various animal and human studies (33, 34). The anti-inflammatory and immunomodulatory activities of saffron and crocin on leukocytes and lymphocyte cells have been demonstrated under OVA-sensitized animals (34). This study revealed that crocin prevented airway eosinophilia and lung inflammation. In addition, in previous studies, the reducing effects of saffron and crocin on Th2 cytokine levels (IL-4, IL-5, and IL-13) also have been reported (34). The results suggest that crocin had induced anti-inflammatory effects in OVA-sensitization status.
In asthma, Th1/Th2 imbalance has been shown to play a critical role in the pathogenesis of the disease (35). CD4 + T cells are divided into Th1 and Th2 based on functional differences and the type of cytokines produced (35). Two transcription factors, GATA-3 and T-bet, determine the differentiation of T cells to Th2 and Th1, respectively (5). IL-4 and INF-γ levels in asthma patients have been associated with the T-bet/GATA-3 ratio, indicating an immune imbalance in asthma conditions (35). In addition, a decrease in the T-bet /GATA3 ratio has been shown in most animal and human studies (36). Another therapeutic goal in asthma patients could be to modify the T-bet/GATA-3 ratio. The results showed that GATA-3 and T-bet expression levels were significantly increased and decreased in OVA-sensitized mice. Interestingly, there was a significant decrease in the T-bet/GATA-3 ratio in OVA-sensitized mice, indicating a significant increase in GATA-3 expression level. The current study results were consistent with the previous study results, which showed a more significant increase in GATA-3 compared with a decrease in T-bet in asthmatic mice (5). In fact, the role of GATA-3 in controlling Th1/Th2 differentiation appears to be greater than that of T-bet, as the direct effect of GATA-3 on IL-5 expression has been reported (5). Crocin treatment prevented T-bet/GATA-3 ratio imbalance, especially at high concentrations. Although there are not many findings regarding the role of crocin on the T-bet/GATA-3 ratio, a recent study by Hosseinzadeh et al. reported the modifying effects of crocin on ConA-treated human lymphocyte proliferation (37). Crocin-treated cells showed slightly lower T-bet/GATA-3 and INF-γ/IL-4 ratios than untreated cells (37). In another study, the effects of crocin on GATA-3 and T-bet expression in mononuclear cells of patients with osteoarthritis (OA) were investigated (38). The results revealed that crocin treatment significantly increased GATA-3 expression in mononuclear cells (38). Contradictory results of crocin effects in inflammatory diseases need further study.
Various studies have shown that mirRs are involved in immune regulation and play a vital role in the therapeutic aspects of immune-related diseases (39). Elevated miR-146a and miR-106a have been reported in OVA-sensitized mice (13, 14). The present study results revealed that OVA-sensitization increased the expression of miR-146a and miR-106a in the lung tissue of mice, which was consistent with previous findings (13, 14). Crocin treatment reduced the expression of miR-146a and miR-106a levels markedly at high concentrations. Inhibition of miR-146a and miR-106 expression in an asthmatic animal model has improved disease severity (40). MiR-146a may exacerbate the disease in asthma patients by increasing IL-1β production and miR-106a by decreasing IL-10 production (13, 14). Interestingly, miR-146a has been reported to play a dual role in asthma. Some studies have shown miR-146a as an anti-inflammatory factor, the reduction of which leads to an increase in neutrophil migration (41). On the other hand, miR-146a expression increases in response to IL-17A, TNF-α, and IL-4, which indicates its negative feedback effects on inflammatory cells such as neutrophils (41, 42). It seems that the results of the current study, on the other hand, confirmed the anti-inflammatory effects of miR-146a in conditions of ovalbumin sensitization. Intervention with crocin may have prevented the increase in miR-146a expression by reducing the number of leukocytes and inflammatory cytokines. In relation to the role of mir-106, it has also been shown that its increased expression was caused by IL-4, which led to inhibitory effects on the expression of Th2 cells (43). The results of the current study also showed that sensitization with ovalbumin led to an increase in the expression of miR-106a, the levels of which treatment with crocin reduced . In fact, reducing the severity of the disease affected the expression of miR-106a
Little is known about the association between mirRs and transcription factors, especially in patients with asthma. In a study, Saki et al. showed that ectopic expression of miR-146a increased the expression of various transcription factors such as PU.1, c-Fos, CCAAT/enhancer-binding protein alpha (C/EBPα), GATA3, Foxp3, and Runx1 in lymphoblastic cells (44). The current study results were in line with the findings of the Saki study. Although the present study did not directly evaluate the effects of miR-146a on GATA-3 expression level, a significant positive correlation between GATA-3 and miR-146a reflects the role of miR-146a in GATA-3 expression, which requires further studies. On the other hand, the role of miR-146a in T-bet expression was observed as an increased expression in peripheral blood mononuclear cells in patients with acute coronary syndrome (45), which was different from the findings of the current study. The results revealed a significant negative association between miR-146a and T-bet expression in lung tissue of OVA-sensitized mice. The differences between the current study’s findings and the previous study may be due to differences like the diseases that have affected immune responses.
GATA-3 and IL-4, which are two specific markers of Th2 cells, as well as miR-106a were revealed to be significantly increased in Th2 cells and unchanged or less expressed in Th17 cells, which actually confirms the specific differentiation (46). The relationship between miR-106a and the transcription factors T-bet and GATA-3 is not clear. Based on the findings of the current study, at least in part, it can be inferred that miR-106 may have indirect effects on the expression and function of the above transcription factors, which requires further studies.
Conclusion
The study had some limitations. First, the study results evaluated the expression of genes and did not specify their post-translation changes. It is better to study the change levels of target genes in future studies. Second, cell line studies should be designed in terms of mechanism evaluation to evaluate the association between miR-146a and miR-106a with transcription factors GATA-3 and T-bet.
In summary, the present study results revealed changes in the expression of transcription factors associated with Th1 and Th2 cells (T-bet and GATA-3, respectively) occurring in OVA-sensitized mice, which treatment with crocin significantly prevented. In addition, increased expression of miR-146a and miR-106a was observed in the lung tissue of OVA-sensitized mice, which was prevented with crocin intervention. Interestingly, there was a significant positive correlation between miR-146a and miR-106a with GATA-3 and a significant negative correlation with T-bet, at least in part, reflecting the role of miRs in the expression of transcription factors.
Authors’ Contributions
MRA and MA Helped with proposal writing, literature search, data collection, interpretation of data, analysis of data, review of manuscript, and manuscript preparation. ZJ, RR, JR, and AD: Provided proposal writing, draft preparation, review of manuscript, and analysis of data.
Funding
This study was supported by a grant (IR.TBZMED.VCR.REC.1399.059) from the Stem Cell Research Center of Tabriz University of Medical Sciences.
Conflicts of Interest
The authors have declared that there are no conflicts of interest.
Acknowledgment
The authors would like to thank the personnel of the Stem Cell Research Center and Drug Applied Research Center of Tabriz University of Medical Sciences for guidance and help.
References
- 1.Aslani MR, Sharghi A, Boskabady MH, Ghobadi H, Keyhanmanesh R, Alipour MR, et al. Altered gene expression levels of IL-17/TRAF6/MAPK/USP25 axis and pro-inflammatory cytokine levels in lung tissue of obese ovalbumin-sensitized rats. Life Sci. 2022;296:120425. doi: 10.1016/j.lfs.2022.120425. [DOI] [PubMed] [Google Scholar]
- 2.Pelaia G, Renda T, Gallelli L, Vatrella A, Busceti MT, Agati S, et al. Molecular mechanisms underlying airway smooth muscle contraction and proliferation: implications for asthma. Respir Med. 2008;102:1173–1181. doi: 10.1016/j.rmed.2008.02.020. [DOI] [PubMed] [Google Scholar]
- 3.Krug N, Hohlfeld JM, Kirsten AM, Kornmann O, Beeh KM, Kappeler D, et al. Allergen-induced asthmatic responses modified by a GATA3-specific DNAzyme. N Engl J Med. 2015;372:1987–1995. doi: 10.1056/NEJMoa1411776. [DOI] [PubMed] [Google Scholar]
- 4.Vale-Pereira S, Todo-Bom A, Geraldes L, Schmidt-Weber C, Akdis CA, Mota-Pinto A. FoxP3, GATA-3 and T-bet expression in elderly asthma. Clin Exp Allergy. 2011;41:490–496. doi: 10.1111/j.1365-2222.2010.03640.x. [DOI] [PubMed] [Google Scholar]
- 5.Kim SH, Hong JH, Lee YC. Oleanolic acid suppresses ovalbumin-induced airway inflammation and Th2-mediated allergic asthma by modulating the transcription factors T-bet, GATA-3, RORγt and Foxp3 in asthmatic mice. Int Immunopharmacol. 2014;18:311–324. doi: 10.1016/j.intimp.2013.12.009. [DOI] [PubMed] [Google Scholar]
- 6.Yuan Y, Yang B, Ye Z, Zhang M, Yang X, Xin C, et al. Sceptridium ternatum extract exerts antiasthmatic effects by regulating Th1/Th2 balance and the expression levels of leukotriene receptors in a mouse asthma model. J Ethnopharmacol. 2013;149:701–706. doi: 10.1016/j.jep.2013.07.032. [DOI] [PubMed] [Google Scholar]
- 7.Kim SH, Hong JH, Yang WK, Kim HJ, An HJ, Lee YC. Cryptotympana pustulata extract and its main active component, oleic acid, inhibit ovalbumin-induced allergic airway inflammation through inhibition of Th2/GATA-3 and Interleukin-17/RORγt signaling pathways in asthmatic mice. Molecules. 2021;26:1854. doi: 10.3390/molecules26071854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ahmadi M, Rahbarghazi R, Aslani MR, Shahbazfar AA, Kazemi M, Keyhanmanesh R. Bone marrow mesenchymal stem cells and their conditioned media could potentially ameliorate ovalbumin-induced asthmatic changes. Biomed Pharmacother. 2017;85:2 8–40. doi: 10.1016/j.biopha.2016.11.127. [DOI] [PubMed] [Google Scholar]
- 9.Guo HW, Yun CX, Hou GH, Du J, Huang X, Lu Y, et al. Mangiferin attenuates TH1/TH2 cytokine imbalance in an ovalbumin-induced asthmatic mouse model. PLoS One. 2014;9:e100394. doi: 10.1371/journal.pone.0100394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ahmadi M, Rahbarghazi R, Shahbazfar A-A, Keyhanmanesh R. Monitoring IL-13 expression in relation to miRNA-155 and miRNA-133 changes following intra-tracheal administration of mesenchymal stem cells and conditioned media in ovalbuminsensitized rats. Thai J Vet Med. 2018;48:347–355. [Google Scholar]
- 11.Rahbarghazi R, Kihanmanesh R, Rezaie J, Mirershadi F, Heiran H, Saghaei Bagheri H, et al. c-kit+ cells offer hopes in ameliorating asthmatic pathologies via regulation of miRNA-133 and -126. Iran J Basic Med Sci. 2021;24:369–376. doi: 10.22038/ijbms.2021.49008.11231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Collison A, Herbert C, Siegle JS, Mattes J, Foster PS, Kumar RK. Altered expression of microRNA in the airway wall in chronic asthma: miR-126 as a potential therapeutic target. BMC Pulmonary Medicine. 2011;11:29–34. doi: 10.1186/1471-2466-11-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Akhavanakbari G, Babapour B, Alipour MR, Keyhanmanesh R, Ahmadi M, Aslani MR. Effect of high fat diet on NF-кB microRNA146a negative feedback loop in ovalbumin-sensitized rats. Biofactors. 2019;45:75–84. doi: 10.1002/biof.1466. [DOI] [PubMed] [Google Scholar]
- 14.Fang C, Lu W, Li C, Peng X, Wang Y, Huang X, et al. MiR-3162-3p Is a Novel MicroRNA That Exacerbates Asthma by Regulating β-Catenin. PLoS One. 2016;11:e0149257–e0149257. doi: 10.1371/journal.pone.0149257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Panganiban RP, Wang Y, Howrylak J, Chinchilli VM, Craig TJ, August A, et al. Circulating microRNAs as biomarkers in patients with allergic rhinitis and asthma. J Allergy Clin Immunol. 2016;137:1423–1432. doi: 10.1016/j.jaci.2016.01.029. [DOI] [PubMed] [Google Scholar]
- 16.Ghasemi Z, Rezaee R, Aslani MR, Boskabady MH. Anti-inflammatory, anti-oxidant, and immunomodulatory activities of the genus Ferula and their constituents: A review. Iran J Basic Med Sci. 2021;24:1613–1623. doi: 10.22038/IJBMS.2021.59473.13204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saadat S, Aslani MR, Ghorani V, Keyhanmanesh R, Boskabady MH. The effects of Nigella sativa on respiratory, allergic and immunologic disorders, evidence from experimental and clinical studies, a comprehensive and updated review. Phytother Res. 2021;35:2968–2996. doi: 10.1002/ptr.7003. [DOI] [PubMed] [Google Scholar]
- 18.Khazdair MR, Saadat S, Aslani MR, Shakeri F, Boskabady MH. Experimental and clinical studies on the effects of Portulaca oleracea L and its constituents on respiratory, allergic, and immunologic disorders, a review. Phytotherapy Research. 2021;35:6813–6842. doi: 10.1002/ptr.7268. [DOI] [PubMed] [Google Scholar]
- 19.Boskabady MH, Aslani MR, Mansuri F, Amery S. Relaxant effect of Satureja hortensis on guinea pig tracheal chains and its possible mechanism (s. DARU Journal of Pharmaceutical Sciences . 2007:6. [Google Scholar]
- 20.Boskabady MH, Aslani MR. Relaxant effect of Crocus sativus (saffron) on guinea-pig tracheal chains and its possible mechanisms. J Pharm Pharmacol. 2006;58:1385–1390. doi: 10.1211/jpp.58.10.0012. [DOI] [PubMed] [Google Scholar]
- 21.Aslani MR, Amani M, Masrori N, Boskabady MH, Ebrahimi HA, Chodari L. Crocin attenuates inflammation of lung tissue in ovalbumin-sensitized mice by altering the expression of endoplasmic reticulum stress markers. Biofactors. 2022;48:204–215. doi: 10.1002/biof.1809. [DOI] [PubMed] [Google Scholar]
- 22.Ghobadi H, Abdollahi N, Madani H, Aslani MR. Effect of crocin from saffron (Crocus sativus L ) supplementation on oxidant/antioxidant markers, exercise capacity, and pulmonary function tests in COPD patients: A randomized, double-blind, placebo-controlled trial. Front Pharmacol. 2022;13:884710. doi: 10.3389/fphar.2022.884710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rahimi G, Shams S, Aslani MR. Effects of crocin supplementation on inflammatory markers, lipid profiles, insulin and cardioprotective indices in women with PCOS: A randomized, double-blind, placebo-controlled trial. Phytother Res. 2022;36:2605–2615. doi: 10.1002/ptr.7474. [DOI] [PubMed] [Google Scholar]
- 24.Nam KN, Park YM, Jung HJ, Lee JY, Min BD, Park SU, et al. Anti-inflammatory effects of crocin and crocetin in rat brain microglial cells. Eur J Pharmacol. 2010;648:110–116. doi: 10.1016/j.ejphar.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 25.Yarijani ZM, Pourmotabbed A, Pourmotabbed T, Najafi H. Crocin has anti-inflammatory and protective effects in ischemia-reperfusion induced renal injuries. Iran J Basic Med Sci. 2017;20:753–759. doi: 10.22038/IJBMS.2017.9005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ahmadi M, Rahbarghazi R, Soltani S, Aslani MR, Keyhanmanesh R. Contributory anti-inflammatory effects of mesenchymal stem cells, not conditioned media, on ovalbumin-induced asthmatic changes in male rats. Inflammation. 2016;39:1960–1971. doi: 10.1007/s10753-016-0431-2. [DOI] [PubMed] [Google Scholar]
- 27.Taghizadeh S, Keyhanmanesh R, Rahbarghazi R, Rezaie J, Delkhosh A, Hassanpour M, et al. Systemic administration of c-Kit+ cells diminished pulmonary and vascular inflammation in rat model of chronic asthma. BMC Molecular and Cell Biology. 2022;23:1–10. doi: 10.1186/s12860-022-00410-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rahbarghazi R, Keyhanmanesh R, Aslani MR, Hassanpour M, Ahmadi M. Bone marrow mesenchymal stem cells and condition media diminish inflammatory adhesion molecules of pulmonary endothelial cells in an ovalbumin-induced asthmatic rat model. Microvasc Res. 2019;121:63–70. doi: 10.1016/j.mvr.2018.10.005. [DOI] [PubMed] [Google Scholar]
- 29.Saadat S, Mohamadian Roshan N, Aslani MR, Boskabady MH. Rosuvastatin suppresses cytokine production and lung inflammation in asthmatic, hyperlipidemic and asthmatic-hyperlipidemic rat models. Cytokine. 2020;128:154993. doi: 10.1016/j.cyto.2020.154993. [DOI] [PubMed] [Google Scholar]
- 30.Lloyd CM, Hessel EM. Functions of T cells in asthma: more than just T(H)2 cells. Nat Rev Immunol. 2010;10:838–848. doi: 10.1038/nri2870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schatz M, Rosenwasser L. The allergic asthma phenotype. J Allergy Clin Immunol Pract. 2014;2:645–648. doi: 10.1016/j.jaip.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 32.Keyhanmanesh R, Alipour MR, Ebrahimi H, Aslani MR. Effects of diet-induced obesity on tracheal responsiveness to methacholine, tracheal visfatin level, and lung histological changes in ovalbumin-sensitized female wistar rats. Inflammation. 2018;41:846–858. doi: 10.1007/s10753-018-0738-2. [DOI] [PubMed] [Google Scholar]
- 33.Korani S, Korani M, Sathyapalan T, Sahebkar A. Therapeutic effects of Crocin in autoimmune diseases: A review. Biofactors. 2019;45:835–843. doi: 10.1002/biof.1557. [DOI] [PubMed] [Google Scholar]
- 34.Zeinali M, Zirak MR, Rezaee SA, Karimi G, Hosseinzadeh H. Immunoregulatory and anti-inflammatory properties of Crocus sativus (Saffron) and its main active constituents: A review. Iran J Basic Med Sci. 2019;22:334–344. doi: 10.22038/ijbms.2019.34365.8158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Du Q, Gu X, Cai J, Huang M, Su M. Chrysin attenuates allergic airway inflammation by modulating the transcription factors T-bet and GATA-3 in mice. Mol Med Rep. 2012;6:100–104. doi: 10.3892/mmr.2012.893. [DOI] [PubMed] [Google Scholar]
- 36.Barrett NA, Austen KF. Innate cells and T helper 2 cell immunity in airway inflammation. Immunity. 2009;31:425–437. doi: 10.1016/j.immuni.2009.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Abdi H, Aganj Z, Hosseinzadeh H, Mosaffa F. Crocin restores the balance of Th1/Th2 immune cell response in ConA-treated human lymphocytes. Pharmacol Rep. 2022;74:513–522. doi: 10.1007/s43440-022-00362-3. [DOI] [PubMed] [Google Scholar]
- 38.Poursamimi J, Shariati-Sarabi Z, Tavakkol-Afshari J, Mohammadi M. A significant increase in the gene expression of GATA-3 following the treatment of osteoarthritis patients with crocin. Iran J Allergy Asthma Immunol. 2022;21:35–43. doi: 10.18502/ijaai.v21i1.8611. [DOI] [PubMed] [Google Scholar]
- 39.Sharma A, Kumar M, Ahmad T, Mabalirajan U, Aich J, Agrawal A, et al. Antagonism of mmu-mir-106a attenuates asthma features in allergic murine model. J Appl Physiol. 2012;113:459–464. doi: 10.1152/japplphysiol.00001.2012. [DOI] [PubMed] [Google Scholar]
- 40.Feng MJ, Shi F, Qiu C, Peng WK. MicroRNA-181a, -146a and -146b in spleen CD4+ T lymphocytes play proinflammatory roles in a murine model of asthma. Int Immunopharmacol. 2012;13:347–353. doi: 10.1016/j.intimp.2012.05.001. [DOI] [PubMed] [Google Scholar]
- 41.Kivihall A, Aab A, Soja J, Sładek K, Sanak M, Altraja A, et al. Reduced expression of miR-146a in human bronchial epithelial cells alters neutrophil migration. Clin Transl Allergy. 2019;9 doi: 10.1186/s13601-019-0301-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Weidner J, Bartel S, Kılıç A, Zissler UM, Renz H, Schwarze J, et al. Spotlight on microRNAs in allergy and asthma. Allergy. 2021;76:1661–1678. doi: 10.1111/all.14646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kılıç A, Santolini M, Nakano T, Schiller M, Teranishi M, Gellert P, et al. A systems immunology approach identifies the collective impact of 5 miRs in Th2 inflammation. JCI Insight. 2018;3 doi: 10.1172/jci.insight.97503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Saki N, Abroun S, Soleimani M, Mortazavi Y, Kaviani S, Arefian E. The roles of miR-146a in the differentiation of Jurkat T-lymphoblasts. Hematology. 2014;19:141–147. doi: 10.1179/1607845413Y.0000000105. [DOI] [PubMed] [Google Scholar]
- 45.Guo M, Mao X, Ji Q, Lang M, Li S, Peng Y, et al. miR-146a in PBMCs modulates Th1 function in patients with acute coronary syndrome. Immunol Cell Biol. 2010;88:555–564. doi: 10.1038/icb.2010.16. [DOI] [PubMed] [Google Scholar]
- 46.Kästle M, Bartel S, Geillinger-Kästle K, Irmler M, Beckers J, Ryffel B, et al. microRNA cluster 106a~363 is involved in T helper 17 cell differentiation. Immunology. 2017;152:402–413. doi: 10.1111/imm.12775. [DOI] [PMC free article] [PubMed] [Google Scholar]