Abstract
Rationale:
Allergic diseases are an increasing public health concern, and early life environment is critical to immune development. Maternal diet during pregnancy has been linked to offspring allergy risk. In turn, maternal diet is a potentially modifiable factor, which could be targeted as an allergy prevention strategy. In this systematic review, we focused on non-allergen-specific modifying factors of the maternal diet in pregnancy on allergy outcomes in their offspring.
Methods:
We undertook a systematic review of studies investigating the association between maternal diet during pregnancy and allergic outcomes (asthma/wheeze, hay fever/allergic rhinitis/seasonal allergies, eczema/atopic dermatitis (AD), food allergies, and allergic sensitization) in offspring. Studies evaluating the effect of food allergen intake were excluded. We searched three bibliographic databases (MEDLINE, EMBASE, and Web of Science) through February 26, 2019. Evidence was critically appraised using modified versions of the Cochrane Collaboration Risk of Bias tool for intervention trials and the National Institute for Clinical Excellence methodological checklist for cohort and case-control studies and meta-analysis performed from RCTs.
Results:
We identified 95 papers: 17 RCTs and 78 observational (case-control, cross-sectional, and cohort) studies. Observational studies varied in design and dietary intakes and often had contradictory findings. Based on our meta-analysis, RCTs showed that vitamin D supplementation (OR: 0.72; 95% CI: 0.56–0.92) is associated with a reduced risk of wheeze/asthma. A positive trend for omega-3 fatty acids was observed for asthma/wheeze, but this did not reach statistical significance (OR: 0.70; 95% CI: 0.45–1.08). Omega-3 supplementation was also associated with a non-significant decreased risk of allergic rhinitis (OR: 0.76; 95% CI: 0.56–1.04). Neither vitamin D nor omega-3 fatty acids were associated with an altered risk of AD or food allergy.
Conclusions:
Prenatal supplementation with vitamin D may have beneficial effects for prevention of asthma. Additional nutritional factors seem to be required for modulating the risk of skin and gastrointestinal outcomes. We found no consistent evidence regarding other dietary factors, perhaps due to differences in study design and host features that were not considered. While confirmatory studies are required, there is also a need for performing RCTs beyond single nutrients/foods.
Keywords: AD, allergic diseases, allergic rhinitis, allergic rhinoconjunctivitis, asthma, children, eczema, food allergy, hay fever, infants, maternal diet, pregnancy, prevention, seasonal allergies, wheeze
1 |. INTRODUCTION
Allergic diseases are an increasing public health concern.1 The most common allergic conditions are asthma, allergic rhinitis/hay fever/seasonal allergies, eczema/atopic dermatitis (AD) (from now on referred to as eczema), and food allergies. Worldwide, an estimated 300 million people suffer from asthma, and an additional 400 million have allergic rhinitis.2 In addition, 570 million children and 116 million adults suffer from eczema, and 200–250 million people suffer from food allergies.3 These allergic diseases may often co-exist in one individual.4 Allergies can affect quality of life and reduce academic performance and income,5 and in rare cases can be fatal.6,7
Maternal diet in pregnancy is an important, potentially modifiable factor in allergy prevention. Several studies have investigated the role of prenatal diet on offspring allergy, but the findings are conflicting, and there is a paucity of randomized controlled trials (RCTs). An evidence synthesis is paramount in order to adequately appreciate the underlying evidence.
A number of review papers have been published focusing on maternal diet during pregnancy, lactation, or early life8–11 and allergy prevention.8–12 Our review follows and extends a recent systematic review and meta-analysis by Garcia-Larsen et al,13 who summarized the evidence up to December 2017. Garcia-Larsen et al 13 considered dietary intake not only during pregnancy, but also in lactation and infancy; their main conclusions 13 were that maternal omega-3 fatty acid supplementation during pregnancy and lactation may reduce the risk of allergic sensitization to egg and peanut. However, only three of the interventional studies regarding omega-3 fatty acids included in that review were conducted in pregnancy only. In addition, the review13 showed that maternal probiotic supplementation in the postnatal period reduced the risk of offspring eczema and allergic sensitization to cow’s milk, and none of the probiotic studies were limited to pregnancy. Since the publication of that systematic review, new studies14–40 evaluating maternal diet during pregnancy and childhood allergic outcomes have been published. We reviewed diet during pregnancy only as not all mothers breastfeed or introduce solids to their infants in a similar fashion. We therefore reviewed literature regarding the pregnancy period only, to investigate specific pregnancy-related dietary associations.
Globally, recommendations on dietary intake during pregnancy for prevention of allergic disease are sparse. The Australasian guideline recommends eating fatty fish or taking omega-3 fatty acid supplements during pregnancy to reduce eczema in the offspring.41 Other guidelines from the UK,42 Europe,43 and the United States44 only give recommendations pertaining to food allergen avoidance in pregnancy. They state that food allergens should not be avoided during pregnancy, but give no further dietary recommendations for allergy prevention. The German allergy prevention guidelines45 recommend that pregnant women should follow a healthy diet, need not avoid food allergens, and should eat fish regularly. In support of this, the UK prevention guidelines state that omega-3 fatty acids (found in oily fish such as salmon, trout, mackerel, and fresh [not canned] tuna) during pregnancy may help reduce the risk of eczema and allergic sensitization (development of antibodies to allergens) in early life.
The goal of this review was to summarize studies which examined the impact of maternal diet during pregnancy on offspring’s allergic outcomes. We also compared the amount of nutrients or foods consumed and food patterns identified, which have been shown to have an allergy preventative effect to current US dietary guidelines.
2 |. METHODS
2.1 |. Studies included in this review
2.1.1 |. Types of studies
We included RCTs, observational (cross-sectional and cohort), and case-control studies, which (a) considered the time period of pregnancy alone, (b) reported allergic outcomes or allergic sensitization status in the offspring, and (c) were written in English. Studies examining food allergen intake were excluded. Studies that included intake of major allergens such as milk or nuts were included, as long as the outcome did not relate to the particular food allergen. As an example, studies on tree nut intake were included only when an association with an allergy outcome other than a tree nut was studied, such as asthma.46 The latest data indicate that early introduction of a food allergen specifically prevents food allergy to that allergen only.47 We were therefore confident that any outcome noted was less likely to be related to the nutritional composition, rather than the allergen content of the food. We also excluded case studies, case series, systematic reviews and meta-analyses, expert reviews and opinions, and editorials.
2.1.2 |. Types of publications
A consensus was reached on including full-text publications only, as there were discrepancies in some of the data published in abstract form (as presented at conferences) when compared to the full paper publications.
2.1.3 |. Types of participants
Pregnant women were the target group for this systematic review, provided there was allergic outcome data published for their offspring. Both general population and populations at high risk of atopy were included.
2.1.4 |. Types of interventions in RCT
Studies that used supplementation or specific foods (eg, fatty fish) during pregnancy alone, irrespective of dose, formulation, or mode of delivery and composition (eg, oil and tablet), were included. Trials were not included if the intervention(s) had been continued beyond pregnancy through breastfeeding or given only to the infant.
2.1.5 |. Types of outcome measures
Studies were included if they reported on allergic outcomes or allergic sensitization status in the offspring, either as a primary end-point or as a secondary end-point. Allergic outcomes were defined as wheeze/asthma, allergic rhinitis/rhinoconjunctivitis/hay fever, food allergy, eczema, allergic sensitization measured by skin prick tests/specific IgE tests to food/aero/any allergen, or total IgE levels. Please see Supplementary File S1 for a list of the search terms used in respective databases. An expert panel from the United States, Europe, and Australia commented on the search strategy and the list of identified and included studies. Additional studies were located through searching the references cited in identified studies and systematic reviews, and through discussion with the expert panel.
2.2 |. Dietary guidelines for food/nutrient intake in pregnancy
To compare our study findings with current dietary guidelines, we used the following approach to calculate food or nutrient intake: For micronutrient comparisons, the US dietary reference intakes (DRI) and the United States Department of Agriculture (USDA) www.choosemyplate.gov guidelines48 for pregnant women were used. Macronutrient requirements and number of portions for the second trimester of pregnancy were calculated, assuming a daily caloric intake of 2400 kcal, comprising 71 g of protein, 53.33–93.33gof total fat, <27 g of saturated fat, 13 g of linoleic acid (LA), 1.4 g of alpha-linolenic acid (AI), 175 g of carbohydrates, 28 g of fiber, <12 g of added sugar, 2 cups (2× approx. 125 g) of fruit, 3 cups (3 × 125 g) of vegetables, 8 oz of grains (8 × 30 g), 6.5 oz of high-protein foods (6.5 × 30 g), and 3 cups (3 × 200–250 mL) of dairy. Recommended fish intake was assumed to be 2–3 servings (in total approx. 8–12 oz/240–360 g) per week as per the United States Food and Drug Administration (FDA) (Best Choices: https://www.fda.gov/food/consumers/eating-fish-what-pregnant-women-and-parents-should-know).
2.3 |. Data collection
2.3.1 |. Ethics application/Institutional Review Board
This study is considered exempt from Institutional Review Board review by the University of Colorado School of Medicine Combined Institutional Review Board.
2.4 |. Study selection
The titles and abstracts of articles considered for inclusion were independently assessed by MBA, MP, and AM and categorized as included, not included, or potentially relevant. CV then checked each assessment. For studies considered potentially relevant, full-text copies were obtained, and inclusion of the studies was discussed by CV, MBA, MP, and AM. Any discrepancies were resolved either by consensus among the authors, or, if there was lack of consensus, by discussion with the expert panel. All the studies in this publication were reviewed and approved by both the authors and the expert panel.
2.5 |. Risk of bias assessment
Risk of bias assessment was undertaken independently by MBA and CV using a similar approach as in Garcia-Larsen et al13 As in the paper by Garcia-Larsen-et al,13 we used modified versions of the Cochrane Collaboration Risk of Bias tool for intervention trials and the National Institute for Clinical Excellence methodological checklist for cohort and case-control studies.49 For intervention trials, risk of bias assessment included assessment of: (a) selection bias, including sequence generation and allocation concealment; (b) assessment bias, included blinding of outcome assessors and ensuring outcomes were determined by validated assessment tools; and (c) attrition bias, which was considered high when outcome data were reported in <70% of randomized participants. Risk of bias for cohort and case-control studies included assessment of: (a) selection bias, which was considered low if cases and controls were recruited from similar populations and had a similar attrition rate <20%; (b) assessment bias, which included blinding of outcome assessors and use of validated assessment tools; and (c) confounding bias (did study design and analysis account for relevant confounders?). Conflicts of interest were noted if industry was involved in any aspect of the study or if authors received funding for other activities from relevant industry partners. Using these components, each study was graded as high, medium, or low. Supplementary File S2 includes the grading for risk of bias assessment. In order to minimize publication bias, we performed a comprehensive search of the literature and included experts in the field to ensure that no relevant study was missed. We did not include gray literature (unpublished information or information that is not produced by commercial publishers) in order to avoid discrepancies with published data. None of the meta-analyses included more than 10 papers, and we therefore did not use funnel plot and Egger tests to formally test for publication bias.
2.6 |. Data extraction and reporting
Data were extracted by three authors (AM, MBA, and MP). New articles were added into the excel sheets downloaded from Garcia-Larsen et al13 Inclusion and exclusion criteria differed between the current review and that of Garcia-Larsen et al13; some entries were edited, modified, or deleted. The edited files were reviewed for quality and completeness by CV. Detailed information on study characteristics was recorded by CV, MP, and MBA and appears in Supplementary File S3. Any discrepancies regarding the classification of dietary measures and offspring allergic outcomes were discussed and resolved within the group of authors. Classification of maternal dietary measures and offspring allergic outcomes were double-checked by the expert panel. The authors’ names, institutions, journals, or data were not blinded during data extraction.
2.7 |. Data analysis
2.7.1 |. Observational studies
For wheeze, studies were split into two categories: infants and young children (0-<3 years) and older children (3 years and older). All studies reporting on allergic rhinitis included offspring with ages over 5 years. All ages were included for food allergy, eczema, and allergic sensitization.
The reported data were plotted. If multiple odds ratios (ORs) were reported for a single study, only the highest exposure compared to the lowest exposure OR was used. Meta-analysis of observational studies was not appropriate because the studies were heterogeneous in focus, design, and target populations. We used forest plots to visually summarize all significant outcomes found by the studies, that is, the adjusted OR, adjusted hazard ratio (HR), or adjusted risk ratio (RR), and their corresponding 95% confidence intervals. If more than one significant outcome was found in a manuscript, then only the largest observed difference was included in this review. For example, if authors compared quartiles, by comparing Q1 vs Q2 and Q1 vs Q4 for one nutrient in the same age-group, only the largest difference would have been included. We understand that it is better to report the estimates regardless of their statistical significance in order to give readers an appreciation of the magnitude of the effect sizes and that statistical significance is purely a function of the sample size of a study and hence provides information only of the random error. It does not give any indication of the magnitude of the estimates or potential systematic errors. However, our goal here was to provide a visual presentation of the data, and the Supplementary Files S1–S9 contain the full set of information. Therefore, findings from observational studies were synthesized narratively by grouping studies according to allergy outcome and nutrient, food, or diet pattern studied: nutrients, including vitamins and minerals, omega-3 fatty acids and fatty fish, fruit and vegetables, other foods, and food patterns.
2.7.2 |. Randomized clinical trial meta-analyses
For each exposure-disease outcome pair included in the RCTs, separate meta-analyses were performed. Because the number of studies was limited, several meta-analyses included fewer than four data points. All meta-analyses included at least two studies. 50 DerSimonian-Laird random-effects models were used to perform the meta-analyses and estimate the pooled ORs using the rmeta (v 3.0)51 package in R. The heterogeneity of the studies was calculated using the Cochran χ2 and I2 statistics. Forest plots were created for each of the diseases using the forestplot (v1.9)52 package in R (v3.5.1). Where studies reported outcomes in the same children at more than one time point, we included each outcome in the meta-analysis as well as the combined outcomes. As the different analyses showed no changes in the outcomes, the overall decision was to use food allergy outcomes at 3 years; eczema at 3 years; allergic sensitization at 3 years; and asthma, rhinitis, and allergic sensitization at 6 years.
3 |. RESULTS
3.1 |. Results of literature search and characteristics of included studies
We included all the relevant published studies on the topic of maternal dietary intake during pregnancy alone identified by Garcia-Larsen et al13 (n = 68 papers).14–40,46,53–119 An additional search was performed using the same search terms to include studies published after, or potentially missed by their search, including observational studies from August 2013 to February 2019 and intervention studies from January 2017 to February 2019 (Figure 1). Based on this additional search, we identified an additional 27 publications. Of them, 25 were cohort or case-control studies 14–38 and 2 were RCTs.39,40 In total, our systematic review is based on 95 papers (17 RCTs and 78 cohort and case-control studies). The numbers of pregnant women and their offspring studied at different time points and presented in different publications are shown in the supplementary Files S1–S9. Supplementary File S4 has information on nutrients, including vitamins and minerals; Supplementary File S5 contains information on fats and fatty fish; Supplementary File S6 is on fruit and vegetables; Supplementary File S7 contains information on other foods; and Supplementary File S8 is on food patterns.
FIGURE 1.
Flow chart for study inclusion
Sufficient numbers (at least two) of RCTs were found for vitamin D, vitamins C and/or E, and omega-3 fatty acids. We performed a total of 9 meta-analyses.
3.2 |. Quality and risk of bias assessment
The 95 papers were generated from 50 studies. The 50 studies included 11 RCTs39,40,53–67 (7/11 normal risk), 2 cross-sectional21,74 (both normal) and 30 cohort studies15,18–20,22–30,32–37,46,68–70,72,73,75–78,80–108,110,112,115–119 (all normal risk), 3 case-control designs nested within cohorts16,17,31,38,79,113,114 (all normal risk), and 4 case-control studies14,71,109,111 (3 normal and 1 high risk). Due to low numbers of RCTs that could be included in the meta-analysis and low numbers of studies including high-risk populations in the observational studies, these were not separated. Of the studies, 8 (7 observational and 1 RCT) (16%;) were considered to be of high quality, 10 (8 observational and 2 RCTs) (20%) were of uncertain quality, and 32 (24 observational and 8 RCTs) (64%) were of low quality. The results for the quality assessment of the studies are shown in Supplementary File S2.
3.3 |. Prevention strategies in pregnant women
Supplementary excel Files S4–S8 summarize the results of all the 95 publications. The excel files contain, if available for each paper, the last name of the first author, publication year, study design, dietary exposure, dietary intake time, outcome type, offspring’s age at assessment, and every relevant point estimate reported with its associated 95% confidence intervals and p-values, as well as the relevant comparison of interest that each point estimate describes. Due to the heterogeneity between papers on the measurement of dietary intake, the analysis of the data, and the reporting of the results, data from observational studies were not pooled in meta-analyses.
3.4 |. Dietary intake
Dietary associations measured in observational studies depend on the validity of the measures used to study dietary intake.120 This important methodological factor has so far been omitted from past systematic reviews. Methods for measuring dietary intake differ greatly between studies. We have summarized the approaches for assessing dietary intake and adherence to study interventions used in the RCTs in Supplementary File S3. Of the 17 publications reporting on RCT outcomes, 8 papers 39,40,59–62,65,66 mentioned that they monitored adherence and 6 papers54,55,57,58,63,64 did not monitor intake. For the observational studies, instruments used to measure food or nutrient intake were validated food frequency questionnaires (FFQs),15,18,21,23,25–27,30,33,34,36,46,70,75–78,80–84,87–107,110,115,118 unvalidated FFQs,16,17,19,28,29,31,32,73,111–114,119 questionnaires,14,20,22,35,38,68,69,71,72,74,79,85,86,108,116 interviews,24 information obtained from medical records,109 3/5-day food records and/or 24-hour recalls and questionnaires37,117 These points are important to take into account when reading the data.
3.5 |. Asthma/wheeze outcomes
Early childhood wheeze may not lead to allergic asthma outcomes in later childhood.121,122 To distinguish between transient wheeze/asthma in infancy and early childhood, and later wheeze/asthma, which may be a stronger predictor of subsequent allergic asthma outcomes, we have separately reported observational study manuscripts on maternal diet and wheeze/asthma into those which considered offspring wheeze/asthma between 0 and <3 years and for offspring 3 years and older. For the meta-analysis, we have included all ages. Allergic outcomes and how these were measured are shown in supplementary File S3.
3.5.1 |. Randomized controlled trials
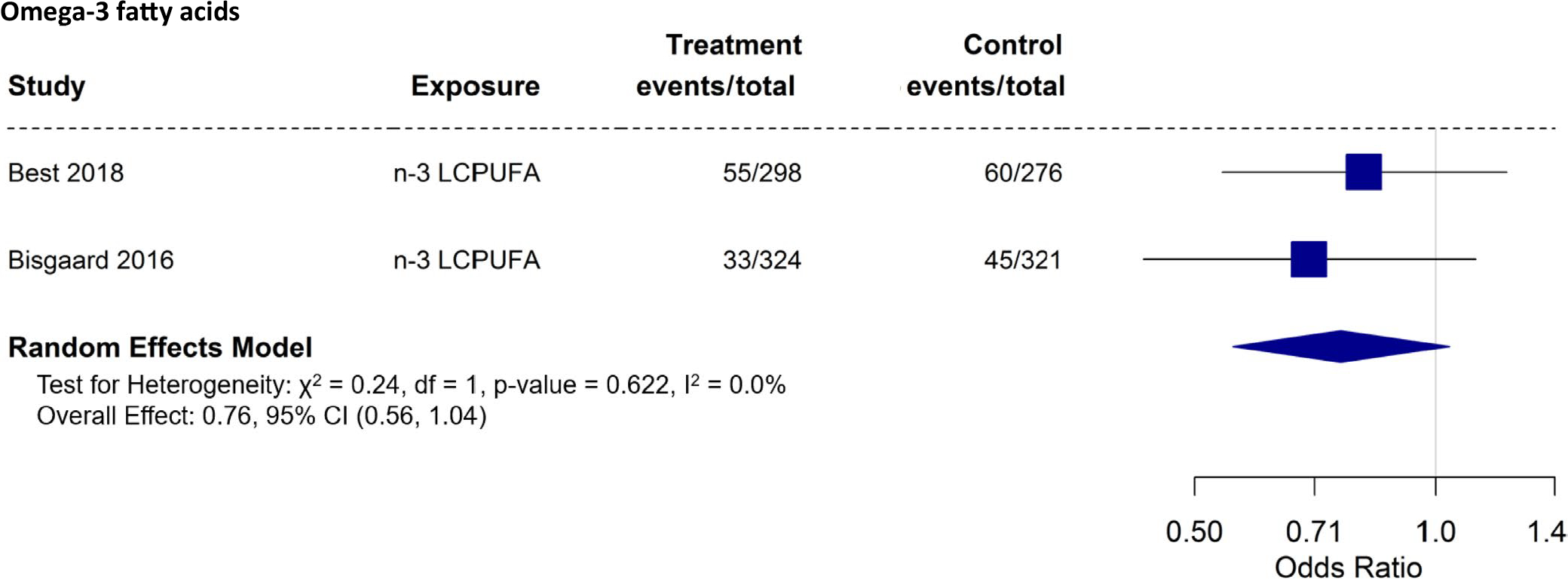
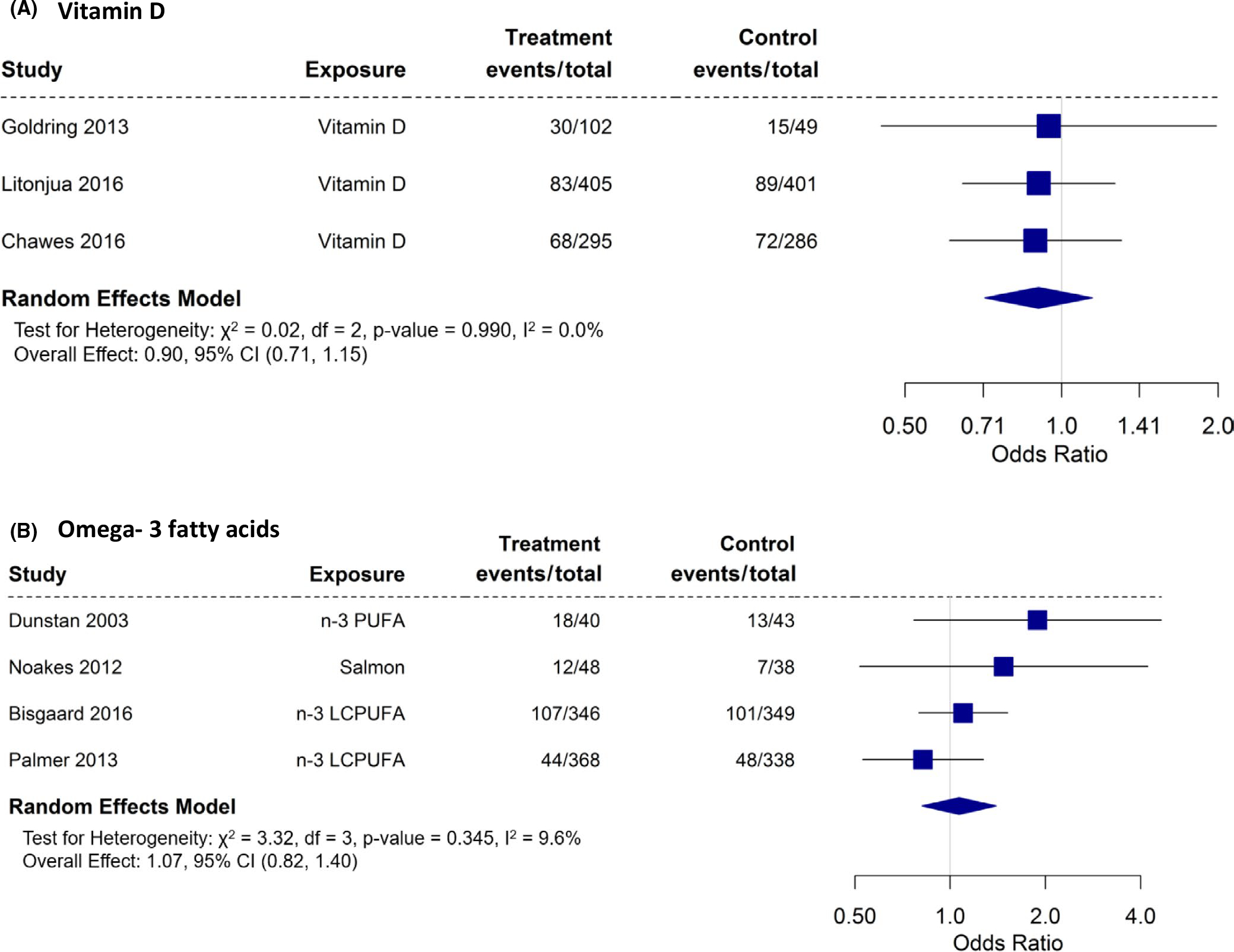
We had a limited number of studies to perform meta-analysis of antioxidant (vitamins C and E; 2 studies—children of 1–2 years),58,61 vitamin D (3 studies—children of 3 years old),55,57,60 and omega-3 fatty acid (4 studies; 5 papers; children of age 6m, 5 – 16 years) 62,63,66,67,72 intake or supplementation against wheeze/asthma outcomes in the offspring (Figure 2A–C). Our meta-analyses indicate that vitamin D during pregnancy had a statistically significant protective effect on wheeze/asthma in the offspring (OR: 0.72; 95% CI: 0.56 – 0.92). Although there was a trend toward a protective effect, maternal supplementation/intake during pregnancy with omega-3 fatty acid (OR: 0.70; 95% CI: 0.45–1.08) did not reach statistical significance on wheeze/asthma outcomes in the offspring. If we remove the study by Noakes et al who reported on wheeze in the first six months of life, significance is still not reached (OR: 0.63; 95% CI: 0.4–1.00—meta-analysis not shown). Using data from children at 1–2 years, anti-oxidant did not show a protective effect against asthma/wheeze (OR: 0.67; 95% CI: 0.29–1.56).
FIGURE 2.
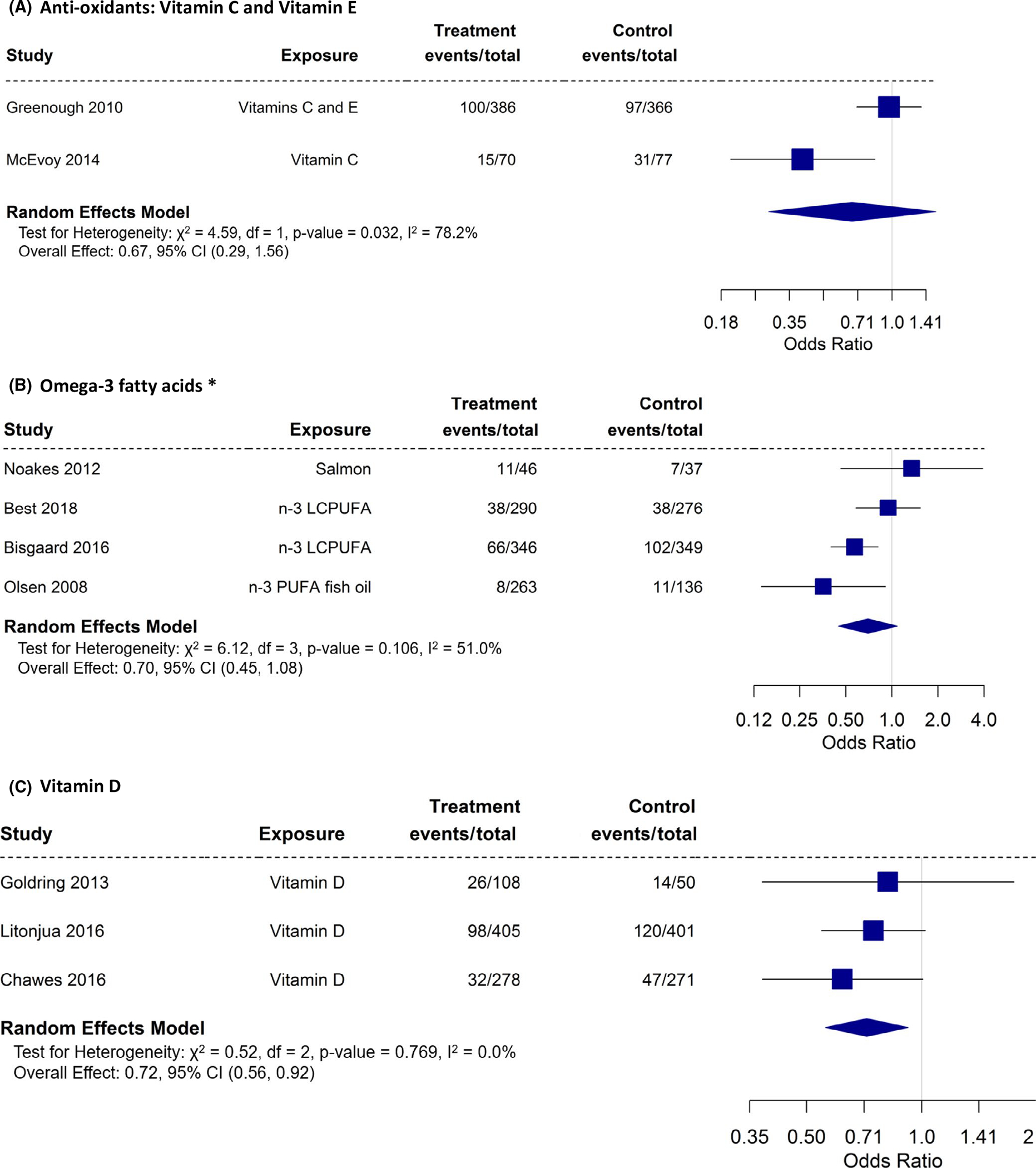
A-C, Randomized controlled trials with asthma/wheeze as an outcome
3.5.2 |. Observational studies
Many different outcomes have been reported as summarized in supplementary File S9. Sixty-two papers from observational studies14–16,19–26,28,30–33,35–38,46,68–70,74–80,82–93,95,96,98–101,103–111,113,117–119 reported on the association between maternal diet during pregnancy and asthma/wheeze as an outcome (Table 1; Figures 3A and B and 4A and B). We summarized all the significant outcomes reported in Figure 3 to give a visual presentation of the outcomes rather than making a statistical conclusion. Figure 4 indicates that a large number of foods/nutrients/food patterns were associated with an increased or reduced risk of asthma/wheeze in children 3 years and over. The most significant protective factors were foods associated with a Mediterranean diet, meat, vitamin D, and fatty fish. The most significant factors associated with an increased risk were pasta, fish sticks, and arachidonic acid. From the summarized data, no firm conclusion can be drawn in regard to the protective effect of nutrients/foods/food patterns studied on allergic disease. However, when considering asthma, reduced offspring allergic outcomes were associated with intake of copper, vitamin D, Mediterranean diet and Western diet, fish/fatty fish, tree nuts, fruit, and meat (type of meat not specified) in the observational studies included.
TABLE 1.
Correct ref numbers
| Food and dietary patterns | Asthma/wheeze | Hayfever/rhinitis | Eczema | Food allergy | Sensitization |
|---|---|---|---|---|---|
| Total | 62 papers14,16,19–26,28,30–33,35–38,46,68–70,74–80,82–93,95,96,98–101,103–111,113,117–119 | 19 papers15,16,18,21,24,25,31,32,46,70,90–93,103,104,106,107,113 | 38 papers15,16,19,20,24,26,29,31,37,68,70,72,76,77,81,82,84–88,90,94–101,103,104,107,108,112,113,116,118 | 5 papers34,71,77,115,118 | 19 papers16–18,24,27,31,68,73,75–77,82,86,102,110,112–114,118 |
| Fruit and/or vegetables (Supplementary excel file 6 Fruits and Vegetables) | 11 papers20,28,36,69,75,78,90,99,106,108 Fruit, vegetables, leafy vegetables, roots and potatoes, citrus fruits, Malaceous fruits, berries, fruit and berry juices, Green and yellow vegetables, Cruciferous vegetables, Folate vegetables, apples |
1 paper106 classified as fruits, vegetables, leafy and root vegetables, berries, malicious, citrus fruit and juices |
4 papers20,99,108,112 fruits, vegetables, citrus fruits, apples, green leafy vegetables, green and yellow vegetables, carrots, spinach, cabbage, celery, tomato, sweet pepper, salad, and juice |
1 papers115 fruit and berries, vegetables and roots |
3 papers17,102,112 fruits, vegetables, malicious fruits, citrus fruits, berries, apples, bananas, strawberry, exotic fruit, root vegetables, carrots, spinach, cabbage, celery, tomatoes, sweet pepper, salad, and fruit and vegetable juices |
| Fats and fatty fish (Supplementary excel file 5 Fish and Fats) Fat and fatty acid intake (4 papers), classified as total fat intake, omega 3 or omega 6 poly-unsaturated fatty acids or other types of fatty acids, and specific fatty acids (we also included fish intake and fish oil supplementation in this group); |
12 papers20,32,36,74,78,85,95,96,104–106,111 | 432,90,104,106 | 920,29,85,95–97,104,112,116 | 1 | 4 papers27,73,102,112 |
| Vitamin and mineral intake (Supplementary excel file 4 vitamins and minerals) | 33 papers14,15,22,23,25,26,28,30,33,35,36,38,68,77,79,83,84,86–89,91,98–100,107,110,117–119 multivitamin, pre-natal vitamins, folic acid, vitamin A, vitamin C, vitamin D, vitamin E, vitamin K,B-vitaminsand their precursors, zinc, calcium, selenium, copper, magnesium, and manganese |
6 papers15,18,25,103,107 folate, riboflavin, retinol, vitamin A, vitamin D, vitamin E, vitamin K, and their precursors, zinc, copper, selenium, magnesium, calcium, manganese |
14 papers15,26,68,77,84,87,88,98–100,103,107,116,118 vitamins A, C, E, and their precursors, vitamin D, vitamin B and folic acid, mineral intake zinc, calcium, copper, magnesium, manganese and selenium |
4 papers71,77,115,118 antioxidant vitamins C, E, and B-carotene, vitamin D and folic acid, mineral intake, copper and zinc |
9 papers17,18,68,77,86,102,110,114,118 Vitamin A, Vitamin C, Vitamin D, Vitamin E, Folic acids, Prenatal vitamins, Multivitamins, Anti-oxidants and their precursors, Zinc, Selenium |
| Other dietary exposures (Supplementary excel file 7 other foods) | 25 papers16,19,20,26,31,32,37,46,69,70,75,76,80,85,92,93,95,96,98,105,106,108,109,111,117 sugar, milk/dairy products, grains (pasta), nuts, meat, fish, chocolate candy, soft drinks/artificial sweeteners, egg, alcohol, glucose and dietary water |
8 papers16,31,32,46,70,92,93,106 sugar, milk/dairy products, cereals/grains, fish, seafood, meat, chocolate candy, nuts, soft drinks/artificial sweetener, alcohol. |
22 papers16,19,20,26,29,31,37,70,72,76,81,85,90,94–98,108,112,116 alcohol, probiotic drinks, fast foods, meat, dairy, cereals, fish and shellfish, chocolate, cheese, natto, eggs, cream, seeds and nuts. |
1 papers115 Cereal and dairy |
7 papers16,27,31,73,76,102,112 sugar, fish, nuts, legumes, seeds, mild/dairy products, cereals/grains, egg, chocolate candy, juices and alcohol |
| Food patterns (Supplementary excel file 8 dietary patterns) | 8 papers20,21,24,74,75,82,101,113 Mediterranean, Health conscious, Vegetarian, Prudent, Western, Confectionery, Traditional, Japanese, Vegetable, Fruit and white Rice, Seafood and Noodles Pasta, Cheese and Processed meat, Alternative Healthy Eating index |
3 papers21,24,113 Mediterranean, Health conscious, Traditional, Processed, Vegetarian, Confectionery, Japanese, Vegetable, Fruit and white Rice, Seafood and Noodles Pasta, Cheese and Processed meat Vegetable, Fruit and white Rice as well as the Alternative Healthy Eating index |
520,24,82,101,113 Mediterranean, Health conscious, Vegetarian, Prudent, Western, Confectionery, Traditional, Japanese, Vegetable, Fruit and white Rice, Seafood and Noodles Pasta, Cheese and Processed meat Vegetable, Fruit and white Rice (VFR) as well as the Alternative Healthy Eating index during pregnancy |
0 | 5 papers17,24,27,75,82,102,112,113 Mediterranean diet, Alternate Healthy Eating Index modified for Pregnancy score, Prudent diet, Western diet, Vegetable, Fruit and white Rice, Seafood and Noodles, Pasta, Cheese and Processed meat, Vegetable, Fruit and white Rice, Seafood and Noodles, Pasta, Cheese and Processed meat, Healthy Conscious, Traditional, Confectionery, Traditional and Vegetarian |
FIGURE 3.
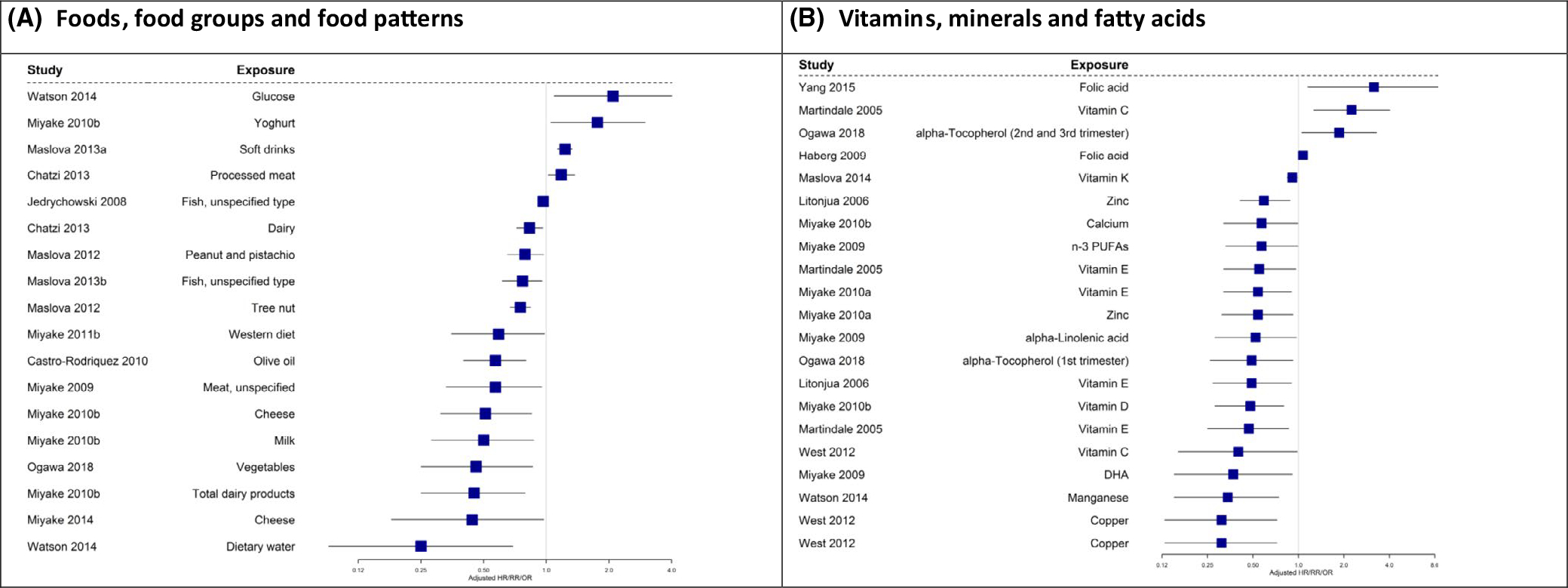
A and B, Observational studies with asthma/wheeze (age 0-<3 y) as an outcome
FIGURE 4.
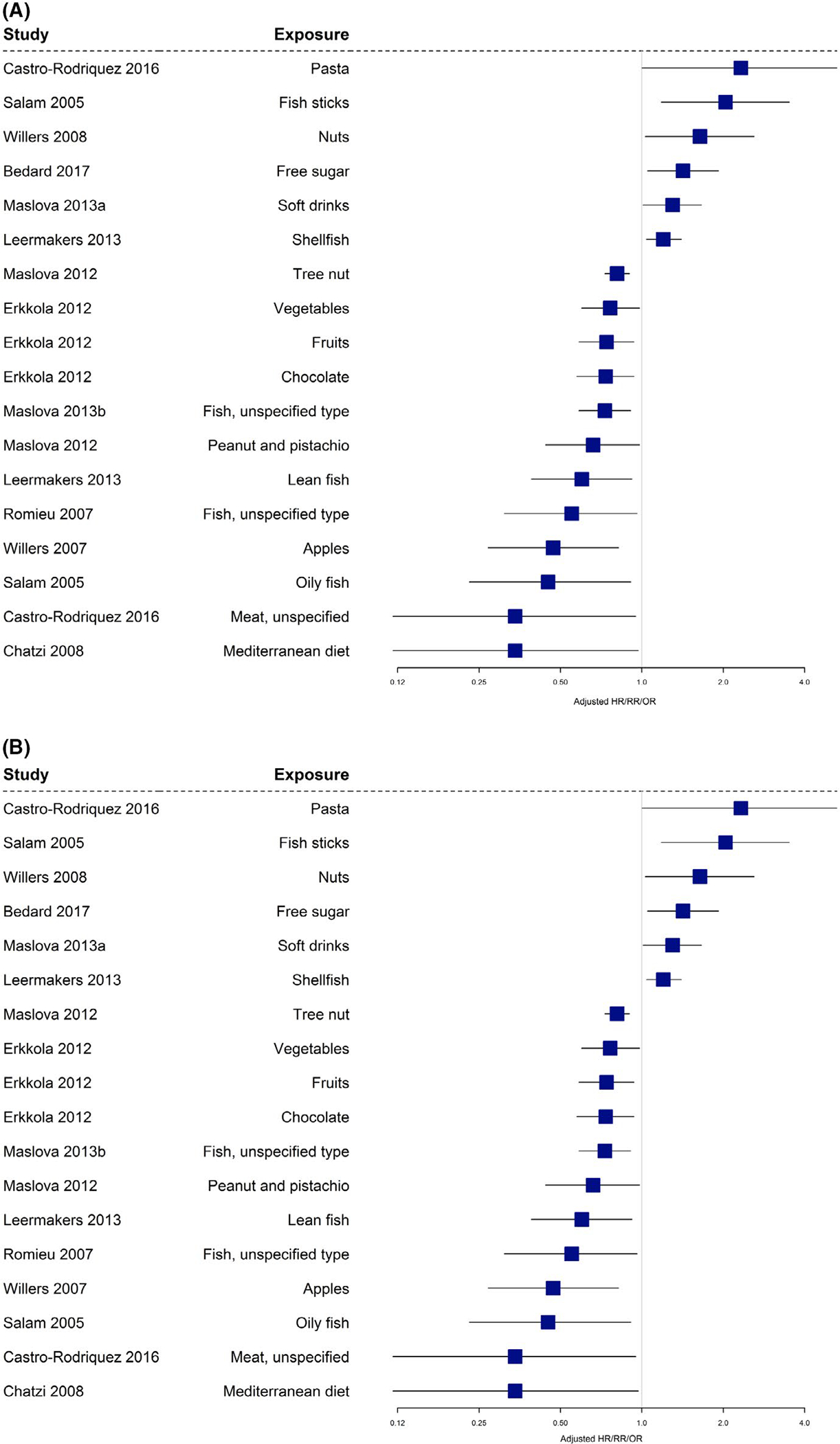
A and B, Observational studies with asthma/wheeze (age 3 y and above) as an outcome
3.6 |. Allergic rhinitis outcomes
Figures 5 and 6 summarize allergic rhinitis outcomes in the offspring for both observational and randomized controlled studies, respectively. All observational studies with significant outcomes are shown in Figure 6A and B. The studies shown in both these figures included results for offspring aged 3 years and above.
FIGURE 5.
Randomized controlled trials with allergic rhinitis/hay fever as an outcome
FIGURE 6.
A and B, Observational studies with allergic rhinitis/hay fever as an outcome
3.6.1 |. Randomized controlled trials
We were able to perform meta-analysis of omega-3 fatty acid supplementation (2 studies, 3 papers—all children over 5 years) 39,54,66,67 against allergic rhinitis outcomes in the offspring (Figure 5). Although we found a potential protective effect based on the meta-analysis combining two studies of omega-3 supplementation during pregnancy on allergic rhinitis in the offspring, the effect showed a trend toward statistical significance (OR: 0.76; 95% CI: 0.56–1.04).
3.6.2 |. Observational studies
Nineteen papers from observational studies15,16,18,21,24,25,31,32,46,70,90–93,103,104,106,107,113 reported an association between maternal diet during pregnancy and offspring allergic rhinitis/rhinoconjunctivitis as an outcome. Different studies reported different terms to describe outcomes and definitions (ie, some studies referred to ever suffering from hay fever and others reported on current allergic rhinitis) were not always similar, though the same disease group was being studied. The results are described and summarized in both Table 1 and Figure 6.
Figure 6A and B indicates that vitamin D, vitamin A, fatty fish, polyunsaturated fatty acids (PUFA), alpha-linolenic acid, peanut, pistachio, and probiotic milk products were associated with reduced risk of allergic rhinitis. Fish (unspecified type), the ratio of omega-6 to omega-3 fatty acids, butter, and fruit were all associated with increased risk of allergic rhinitis. As above, due to heterogeneity between studies, these data were summarized narratively and not pooled for meta-analysis. Vitamin A, vitamin D, dairy, nuts, and probiotic-containing foods showed associations with reduced allergic rhinitis outcomes.
3.7 |. Eczema outcomes
Eczema usually develops in infancy123 prior to food allergy and other allergic manifestations, making it a target for allergy prevention studies. Data presented in this systematic review include offspring data from 6 months onwards.
3.7.1 |. Randomized controlled trials
We were able to perform meta-analysis on the effects of vitamin D55,57,60 (3 studies) and omega-3 fatty acid intake62 or supplementation39,53,54,56,65–67 (4 studies, 8 papers; 4 used in meta-analysis) on offspring eczema outcomes (Figure 7A,B). None of the dietary exposures during pregnancy had a significant effect on offspring eczema (vitamin D [OR: 0.90; 95% CI: 0.71 – 1.15] and omega-3 [OR: 1.07; 95% CI: 0.82–1.40]).
FIGURE 7.
A and B, Randomized controlled trials with atopic dermatitis as an outcome
3.7.2 |. Observational studies
Thirty-eight papers from observational studies15,16,19,20,24,26,29,31,37,68,70,72,76,77,81,82,84–88,90,94–101,103,104,107,108,112,113,116,118 reported the association between maternal diet during pregnancy and eczema in their offspring (Table 1; Figure 8). Due to heterogeneity between studies, these data were not pooled for meta-analysis.
FIGURE 8.
A and B, Observational studies with atopic dermatitis as an outcome
Figure 8A and B summarize the significant associations published between various components of maternal diet during pregnancy and offspring eczema that were reported from observational studies. In terms of vitamins and minerals, studies presented significant associations for a decreased risk of offspring eczema with maternal intake of beta-carotene, vitamin E, zinc, calcium, magnesium, and copper during pregnancy. Paradoxically, studies showed both positive and protective associations between maternal intake of vitamins C and D and offspring eczema. Intake of fatty fish, margarine, vegetable oil, total fat, omega-6 fatty acid, omega-3/omega-6 ratio, linoleic acid, alpha-linoleic acid, natto, and MUFAs all was positively associated with offspring risk of eczema. Cholesterol and arachidonic acid showed a decreased association with eczema. Looking at other foods, fast foods, shellfish, alcohol, and meat (not defined) were associated with an increased risk of offspring dermatitis. Maternal intake of milk, fish, vegetables, and apples was associated with a reduced risk of developing AD. Intake of copper, vitamin E, calcium, zinc, beta-carotene, magnesium, dairy/probiotic foods, vegetables, and fruit was associated with reduced eczema outcomes.
3.8 |. Food allergy outcomes
3.8.1 |. Randomized controlled trials
There were two (three papers)56,65,66 RCTs that studied the effect of maternal intake of omega-3 fatty acid during pregnancy and food allergy outcomes in the offspring. The meta-analysis is shown in Figure 9, revealing that maternal intake/supplementation of omega-3 fatty acids during pregnancy had no significant effect on food allergy in offspring (OR: 1.18; 95% CI: 0.56 – 2.46).
FIGURE 9.
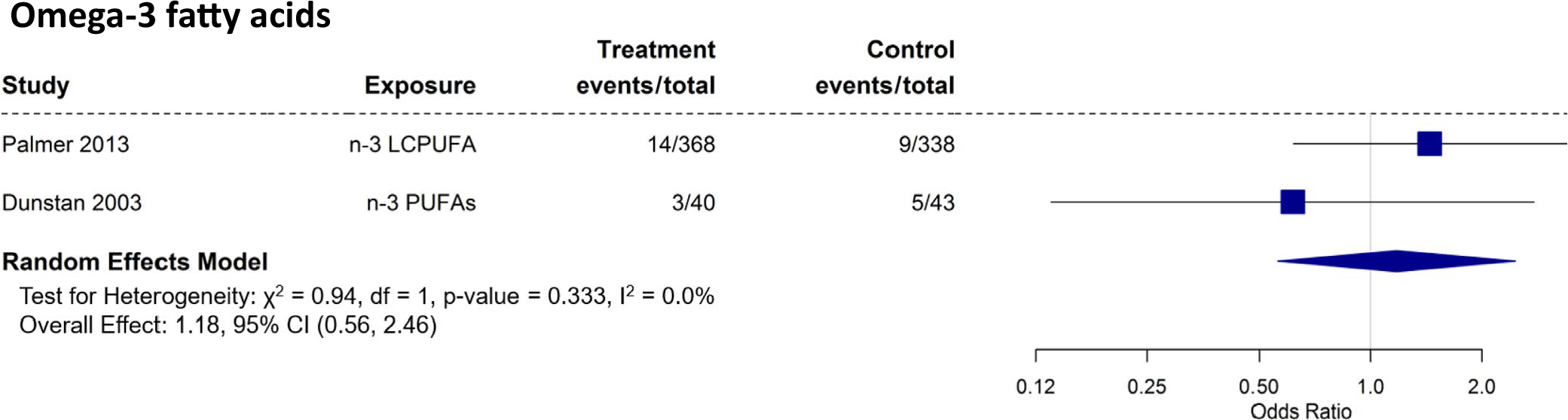
Randomized controlled trials with food allergy as an outcome
3.8.2 |. Observational studies
Five publications from observational studies34,71,77,115,118 discussed associations between maternal diet during pregnancy and food allergy in their offspring (Table 1). Figure 10 summarizes significant results from observational studies regarding the association between various components of maternal diet during pregnancy and food allergy in offspring. The studies reported protective associations between maternal intake of copper and vitamin C and risk of food allergy outcomes in the offspring. Maternal intake of vegetables and vitamin D was associated with increased risk of food allergy in the offspring.
FIGURE 10.
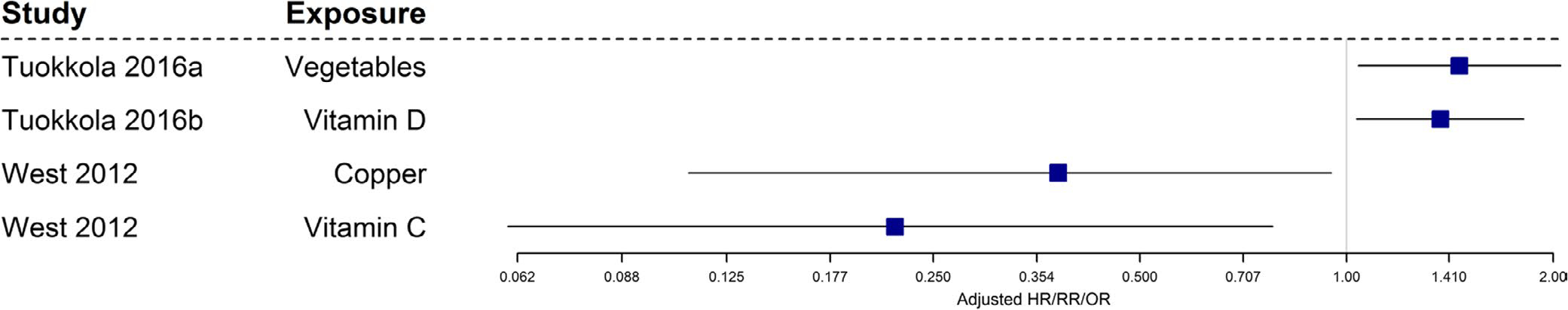
Observational studies with food allergy as an outcome
3.9 |. Allergic sensitization outcomes
Allergic sensitization in the included studies measured both allergic sensitization to food and/or aero-allergens, using skin prick test (SPT) and/or specific IgE levels. Different outcome measures were used, including increased total IgE, a range of specific IgE or SPT to foods and aero-allergens, and amalgamation using the term atopy to indicate any level of allergic sensitization.
3.9.1 |. Randomized controlled studies
Three55,57,60 RCTs reported results on the effects of vitamin D on allergic sensitization in the offspring, and five reported on the effects of omega-3 fatty acid intake62 or supplementation39,54,56,65–67 on the same outcome. The meta-analysis is shown in Figure 11A, B. The meta-analyses showed no effects for either maternal intake of vitamin D (OR: 0.91; 95% CI: 0.68 – 1.21) or intake/supplementation of omega-3 fatty acid (OR: 01.02; 95% CI: 0.77 – 1.36) during pregnancy with offspring allergic sensitization outcomes.
FIGURE 11.
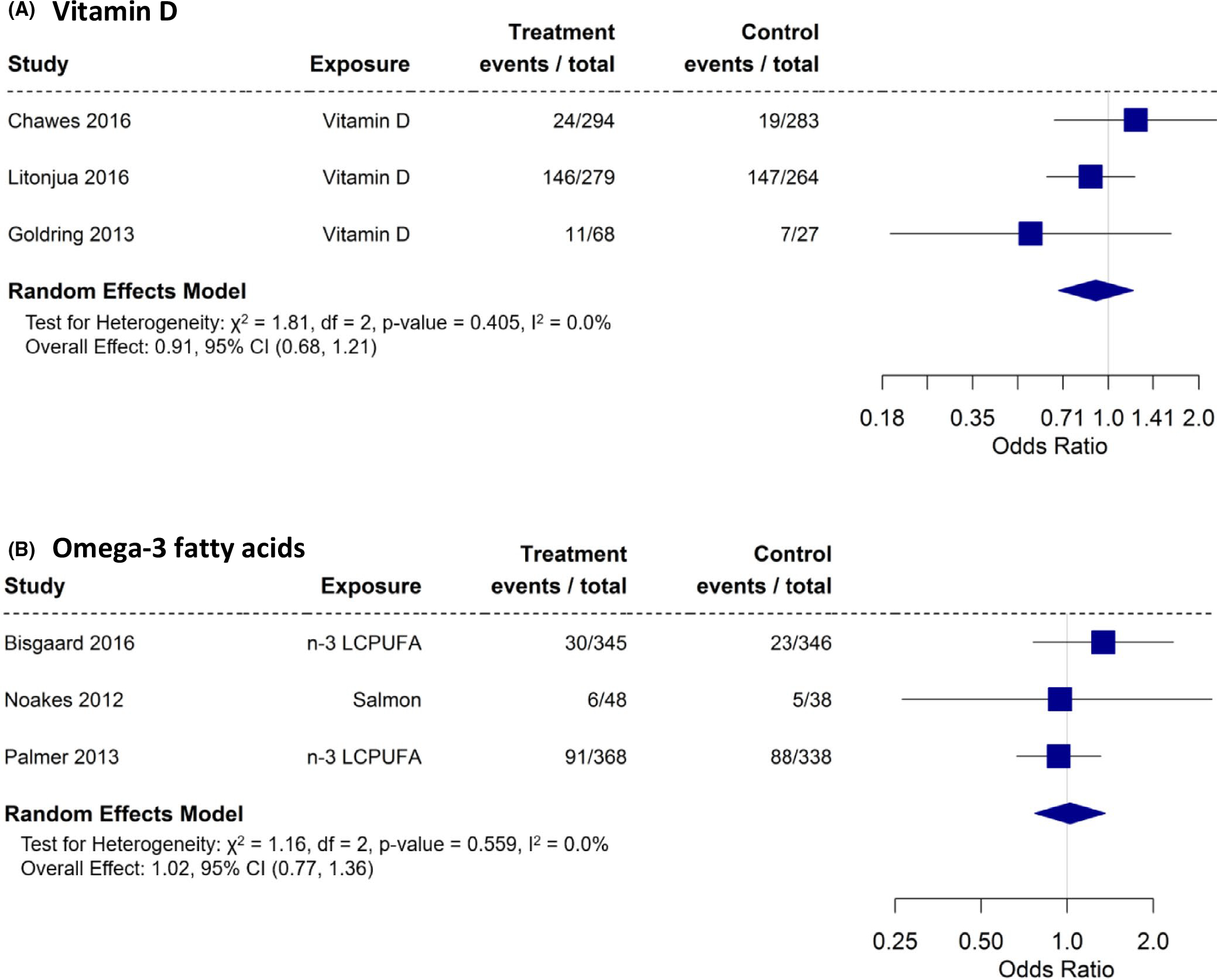
A and B, Randomized controlled trials with “any” sensitization as an outcome
3.9.2 |. Observational studies
Nineteen papers from observational studies 16–18,24,27,31,68,73,75–77,82,86,102,110,112–114,118 studied the association between maternal diet during pregnancy and allergic sensitization as an outcome.
Data shown in Figure 12 and B and summarized in Table 1 indicate that vitamin C, vitamin D, copper, omega-6 PUFAs, PUFA, butter, fish, the Mediterranean diet, and the seafood and noodle diet pattern were associated with reduced allergic sensitization. Fruit, celery, sweet pepper, free sugar, fatty fish, vegetable fat, and vegetarian and health-conscious diet were associated with increased prevalence of allergic sensitization. Intake of vitamin D, copper, vitamin C, and diet containing soy, fish, and nuts was associated with reduced prevalence of sensitization.
FIGURE 12.
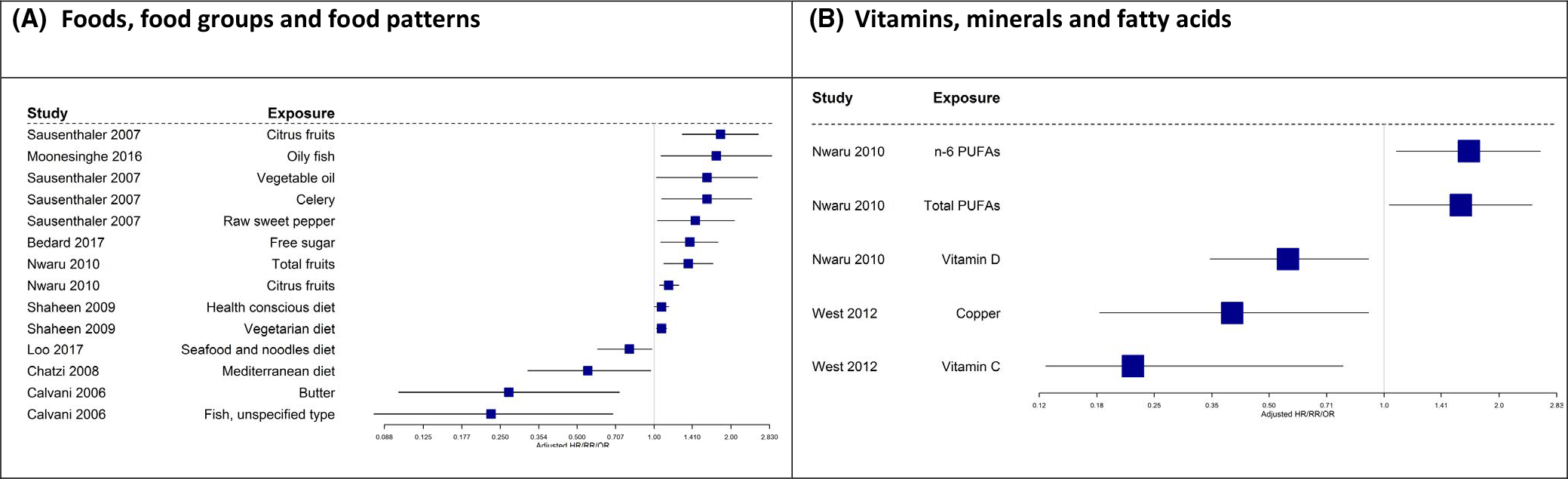
A and B, Observational studies with “any” sensitization as an outcome
3.10 |. Dietary intake and its association with allergy outcomes
Where data allowed, we summarized the dose of nutrients/food intake associated with allergy outcomes in observational studies (Tables 2 and 3). We find no clear association between dose of nutrient/food required to reduce or increase any specific allergy outcomes. Of note is that the dose/amount showing an association frequently does not comply with national dietary recommendations from the United States.124 The following associations between diet pattern, food and/or nutrient intake and allergic outcomes was reported in the observational studies: Vitamin A (allergic rhinitis), vitamin D (asthma, allergic rhinitis, and sensitization), copper (food allergy, eczema, and allergic sensitization), vitamin C (food allergy and allergic sensitization), vitamin E (eczema and asthma), calcium (eczema), zinc (eczema and asthma), beta-carotene (eczema), magnesium (eczema), dairy/probiotic-containing foods (eczema and allergic rhinitis), Mediterranean diet and Western diet (asthma/ wheeze), Mediterranean and soy/fish/nuts diet (allergic sensitiza- tion), fruit and vegetables (asthma and eczema), fish (sensitization), nuts (asthma and allergic rhinitis), and meat (asthma). We suggest that researchers use nutrition guidelines to set predetermined cutoffs for food intake to (a) compare outcomes in observational studies and (b) determine dose given during RCTs. This will help ensure safe dietary interventions and will also allow comparison of future studies.
TABLE 2.
Comparison of food and nutrient (where available) against RDA or AI
| Wheeze/asthma |
||||
|---|---|---|---|---|
| Food/nutrient intake | Referencea | Intake | RDI or AI | % RDI or AI |
| Alpha-tocopherol28 | Increase | 6.5 mg | 15 mg | 43% |
| Calcium98 | Reduce | 571.1 mg | 1000 mg | 57.1% |
| Folate30 | Increase | >578 | 600 μg | >96.3% |
| Vitamin D15 | Reduce | 4.69–35 μg | 15 μg | 31.2%–233% |
| Vitamin D15 | Reduce | 3.51–4.68 μg | 15 μg | 23.4%–31.2% |
| Vitamin D84 | Reduce | 724 IU (18.1 μg) | 15 μg | 120% |
| Vitamin D23 | Reduce | 4.86–17 | 15 μg | 32.4%–113% |
| Vitamin D23 | Reduce | 3.44–4.85 | 15 μg | 22.9–32.3 |
| Vitamin D91 | Increase | 8.2, 13, 16.5 | 15 μg | 54.7%, 86.7%, 110% |
| Vitamin D98 | Reduce | 5.1, 6.4 | 15 μg | 34%, 42.7% |
| Vitamin E15 | Reduce | 6.22–7.08 mg | 15 mg | 41.5%, 47.2% |
| Vitamin E88 | Reduce | 7.07–30.30 | 15 mg | 47.1%–202% |
| Vitamin E23 | Reduce | 10.3–29.4 | 15 mg | 68.7%–98% |
| Vitamin E99 | Reduce | 7.3 mg | 15 mg | 48.6% |
| Zinc88 | Reduce | 14.25–30.30 mg | 11 mg | 126%–268% |
| Zinc99 | Reduce | 7, 8.5 mg | 11 mg | 61.9%–75.2% |
| Omega 6105 | Reduce | <8.03 g | 13 g | 61.8% |
| Alpha-Linolenic acid105 | Increase | <7.78 g | 13 g | 58.9% |
| N-3 poly-unsaturated fatty acid105 | Increase | <2.24 g | 1.4 g | 160% |
| n-3 poly-unsaturated fatty acid96 | Reduce | 2.2 g | 1.4 g | 157% |
| Alpha-Linolenic96 | Reduce | 2.3 g | 1.4 g | 164% |
| Vegetables106 | Increase | <149.1 | 375 g | 39.7% |
| Fruit106 | Increase | 326.2 | 250 g | 130.5% |
| Vegetables28 | Reduced | 206–286 g/d | 375 g | 54.9%–76.3% |
| Free sugar16 | Increase | 82.4–345.1 g/d | <12 g | 687%–2875.8% |
| Meat96 | Reduce | 63.6 g/d | 71 g | 89.6% |
| Dairy products98 | Reduce | 280.7 g/d | 250 g | 115.% |
| Allergic rhinitis | ||||
| Vitamin D - food107 | Reduce | 4.4–5.72 mg | 15 μg | 29%–38.1% |
| Alpha-linolenic acid104 | Reduce | 6.4–8.9 g | 1.4 g | 457%–635.7% |
| Fruit106 | Increase | 145.7–1700 g/d | 250 g | 58.3%–680% |
| Atopic dermatitis | ||||
| Beta-carotene99 | Reduce | 1923.9 and 4218.0 mg | 770 μg | 41.5% and 91.3% |
| Vitamin D26 | Increase | 8.6 μg/d | 15 μg | 57% |
| Vitamin E15 | Reduce | 2.32–2.9 mg | 15 mg | 15%–0.19% |
| Vitamin E99 | Reduce | 86.7 mg/d | 15 mg | 578% |
| Zinc88 | Reduce | 10.81, 13.4, 22.27 mg/d | 11 mg | 95%, 117% 194% |
| Calcium26 | Reduce | 677.6 mg/d | 1000 mg | 67.8% |
| Omega - 696 | Increase | 14.1 | 13 g | 107% |
| Linolenic acid96 | Increase | 10.4–11.3 g | 1.4 g | 743%–807% |
| Omega 697 | Increase | 10.6 g/d | 13 g/d | 81.5% |
| Total fat97 | Increase | 58.6/d | 80 g/d (range 53–93 g) | 3.25% |
| Alpha Linolenic acid97 | Increase | 1.9 | 1.4 g/d | 136% |
| Vegetables99 | Reduce | Total Veg 144.4 g | 3 × 125 g | 39% |
| Total dairy products98 | Reduce | 120.8 g/d | 500 ml/g/d | 24% |
| Total dairy products26 | Reduce | 255.3 g/d | 500 ml/g/d | 50% |
| Total fish90 | Reduce | ≥1×/wk vs no intake | 2–3 times per week | 33%–50% |
| Food allergy | ||||
| Vitamin C118 | Increase | 102–201 (mg/d)2 | 85 mg | 120%–236% |
| Copper118 | Increase | >2.6 (mg/d)2 | 1 mg | 260% |
| Vegetable115 | Reduce | >324 g/d (21/2 cups) vs 153–324 g/d (1–2.5 cups) | 3 cups | 75% to >100% |
| Cereal115 | Reduce | Both having less than 143 g/d (5 × 1 oz) and more than 230 g/d (8 × 1 oz) | 240 g | Decrease if intake is <60% and >95% |
| Sensitization | ||||
| Vitamin C118 | Increase | 130 | 85 mg | 216.7% |
| Copper118 | Increase | 1.6 | 1 g | 160% |
| Free sugar16 | Increase | 82.4–345.1 g/d | <12 g | 687%–2875.8% |
Note: Italic = below recommended intake; bold = above recommended intake; normal – stretch across lower than higher than recommended intake.
Listing only significant OR/RR/HR.
TABLE 3.
Summary table for systematic review
|
Decreased
|
Increased
|
|||||||
| Observational trials | Vit/Min | Fats/fatty fish | Foods | Diet Pattern | Vit/Min | Fats/fatty fish | Foods | Diet Pattern |
| Food allergy |
Copper Vitamin C |
NA | NA | NA | Vitamin D | NA | Vegetables | NA |
| Atopic dermatitis |
Vitamin E
Copper Calcium Zinc Beta-carotene Magnesium |
Cholesterol Arachidonic acid |
Green vegetables Dairy Fruit (apples) Fish Probiotic milk |
NA | Vitamin D Vitamin C |
Vegetable oil Fatty fish Margarine Linoleic acid Omega-6 fatty acid Ratio of omega-3/omega-6 Alpha-linolenic acid Total fat Monounsaturated fatty acids |
Alcohol Shellfish Meat Fast food Natto |
NA |
| Asthma/wheeze |
Vitamin E Zinc Copper Manganese Folic acid Vitamin D Vitamin C Alpha-tocopherol Calcium Vitamin K |
DHA Fatty fish Palmitic acid Arachidonic acid Alpha-linolenic acid Saturated fatty acid Olive oil Omega-3 fatty acid |
Vegetables Fruit (apples) |
Mediterranean
Western |
Folic acid Vitamin D Vitamin A Vitamin K Alpha-tocopherol Vitamin C |
Omega-3 fatty acid Alpha-linolenic acid |
Fruit Vegetables |
NA |
| Rhinoconjunctivitis | Vitamin D Vitamin A |
Fatty fish Polyunsaturated fatty acid Alpha-linolenic acid |
Alcohol Fish Probiotic foods Peanut and pistachio |
NA | Vitamin D | Butter Omega-6/omega-3 ratio |
Fruit | NA |
| Sensitization |
Vitamin C Copper Vitamin D |
Butter Omega-6 fatty acids Polyunsaturated fatty acid |
fish |
Mediterranean
Soy, Fish, nuts |
NA | Vegetable fat Oily fish |
Fruit Vegetable (celery and sweet pepper) Free sugar |
Health-conscious Vegetarian |
| Decreased | Increased | |||||||
| RCTs | Vit/Min | Fats/fatty fish | Foods | Diet Pattern | Vit/Min | Fats/fatty fish | Foods | Diet Pattern |
| Food allergy | NA | No effect | NA | NA | NA | No effect | NA | NA |
| Atopic dermatitis | No effect | No effect | NA | NA | No effect | No effect | NA | NA |
| Asthma/wheeze | Vitamin D | No effect | NA | NA | NA | No effect | NA | NA |
| Rhinoconjunctivitis | NA | No effect | NA | NA | NA | NA | NA | NA |
| Sensitization | No effect | No effect | NA | NA | No effect | No effect | NA | NA |
Note: Italic = only associated with reduced outcomes.
4 |. DISCUSSION
4.1 |. Summary of findings
We find insufficient evidence to provide guidance on diet diversity (no published reports), diet patterns, diet indices or specific foods, food groups, and macro- or micronutrients that should be consumed or avoided during pregnancy for the prevention of allergic diseases in the offspring. However, based on 3 RCTs, we did find that intake of vitamin D, higher than EFSA and IOM recommendations, was significantly associated with a reduced risk of wheeze/asthma. This needs to be further studied before guidelines are changed, particularly as intake of vitamin D in both lactation125 and early life is associated with increased food allergy in the offspring.
4.2 |. Strengths and limitations
We included the most up-to-date studies on dietary intake during pregnancy and prevention of allergic disease in their infants. Studies from Europe, North America, Asia, and Australia were included. We used the stringent standard employed by Garcia-Larsen et al13 to conduct the search and perform the risk of bias assessment and data extraction. We brought together a panel of international experts to review the data and conclusions of this review.
The review was limited by the quality of studies included. Some 64% of the studies included were deemed to be of low quality. The pool of data is also relatively small with 50 studies contributing to 95 papers. The observational studies were highly heterogeneous and were therefore not considered to be suitable for meta-analysis. There is a paucity of RCTs in this field; only four meta-analyses were performed using data from RCTs. Finally, none of the studies looked at diet diversity during pregnancy, this may be partly due to the uncertainties in defining diet diversity, which has now been addressed in an EAACI position paper.9
4.3 |. Comparison of findings to previous studies
The systematic review by Vadhaninia et al126 based on RCTs assessed the impact of omega-3 fatty acid supplementation solely in pregnancy and found that based on pooled estimates, omega-3 fatty acid intake significantly reduces allergic sensitization to egg and peanut in the offspring. We did not find a significant effect on any allergic sensitization outcome. The discrepancy may be attributed to the fact that one of the studies included by Vadhaninia et al126 was excluded from our review, as maternal supplementation continued up to 2.5 months post-delivery.127 Our findings are also different from the conclusions drawn by Garcia-Larsen et al.13 The difference probably lies in the choice of studies included in each systematic review. Garcia-Larsen et al13 included studies in pregnancy, lactation, and early life. We considered studies in pregnancy alone. It is therefore possible that supplementation with omega-3 fatty acid, which continues during breastfeeding and early life, may be more effective than just during pregnancy alone to prevent allergic outcomes in the offspring.
In terms of probiotic supplementation, published literature suggests that probiotic supplementation during late pregnancy, breastfeeding, and early life13,43,128–135 may be associated with reduced risk of the development of eczema. Substantial uncertainties remain regarding the specific strain required and the optimal timing of supplementation. We were unable to find any RCTs using probiotic supplementation in pregnancy only. We did find only one observational study that examined the association between maternal intake of probiotics and offspring allergy, but the authors found no association with allergic outcomes.106
We were able to demonstrate that vitamin D supplementation in pregnancy may reduce the risk of wheeze/asthma.55,57,60 Vadhadania et al136 also concluded that prenatal supplementation of vitamin D might be associated with reduced odds of recurrent wheezing in children. We were unable to find any RCT on folic acid supplementation in pregnancy only and observational studies provided conflicting evidence.14,22,28,30,33,35,38,68,71,77,79,83,100,103,114,115,119 There is lack of evidence on the effect of other vitamins for the prevention of respiratory and/or allergic outcomes.
Many systematic reviews have made recommendations, which relate to maternal diet in pregnancy and allergy prevention in the offspring. The review by Lodge et al137 did not identify any nutritional factors during pregnancy that reduced the risk of food allergy in the offspring. Four reviews recommended omega-3 fatty acid intake in pregnancy, vitamins A, D, and E; zinc; fruits and vegetables; and a Mediterranean diet for the prevention of asthma.43,137–140 Reviews focusing on allergy prevention in general concluded that pregnant women should consume their normal diet and that maternal intake of Mediterranean dietary patterns, diets rich in fruits and vegetables, fish, and vitamin D-containing foods may be associated with reduction of offspring allergic disease.10,43,131,132,137,140–146 We were unable to make generalized recommendations from the current review. Another factor that needs to be considered is whether family, paternal, and/or specifically maternal history of allergy play a role in the outcomes measured.
Since the review was performed, one study has shown an association between Diet inflammatory index (DII) scores in pregnancy and childhood asthma outcomes over 10 years.147 Another study found an association with wheeze trajectories in the child, but not asthma up to 7.5 years of age148 indicating that perhaps, the inflammatory potential of the diet needs to be assessed when focusing on asthma and/or wheeze outcomes.
4.4 |. Interpretation of the evidence
The heterogeneity of the results from observational studies yields little guidance regarding changes in the maternal diet that may be associated with reduction in offspring allergic diseases. Several nutrients, foods and food patterns such as vitamin A, vitamin D, copper, vitamin C, vitamin E, calcium, zinc, beta-carotene, magnesium, dairy/probiotic-containing foods, Mediterranean diet and Western diet, Mediterranean and soy/fish/nuts diet, fruit and vegetables, fish, nuts, fish/fatty fish, and meat showed associations with reduced allergy outcomes in the observational studies. But amounts consumed do not relate to current nutrition guidelines. Yet, our meta-analysis of RCTs conducted in pregnancy does show some significant and replicable results. Randomized intervention trials in pregnancy show a preventative effect of vitamin D supplementation of 800,57 2400,55 or 400060 IU per day on wheeze/asthma; though, EFSA and the IOM recommend only 600 IU of vitamin D per day during pregnancy.
4.5 |. Clinical, policy, and research implications of the findings
The current systematic review does not lead to any changes in clinical practice or policies but clearly highlights the research need for more RCTs in this field, particularly focusing on diet patterns or total dietary intake. There may be a particular need to review vitamin D recommendations during pregnancy.
5 |. CONCLUSIONS
The lack of consistent results across the different studies presented in this review may largely be influenced by the lack of a standardized approach to supplementation in terms of dose and duration, disease definition/outcomes used, and individual host features that are difficult to compare across studies. Polymorphisms in genes associated with catabolism and utilization will influence nutrient requirements and function. GWAS-led prevention and intervention studies, including functional microbiome, immunologic, metabolomic, and lipidomic assessments, are required and will increase our understanding of the importance of specific nutrients in the natural course of allergies and asthma. It is likely that a custom-individual-tailored approach to nutrition, focusing on overall dietary intake which may include supplementation, is required to observe the optimal benefits that can potentially be derived from dietary factors in the prevention and treatment of allergies and asthma. Future research and clinical efforts should be focused on large, adequately powered human studies of rigorous methodological design. These studies should focus on identifying the key host characteristics (ie, genetics, environmental factors, microbiome, biochemical and inflammatory parameters, and functional clinical characterization) that influence responses, while also taking levels of nutrient intake, history of allergic diseases, and the composition of the total underlying diet and nutrient interactions into account. Improving our understanding of potential strategies to prevent the development of allergic diseases would significantly reduce morbidity, mortality, and costs from allergic diseases. The current evidence mainly focused on single dietary factors, and the data from observational studies were very heterogeneous. Meta-analysis based on 3 RCTs showed some benefits of vitamin D supplementation on asthma/wheeze, but doses used were higher than recommended by EFSA and the IOM. Before routine supplementation during pregnancy for the prevention of allergic disease can be advised, more studies are required.
Supplementary Material
Key Message.
The systematic review focused on diet during pregnancy only, as not all mothers breastfeed or introduce solids to their infants in a similar fashion. Thus, we hence provide here pregnancy-specific dietary recommendations. Based on RCTs, we found that prenatal supplementation with vitamin D in doses higher than recommended by most countries may have beneficial effects for prevention of asthma in the offspring. Doses used in RCTs were 800 IU, 2400 IU, and 4000 IU. The European Food Safety Authority (EFSA) (https://www.efsa.europa.eu/en/press/news/161028) and the Institute of Medicine (https://www.aafp.org/news/health-of-the-public/20101201iomrpt-vitdcal.html) recommend 600 IU per day for pregnant women. Summarizing the data from observational studies, we found that the following nutrients, foods, and diet patterns appear to be associated with reduced allergic outcomes: vitamin A (allergic rhinitis), vitamin D (asthma, allergic rhinitis, and sensitization), copper (food allergy, eczema, and allergic sensitization), vitamin C (food allergy and allergic sensitization), vitamin E (eczema and asthma), calcium (eczema), zinc (eczema and asthma), beta-carotene (eczema), magnesium (eczema), dairy/probiotic-containing foods (eczema and allergic rhinitis), Mediterranean and Western diet (asthma/wheeze), Mediterranean and soy/fish/nuts diet (allergic sensitization), fruit and vegetables (asthma and eczema), fish (sensitization), nuts (asthma and allergic rhinitis), fish/fatty fish (asthma), and meat (asthma). However, the doses of foods or nutrients studied were heterogeneous and we were unable to relate these to current nutrition guidelines. We found no consistent evidence regarding other dietary factors, perhaps due to differences in study design and host features such as nutrient intake/levels at the start of the study that were not considered. There is a need for confirmatory studies and RCTs beyond single nutrients.
Acknowledgments
CONFLICT OF INTEREST
Venter C provided educational material or reviewed educational materials for Abbott Laboratories, Danone, and Reckitt Benckiser. Agostoni C is an advisor for Ferrero and Dicofarm. Fleischer D is a consultant to Aquestive, Aravax, Genentech, Nasus, AllerGenis, Intrommune, and DOTS Technology Corp. He has provided educational material for Nutricia. He has the following organizational declarations: DBV Technologies—Clinical Medical Advisory Board; Food Allergy & Anaphylaxis Connection Team—Medical Advisory Board; Food Allergy Research & Education—Clinical Advisory Board; and National Peanut Board—Allergy Education Advisory Council. Greenhawt M is supported by grant #5K08HS024599–02 from the Agency for Healthcare Research and Quality; is an expert panel and coordinating committee member of the NIAID-sponsored Guidelines for Peanut Allergy Prevention; has served as a consultant for the Canadian Transportation Agency, Thermo Fisher, Intrommune, and Aimmune Therapeutics; is a member of physician/medical advisory boards for Aimmune Therapeutics, DBV Technologies, Sanofi Genzyme, Genentech, Nutricia, Kaleo Pharmaceutical, Nestle, Aquestive, Allergy Therapeutics, AllerGenis, Aravax, GlaxoSmithKline, Prota, and Monsanto; is a member of the scientific advisory council for the National Peanut Board; has received honorarium for lectures from Thermo Fisher, Aimmune, DBV, Before Brands, multiple state allergy societies, the American College of Allergy, Asthma, and Immunology, and the European Academy of Allergy and Clinical Immunology; is an associate editor for the Annals of Allergy, Asthma, and Immunology; and is a member of the Joint Task Force on Allergy Practice Parameters.Meyer R has grants from Danone, personal fees from Nutricia and Mead Johnson, and is an advisor to CoMiSS group from Nestle. Netting MJ has received honorarium to her institution for speaker fees from Nestle. Smith PK has provided lecture material and/or consultancy to Abbot, NNI, and Danone. He is a PI for DBV and has received investigator-initiated funding from Nestle. O’Mahony L has provided consultancy with Alimentary Health and research grant from GSK. The following authors have no conflict of interest: Vanderlinden L, Untersmayr E, Roberts G, Roduit C’ Palumbo MP, Palmer DJ, Nwaru BI, Muraro A, Maiorella A, Maslin K, Lunjani N, Groetch M, Glueck DH, Du Toit G, Ben-Abdallah M, and Arshad SH
Abbreviations:
- AD
Atopic dermatitis
- AI
Adequate intake
- FA
Food allergy
- PC
Prospective cohort study
- RDI
Recommended dietary intake
Footnotes
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section.
REFERENCES
- 1.Institute of Medicine. Food Allergies: Global Burden, Causes, Treatment, Prevention and Public Policy. National Academy of Sciences. http://www.nationalacademies.org/hmd/Activities/Nutrition/FoodAllergies.aspx. Published 2016. Updated 28 March 2017. Accessed March, 2017. [Google Scholar]
- 2.World Allergy Organization. World Allergy Organization White Book on Allergy. 2011: https://www.worldallergy.org/UserFiles/file/WAO-White-Book-on-Allergy_web.pdf
- 3.Pawankar RCG, Holgate ST, Lockey RF, Blaiss M. The WAO White Book on Allergy. 2013. https://www.worldallergy.org/UserFiles/file/WhiteBook2-2013-v8.pdf
- 4.Agache I, Akdis CA. Precision medicine and phenotypes, endotypes, genotypes, regiotypes, and theratypes of allergic diseases. J Clin Invest. 2019;130:1493–1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Muraro A, Dubois AEJ, DunnGalvin A, et al. EAACI Food Allergy and Anaphylaxis Guidelines. Food allergy health-related quality of life measures. Allergy. 2014;69(7):845–853. [DOI] [PubMed] [Google Scholar]
- 6.Dwyer-Lindgren L, Bertozzi-Villa A, Stubbs RW, et al. Trends and patterns of differences in chronic respiratory disease mortality among US counties, 1980–2014. JAMA. 2017;318(12):1136–1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Turner PJ, Gowland MH, Sharma V, et al. Increase in anaphylaxis-related hospitalizations but no increase in fatalities: an analysis of United Kingdom national anaphylaxis data, 1992–2012. J Allergy Clin Immunol. 2015;135(4):956–963.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Venter C, Greenhawt M, Meyer RW, et al. EAACI position paper on diet diversity in pregnancy, infancy and childhood: Novel concepts and implications for studies in allergy and asthma. Allergy. 2019;75(3):497–523. [DOI] [PubMed] [Google Scholar]
- 9.Venter C, Meyer RW, Nwaru BI, et al. EAACI position paper: Influence of dietary fatty acids on asthma, food allergy, and atopic dermatitis. Allergy. 2019;74(8):1429–1444. [DOI] [PubMed] [Google Scholar]
- 10.de Silva D, Geromi M, Halken S, et al. Primary prevention of food allergy in children and adults: systematic review. Allergy. 2014;69(5):581–589. [DOI] [PubMed] [Google Scholar]
- 11.de Silva D, Halken S, Singh C, et al. Preventing immediate-onset food allergy in infants, children and adults: Systematic review protocol. Pediatr Allergy Immunol. 2020;31(3):243–249. [DOI] [PubMed] [Google Scholar]
- 12.Venter C, Brown KR, Maslin K, Palmer DJ. Maternal dietary intake in pregnancy and lactation and allergic disease outcomes in offspring. Pediatr Allergy Immunol. 2017;28(2):135–143. [DOI] [PubMed] [Google Scholar]
- 13.Garcia-Larsen V, Ierodiakonou D, Jarrold K, et al. Diet during pregnancy and infancy and risk of allergic or autoimmune disease: A systematic review and meta-analysis. PLoS Medicine. 2018;15(2):e1002507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alfonso VH, Bandoli G, von Ehrenstein O, Ritz B. Early folic acid Supplement initiation and risk of adverse early childhood respiratory health: a population-based study. Matern Child Health J. 2018;22(1):111–119. [DOI] [PubMed] [Google Scholar]
- 15.Allan KM, Prabhu N, Craig LC, et al. Maternal vitamin D and E intakes during pregnancy are associated with asthma in children. Eur Respir J. 2015;45(4):1027–1036. [DOI] [PubMed] [Google Scholar]
- 16.Bedard A, Northstone K, Henderson AJ, Shaheen SO. Maternal intake of sugar during pregnancy and childhood respiratory and atopic outcomes. Eur Respir J. 2017;50(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bedard A, Northstone K, Holloway JW, Henderson AJ, Shaheen SO. Maternal dietary antioxidant intake in pregnancy and childhood respiratory and atopic outcomes: birth cohort study. Eur Respir J. 2018;52(2):1800507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bunyavanich S, Rifas-Shiman SL, Platts-Mills TA, et al. and childhood vitamin D exposure and their association with childhood allergic rhinitis and allergic sensitization. J Allergy Clin Immunol. 2016;137(4):1063–1070.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Castro-Rodriguez JA, Ramirez-Hernandez M, Padilla O, Pacheco-Gonzalez RM, Perez-Fernandez V, Garcia-Marcos L. Effect of foods and Mediterranean diet during pregnancy and first years of life on wheezing, rhinitis and dermatitis in preschoolers. Allergol Immunopathol. 2016;44(5):400–409. [DOI] [PubMed] [Google Scholar]
- 20.Chatzi L, Garcia R, Roumeliotaki T, et al. Mediterranean diet adherence during pregnancy and risk of wheeze and eczema in the first year of life: INMA (Spain) and RHEA (Greece) mother-child cohort studies. Br J Nutr. 2013;110(11):2058–2068. [DOI] [PubMed] [Google Scholar]
- 21.de Batlle J, Garcia-Aymerich J, Barraza-Villarreal A, Anto JM, Romieu I. Mediterranean diet is associated with reduced asthma and rhinitis in Mexican children. Allergy. 2008;63(10):1310–1316. [DOI] [PubMed] [Google Scholar]
- 22.den Dekker HT, Jaddoe VWV, Reiss IK, de Jongste JC, Duijts L. Maternal folic acid use during pregnancy, methylenetetrahydrofolate reductase gene polymorphism, and child’s lung function and asthma. Clin Exp Allergy. 2018;48(2):175–185. [DOI] [PubMed] [Google Scholar]
- 23.Devereux G, Craig L, Seaton A, Turner S. Maternal vitamin D and E intakes in pregnancy and asthma to age 15 years: a cohort study. Pediatr Pulmonol. 2019;54(1):11–19. [DOI] [PubMed] [Google Scholar]
- 24.Loo EXL, Ong L, Goh A, et al. Effect of Maternal Dietary Patterns during Pregnancy on Self-Reported Allergic Diseases in the First 3 Years of Life: Results from the GUSTO Study. Int Arch Allergy Immunol. 2017;173(2):105–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maslova E, Hansen S, Strom M, Halldorsson TI, Olsen SF. Maternal intake of vitamins A, E and K in pregnancy and child allergic disease: a longitudinal study from the Danish National Birth Cohort. Br J Nutr. 2014;111(6):1096–1108. [DOI] [PubMed] [Google Scholar]
- 26.Miyake Y, Tanaka K, Okubo H, Sasaki S, Arakawa M. Maternal consumption of dairy products, calcium, and vitamin D during pregnancy and infantile allergic disorders. Ann Allergy Asthma Immunol. 2014;113(1):82–87. [DOI] [PubMed] [Google Scholar]
- 27.Moonesinghe H, Patil VK, Dean T, et al. Association between healthy eating in pregnancy and allergic status of the offspring in childhood. Ann Allergy Asthma Immunol. 2016;116(2):163–165. [DOI] [PubMed] [Google Scholar]
- 28.Ogawa K, Morisaki N, Kobayashi M, et al. Maternal vegetable intake in early pregnancy and wheeze in offspring at the age of 2 years. Eur J Clin Nutr. 2018;72(5):761–771. [DOI] [PubMed] [Google Scholar]
- 29.Ozawa N, Shimojo N, Suzuki Y, et al. Maternal intake of Natto, a Japan’s traditional fermented soybean food, during pregnancy and the risk of eczema in Japanese babies. Allergol Int. 2014;63(2):261–266. [DOI] [PubMed] [Google Scholar]
- 30.Parr CL, Magnus MC, Karlstad O, et al. Maternal folate intake during pregnancy and childhood asthma in a population-based cohort. Am J Respir Crit Care Med. 2017;195(2):221–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shaheen SO, Rutterford C, Zuccolo L, et al. Prenatal alcohol exposure and childhood atopic disease: a Mendelian randomization approach. J Allergy Clin Immunol. 2014;133(1):225–232.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stratakis N, Roumeliotaki T, Oken E, et al. Fish and seafood consumption during pregnancy and the risk of asthma and allergic rhinitis in childhood: a pooled analysis of 18 European and US birth cohorts. Int J Epidemiol. 2017;46(5):1465–1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Trivedi MK, Sharma S, Rifas-Shiman SL, et al. Folic Acid in Pregnancy and Childhood Asthma: A US Cohort. Clin Pediatr (Phila). 2018;57(4):421–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tuokkola J, Luukkainen P, Kaila M, et al. Maternal dietary folate, folic acid and vitamin D intakes during pregnancy and lactation and the risk of cows’ milk allergy in the offspring. Br J Nutr. 2016;116(4):710–718. [DOI] [PubMed] [Google Scholar]
- 35.Veeranki SP, Gebretsadik T, Mitchel EF, et al. Maternal folic acid supplementation during pregnancy and early childhood asthma. Epidemiology. 2015;26(6):934–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Viljoen K, Segurado R, O’Brien J, et al. Pregnancy diet and offspring asthma risk over a 10-year period: the lifeways cross generation cohort study, Ireland. BMJ Open. 2018;8(2):e017013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wada K, Konishi K, Tamura T, Shiraki M, Iwasa S, Nagata C. Alcohol intake during pregnancy and offspring’s atopic eczema risk. Alcohol Clin Exp Res. 2016;40(5):1037–1043. [DOI] [PubMed] [Google Scholar]
- 38.Yang L, Jiang L, Bi M, et al. High dose of maternal folic acid supplementation is associated to infant asthma. Food Chem Toxicol. 2015;75:88–93. [DOI] [PubMed] [Google Scholar]
- 39.Best KP, Sullivan TR, Palmer DJ, et al. Prenatal omega-3 LCPUFA and symptoms of allergic disease and sensitization throughout early childhood - a longitudinal analysis of long-term follow-up of a randomized controlled trial. World Allergy Organ J. 2018;11(1):10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wolsk HM, Chawes BL, Litonjua AA, et al. Prenatal vitamin D supplementation reduces risk of asthma/recurrent wheeze in early childhood: A combined analysis of two randomized controlled trials. PLoS One. 2017;12(10):e0186657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Allergy ASoCIa. Australasian Society of Clinical Immunology and Allergy guidelines for infant feeding and allergy prevention. https://www.allergy.org.au/patients/allergy-prevention/ascia-guidelines-for-infant-feeding-and-allergy-prevention Published 2016. Accessed. [DOI] [PubMed]
- 42.British Dietetic Association. Early Feeding Guidance. https://www.bsaci.org/about/early-feeding-guidance. Published 2018. Accessed April, 2019.
- 43.Muraro A, Halken S, Arshad SH, et al. EAACI Food Allergy and Anaphylaxis Guidelines. Primary prevention of food allergy. Allergy. 2014;69(5):590–601. [DOI] [PubMed] [Google Scholar]
- 44.Greer FR, Sicherer SH, Burks AW. The effects of early nutritional interventions on the development of atopic disease in infants and children: the role of maternal dietary restriction, breastfeeding, hydrolyzed formulas, and timing of introduction of allergenic complementary foods. Pediatrics. 2019;143(4):e20190281. [DOI] [PubMed] [Google Scholar]
- 45.Schafer T, Bauer CP, Beyer K, et al. S3-Guideline on allergy prevention: 2014 update: Guideline of the German Society for Allergology and Clinical Immunology (DGAKI) and the German Society for Pediatric and Adolescent Medicine (DGKJ). Allergo J Int. 2014;23(6):186–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Maslova E, Granstrom C, Hansen S, et al. Peanut and tree nut consumption during pregnancy and allergic disease in children-should mothers decrease their intake? Longitudinal evidence from the Danish National Birth Cohort. J Allergy Clin Immunol. 2012;130(3):724–732. [DOI] [PubMed] [Google Scholar]
- 47.du Toit G, Sayre PH, Roberts G, et al. The allergen-specificity of early peanut consumption and the impact on the development of allergic disease in the LEAP Study Cohort. J Allergy Clin Immunol. 2018;141(4):1343–1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.National Academies Press. Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids. 2002. [DOI] [PubMed]
- 49.National Institute of Health and Care Excellence. Methods for development of NICE public health guidance. https://www.nice.org.uk/process/pmg4/chapter/introduction. Accessed April, 2019. [PubMed]
- 50.Grade Handbook. Introduction to Grade Handbook. https://gdt.gradepro.org/app/handbook/handbook.html#h.ygojbnr1bi5y. Published 2019. Accessed August 2019.
- 51.T. L. Meta-Analysis. R package version 3.0. https://CRAN.R-project.org/package=rmeta.Published 2018. Accessed August 2019.
- 52.Gordon M LT Advanced Forest Plot Using ‘grid’ Graphics. R package version 1.9. https://CRAN.R-project.org/package=forestplot. Published 2019. Accessed 2019. [Google Scholar]
- 53.Berman D, Clinton C, Limb R, Somers EC, Romero V, Mozurkewich E. Prenatal omega-3 supplementation and eczema risk among offspring at age 36 months. Insights Allergy Asthma Bronchitis. 2016;2(1). 10.21767/2471-304X.100014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bisgaard H, Stokholm J, Chawes BL, et al. Fish oil-derived fatty acids in pregnancy and wheeze and asthma in offspring. N Engl J Med. 2016;375(26):2530–2539. [DOI] [PubMed] [Google Scholar]
- 55.Chawes BL, Bonnelykke K, Stokholm J, et al. Effect of vitamin D3 supplementation during pregnancy on risk of persistent wheeze in the offspring: a randomized clinical trial. JAMA. 2016;315(4):353–361. [DOI] [PubMed] [Google Scholar]
- 56.Dunstan JA, Mori TA, Barden A, et al. Fish oil supplementation in pregnancy modifies neonatal allergen-specific immune responses and clinical outcomes in infants at high risk of atopy: a randomized, controlled trial. J Allergy Clin Immunol. 2003;112(6):1178–1184. [DOI] [PubMed] [Google Scholar]
- 57.Goldring ST, Griffiths CJ, Martineau AR, et al. Prenatal vitamin d supplementation and child respiratory health: a randomised controlled trial. PLoS One. 2013;8(6):e66627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Greenough A, Shaheen SO, Shennan A, Seed PT, Poston L. Respiratory outcomes in early childhood following antenatal vitamin C and E supplementation. Thorax. 2010;65(11):998–1003. [DOI] [PubMed] [Google Scholar]
- 59.Imhoff-Kunsch B, Stein AD, Martorell R, Parra-Cabrera S, Romieu I, Ramakrishnan U. Prenatal docosahexaenoic acid supplementation and infant morbidity: randomized controlled trial. Pediatrics. 2011;128(3):505–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Litonjua AA, Carey VJ, Laranjo N, et al. Effect of prenatal supplementation with vitamin D on asthma or recurrent wheezing in offspring by age 3 years: the VDAART randomized clinical trial. JAMA. 2016;315(4):362–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.McEvoy CT, Schilling D, Clay N, et al. Vitamin C supplementation for pregnant smoking women and pulmonary function in their newborn infants: a randomized clinical trial. JAMA. 2014;311(20):2074–2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Noakes PS, Vlachava M, Kremmyda LS, Diaper ND, Miles EA, Erlewyn-Lajeunesse M. Increased intake of oily fish in pregnancy: Effects on neonatal immune responses and on clinical outcomes in infants at 6 mo. Am J Clin Nutr. 2012;95(2):395–404. [DOI] [PubMed] [Google Scholar]
- 63.Olsen SF, Osterdal ML, Salvig JD, et al. Fish oil intake compared with olive oil intake in late pregnancy and asthma in the offspring: 16 y of registry-based follow-up from a randomized controlled trial. Am J Clin Nutr. 2008;88(1):167–175. [DOI] [PubMed] [Google Scholar]
- 64.Hansen S, Strom M, Maslova E, et al. Fish oil supplementation during pregnancy and allergic respiratory disease in the adult offspring. J Allergy Clin Immunol. 2017;139(1):104–111.e4. [DOI] [PubMed] [Google Scholar]
- 65.Palmer DJ, Sullivan T, Gold MS, et al. Effect of n-3 long chain polyunsaturated fatty acid supplementation in pregnancy on infants’ allergies in first year of life: randomised controlled trial. BMJ. 2012;344:184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Palmer DJ, Sullivan T, Gold MS, et al. Randomized controlled trial of fish oil supplementation in pregnancy on childhood allergies. Allergy. 2013;68(11):1370–1376. [DOI] [PubMed] [Google Scholar]
- 67.Best KP, Sullivan T, Palmer D, et al. Prenatal fish oil supplementation and allergy: 6-year follow-up of a randomized controlled trial. Pediatrics. 2016;137(6):e20154443. [DOI] [PubMed] [Google Scholar]
- 68.Bekkers MB, Elstgeest LE, Scholtens S, et al. Maternal use of folic acid supplements during pregnancy, and childhood respiratory health and atopy. Eur Respir J. 2012;39(6):1468–1474. [DOI] [PubMed] [Google Scholar]
- 69.Willers SM, Wijga AH, Brunekreef B, et al. Maternal food consumption during pregnancy and the longitudinal development of childhood asthma. Am J Respir Crit Care Med. 2008;178(2):124–131. [DOI] [PubMed] [Google Scholar]
- 70.Bertelsen RJ, Brantsaeter AL, Magnus MC, et al. Probiotic milk consumption in pregnancy and infancy and subsequent childhood allergic diseases. J Allergy Clin Immunol. 2014;133(1):165–171.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Binkley KE, Leaver C, Ray JG. Antenatal risk factors for peanut allergy in children. Allergy Asthma Clin Immunol. 2011;7(1):17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bisgaard H, Halkjaer LB, Hinge R, et al. Risk analysis of early childhood eczema. J Allergy Clin Immunol. 2009;123(6):1355–1360. e1355. [DOI] [PubMed] [Google Scholar]
- 73.Calvani M, Alessandri C, Sopo SM, et al. Consumption of fish, butter and margarine during pregnancy and development of allergic sensitizations in the offspring: role of maternal atopy. Pediatr Allergy Immunol. 2006;17(2):94–102. [DOI] [PubMed] [Google Scholar]
- 74.Castro-Rodriguez JA, Garcia-Marcos L, Sanchez-Solis M, Perez-Fernandez V, Martinez-Torres A, Mallol J. Olive oil during pregnancy is associated with reduced wheezing during the first year of life of the offspring. Pediatr Pulmonol. 2010;45(4):395–402. [DOI] [PubMed] [Google Scholar]
- 75.Chatzi L, Torrent M, Romieu I, et al. Mediterranean diet in pregnancy is protective for wheeze and atopy in childhood. Thorax. 2008;63(6):507–513. [DOI] [PubMed] [Google Scholar]
- 76.Romieu I, Torrent M, Garcia-Esteban R, et al. Maternal fish intake during pregnancy and atopy and asthma in infancy. Clin Exp Allergy. 2007;37(4):518–525. [DOI] [PubMed] [Google Scholar]
- 77.Dunstan JA, West C, McCarthy S, et al. The relationship between maternal folate status in pregnancy, cord blood folate levels, and allergic outcomes in early childhood. Allergy. 2012;67(1):50–57. [DOI] [PubMed] [Google Scholar]
- 78.Fitzsimon N, Fallon U, O’Mahony D, et al. Mothers’ dietary patterns during pregnancy and risk of asthma symptoms in children at 3 years. Ir Med J. 2007;100(8):27–32. [PubMed] [Google Scholar]
- 79.Haberg SE, London SJ, Stigum H, Nafstad P, Nystad W. Folic acid supplements in pregnancy and early childhood respiratory health. Arch Dis Child. 2009;94(3):180–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jedrychowski W, Flak E, Mroz E, et al. Modulating effects of maternal fish consumption on the occurrence of respiratory symptoms in early infancy attributed to prenatal exposure to fine particles. Ann Nutr Metab. 2008;52(1):8–16. [DOI] [PubMed] [Google Scholar]
- 81.Jedrychowski W, Perera F, Maugeri U, et al. Effects of prenatal and perinatal exposure to fine air pollutants and maternal fish consumption on the occurrence of infantile eczema. Int Arch Allergy Immunol. 2011;155(3):275–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lange NE, Rifas-Shiman SL, Camargo CA Jr, Gold DR, Gillman MW, Litonjua AA. Maternal dietary pattern during pregnancy is not associated with recurrent wheeze in children. J Allergy Clin Immunol. 2010;126(2):250–255, 255 e251–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Litonjua AA, Rifas-Shiman SL, Ly NP, et al. Maternal antioxidant intake in pregnancy and wheezing illnesses in children at 2 y of age. Am J Clin Nutr. 2006;84(4):903–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Camargo CA Jr, Rifas-Shiman SL, Litonjua AA, et al. Maternal intake of vitamin D during pregnancy and risk of recurrent wheeze in children at 3 y of age. Am J Clin Nutr. 2007;85(3):788–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Leermakers ET, Sonnenschein-van der Voort AM, Heppe DH, et al. Maternal fish consumption during pregnancy and risks of wheezing and eczema in childhood: the Generation R Study. Eur J Clin Nutr. 2013;67(4):353–359. [DOI] [PubMed] [Google Scholar]
- 86.Magdelijns FJ, Mommers M, Penders J, Smits L, Thijs C. Folic acid use in pregnancy and the development of atopy, asthma, and lung function in childhood. Pediatrics. 2011;128(1):e135–e144. [DOI] [PubMed] [Google Scholar]
- 87.Martindale S, McNeill G, Devereux G, Campbell D, Russell G, Seaton A. Antioxidant intake in pregnancy in relation to wheeze and eczema in the first two years of life. Am J Respir Crit Care Med. 2005;171(2):121–128. [DOI] [PubMed] [Google Scholar]
- 88.Devereux G, Turner SW, Craig LC, et al. Low maternal vitamin E intake during pregnancy is associated with asthma in 5-year-old children. Am J Respir Crit Care Med. 2006;174(5):499–507. [DOI] [PubMed] [Google Scholar]
- 89.Devereux G, Litonjua AA, Turner SW, et al. Maternal vitamin D intake during pregnancy and early childhood wheezing. Am J Clin Nutr. 2007;85(3):853–859. [DOI] [PubMed] [Google Scholar]
- 90.Willers SM, Devereux G, Craig LC, et al. Maternal food consumption during pregnancy and asthma, respiratory and atopic symptoms in 5-year-old children. Thorax. 2007;62(9):773–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Maslova E, Hansen S, Jensen CB, Thorne-Lyman AL, Strom M, Olsen SF. Vitamin D intake in mid-pregnancy and child allergic disease - a prospective study in 44,825 Danish mother-child pairs. BMC Pregnancy Childbirth. 2013;13:199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Maslova E, Strom M, Olsen SF, Halldorsson TI. Consumption of artificially-sweetened soft drinks in pregnancy and risk of child asthma and allergic rhinitis. PLoS One. 2013;8(2):e57261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Maslova E, Strom M, Oken E, et al. Fish intake during pregnancy and the risk of child asthma and allergic rhinitis - longitudinal evidence from the Danish National Birth Cohort. Br J Nutr. 2013;110(7):1313–1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Linneberg A, Petersen J, Gronbaek M, Benn CS. Alcohol during pregnancy and atopic dermatitis in the offspring. Clin Exp Allergy. 2004;34(11):1678–1683. [DOI] [PubMed] [Google Scholar]
- 95.Miyake Y, Tanaka K, Okubo H, Sasaki S, Arakawa M. Maternal fat intake during pregnancy and wheeze and eczema in Japanese infants: the Kyushu Okinawa Maternal and Child Health Study. Ann Epidemiol. 2013;23(11):674–680. [DOI] [PubMed] [Google Scholar]
- 96.Miyake Y, Sasaki S, Tanaka K, Ohfuji S, Hirota Y. Maternal fat consumption during pregnancy and risk of wheeze and eczema in Japanese infants aged 16–24 months: the Osaka Maternal and Child Health Study. Thorax. 2009;64(9):815–821. [DOI] [PubMed] [Google Scholar]
- 97.Saito K, Yokoyama T, Miyake Y, et al. Maternal meat and fat consumption during pregnancy and suspected atopic eczema in Japanese infants aged 3–4 months: the Osaka Maternal and Child Health Study. Pediatr Allergy Immunol. 2010;21(1 Pt 1):38–46. [DOI] [PubMed] [Google Scholar]
- 98.Miyake Y, Sasaki S, Tanaka K, Hirota Y. Dairy food, calcium and vitamin D intake in pregnancy, and wheeze and eczema in infants. Eur Respir J. 2010;35(6):1228–1234. [DOI] [PubMed] [Google Scholar]
- 99.Miyake Y, Sasaki S, Tanaka K, Hirota Y. Consumption of vegetables, fruit, and antioxidants during pregnancy and wheeze and eczema in infants. Allergy. 2010;65(6):758–765. [DOI] [PubMed] [Google Scholar]
- 100.Miyake Y, Sasaki S, Tanaka K, Hirota Y. Maternal B vitamin intake during pregnancy and wheeze and eczema in Japanese infants aged 16–24 months: the Osaka Maternal and Child Health Study. Pediatr Allergy Immunol. 2011;22(1 Pt 1):69–74. [DOI] [PubMed] [Google Scholar]
- 101.Miyake Y, Okubo H, Sasaki S, Tanaka K, Hirota Y. Maternal dietary patterns during pregnancy and risk of wheeze and eczema in Japanese infants aged 16–24 months: the Osaka Maternal and Child Health Study. Pediatr Allergy Immunol. 2011;22(7):734–741. [DOI] [PubMed] [Google Scholar]
- 102.Nwaru BI, Ahonen S, Kaila M, et al. Maternal diet during pregnancy and allergic sensitization in the offspring by 5 yrs of age: a prospective cohort study. Pediatr Allergy Immunol. 2010;21(1 Pt 1):29–37. [DOI] [PubMed] [Google Scholar]
- 103.Nwaru BI, Erkkola M, Ahonen S, et al. Intake of antioxidants during pregnancy and the risk of allergies and asthma in the offspring. Eur J Clin Nutr. 2011;65(8):937–943. [DOI] [PubMed] [Google Scholar]
- 104.Nwaru BI, Erkkola M, Lumia M, et al. Maternal intake of fatty acids during pregnancy and allergies in the offspring. Br J Nutr. 2012;108(4):720–732. [DOI] [PubMed] [Google Scholar]
- 105.Lumia M, Luukkainen P, Tapanainen H, et al. Dietary fatty acid composition during pregnancy and the risk of asthma in the offspring. Pediatr Allergy Immunol. 2011;22(8):827–835. [DOI] [PubMed] [Google Scholar]
- 106.Erkkola M, Nwaru BI, Kaila M, et al. Risk of asthma and allergic outcomes in the offspring in relation to maternal food consumption during pregnancy: a Finnish birth cohort study. Pediatr Allergy Immunol. 2012;23(2):186–194. [DOI] [PubMed] [Google Scholar]
- 107.Erkkola M, Kaila M, Nwaru BI, et al. Maternal vitamin D intake during pregnancy is inversely associated with asthma and allergic rhinitis in 5-year-old children. Clin Exp Allergy. 2009;39(6):875–882. [DOI] [PubMed] [Google Scholar]
- 108.Oien T, Storro O, Johnsen R. Do early intake of fish and fish oil protect against eczema and doctor-diagnosed asthma at 2 years of age? A cohort study. J Epidemiol Community Health. 2010;64(2):124–129. [DOI] [PubMed] [Google Scholar]
- 109.Oliveti JF, Kercsmar CM, Redline S. Pre- and perinatal risk factors for asthma in inner city African-American children. Am J Epidemiol. 1996;143(6):570–577. [DOI] [PubMed] [Google Scholar]
- 110.Pike KC, Inskip HM, Robinson S, et al. Maternal late-pregnancy serum 25-hydroxyvitamin D in relation to childhood wheeze and atopic outcomes. Thorax. 2012;67(11):950–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Salam MT, Li YF, Langholz B, Gilliland FD. Maternal fish consumption during pregnancy and risk of early childhood asthma. J Asthma. 2005;42(6):513–518. [DOI] [PubMed] [Google Scholar]
- 112.Sausenthaler S, Koletzko S, Schaaf B, et al. Maternal diet during pregnancy in relation to eczema and allergic sensitization in the offspring at 2 y of age. Am J Clin Nutr. 2007;85(2):530–537. [DOI] [PubMed] [Google Scholar]
- 113.Shaheen SO, Northstone K, Newson RB, Emmett PM, Sherriff A, Henderson AJ. Dietary patterns in pregnancy and respiratory and atopic outcomes in childhood. Thorax. 2009;64(5):411–417. [DOI] [PubMed] [Google Scholar]
- 114.Granell R, Heron J, Lewis S, Davey Smith G, Sterne JA, Henderson J. The association between mother and child MTHFR C677T polymorphisms, dietary folate intake and childhood atopy in a population-based, longitudinal birth cohort. [Erratum appears in Clin Exp Allergy. 2008 Apr; 38(4):699 Note: Smith, G D [corrected to Davey Smith, G]]. Clin Exp Allergy. 2008;38(2):320–328. [DOI] [PubMed] [Google Scholar]
- 115.Tuokkola J, Luukkainen P, Tapanainen H, et al. Maternal diet during pregnancy and lactation and cow’s milk allergy in offspring. Eur J Clin Nutr. 2016;70(5):554–559. [DOI] [PubMed] [Google Scholar]
- 116.Wang IJ, Guo YL, Weng HJ, et al. Environmental risk factors for early infantile atopic dermatitis. Pediatr Allergy Immunol. 2007;18(5):441–447. [DOI] [PubMed] [Google Scholar]
- 117.Watson PE, McDonald BW. Water and nutrient intake in pregnant New Zealand women: association with wheeze in their infants at 18 months. Asia Pacific J Clin Nutr. 2014;23(4):660–670. [DOI] [PubMed] [Google Scholar]
- 118.West CE, Dunstan J, McCarthy S, et al. Associations between maternal antioxidant intakes in pregnancy and infant allergic outcomes. Nutrients. 2012;4(11):1747–1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Whitrow MJ, Moore VM, Rumbold AR, Davies MJ. Effect of supplemental folic acid in pregnancy on childhood asthma: a prospective birth cohort study. Am J Epidemiol. 2009;170(12):1486–1493. [DOI] [PubMed] [Google Scholar]
- 120.Ioannidis JP. Implausible results in human nutrition research. BMJ. 2013;347:f6698. [DOI] [PubMed] [Google Scholar]
- 121.Scott M, Kurukulaaratchy RJ, Raza A, Arshad SH. Understanding the nature and outcome of childhood wheezing. Eur Respir J. 2009;33(3):700–701. [DOI] [PubMed] [Google Scholar]
- 122.Raza A, Kurukulaaratchy RJ, Grundy JD, et al. What does adolescent undiagnosed wheeze represent? Findings from the Isle of Wight Cohort. Eur Respir J. 2012;40(3):580–588. [DOI] [PubMed] [Google Scholar]
- 123.European Academy of Allergy CIinical Immunology.Global atlas of Allergy. http://www.eaaci.org/globalatlas/GlobalAtlasAllergy.pdf. Published 2014. Accessed 2018.
- 124.Kominiarek MA, Rajan P. Nutrition Recommendations in Pregnancy and Lactation. Med Clin North Am. 2016;100(6):1199–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Norizoe C, Akiyama N, Segawa T, et al. Increased food allergy and vitamin D: randomized, double-blind, placebo-controlled trial. Pediatr Int. 2014;56(1):6–12. [DOI] [PubMed] [Google Scholar]
- 126.Vahdaninia M, Mackenzie H, Dean T, Helps S. omega-3 LCPUFA supplementation during pregnancy and risk of allergic outcomes or sensitization in offspring: A systematic review and meta-analysis. Ann Allergy Asthma Immunol. 2019;122(3):302–313 e302. [DOI] [PubMed] [Google Scholar]
- 127.Furuhjelm C, Warstedt K, Fageras M, et al. Allergic disease in infants up to 2 years of age in relation to plasma omega-3 fatty acids and maternal fish oil supplementation in pregnancy and lactation. Pediatr Allergy Immunol. 2011;22(5):505–514. [DOI] [PubMed] [Google Scholar]
- 128.Azad MB, Coneys JG, Kozyrskyj AL, et al. Probiotic supplementation during pregnancy or infancy for the prevention of asthma and wheeze: systematic review and meta-analysis. BMJ. 2013;347:f6471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Cuello-Garcia CA, Brozek JL, Fiocchi A, et al. Probiotics for the prevention of allergy: a systematic review and meta-analysis of randomized controlled trials. J Allergy Clin Immunol. 2015;136(4):952–961. [DOI] [PubMed] [Google Scholar]
- 130.Doege K, Grajecki D, Zyriax BC, Detinkina E, Zu Eulenburg C, Buhling KJ. Impact of maternal supplementation with probiotics during pregnancy on atopic eczema in childhood–a meta-analysis. Br J Nutr. 2012;107(1):1–6. [DOI] [PubMed] [Google Scholar]
- 131.Lis-Swiety A, Milewska-Wrobel D, Janicka I. Dietary strategies for primary prevention of atopic diseases - what do we know? Med Wieku Rozwoj. 2016;20(1):68–74. [PubMed] [Google Scholar]
- 132.Mansfield JA, Bergin SW, Cooper JR, Olsen CH. Comparative probiotic strain efficacy in the prevention of eczema in infants and children: a systematic review and meta-analysis. Mil Med. 2014;179(6):580–592. [DOI] [PubMed] [Google Scholar]
- 133.Pelucchi C, Chatenoud L, Turati F, et al. Probiotics supplementation during pregnancy or infancy for the prevention of atopic dermatitis: a meta-analysis. Epidemiology. 2012;23(3):402–414. [DOI] [PubMed] [Google Scholar]
- 134.Tang ML, Lahtinen SJ, Boyle RJ. Probiotics and prebiotics: clinical effects in allergic disease. Curr Opin Pediatrics. 2010;22(5):626–634. [DOI] [PubMed] [Google Scholar]
- 135.Zuccotti G, Meneghin F, Aceti A, et al. Probiotics for prevention of atopic diseases in infants: systematic review and meta-analysis. Allergy. 2015;70(11):1356–1371. [DOI] [PubMed] [Google Scholar]
- 136.Vahdaninia M, Mackenzie H, Helps S, Dean T. Prenatal intake of vitamins and allergic outcomes in the offspring: a systematic review and meta-analysis. J Allergy Clin Immunol Pract. 2017;5(3):771–778.e5. [DOI] [PubMed] [Google Scholar]
- 137.Lodge CJ, Allen KJ, Lowe AJ, Dharmage SC. Overview of evidence in prevention and aetiology of food allergy: a review of systematic reviews. Int J Environ Res Public Health. 2013;10(11):5781–5806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Lv N, Xiao L, Ma J. Dietary pattern and asthma: a systematic review and meta-analysis. J Asthma Allergy. 2014;7:105–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Yang H, Xun P, He K. Fish and fish oil intake in relation to risk of asthma: a systematic review and meta-analysis. PLoS One. 2013;8(11):e80048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Nurmatov U, Devereux G, Sheikh A. Nutrients and foods for the primary prevention of asthma and allergy: systematic review and meta-analysis. J Allergy Clin Immunol. 2011;127(3):724–733.e30. [DOI] [PubMed] [Google Scholar]
- 141.Beckhaus AA, Garcia-Marcos L, Forno E, Pacheco-Gonzalez RM, Celedon JC, Castro-Rodriguez JA. Maternal nutrition during pregnancy and risk of asthma, wheeze, and atopic diseases during childhood: a systematic review and meta-analysis. Allergy. 2015;70(12):1588–1604. [DOI] [PubMed] [Google Scholar]
- 142.Best KP, Gold M, Kennedy D, Martin J, Makrides M. Omega-3 long-chain PUFA intake during pregnancy and allergic disease outcomes in the offspring: a systematic review and meta-analysis of observational studies and randomized controlled trials. Am J Clin Nutr. 2016;103(1):128–143. [DOI] [PubMed] [Google Scholar]
- 143.Brown SB, Reeves KW, Bertone-Johnson ER. Maternal folate exposure in pregnancy and childhood asthma and allergy: a systematic review. Nutr Rev. 2014;72(1):55–64. [DOI] [PubMed] [Google Scholar]
- 144.Crider KS, Cordero AM, Qi YP, Mulinare J, Dowling NF, Berry RJ. Prenatal folic acid and risk of asthma in children: a systematic review and meta-analysis. Am J Clin Nutr. 2013;98(5):1272–1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Gunaratne AW, Makrides M, Collins CT. Maternal prenatal and/or postnatal n-3 long chain polyunsaturated fatty acids (LCPUFA) supplementation for preventing allergies in early childhood. Cochrane Database Syst Rev. 2015;7:CD010085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Netting MJ, Middleton PF, Makrides M. Does maternal diet during pregnancy and lactation affect outcomes in offspring? A systematic review of food-based approaches. Nutrition. 2014;30(11–12):1225–1241. [DOI] [PubMed] [Google Scholar]
- 147.Chen LW, Lyons B, Navarro P, et al. Maternal dietary inflammatory potential and quality are associated with offspring asthma risk over 10-year follow-up: the Lifeways Cross-Generation Cohort Study. Am J Clin Nutr. 2020;111(2):440–447. [DOI] [PubMed] [Google Scholar]
- 148.Hanson C, Rifas-Shiman SL, Shivappa N, et al. Associations of prenatal dietary inflammatory potential with childhood respiratory outcomes in project viva. J Allergy Clin Immunol Pract. 2020;8(3):945–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


