To the Editor
Nonalcoholic fatty liver disease (NAFLD) affects approximately one-quarter of the global adult population1. A subset of affected individuals worldwide have nonalcoholic steatohepatitis (NASH), a more progressive form of the disease that has a higher risk of advancing to cirrhosis and end-stage liver disease (ESLD). Given the enormous number of afflicted patients, identification of the subset at risk of disease progression is critically important for efficient therapy allocation.
NASH patients with fibrosis stage 2 or higher have elevated all-cause and liver-related mortality2, and those with high disease activity scores are at greater risk of fibrosis progression3. These at-risk NASH patients are the target population for therapeutic intervention beyond lifestyle improvement. Histopathological assessment is the current reference standard for diagnosis, risk stratification and therapeutic efficacy evaluation for NAFLD. Unfortunately, liver biopsy for histological assessment carries risks, including even rare mortality. Histopathological assessment is also susceptible to sampling error and intra- and inter-reader variability. These drawbacks of liver biopsy have limited biopsy-based assessment in routine practice and pose challenges in clinical trial design and interpretation. There is therefore a need for reliable non-invasive tools for the diagnosis, risk stratification and monitoring of the course of NAFLD.
The integration of non-invasive tools into routine practice and in trial design requires both acceptance by the scientific community and, ideally, regulatory endorsement. The latter is particularly relevant to the use of non-invasive tools for defining trial populations and monitoring the course of disease with or without therapeutic intervention. An important approval pathway for non-invasive tools by the US Food and Drug Administration (FDA) is the Biomarker Qualification Program (BQP)4, which allows multiple stakeholders to come together to establish the utility of a non-invasive tool for its specific intended use. Substantial resources are needed for planning, data acquisition and analysis to meet the evidentiary burden for a full qualification package. This often requires a collaborative approach, and the likelihood of success can be maximized by pooling resources and expertise in a multi-stakeholder public–private partnership4.
The NIMBLE (Non-invasive Biomarkers of Metabolic Liver Disease) consortium is a comprehensive, multi-year, pre-competitive public–private partnership collaboration conducted under the auspices of the Foundation for the NIH (FNIH) Biomarkers Consortium. The Biomarkers Consortium brings together partners from academia, industry, regulatory bodies and nonprofit organizations to identify, develop and qualify potential biomarkers to improve drug development and regulatory decision-making5. The Metabolic Disorders Steering Committee and the Biomarker Consortium Executive Committee provide oversight for NIMBLE. NIMBLE is led by academic and industry co-chairs for the entire project and has two workstreams, for circulating biomarkers and for imaging biomarkers. NIMBLE is supported by a project team whose membership includes researchers from academia and industry and designated members from the FDA, who advise on project strategy without participating in the approval process for non-invasive tools. A central goal of NIMBLE is to systematically address and eliminate gaps in the existing scientific literature, thereby advancing FDA BQP qualification of one or more biomarkers for diagnosis and disease monitoring.
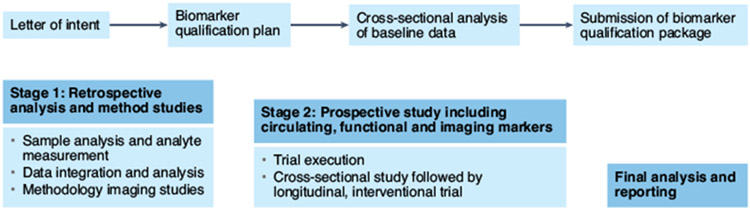
NIMBLE is structured as a two-stage project (Fig. 1). In stage 1, which is currently underway, the circulating biomarkers workstream will evaluate select biomarker panels for their ability to diagnose NASH and its activity, fibrosis stage or the presence of at-risk NASH (a composite including the presence of NASH with a NAFLD activity score of 4 or higher and fibrosis stage 2 or higher). The circulating workstream will evaluate the following biomarker panels for their specific intended use: NIS4 (Genfit) for at-risk NASH; OWLiver (one-way lipidomics) for diagnosis of NASH and for high disease activity (NAFLD activity score ≥ 4); PROC3 (Nordic Biosciences); the enhanced liver fibrosis (ELF) test (Siemens); and the FibroMeter test (Echosens) for fibrosis stages. For assessment of fibrosis, the ability to identify stage 2 or higher fibrosis, advanced fibrosis (stages 3 + 4) or cirrhosis (stage 4) will be evaluated. Work for the stage 1 circulating biomarkers workstream for blood-based biomarker panels is being conducted in collaboration with the NIDDK NASH Clinical Research Network and will simultaneously evaluate the performance of biomarker panels in the same serum sample. Samples are obtained within 90 days of a liver biopsy demonstrating NAFLD, in a cohort of appropriate size, with the full spectrum of disease.
Fig. 1 ∣. The FNIH NIMBLE project plan.
The biomarker qualification process was initiated by approval of the letter of intent submitted by the NIMBLE consortium to the FDA. This will be complemented by cross-sectional studies of circulating biomarkers and methodological studies of MRI and ultrasound-based biomarkers in stage 1. After completion of stage 1, a full biomarker qualification plan will be submitted for review by the FDA. The results of stage 1 will also trigger stage 2, which will include a prospective evaluation of selected biomarkers for final and full qualification combining data from both stage 1 and stage 2.
In stage 1, the imaging biomarkers workstream will also fill important knowledge gaps by characterizing the test–retest repeatability and reproducibility of the leading imaging biomarkers across vendor platforms and across the spectrum of disease severity. Tests include ultrasound-based elastography measurements, magnetic resonance (MR)-based elastography measurements and MR-based measurements of liver fat content6,7. The imaging biomarkers workstream effort incorporates and builds upon rigorous methods developed by the Radiological Society of North America Quantitative Imaging Biomarker Alliance to collect technical performance data on the selected imaging biomarkers.
The first step in biomarker qualification via the FDA’s BQP is the approval of a letter of intent describing the specific non-invasive tools proposed and their intended context of use. This letter of intent process provides a road map for subsequent biomarker qualification. Letters of intent for the circulating8 and imaging9 biomarkers have already been accepted by the FDA biomarker qualification program.
Based on the overall evidence from stage 1, a select group of non-invasive tools whose performance characteristics meet approved prespecified criteria will be advanced for further testing in stage 2 in patients with clinical risk factors for at-risk NASH in specific intended use populations. These criteria include the ability of selected biomarkers to outperform the alanine transaminase (ALT) level for the diagnosis of NASH and the fibrosis-4 (FIB4) score for assessment of fibrosis severity. Further, additional contexts of use may be included in stage 2, such as measurement of fibrogenesis and other dynamic measures of treatment response that can only be assessed prospectively. These studies and data will be part of the biomarker qualification plan for disease monitoring contexts of use and will also further support the qualification of non-invasive tests for diagnostic contexts of use.
It is critically important that data for biomarker qualification studies meet the highest standards for rigor and transparency of reporting. NIMBLE activities are governed by pre-established FNIH data sharing and conflict of interest policies. Furthermore, NIMBLE has established rigorous protocols and policies governing the chain of custody of both samples and data so that individual commercial entities that own biomarker technologies are appropriately firewalled from the data analyses and interpretation process. Data analyses are performed by an independent statistics center following stringent processes to maintain data integrity. Standards set by the FNIH Biomarkers Consortium mandate that data will be shared within the project team and results disseminated via peer-reviewed literature, or other data sharing mechanisms as determined by the project team and the FNIH.
The NIMBLE paradigm aims to qualify one or more non-invasive tools, singly or in combination, for NASH with increasing fibrosis, to allow the identification of patients who should be prioritized for therapeutic intervention and for the monitoring of treatment responses. These tools will facilitate selection of the right patients for clinical trials and increase identification of at-risk NASH and access to care in the clinical setting.
Acknowledgements
We acknowledge support from the Global Liver Institute, USA, and the US FDA. The NIMBLE project is sponsored by the FNIH and is a public–private partnership supported by multiple entities including AbbVie, AstraZeneca, Boehringer Ingelheim, Bristol Myers Squibb, Echosens, GE Healthcare, Genentech, Gilead Sciences, Intercept Pharmaceuticals, Novo Nordisk, Pfizer and Takeda Development Center Americas. Key collaborators who provide in-kind support to NIMBLE include AMRA Medical, Canon Medical Systems USA, Echosens, GENFIT, GE Healthcare, Nordic Bioscience, OWL Metabolomics, Philips Ultrasound, P-Value, Hologic SuperSonic Imagine, Siemens Healthineers and Siemens Medical Solutions USA.
Footnotes
Competing interests
A.J.S. is President of Sanyal Biotechnology, has stock options in Genfit, Akarna, Tiziana, Indalo, Exhalenz and Durect; receives royalties from Elsevier and UptoDate; and has served as a consultant to Astra Zeneca, Janssen, Gilead, Terns, Histoindex, Path-AI, Inventiva, Amgen, Regeneron, Genentech, Merck, Valeant, Boehringer-Ingelheim, Bristol Myers Squibb, Fractyl, Lilly, Hemoshear, Novartis, Novo Nordisk, Pfizer, Intercept and Genfit. Virginia Commonwealth University has received grant support from Gilead, Salix, Tobira, Eli Lilly, Novo Nordisk, Celgene, Viking, Madrigal, Galmed, Pfizer, Bristol Myers, Intercept, Merck, Astra Zeneca, Mallinckrodt and Novartis. S.S.S. is an employee of Astra Zeneca. R.A.C. is employed by Regeneron Pharmaceuticals and is owner of restricted stock units from Regeneron and Pfizer. A.E.S. has served as a compensated consultant for Astra Zeneca, Bracco Diagnostics, Bristol Myers Squibb, General Electric, Gerson Lehman Group, Guidepoint Global Advisors, Supersonic Imagine, Novartis, Pfizer, Philips, Parexel Informatics and WorldCare Clinical; holds stock options in Remedy Medical, Rhino Healthtech and Ochre Bio; is a co-founder of, and holds equity in, Avira, Autonomus, Evidence Based Psychology, Klea, Katharos Laboratories, Quantix Bio and Sonoluminous; receives royalties from Elsevier and Katharos Laboratories; holds advisory board or committee memberships for General Electric, the FNIH and the Sano Center for Computational Personalized Medicine; and is a member of the Board of Governors of the American Institute for Ultrasound in Medicine. Massachusetts General Hospital has received research grant support and/or equipment for projects that A.E.S. has led from the Analogic Corporation, Canon, Echosens, General Electric, Hitachi, Philips, Siemens, Supersonic Imagine/Hologic, Toshiba Medical Systems, the US Department of Defense, Fujifilm Healthcare, the Foundation for the National Institutes of Health, Partners Healthcare, Toshiba Medical Systems and Siemens Medical Systems. C.B.S. reports grants from General Electric, Siemens, Philips, Bayer, the FNIH, Gilead and Pfizer (grant is to University of Wisconsin–Madison (UW-Madison); the University of California, San Diego (UCSD), is a subcontract to UW-Madison); personal consultation fees from Blade, Boehringer and Epigenomics; consultation under the auspices of the University to AMRA, BMS, Exact Sciences, GE Digital, IBM-Watson, and Pfizer; lab service agreements from Enanta, Gilead, ICON, Intercept, Nusirt, Shire, Synageva and Takeda; royalties from Wolters Kluwer for educational material outside the submitted work; honoraria to his institution, UCSD, from Medscape for educational material outside the submitted work; ownership of stock options in Livivos; and an unpaid position on an advisory board to Quantix Bio. S.P.S. is employed by Pfizer and is owner of restricted stock units from Pfizer. R.L. is co-founder of Liponexus, and serves as a consultant for Alnylam/Regeneron, Amgen, Arrowhead Pharmaceuticals, Astra Zeneca, Bristol-Myer Squibb, CohBar, Eli Lilly, Galmed, Gilead, Glympse bio, Inipharm, Intercept, Ionis, Janssen, Madrigal, Metacrine, NGM Biopharmaceuticals, Novartis, Novo Nordisk, Pfizer, Sagimet, 89 bio and Viking Therapeutics. UCSD has received grant support from Allergan, AstraZeneca, Boehringer-Ingelheim, Bristol-Myers Squibb, Eli Lilly, Galectin Therapeutics, Galmed Pharmaceuticals, Genfit, Gilead, Intercept, Inventiva, Janssen, Madrigal Pharmaceuticals, Merck, NGM Biopharmaceuticals, Pfizer and Siemens. K.J.F. reports grant support from General Electric, Bayer, Pfizer and Median; institutional consulting with General Electric; personal consulting with Bayer; an unpaid position on the advisory board of Quantix Bio; and lab service agreements from Enanta, Gilead, ICON, Intercept, Nusirt, Shire, Synageva and Takeda.
References
- 1.Younossi ZM J. Hepat 70, 531–544 (2019). [DOI] [PubMed] [Google Scholar]
- 2.Dulai PS et al. Hepatology 65, 1557–1565 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kleiner DE et al. JAMA Netw. Open 2, e1912565 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.U.S. Food and Drug Administration. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/biomarker-qualification-evidentiary-framework (USDA, 2018). [Google Scholar]
- 5.Menetski JP et al. Nat. Rev. Drug Discov 18, 567–568 (2019). [DOI] [PubMed] [Google Scholar]
- 6.Zhang YN et al. J. Magn. Reson. Imaging 51, 25–42 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li Q, Dhyani M, Grajo JR, Sirlin C & Samir AE World J. Hepatol 10, 530–542 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.FDA. Biomarker Qualification Submission DDTBMQ000084: Circulating Biomarkers for Diagnosis of Nonalcoholic Steatohepatitis (NASH) (FDA, 2020). [Google Scholar]
- 9.FDA. Biomarker Qualification Submission DDTBMQ000112: Imaging Biomarkers for Diagnosis of Nonalcoholic Steatohepatitis (FDA, 2021). [Google Scholar]