Abstract
Inspired by the discovery of an S=N bond in the collagen IV network and its essential role in stabilizing basement membranes, sulfilimines have drawn much attention in the fields of chemistry and biology. However, its further uptake is hindered by the lack of mild, efficient and environmentally benign protocols by which sulfilimines can be constructed under biomolecule-compatible conditions. Here, we report a terminal oxidant-free copper-catalyzed dehydrogenative Chan-Lam coupling of free diaryl sulfilimines with arylboronic acids with excellent chemoselectivity and broad substrate compatibility. The mild reaction conditions and biomolecule-compatible nature allow the employment of this protocol in the late-stage functionalization of complex peptides, and more importantly, as an effective bioconjugation method as showcased in a model protein. A combined experimental and computational mechanistic investigation reveals an inner sphere electron transfer process circumvents the sacrificial oxidant employed in traditional Chan-Lam coupling reactions. An energetically viable concerted pathway was located wherein a copper hydride facilitates hydrogen atom abstraction from the isopropanol solvent to produce dihydrogen via a four-membered transition state.
Graphical Abstract
INTRODUCTION
Sulfilimines, the aza-analogues of sulfoxides, are a class of sulfur (IV)-derived scaffolds, which possess a unique sulfurstereocentre if two carbon-based substituents on sulfur are not identical. They have been exploited in organic chemistry as building blocks, directing groups, chiral axillaries, among others.1 Moreover, chiral sulfilimines have also been developed as ligands for transition-metal catalysts.2 In recent years, sulfilimines have been added to the toolbox used by medicinal chemists owing to their structural similarity to sulfoxides yet possessing an additional site for derivatization.2–3
In 2009, Hudson and coworkers discovered a surprising sulfilimine bond covalently crosslinking hydroxylysine-211 and methionine-93 to adjoin triple-helical protomers of collagen IV, which is a highly conserved major component of basement membranes across the animal kingdom.4 Moreover, this unique bond plays a key role in the structural integrity of basement membranes.5 The discovery of the first sulfilimine bond in naturally occurring biomacromolecules has aroused tremendous interest in this unusual structural motif in biological contexts. For example, Chang, Toste and coworkers have introduced an elegant biocompatible tagging strategy to oxidize methionines to the corresponding sulfilimines by treatment of oxaziridines, and successfully achieved antibody-drug conjugates as well as identification of reactive methionine residues in whole proteomes.6 In addition, the Tang group has developed a molecular probe which can image HOBr based on formation of an intramolecular S=N bond in live cells and zebrafish.7
Considering the unique and intriguing properties of collagen IV derived from sulfilimine crosslinks, derivatization of peptides and proteins with this S(IV)-derived motif could endow them with novel features. In this regard, N-Ar diaryl sulfilimines, in sharp contrast to their S-alkyl counterparts, represent a promising scaffold of great value, since they are more stable toward acid, base, water, or elevated temperature.1a However, their uptake in medicinal chemistry as well as chemical biology was hampered by the lack of general and biocompatible synthetic methods. Conventionally, N-Ar diaryl sulfilimines could be prepared via oxidative imination of the corresponding sulfides,1h,8 but the requisite strong oxidants jeopardize its application on biomolecules. Friedel-Crafts-typed arylation of NH-sulfilimines with aryl fluorides, chlorides or nitrates successfully leads to the formation of arylated products,9 but substrate scope is limited to the electron-deficient arenes, thereby preventing its application on peptides or proteins. In 2019, the Hashmi group has disclosed an elegant palladium-catalyzed arylation strategy of NH-sulfilimines with aryl bromides or iodides.1h Unfortunately, the strongly basic condition (KOtBu) as well as the high reaction temperature (110 °C) renders this protocol unsuitable for functionalization of peptides or proteins.
Therefore, a general, mild and efficient synthetic route for N-Ar diaryl sulfilimines that could be applied to peptides and proteins is an unmet challenge.
With inexpensive and bio-friendly catalysts, an absence of acid, base, or elaborate ligands, mild and environmentally benign reaction conditions, as well as the excellent functional group compability, we hypothesized that copper-catalyzed Chan-Lam coupling10 of free sulfilimines could be a powerful tool to install diaryl sulfilimines on complex peptides or even proteins. Indeed, the Bolm group has developed Chan-Lam couplings of sulfur(VI)-based sulfoximines11 and sulfondiimines12 using dry air as external oxidant in alcoholic solvents at ambient temperature. Ball and coworkers have introduced a seminal copper-catalyzed Chan-Lam coupling of the pyroglutamate amide N-H bond at a naturally encoded pyroglutamate-histidine dipeptide sequences.13 However, due to the cross-nucleophile coupling nature, conventional Chan-Lam couplings typically require the use of oxidants to facilitate the oxidation of Cu(I) to Cu(II),14 which could be problematic especially in biological contexts, wherein proteins and cells are sensitive to oxidative stress.15 In this regard, a terminal oxidant-free Chan-Lam coupling process is an appealing alternative (Figure 1a). A source of inspiration of our design came from the classic electron-transfer triggered sulfoxide isomerization in metal complexes, in which an electron could be transferred from a metal center to sulfur(IV) atom to form sulfur-based radical anion (Figure 1b).16 Though this process, which also serves as a key step in the transformation of cytochrome c from an electron-transfer carrier to a peroxidase associated with the methionine 80 sulfoxide ligation, has been known for decades,17 it has not been previously explored in small molecule catalysis. We envisioned that electron transfer process from copper catalyst to sulfur (IV) atom of sulfilimines, which are structurally similar to sulfoxides, could be leveraged to circumvent the sacrificial oxidant required in traditional Chan-Lam coupling reactions. As part of our investigations into Chan-Lam coupling,18 herein we report an unprecedented copper-catalyzed dehydrogenative Chan-Lam coupling of free diaryl sulfilimines with arylboronic acids, which does not require a terminal oxidant. It permits facile access to a myriad of NAr-diaryl sulfilimines and allows the synthesis of sulfilimine-modified peptides and protein under biomolecule-compatible conditions.
Figure 1. Conceptual Design of Terminal Oxidant-Free Process.
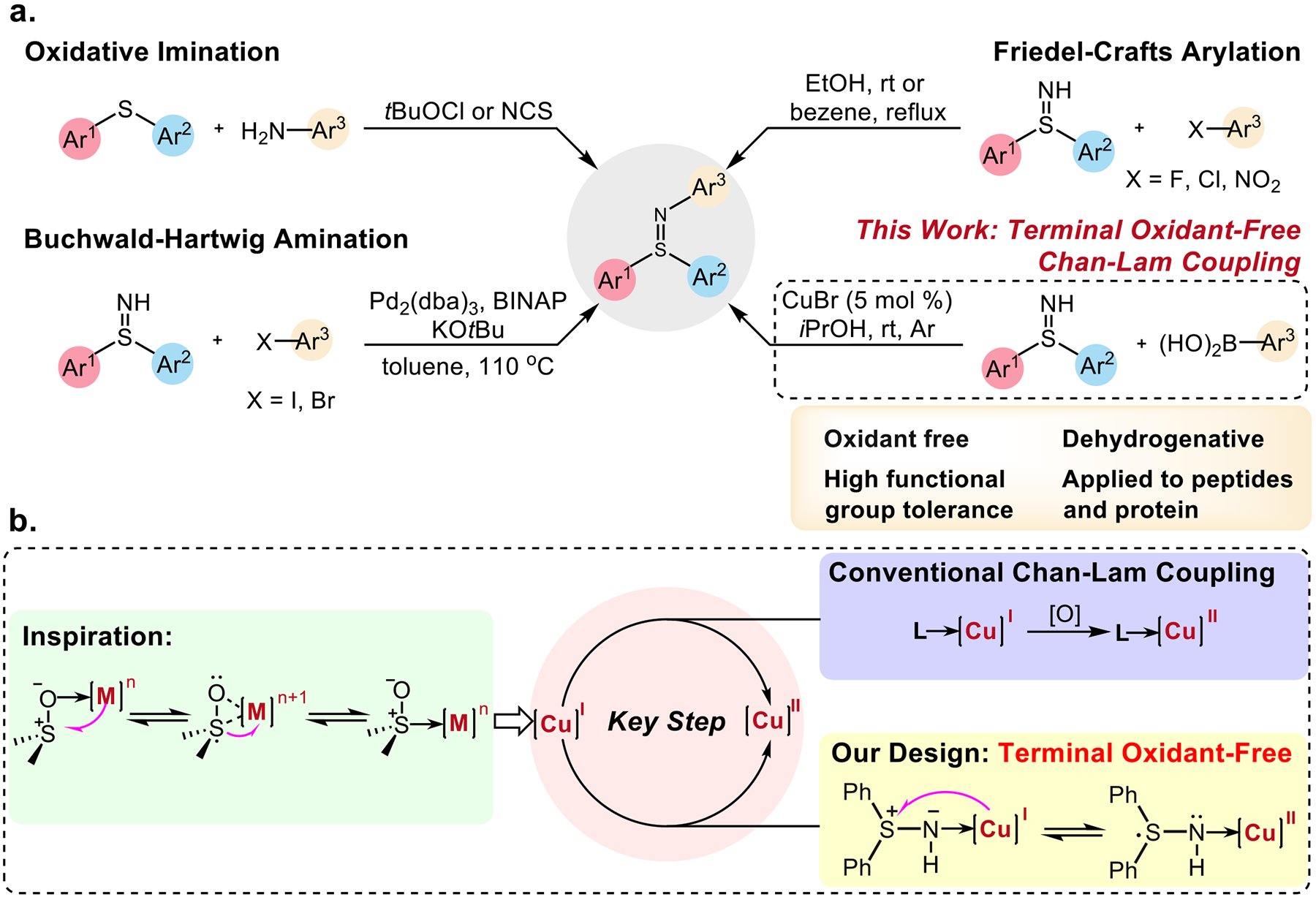
a, Preparative routes towards N-Ar diaryl sulfilimines. b, Design of Cu(I) to Cu(II) transformation via an electron-transfer pathway.
RESULTS AND DISCUSSION
Condition Optimization.
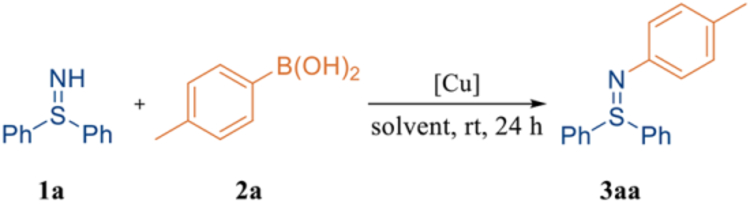
In pursuit of a terminal oxidant-free coupling process, the model reaction between NH-diphenyl sulfilimine 1a and p-tolylboronic acid 2a was carried out under an atmosphere of argon to preclude the influence of air. While the model reaction proceeded in ethanol (0.3 M) with Cu(OAc)2 (10 mol %), only 11% assay yield of desired product 3aa was obtained (entry 1, Table 1). Even so, an analysis of the redox balance of this process is consistent with two turnovers which was supported by results with other copper sources (entries 1–5). After a survey of copper catalyst sources, CuBr was determined to be superior with 71% yield of 3aa obtained (compare entry 6 vs entries 1–5). Forging ahead with CuBr as the optimal copper source, three other commonly used alcoholic solvents (MeOH, iPrOH and tBuOH) were investigated (entries 7–9). When iPrOH was employed as the solvent, 3aa was obtained in 86% yield (entry 8). Other alcohols, unfortunately, produced 3aa in much lower yields (entries 7, 9). Concentration was also discovered to be a key factor. Interestingly, when the reaction concentration was decreased from 0.3 M to 0.1 M, the assay yield of 3aa could be improved from 86% (entry 8) to 99% (entry 11). Subsequently, the amount of 2a could be successfully decreased to 1.5 equivalents, and the assay yield of 3aa remained the same (entry 12). However, utilization of 1.2 equivalents of 2a resulted in slightly diminished yield (85%, entry 13). Gratifyingly, the copper loading could be successfully lowered to 5 mol %, and 3aa could be generated in 99% assay yield and 99% isolated yield (entry 14). Further reduction of the copper source to 2.5 mol %, however, led to lower yield (75% yield 3aa, entry 15). A control experiment was also conducted in the absence of any copper source, and no desired product formed, which confirmed the essential role of the copper species in the transformation (entry 16). Therefore, the optimal condition for copper-catalyzed Chan-Lam coupling of free sulfilimines was determined to be: free sulfilimine 1a as limiting reagent, boronic acid 2a (1.5 equiv) as coupling partner, CuBr (5 mol %) as catalyst, in iPrOH (0.1 M) under an argon atmosphere at room temperature for 24 h. Interestingly, despite no need for external oxidant, the model reaction led to formation of 3aa in slightly diminished yield (81%) under an air atmosphere (entry 17), which paves the road for convenient operation of our method on bioconjugation of proteins.
Table 1.
Optimization of the Copper-Catalyzed Cross-Coupling Reaction of 1a and 2a.a
| entry | [Cu]/mol % | solvent | conc./M | assay yieldb/% |
|---|---|---|---|---|
| 1 | Cu(OAc)2/10 | EtOH | 0.3 | 11 |
| 2 | Cu(acac)2/10 | EtOH | 0.3 | 39 |
| 3 | CuCl2/10 | EtOH | 0.3 | 62 |
| 4 | CuCl/10 | EtOH | 0.3 | 63 |
| 5 | CuBr2/10 | EtOH | 0.3 | 57 |
| 6 | CuBr/10 | EtOH | 0.3 | 71 |
| 7 | CuBr/10 | MeOH | 0.3 | 41 |
| 8 | CuBr/10 | iPrOH | 0.3 | 86 |
| 9 | CuBr/10 | tBuOH | 0.3 | 53 |
| 10 | CuBr/10 | iPrOH | 0.2 | 77 |
| 11 | CuBr/10 | iPrOH | 0.1 | 99 |
| 12c | CuBr/10 | iPrOH | 0.1 | 99 |
| 13d | CuBr/10 | iPrOH | 0.1 | 85 |
| 14c | CuBr/5 | iPrOH | 0.1 | 99(99e) |
| 15c | CuBr/2.5 | iPrOH | 0.1 | 75 |
| 16c | — | iPrOH | 0.1 | 0 |
| 17f | CuBr/5 | iPrOH | 0.1 | 81 |
Unless otherwise stated, reactions were carried out with 1a (0.3 mmol), 2a (2.3 equiv), copper source (10 mol %) in solvent at room temperature under an argon atmosphere for 24 h.
Assay yields determined by 1H NMR spectroscopy of unpurified reaction mixtures using 0.1 mmol (7.0 μL) of CH2Br2 as internal standard.
2a (1.5 equiv).
2a (1.2 equiv).
Isolated yield.
Under air atmosphere.
Substrate Scope.
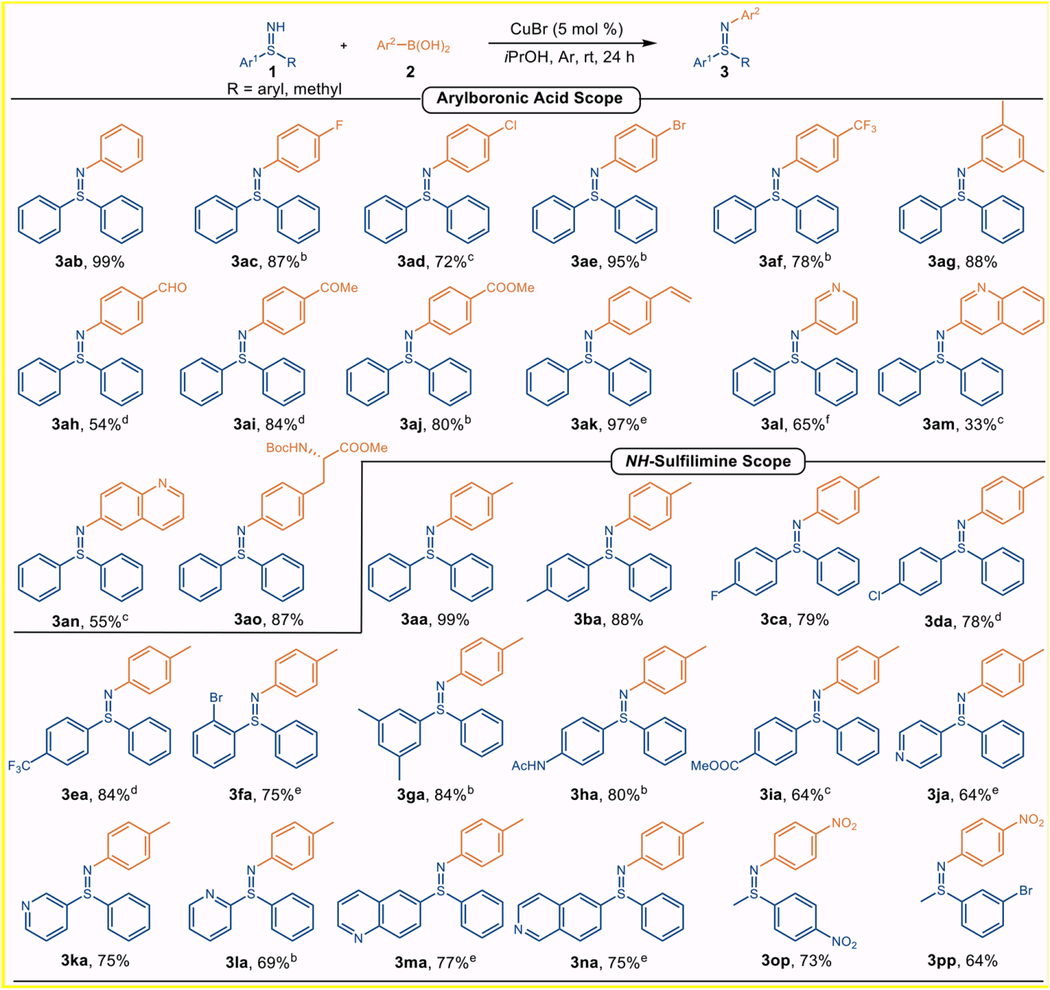
With the optimal conditions established, generality of arylboronic acid substrates was first investigated (Table 2). The parent phenylboronic acid 2b coupled with 1a to yield the corresponding sulfilimines 3ab near quantitatively. Arylboronic acids possessing electron-withdrawing groups, such as 4-F (2c), 4-Cl (2d), 4-Br (2e), or 4-CF3 (2f), proved to be compatible coupling partners under the optimal conditions, providing 3ac–af in 72–95% yields. A meta-substituted arylboronic acid 2g was also well-suited. The neutral and oxidant-free conditions enabled a range of functionalized arylboronic acids to be tolerated, including those contained aldehyde (2h), ketone (2i), ester (2j), and even the polymerizable vinyl group (2k) (54–97%). To our delight, heteroarylboronic acids, such as 3-pyridyl (2l), 3-quinolinyl (2m) and 6-quinolinyl (2n), proceeded smoothly to furnish the corresponding products 3al–an in usable yields (33–65%) by increasing the stoichiometry of 2a and extending the reaction time. Moreover, a tyrosine-derived boronic acid 2o was also compatible with the transformation, providing 3ao in 87% yield. Remarkably, the coupling protocol displayed excellent chemoselectivity favoring arylation of the NH-sulfilimine 1a over arylation of the amide N–H bond.
Table 2.
Substrate Scope of Arylboronic Acids and Sulfilimines in Copper-Catalyzed Chan-Lam Coupling.a
|
Reaction conditions:
1 (0.3 mmol), 2 (1.5 equiv), 5 mol % CuBr in iPrOH (3.0 mL) under an argon atmosphere at room temperature for 24 h.
36 h.
2 (2.0 equiv), 48 h.
48 h.
2 (2.0 equiv), 36 h.
2 (2.0 equiv), 72 h.
We then turned our attention to the substrate scope of diaryl sulfilimines using 4-tolylboronic acid (2a) as the coupling partner. Electron-neutral 1a and 1b furnished 3aa and 3ba in 99% and 88% yield, respectively. NH-Sulfilimines bearing electron-withdrawing substituents, such as 4-F (1c), 4-Cl (1d), 4-CF3 (1e) and 2-Br (1f) performed very well, furnishing the corresponding N-aryl sulfilimines (3ca–fa) in yields ranging from 75% to 84% under slightly modified conditions. The coupling chemistry proceeded smoothly with 3,5-dimethyl substituent appended to the sulfilimine, giving 3ga in 84% yield, albeit with an extended reaction time. Other functional groups, such as 4-NHAc (1h) and 4-COOMe (1i) were also viable, providing 3ha and 3ia in 80% and 64% yield respectively. This chemistry was also well accommodated by heteroaryl NH-sulfilimines to generate 3ja–na in 64–77% yields. Notably, the substrate scope of this chemistry could even be expanded to S-aryl-S-alkyl sulfilimines, and 3op and 3pp were successfully obtained in 73% and 64% yield respectively.
Owing to the instability of the N-arylated sulfilimines bearing an ortho-substituted aryl group on nitrogen, such as 1-naphthyl or 2-tolyl, we have successfully developed the sequential coupling/oxidation cascade to directly afford the corresponding N-aryl sulfoximines 4aq and 4ar (Figure 2), which provides a step-economic approach to prepare NAr-sulfoximines.
Figure 2.
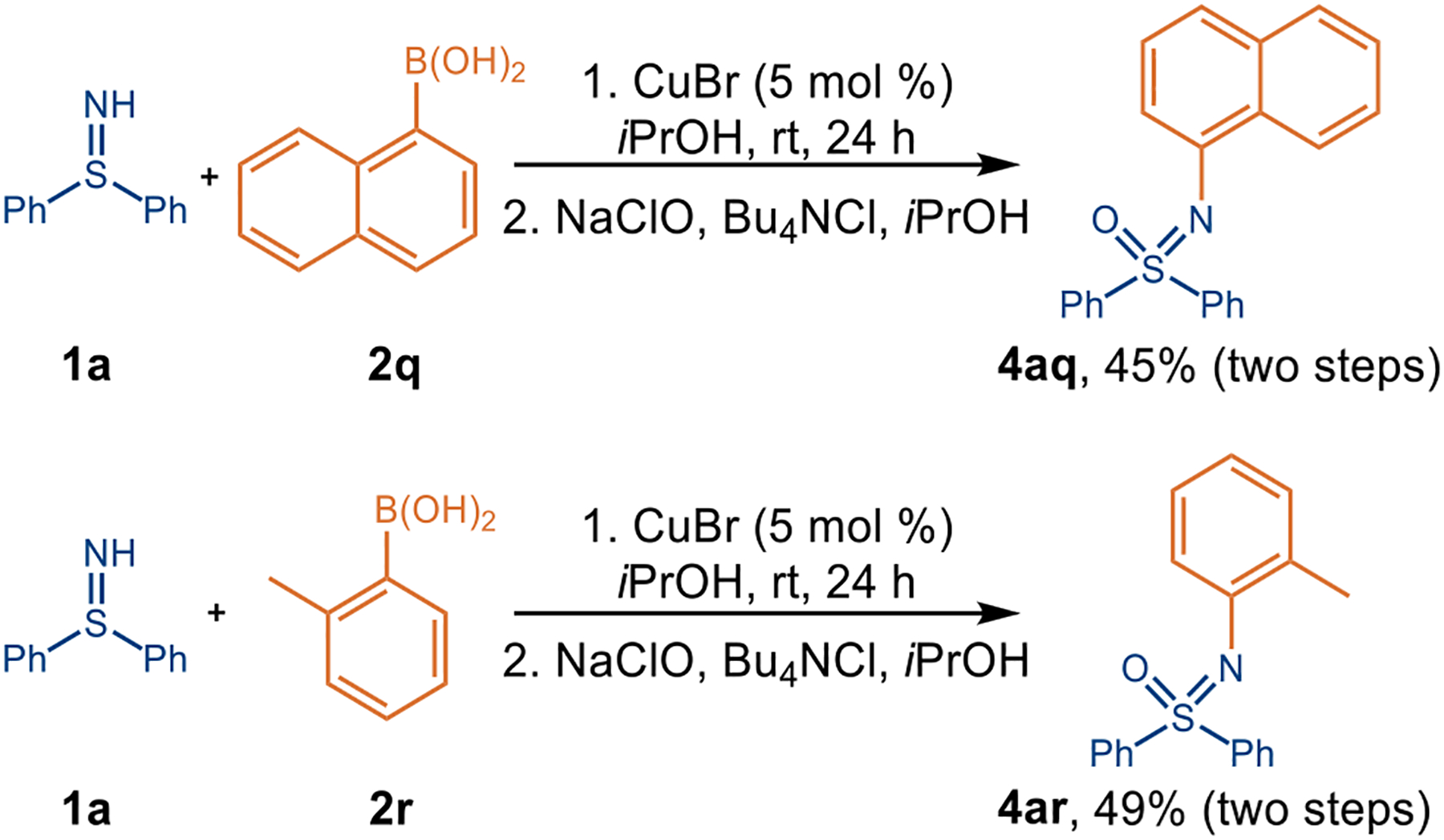
Sequential Coupling/Oxidation Cascade to Prepare N-Aryl Sulfoximines Directly from Free Sulfilimines and Arylboronic Acids.
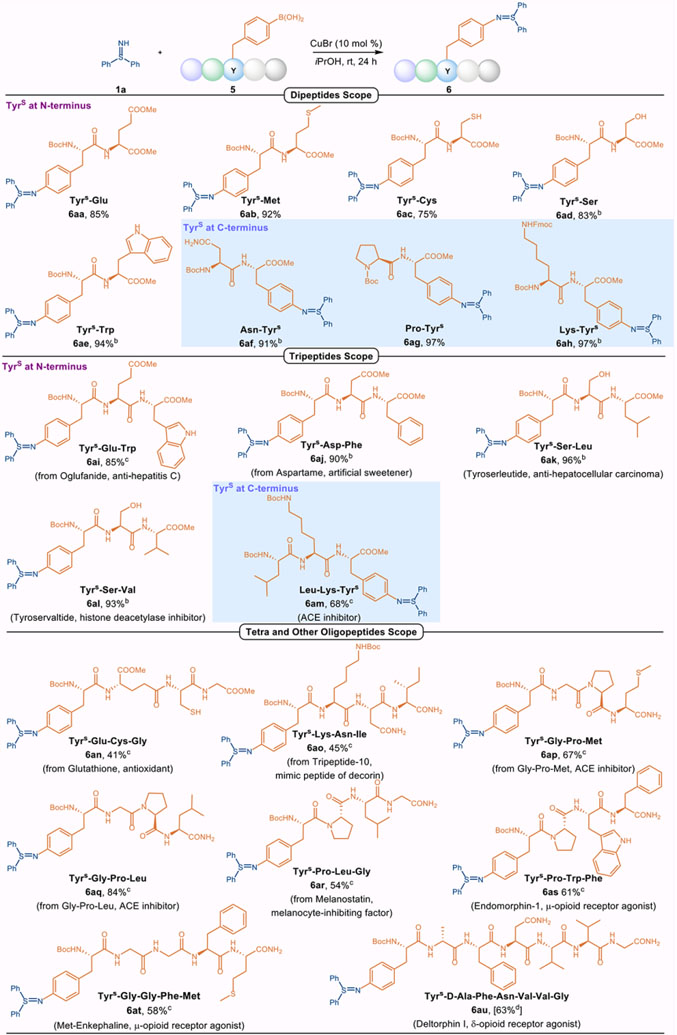
Inspired by the reactivity as well as the excellent chemoselectivity observed with tyrosine-derived substrate 3ao, the suitability of the newly devised copper-catalyzed Chan-Lam coupling for use in sulfilimine-modified peptides was investigated (Table 3). The introduction of second amino acid to the tyrosine-based boronic acid 2o was nitially investigated. As depicted in Table 3, Tyr containing amino acids at N-terminal positions bearing diverse functionalities were not detrimental, affording dipeptides 6aa–ae in good to excellent yields. These results highlight the excellent chemoselectivity of our protocol, favoring C-N bond coupling of sulfilimine N-H bonds over C-O, C-S, or C-N bond formation of hydroxyls O-H bonds, thiol S-H bonds, amide N-H bonds, or indole N-H bonds. It is noteworthy that methionine (Met, 5b) and cysteine (Cys, 5c), which were not amendable in the previous sulfide imination strategy,6 could be successfully employed in our coupling protocol. It is also apparent that the position of the phenylboronic acid containing residue at either the N-terminus or C-terminus (6af–ah) does not significantly affect the efficiency. We next investigated the compatibility and superiority of this Chan-Lam coupling to modify more complex peptides. The Tyr(B(OH)2) derivative of oglufanide, a tripeptide immuno-modulator in the treatment of hepatitis C (5i),19 coupled with 1a to furnish 6ai in 85% yield. The aspartame derivate 5j, as well as two anti-cancer tripeptides, tyroserleutide and tyroservatide (5k–l),20 were also well-suited to the coupling, providing 6aj–l in good yields. Of note, an angiotensin converting enzyme (ACE) inhibitor tripeptide used to decrease angiotensin I21 performed well under the optimal conditions to produce 6am in 68% yield. To evaluate the robustness of our coupling protocol, even more complex Tyr((B(OH)2)-containing oligopeptides beyond tripeptides were explored. At the outset, a series of bioactive tripeptides [glutathione, tripeptide-10,22 melanostatin,23 and two ACE inhibitors (Gly-Pro-Met-NH2 and Gly-Pro-Leu-NH2)24] were decorated with a Tyr((B(OH)2) residue at the N-terminus to afford 5n–r. These substrates proved successful partners in the coupling reactions with 1a to generate the corresponding products 6an–r. The terminal oxidant-free Chan-Lam coupling was an effective tool to install diphenyl sulfilimine moieties on two endogenous opioid receptor agonists endomorphin-1 (6as) and met-enkephalin (6at) in 61% and 58% yield, respectively. Remarkably, the chemistry was well accommodated even with deltorphin I (5u), an exogenous opioid receptor agonist,25 providing the heptapeptide derivative 6au in 63% yield under slightly modified reaction condition, highlighting the breadth and expediency of this procedure.
Table 3.
Scope of Peptides in Copper-Catalyzed Chan-Lam Coupling with 1a.a
|
Reaction conditions:
1a (0.1 mmol), 5 (1.5 equiv), 10 mol % CuBr in iPrOH (1.0 mL) under an argon atmosphere at room temperature for 24 h.
48 h.
1a (0.1 mmol), 5 (1.5 equiv), CuBr (10 mol %), MeOH (1.0 mL), 48 h.
1a (0.05 mmol), 5 (1.5 equiv), CuBr (10 mol %), MeOH (1.0 mL), H2O (1.0 mL), DMF (0.15 mL), 48 h. Yields provided in square brackets are reported as a % conversion determined from reverse-phase HPLC. Tyrs: 4-diphenylsulfiliminyl Tyrosine.
Chan-Lam Coupling-Based Bioconjugation in Model Protein Halotag 7.
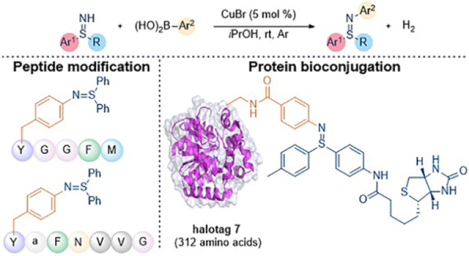
Encouraged by the broad tolerance to different peptides as depicted in Table 3, we then sought to develop the copper-catalyzed Chan-Lam coupling of free sulfilimines as a selective protein conjugation method based on C-N bond formation of free sulfilimines. A major chemical challenge for such an unprecedented bioconjugation method under pH-neutral physiological conditions is the relatively weak nucleophilicity of free sulfilimines, which demands exceptional level of chemoselectivity relative to more nucleophilic amino acids, such as cysteine, lysine, tyrosine, or serine. Halotag 7, the 33 kDa monomeric protein, is a genetically engineered derivative of a dehalogenase, which can efficiently and specifically form a covalent bond with a synthetic ligand.26 Therefore, it was selected as a model protein substrate for this study. As illustrated in Figure 3a, we initialized the investigation by installing a PEG linker tethered with a phenylboronic acid motif on D106 of halotag 7, to provide the viable protein substrate, namely halotag 7’, for the subsequent coupling protocol (see details in Figure S4 and S5). The boronic acid group serves as a point for further functionalization of the protein via the copper-catalyzed Chan-Lam coupling with free sulfilimine 1q. In practice, exposure of 330 μM halotag 7’ to the standard catalytical condition resulted in the formation of cross-coupling product 7 with 43% conversion based on intact protein mass analysis.
Figure 3. Cross Coupling Reaction of Halotag 7’ with Biotin Group Containing NH-sulfilimine 1q.
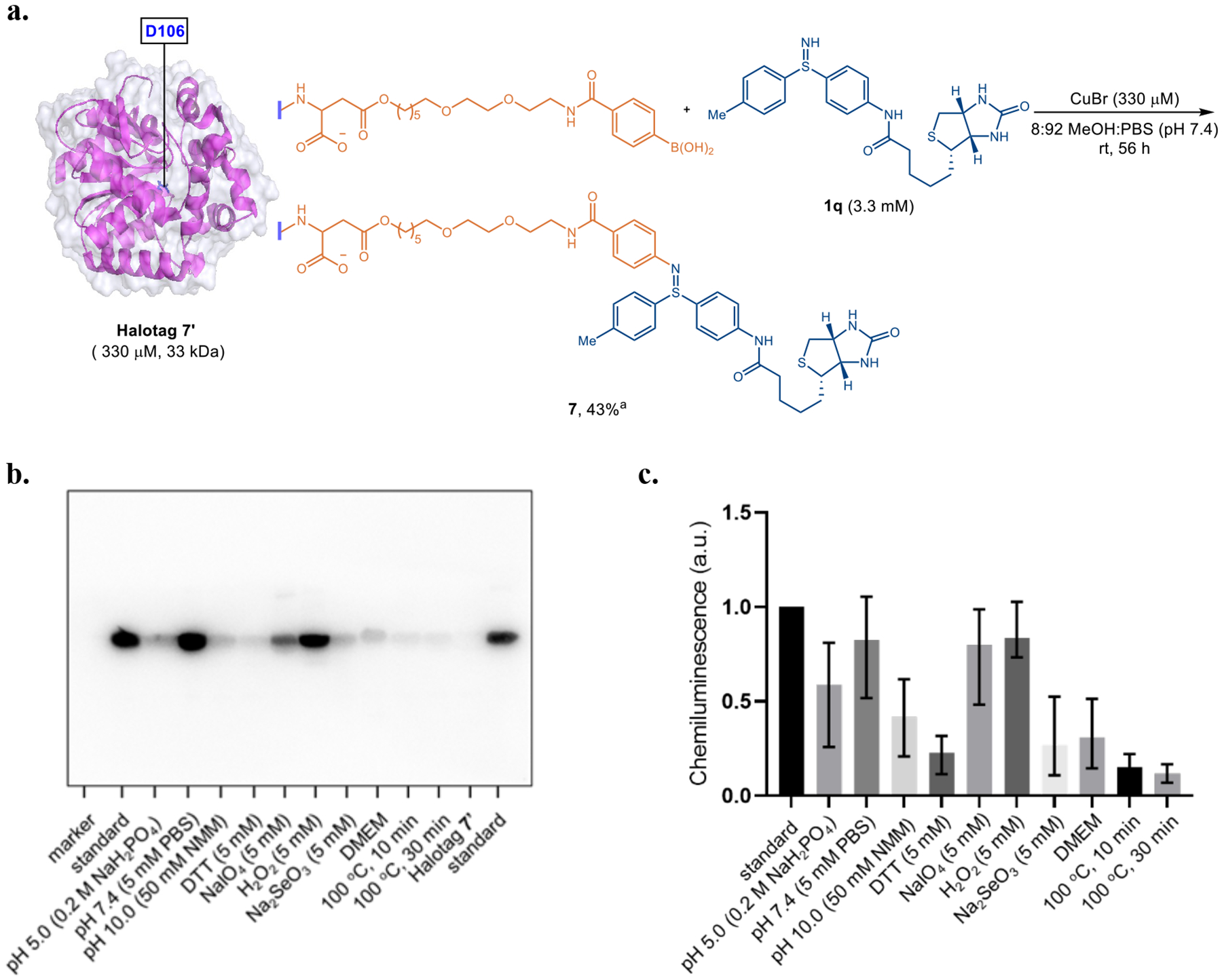
a, Scheme for the reaction of halotag 7’ with the biotin containing NH-sulfilimine 1q. General reaction conditions: halotag 7’ (330 μmol) treated with 1q (10.0 equiv) using CuBr (1.0 equiv) in MeOH/PBS solvent (v/v = 8/92, pH 7.4) for 56 h at room temperature. b, Western blot image of stability experiments on purified protein 7 after incubating under the given conditions at room temperature for 12 h. c, Quantification of the western blot results with image J software. The untreated purified protein 7 was used as a standard (chemiluminescence intensity = 1.0 in Figure 3c). Average of two standard bands was used for each experiment.
As discovered by Hudson group, the sulfilimine covalent crosslink in collagen IV appears as an adaptation of the extracellular matrix in response to mechanical stress in metazoan evolution, and thus serves as a key reinforcement that stabilizes networks as well as basement membranes.5 Thus, the stability of sulfilimines in the context of proteins under various physiologically relevant conditions stands as an interesting problem. Nevertheless, our biomolecule-compatible terminal oxidant-free Chan-Lam coupling offers an enabling platform to tackle this issue. Purified protein 7 was incubated under a variety of biologically-relevant conditions at room temperature. As disclosed in Figure 3b and 3c, the sulfilimine bond in protein 7 (50 μM) was relatively stable in PBS buffer (pH 7.4) or under oxidative conditions (5 mM sodium periodate and 5 mM hydrogen peroxide), as evidenced by no statistically significant variation in biotinylation levels after 12 h as quantified by Western blots. Nevertheless, the S=N bond is sensitive to acidic (pH 5.0, 0.2 mM NaH2PO4), basic (pH 10.0, 50 mM NMM), reductive (5.0 mM DTT), and Dulbecco’s Modified Eagle Medium (DMEM, with 10% v/v fetal bovine serum) conditions, with a considerable decrease of biotinylated protein 7 (25 to 60% retained). Among all of the factors examined, high temperature caused the most degradation of the sulfilimine motif, leading to significant cleavage of sulfilimine bond in very short period of times (10 or 30 min), which is consistent with the previous observations from Wells.27 In addition, significant degradation of sulfilimine-based covalent linker in protein 7 occurred upon treatment with sodium selenite (5 mM), attesting to selective reduction of S=N bond in protein under physiological conditions based on Tang’s pioneering report.7b
Mechanistic Studies.
To gain mechanistic insight into the unprecedented terminal oxidant-free Chan-Lam cross-coupling reaction, several control experiments were performed. The oxidant in the conventional Chan-Lam coupling is believed to facilitate the oxidation of Cu(I) to Cu(II) or Cu(III) in the catalytic cycle.10 Due to the terminal oxidant-free nature of the Chan-Lam coupling of free sulfilimines,28 along with our report on oxidant-free photoredox Chan-Lam coupling of free sulfoximines,18a the impact of light on the newly devised coupling reaction was initially explored. When the model reaction between 1a and 2a was performed in the dark under otherwise identical conditions, 3aa was still obtained in 94% yield (Figure 4a), suggesting that light is not integral to this process. To discover the oxidation states of the copper species, spectroscopic studies were conducted under an atmosphere of argon. Electron paramagnetic resonance spectroscopy (EPR) (Figure 5a) of the reaction mixture revealed a Cu(II) signal. When CuBr was only dissolved in iPrOH, or mixed with p-tolylboronic acid 2a, no noticeable signal was observed via EPR, in agreement with the existence of a Cu(I) species. Surprisingly, a Cu(II) peak appeared when CuBr was mixed with free sulfilimine 1a together in iPrOH. This unexpected result supports that 1a could facilitate the oxidation of Cu(I) to Cu(II), replacing the role of oxidant in classical Chan-Lam coupling. To further verify this finding, a series of UV-Vis experiments was performed (Figure 5b). There was no obvious UV absorption of CuBr solution above 400 nm, whereas a CuBr2 solution exhibited a characteristic band on 590 nm, which is consistent with the d−d transition of Cu(II) species. In addition, the UV band shifted to 760 nm after 1a was added into CuBr2 solution, presumably due to coordination of 1a to the Cu(II). As expected, a characteristic UV absorption of Cu(II) species appeared at 690 nm when CuBr was mixed with 1a, supporting the pivotal role of 1a in the transformation of Cu(I) to Cu(II). Furthermore, addition of radical scavengers [2.0 equivalents of tetramethylpiperidine N-oxide (TEMPO) or 1,4-dinitrobenzene (p-DNB)] to the model reactions only had a modest effect affording 3aa in 89% and 74% yield, respectively (Figure 4b). This result implies that free radicals are not major intermediates in the transformation, although caged radical pairs are still plausible. We hypothesized that the byproduct of the transformation might be dihydrogen gas, which was confirmed by the examination of the reaction headspace via gas chromatography (GC) (see details in Table S2 and Figure S7–S10). To shed light on the source of H2, mass spectroscopy was used to monitor the gaseous byproduct formed while deuterated methanol was employed as the solvent. A signal corresponding to HD was observed, supporting that one hydrogen atom of H2 arises from the alcoholic solvent. When competitive external ligands, such as 4,4-di-tert-butyl bipyridine (dtbpy) or 2,9-dimethyl-1,10-phenanthroline (neocuproine), were added to the reaction system containing CuBr, the reaction rates were inhibited dramatically (Figure 4c). Together with UV-vis studies (see above), this result supports coordination of sulfilimine 1a to copper catalyst playing a pivotal role in the catalytic cycles. All told, the data is consistent with an inner sphere electron transfer process enabling the formal oxidation of Cu(I) to Cu(II).
Figure 4.
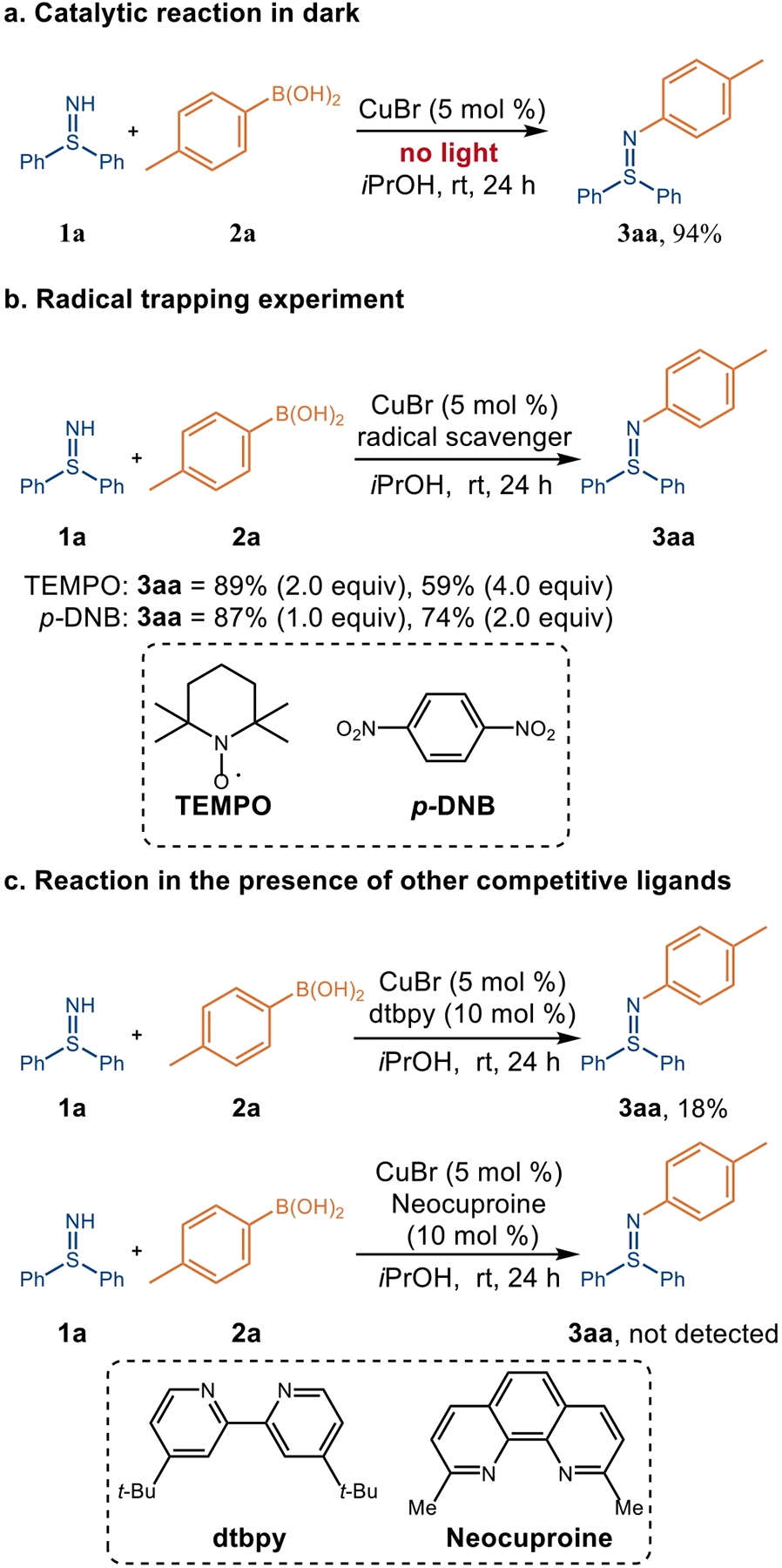
Control Experiments of the Copper-Catalyzed Arylation of Sulfilimines.
Figure 5. Mechanistic Studies.
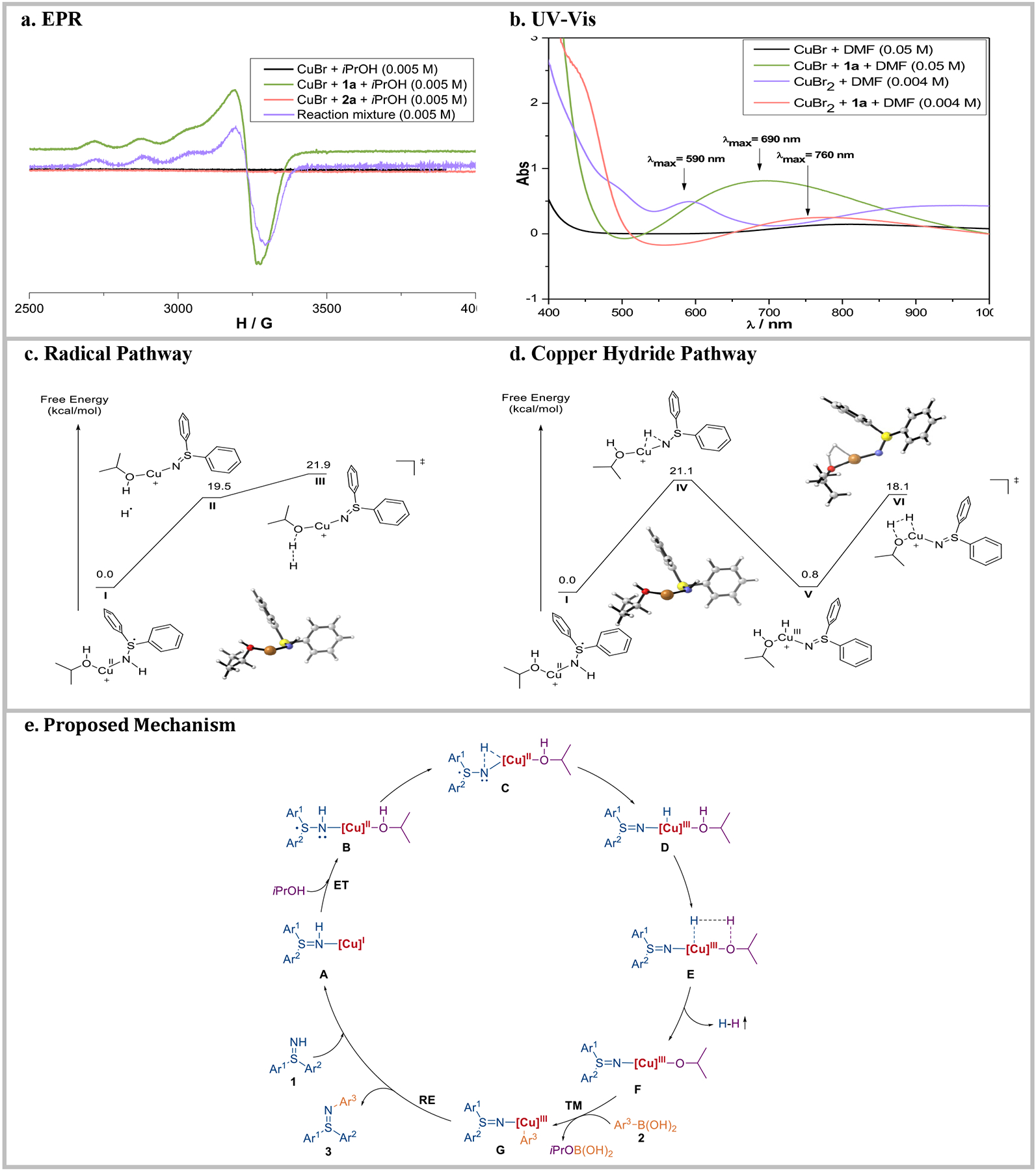
a, EPR spectra. b, UV-Vis spectra. c, Energy profile for the formation of hydrogen gas through a radical mechanism. d, Energy profile for the formation of hydrogen gas through a copper hydride species. Free energy for both pathways were computed using SMD-2-propanol-BP86-d3/6-311G(d,p), Cu:SDD//BP86-d3/6-311G(d,p), Cu:SDD. e, Proposed mechanism of the terminal oxidant-free Chan-Lam coupling.
To understand the origin of the hydrogen evolution in this reaction, a DFT study was initiated. Initial calculations were conducted using Gaussian 1629 with B3LYP/6-31G(d), Cu:SDD30 to explore different pathways. Key steps were further evaluated with SMD-2-propanol-BP86-d3/6-311G(d,p), Cu:SDD//BP86-d3/6-311G(d,p), Cu:SDD,31 a method previously used to calculate pathways with copper hydrides.32
With the presence of copper (II) confirmed through EPR and UV-Vis, a Born-Haber cycle (Figure S13) was used to calculate the redox potential of Cu+ and 1a to Cu2+ and the anion radical of 1a.33 The favorable potential of 0.5 V vs SHE indicates that NH-sulfilimines can oxidize Cu(I) to Cu(II) providing a possible explanation for the presence of Cu(II) in the reaction solution.
Three possible mechanisms for the formation of hydrogen gas were considered. The first formed hydrogen through a five-membered transition state; the second formed hydrogen through an H• arising from isopropanol (iPrOH); the third formed hydrogen through a four-membered transition state arising from initial formation of a copper hydride. The five-membered pathway formed hydrogen gas directly through a union between the hydrogen atoms of iPrOH and 1a when coordinated to copper (Figure S14). This pathway proceeds from a Cu(I) to a Cu(III) species. The transition state barrier for this pathway is 91.1 kcal/mol, which is far too high to be feasible. From Cu(II), the corresponding five-membered transition state could not be located, and versions with a frozen core indicated any such transition state would be very high in energy.
The remaining pathways were investigated commencing from the triplet Cu(II) species I as illustrated in Figure 5c and 5d. A radical pathway (Figure 5c) can form H• by dissociation from the sulfilimine (II). The H• then abstracts the hydrogen from iPrOH via transition state III with a barrier of 21.9 kcal/mol. While transition state III is accessible at room temperature, the formation of a radical is inconsistent with the experimental results showing that TEMPO does not inhibit the reaction. Another pathway (Figure 5d) forms hydrogen gas through a copper hydride species. The copper hydride (V) arises from insertion into the N-H of the sulfilimine via transition state IV. From V, a four-membered transition VI allows the copper hydride to abstract H+ from coordinated iPrOH to form hydrogen gas. The formation of copper hydride and the further formation of hydrogen gas is plausible at room temperature, since the largest barrier in the pathway is 21.1 kcal/mol (IV).
On the base of the combined experimental results and computational studies, a plausible mechanism is outlined in Figure 5e. Initially, CuBr binds to NH-sulfilimines 1 to give Cu(I) species A, which undergoes an inner-sphere electron transfer process to yield Cu(II) intermediate B featuring a radical anion NH-sulfilimine ligand. Meanwhile, the solvent iPrOH coordinates to the copper centre to generate B. Subsequently, an insertion of Cu(II) into the N-H bond of the sulfilimine leads to the formation of three-membered transition state C. Cu(II)-facilitated homolysis of O–H bond of iPrOH occurs to give a formal Cu(III) hydride species D, which can release the hydrogen gas, as revealed by headspace GC analysis, via a feasible four-membered transition state. The resultant Cu(III) species F can undergo transmetallation with boronic acid 2 to produce arylated Cu(III) species G, followed by reductive elimination and ligand exchange with 1 to generate the product 3, and close the overall catalytic cycle.
CONCLUSION
In summary, a terminal oxidant-free copper-catalyzed dehydrogenative Chan-Lam cross coupling of NH-sulfilimines with arylboronic acids has been achieved. This method obviates the need for stoichiometric oxidants of classic Chan-Lam couplings, enables the facile syntheses of a variety of N-arylated diaryl sulfilimines, and is also compatible with complex peptide scaffolds. Furthermore, leveraging the exceptional chemoselectivity, we have showcased its capability as an efficient bioconjugation tool on a model protein under biomolecule-compatible conditions. A combined experimental and computational investigation reveals that the free sulfilimines could facilitate the oxidation of Cu(I) to Cu(II) via an inner sphere electron transfer process, which allows the C-N bond formation in the absence of external oxidants. The protocol described herein represents an appealing alternative to classic oxidative C-N coupling strategies, enabling greater substrate generality and eliminating byproducts from oxidants. Our simple copper catalytic system provides a promising solution toward addressing the challenge associated with construction of sulfilimine covalent crosslink in the context of proteins, which is currently under investigation in our laboratory.
EXPERIMENTAL SECTION
Typical Procedure for the Terminal Oxidant-Free Chan-Lam Coupling Reaction.
To an oven-dried microwave vial equipped with a stir bar was added free sulfilimine 1 (0.3 mmol, 1.0 equiv), boronic acid 2 (0.45 mmol, 1.5 equiv), CuBr (2.2 mg, 0.015 mmol, 5 mol %) under an argon atmosphere in a dry box. The vial was capped with a septum and removed from the dry box. iPrOH (3.0 mL) was added into the reaction vial via syringe, and the reaction solution was stirred at room temperature under an argon atmosphere for 24 h. Upon completion of the reaction, the vial was opened to air, and the reaction mixture was passed through a short pad of silica gel. The pad was then rinsed with 20:1 dichloromethane:methanol (20.0 mL). The solvent was removed under reduced pressure. The residue was purified by flash chromatography to afford the purified product.
Supplementary Material
ACKNOWLEDGMENT
T.J. thanks Guangdong-Joint Foundation of Shenzhen (2021B1515120046), Natural Science Foundation of Guangdong Province (2022A1515011770), Shenzhen Nobel Prize Scientists Laboratory Project (C17783101), and Guangdong Provincial Key Laboratory of Catalysis (2020B121201002) for financial support. M.C.K. thanks the NIH (R35 GM131902) for financial support and XSEDE (TG-CHE120052) for computational support. We are also very grateful to Prof. Lele Duan and Mr. Haiyuan Zou (SUSTech) for assistance of GC and Dr. Yang Yu (SUSTech) for HRMS. We acknowledge the assistance of SUSTech Core Research Facilities.
Footnotes
Supporting Information
Detailed experimental procedures, characterization data, NMR spectra of new compounds, detailed computational study, and calculated structures are included. This material is available free of charge via the Internet at http://pubs.acs.org.
The authors declare no competing interests.
REFERENCES
- (1).For reviews, see:; (a) Gilchrist TL; Moody CJ The chemistry of sulfilimines. Chem. Rev 1977, 77, 409; [Google Scholar]; (b) Furukawa N; Oae S Sulfilimines. Synthetic applications and potential utilizations. Ind. Eng. Chem. Res 1981, 20, 260; [Google Scholar]; (c) Koval IV Advances in the chemistry of sulfimides and related compounds. Sulfur Rep. 1993, 14, 149; [Google Scholar]; (d) Taylor PC Sulfimides (sulfilimines): Applications in stereoselective synthesis. Sulfur Rep. 1999, 21, 241; [Google Scholar]; (e) Tian X; Song L; Hashmi ASK Alpha-imino gold carbene intermediates from readily accessible sulfilimines: Intermolecular access to structural diversity. Chem. Eur. J 2020, 26, 3197. [DOI] [PMC free article] [PubMed] [Google Scholar]; For recent examples, see:; (f) Yoshida S; Yano T; Misawa Y; Sugimura Y; Igawa K; Shimizu S; Tomooka K; Hosoya T Direct thioamination of arynes via reaction with sulfilimines and migratory N-arylation. J. Am. Chem. Soc 2015, 137, 14071; [DOI] [PubMed] [Google Scholar]; (g) Grange RL; Clizbe EA; Counsell EJ; Evans PA Enantioselective construction of C-chiral allylic sulfilimines via the iridium-catalyzed allylic amination with S,S-diphenylsulfilimine: Asymmetric synthesis of primary allylic amines. Chem. Sci 2015, 6, 777; [DOI] [PMC free article] [PubMed] [Google Scholar]; (h) Tian X; Song L; Rudolph M; Rominger F; Oeser T; Hashmi ASK Sulfilimines as versatile nitrene transfer reagents: Facile access to diverse azaheterocycles. Angew. Chem., Int. Ed 2019, 58, 3589; [DOI] [PubMed] [Google Scholar]; (i) Zhang ZX; Davies TQ; Willis MC Modular sulfondiimine synthesis using a stable sulfinylamine reagent. J. Am. Chem. Soc 2019, 141, 13022; [DOI] [PMC free article] [PubMed] [Google Scholar]; (j) Tian X; Song L; Hashmi ASK Synthesis of carbazoles and related heterocycles from sulfilimines by intramolecular C-H aminations. Angew. Chem., Int. Ed 2020, 59, 12342; [DOI] [PMC free article] [PubMed] [Google Scholar]; (k) Xie X; Sun J [4+3]-cycloaddition reaction of sulfilimines with cyclobutenones: Access to benzazepinones. Org. Lett 2021, 23, 8921. [DOI] [PubMed] [Google Scholar]
- (2).Otocka S; Kwiatkowska M; Madalinska L; Kielbasinski P Chiral organosulfur ligands/catalysts with a stereogenic sulfur atom: Applications in asymmetric synthesis. Chem. Rev 2017, 117, 4147. [DOI] [PubMed] [Google Scholar]
- (3).(a) Milaeva ER; Shpakovsky DB; Maklakova IA; Rufanov KA; Neganova ME; Shevtsova EF; Churakov AV; Babkova VA; Babkov DA; Kosolapov VA; Spasov AA Novel diphenylsulfimide antioxidants containing 2,6-di-tert-butylphenol moieties. Russ. Chem. Bull 2018, 67, 2025; [Google Scholar]; (b) Lücking U; Nguyen D; Von Bonin A; Von Ahsen O; Siemeister G; Jautelat R; Doecke W-D Sulphimides as protein kinase inhibitors. WO2007140957 A1, 2007. [Google Scholar]
- (4).Vanacore R; Ham A-JL; Voehler M; Sanders CR; Conrads TP; Veenstra TD; Sharpless KB; Dawson PE; Hudson BG A sulfilimine bond identified in collagen IV. Science 2009, 325, 1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Fidler A; Vanacore R; Chetyrkin S; Pedchenko V; Bhave G; Yin V; Stothers C; Rose K; McDonald W; Clark T; Borza D-B; Steele R; Ivy M; Hudson J; Hudson B A unique covalent bond in basement membrane is a primordial innovation for tissue evolution. Proc. Natl. Acad. Sci. U. S. A 2014, 111, 331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Lin S; Yang X; Jia S; Weeks AM; Hornsby M; Lee PS; Nichiporuk RV; Iavarone AT; Wells JA; Toste FD; Chang CJ Redox-based reagents for chemoselective methionine bioconjugation. Science 2017, 355, 597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).(a) Xu K; Luan D; Wang X; Hu B; Liu X; Kong F; Tang B An ultrasensitive cyclization-based fluorescent probe for imaging native HOBr in live cells and zebrafish. Angew. Chem., Int. Ed 2016, 55, 12751; [DOI] [PubMed] [Google Scholar]; (b) Luan D; Gao X; Kong F; Song X; Zheng A; Liu X; Xu K; Tang B Cyclic regulation of the sulfilimine bond in peptides and NC1 hexamers via the HOBr/H2Se conjugated system. Anal. Chem 2018, 90, 9523. [DOI] [PubMed] [Google Scholar]
- (8).(a) Koval IV Sulfides: Synthesis and properties. Russ. Chem. Rev 1994, 63, 323; [Google Scholar]; (b) Claus PK; Rieder W; Hofbauer P; Vilsmaier E N-aryl sulfimides. Tetrahedron 1975, 31, 505. [Google Scholar]
- (9).(a) Tamura Y; Sumoto K; Matsushima H; Taniguchi H; Ikeda M Reactions of N-substituted arylsulfilimines with acylating agents and with activated halobenzenes, alkynes, and alkenes. J. Org. Chem 1973, 38, 4324; [Google Scholar]; (b) Abou-Gharbia M; Ketcha DM; Zacharias DE; Swern D Reactions of ketenes with sulfilimines. Synthetic routes to oxazolinones and indolinones. J. Org. Chem 1985, 50, 2224; [Google Scholar]; (c) Claridge RP; Millar RW; Sandall JPB; Thompson C Preparation of N-aryl-S,S-diphenylsulfilimines by nucleophilic attack of N-lithio-S,S-diphenylsulfilimine on aromatic compounds. Tetrahedron 1999, 55, 10243; [Google Scholar]; (d) Vlasova OG; Rakitin OA; Khmelnitski LI A simple synthesis of the heterocyclic S,S-diphenylsulfilimines. Org. Prep. Proced. Int 1994, 26, 331. [Google Scholar]
- (10).(a) West MJ; Fyfe JWB; Vantourout JC; Watson AJB Mechanistic development and recent applications of the Chan-Lam amination. Chem. Rev 2019, 119, 12491; [DOI] [PubMed] [Google Scholar]; (b) Chen JQ; Li JH; Dong ZB A review on the latest progress of Chan-Lam coupling reaction. Adv. Synth. Catal 2020, 362, 3311. [Google Scholar]
- (11).Moessner C; Bolm C Cu(OAc)2-catalyzed N-arylations of sulfoximines with aryl boronic acids. Org. Lett 2005, 7, 2667. [DOI] [PubMed] [Google Scholar]
- (12).Bohmann RA; Bolm C Copper-catalyzed C-N cross-coupling of sulfondiimines with boronic acids. Org. Lett 2013, 15, 4277. [DOI] [PubMed] [Google Scholar]
- (13).Ohata J; Zeng Y; Segatori L; Ball ZT A naturally encoded dipeptide handle for bioorthogonal Chan-Lam coupling. Angew. Chem., Int. Ed 2018, 57, 4015. [DOI] [PubMed] [Google Scholar]
- (14).(a) King AE; Brunold TC; Stahl SS Mechanistic study of copper-catalyzed aerobic oxidative coupling of arylboronic esters and methanol: Insights into an organometallic oxidase reaction. J. Am. Chem. Soc 2009, 131, 5044; [DOI] [PubMed] [Google Scholar]; (b) Vantourout JC; Miras HN; Isidro-Llobet A; Sproules S; Watson AJ Spectroscopic studies of the Chan-Lam amination: A mechanism-inspired solution to boronic ester reactivity. J. Am. Chem. Soc 2017, 139, 4769. [DOI] [PubMed] [Google Scholar]
- (15).Sies H; Berndt C; Jones DP Oxidative stress. Annu. Rev. Biochem 2017, 86, 715. [DOI] [PubMed] [Google Scholar]
- (16).Rack J Electron transfer triggered sulfoxide isomerization in ruthenium and osmium complexes. Coord. Chem. Rev 2009, 253, 78. [Google Scholar]
- (17).Alvarez-Paggi D; Hannibal L; Castro MA; Oviedo-Rouco S; Demicheli V; Tortora V; Tomasina F; Radi R; Murgida DH Multifunctional cytochrome c: Learning new tricks from an old dog. Chem. Rev 2017, 117, 13382. [DOI] [PubMed] [Google Scholar]
- (18).(a) Wang C; Zhang H; Wells LA; Liu T; Meng T; Liu Q; Walsh PJ; Kozlowski MC; Jia T Autocatalytic photoredox Chan-Lam coupling of free diaryl sulfoximines with arylboronic acids. Nat. Commun 2021, 12, 932; [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Liang Q; Wells LA; Han K; Chen S; Kozlowski MC; Jia T Synthesis of sulfilimines enabled by copper-catalyzed S-arylation of sulfenamides. November 1, 2021. Research Square. https://www.researchsquare.com/article/rs-1037799/v1. (accessed 2021-11-11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Simbirtsev A; Kolobov A; Zabolotnych N; Pigareva N; Konusova V; Kotov A; Variouchina E; Bokovanov V; Vinogradova T; Vasilieva S; Tuthill C Biological activity of peptide SCV-07 against murine tuberculosis. Russ. J. Immunol 2003, 8, 11. [PubMed] [Google Scholar]
- (20).Ding W; Zhang J; Yao Z; Lu R; Wu D; Li G; Shen Z; Sun Y; Lin G; Wang C; Zhao M; Peng S The synthesis, distribution, and anti-hepatic cancer activity of YSL. Bioorg. Med. Chem. Lett 2004, 12, 4989. [DOI] [PubMed] [Google Scholar]
- (21).Kawakami A; Kayahara H; Tadasa K Taste evaluations of angiotensin I converting enzyme inhibitors, Leu-Lys-Tyr analogues. Biosci. Biotechnol. Biochem 1995, 59, 709. [DOI] [PubMed] [Google Scholar]
- (22).Puig A; Antón JM; Mangues M A new decorin-like tetrapeptide for optimal organization of collagen fibres. Int. J. Cosmet. Sci 2008, 30, 97. [DOI] [PubMed] [Google Scholar]
- (23).Ishihara Y; Oka M; Tsunakawa M; Tomita K; Hatori M; Yamamoto H; Kamei H; Miyaki T; Konishi M; Oki T Melanostatin, a new melanin synthesis inhibitor. Production, isolation, chemical properties, structure and biological activity. J. Antibiot 1991, 44, 25. [DOI] [PubMed] [Google Scholar]
- (24).Byun H-G; Kim S Purification and characterization of angiotensin I converting enzyme (ACE) inhibitory peptides from alaska pollack (theragra chalcogramma) skin. Process Biochem. 2001, 36, 1155. [Google Scholar]
- (25).Erspamer V; Melchiorri P; Falconieri-Erspamer G; Negri L; Corsi R; Severini C; Barra D; Simmaco M; Kreil G Deltorphins: A family of naturally occurring peptides with high affinity and selectivity for delta opioid binding sites. Proc. Natl. Acad. Sci. U. S. A 1989, 86, 5188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Los GV; Encell LP; McDougall MG; Hartzell DD; Karassina N; Zimprich C; Wood MG; Learish R; Ohana RF; Urh M; Simpson D; Mendez J; Zimmerman K; Otto P; Vidugiris G; Zhu J; Darzins A; Klaubert DH; Bulleit RF; Wood KV Halotag: A novel protein labeling technology for cell imaging and protein analysis. ACS Chem. Biol 2008, 3, 373. [DOI] [PubMed] [Google Scholar]
- (27).Elledge SK; Tran HL; Christian AH; Steri V; Hann B; Toste FD; Chang CJ; Wells JA Systematic identification of engineered methionines and oxaziridines for efficient, stable, and site-specific antibody bioconjugation. Proc. Natl. Acad. Sci. U. S. A 2020, 117, 5733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).According to ideal gas law (PV=nRT), even if entire headspace (6 mL from a 9 mL vial) was air, there would not be sufficient O2 (18.5 mL air) to provide the observed yield for reaction of 87.0 mg (0.3 mmol) of substrate 3aa.
- (29).Frisch MJ; Trucks GW; Schlegel HB; Scuseria GE; Robb MA; Cheeseman JR; Scalmani G; Barone V; Petersson GA; Nakatsuji H; Li X; Caricato M; Marenich AV; Bloino J; Janesko BG; Gomperts R; Mennucci B; Hratchian HP; Ortiz JV; Izmaylov AF; Sonnenberg JL; Williams; Ding F; Lipparini F; Egidi F; Goings J; Peng B; Petrone A; Henderson T; Ranasinghe D; Zakrzewski VG; Gao J; Rega N; Zheng G; Liang W; Hada M; Ehara M; Toyota K; Fukuda R; Hasegawa J; Ishida M; Nakajima T; Honda Y; Kitao O; Nakai H; Vreven T; Throssell K; Montgomery JA Jr.; Peralta JE; Ogliaro F; Bearpark MJ; Heyd JJ; Brothers EN; Kudin KN; Staroverov VN; Keith TA; Kobayashi R; Normand J; Raghavachari K; Rendell AP; Burant JC; Iyengar SS; Tomasi J; Cossi M; Millam JM; Klene M; Adamo C; Cammi R; Ochterski JW; Martin RL; Morokuma K; Farkas O; Foresman JB; Fox DJ Gaussian 16, Revision B.01. 2016.
- (30).(a) Becke AD A new mixing of hartree-fock and local density-functional theories. J. Chem. Phys 1993, 98, 1372; [Google Scholar]; (b) Becke AD Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys 1993, 98, 5648; [Google Scholar]; (c) Lee C; Yang W; Parr RG Development of the colle-salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785. [DOI] [PubMed] [Google Scholar]
- (31).(a) Perdew JP Density-functional approximation for the correlation energy of the inhomogeneous electron gas. Phys. Rev. B 1986, 33, 8822; [DOI] [PubMed] [Google Scholar]; (b) Marenich AV; Cramer CJ; Truhlar DG Universal solvation model based on solute electron density and on a continuum model of the solvent defined by the bulk dielectric constant and atomic surface tensions. J. Phys. Chem. B 2009, 113, 6378. [DOI] [PubMed] [Google Scholar]
- (32).Wahidur Rahaman SM; Matyjaszewski K; Poli R Cobalt(III) and copper(II) hydrides at the crossroad of catalysed chain transfer and catalysed radical termination: A DFT study. Polym. Chem 2016, 7, 1079. [Google Scholar]
- (33).Yan L; Lu Y; Li X A density functional theory protocol for the calculation of redox potentials of copper complexes. Phys. Chem. Chem. Phys 2016, 18, 5529. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.