Abstract
We present a case of right ventricle to pulmonary artery hybrid perforation and stenting in a patient with pulmonary atresia with ventricular septal defect major aortopulmonary collaterals and diminutive native pulmonary arteries, then discuss how it compares with established approaches. (Level of Difficulty: Advanced.)
Key Words: periventricular right ventricular outflow tract stent, pulmonary atresia
Abbreviations and Acronyms: LPA, left pulmonary artery; MAPCA, major aortopulmonary collateral; MPA, main pulmonary artery; PA VSD, pulmonary atresia with ventricular septal defect; PA, pulmonary artery; RPA, right pulmonary artery; RVOT, right ventricular outflow tract; RV-PA, right ventricle to pulmonary artery; SPS, surgical pulmonary shunt
Central Illustration
Pulmonary atresia with ventricular septal defect (PA VSD) can be split into 2 extremes: well-developed branch pulmonary arteries (PAs) that are supplied by a patent ductus arteriosus and pulmonary atresia with main pulmonary blood supply through major aortopulmonary collaterals (MAPCAs).
Learning Objectives
-
•
To understand challenges in treatment of PA VSD MAPCAs.
-
•
To explore nonconventional transcatheter options to palliate infants with PA VSD, to promote native PAs growth, and to understand the challenges of a hybrid approach.
The first group has traditionally been palliated in the neonatal period with a surgical pulmonary shunt (SPS), which has been replaced in many centers with ductal stenting as a preferred alternative due to greater postprocedural stability and improved patient survival to destination surgical treatment.1
Treatment options for PA VSD MAPCAs depend on the development of native PAs. The surgical management has focused on unifocalization of MAPCAs and rehabilitation of native PAs by SPS and right ventricle to pulmonary artery (RV-PA) connection with either a right ventricular outflow tract (RVOT) patch or RV-PA conduit.2
With known morbidity and mortality burden of cardiopulmonary bypass in the neonatal period and early infancy,3 as well as difficulties in pulmonary blood flow regulation using central shunts,4 it is worth considering whether we should be pursuing transcatheter options for early palliation of PA VSD (MAPCAs), and at what level of acceptable risk. We present a hybrid approach to establish continuity of the right ventricle to the pulmonary artery blood flow in a patient with PA VSD MAPCAs and diminutive native PAs.
History of Presentation
The patient is a term 2.78-kg male infant with antenatal diagnosis of PA VSD MAPCAs and severely hypoplastic PAs, with low percutaneous saturations (60%-70%).
Medical History
He was initially started on alprostadil, but this was discontinued in view of no patent ductus arteriosus present on computed tomography.
Differential Diagnosis
High pulmonary vascular resistance or stenosis of MAPCAs was considered as the cause of hypoxemia.
Investigations
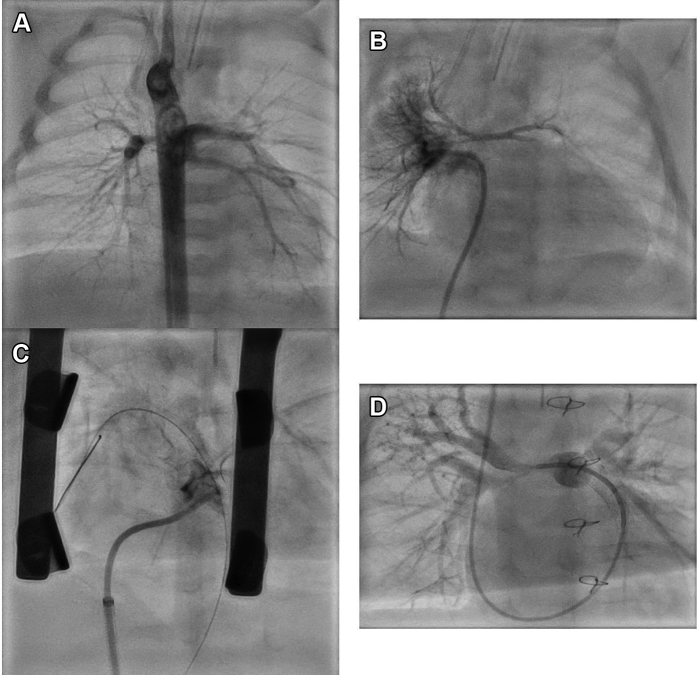
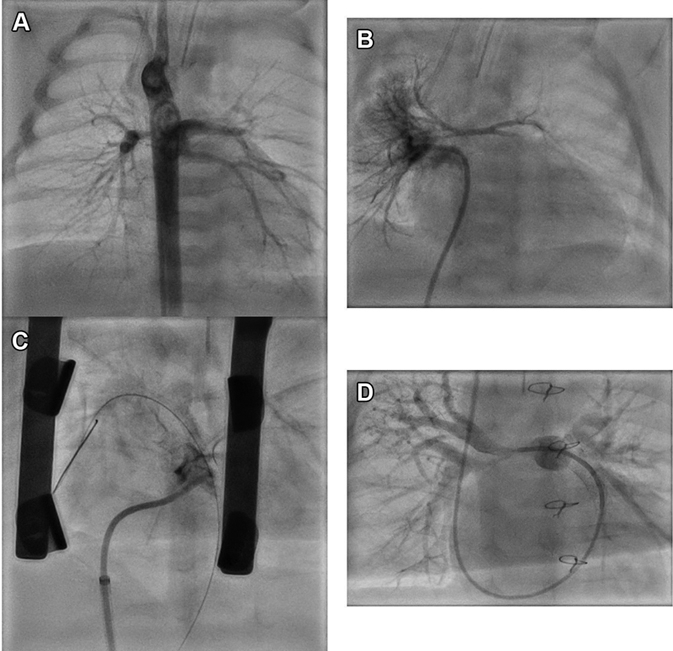
Cardiac computed tomography at the age of 2 days showed that the pulmonary blood supply was provided by 3 MAPCAs with a single origin from descending aorta (Video 1); main PAs were diminutive. At cardiac catheterization, pulmonary vein wedge injections demonstrated native 1-mm right pulmonary artery (RPA) and 1.5-mm left pulmonary artery (LPA) (Nakata index 13.4 mm2/m2) with miniscule main pulmonary artery (MPA) ending almost 10 mm from RVOT (Figures 1A and 1B, Video 2). There was a mild stenosis at the common origin of all 3 MAPCAs from the anterior thoracic aorta. The contrast transit time through the lungs was very prolonged, consistent with elevated pulmonary vascular resistance as the cause of low pulmonary blood flow and saturations.
Figure 1.
Hybrid Periventricular Right Ventricular Outflow Tract Stent in Pulmonary Atresia With Ventricular Septal Defect With Diminutive Branch Pulmonary Arteries
(A) Angiogram in descending aorta showing a major aortopulmonary artery collateral giving off 3 branches that supplied most lung segments. (B) Pulmonary venous wedge angiogram demonstrating diminutive central pulmonary arteries. (C) Hybrid coronary covered stent placement between the right ventricle outflow tract and pulmonary artery with good forward flow into native pulmonary arteries. (D) At last catheterization, distal branch pulmonary arteries have developed well, while severe proximal stenosis persists.
Management
Due to severe native PA hypoplasia, SPS or RVOT patch were not considered a viable option. A hybrid periventricular RV-PA stent was performed at the age of 6 weeks to establish antegrade pulmonary blood flow. After a midline sternotomy, a micropuncture needle (Cook Medical) was advanced through the RV and into the MPA, and an 0.014 coronary guidewire was advanced. A bolus of heparin 100 U/kg was given. A 4-mm coronary balloon was used to dilate the RVOT-MPA tract because there seemed to be an established RV-MPA connection on inspection. Significant bleeding occurred (confirming true pulmonary atresia) but was controllable, while a 4- x 16-mm coronary covered stent (BeGraft Coronary Stent, Bentley) was positioned directly under fluoroscopy guidance and deployed (Figure 1C). The branch PAs filled well antegradely and the bleeding resolved (Video 3). The chest was closed and the patient was moved to pediatric intensive care unit on a therapeutic heparin infusion 20 U/kg/h.
The patient’s condition deteriorated after a period of instability (hypotension and acidosis) with profound hypoxemia 6 h after the procedure. He returned urgently to the cardiac catheterization laboratory. The angiogram showed complete thrombosis of the stent. The stent was crossed with a microcatheter from a jugular approach and angioplasty performed with a 4.5- × 15-mm coronary balloon. Two boluses of heparin 100 U/kg were given during the procedure, with final activated clotting time 240 seconds.
Acute management post-stent recanalization focused on maintaining adequate systolic blood pressure, heparin infusion (target activated clotting time 200-240 seconds for the first night), and dual antiplatelet agents administration.
He underwent 2 further percutaneous reinterventions: restenting with coronary uncovered stent (4.5- × 15-mm Resolute Onyx DES, Medtronic) due to proximal stent stenosis at the age of 3 months and stent redilation at the age of 6 months with 6-mm high-pressure balloon (Emerge PTCA Dilatation Catheter, Boston Scientific). Branch PAs at last catheterization (Figure 1D, Video 4) showed encouraging development with distal RPA of 3.1 mm and distal LPA of 4.2 mm (Nakata index 61 mm2/m2). Both proximal RPA and LPA remain hypoplastic. The common MAPCA from the descending aorta has a proximal stenosis that offers pulmonary blood flow protection (mean PA pressure: 13 mm Hg).
Discussion
Watterson et al5 described the Melbourne experience using central end-to-side shunt (Mee shunt) in 28 patients with PA VSD MAPCAs and very small pulmonary arteries. Although they managed to achieve a satisfactory pulmonary artery growth in 16 of 24 survivors, the rate of complications was high: central shunt-related complications (congestive heart failure, endocarditis) manifested in 12 patients (50% of survivors), with significant occurrence of proximal branch PA stenosis (75% and 50% for RPA and LPA, respectively).
Zhao et al2 have published a large study comparing the outcomes of SPS vs surgical RV-PA connection (patch or conduit) to promote the growth of native branch PAs in PA VSD MAPCAs. The probability of complete repair was significantly lower in the SPS group (56.0% vs 74.5% after 5 years in the RV-PA group). The increase in the mean Nakata index was significantly lower after SPS. The in-hospital morbidity and mortality after complete repair were similar between the 2 groups. However, suturing either SPS or RV-PA conduit on diminutive branch PAs comes with a high risk of distortion and thrombosis.
Hybrid periventricular RV-PA stenting is an option in these patients and has been reported previously in the literature.6 It has been described in higher-risk low–birth weight infants,7,8 who are not candidates for ductal stenting, and have unfavorable anatomy for SPS (hypoplastic branch PAs). Establishing more physiological forward RV-PA flow may promote better growth of the pulmonary tree than a surgical shunt similar to that demonstrated for tetralogy of Fallot patients after RVOT stenting.9 Choice of the stent used depends on the underlying anatomy; uncovered coronary stent is an option where there is a membranous atresia of the pulmonary valve. In our case, however, use of the covered coronary stent was essential because there was true fibrotic gap between RVOT and MPA), as proven by bleeding after the initial balloon dilatation of the RV-PA segment.
Our case shows that we can achieve a promising result with this approach even in extremely diminutive branch pulmonary arteries, but it also highlights the technical difficulties, delicate periprocedural management, and expected reinterventions to promote optimal PA growth.
Follow-up
Our patient is now 10 months old and awaiting surgical RV-PA conduit, MAPCAs unifocalization, and fenestrated VSD closure with patch augmentation of the proximal branch pulmonary arteries. We have not decided to occlude MAPCAs because they serve as single pulmonary blood supply to selected lung segments; where dual supply is present, we have no concerns regarding excessive pulmonary blood flow due to MAPCA origin stenosis offering lung protection.
Conclusions
Transcatheter options to secure pulmonary blood flow in infants with PA VSD are expanding. Further studies are warranted to decide whether these could become the treatment of choice, as has been shown for ductal stenting instead of a surgical shunt. The idea of promoting optimal growth of branch PAs by establishing the continuity between RV and MPA by percutaneous or hybrid stent placement is attractive and should be kept in mind when making an initial management plan.
Funding Support and Author Disclosures
The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For supplemental videos, please see the online version of this paper.
Appendix
Selective MAPCA Injection
Selective MAPCA injection demonstrates pulmonary blood supply by single MAPCA dividing into 3 branches supplying almost all lung segments.
Wedge Balloon Catheter
When using a wedge balloon catheter with the balloon inflated in the right upper pulmonary vein, native pulmonary arteries fill retrogradely. The angiogram shows diminutive native RPA and LPA with a small MPA ending almost 10 mm from where the RVOT should be. RPA measures 1.4 mm and the LPA measures 1 mm. Dual supply is unlikely particularly to the left lingular and right middle lobe.
Final Angiogram
The final angiogram at the end of the hybrid periventricular RV-PA stent placement confirms good stent position with MPA well covered and proximal fourth of the stent in the RV, free. Branch PAs now fill antegradely.
Last Cardiac Catheterization
At the last cardiac catheterization before referring for full repair, both branch pulmonary arteries remain to have tight proximal stenosis, however, the intermediate and distal vessels have become well developed with good flow to the distal pulmonary vascular bed.
References
- 1.Bentham J.R., Zava N.K., Harrison W.J., et al. Duct stenting versus modified Blalock-Taussig shunt in neonates with duct-dependent pulmonary blood flow: associations with clinical outcomes in a multicenter national study. Circulation. 2018;137:581–588. doi: 10.1161/CIRCULATIONAHA.117.028972. [DOI] [PubMed] [Google Scholar]
- 2.Zhao D., Yang K., Li S., et al. Outcomes of different rehabilitative procedures in patients with pulmonary atresia, ventricular septal defect and major aortopulmonary collateral arteries. Eur J Cardiothorac Surg. 2019;55:837–844. doi: 10.1093/ejcts/ezy375. [DOI] [PubMed] [Google Scholar]
- 3.Brown K.L., Pagel C., Ridout D. Cardiac Impact Study Group, et al. What are the important morbidities associated with paediatric cardiac surgery? A mixed methods study. BMJ Open. 2019;9 doi: 10.1136/bmjopen-2018-028533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sasikumar N., Hermuzi A., Fan C.S., et al. Outcomes of Blalock-Taussig shunts in current era: a single center experience. Congenit Heart Dis. 2017;12:808–814. doi: 10.1111/chd.12516. [DOI] [PubMed] [Google Scholar]
- 5.Watterson K.G., Wilkinson J.L., Karl T.R., Mee R.B. Very small pulmonary arteries: central end-to-side shunt. Ann Thorac Surg. 1991;52:1132–1137. doi: 10.1016/0003-4975(91)91294-6. [DOI] [PubMed] [Google Scholar]
- 6.Bondanza S., Calevo M.G., Derchi M.E., Santoro F., Marasini M. Hybrid procedure of right ventricle outflow tract stenting in small infants with pulmonary atresia and ventricular septal defect: early and mid-term results from a single centre. Cardiol Young. 2019;29:375–379. doi: 10.1017/S1047951118002482. [DOI] [PubMed] [Google Scholar]
- 7.Zampi J.D., Armstrong A.K., Hirsch-Romano J.C. Hybrid perventricular pulmonary valve perforation and right ventricular outflow stent placement: a case report of a premature, 1.3-kg neonate with tetralogy of Fallot and pulmonary atresia. World J Pediatr Congenit Heart Surg. 2014;5:338–341. doi: 10.1177/2150135113512136. [DOI] [PubMed] [Google Scholar]
- 8.Cools B., Boshoff D., Heying R., Rega F., Meyns B., Gewillig M. Transventricular balloon dilation and stenting of the RVOT in small infants with tetralogy of Fallot with pulmonary atresia. Catheter Cardiovasc Interv. 2013;82:260–265. doi: 10.1002/ccd.24548. [DOI] [PubMed] [Google Scholar]
- 9.Quandt D., Ramchandani B., Stickley J., et al. Stenting of the right ventricular outflow tract promotes better pulmonary arterial growth compared with modified Blalock-Taussig shunt palliation in tetralogy of Fallot-type lesions. J Am Coll Cardiol Intv. 2017;10:1774–1784. doi: 10.1016/j.jcin.2017.06.023. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Selective MAPCA Injection
Selective MAPCA injection demonstrates pulmonary blood supply by single MAPCA dividing into 3 branches supplying almost all lung segments.
Wedge Balloon Catheter
When using a wedge balloon catheter with the balloon inflated in the right upper pulmonary vein, native pulmonary arteries fill retrogradely. The angiogram shows diminutive native RPA and LPA with a small MPA ending almost 10 mm from where the RVOT should be. RPA measures 1.4 mm and the LPA measures 1 mm. Dual supply is unlikely particularly to the left lingular and right middle lobe.
Final Angiogram
The final angiogram at the end of the hybrid periventricular RV-PA stent placement confirms good stent position with MPA well covered and proximal fourth of the stent in the RV, free. Branch PAs now fill antegradely.
Last Cardiac Catheterization
At the last cardiac catheterization before referring for full repair, both branch pulmonary arteries remain to have tight proximal stenosis, however, the intermediate and distal vessels have become well developed with good flow to the distal pulmonary vascular bed.