Abstract
Background
People with HIV (PWH) are more likely to experience depression, a highly morbid disease. More evidence is needed to better understand mechanisms of depression in PWH. We evaluated a panel of blood biomarkers in relation to depression symptoms in the Multicenter AIDS Cohort Study (MACS).
Setting
Four sites in the United States
Methods
A cross-sectional analysis was performed within the MACS, a prospective study of cisgender men with and without HIV. Depression was assessed with the Center for Epidemiological Studies-Depression (CES-D) scale, and six blood biomarkers were measured: GlycA, high sensitivity C-reactive protein (CRP), interleukin-6 (IL-6), CCL2, soluble CD14 (sCD14), and soluble CD163 (sCD163). Using univariable and multivariable logistic regression, the biomarkers and other factors were evaluated in relation to significant depression symptoms (SDS) by CES-D score ≥16.
Results
784 men were analyzed, the majority of whom (63%) were PWH. PWH were more likely to have SDS (32% versus 21%). In univariable analysis, higher GlycA, CRP, and sCD163 concentration were associated with SDS. In multivariable analysis, however, only higher sCD163 concentration was associated with SDS (OR=2.30, 95%CI=1.11, 4.76). This relationship was driven by the PWH group (OR=2.72, 95%CI=1.12, 6.58) and remained significant when controlling for antidepressant use. Lack of college education was also associated with SDS.
Conclusions
Higher sCD163, a marker of macrophage activation, was significantly associated with significant depression symptoms in the MACS. Further research on this biomarker and macrophage activation in general is warranted to better understand and treat depression in PWH.
Keywords: HIV, Depression, sCD163, Antiretroviral Therapy
Introduction
While people with HIV (PWH) have experienced dramatic improvements in survival and quality of life in the combination antiretroviral therapy (ART) era, end organ diseases remain common, including those that involve the central nervous system (CNS). Among these, depression is a condition that has long been recognized to be highly prevalent among PWH.1 For instance, the Women’s Interagency HIV Study (WIHS) in the United States found that 20% of women with HIV have Major Depressive Disorder (MDD) based on structured diagnostic interview (compared to 10% nationally). Additionally, 32.4% experienced MDD in their lifetime (versus 22.9% nationally).2,3 The medical monitoring project found that depression prevalence is significantly higher in men with HIV (prevalence ratio=3.1) compared to a cohort of individuals from the general population.4 Depression in PWH is associated with multiple adverse clinical outcomes, including worse ART adherence and virologic control.5,6 PWH with chronic depression symptoms are approximately twice as likely to die compared to PWH with few or no depression symptoms.7 A stepwise relationship between depression persistence and adverse outcomes has been demonstrated. Specifically, each 25% increase in days with depression is associated with significant increases in missed appointments, detectable plasma HIV RNA, and a 19% increase in mortality hazard.8
A better understanding of the mechanisms that underpin depression in PWH is needed. In people without HIV (PWOH), a significant association between inflammation and depression has been identified. For example, individuals with MDD who do not have significant medical comorbidities have higher plasma concentrations of tumor necrosis factor alpha (TNFα) and interleukin 6 (IL-6) compared to individuals without MDD.9 Studies focusing on PWH have also shown a relationship between depression and higher concentrations of inflammatory biomarkers, including TNFα, IL-6, and C-reactive protein (CRP).10–12 Larger cohorts that involve both PWH and PWOH are needed to better understand these relationships across both groups. Evaluation of newer biomarkers is needed, including composite biomarkers that may reflect multiple physiologic processes. For example, the composite marker GlycA reflects the enzymatic glycosylation of circulating proteins.13 The proteins with the highest representation within the GlycA signal include α1-acid glycoprotein, haptoglobin, α1-antitrypsin, α1-antichymotrypsin, and transferrin. Given that most of these molecules are acute phase reactants, GlycA appears to be a composite biomarker of systemic inflammation. Outside of HIV, blood GlycA level has been associated with significant depression symptoms in multiple studies.14,15 Markers of monocyte and macrophage activation which have been found to be important in HIV complications16,17 also merit examination in relation to depression symptoms. For this analysis, we leveraged GlycA and other inflammatory biomarker data within the Multicenter AIDS Cohort Study (MACS) to evaluate a potential relationship with depression symptoms, which are regularly assessed.
Methods
MACS, a multicenter prospective observational study of men who have sex with men (including those with HIV and HIV-seronegatives at risk for HIV acquisition), has been conducted at multiple cities across the U.S., including Pittsburgh, Pennsylvania; Baltimore, Maryland; Chicago, Illinois; Los Angeles, California; Columbus, Ohio; and Washington, DC. There were four enrollment waves, including two pre-ART (1984–1985 and 1987–1991) and two post-ART (2001–2003 and 2010–2018) time periods. The MACS cardiovascular disease substudy took place between 2010 and 2013 (including Pittsburgh, Baltimore, Chicago, and Los Angeles sites) and represents the period of time when the blood draw for biomarkers and depression testing were performed.18,19 Participants were excluded for a history of cardiac surgery or percutaneous coronary intervention. Depression symptoms were assessed with the Center for Epidemiologic Studies Depression Scale (CES-D), a widely used 20-item questionnaire that has been commonly used in HIV research.20,21 Previous research supports that a cutoff score of 16 or higher on the CES-D is reflective of major depression,22,23 so this was used to define significant depression symptoms (SDS), the primary outcome for our analysis. A supplementary analysis using the cutoff score of 20 or higher was performed as well. For the purposes of the current analysis, we also excluded individuals with CNS comorbidities that could predispose to depression symptoms,24,25 including stroke, traumatic brain injury, and a previous diagnosis of CNS opportunistic infection.
Laboratory testing
GlycA from plasma was quantified using nuclear magnetic resonance as previously described.13 A panel of other blood biomarkers was measured26,27 including high sensitivity C-reactive protein (hsCRP), IL-6, CCL2, soluble CD14 (sCD14), and soluble CD163 (sCD163). IL-6 was measured using chemiluminescent enzyme linked immunosorbent assay (ELISA, R&D Systems), hsCRP was measured using the BNII Nephelometer (Siemens Healthcare Diagnostics) and CCL2 was measured using Luminex-based singleplex cytokine panel (Millipore). sCD14 and sCD163 were measured with ELISA (R&D systems).
Statistical analysis
Continuous and categorical baseline characteristics were reported for the whole study population and by HIV serostatus using Wilcoxon and chi-square tests, respectively. Logistic regressions were used to assess associations with CES-D (≥16 versus <16). In addition to biomarkers, covariates that were first evaluated in univariable analysis included: age, race, education, pre-ART versus post-ART era MACS enrollment wave (wave 1 [1984–1985] and 2 [1987–1991] versus wave 3 [2001–2003] and 4 [2010–2018]), current antidepressant therapy, active RNA-positive hepatitis C infection (HCV), heavy alcohol use in the last six months (>13 alcoholic drinks/week), and cocaine use in the last six months. Other substance use variables such as marijuana use, poppers, and injection drug use were also evaluated independently with the outcome but were not statistically significant and therefore not included in the multivariate analysis. Additionally, body mass index (BMI) and estimated glomerular filtration rate (eGFR), two factors shown to be associated with cognition in previous MACS studies,28,29 were also evaluated as covariates. For the PWH group, additional variables evaluated were nadir CD4, cumulative plasma HIV RNA (area under the curve, log 10-transformed), currently suppressed plasma HIV RNA (<200 copies/milliliter [ml]), history of AIDS-defining illness, and categories of CD4+ T cells by cells/microliter (CD4<200, 200 ≤CD4≤499, CD4≥500). IL-6 and CCL2 were scaled to ng/dl and sCD163 and sCD14 were scaled to ug/ml. Interaction terms were evaluated between HIV status and biomarkers associated with SDS in multivariable analysis. Stratified analysis was based on interactions and based on HIV status given the possibility that SDS in PWH may be influenced by factors different from PWOH. As a supplementary analysis, a SDS cutoff of CES-D ≥20 was used. A supplementary analysis was also performed that included only PWH with undetectable plasma HIV RNA (<20 copies/ml) and accounted for cumulative use of efavirenz, an antiretroviral with a significant signal with respect to depression symptoms.30 Data management was done in SAS 9.4, and Statistical analysis was done in STATA® Version 15.1
Results
A total of 784 men were analyzed, with 493 (63%) being PWH (see Table 1). Hypertension was present in 47%, 32% were on statin therapy, 28% were current smokers, and 13% had diabetes. Fifty percent were college graduates. In the PWH group, 92% were on ART and 87% had plasma HIV RNA < 200 copies/ml. 95.5% had CD4+ T cell count ≥ 200 cells/microliter. Multiple demographic and disease characteristics were significantly different (P<0.05) by HIV serostatus. All inflammatory biomarkers were higher in the PWH group, particularly CCL2, sCD14, and sCD163 (P<0.001 for each). The median time between depression assessment and GlycA draw was 0 days (IQR=0–36.5) and the median time between depression assessment and draw for other biomarkers was 36.5 days (IQR=36.5–73.1). The PWH group had higher CES-D scores and were more likely to have SDS by CES-D ≥16 compared to PWOH (32% versus 21%). See supplementary table 1 for participant data by SDS status. In univariable analysis (Table 2), lower age, white race, MACS enrollment wave, and active HCV were associated with SDS. Additionally, lack of college education, antidepressant therapy, and crack/cocaine use since last visit were significantly associated with the presence of SDS. For the biomarkers, higher sCD163 was significantly associated (P<0.001) with SDS, driven by the PWH group. Higher GlycA and hsCRP were also associated (P<0.05) with SDS. In the PWH group specifically, currently suppressed HIV RNA<200 copies/ml was associated with the absence of SDS.
Table 1:
Characteristics of participants
| Overall | HIV-uninfected | HIV+ | |
|---|---|---|---|
| N | 784 | 291 | 493 |
| Age in years * | 53 (48, 59) | 54 (50, 61) | 52 (47, 57) |
| Race * | |||
| White, non-Hispanic | 447 (57.0%) | 192 (66.0%) | 255 (51.7%) |
| Black, non-Hispanic | 248 (31.6%) | 73 (25.1%) | 175 (35.5%) |
| Other | 89 (11.4%) | 26 (8.9%) | 63 (12.8%) |
| MACS Enrollment Wave * | |||
| Wave 1,2 | 415 (52.9%) | 179 (61.5%) | 236 (47.9%) |
| Wave 3,4 | 369 (47.1%) | 112 (38.5%) | 257 (52.1%) |
| Education * | |||
| High school | 160 (20.4%) | 36 (12.4%) | 124 (25.2%) |
| Some college | 230 (29.3%) | 73 (25.1%) | 157 (31.8%) |
| College Graduate | 394 (50.3%) | 182 (62.5%) | 212 (43.0%) |
| Diabetes | 99 (13.2%) | 28 (10.2%) | 71 (15.0%) |
| Statin therapy | 237 (32.0%) | 89 (33.1%) | 148 (31.4%) |
| Hypertension | 355 (46.8%) | 121 (43.1%) | 234 (49.0%) |
| BMI (kg/meter2) | 25.9 (23.5, 29.4) | 26.7 (24.0, 30.2) | 25.5 (23.0, 28.6) |
| eGFR (ml/min/1.73 m2), median (IQR) | 88.3 (74.7, 102.0) | 88.6 (76.9, 101.5) | 88.1 (73.3, 102.6) |
| Active hepatitis C virus infection * | 64 (8.2%) | 11 (3.8%) | 53 (10.8%) |
| Depressive symptoms (CES-D ≥16) * | 218 (27.8%) | 61 (21.0%) | 157 (31.8%) |
| CES-D score * | 7.0 (2.0, 17.0) | 6.0 (1.0, 13.0) | 8.0 (3.0, 19.0) |
| Antidepressant use at depression assessment * | 185 (23.6%) | 52 (17.9%) | 133 (27.0%) |
| Smoking status * | |||
| Never | 233 (29.7%) | 92 (31.6%) | 141 (28.6%) |
| Former | 331 (42.2%) | 137 (47.1%) | 194 (39.4%) |
| Current | 220 (28.1%) | 62 (21.3%) | 158 (32.0%) |
| Alcohol use last six months * | |||
| None | 156 (19.9%) | 40 (13.7%) | 116 (23.5%) |
| 1 to 3 drinks/week | 406 (51.8%) | 145 (49.8%) | 261 (52.9%) |
| 4 to 13 drinks/week | 161 (20.5%) | 75 (25.8%) | 86 (17.4%) |
| >13 drinks/week | 61 (7.8%) | 31 (10.7%) | 30 (6.1%) |
| Marijuana/hash last six months | |||
| Daily | 62 (7.9%) | 21 (7.2%) | 41 (8.3%) |
| Weekly | 58 (7.4%) | 17 (5.8%) | 41 (8.3%) |
| Monthly | 24 (3.1%) | 11 (3.8%) | 13 (2.6%) |
| Less often/None | 640 (81.6%) | 242 (83.2%) | 398 (80.7%) |
| Poppers last six months | |||
| Daily | 7 (0.9%) | 2 (0.7%) | 5 (1.0%) |
| Weekly | 50 (6.4%) | 20 (6.9%) | 30 (6.1%) |
| Monthly | 47 (6.0%) | 15 (5.2%) | 32 (6.5%) |
| Less often/None | 680 (86.7%) | 254 (87.3%) | 426 (86.4%) |
| Crack/cocaine last six months | |||
| Daily | 3 (0.4%) | 3 (1.1%) | 0 (0.0%) |
| Weekly | 20 (2.6%) | 5 (1.8%) | 15 (3.1%) |
| Monthly | 9 (1.2%) | 2 (0.7%) | 7 (1.5%) |
| Less often/None | 723 (95.8%) | 268 (96.4%) | 455 (95.4%) |
| Injection drug use last six months * | 15 (1.9%) | 1 (0.3%) | 14 (2.8%) |
| GlycA (umol/cl) * | 3.8 (3.5, 4.2) | 3.8 (3.4, 4.1) | 3.8 (3.5, 4.3) |
| CRP (ug/ml) * | 1.2 (0.6, 2.5) | 1.0 (0.5, 1.9) | 1.2 (0.7, 2.7) |
| IL6 (pg/ml) * | 1.4 (0.9, 2.3) | 1.3 (0.9, 2.1) | 1.5 (1.0, 2.5) |
| CCL2 (pg/ml) * | 263.4 (199.9, 336.0) | 237.1 (184.2, 312.3) | 273.3 (210.0, 349.7) |
| sCD163 (ng/ml) * | 618.4 (476.5, 801.1) | 544.5 (426.2, 689.1) | 679.3 (519.2, 872.0) |
| sCD14(ng/ml) * | 1497.2 (1255.8, 1755.5) | 1281.3 (1129.3, 1470.1) | 1614.0 (1409.8, 1898.6) |
| Current CD4+ T-lymphocytes/microliter | 593.0 (417.0, 766.0) | - | 593.0 (417.0, 766.0) |
| Nadir CD4+ | 145 (29.4%) | - | 145 (29.4%) |
| Log 10 Cumulative viral RNA | 1.5 (1.0, 1.6) | - | 1.5 (1.0, 1.6) |
| HIV RNA <200 copies/ml | 430 (87.2%) | - | 430 (87.2%) |
| ART at visit | 452 (91.7%) | - | 452 (91.7%) |
| Cumulative years of ART | 9.2 (6.0, 12.2) | - | 9.2 (6.0, 12.2) |
| Clinical AIDS history | 68 (13.8%) | - | 68 (13.8%) |
Denotes p value <0.05 between HIV+ and HIV-negative groups
Values are reported as either Median (interquartile range) or number (percentage)
Abbreviations: BMI=body mass index; eGFR=estimated glomerular filtration rate; ART=antiretroviral therapy; kg=kilograms; ml=milliliters; umol=micromoles; cl=centiliters; ug=micrograms; pg=picograms; ng=nanograms.
Table 2:
Univariable models for association with significant depression symptoms (CES-D ≥16)
| Variable | Overall | HIV-negative | HIV+ |
|---|---|---|---|
| OR (95%CI) | OR (95%CI) | OR (95%CI) | |
| Age (years) | 0.96***[0.94,0.98] | 0.99[0.96,1.03] | 0.95**[0.92,0.98] |
| MACS Enrollment (cohort 1,2 vs. 3,4) | 1.77***[1.29,2.43] | 1.24[0.70,2.21] | 1.92***[1.30,2.84] |
| Race (White, non-Hispanic vs. Black, non-Hispanic/Other) | 1.87***[1.36,2.56] | 1.46[0.82,2.62] | 1.91***[1.30,2.81] |
| Education | |||
| College graduate | Ref | Ref | Ref |
| Some college | 1.32[0.91,1.93] | 1.17[0.59,2.31] | 1.28[0.81,2.03] |
| High school | 2.79***[1.88,4.13] | 2.55*[1.17,5.55] | 2.55***[1.59,4.09] |
| Antidepressant therapy at depression assessment | 2.24***[1.58,3.18] | 2.70**[1.40,5.20] | 1.95**[1.29,2.95] |
| BMI (kg/meter 2 ) | 1.01[0.98,1.04] | 1.04[0.98,1.09] | 1.01[0.97,1.05] |
| eGFR (ml/minute/1.73 m 2 ) | 1.00[1.00,1.01] | 1.00[0.98,1.02] | 1.01[1.00,1.01] |
| Active HCV | 3.08***[1.83,5.17] | 2.24[0.63,7.90] | 2.95***[1.65,5.25] |
| Crack/cocaine last six months | 1.76*[1.10,2.81] | 1.58[0.66,3.80] | 1.80*[1.03,3.16] |
| Heavy alcohol use (>13 drinks/week) | 1.00[0.56,1.80] | 1.11[0.46,2.72] | 1.07[0.49,2.35] |
| GlycA (umol/cl) | 1.31*[1.04,1.66] | 1.86**[1.18,2.95] | 1.10[0.84,1.46] |
| CRP (ug/ml) | 1.05*[1.01,1.09] | 1.07[0.98,1.16] | 1.04[1.00,1.08] |
| IL-6 (ng/dl) | 1.09[0.88,1.36] | 0.98[0.68,1.42] | 1.21[0.85,1.71] |
| CCL2 (ng/dl) | 1.01[0.99,1.02] | 1.00[0.99,1.02] | 1.00[0.99,1.02] |
| sCD163 (ug/ml) | 3.41***[1.82,6.40] | 1.31[0.32,5.39] | 3.38**[1.60,7.13] |
| sCD14 (ug/ml) | 1.36[0.96,1.94] | 2.19[0.79,6.11] | 0.94[0.62,1.44] |
| Cumulative HIV RNA (log10 copies/ml) | - | - | 1.34**[1.11,1.61] |
| Suppressed HIV RNA (<200 copies/ml) | - | - | 0.43**[0.25,0.73] |
| Clinical AIDS history | - | - | 1.20[0.70,2.05] |
| Current CD4+ | - | - | |
| CD4<200 | - | - | Ref |
| 200 ≤CD4≤499 | - | - | 1.14[0.44,2.96] |
| CD4≥500 | - | - | 0.94[0.37,2.38] |
| Nadir CD4+ | - | - | 0.83[0.54,1.26] |
| Observations | 784 | 291 | 493 |
Exponentiated coefficients; 95% confidence intervals in brackets;
p < 0.05,
p < 0.01,
p < 0.001
OR=Odds Ratio; CI=Confidence Interval; BMI=body mass index; eGFR=estimated glomerular filtration rate; kg=kilograms; ml=milliliters; umol=micromoles; cl=centiliters; ug=micrograms; pg=picograms; ng=nanograms.
In the multivariable analysis that included significant univariable associations, MACS enrollment wave, race, and active HCV infection were no longer associated with SDS. Antidepressant therapy (Odds ratio [OR]=2.25, 95% Confidence Interval [CI]=1.55,3.25) and lack of college education (OR=1.73, 95% CI=1.09,2.76) continued to be associated with SDS in the entire group. GlycA and hsCRP were no longer associated with SDS, but the relationship between sCD163 and significant symptoms remained significant (OR=2.30, 95%CI=1.11, 4.76, see Figure 1 for forest plot of overall group). For the interaction analysis, the first model used the main effects of HIV positive status, sCD163, and the interaction between sCD163 and HIV+. The interaction result was OR=2.58, 95% CI=0.52,12.72. The second interaction model included each of the same variables as well as the other medical and demographic variables that were shown to be associated with SDS in univariable analysis (age, MACS Enrollment cohort, Race, Education, Antidepressant therapy, HCV status, Crack/cocaine since last visit, and CRP). For this model, the interaction between HIV+ and sCD163 was OR=1.61, 95% CI=0.32,8.20. These two interaction results, while associated with wide confidence intervals, were large enough (1.5–2.5 relative increase in odds ratio), that stratified analysis based on HIV status was justified.
Figure 1:
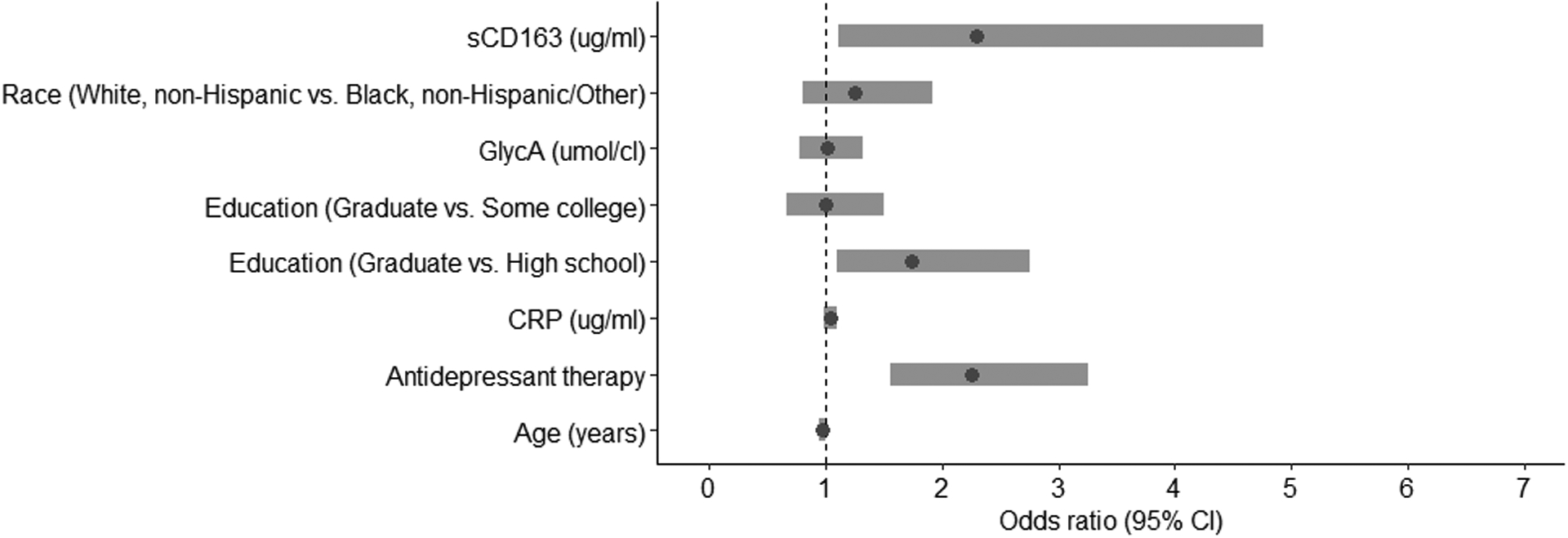
Forest plot of multivariable odds ratios (OR) in association with significant depression symptoms for overall group (n=784)
ug=micrograms; ml=milliliters; umol=micromoles; cl=centiliters; CRP=C-reactive protein
The relationship between SDS and sCD163 was driven by the PWH group (OR=2.72, 95%CI=1.12, 6.58) and was not statistically significant for PWOH (see Figure 2 for stratification by HIV status). In the analysis using an SDS cutoff of CES-D ≥20, higher sCD163 remained associated with significant depression symptoms in the whole study group in multivariable regression (OR=2.41, 95% CI=1.08,5.37, see supplementary table 2). In the analysis which only included PWH with plasma HIV RNA <20 copies/ml, 229 participants remained. As seen in the supplementary table 3, only lower age was associated with SDS in this subgroup.
Figure 2:

Forest plot of multivariable odds ratios (OR) in association with significant depression symptoms stratified by HIV+ group (n=493) and HIV− group (n=291)
ug=micrograms; ml=milliliters; umol=micromoles; cl=centiliters; CRP=C-reactive protein
Discussion
PWH have significantly higher rates of depression compared to individuals without HIV.2–4 Given that depression in PWH is associated with multiple adverse outcomes, including lack of virologic suppression and higher mortality,5–8,31 there is a pressing need to better understand the mechanisms that may underlie depression in PWH. In the current study nested within the MACS, we evaluated significant depression symptoms (SDS) in relation to a panel of six blood biomarkers, including the novel inflammatory marker GlycA. The biomarker that was associated with significant depression symptoms in multivariable analysis was sCD163, even when controlling for current antidepressant therapy. This relationship was particularly strong in the PWH group. The CD163 molecule is highly expressed by macrophages and serves as a scavenger molecule to prevent hemoglobin toxicity.32 sCD163 is the soluble form of this antigen and appears to reflect macrophage activation during inflammatory disease states.33 This marker remains elevated in the blood of PWH despite virologic suppression.34 For many years, there has been speculation of a possible association between macrophage activity and depression.35 For example, the cytokine interferon alpha (IFNα) is expressed by macrophages. This cytokine has a clear link with depression based on studies showing that exogenous administration is commonly associated with depression symptoms.36 Macrophages express several other inflammatory markers including tumor necrosis factor alpha (TNFα), which also has been linked to depression in PWH.10,12 Further investigations should include markers such as IFNα and TNFα to evaluate if these have a mediation effect between macrophage activation and depression symptoms. Although a population of CD163+ macrophages resides in the CNS,37 peripheral macrophage activation may contribute to pathology based on the fact that measurements were from blood in the study.
There is an established body of literature suggesting a link between reactivity of monocyte lineage cells and depression in the general population.38,39 Depressed individuals demonstrate a shift towards a pro-inflammatory phenotype characterized by higher frequency and higher absolute numbers of non-classical monocytes.40 However, other results from our study suggest that some aspects of monocyte lineage activation may not be linked with depression symptoms in PWH. The marker sCD14, which reflects monocyte activation, was not associated with SDS. Additionally, we found that CCL2, a chemokine involved in monocyte recruitment, was not associated with SDS. While we found a relationship between significant depression symptoms and both higher GlycA and higher hsCRP in univariable analysis, these relationships were not statistically significant in the multivariable analysis. The lack of association with GlycA suggests that depression symptoms in PWH may have a slightly different pathogenesis than in people without HIV.14,15 Given that hsCRP is a relatively non-specific marker of inflammation, this may explain why it became non-significant with the inclusion of more specific biomarkers. While we did not find an association between IL-6 and depression, there were some methodologic differences between our study and ones that have identified a relationship with this cytokine. In a study of 102 PWH,41 the patient health questionnaire (PHQ)-9 was used, which has half the questions of the CES-D. Higher blood IL-6 concentration was associated with somatic symptoms (but not cognitive-affective symptoms). In another study of 201 PWH,10 higher blood IL-6 concentration was associated with the presence of depression determined by Mini International Neuropsychiatric Interview (MINI), a structured diagnostic interview based on criteria outlined in the Diagnostic and Statistical Manual of Mental Disorders (DSM). However, participants in that study were ART-naïve and 80% were women, meaning that the study population was significantly different. Finally, the current analysis differs from a previous MACS analysis utilizing data from a different period of time (1984–2010).42 That study utilized exploratory factor analysis to group biomarkers together and found that one particular factor was associated with depression symptoms based on CES-D. The factor included nine different biomarkers, making determination of the relative contribution of individual biomarkers difficult. Additionally, less than 40% of participants with HIV had virologic suppression, and the subgroup analysis limited to participants on ART yielded statistically non-significant results.
We acknowledge the limitations of the current study. Only men were included in MACS, and therefore our findings may not be generalizable to women. While HIV in the United States is predominantly a disease of men,43 the population of PWH worldwide is approximately 50% women.44 Also, given that women are particularly susceptible to depression,45 more inclusion of women in studies of depression in PWH is needed. The newly unified MACS-WIHS cohort (MWCCS) will allow for exploration of sex-based differences in the depression in men and women with and without HIV. The association between depression and certain aspects of inflammation in HIV may be stronger among men. For example, in a study of 316 PWH, there was a stronger association between CRP and depression in men compared to women. Specifically, a CRP concentration of >3mg/L was associated with a 3.6-fold higher odds of depression in men (p=0.002), but only 1.7-fold higher odds (p=0.33) in women.11 In another study that utilized factor analysis to group biomarkers in relation to depression symptoms in PWH, a factor including CRP, IL-6, and d-dimer was associated with increased depression symptoms. However, the finding was again only statistically significant among the men in the study and not among the women.46 Collection of blood for biomarker measurement was typically not performed on the same day as depression assessment in this study.
We acknowledge that several potential biomarkers were not included in the present study. There is literature suggesting a relationship between certain amino acids and depression in PWH. Greater depression symptoms in PWH are associated with both lower plasma tryptophan and higher plasma kynurenine/tryptophan ratio (indicating inflammation associated tryptophan degradation).47,48 Meanwhile, lower concentration of homovanillic acid (the primary metabolic of dopamine) is associated with increased depression symptoms in PWH. This may be from decreased turnover of phenylalanine (which is a precursor to dopamine) during HIV.49 In one pilot study, PWH with at least mild symptoms of depression had significantly higher plasma concentrations of CXCL10, IL-15, granulocyte colony stimulation factor, and IL-12 p40/p70.50 In a Veterans Aging Cohort Study (VACS) analysis from the United States, in which the vast majority of participants were male, higher D-dimer concentration was associated with a slight increase in somatic depression symptoms among PWH.51 However, this relationship became not statistically significant after incorporating other factors such as alcohol and drug use into the models. Another recent pilot study showed that plasma TNFα along with age, glucose levels, and glycosylated hemoglobin most strongly predicted depression based on the Patient Health Questionnaire (PHQ)-9.12 A larger study, which showed that PWH with markedly elevated blood TNFα concentrations were more likely to have major depression, supported this finding.10 Therefore, future studies should incorporate a broader panel of biomarkers. While this study suggests a relationship between macrocyte activation and depressive symptoms in HIV, other processes are likely to be involved. While our study did not find an association between depression and CRP (a marker of general inflammation), other studies of PWH have.12,52 The gut microbiome may play a role, particularly in PWH who are co-infected with hepatitis C virus.53,54 PWH with depression have lower neuroactive steroid levels.55 Depression in HIV is associated with decreased hippocampal volume, suggesting a possible structural component.56 Being an observational study, we cannot determine if there is a causal relationship between sCD163 and depressive symptoms; residual confounding may in part explain the associations found. We acknowledge that the study might have been stronger with the including of cerebrospinal fluid (CSF) biomarkers. However, CSF collection is not routinely performed in the MACS. Lastly, the association we found in this study was with depression symptoms, not the clinical diagnosis of depression by structured interview.
In conclusion, higher blood concentration of sCD163 was associated with significant depression symptoms in a population of men, the majority of whom were PWH. This suggests that depression symptoms and macrophage activation may be linked. Based on our review of the literature, there are no published studies evaluating sCD163 in relation to depression symptoms in PWH. This is a significant finding because it may reflect a different part of the immune system than do markers of overall inflammation such as cytokines. This is also significant because it sets the stage for the study of therapies that target macrophage activation to treat depression in PWH. Given the complexity of macrophage activation,57 further investigation will be required to better define this relationship. More research is clearly needed given the significant morbidity and mortality of the HIV and depression combination.
Sources of funding:
Data in this manuscript were collected by the Multicenter AIDS Cohort Study (MACS), now the MACS/WIHS Combined Cohort Study (MWCCS).
The contents of this publication are solely the responsibility of the authors and do not represent the official views of the National Institutes of Health (NIH). MWCCS (Principal Investigators): Atlanta CRS (Ighovwerha Ofotokun, Anandi Sheth, and Gina Wingood), U01-HL146241; Baltimore CRS (Todd Brown and Joseph Margolick), U01-HL146201; Bronx CRS (Kathryn Anastos and Anjali Sharma), U01-HL146204; Brooklyn CRS (Deborah Gustafson and Tracey Wilson), U01-HL146202; Data Analysis and Coordination Center (Gypsyamber D’Souza, Stephen Gange and Elizabeth Golub), U01-HL146193; Chicago-Cook County CRS (Mardge Cohen and Audrey French), U01-HL146245; Chicago-Northwestern CRS (Steven Wolinsky), U01-HL146240; Northern California CRS (Bradley Aouizerat, Jennifer Price, and Phyllis Tien), U01-HL146242; Los Angeles CRS (Roger Detels and Matthew Mimiaga), U01-HL146333; Metropolitan Washington CRS (Seble Kassaye and Daniel Merenstein), U01-HL146205; Miami CRS (Maria Alcaide, Margaret Fischl, and Deborah Jones), U01-HL146203; Pittsburgh CRS (Jeremy Martinson and Charles Rinaldo), U01-HL146208; UAB-MS CRS (Mirjam-Colette Kempf, Jodie Dionne-Odom, and Deborah Konkle-Parker), U01-HL146192; UNC CRS (Adaora Adimora), U01-HL146194. The MWCCS is funded primarily by the National Heart, Lung, and Blood Institute (NHLBI), with additional co-funding from the Eunice Kennedy Shriver National Institute Of Child Health & Human Development (NICHD), National Institute On Aging (NIA), National Institute Of Dental & Craniofacial Research (NIDCR), National Institute Of Allergy And Infectious Diseases (NIAID), National Institute Of Neurological Disorders And Stroke (NINDS), National Institute Of Mental Health (NIMH), National Institute On Drug Abuse (NIDA), National Institute Of Nursing Research (NINR), National Cancer Institute (NCI), National Institute on Alcohol Abuse and Alcoholism (NIAAA), National Institute on Deafness and Other Communication Disorders (NIDCD), National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), National Institute on Minority Health and Health Disparities (NIMHD), and in coordination and alignment with the research priorities of the National Institutes of Health, Office of AIDS Research (OAR). MWCCS data collection is also supported by UL1-TR000004 (UCSF CTSA), UL1-TR003098 (JHU ICTR), UL1-TR001881 (UCLA CTSI), P30-AI-050409 (Atlanta CFAR), P30-AI-073961 (Miami CFAR), P30-AI-050410 (UNC CFAR), P30-AI-027767 (UAB CFAR), and P30-MH-116867 (Miami CHARM).
The authors gratefully acknowledge funding from the following sources: K23 MH095679, R21 MH118092, R01 AG062387 (Principal Investigator: A. Anderson); RO1 HL095129 (Principal Investigator: W. Post), P30AI094189 (Johns Hopkins Center for AIDS Research) and P30AI050409 (Emory Center for AIDS Research). The study sponsors had no involvement in the design or performance of the project or the analysis of the results.
Supplementary Material
Table 3:
Multivariable model with outcome of significant depression symptoms (CES-D ≥16)
| Variable | Overall | HIV− | HIV+ |
|---|---|---|---|
| OR (95%CI) | OR (95%CI) | OR (95%CI) | |
| Age (years) | 0.97[0.94,1.00] | 1.00[0.95,1.05] | 0.96*[0.92,1.00] |
| MACS Enrollment (cohort 1,2 vs. 3,4) | 1.03[0.66,1.61] | 0.84[0.35,2.01] | 1.13[0.67,1.91] |
| Race (White, non-Hispanic vs. Black, non-Hispanic/Other) | 1.25[0.81,1.92] | 1.18[0.51,2.72] | 1.25[0.75,2.11] |
| Education | |||
| College Graduate | Ref | Ref | Ref |
| Some college | 1.00[0.67,1.50] | 0.90[0.43,1.90] | 0.96[0.58,1.58] |
| High school | 1.73*[1.09,2.76] | 2.27[0.89,5.84] | 1.43[0.82,2.49] |
| Antidepressant therapy | 2.25***[1.55,3.25] | 2.94**[1.46,5.89] | 2.00**[1.28,3.12] |
| HCV status | 1.64[0.86,3.10] | 1.79[0.42,7.72] | 1.67[0.81,3.46] |
| Crack/cocaine last six months | 1.22[0.73,2.04] | 1.11[0.41,2.98] | 1.32[0.71,2.44] |
| GlycA (umol/cl) | 1.01[0.78,1.32] | 1.48[0.87,2.51] | 0.87[0.64,1.19] |
| CRP (ug/ml) | 1.04[0.99,1.09] | 1.02[0.92,1.13] | 1.04[0.99,1.10] |
| sCD163 (ug/ml) | 2.30*[1.11,4.76] | 1.18[0.25,5.67] | 2.72*[1.12,6.58] |
| Observations | 784 | 291 | 493 |
Exponentiated coefficients; 95% confidence intervals in brackets;
p < 0.05,
p < 0.01,
p < 0.001
OR=Odds Ratio; CI=Confidence Interval; umol=micromoles; cl=centiliters; ug=micrograms; ml=milliliters.
Footnotes
Conflicts of Interest:
None declared
References
- 1.Ciesla JA, Roberts JE. Meta-analysis of the relationship between HIV infection and risk for depressive disorders. Am J Psychiatry 2001;158:725–30. [DOI] [PubMed] [Google Scholar]
- 2.Cook JA, Burke-Miller JK, Steigman PJ, et al. Prevalence, Comorbidity, and Correlates of Psychiatric and Substance Use Disorders and Associations with HIV Risk Behaviors in a Multisite Cohort of Women Living with HIV. AIDS and behavior 2018;22:3141–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rubin LH, Maki PM. HIV, Depression, and Cognitive Impairment in the Era of Effective Antiretroviral Therapy. Curr HIV/AIDS Rep 2019;16:82–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Do AN, Rosenberg ES, Sullivan PS, et al. Excess burden of depression among HIV-infected persons receiving medical care in the united states: data from the medical monitoring project and the behavioral risk factor surveillance system. PLoS One 2014;9:e92842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Horberg MA, Silverberg MJ, Hurley LB, et al. Effects of depression and selective serotonin reuptake inhibitor use on adherence to highly active antiretroviral therapy and on clinical outcomes in HIV-infected patients. Journal of acquired immune deficiency syndromes 2008;47:384–90. [DOI] [PubMed] [Google Scholar]
- 6.Kacanek D, Jacobson DL, Spiegelman D, Wanke C, Isaac R, Wilson IB. Incident depression symptoms are associated with poorer HAART adherence: a longitudinal analysis from the Nutrition for Healthy Living study. Journal of acquired immune deficiency syndromes 2010;53:266–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ickovics JR, Hamburger ME, Vlahov D, et al. Mortality, CD4 cell count decline, and depressive symptoms among HIV-seropositive women: longitudinal analysis from the HIV Epidemiology Research Study. JAMA : the journal of the American Medical Association 2001;285:1466–74. [DOI] [PubMed] [Google Scholar]
- 8.Pence BW, Mills JC, Bengtson AM, et al. Association of Increased Chronicity of Depression With HIV Appointment Attendance, Treatment Failure, and Mortality Among HIV-Infected Adults in the United States. JAMA Psychiatry 2018;75:379–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dowlati Y, Herrmann N, Swardfager W, et al. A meta-analysis of cytokines in major depression. Biol Psychiatry 2010;67:446–57. [DOI] [PubMed] [Google Scholar]
- 10.Musinguzi K, Obuku A, Nakasujja N, et al. Association between major depressive disorder and pro-inflammatory cytokines and acute phase proteins among HIV-1 positive patients in Uganda. BMC Immunol 2018;19:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Poudel-Tandukar K, Bertone-Johnson ER, Palmer PH, Poudel KC. C-reactive protein and depression in persons with Human Immunodeficiency Virus infection: the Positive Living with HIV (POLH) Study. Brain, behavior, and immunity 2014;42:89–95. [DOI] [PubMed] [Google Scholar]
- 12.Zuniga JA, Harrison ML, Henneghan A, Garcia AA, Kesler S. Biomarkers panels can predict fatigue, depression and pain in persons living with HIV: A pilot study. Appl Nurs Res 2020;52:151224. [DOI] [PubMed] [Google Scholar]
- 13.Otvos JD, Shalaurova I, Wolak-Dinsmore J, et al. GlycA: A Composite Nuclear Magnetic Resonance Biomarker of Systemic Inflammation. Clin Chem 2015;61:714–23. [DOI] [PubMed] [Google Scholar]
- 14.Brunoni AR, Salum GA, Hoffmann MS, et al. Prospective associations between hsCRP and GlycA inflammatory biomarkers and depression: The Brazilian longitudinal study of adult health (ELSA-Brasil). J Affect Disord 2020;271:39–48. [DOI] [PubMed] [Google Scholar]
- 15.Huckvale S, Reyes S, Kulikova A, Rohatgi A, Riggs KA, Brown ES. An Association Between the Inflammatory Biomarker GlycA and Depressive Symptom Severity. J Clin Psychiatry 2020;82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pereyra F, Lo J, Triant VA, et al. Increased coronary atherosclerosis and immune activation in HIV-1 elite controllers. AIDS 2012;26:2409–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sandler NG, Wand H, Roque A, et al. Plasma levels of soluble CD14 independently predict mortality in HIV infection. The Journal of infectious diseases 2011;203:780–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tibuakuu M, Fashanu OE, Zhao D, et al. GlycA, a novel inflammatory marker, is associated with subclinical coronary disease. AIDS 2019;33:547–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Post WS, Budoff M, Kingsley L, et al. Associations between HIV infection and subclinical coronary atherosclerosis. Annals of internal medicine 2014;160:458–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kagee A, Bantjes J, Saal W, Sterley A. Predicting caseness of major depressive disorder using the Center for Epidemiological Studies Depression Scale (CESD-R) among patients receiving HIV care. Gen Hosp Psychiatry 2020;67:70–6. [DOI] [PubMed] [Google Scholar]
- 21.Nagasawa T CXC chemokine ligand 12 (CXCL12) and its receptor CXCR4. Journal of molecular medicine 2014;92:433–9. [DOI] [PubMed] [Google Scholar]
- 22.Lewinsohn PM, Seeley JR, Roberts RE, Allen NB. Center for Epidemiologic Studies Depression Scale (CES-D) as a screening instrument for depression among community-residing older adults. Psychol Aging 1997;12:277–87. [DOI] [PubMed] [Google Scholar]
- 23.Pettit JW, Lewinsohn PM, Seeley JR, Roberts RE, Hibbard JH, Hurtado AV. Association between the Center for Epidemiologic Studies Depression Scale (CES-D) and mortality in a community sample: An artifact of the somatic complaints factor? Int J Clin Health Psychol 2008;8:383–97. [PMC free article] [PubMed] [Google Scholar]
- 24.Fann JR, Hart T, Schomer KG. Treatment for depression after traumatic brain injury: a systematic review. J Neurotrauma 2009;26:2383–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gaete JM, Bogousslavsky J. Post-stroke depression. Expert Rev Neurother 2008;8:75–92. [DOI] [PubMed] [Google Scholar]
- 26.Bahrami H, Budoff M, Haberlen SA, et al. Inflammatory Markers Associated With Subclinical Coronary Artery Disease: The Multicenter AIDS Cohort Study. J Am Heart Assoc 2016;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McKibben RA, Margolick JB, Grinspoon S, et al. Elevated levels of monocyte activation markers are associated with subclinical atherosclerosis in men with and those without HIV infection. The Journal of infectious diseases 2015;211:1219–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rubin LH, Gustafson D, Hawkins KL, et al. Midlife adiposity predicts cognitive decline in the prospective Multicenter AIDS Cohort Study. Neurology 2019;93:e261–e71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Becker JT, Kingsley L, Mullen J, et al. Vascular risk factors, HIV serostatus, and cognitive dysfunction in gay and bisexual men. Neurology 2009;73:1292–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Checa A, Castillo A, Camacho M, Tapia W, Hernandez I, Teran E. Depression is associated with efavirenz-containing treatments in newly antiretroviral therapy initiated HIV patients in Ecuador. AIDS research and therapy 2020;17:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Regan M, Muhihi A, Nagu T, et al. Depression and Viral Suppression Among Adults Living with HIV in Tanzania. AIDS and behavior 2021;25:3097–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fabriek BO, Dijkstra CD, van den Berg TK. The macrophage scavenger receptor CD163. Immunobiology 2005;210:153–60. [DOI] [PubMed] [Google Scholar]
- 33.Bleesing J, Prada A, Siegel DM, et al. The diagnostic significance of soluble CD163 and soluble interleukin-2 receptor alpha-chain in macrophage activation syndrome and untreated new-onset systemic juvenile idiopathic arthritis. Arthritis Rheum 2007;56:965–71. [DOI] [PubMed] [Google Scholar]
- 34.Burdo TH, Lentz MR, Autissier P, et al. Soluble CD163 made by monocyte/macrophages is a novel marker of HIV activity in early and chronic infection prior to and after anti-retroviral therapy. The Journal of infectious diseases 2011;204:154–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smith RS. The macrophage theory of depression. Med Hypotheses 1991;35:298–306. [DOI] [PubMed] [Google Scholar]
- 36.Hoyo-Becerra C, Schlaak JF, Hermann DM. Insights from interferon-alpha-related depression for the pathogenesis of depression associated with inflammation. Brain, behavior, and immunity 2014;42:222–31. [DOI] [PubMed] [Google Scholar]
- 37.Prinz M, Priller J. Microglia and brain macrophages in the molecular age: from origin to neuropsychiatric disease. Nature reviews Neuroscience 2014;15:300–12. [DOI] [PubMed] [Google Scholar]
- 38.Lisi L, Camardese G, Treglia M, et al. Monocytes from depressed patients display an altered pattern of response to endotoxin challenge. PLoS One 2013;8:e52585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ding KQ, Lai ZH, Zhang Y, Yang GY, He JR, Zeng LL. Monocyte-to-Lymphocyte Ratio is Associated with Depression 3 Months After Stroke. Neuropsychiatr Dis Treat 2021;17:835–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hasselmann H, Gamradt S, Taenzer A, et al. Pro-inflammatory Monocyte Phenotype and Cell-Specific Steroid Signaling Alterations in Unmedicated Patients With Major Depressive Disorder. Front Immunol 2018;9:2693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Norcini Pala A, Steca P, Bagrodia R, et al. Subtypes of depressive symptoms and inflammatory biomarkers: An exploratory study on a sample of HIV-positive patients. Brain, behavior, and immunity 2016;56:105–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lu H, Surkan PJ, Irwin MR, et al. Inflammation and Risk of Depression in HIV: Prospective Findings From the Multicenter AIDS Cohort Study. Am J Epidemiol 2019;188:1994–2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Centers for Disease Control. HIV in the United States at a Glance. https://www.cdc.gov/hiv/statistics/overview/ataglance.html. Accessed April 6/2021.
- 44.WorldHealthOrganization. World Health Organization HIV/AIDS fact sheet: http://www.who.int/mediacentre/factsheets/fs360/en/. 2016.
- 45.Whiteford HA, Degenhardt L, Rehm J, et al. Global burden of disease attributable to mental and substance use disorders: findings from the Global Burden of Disease Study 2010. Lancet 2013;382:1575–86. [DOI] [PubMed] [Google Scholar]
- 46.Ellis RJ, Letendre SL, Atkinson JH, et al. Higher levels of plasma inflammation biomarkers are associated with depressed mood and quality of life in aging, virally suppressed men, but not women, with HIV. Brain Behav Immun Health 2020;7:100121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Martinez P, Tsai AC, Muzoora C, et al. Reversal of the Kynurenine pathway of tryptophan catabolism may improve depression in ART-treated HIV-infected Ugandans. Journal of acquired immune deficiency syndromes 2014;65:456–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lee JY, Glynn TR, Moskowitz JT, et al. Tryptophan depletion predicts lower positive affect in sexual minority men living with HIV who use methamphetamine. Journal of neurovirology 2021;27:178–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gostner JM, Becker K, Kurz K, Fuchs D. Disturbed Amino Acid Metabolism in HIV: Association with Neuropsychiatric Symptoms. Front Psychiatry 2015;6:97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rivera-Rivera Y, Garcia Y, Toro V, et al. Depression Correlates with Increased Plasma Levels of Inflammatory Cytokines and a Dysregulated Oxidant/Antioxidant Balance in HIV-1-Infected Subjects Undergoing Antiretroviral Therapy. J Clin Cell Immunol 2014;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stewart JC, Polanka BM, So-Armah KA, et al. Associations of Total, Cognitive/Affective, and Somatic Depressive Symptoms and Antidepressant Use With Cardiovascular Disease-Relevant Biomarkers in HIV: Veterans Aging Cohort Study. Psychosom Med 2020;82:461–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Saloner R, Paolillo EW, Heaton RK, et al. Chronically elevated depressive symptoms interact with acute increases in inflammation to predict worse neurocognition among people with HIV. Journal of neurovirology 2021;27:160–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Taylor BC, Weldon KC, Ellis RJ, et al. Depression in Individuals Coinfected with HIV and HCV Is Associated with Systematic Differences in the Gut Microbiome and Metabolome. mSystems 2020;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Perez-Santiago J, Marquine MJ, Cookson D, et al. Gut microbiota dysbiosis is associated with worse emotional states in HIV infection. Journal of neurovirology 2021;27:228–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mukerji SS, Misra V, Lorenz DR, et al. Low Neuroactive Steroids Identifies a Biological Subtype of Depression in Adults with Human Immunodeficiency Virus on Suppressive Antiretroviral Therapy. The Journal of infectious diseases 2021;223:1601–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bronshteyn M, Yang FN, Shattuck KF, et al. Depression is associated with hippocampal volume loss in adults with HIV. Hum Brain Mapp 2021;42:3750–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nature reviews Immunology 2008;8:958–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


