Abstract
An indirect fluorescence antibody (IFA) procedure was used for the rapid detection of respiratory viruses in direct clinical specimens and for determining the epidemiology of viruses in a community hospital setting. Viral respiratory diseases were monitored for 10 consecutive respiratory seasons. The Bartels Viral Respiratory Screening and Identification Kit is an IFA method that contains pooled and individual monoclonal antibodies for seven common respiratory viruses. Compared with 8,670 conventional tube cell cultures, IFA staining of direct patient specimens had an overall sensitivity of 84.2% and a specificity of 87.7%. Yearly epidemics of respiratory syncytial virus were seen with alternating short and long intervals between successive periods when virus was isolated. Epidemics following short intervals were more severe. Influenza A virus epidemics occurred yearly, and influenza B virus activity was seen generally every other year. When influenza A and influenza B viruses were cocirculating in a given season, the months of peak activity of one virus were always within 1 month of the peak activity of the other virus. Parainfluenza virus type 1 was detected in the autumn of odd-numbered years, and parainfluenza type 2 virus was seen usually in the autumn of even-numbered years. Parainfluenza type 3 virus and adenovirus were the most ubiquitous agents, with peak incidence occurring in the late winter to spring.
Community hospitals are faced with the need to determine the type of viral diagnostic services to offer. Cost constraints must be weighed against medical necessity. Virus isolation in cell culture is not usually an answer since it is labor intensive, demands a high level of expertise, and may require several days to weeks to complete. Rapid viral diagnostic procedures are expensive and have limited utility because they generally target a single virus type. Physicians request virology services because nearly half of ill children seen by the primary care physician have acute respiratory ailments (9). A significant number of such illnesses are of viral origin (9). The decision to use antiviral agents or antibiotics can be made only on clinical and epidemiologic grounds if viral diagnostic services are not available. Such treatment decisions are problematic because there is a significant overlap in the clinical syndromes caused by different infectious organisms (4, 11, 16). Although diagnostic assumptions can be made from general epidemiologic patterns, the epidemiologic aspect most variable and most likely to differ among geographic locations is the seasonal occurrence of infections with specific agents (4). The decision to offer viral diagnostic services depends on the availability of reliable, easy to perform, cost-effective, and rapid viral diagnostic tests. These tests should be broad enough so that epidemiologic patterns for specific viruses can be determined and appropriate antiviral therapy can be initiated. The Bartels indirect fluorescence antibody (IFA) kit has been evaluated in university hospital settings (14, 17) for identifying viruses in direct clinical specimens and in cell culture. The sensitivity and specificity of the kit compared to shell vial cultures were 85.9 and 87.1%, respectively. Compared to conventional tube cell culture, the sensitivity and specificity were 69 and 97%, respectively. These studies concluded that the kit provided potentially cost-effective, useful same-day screening of respiratory specimens for viruses.
The Bartels kit contains monoclonal antibodies to seven common respiratory viruses (respiratory syncytial virus [RSV]; influenza A and B viruses [FLUA and FLUB]; parainfluenza virus types 1, 2, and 3 [PIV1, -2, and -3]; and adenovirus [ADENO]), which broadens the potential of this product as an epidemiologic tool. Epidemiologic patterns have been determined for respiratory viruses in many communities (4, 9, 12, 13, 16), and the occurrence of these viruses in different age groups defines the populations in which specific antiviral therapy, infection control practices, and prophylaxis could be beneficial. Determination of viral epidemiology for a specific geographical region significantly enhances the treatment guidelines for clinicians.
Medcenter One Health Systems is located in Bismarck, N.D., and is comprised of a 241-bed, acute-care hospital; a 100-physician multispecialty clinic (Quain and Ramstad Clinic); and 14 regional clinics serving the surrounding community. For 10 years, the rapid direct detection of respiratory viruses using the Bartels kit has been offered to the clinicians in the community. The efficiency of this IFA procedure for detecting respiratory virus antigens in direct clinical specimens was examined, and the results were analyzed to determine the epidemiology of respiratory viral disease in the Bismarck area.
MATERIALS AND METHODS
Specimens.
Respiratory specimens included in this study were submitted to the Microbiology Laboratory at Medcenter One Health Systems–Quain and Ramstad Clinic, Bismarck, N.D., from December 1987 through July 1998. Nasopharyngeal swab (NPS), nasopharyngeal aspirate (NPA), and nasopharyngeal wash (NPW) specimens were collected by physicians and nursing personnel from clinic outpatients or hospital inpatients, or specimens were referred to the laboratory from neighboring clinics and hospitals. The method described by Hall and Douglas (9) was used to collect NPW specimens. At the patient's bedside, a 1-oz. rubber ear and ulcer syringe (Davol, Inc., Cranston, R.I.) was loaded with 3 to 5 ml of sterile phosphate-buffered saline (PBS). With the patient's head tipped back at about 70°, the bulb was inserted until it occluded the nostril. With one complete squeeze and release, the nasal wash was collected in the bulb. The bulb contents were then emptied into a sterile screw-capped tube and transported to the laboratory. NPA specimens were collected using a pediatric Safe-T-Vac suction catheter (Kendall Healthcare Products, Mansfield, Mass.). Normal saline was instilled into the nasopharynx, and the specimen was aspirated into a sterile specimen trap. NPS specimens were collected on Mini-Tip culturettes (Becton Dickinson Microbiology Systems, Cockeysville, Md.) by inserting the swab 4 to 5 cm into the nostril. The swab was left in place for approximately 5 s, carefully removed, and immediately placed into the culturette container with modified Stuart's medium for transport to the laboratory. Collection of two NPS specimens was strongly encouraged in order to ensure a sufficient number of ciliated epithelial cells for microscopic examination. Specimens containing fewer than two ciliated epithelial cells/×400 magnification field were judged to be unsuitable for immunofluorescent assay. All respiratory specimens were stored at 4°C until processed, usually within 24 h of receipt.
Specimen processing.
NPW, NPA, and NPS specimens in 1 ml of PBS were vortexed (10 s, highest speed setting) to create a primary cell suspension and then centrifuged (5 min, 1,500 × g) to pellet the cells. The supernatant was used to inoculate cell culture. The concentrated cells were resuspended in approximately 0.5 ml of PBS, and direct patient slides were prepared by spotting one drop of the suspension onto each well of a 10-well glass slide (wettable, acetone resistant; Cel-line Associates, Inc., Newfield, N.J.). The direct patient slides were then air dried, fixed in cold acetone for 10 min, and stained by IFA.
Cell culture isolations.
Primary monkey kidney (PMK), human lung laryngeal epidermoid carcinoma (HEp-2), and human embryonal diploid lung (MRC-5) cells in 16-by-125-mm glass culture tubes were obtained from Bartels, Inc. (Issaquah, Wash.), or Viromed Laboratories (Minneapolis, Minn.). Culture refeeding medium consisted of Earle's minimal essential medium containing gentamicin (10 μg/ml), streptomycin (50 μg/ml), penicillin (50 μg/ml), and amphotericin B (4 μg/ml). The maintenance medium was decanted from the culture cells, and each tube was inoculated with 0.3 ml of supernatant from the primary cell suspension. After adding 2 ml of refeeding medium to each tube, the cultures were incubated at 35°C and examined every other day for cytopathic effect (CPE). After 7 to 10 days of incubation or when CPE was evident, the medium was decanted, and the cell monolayers were washed with 2 ml of sterile PBS. The PBS was decanted, and the cell monolayers were scraped into the residual PBS with a sterile disposable pipette. The cells were spotted onto a glass slide, air dried, fixed in cold acetone for 10 min, and stained by IFA.
Immunofluorescence staining.
The Bartels Viral Respiratory Screening and Identification Kit was used for staining direct patient slides and cell monolayers. The kit contains individual murine monoclonal antibodies specific for seven common respiratory viruses: RSV, FLUA, FLUB, PIV1, PIV2, PIV3, and ADENO; a murine monoclonal antibody pool; a fluorescein isothiocyanate-labeled rabbit anti-mouse antibody with Evan's blue counterstain (conjugate); and a negative control containing nonimmune rabbit antibodies. The pool reagent contained monoclonal antibodies specific for all seven virus types.
Direct patient slides were stained simultaneously with the pool reagent, the negative control, and the individual virus-specific reagents. Cell monolayers were stained first with the pool reagent and, if positive, with the individual virus-specific reagents. One drop (approximately 30 μl) of pool reagent, negative control, and virus-specific reagent was added to each well. The slides were incubated for 30 min at 35°C in a humidified chamber, washed in PBS with gentle agitation for 10 min, and air dried. One drop (approximately 30 μl) of conjugate was added to each well, and the slide was incubated for 30 min in a humidified chamber. After a wash with agitation in PBS for 10 min, the slides were air dried and coverslipped with buffered glycerol.
Immunofluorescence microscopy.
Slides were examined at ×200 or ×400 magnifications with incident light illumination using a Zeiss fluorescent microscope (Carl Zeiss, Inc., Thornwood, N.Y.). A positive immunofluorescence result (virus antigen present) was dependent upon observation of specific intracellular fluorescence patterns: cytoplasmic for RSV and the parainfluenza viruses, nuclear for INFA and INFB, and cytoplasmic and nuclear for ADENO. All wells were observed for IFA-positive cells, with the final interpretation made from the wells stained with virus-specific reagents. At least two fluorescent cells per well were required for a positive result when reading direct patient slides and cell monolayers.
Data analysis.
The relative efficiencies of immunofluorescence reagents were determined by using isolation in a conventional tube cell culture as the standard. Sample pairs which were both culture positive and immunofluorescence positive were considered to be true positive (TP), culture-negative and immunofluorescence-positive pairs were false positive (FP), culture-positive and immunofluorescence-negative pairs were false negative (FN), and specimen pairs that were culture negative and immunofluorescence negative were true negative (TN). Sensitivity (SENS), specificity (SPEC), and predictive values were calculated as follows: SENS = (TP/TP + FN) × 100; SPEC = (TN/TN + FP) × 100; positive predictive value (PPV) = (TP/TP + FP) × 100; and negative predictive value (NPV) = (TN/TN + FN) × 100.
RESULTS
Study population.
A total of 10,495 respiratory specimens were included in the study; 8,670 specimens were tested by IFA and conventional tube cell culture, and 1,825 specimens were tested by IFA or tube cell culture only. A total of 367 specimens were rejected because of an insufficient number of ciliated epithelial cells. Patients were grouped by age as follows: infants ≤1 year of age, children >1 to 6 years of age, adolescents and young adults 7 to 25 years of age, adults 26 to 49 years of age, and adults ≥50 years of age. The majority (6,384 or 60.5%) of the specimens was collected from the youngest patients (<6 years of age); however, the older groups of 7 to 25 years, 26 to 49 years, and ≥50 years were well represented (13.7, 9.6, and 15.9% of all specimens, respectively).
Viral incidence.
The study involves the composite analysis of 10 consecutive respiratory seasons; a respiratory season being defined as July of one year through June of the next year. The annual incidence for each age group and the yearly incidence of respiratory viruses in the Bismarck area for each viral type are given in Tables 1 and 2, respectively. Virus identifications include identifications made by IFA and/or conventional tube cell culture. Patients in the ≤1-year and the >1- to 6-year age groups had the highest viral attack rates, with almost half of these patients (45.2 and 46.7%, respectively) showing evidence of viral infection. Significant viral morbidity was also seen in the other age groups; 33.1% in the 7- to 25-year age group, 24.3% in the 26- to 49-year age group, and 21.1% in the ≥50-year age group. RSV was the most frequently encountered virus in the age groups ≤1 and >1 to 6 years. Influenza viruses were the most frequently identified viruses in the remaining age groups. The annual viral incidence varied from 30.9 to 45.7%, with an average of 38.1%. RSV was the most frequently identified virus (15.9%), followed by influenza viruses (15.0%), parainfluenza viruses (5.0%), and ADENO (2.2%).
TABLE 1.
Annual incidence of viruses by age group in the Bismarck, N.D., area
| Age (yr) | Yr | No. of samples | No. of virus identifications (%)a
|
Incidence (%)b | |||
|---|---|---|---|---|---|---|---|
| RSV | Influenza virus | Parainfluenza virus | ADENO | ||||
| 1 | 87–88 | 76 | 31 (40.8) | 3 (3.9) | 8 (10.5) | 2 (2.6) | 44 (57.9) |
| 88–89 | 143 | 44 (30.8) | 16 (4.2) | 4 (2.8) | 3 (2.1) | 57 (39.9) | |
| 89–90 | 308 | 107 (34.7) | 15 (4.9) | 22 (7.1) | 13 (4.2) | 157 (51.0) | |
| 90–91 | 415 | 117 (28.2) | 15 (3.6) | 42 (10.1) | 20 (4.8) | 197 (46.8) | |
| 91–92 | 470 | 169 (36.0) | 28 (6.0) | 29 (6.2) | 9 (1.9) | 235 (50.0) | |
| 92–93 | 274 | 20 (7.3) | 27 (9.9) | 26 (9.5) | 12 (4.4) | 85 (31.0) | |
| 93–94 | 421 | 137 (32.5) | 36 (8.6) | 29 (6.0) | 13 (3.1) | 215 (51.1) | |
| 94–95 | 282 | 70 (24.8) | 10 (3.6) | 33 (11.7) | 7 (4.5) | 120 (42.6) | |
| 95–96 | 316 | 102 (32.3) | 26 (8.2) | 30 (9.5) | 11 (3.5) | 169 (53.5) | |
| 96–97 | 226 | 16 (7.1) | 28 (12.4) | 22 (9.7) | 4 (1.8) | 70 (31.0) | |
| 97–98 | 445 | 138 (31.0) | 31 (7.0) | 7 (1.6) | 2 (0.5) | 178 (40.0) | |
| Total | 3,376 | 951 (28.2) | 225 (6.7) | 252 (7.5) | 96 (2.8) | 1,524 (45.2) | |
| 1–6 | 87–88 | 38 | 13 (34.2) | 6 (15.8) | 1 (2.6) | 1 (2.6) | 21 (55.3) |
| 88–89 | 77 | 12 (15.6) | 13 (16.9) | 5 (6.5) | 5 (6.5) | 35 (45.3) | |
| 89–90 | 324 | 89 (27.5) | 44 (13.6) | 26 (8.0) | 12 (3.7) | 171 (52.8) | |
| 90–91 | 315 | 57 (18.1) | 46 (14.6) | 21 (6.7) | 15 (4.8) | 139 (44.1) | |
| 91–92 | 397 | 122 (30.7) | 38 (9.6) | 36 (9.1) | 7 (1.8) | 203 (51.1) | |
| 92–93 | 318 | 9 (2.8) | 69 (21.7) | 19 (6.0) | 11 (3.5) | 108 (40.0) | |
| 93–94 | 410 | 94 (22.9) | 120 (29.3) | 29 (7.1) | 6 (1.5) | 249 (60.7) | |
| 94–95 | 266 | 45 (16.9) | 16 (6.0) | 19 (7.1) | 27 (10.2) | 107 (40.2) | |
| 95–96 | 239 | 65 (27.2) | 29 (21.1) | 26 (10.9) | 1 (0.4) | 121 (50.6) | |
| 96–97 | 228 | 5 (2.2) | 77 (33.8) | 16 (7.0) | 3 (1.3) | 101 (44.3) | |
| 97–98 | 396 | 83 (20.1) | 48 (12.1) | 10 (2.5) | 9 (2.3) | 150 (37.9) | |
| Total | 3,008 | 594 (19.8) | 506 (16.8) | 208 (6.9) | 97 (3.2) | 1,405 (46.7) | |
| 7–25 | 87–88 | 18 | 0 0 | 6 (33.0) | 2 (11.0) | 2 (11.0) | 10 (55.6) |
| 88–89 | 34 | 1 (2.9) | 7 (20.6) | 0 0 | 0 0 | 8 (23.5) | |
| 89–90 | 115 | 13 (11.3) | 20 (17.4) | 0 0 | 0 0 | 33 (28.7) | |
| 90–91 | 117 | 3 (2.6) | 23 (19.7) | 0 0 | 2 (1.7) | 28 (23.9) | |
| 91–92 | 125 | 9 (7.2) | 25 (20.0) | 1 (0.8) | 1 (0.8) | 36 (28.8) | |
| 92–93 | 191 | 1 (0.5) | 63 (33.0) | 5 (2.6) | 4 (2.1) | 73 (38.2) | |
| 93–94 | 158 | 6 (3.8) | 48 (30.4) | 1 (0.6) | 0 0 | 55 (34.8) | |
| 94–95 | 116 | 3 (2.6) | 20 (17.2) | 4 (3.5) | 6 (5.2) | 33 (28.5) | |
| 95–96 | 132 | 2 (1.5) | 54 (40.9) | 3 (2.3) | 5 (3.8) | 64 (48.5) | |
| 96–97 | 247 | 0 0 | 93 (37.7) | 3 (1.2) | 0 0 | 96 (38.9) | |
| 97–98 | 189 | 6 (3.2) | 33 (17.4) | 2 (1.1) | 0 0 | 41 (22.0) | |
| Total | 1,442 | 44 (3.1) | 392 (27.2) | 21 (2.1) | 20 (1.4) | 477 (33.1) | |
| 26–49 | 87–88 | 11 | 0 0 | 2 (18.2) | 0 0 | 0 0 | 2 (18.2) |
| 88–89 | 34 | 0 0 | 2 (5.9) | 0 0 | 0 0 | 2 (5.9) | |
| 89–90 | 44 | 1 (2.3) | 6 (13.6) | 0 0 | 0 0 | 7 (15.9) | |
| 90–91 | 30 | 0 0 | 5 (16.7) | 0 0 | 0 0 | 5 (16.7) | |
| 91–92 | 71 | 4 (5.6) | 9 (12.7) | 1 (1.4) | 0 0 | 14 (19.7) | |
| 92–93 | 95 | 0 0 | 24 (25.3) | 4 (4.2) | 0 0 | 28 (29.5) | |
| 93–94 | 166 | 4 (2.4) | 43 (25.9) | 4 (2.4) | 1 (0.6) | 52 (31.3) | |
| 94–95 | 81 | 0 0 | 3 (3.7) | 0 0 | 8 (9.9) | 11 (13.8) | |
| 95–96 | 108 | 0 0 | 35 (32.4) | 2 (1.9) | 0 0 | 37 (34.3) | |
| 96–97 | 230 | 0 0 | 57 (24.8) | 1 (0.4) | 1 (0.4) | 59 (25.7) | |
| 97–98 | 133 | 4 (3.0) | 23 (17.3) | 0 0 | 0 0 | 27 (20.3) | |
| Total | 1,003 | 13 (1.3) | 209 (20.8) | 12 (1.2) | 10 (1.0) | 244 (24.3) | |
| >50 | 87–88 | 53 | 1 (1.9) | 5 (9.4) | 0 0 | 1 (1.9) | 7 (13.2) |
| 88–89 | 70 | 3 (4.3) | 8 (11.4) | 0 0 | 0 0 | 11 (15.7) | |
| 89–90 | 128 | 8 (6.3) | 14 (10.9) | 0 0 | 1 (0.8) | 23 (18.0) | |
| 90–91 | 36 | 0 0 | 3 (8.3) | 2 (5.6) | 0 0 | 5 (13.9) | |
| 91–92 | 147 | 12 (8.2) | 25 (17.0) | 4 (2.7) | 0 0 | 41 (27.9) | |
| 92–93 | 123 | 1 (0.8) | 17 (13.8) | 4 (3.3) | 0 0 | 22 (17.9) | |
| 93–94 | 247 | 16 (6.5) | 46 (18.6) | 8 (3.2) | 0 0 | 70 (28.3) | |
| 94–95 | 154 | 2 (1.3) | 13 (8.4) | 5 (3.2) | 0 0 | 20 (13.0) | |
| 95–96 | 188 | 13 (6.9) | 16 (8.5) | 3 (1.6) | 2 (1.1) | 33 (18.1) | |
| 96–97 | 300 | 0 0 | 46 (15.3) | 5 (1.7) | 3 (1.0) | 54 (18.0) | |
| 97–98 | 220 | 14 (6.4) | 48 (21.8) | 2 (0.9) | 0 0 | 64 (29.1) | |
| Total | 1,666 | 70 (4.2) | 241 (14.5) | 33 (2.0) | 7 (0.4) | 351 (21.1) | |
Number of virus identifications/number of samples tested.
Total number of viruses identified/number of samples tested.
TABLE 2.
Total respiratory isolations by year in the Bismarck, N.D., area
| Yr | No. of samples | No. of virus identifications (%)a
|
Incidence (%)b | |||
|---|---|---|---|---|---|---|
| RSV | Influenza virus | Parainfluenza virus | ADENO | |||
| 87–88 | 196 | 45 (23.1) | 21 (10.8) | 12 (6.2) | 5 (6.9) | 83 (42.6) |
| 88–89 | 358 | 60 (16.8) | 36 (10.1) | 9 (2.5) | 8 (2.2) | 113 (31.6) |
| 89–90 | 919 | 218 (23.7) | 99 (10.8) | 48 (5.2) | 26 (2.2) | 391 (42.5) |
| 90–91 | 913 | 177 (12.8) | 92 (10.1) | 65 (7.1) | 37 (4.1) | 371 (40.6) |
| 91–92 | 1,210 | 316 (26.1) | 125 (10.3) | 71 (5.9) | 17 (1.4) | 529 (43.7) |
| 92–93 | 1,001 | 31 (3.1) | 200 (20.0) | 58 (5.8) | 27 (2.7) | 316 (31.6) |
| 93–94 | 1,402 | 257 (18.3) | 293 (20.9) | 71 (5.1) | 20 (1.4) | 641 (45.7) |
| 94–95 | 899 | 120 (12.2) | 62 (6.9) | 61 (6.8) | 48 (5.3) | 291 (32.4) |
| 95–96 | 983 | 182 (18.5) | 160 (16.3) | 64 (6.5) | 19 (1.9) | 425 (43.2) |
| 96–97 | 1,231 | 21 (1.7) | 301 (24.5) | 47 (3.8) | 11 (0.9) | 380 (30.9) |
| 97–98 | 1,383 | 245 (17.7) | 182 (13.2) | 22 (1.6) | 11 (0.8) | 461 (33.3) |
| Total | 10,495 | 1,672 (15.9) | 1,572 (15.0) | 528 (5.0) | 229 (2.2) | 4,001 (38.1) |
Number of specific virus identifications/number of samples tested.
Total number of viruses identified/number of samples tested.
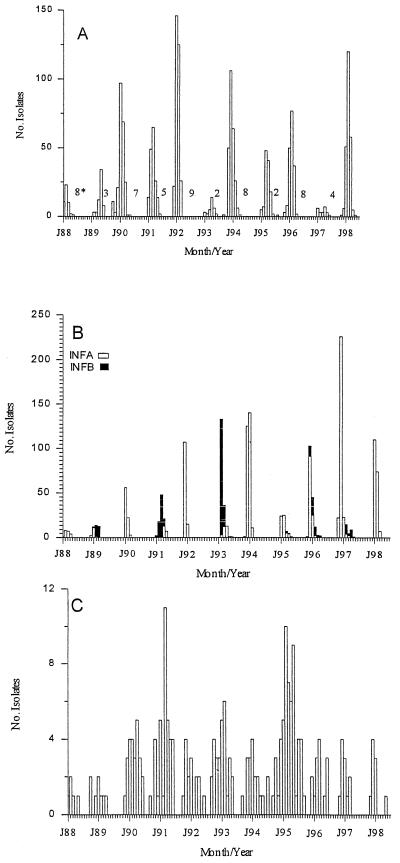
Epidemics of RSV were seen in each of the respiratory seasons (Fig. 1). An initial appearance in the late fall or early winter was followed by peak prevalence 2 to 4 months later. Alternating short (2- to 5-month) and long (7- to 9-month) intervals occurred between successive periods when virus was recovered. There was a tendency for the incidence of RSV to be higher during the epidemic immediately following a short interval. The month of peak RSV activity seemed to occur earlier in the year (December through February) following a short interval and for peak activity to occur later in the season (March through May) following a long interval. RSV was rarely seen between July and October.
FIG. 1.
Seasonality of RSV (A), influenza viruses (B), and ADENO (C). The time scale on the x axis is from January 1988 to July 1998. Numbers within the figure (∗) give alternating long and short intervals between outbreaks.
Influenza epidemics were seen each year of the study (Fig. 1). With the exception of the 1996 to 1997 season, there was an alternating high and low annual incidence of INFA. The seasons with high INFA activity were usually devoid of INFB activity. The seasons with lower INFA activity usually coincided with outbreaks of INFB. INFB activity was generally seen every other year. In the seasons when INFA and INFB were cocirculating, the months of peak activity of one virus was always within 1 month of the peak activity of the other virus. The activity of one virus would rapidly wane with the appearance of the other influenza virus type. Even if both influenza viruses types were circulating in the community, the influenza season never lasted longer than 5 months, with most activity occurring within a 2-month period.
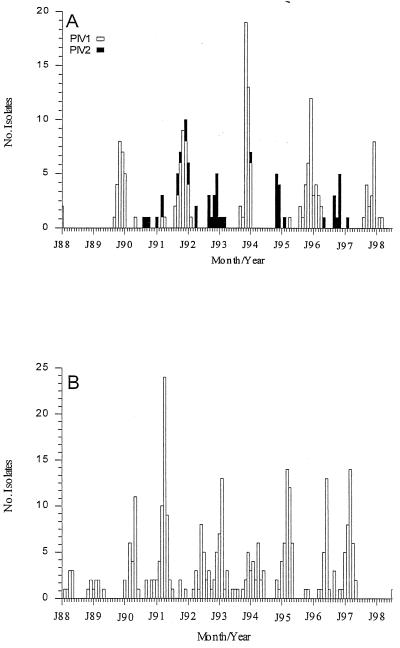
PIV1 was detected in the autumn of odd-numbered years (Fig. 2), with peak activity occurring in November or December. PIV2 was seen in 7 of the 11 seasons of the study (Fig. 2), with only a single laboratory diagnosis in 1994 to 1995 and 1996 to 1997. Many of the peak outbreaks of PIV2 occurred during December of even-numbered years, which were devoid of PIV1 activity. PIV3 was seen in each of the study years with the exception of 1997 to 1998 (Fig. 2). In contrast to PIV1 and PIV2, the months of peak PIV3 activity varied between February and June of each year.
FIG. 2.
Seasonality of PIV1 and PIV2 (A) and PIV3 (B).
ADENO were detected in each year of the study with the peak incidence occurring in midwinter (Fig. 1). PIV3 and ADENO were the most ubiquitous of the agents, occurring in most months of the year. ADENO were much less common in school age children and adults than in children 6 years of age or younger (Table 1).
There was a tendency for major viral agents not to cause simultaneous epidemics (Table 3). Peak RSV activity and peak FLUA activity occurred during the same month only three times (respiratory seasons 1987 to 1988, 1989 to 1990, and 1992 to 1993). RSV and FLUB peaked together only twice (1990 to 1991 and 1994 to 1995). Only rarely did RSV peak activity occur concurrently with the parainfluenza viruses; never with PIV1, once with PIV2 (1990 to 1991) and once with PIV3 (1994 to 1995).
TABLE 3.
Peak month of viral activity
| Yr | Peak mo of virus activity
|
||||||
|---|---|---|---|---|---|---|---|
| RSV | FLUA | FLUB | PIV1 | PIV2 | PIV3 | ADENO | |
| 87–88 | Feb | Feb | –a | Jan | – | Apr | Feb |
| 88–89 | May | Jan | Feb | – | – | Feb | Apr |
| 89–90 | Jan | Jan | – | Nov | – | May | Apr |
| 90–91 | Mar | Apr | Mar | NPMb | Mar | Apr | Jan |
| 91–92 | Jan | Dec | – | Nov | NPM | Jun | Nov |
| 92–93 | Apr | Apr | Feb | – | Dec | Feb | Feb |
| 93–94 | Dec | Jan | – | Nov | – | Apr | Jan |
| 94–95 | Mar | Feb | Mar | – | Dec | Mar | Feb |
| 95–96 | Feb | Dec | Jan | Dec | – | Jun | Mar |
| 96–97 | Apr | Dec | Feb | – | Dec | Mar | Dec |
| 97–98 | Feb | Jan | – | Dec | – | – | Dec |
–, no isolations.
NPM, no predominant month of activity.
Efficiency of immunofluorescence.
The efficiency of the Bartels IFA procedure was determined for each viral type (Table 4). Results are based upon interpretation of 8,670 direct patient specimens stained with pool and individual virus-specific reagents and isolation with IFA detection in conventional tube cell culture. Overall, 1,903 specimens were IFA positive and culture positive (TP), 358 specimens were IFA negative and culture positive (FN), 788 specimens were IFA positive and culture negative (FP), and 5,621 specimens were IFA negative and culture negative (TN). The overall SENS, SPEC, PPV, and NPV values were 84.2, 87.7, 70.7, and 94.0%, respectively. The sensitivities for individual viruses were 90.6% for RSV, 84.3% for FLUA, 82.6% for FLUB, 82.0% for PIV1, 41.3% for PIV2, 87.0% for PIV3, and 64.8% for ADENO. The SPEC values of the procedure for individual viruses were as follows: RSV, 93.3%; FLUA, 95.1%; FLUB, 99.0%; PIV1, 99.8%; PIV2, 99.9%; PIV3, 99.6%; and ADENO, 99.9%.
TABLE 4.
Efficiency of monoclonal antibodiesa
| Virus | Yr | No. of IFA tests found to be:
|
SENS (%) | SPEC (%) | PPV (%) | NPV (%) | |||
|---|---|---|---|---|---|---|---|---|---|
| TP | FN | FP | TN | ||||||
| RSV | 87–89b | 77 | 12 | 52 | 304 | 86.5 | 85.4 | 60.0 | 96.4 |
| 89–90 | 82 | 6 | 109 | 526 | 95.3 | 82.8 | 42.9 | 98.9 | |
| 90–91 | 89 | 6 | 62 | 523 | 93.7 | 89.4 | 58.9 | 98.9 | |
| 91–92 | 176 | 23 | 71 | 777 | 88.4 | 91.6 | 71.3 | 97.1 | |
| 92–93 | 26 | 1 | 5 | 685 | 96.3 | 99.3 | 83.9 | 99.9 | |
| 93–94 | 111 | 9 | 54 | 748 | 92.5 | 93.3 | 67.3 | 98.8 | |
| 94–95 | 55 | 8 | 15 | 617 | 87.3 | 97.6 | 78.6 | 98.7 | |
| 95–96 | 109 | 10 | 33 | 540 | 91.6 | 94.2 | 76.8 | 98.2 | |
| 96–97 | 18 | 2 | 1 | 901 | 90.0 | 99.9 | 94.7 | 99.8 | |
| Total | 743 | 77 | 402 | 5,621 | 90.6 | 93.3 | 64.9 | 98.7 | |
| INFA | 87–89 | 13 | 0 | 3 | 304 | 100 | 99.0 | 81.3 | 100 |
| 89–90 | 32 | 2 | 47 | 526 | 94.1 | 91.2 | 40.5 | 99.6 | |
| 90–91 | 7 | 13 | 0 | 523 | 35.0 | 100 | 100 | 97.6 | |
| 91–92 | 90 | 5 | 17 | 777 | 94.7 | 97.9 | 84.1 | 99.4 | |
| 92–93 | 17 | 7 | 2 | 685 | 70.1 | 97.4 | 89.5 | 99.0 | |
| 93–94 | 120 | 15 | 66 | 748 | 88.9 | 91.9 | 64.5 | 98.0 | |
| 94–95 | 25 | 0 | 16 | 617 | 100 | 97.5 | 61.0 | 100 | |
| 95–96 | 49 | 32 | 10 | 540 | 60.5 | 98.2 | 83.1 | 94.4 | |
| 96–97 | 134 | 17 | 127 | 901 | 88.7 | 87.8 | 51.3 | 98.2 | |
| Total | 487 | 91 | 288 | 5,621 | 84.3 | 95.1 | 62.8 | 98.4 | |
| INFB | 87–89 | 5 | 4 | 14 | 304 | 55.6 | 95.6 | 26.3 | 98.7 |
| 89–90 | 0 | 0 | 0 | 526 | |||||
| 90–91 | 52 | 7 | 16 | 523 | 88.1 | 97.0 | 76.5 | 98.7 | |
| 91–92 | 0 | 0 | 0 | 777 | |||||
| 92–93 | 132 | 27 | 18 | 685 | 83.0 | 97.4 | 88.0 | 96.3 | |
| 93–94 | 0 | 0 | 0 | 748 | |||||
| 94–95 | 0 | 0 | 0 | 617 | |||||
| 95–96 | 26 | 7 | 8 | 540 | 78.8 | 98.5 | 76.5 | 98.7 | |
| 96–97 | 17 | 4 | 1 | 901 | 80.9 | 99.9 | 94.4 | 99.6 | |
| Total | 232 | 49 | 57 | 5,621 | 82.6 | 99.0 | 80.5 | 99.1 | |
| PIV1 | 87–89 | 1 | 0 | 0 | 319 | 100 | 100 | 100 | 100 |
| 89–90 | 23 | 1 | 0 | 526 | 95.8 | 100 | 100 | 99.9 | |
| 90–91 | 2 | 0 | 0 | 523 | 100 | 100 | 100 | 100 | |
| 91–92 | 16 | 9 | 7 | 777 | 64.0 | 99.1 | 69.6 | 98.9 | |
| 92–93 | 0 | 0 | 0 | 685 | |||||
| 93–94 | 21 | 7 | 2 | 748 | 75.0 | 99.7 | 91.3 | 99.1 | |
| 94–95 | 0 | 0 | 0 | 617 | |||||
| 95–96 | 28 | 2 | 1 | 540 | 93.3 | 98.5 | 96.6 | 99.6 | |
| 96–97 | 0 | 0 | 0 | 901 | |||||
| Total | 91 | 19 | 10 | 5,621 | 82.0 | 99.8 | 89.2 | 99.7 | |
| PIV2 | 87–89 | 0 | 0 | 0 | 304 | ||||
| 89–90 | 0 | 0 | 0 | 526 | |||||
| 90–91 | 4 | 2 | 0 | 523 | 66.7 | 100 | 100 | 99.6 | |
| 91–92 | 4 | 2 | 0 | 777 | 66.7 | 100 | 100 | 99.7 | |
| 92–93 | 1 | 13 | 0 | 685 | 7.1 | 100 | 100 | 98.1 | |
| 93–94 | 0 | 0 | 1 | 748 | 100 | 99.9 | 100 | 99.9 | |
| 94–95 | 4 | 6 | 0 | 617 | 40.0 | 100 | 100 | 99.0 | |
| 95–96 | 1 | 0 | 0 | 540 | 100 | 100 | 100 | 100 | |
| 96–97 | 5 | 4 | 1 | 901 | 55.6 | 99.9 | 83.3 | 99.6 | |
| Total | 19 | 27 | 2 | 5,621 | 41.3 | 99.9 | 90.5 | 99.8 | |
| PIV3 | 87–89 | 9 | 3 | 3 | 304 | 75.0 | 99.0 | 75.0 | 99.0 |
| 89–90 | 13 | 2 | 7 | 526 | 86.7 | 98.7 | 65.0 | 99.6 | |
| 90–91 | 48 | 6 | 3 | 523 | 88.5 | 99.4 | 94.1 | 98.9 | |
| 91–92 | 18 | 4 | 0 | 777 | 81.8 | 100 | 100 | 99.5 | |
| 92–93 | 35 | 5 | 4 | 685 | 87.5 | 99.4 | 89.7 | 99.3 | |
| 93–94 | 15 | 4 | 2 | 748 | 78.9 | 99.7 | 88.2 | 99.5 | |
| 94–95 | 24 | 3 | 3 | 617 | 88.9 | 99.5 | 88.9 | 99.5 | |
| 95–96 | 19 | 3 | 1 | 540 | 86.4 | 99.8 | 95.0 | 99.5 | |
| 96–97 | 34 | 2 | 2 | 901 | 94.4 | 99.8 | 94.4 | 99.8 | |
| Total | 215 | 32 | 25 | 5,621 | 87.0 | 99.6 | 89.6 | 99.4 | |
| ADENO | 87–89 | 3 | 5 | 0 | 304 | 37.5 | 100 | 100 | 98.4 |
| 89–90 | 18 | 6 | 0 | 526 | 75.0 | 100 | 100 | 98.9 | |
| 90–91 | 26 | 11 | 0 | 523 | 70.3 | 100 | 100 | 97.9 | |
| 91–92 | 7 | 8 | 1 | 777 | 46.7 | 99.9 | 87.5 | 99.0 | |
| 92–93 | 13 | 7 | 1 | 685 | 65.0 | 99.8 | 92.9 | 99.0 | |
| 93–94 | 10 | 6 | 1 | 748 | 62.5 | 99.9 | 90.9 | 99.2 | |
| 94–95 | 22 | 9 | 0 | 617 | 71.0 | 100 | 100 | 98.6 | |
| 95–96 | 10 | 7 | 1 | 540 | 58.8 | 98.5 | 90.9 | 98.7 | |
| 96–97 | 7 | 4 | 0 | 901 | 63.6 | 100 | 100 | 99.6 | |
| Total | 116 | 63 | 4 | 5,621 | 64.8 | 99.9 | 96.7 | 98.9 | |
| Overall | 1,903 | 358 | 788 | 5,621 | 84.2 | 87.7 | 70.7 | 94.0 | |
Values in boldface are averages rather than totals.
Years were combined because of low numbers.
DISCUSSION
The objectives of this study were twofold: (i) to determine the epidemiology of respiratory viruses in the Bismarck, N.D., area and (ii) to determine the efficiency of commercial monoclonal antibodies to detect common respiratory virus antigens in clinical specimens.
The overall virus incidence rate of 37.9% emphasizes the scope of viral infections in the community and explains the heavy burden of morbidity due to viral respiratory illness. Infants ≤1 year old and children >1 to 6 years of age had the highest incidence of viral infection (45.2 and 46.7%, respectively). Significant virus morbidity was seen in the 7- to 25-year-old group (33.1%), the 26- to 49-year-old group (24.3%), and the ≥50-year-old group (21.1%). The importance of RSV as the major viral pathogen of infancy and early childhood is supported by a 19.8% incidence in the >1- to 6-year-old age group and a 28.2% incidence in the ≤1-year-old age group. RSV and the parainfluenza viruses were the most frequently identified viruses in the ≤1-year-old age group. RSV and influenza viruses were the most frequently isolated viruses in the remaining four age groups. It has been stated that reinfection with RSV and parainfluenza viruses occurs with appreciable frequency in older children and adults and probably plays a major role in the spread of virus to the young infant (5). We found a low viral incidence in these age groups in the Bismarck community that tends not to support this statement. However, RSV and parainfluenza virus infections in older children and adults cause mild upper respiratory illnesses that generally would not result in physician visitation. Because the only participants included in this study were patients exhibiting respiratory illness serious enough to seek medical attention, it is probable that the numbers of RSV and parainfluenza virus infections in older children and adults were underestimated. The incidence of RSV and parainfluenza viruses in adults ≥50 years of age were 4.2 and 2.0%, respectively, thus reinforcing the fact that these viruses cause illness throughout life and that a closer surveillance of our elderly population is warranted. The seasonality data show the emergence of consistent epidemiologic patterns that could provide clinical guidelines for estimation of an etiologic diagnosis.
Epidemics of RSV occurred in all years of the study and appeared with an interesting periodicity. Although RSV was epidemic in all years, the month of peak viral activity and the month of initial RSV detection alternated from year to year. This finding is consistent with previous studies reporting yearly epidemics of RSV which alternated in their occurrence between midwinter and early spring (1, 8, 12). This predictable behavior of RSV would be useful to clinicians in preparing for upcoming RSV seasons. A milder RSV epidemic would be expected following a long interval between successive epidemics. Conversely, a more severe RSV epidemic would be expected following a short interval between epidemics. RSV epidemics were primarily confined to 5-month periods. The intensity of RSV activity was very similar to that described by Brandt et al. (3) in that 44.1% of the RSV infections occurred during the peak month of the epidemic, 87.7% of the RSV infections occurred during the peak month ±1 month, and 97.6% of the RSV infections occurred during the peak month ±2 months.
Influenza epidemics were seen each year of the study, with peak activity from December through April. This underscores the well-recognized yearly variation in influenza epidemics and the importance of annual monitoring. The influenza viruses were a significant cause of morbidity in the ≤1-year-old age group, causing 6.7% of infections. In the >1- to 6-year-old group, influenza viruses were identified in 16.8% of children, a prevalence second only to RSV (19.8%). Influenza viruses were the most frequently encountered virus group in the remaining three age groups.
PIV1 epidemics occurred in the autumn of odd-numbered years, and PIV2 epidemics occurred in the autumn of even-numbered years. This is in contrast to previous reports of epidemics of PIV1 virus occurring in the autumn of even-numbered years and peak activity of PIV2 virus occurring during the autumn of odd-numbered years (8, 10, 18). Information concerning viral epidemiology cannot necessarily be extrapolated from one geographic region to another.
PIV3 and ADENO were the most ubiquitous viruses. During the 137 months of the study, PIV3 was isolated in 75 of the months, and ADENO was isolated in 79 of the months. The peak incidence of PIV3 occurred between midwinter and early summer, and ADENO peak activity was usually seen during midwinter.
A type of interference phenomenon has been described that appeared to influence the occurrence of lower respiratory tract infections with the major respiratory viruses (8). In general, when the peak incidence of one major virus occurred, the other major viruses were absent. We observed a tendency for major viral agents not to cause simultaneous epidemics in the Bismarck area. RSV had a dampening effect on the occurrence of paramyxovirus activity in the community. Only rarely did RSV peak activity occur concurrently with the parainfluenza viruses. Discrete outbreaks of RSV and influenza virus infection usually occurred at times when other agents were present only in small numbers. The viral incidence did not vary more than 15% from year to year regardless of the number of types of respiratory viruses present. The high and low variations in annual viral incidence was most influenced by the rate of RSV recovery.
The rapid availability of information concerning the presence of viruses in the community prompted us to post a weekly “virus watch” notice. This informed clinicians about the number and types of viruses present from week to week during the respiratory virus season and allowed them to estimate the viral etiology in their patients.
The overall SENS, SPEC, PPV, and NPV of the monoclonal antibodies (84.2, 87.7, 70.7, and 94.0%, respectively) compare favorably with percentages previously reported for these monoclonal antibodies (13, 16). The IFA procedure resulted in a high number of FP tests when compared to a concomitant isolation in conventional tube cell culture. Immunofluorescence has distinct advantages over culture in some situations. Fulton and Middleton have found that immunofluorescence had a diagnostic advantage over tube cell culture isolation when specimens were taken in the late stages of infection (6). Other investigators have suggested that immunofluorescence assays may be positive in samples taken up to 1 week after the onset of infection, when specimens obtained at the same time for cell culture isolation are negative (7, 17, 19). Immunofluorescence-positive versus culture-negative results could be explained by the presence of secretory antibodies which reduce the infectivity of free virus even though viral antigens may still be present in exfoliated epithelial cells.
The efficiency of conventional tube cell culture to isolate all viruses should be considered in the discussion of FP immunofluorescence results. Since the immunofluorescence reagents were quality controlled and specific cellular patterns of fluorescence were observed, are the immunofluorescence results FP or are the culture results FN? Investigators have reported the inefficiency of tube cell culture for the isolation of RSV (7), influenza viruses (11), parainfluenza viruses (19), and ADENO (2). The inefficiency of conventional tube cell culture was suggested in our data by sensitivities ranging from 35.0 to 100% for INFA, 55.6 to 88.1% for INFB, 64.0 to 100% for PIV1, 7.1 to 100% for PIV2, 75.0 to 94.4% for PIV3, and 37.5 to 75.0% for ADENO. Reconsideration of the FP immunofluorescence results as true indicators of viral infection resulted in significant improvement in the diagnostic power of the IFA. Examination of the IFA and tube cell culture comparative data indicated that 2,261 identifications were made by culture alone, whereas 2,691 identifications were made by immunofluorescence alone, an increase of 430 (19.0%) identifications. Use of both IFA and tube cell culture resulted in the greatest diagnostic efficiency, with 3,049 identifications.
A positive IFA result has the potential to reduce or eliminate the need for routine cell culture, which is labor-intensive and requires considerable clinical expertise. Minnich and Ray calculated the hands-on time for an individual IFA to be 18.3 min and for a routine tube cell culture with identification by neutralization to be 82.1 min (14). It is clear that elimination of cell culture in select situations such as during periods of high viral transmission in the community would result in significant savings for the virology laboratory. The high specificities of the monoclonal antibodies provided for a confident presumptive diagnosis by IFA and a selective culture policy was instituted at the beginning of the 1997 to 1998 respiratory season. A conventional tube cell culture would not be routinely performed upon receipt of a respiratory specimen; rather, the IFA result was reported with a comment instructing the clinician to call the laboratory if a culture was desired. This action resulted in a decrease in the number of virus cultures performed; 1,281 cultures during the 1996 to 1997 respiratory season compared to 313 cultures performed during the 1997 to 1998 season. The viral incidence rates, however, were very similar for both respiratory seasons, suggesting the utility of selective virus culture. It should be noted that decisions concerning the deletion of cell culture based on rapid detection results must be made with caution since viral agents other than those tested for by immunofluorescence may be present (14).
In conclusion, an IFA procedure using commercial monoclonal antibodies was practical and efficient for the rapid identification of respiratory viruses in direct clinical specimens and for determining the epidemiology of respiratory viruses in the Bismarck, N.D., region. The prompt reporting to clinicians of a positive IFA result for a specific virus has the potential to negate the need for virus culture, saving technical time and hopefully optimizing treatment. Timely dissemination of epidemiologic data for specific respiratory viruses in our community provided the information to help clinicians make an informed decision about a potential viral etiology.
REFERENCES
- 1.Anderson L J, Parker R A, Strikas R L. Association between respiratory syncytial virus outbreaks and lower respiratory tract deaths of infants and young children. J Infect Dis. 1990;161:640–646. doi: 10.1093/infdis/161.4.640. [DOI] [PubMed] [Google Scholar]
- 2.August J J, Warford A L. Evaluation of a commercial monoclonal antibody for detection of adenovirus antigen. J Clin Microbiol. 1987;25:2233–2235. doi: 10.1128/jcm.25.11.2233-2235.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brandt C D, Kim H W, Arrobio J O, Jeffries B C, Wood S C, Chanock R M, Parrott R H. Epidemiology of respiratory syncytial virus infection in Washington, D.C. III. Composite analysis of eleven consecutive yearly epidemics. Am J Epidemiol. 1973;98:355–364. doi: 10.1093/oxfordjournals.aje.a121565. [DOI] [PubMed] [Google Scholar]
- 4.Denny F W, Clyde W A. Acute lower respiratory tract infections in nonhospitalized children. J Pediatr. 1986;108:635–646. doi: 10.1016/s0022-3476(86)81034-4. [DOI] [PubMed] [Google Scholar]
- 5.Evans A S. Epidemiologic concepts and methods. In: Evans A S, editor. Viral infections of humans. New York, N.Y: Plenum Publishing Corp.; 1989. pp. 1–29. [Google Scholar]
- 6.Fulton R E, Middleton P J. Comparison of immunofluorescence and isolation techniques in the diagnosis of respiratory infections in children. Infect Immun. 1974;10:92–101. doi: 10.1128/iai.10.1.92-101.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gardner P S, McQuillan J, McGuckin R. The late detection of respiratory syncytial virus in cells of respiratory tract by immunofluorescence. J Hyg. 1970;68:575–580. doi: 10.1017/s0022172400042509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Glezen W P, Denny F S. Epidemiology of acute lower respiratory disease in children. N Engl J Med. 1973;288:498–505. doi: 10.1056/NEJM197303082881005. [DOI] [PubMed] [Google Scholar]
- 9.Hall C B, Douglas R G. Clinically useful method for the isolation of respiratory syncytial virus. J Infect Dis. 1975;131:1–5. doi: 10.1093/infdis/131.1.1. [DOI] [PubMed] [Google Scholar]
- 10.Hemming V G. Viral respiratory diseases in children: Classification, etiology, epidemiology, and risk factors. J Pediatr. 1994;124:S13–S16. doi: 10.1016/S0022-3476(94)70185-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim J W, Brandt C D, Arrobio J O, Murphy B, Chanock R M, Parrott R H. Influenza A and B virus infection in infants and young children during the years 1957–1976. Am J Epidemiol. 1979;109:464–479. doi: 10.1093/oxfordjournals.aje.a112704. [DOI] [PubMed] [Google Scholar]
- 12.Kim H W, Arrobio J O, Brandt C D, Jeffries B C, Pyles G, Reid J L, Chanock R M, Parrott R H. Epidemiology of respiratory syncytial virus infection in Washington, D.C. I. Importance of the virus in different respiratory tract disease syndromes and temporal distribution of infection. Am J Epidemiol. 1973;98:216–225. doi: 10.1093/oxfordjournals.aje.a121550. [DOI] [PubMed] [Google Scholar]
- 13.Matthey S, Nicholson D, Ruhs S, Alden B, Knock M, Schultz K, Schmuecker A. Rapid detection of respiratory viruses by shell vial culture and direct staining using pooled and individual monoclonal antibodies. J Clin Microbiol. 1992;30:540–544. doi: 10.1128/jcm.30.3.540-544.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Minnich L L, Ray C G. Early testing of cell cultures for detection of hemadsorbing viruses. J Clin Microbiol. 1987;25:421–422. doi: 10.1128/jcm.25.2.421-422.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Monto A S, Ullman B M. The Tecumseh study: acute respiratory illness in an American community. JAMA. 1974;227:164–169. [PubMed] [Google Scholar]
- 16.Stout C, Murphy M D, Lawrence S, Julian S. Evaluation of a monoclonal antibody pool for rapid diagnosis of respiratory viral infections. J Clin Microbiol. 1989;27:448–452. doi: 10.1128/jcm.27.3.448-452.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Swenson P D, Kaplan M H. Rapid detection of influenza virus in cell culture by indirect immunoperoxidase staining with type-specific monoclonal antibodies. Diagn Microbiol Infect Dis. 1987;7:265–268. doi: 10.1016/0732-8893(87)90142-8. [DOI] [PubMed] [Google Scholar]
- 18.Tyeryarm F J, Richardson L S, Belshe R B. Report of a workshop on respiratory syncytial virus and parainfluenza viruses [National Institutes of Health] J Infect Dis. 1978;137:835–846. doi: 10.1093/infdis/137.6.835. [DOI] [PubMed] [Google Scholar]
- 19.Wong D T, Welliver R T, Riddlesberger K R, Sin M S, Ogra P L. Rapid diagnosis of parainfluenza virus infections in children. J Clin Microbiol. 1982;16:164–167. doi: 10.1128/jcm.16.1.164-167.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]