Abstract
Background
It is not known whether older adults' willingness to deprescribe is associated with their health outcome priorities related to medications.
Methods
A cross‐sectional survey was conducted from March–April 2020 using a nationally representative online panel. The survey presented two vignettes: (1) a preventive medicine; and (2) a symptom‐relief medicine. Participants were asked whether they would be willing to stop each medicine if their doctor recommended it, and to rate their level of agreement with two health outcome priorities statements: “I am willing to accept the risk of future side effects … to feel better now,” and “I would prefer to take fewer medicines, even if … I may not live as long or may have bothersome symptoms sometimes.” Ordinal logistic regression was used to examine associations between willingness to stop each medicine, baseline characteristics and health outcome priorities.
Results
Of 1193 panel members ≥65 years invited to participate, 835 (70%) completed the survey. Mean (SD) age was 73 years; 496 (59%) had taken a statin and 124 (15%) a prescription sedative‐hypnotic. 507 (61%) were willing to stop preventive medicines; 276 (33%) were maybe willing. 419 (50%) were willing to stop symptom‐relief medicines; 380 (46%) were maybe willing. Prioritizing fewer medicines was associated with higher odds of being willing to stop symptom‐relief medicines (aOR 1.43 [95% CI 1.02–2.00]) and preventive medicines (aOR 1.52 [95% CI 1.05–2.18]). Prioritizing now over future was associated with lower odds of being willing to stop symptom‐relief medicines (aOR 0.62 [95% CI 0.39–1.00]). Current/prior use of statins was associated with lower willingness to stop preventive medicines (aOR 0.66 [95% CI 0.48–0.91]).
Conclusions
Older adults' health outcome priorities related to medication use are associated with their willingness to consider deprescribing. Future research should determine how best to elicit patients' health outcome priorities to facilitate goal‐concordant decisions about medication use.
Keywords: deprescribing, health outcome priorities, preventive medicines, symptom‐relief medicines
Key points
This national survey found that high rates of willingness to deprescribe both preventive and symptom‐relief medicines among U.S. older adults.
Many older adults prioritized taking fewer medicines, and avoiding future side effects over “feeling better now”; these sentiments were associated with higher willingness to deprescribe.
Why does this paper matter?
Linking deprescribing messages to patients' health outcome priorities related to medication use may increase uptake of deprescribing.
INTRODUCTION
In the United States, 36% of patients aged 65 or older use at least five prescription medicines concurrently. 1 Polypharmacy is associated with high risk of drug interactions, treatment burden, and adverse drug events—including hospitalization, disability, negative effects on cognitive function, and death. 2 , 3 , 4 , 5 Approximately one in five drugs commonly used in older people may be inappropriate, 6 meaning that the potential harms outweigh the expected clinical benefits or that safer alternatives are available. 7
Deprescribing is the physician‐supervised process of reducing or stopping a medication that is inappropriate or no longer necessary, with the goal of improving health and quality‐of‐life outcomes. 8 , 9 Deprescribing is a promising strategy to reduce high rates of iatrogenic harm for older adults with multiple chronic conditions. Most older adults in the United States are open to deprescribing, with 92% of Medicare beneficiaries in a national survey reporting that they would be willing to deprescribe if their physician said it was possible, and 66% wanting to reduce the number of medicines that they were taking. 10 Yet in spite of these findings, uptake of deprescribing in the United States remains suboptimal. How clinicians communicate about deprescribing may affect to what extent older adults are willing to do it. Deprescribing, including the determination of whether a given medication is suitable for withdrawal, should occur within the framework of shared decision making between patient and clinician. 8
An important part of shared decision making is the integration of older adults' health outcome priorities—the health outcome goals a person most desires in the context of what they are willing and able to do to achieve those outcomes. 11 A small body of literature has explored how older adults consider competing priorities in healthcare decision making (e.g., are they willing to compromise on stroke risk reduction to avoid falls when medications that lower stroke risk, such as anti‐hypertensives, are linked to increased fall risk), 12 , 13 , 14 but it is not known how these perspectives may translate to willingness to deprescribe a medication that they are already taking, particularly a symptom‐relief medication. Studies have suggested that incorporating older adults' health outcome goals and priorities into clinical decision making may be associated with reductions in unwanted healthcare and treatment burden. 15 , 16 , 17 For these reasons, we sought to investigate whether patients' health outcome priorities related to medication use are associated with their willingness to consider deprescribing.
This study used a national survey to investigate two objectives. First, we characterized older adults' health outcome priorities related to medication use and how these varied by individual characteristics. Second, we examined associations between willingness to deprescribe two types of medications (preventive and symptom‐relief), individual health outcome priorities related to medication use, and other individual characteristics, in order to determine whether incorporating health outcome priorities into deprescribing communication may be a promising strategy to enhance uptake of deprescribing.
METHODS
Study design and sample
We conducted a cross‐sectional survey of non‐institutionalized U.S. adults aged ≥65 years in 2020. We used the Ipsos KnowledgePanel, an online research panel with approximately 60,000 members that is designed to be representative of the U.S. adult population. Complete details of the survey methodology have been previously reported (Supplementary Text S1). 18 Among 1193 eligible panel members (aged ≥65 years, English‐speaking) invited to participate, 835 (70%) completed the survey between March 25, 2020 and April 19, 2020. The study was approved by the Johns Hopkins University School of Medicine Institutional Review Board. Informed consent of study participants was indicated by voluntary completion of the survey. The study followed the American Association for Public Opinion Research reporting guideline. 19
Survey instrument
The survey (Supplementary Text S1) was developed by the study team, which included three geriatricians, and piloted with nine older adults who were not included in the study. Pilot testers reviewed a pen‐and‐paper version of the survey and provided feedback. We pilot tested with one individual at a time. We iteratively revised the survey after each round of feedback and then tested the updated survey with additional older adults. We specifically probed if the survey questions were clear, if they were relevant to their experience and if the questions were burdensome. Overall, the pilot testing resulted only in minor wording changes. We separately examined two scenarios: One was a statin being taken for primary prevention by an older adult with functional impairment, multimorbidity and polypharmacy. Given the uncertainties in the evidence for such patients, the decision to continue or discontinue a statin must incorporate individual patient preference. 20 , 21 , 22 We briefly described the benefits of statins (lowering the risk of heart attacks and strokes) and the risks (muscle pain or weakness, nausea, constipation, diarrhea and drug interactions). Then, participants were asked about their willingness to deprescribe: “If your doctor recommended that you stop taking a medicine, such as a statin, that could lower your risk of future health problems, such as heart attacks and strokes, but may cause side effects, would you be willing?”
The second scenario was a sedative‐hypnotic in the benzodiazepine receptor agonist class (zolpidem) being taken for insomnia by a fairly healthy older adult. Although sedative‐hypnotics are generally contraindicated in older adults, 23 they are especially high risk for frail patients with multicomplexity, while the benefit/harm ratio may be less clear in healthy older adults. 24 Prescription sedative‐hypnotics were described as a type of medicine that many people take to help with sleep but that may cause “problems such as falls, memory problems, hospitalizations and death for older adults.” After this description, participants were asked, “If your doctor recommended that you stop taking a medicine even though it helps you with a bothersome (but not life‐threatening) symptom, would you be willing?”
Participants were then asked to rate their level of agreement with two statements adapted from prior studies on health outcome prioritization among older adults with multiple chronic conditions. 25 , 26 The statements focused on trade‐offs between quantity and quality of life, and between current and future health: (1) I am willing to accept the risk of future side effects, such as falls or memory problems, to feel better now (hereafter referred to as prioritizing “now over future”), and (2) I would prefer to take fewer medicines, even if it meant that I may not live as long or may have bothersome symptoms sometimes (hereafter referred to as prioritizing “fewer medicines”).
KnowledgePanel provided information on age, sex, race/ethnicity, education, and income of the study participants. The survey also collected information on self‐reported overall health status, health literacy, 27 and general attitudes and experiences relating to medicines.
Statistical analysis
Participant characteristics and responses were analyzed descriptively. Our two‐step analysis investigated (1) association between health outcome priorities and respondent characteristics; and (2) association between willingness to deprescribe and health outcome priorities, while accounting for respondent characteristics.
Association between health outcome priorities related to medicines and respondent characteristics
Two separate ordinal logistic regression models were constructed. The variables included in each model were chosen a priori based on the literature and the investigators' clinical experience. In the first model, the outcome was agreement with the statement that prioritized now over future. Independent variables included age, history of memory concerns or falls in the past year, and prior or current use of prescription sedative‐hypnotics. In the second model, the outcome was preference for taking fewer medicines. Independent variables included age, number of daily medicines, self‐reported health status, current or prior use of prescription sedative‐hypnotics and statins, difficulty affording medications, and prior emergency department visit related to medication side effects.
Association between willingness to deprescribe and health outcome priorities related to medicines
Again, two separate ordinal logistic regression models were constructed. In the first model, the outcome was willingness to stop a symptom‐relief medication (zolpidem), in the second model, it was willingness to stop a preventive medication (statin). We included the health outcome priority of taking fewer medications in both models. For the symptom‐relief medicine, we also included the health outcome priority of prioritizing now over future. In addition, each model included the following respondent characteristics: current or prior use of the medication class in question, age, race/ethnicity, education, self‐reported overall health status, response to the question, “How do you feel about the number of medicines you take?,” and difficulty affording medications (statin model only). All analyses were performed using R version 3.6.3. 28 The ordinal logistic regression used the polr function of the MASS package. 29 A 2‐sided p < 0.05 was considered to be statistically significant.
RESULTS
Respondent characteristics
The mean (SD) age of respondents was 73 (6) years; 414 [50%] were female, 671 [80%] were White and 164 [20%] were non‐White, more than one race or other; 496 (59%) had ever taken a statin (prior or current use), and 124 (15%) had ever taken a sedative‐hypnotic (Table 1). The majority of respondents were willing to stop a preventive medicine (507 [61%], and half (419 [50%]) were willing to stop a symptom‐relief medicine (Figure 1A).
TABLE 1.
Sociodemographic and clinical characteristics of 835 study participants
| Characteristic | No. (%) a |
|---|---|
| Age, mean (SD), years | 73 (6) |
| 65–69 | 311 (37.2) |
| 70–74 | 243 (29.1) |
| 75–79 | 151 (18.1) |
| 80+ | 130 (15.6) |
| Female sex | 414 (49.6) |
| Race/ethnicity | |
| White, non‐Hispanic | 671 (80.4) |
| African American, non‐Hispanic | 61 (7.3) |
| Hispanic | 58 (6.9) |
| More than one race or other | 45 (5.4) |
| Educational level | |
| Did not complete high school | 46 (5.5) |
| Completed high school | 252 (30.2) |
| <4 year college | 232 (27.8) |
| College graduate or postgraduate degree | 305 (36.5) |
| Confidence filling out medical forms | |
| Extremely confident | 558 (67.1) |
| Quite a bit confident | 174 (20.9) |
| Somewhat confident | 69 (8.3) |
| A little bit confident | 20 (2.40) |
| Not at all confident | 11 (1.32) |
| Difficulty paying for medicines | |
| Extremely difficult | 10 (1.2) |
| Somewhat difficult | 80 (9.6) |
| Not at all difficult | 706 (85.0) |
| Unsure | 16 (1.9) |
| Do not wish to answer | 19 (2.3) |
| Current or prior use of statin | 496 (59.8) |
| Current or prior use of prescription sedative‐hypnotic | 124 (14.9) |
| Prior ED b visit for side effects of a medicine | 37 (4.4) |
| Self‐reported health | |
| Excellent | 64 (7.7) |
| Very good | 296 (35.5) |
| Good | 328 (39.4) |
| Fair | 127 (15.2) |
| Poor | 18 (2.2) |
The number of respondents varied between 831 and 835 as some respondents did not answer all questions.
Emergency department.
FIGURE 1.
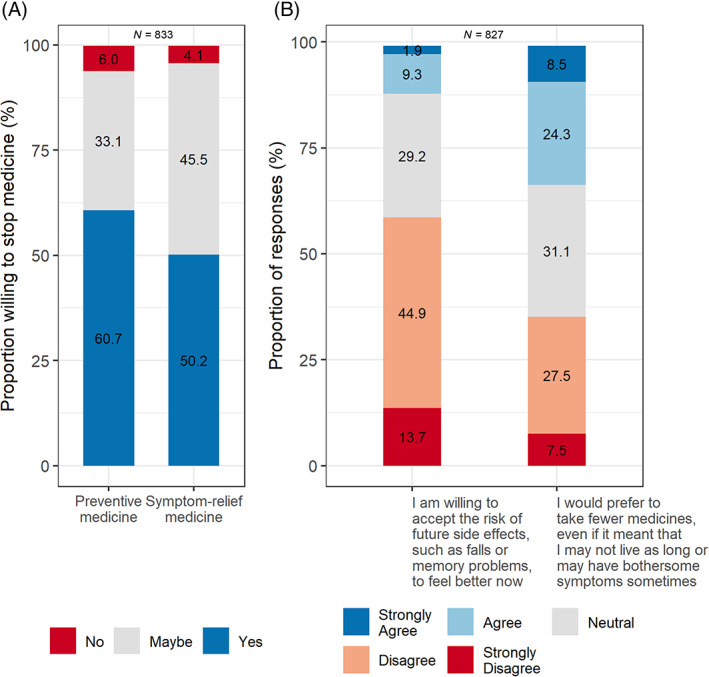
(A) Willingness to stop medicines. (B) Health outcome priorities related to medicines. Percentages do not add to 100% because of missing responses.
Association between health outcome priorities related to medicines and respondent characteristics
The majority of respondents (489 [59%]) disagreed or strongly disagreed with the statement that prioritized now over future. For the statement that prioritized fewer medicines, 274 (33%) agreed or strongly agreed, 260 (31%) neither agreed nor disagreed, and 293 (35%) disagreed or strongly disagreed (Figure 1B). Current or prior use of a prescription sedative‐hypnotic such as zolpidem was associated with higher odds of prioritizing now over future (aOR 1.80 [95% CI 1.26, 2.58). Higher numbers of medications per day was associated with lower odds of prioritizing fewer medicines (aOR 0.70 [95% CI 0.60, 0.81] per 3 additional medications, reference 0 medications per day). There was little or no evidence for associations of other respondent characteristics with health outcome priorities related to medicines (Table 2).
TABLE 2.
Association between health outcome priorities related to medicines and respondent characteristics
| Characteristic | Adjusted odds ratio (95% CI) a |
|---|---|
| Prioritizing now over future | |
| Current or prior use of prescription sedative‐hypnotic | 1.80 (1.26, 2.58) |
| History of falls or memory concerns | 1.06 (0.82, 1.38) |
| Age b (reference: age 65) | 1.06 (0.96, 1.18) |
| Prioritizing fewer medicines | |
| Number of medications per day c | 0.70 (0.60, 0.81) |
| Self‐reported health (excellent/very good/good vs poor or fair) | 0.94 (0.66, 1.34) |
| Age b (reference: age 65) | 1.00 (0.90, 1.11) |
| Current or prior use of statin | 0.78 (0.58, 1.04) |
| Current or prior use of prescription sedative‐hypnotic | 0.77 (0.54, 1.10) |
| Difficulty affording medicines (somewhat or extremely difficult vs not at all difficult) | 1.15 (0.76, 1.75) |
| Prior ED d visit for side effects of a medicine | 1.25 (0.67, 2.31) |
Adjusted for all other variables listed.
OR (CI) based on increments of 5 years; age was included as a scaled, continuous variable in the regression.
OR (CI) based on increments of three medications, reference zero medications; number of daily medications was included as a scaled, continuous variable in the regression.
ED, emergency department.
Association between willingness to deprescribe and respondent characteristics
Prioritizing taking fewer medicines was associated with higher odds of being willing to stop both types of medicines: symptom‐relief medicine (aOR 1.43 [95% CI 1.02–2.00]), preventive medicine (aOR 1.52 [95% CI 1.05–2.18]). (Table 3). Prioritizing now over future was associated with lower odds of being willing to stop a symptom‐relief medicine, (aOR 0.62 [95% CI 0.39–1.00]). Current or prior use of statins was associated with lower willingness to stop preventive medicines (aOR 0.66 [95% CI 0.48–0.91]) (Table 3). Other respondent characteristics were not associated with willingness to deprescribe preventive or symptom‐relief medications.
TABLE 3.
Association between willingness to deprescribe and respondent characteristics
| Characteristic | Adjusted odds ratio (95% CI) a |
|---|---|
| Symptom‐relief medicine | |
| Current or prior use of prescription sedative‐hypnotic (ever vs never) | 0.72 (0.49, 1.08) |
| Perception about number of medicines being taken (too many vs need additional or just right) | 0.95 (0.68, 1.32) |
| Willing to accept future side effects, such as falls or memory problems, to feel better now: | |
| Neutral vs strongly disagree/disagree | 0.70 (0.51, 0.95) |
| Strongly agree/agree vs strongly disagree/disagree | 0.62 (0.39, 1.00) b |
| Prefer to take fewer medicines even if it means not living as long or experiencing bothersome symptoms: | |
| Neutral vs strongly disagree/disagree | 1.22 (0.87, 1.71) |
| Strongly agree/agree vs strongly disagree/disagree | 1.43 (1.02, 2.00) |
| Race/ethnicity (non‐Hispanic White vs all other) c | 1.29 (0.90, 1.84) |
| Education (some college or higher vs high school or less) d | 0.97 (0.72, 1.31) |
| Age e (reference: age 65) | 1.07 (0.95, 1.20) |
| Self‐reported health (excellent/very good/good vs poor or fair)f | 1.04 (0.71, 1.52) |
| Preventive medicine | |
| Current or prior use of statins (ever vs never) | 0.66 (0.48, 0.91) |
| Perception about number of medicines (too many vs need additional or just right) | 1.15 (0.80, 1.66) |
| Prefer to take fewer medicines even if it means not living as long or experiencing bothersome symptoms: | |
| Neutral vs strongly disagree/disagree | 0.94 (0.66, 1.33) |
| Strongly agree/agree vs strongly disagree/disagree | 1.52 (1.05, 2.18) |
| Race/ethnicity (non‐Hispanic White vs all other) c | 1.00 (0.69, 1.46) |
| Education (some college or higher vs high school or less) d | 0.94 (0.68, 1.29) |
| Age (reference: age 65) e | 1.08 (0.96, 1.22) |
| Self‐reported health (excellent/very good/good vs poor or fair) | 0.90 (0.60, 1.35) |
| Difficulty affording medicines (somewhat or extremely difficult vs not difficult) | 0.98 (0.61, 1.57) |
Adjusted for all other variables listed.
The upper limit of the confidence interval is 1.00 due to rounding. The more precise estimate is 0.623 [95% CI 0.389–0.997].
Categories were: non‐Hispanic White; non‐Hispanic African American; Hispanic; More than one race or other.
Categories were: bachelor's degree or higher, some college, high school, and less than high school.
OR (CI) based on 5‐year increments; age was included as a scaled, continuous variable in the regression.
DISCUSSION
This national survey had several key findings. First, the majority of older adults surveyed were willing to deprescribe if their doctor recommended it. Second, willingness to deprescribe aligned with individuals' health outcome priorities related to medication use. We found that more respondents were willing to stop preventive medicines as compared with symptom‐relief medicines, though many respondents were willing to stop both. To our knowledge, this is the first national study of older adults' willingness to deprescribe preventive vs symptom‐relief medications, and the first assessment of older adults' health outcome priorities related to medication use and their association with deprescribing. A considerable percentage—59%—prioritized avoiding future side effects over “feeling better now”; this was associated with higher willingness to stop symptom‐relief medications. One‐third of respondents prioritized taking fewer medicines over not living as long or sometimes having bothersome symptoms. This was associated with higher willingness to stop both preventive and symptom‐relief medications.
Our finding that many older adults are willing to have a medicine deprescribed aligns with prior research that found strong support for deprescribing among older Medicare beneficiaries. 10 A larger proportion of respondents were willing to stop preventive medicines as compared with symptom‐relief medicines. This is in contrast with qualitative research showing that primary care providers feel more confident about deprescribing symptom‐relief medicines than preventive ones such as aspirin and statins. 30 , 31 , 32 This disparity between patients and clinicians may pose a barrier to deprescribing. Clinicians often feel that professional guidelines compel them to prescribe preventive medicines, worry that patients may be harmed by stopping preventive medicines, and believe that patients may interpret a recommendation to stop a preventive medicine as “giving up.” 30 , 31 , 32 The finding that older adults are willing to stop a preventive medication if the doctor recommends it—in the context of a trusting relationship with the clinician 33 —should be reassuring to clinicians concerned about encountering patient resistance to deprescribing.
A key finding was that many respondents (one‐third) prioritized taking fewer medicines over not living as long or having bothersome symptoms sometimes, and that this sentiment was significantly associated with willingness to stop both medication classes. This finding represents an opportunity to initiate deprescribing conversations. Clinicians should also be attuned to recognize and act on cues that patients may be interested in deprescribing, such as comments about the inconvenience, potential adverse effects or costs of taking medications. 34 , 35 Future research should identify approaches to elicit patient health outcome priorities related to medication use and align deprescribing recommendations with them, 36 , 37 , 38 acknowledging that people often have conflicting preferences: They may want to minimize their medication use in theory, yet be willing to accept the hassles and potential harms of treatment to pursue a modest degree of benefit. Or, they may have difficulty envisioning how their current medication use may affect them in the future. Deprescribing interventions will need to use language and approaches that balance these realities and conflicting preferences and help patients make informed decisions about medication use. An approach guided by the “5Ms”—a framework for providing high‐quality care to older adults by focusing on mind, mobility, medications, multicomplexity, and what matters most to the patient—can help facilitate individualized decisions regarding continuing or stopping medications. 22 , 39
Another important finding was that 59% of respondents prioritized avoiding future side effects over “feeling better now”; this was associated with higher willingness to stop symptom‐relief medicines. This aligns with prior research on preferences related to preventive medicines, showing that a considerable proportion of older adults place greater importance on avoiding fall injuries and other medication‐related symptoms than on optimizing blood pressure control or reducing risk of cardiovascular events. 14 Although patients and caregivers highly value avoidance of adverse effects, many are not aware that their medications carry potential harms. 40 This may in part be due to direct to consumer advertising (DTCA), which tends to provide insufficient information to patients regarding potential harms of medications. 41 Clinicians may also have incomplete understanding of benefits and harms of medicines because professional guidelines and review articles may emphasize the effectiveness of symptom‐relief medications, even when benefits are uncertain, and downplay the risks. 42 Prior research shows that anticholinergic overactive bladder medications are often continued even after ED visits and hospitalizations for falls or delirium, which could be adverse effects of this medication class. 43 Future research should develop deprescribing interventions to educate patients about potential adverse effects of symptom‐relief medications. 18 These could incorporate icon arrays or bar graphs, simple graphical representations to help patients understand the probability of benefit vs harm from a medication. 44 Such tools have been shown to help patients accurately interpret risk information in the context of cancer screening or cardiovascular risk reduction, 45 , 46 for example, but they have not been studied in the context of deprescribing interventions.
Lastly, we found that respondents who prioritized taking fewer medications tended to have fewer prescriptions. The study was cross‐sectional, so we cannot determine whether patients who took fewer medications did so as a result of expressing their preference to clinicians. Since the number of medications was associated with self‐reported health, where people with poor or fair self‐reported health took more medications, it is also possible that those who were healthier and needed fewer medications valued medications less than those who were in poorer health and who required more medications. Future studies are needed to better understand the rationale underlying this finding.
This study should be interpreted in the context of several limitations. First, participants' responses may not reflect the choices they would actually make. However, research on other health decisions has shown high consistency between stated preferences in hypothetical situations and actual behavior. 47 , 48 Second, the questions about health outcome priorities were not based on validated instruments. The phrases were adapted from prior research to identify older adults' preferred tools for eliciting health outcome priorities. 25 The survey and hypothetical scenarios were developed by the study team and feedback during pilot testing suggested that they were acceptable and clinically meaningful. However, it is possible that some of the phrases may have biased participants' responses (e.g., referring to zolpidem as a “bothersome (but not life‐threatening) symptom”). Third, the survey asked if respondents had ever taken a prescription sedative‐hypnotic, although distinguishing current from prior use may be important, because someone who successfully stopped taking a sleeping pill might be more agreeable to stopping it in the hypothetical scenario compared to someone who is currently taking one. Fourth, our sample may not be representative of older adults with serious illness, cognitive impairment or low health literacy, although a considerable proportion of respondents reported concerns about their memory or ranked their health as fair or poor.
CONCLUSION
Older adults' health outcome priorities related to medication use are associated with their willingness to consider deprescribing. Future research should determine how best to elicit patients' health outcome priorities related to medicines to facilitate informed, goal‐concordant decisions about medication use for older adults.
AUTHOR CONTRIBUTIONS
Concept and design: All authors. Acquisition, analysis, or interpretation of data: All authors. Drafting of the manuscript: Ariel R. Green. Critical revision of the manuscript for important intellectual content: All authors. Statistical analysis: Hélène Aschmann. Obtained funding: Ariel R. Green, Nancy Schoenborn. Administrative, technical, or material support: Ariel R. Green, Cynthia M. Boyd, Nancy Schoenborn. Supervision: Ariel R. Green, Cynthia M. Boyd, Nancy Schoenborn.
CONFLICT OF INTEREST
Dr. Cynthia M. Boyd writes a chapter on multimorbidity for UpToDate, for which she receives a royalty, and reviews a chapter on falls for an ACP—Dynamed collaboration. The other authors have no conflicts of interest that are directly relevant to the content of this article.
SPONSOR'S ROLE
The funding organizations had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
FINANCIAL DISCLOSURE
This research was supported by the U.S. Deprescribing Research Network (R24AG064025). In addition, Dr. Ariel R. Green was supported by grant K23AG054742, Dr. Cynthia M. Boyd by K24AG056578, and Dr. Nancy Schoenborn by K76AG059984, all from the National Institute on Aging. Dr. Hélène Aschmann was supported by a Swiss National Science Foundation Early Postdoc Mobility fellowship.
Supporting information
Supplementary Text S1 (A) Deprescribing communication survey. (B) Additional details on knowledge networks methodology
Green AR, Aschmann H, Boyd CM, Schoenborn N. Association between willingness to deprescribe and health outcome priorities among U.S. older adults: Results of a national survey. J Am Geriatr Soc. 2022;70(10):2895‐2904. doi: 10.1111/jgs.17917
Prior presentations: This work was presented at the annual scientific meeting of the American Geriatrics Society in May 2021.
Funding information National Institute on Aging, Grant/Award Numbers: K23AG054742, K24AG056578, K76AG059984, R24AG064025; Schweizerischer Nationalfonds zur Förderung der Wissenschaftlichen Forschung
REFERENCES
- 1. Qato DM, Wilder J, Schumm LP, Gillet V, Alexander GC. Changes in prescription and over‐the‐counter medication and dietary supplement use among older adults in the United States, 2005 vs 2011. JAMA Intern Med. 2016;176(4):473‐482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wimmer BC, Cross AJ, Jokanovic N, et al. Clinical outcomes associated with medication regimen complexity in older people: a systematic review. J Am Geriatr Soc. 2017;65(4):747‐753. doi: 10.1111/jgs.14682 [DOI] [PubMed] [Google Scholar]
- 3. Opondo D, Eslami S, Visscher S, et al. Inappropriateness of medication prescriptions to elderly patients in the primary care setting: a systematic review. PLoS One. 2012;7(8):e43617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kalisch LM, Caughey GE, Barratt JD, et al. Prevalence of preventable medication‐related hospitalizations in Australia: an opportunity to reduce harm. Int J Qual Health Care. 2012;24(3):239‐249. [DOI] [PubMed] [Google Scholar]
- 5. Jyrkkä J, Enlund H, Korhonen MJ, Sulkava R, Hartikainen S. Polypharmacy status as an indicator of mortality in an elderly population. Drugs Aging. 2009;26(12):1039‐1048. [DOI] [PubMed] [Google Scholar]
- 6. Roughead EE, Anderson B, Gilbert AL. Potentially inappropriate prescribing among Australian veterans and war widows/widowers. Intern Med J. 2007;37(6):402‐405. [DOI] [PubMed] [Google Scholar]
- 7. Beers MH. Explicit criteria for determining potentially inappropriate medication use by the elderly. An update. Arch Intern Med. 1997;157(14):1531‐1536. [PubMed] [Google Scholar]
- 8. Reeve E, Shakib S, Hendrix I, Roberts MS, Wiese MD. Review of deprescribing processes and development of an evidence‐based, patient‐centred deprescribing process. Br J Clin Pharmacol. 2014;78(4):738‐747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Scott IA, Hilmer SN, Reeve E, et al. Reducing inappropriate polypharmacy: the process of deprescribing. JAMA Intern Med. 2015;175(5):827‐834. [DOI] [PubMed] [Google Scholar]
- 10. Reeve E, Wolff JL, Skehan M, Bayliss EA, Hilmer SN, Boyd CM. Assessment of attitudes toward deprescribing in older Medicare beneficiaries in the United States. JAMA Intern Med. 2018;178:1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tinetti ME, Esterson J, Ferris R, Posner P, Blaum CS. Patient priority‐directed decision making and care for older adults with multiple chronic conditions. Clin Geriatr Med. 2016;32(2):261‐275. [DOI] [PubMed] [Google Scholar]
- 12. Fried TR, Street RL Jr, Cohen AB. Chronic disease decision making and “what matters most”. J Am Geriatr Soc. 2020;68(3):474‐477. doi: 10.1111/jgs.16371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Case SM, O'Leary J, Kim N, Tinetti ME, Fried TR. Older adults' recognition of trade‐offs in healthcare decision‐making. J Am Geriatr Soc. 2015;63(8):1658‐1662. doi: 10.1111/jgs.13534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tinetti ME, McAvay GJ, Fried TR, et al. Health outcome priorities among competing cardiovascular, fall injury, and medication‐related symptom outcomes. J Am Geriatr Soc. 2008;56(8):1409‐1416. doi: 10.1111/j.1532-5415.2008.01815.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tinetti ME, Naik AD, Dindo L, et al. Association of patient priorities–aligned decision‐making with patient outcomes and ambulatory health care burden among older adults with multiple chronic conditions: a nonrandomized clinical trial. JAMA Intern Med. 2019;179(12):1688‐1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Moorhouse P, Theou O, Fay S, McMillan M, Moffatt H, Rockwood K. Treatment in a geriatric day hospital improve individualized outcome measures using goal attainment scaling. BMC Geriatr. 2017;17(1):9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Toto PE, Skidmore ER, Terhorst L, Rosen J, Weiner DK. Goal attainment scaling (GAS) in geriatric primary care: a feasibility study. Arch Gerontol Geriatr. 2015;60(1):16‐21. [DOI] [PubMed] [Google Scholar]
- 18. Green AR, Aschmann H, Boyd CM, Schoenborn N. Assessment of patient‐preferred language to achieve goal‐aligned deprescribing in older adults. JAMA Netw Open. 2021;4(4):e212633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. American Association for Public Opinion Research . Disclosure Standards . https://www.aapor.org/Standards-Ethics/AAPOR-Code-of-Ethics/Disclosure-Standards.aspx. Accessed January 21, 2021.
- 20. Singh S, Zieman S, Go AS, et al. Statins for primary prevention in older adults‐moving toward evidence‐based decision‐making. J Am Geriatr Soc. 2018;66(11):2188‐2196. doi: 10.1111/jgs.15449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yebyo HG, Aschmann HE, Menges D, Boyd CM, Puhan MA. Net benefit of statins for primary prevention of cardiovascular disease in people 75 years or older: a benefit‐harm balance modeling study. Ther Adv Chronic Dis. 2019;10:2040622319877745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hawley CE, Roefaro J, Forman DE, Orkaby AR. Statins for primary prevention in those aged 70 years and older: a critical review of recent cholesterol guidelines. Drugs Aging. 2019;36(8):687‐699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. American Geriatrics Society 2019 updated AGS Beers Criteria® for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2019;67(4):674‐694. doi: 10.1111/jgs.15767 [DOI] [PubMed] [Google Scholar]
- 24. Markota M, Rummans TA, Bostwick JM, Lapid MI. Benzodiazepine use in older adults: dangers, management, and alternative therapies. Mayo Clin Proc. 2016;91(11):1632‐1639. [DOI] [PubMed] [Google Scholar]
- 25. Case SM, Fried TR, O'Leary J. How to ask: older adults' preferred tools in health outcome prioritization. Patient Educ Couns. 2013;91(1):29‐36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Fried TR, Tinetti ME, Iannone L, O'Leary JR, Towle V, Van Ness PH. Health outcome prioritization as a tool for decision making among older persons with multiple chronic conditions. Arch Intern Med. 2011;171(20):1856‐1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chew LD, Bradley KA, Boyko EJ. Brief questions to identify patients with inadequate health literacy. Fam Med. 2004;36(8):588‐594. [PubMed] [Google Scholar]
- 28. R Core Team . R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; 2021. https://www.R-project.org/ [Google Scholar]
- 29. Venables WN, Ripley BD. Modern Applied Statistics with S. 4th ed. Springer; 2002. ISBN 0‐387‐95457‐0, https://www.stats.ox.ac.uk/pub/MASS4/. Accessed January 21, 2021. [Google Scholar]
- 30. Ailabouni NJ, Nishtala PS, Mangin D, Tordoff JM. Challenges and enablers of deprescribing: a general practitioner perspective. PLoS One. 2016;11(4):e0151066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Schuling J, Gebben H, Veehof LJ, Haaijer‐Ruskamp FM. Deprescribing medication in very elderly patients with multimorbidity: the view of Dutch GPs. A qualitative study. BMC Fam Pract. 2012;13:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Green AR, Lee P, Reeve E, et al. Clinicians' perspectives on barriers and enablers of optimal prescribing in patients with dementia and coexisting conditions. J Am Board Fam Med. 2019;32(3):383‐391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Green A, Boyd C, Gleason KS, et al. Designing a primary‐care based deprescribing intervention for patients with dementia and multiple chronic conditions: a qualitative study. J Gen Intern Med. 2020;35(12):3556‐3563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Green AR, Wolff JL, Echavarria DM, et al. How clinicians discuss medications during primary care encounters among older adults with cognitive impairment. J Gen Intern Med. 2020;35(1):237‐246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Reeve E, Wiese MD, Hendrix I, Roberts MS, Shakib S. People's attitudes, beliefs, and experiences regarding polypharmacy and willingness to deprescribe. J Am Geriatr Soc. 2013;61(9):1508‐1514. doi: 10.1111/jgs.12418 [DOI] [PubMed] [Google Scholar]
- 36. Verdoorn S, Kwint HF, Blom J, Gussekloo J, Bouvy ML. DREAMeR: drug use reconsidered in the elderly using goal attainment scales during medication review; study protocol of a randomised controlled trial. BMC Geriatr. 2018;18(1):190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Verdoorn S, Kwint HF, Blom JW, Gussekloo J, Bouvy ML. Effects of a clinical medication review focused on personal goals, quality of life, and health problems in older persons with polypharmacy: a randomised controlled trial (DREAMeR‐study). PLoS Med. 2019;16(5):e1002798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Verdoorn S, van de Pol J, Hövels AM, et al. Cost‐utility and cost‐effectiveness analysis of a clinical medication review focused on personal goals in older persons with polypharmacy compared to usual care: economic evaluation of the DREAMeR study. Br J Clin Pharmacol. 2021;87(2):588‐597. [DOI] [PubMed] [Google Scholar]
- 39. Tinetti M, Huang A, Molnar F. The geriatrics 5M's: a new way of communicating what we do. J Am Geriatr Soc. 2017;65(9):2115. doi: 10.1111/jgs.14979 [DOI] [PubMed] [Google Scholar]
- 40. Kerns JW, Winter JD, Winter KM, Kerns CC, Etz RS. Caregiver perspectives about using antipsychotics and other medications for symptoms of dementia. Gerontologist. 2017;58:e35‐e45. [DOI] [PubMed] [Google Scholar]
- 41. Franquiz MJ, McGuire AL. Direct‐to‐consumer drug advertisement and prescribing practices: evidence review and practical guidance for clinicians. J Gen Intern Med. 2021;36(5):1390‐1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Goodman CW, Brett AS. A clinical overview of off‐label use of gabapentinoid drugs. JAMA Intern Med. 2019;179(5):695‐701. [DOI] [PubMed] [Google Scholar]
- 43. Green AR, Segal J, Boyd CM, Huang J, Roth DL. Patterns of potentially inappropriate bladder antimuscarinic use in people with dementia: a retrospective cohort study. Drugs Real World Outcomes. 2020;7(2):151‐159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Agency for Healthcare Research and Quality . The SHARE Approach—Communicating Numbers to Your Patients: A Reference Guide for Health Care Providers . https://www.ahrq.gov/health-literacy/professional-training/shared-decision/tool/resource-5.html. Content last reviewed September 2020. Accessed January 28, 2022.
- 45. Sepucha KR, Borkhoff CM, Lally J, et al. Establishing the effectiveness of patient decision aids: key constructs and measurement instruments. BMC Med Inform Decis Mak. 2013;13(Suppl 2):S12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Stacey D. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev. 2017;4(4):CD001431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lambooij MS, Harmsen IA, Veldwijk J, et al. Consistency between stated and revealed preferences: a discrete choice experiment and a behavioural experiment on vaccination behaviour compared. BMC Med Res Methodol. 2015;15:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Salampessy BH, Veldwijk J, Jantine Schuit A, et al. The predictive value of discrete choice experiments in public health: an exploratory application. Patient. 2015;8(6):521‐529. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Text S1 (A) Deprescribing communication survey. (B) Additional details on knowledge networks methodology


