Abstract
Background:
Current evidence is inconsistent on the benefits of aerobic exercise training for preventing or attenuating age-related cognitive decline in older adults.
Objective:
To investigate the effects of a one-year progressive, moderate-to-high intensity aerobic exercise intervention on cognitive function, brain volume, and cortical thickness in sedentary but otherwise healthy older adults.
Methods:
We randomized 73 older adults to a one-year aerobic exercise or stretching-and-toning (active control) program. The primary outcome was a cognitive composite score calculated from eight neuropsychological tests encompassing inductive reasoning, long-term and working memory, executive function, and processing speed. Secondary outcomes were brain volume and cortical thickness assessed by MRI, and cardiorespiratory fitness measured by peak oxygen uptake (VO2).
Results:
One-year aerobic exercise increased peak VO2 by ~10% (p<0.001) while it did not change with stretching (p=0.241). Cognitive composite scores increased in both the aerobic and stretching groups (p<0.001 for time effect), although no group difference was observed. Total brain volume (p<0.001) and mean cortical thickness (p=0.001) decreased in both groups over time while the reduction in hippocampal volume was smaller in the stretching group compared with the aerobic group (p=0.040 for interaction). Across all participants, improvement in peak VO2 was positively correlated with increases in cognitive composite score (r=0.282, p=0.042) and regional cortical thickness at the inferior parietal lobe (p=0.016).
Conclusions:
One-year aerobic exercise and stretching interventions improved cognitive performance but did not prevent age-related brain volume loss in sedentary healthy older adults. Cardiorespiratory fitness gain was positively correlated with cognitive performance and regional cortical thickness.
Keywords: aerobic exercise, aging, neurocognitive function, brain volume and cortical thickness, cardiorespiratory fitness
Introduction
The prevalence of dementia (e.g., Alzheimer’s disease [AD]) is rapidly increasing as the average age of the global population continues to rise. Nevertheless, we lack effective strategies to prevent or treat dementia [1, 2]. Because age-related cognitive decline is one of the strongest risk factors for cognitive impairment, preventing or slowing its decline may prevent dementia in later life [3, 4]. With age, there is deterioration in fluid cognitive function—including executive function, long-term and working memory, and processing speed—from early mid-life, while crystallized intelligence such as general knowledge and verbal ability is relatively spared until late adulthood [5, 6]. These age-related cognitive changes may be associated with reductions in cerebral gray matter volume, particularly the prefrontal cortex and the medial temporal and parietal lobes [7]. Notably, age-related reduction in hippocampal volume associated with memory deterioration is an established risk factor for AD dementia [8].
Epidemiological evidence suggests that higher levels of physical activity and fitness are associated with better cognitive performance in older adults [9–12] and reduced risk of late-life dementia [13, 14]. However, the findings from randomized controlled trials (RCT) have been inconsistent [15, 16]. Several meta-analyses and systematic reviews showed small to moderate benefits of moderate-intensity aerobic exercise training on cognitive performance in older adults, but the clinical significance of these changes remains to be determined [16–18]. Moreover, the effects of exercise interventions on brain volume—particularly the hippocampus—remain controversial [19, 20]. Some studies showed small benefits of aerobic exercise for preserving or reversing age-related hippocampal atrophy [19], but others showed no significant effect [20]. Recent meta-analyses suggested that exercise interventions, regardless of modality used, do not have significant impact on brain volume in older adults [21, 22].
These inconsistent results from the exercise RCTs are potentially attributed to the differences in the duration and intensity of training and/or the resultant physiological adaptations in a particular study population. Specifically, change in cardiorespiratory fitness (CRF) in response to exercise training is likely to play a pivotal role in the effect on cognitive function and/or brain volume [23]. Change in CRF, as measured by peak oxygen uptake (peak VO2), may exhibit significant individual variabilities ranging from no changes to significant improvements in those who have undergone similar levels of exercise training [24, 25]. In addition, observational studies which showed positive associations of higher peak VO2 with cognitive performance and gray matter volume [26, 27] may have included individuals who had active lifestyles or exercise training for many years. Conversely, interventional studies commonly enrolled participants with low baseline physical activity levels, only lasted for a few months, and/or implemented a low-to-moderate intensity exercise program which may not improve CRF. When combined, these confounding factors may lead to the inconsistency observed in the current literature.
The purpose of the present study was to investigate the effect of a one-year progressive, moderate-to-high-intensity aerobic exercise training program on neurocognitive function in cognitively normal older adults who previously had a sedentary lifestyle. Our primary hypothesis was that compared with an active control group of stretching-and-toning, which was commonly used in the exercise RCTs [28–30], one-year of aerobic exercise would improve peak VO2 and cognitive performance while reducing the age-related brain volume loss and cortical thinning. Secondly, we hypothesized that improved peak VO2 would be positively correlated with changes in cognitive performance, brain volume, and cortical thickness.
Materials and methods
Study design
This study was a 12-month open label RCT comparing the effects between aerobic exercise training and stretching-and-toning in cognitively normal older adults. Neuropsychological and CRF assessments were conducted at baseline, midpoint (six month), and trial completion. Brain MRI data were collected at baseline and trial completion. The study was approved by the Institutional Review Board of the University of Texas Southwestern Medical Center and Texas Health Presbyterian Hospital Dallas in accordance with the guidelines of the Declaration of Helsinki and Belmont Report. All participants gave written informed consent before participation. This trial was not registered because at the time of trial initiation, registration of non-pharmacological interventional studies in healthy adults in a public database was neither required nor typical.
Participants
This study enrolled cognitively normal sedentary but otherwise healthy men and women aged 60–80 years. Recruitment was conducted in the Dallas-Fort Worth metropolitan area using community-based advertisements. An initial telephone screening was conducted to ask participants if they had subjective cognitive complaints or a history of major clinical conditions, regularly engaged in a structured exercise program, and could participate in a one-year aerobic exercise or stretching program including the required visits for data collection. Subsequently, participants were asked to visit our clinical office and screened for the following exclusion criteria: 1) clinical diagnosis of major psychiatric or neurological disorders or medications causing major effects on cognition; 2) a history of active alcoholism or drug abuse; 3) a history of recurrent epilepsy, stroke, or head injury/trauma with a loss of consciousness ≥30 minutes; 4) Mini-Mental Status Examination (MMSE) score <26 to exclude dementia; 5) uncontrolled hypertension (averaged three measurements of sitting systolic blood pressure ≥140 or diastolic pressure ≥90 mmHg confirmed by 24-h ambulatory blood pressure monitoring); 6) a diagnosis of diabetes mellitus (fasting glucose >126 mg/dL or taking antidiabetic medications); 7) severe obesity with body mass index (BMI) ≥35; 8) smoking within the past 5 years of the study, and 9) other major or unstable medical conditions such as a history of coronary bypass surgery or heart attack within the past year, ongoing chemotherapy, or severe lung, kidney and liver disease. (10) Individuals who spent more than 90 minutes of moderate-to-vigorous physical activity (>4.0 METs) per week were excluded, as determined by a one-week physical activity monitoring using an accelerometer (Actical, Philips Respironics, USA); and (11) individuals with physical disability, metal implants in the body, or claustrophobia precluding MRI scans were excluded. Fluency in English and a minimum of a 10th grade education were required for neuropsychological testing.
Randomization and Blinding
Randomization was performed in SAS V9.2 using two stratification groups: sex (men and women) and education (10–14 years and 15–20 years), using a blocking factor of four. The randomization assignments were generated by the study statistician (LH) and placed in a sealed envelope so that the study personnel was blinded until opening the envelope for treatment assignment of an individual subject. Investigators conducting the primary and secondary outcome measurements were blinded to treatment assignment throughout the study. Participants were instructed to maintain normal daily activities aside from the assigned interventions and were instructed not to disclose group assignment or interventions during outcome measurements or meeting with other participants.
Intervention
Aerobic exercise training
The dose of aerobic exercise training (i.e., intensity, duration, and frequency) was based on individual fitness level assessed with peak VO2 testing and progressively increased as the participant adapted to previous workloads. Specifically, the program started with a frequency of three exercise sessions per week for 25–30 minutes per session at the intensity of 75%−85% of maximal heart rate that was measured during the peak VO2 test at baseline. Between weeks 11–25, participants started alternating between three and four exercise sessions per week for 30–35 minutes per session. At the weeks in which they performed three exercise sessions per week, a high-intensity exercise session was introduced which comprised 30 minutes of walking at the intensity of 85%−90% of maximal heart rate (e.g., brisk uphill walking). After week 26, participants performed four to five exercise sessions per week for 30–40 minutes, including two high-intensity sessions. Each exercise session included a five-minute warm-up and a five-minute cool-down. Any modes of aerobic exercise were allowed if participants maintained the prescribed exercise training dose, as monitored by changes in heart rate during each of the exercise sessions (Polar RS400, Polar Electro, USA). This exercise program meets the national physical activity guidelines for older adults [31] and has been shown to significantly improve CRF in sedentary older adults with mild cognitive impairment [32].
Stretching-and-toning
The stretching program created an active control group to keep participants engaged, and provided the same level of attention from the investigators as those in the aerobic group. The frequency and duration of the stretching program were the same as the aerobic program. Participants in this active control group performed a series of upper- and lower-body stretching-and-toning procedures without significantly increasing heart rate during each session (e.g., to keep heart rate below 50% of maximal heart rate). At week 19, a second set of more advanced full body stretches were introduced corresponding to the training changes in the aerobic program. After week 26, we introduced a set of low resistance exercises that focused on strengthening the upper and lower body using resistance bands (TheraBand).
In both intervention programs, each participant was supervised for the first several weeks until they could comfortably exercise by themselves at home. During the study period, they were asked to perform the assigned training interventions on top of their regular physical activities (e.g., gardening or leisure walking). To ensure adherence to each program, participants were required to keep a training log in addition to heart rate monitoring. Each month, participants visited the clinic to download heart rate data and review the training log with an exercise physiologist to ensure implementation of the prescribed training programs. When adherence to exercise programs was not met with the prescribed intensity, duration, or frequency, in-person and/or telephone meetings were conducted to mitigate reoccurring training issues and encourage continuation in the program.
Outcomes
Primary outcome
The primary outcome was a cognitive composite score focused on fluid cognitive ability sensitive to age and calculated from a comprehensive neuropsychological test battery encompassing inductive reasoning, long-term and working memory, executive function, and processing speed. Specifically, the following eight tests were used to calculate the composite score: 1) ETS Letter Sets [33], 2) Raven’s Progressive Matrices accuracies [34], 3) Logical Memory Immediate and Delayed recalls [33], 4) Woodcock-Johnson Immediate and Delayed recalls [35], 5) Digit Comparison [36], 6) Letter Number Sequencing [37], 7) Operation Span [38], and 8) Controlled Word Association FAS [39]. These tests have been shown to have credible validity, reliability, and sensitivity to cognitive decline in older adults based on our previous studies [5, 6].
A cognitive composite score was calculated by converting individual scores to standardized z-scores by subtracting the baseline group mean and dividing by the baseline group standard deviation (SD). Z-scores from each test were subsequently averaged with equal weighting. Scores of multiple test components (i.e., immediate and delayed recall scores from the Logical Memory and Woodcock-Johnson tests) were first averaged within each test, so that all tests were equally weighted when calculating the composite score. Higher composite scores indicate better cognitive performance. All participants had at least seven test scores to calculate the composite score at each time point. Additionally, crystallized cognitive ability was assessed by the ETS Vocabulary [33]. The same test forms were used during the follow-up assessments.
Secondary outcomes
The secondary outcomes were brain volume and cortical thickness assessed by MRI, and CRF assessed by peak VO2.
Brain volume and cortical thickness.
High-resolution 3D T1-weighted magnetization-prepared rapid acquisition gradient-echo (MPRAGE) images were collected using a 3-tesla scanner (Achieva 3.0T, Philips Medical System, the Netherlands) with the following parameters: TE/TR = 3.7/8.1 ms, flip angle = 12°, FOV = 256×256 mm, number of slices = 160 (no gap), resolution= 1×1×1 mm3, and SENSE factor = 2. To extract reliable volume and thickness estimates, images were automatically processed by the longitudinal stream in FreeSurfer v6.0 with default settings [40]. Specifically, an unbiased within-subject template space and image [41] were created using robust, inverse consistent registration [42]. Several processing steps, such as skull stripping, Talairach transforms, atlas registration as well as spherical surface maps and parcellations were then initialized with common information from the within-subject template, significantly increasing reliability and statistical power [40]. Individual images were visually inspected for segmentation errors, and no manual correction was required. The Desikan-Killiany atlas was used to generate the total and regional measures of brain volume and cortical thickness.
The total brain and hippocampal volumes were selected as a priori regions of interest (ROI) relevant to aging and AD pathologies [43]. These volumetric data were normalized to intracranial volume at each time point and reported as percent intracranial volume (%ICV). The total brain volume (i.e., BrainSegVolNotVent) included the cerebellum but excluded the brainstem, ventricles, cerebrospinal fluid, and choroid plexus. The cortical ROIs were also selected based on their reported vulnerability to aging and AD pathologies [7], which includes the prefrontal, medial temporal (entorhinal cortex and parahippocampal gyrus), and parietal (posterior cingulate cortex and precuneus) cortical areas. The prefrontal cortical thickness was calculated by averaging the following regions [44]: caudal and rostral anterior cingulate; caudal and rostral middle frontal; medial and lateral orbitofrontal; frontal pole; and superior frontal cortices, as well as pars opercularis, triangularis, and orbitalis. Furthermore, the primary motor (precentral) and visual (pericalcarine) cortices were included because voluntary movement and visual stimulus during exercise training may induce brain structural adaptations specific to these areas [45, 46]. Additionally, mean cortical thickness was calculated as the average of all cortical regions. To reduce the number of comparisons, brain volumes from the left and right sides were summed while their cortical thickness was averaged.
Cardiorespiratory fitness (CRF).
Peak VO2, the gold standard index of CRF, was measured to determine the cardiovascular adaptation to the aerobic exercise and stretching programs. Peak VO2 was measured by a modified Astrand-Saltin protocol using a treadmill [47]. The treadmill grade was increased by 2% every two minutes until exhaustion while participants walked or jogged at a fixed speed determined by individual fitness level. VO2, carbon dioxide production, and respiratory exchange ratio (RER) were measured during the second minute of each stage using the Douglas bag method [48]. Gas fractions were analyzed by mass spectrometry (Marquette MGA 1100), and ventilatory volume was measured by a Tissot spirometer. During each testing, blood pressure, 12-lead electrocardiogram, and heart rate were continuously monitored to assess cardiovascular response to exercise and to monitor participants’ safety [32].
Peak VO2 was defined as the highest VO2 measured from a >30-second Douglas bag during the last stage of testing. The criteria to confirm that peak VO2 was achieved included an increase in VO2 <150 ml despite increasing work rate of 2% grade, a RER >1.1, and heart rate <5 beats/min of age-predicted maximal values. In all cases, at least two of these criteria were achieved, confirming the identification of peak VO2 based on the American College of Sports Medicine guidelines [31]. Our previous study showed that by using these methods, peak VO2 can be measured reliably in sedentary older adults with mild cognitive impairment [32]. Peak VO2 are reported as milliliter per kilogram per minute and percentage change to account for body mass and baseline level, respectively.
Sample size estimate
Sample size estimation for this proof-of-concept RCT was based on a meta-analysis showing that aerobic exercise may improve neuropsychological performance in cognitively normal healthy older adults [49]. We anticipated that cognitive composite scores would improve by ~0.6 SD after one year of aerobic exercise (treatment) compared with the stretching group (control). Assuming a 15% attrition rate and an α-level of <0.05, 70 participants provide 80% power to detect an effect size of 0.60.
Statistical analysis
The primary analysis of the study outcomes was based on the intent-to-treat, maximum likelihood estimation using all available data from randomized subjects (n=73). A linear mixed model (LMM) was used to analyze the interaction effect of group (aerobic exercise and stretching) by time (baseline, six month, and one year). In LMM, cognitive and brain volumetric measures were adjusted for baseline age, sex, and years of education, while peak VO2 was adjusted for baseline age and sex. These covariates were selected based on evidence in the literature [5, 7] and their associations with outcomes in the current study (see the Results section). In each model, individual intercept was specified as a random effect, and a compound symmetry covariance structure was used to account for within-individual correlations across time points. To confirm the results from intent-to-treat analysis, LMM analysis on complete outcome data was also performed to compare change scores between the aerobic and stretching groups at each time point. Post-hoc multiple pairwise comparisons were corrected by the Bonferroni method.
Additionally, the Pearson product-moment correlation was used to examine the associations among individual changes in outcomes and the rate of adherence to interventions. Baseline group characteristics were compared by the independent samples t-test and chi-square test for continuous and categorical variables, respectively. Intraclass correlation coefficients (ICC) were calculated to assess test-retest reliability compared between the baseline and 12-month follow-up measurements based on a two-way mixed, absolute agreement model [50]. Data normality was assessed by the Shapiro-Wilk test and the visual inspections of histogram and Q-Q plots. Results from the LMM analysis are presented as estimated marginal means and 95% confidence intervals (CI) while baseline group characteristics are shown as means and SDs. Statistical significance was set a priori at p<0.05. All analyses above were performed by SPSS 25 (IBM Corporation, Armonk, NY, USA, 2011).
Cortical thickness data were further analyzed by FreeSurfer’s QDEC software (Query, Design, Estimate, Contrast, version 1.5) based on the longitudinal two-stage model (https://surfer.nmr.mgh.harvard.edu/fswiki/LongitudinalTwoStageModel). The symmetrized percent change (SPC), a dimensionless measure of variability in cortical thickness, was calculated at each vertex from two time points witheach participant. SPC is defined as follows [40]:
where Thick 1 is cortical thickness at baseline and Thick 2 is the measure at one-year follow-up. Group comparison of the whole-brain SPC and the correlation with change in peak VO2 were examined by a general linear model. Cortical maps were smoothed with a 10-mm full-width-at-half-maximum Gaussian kernel; multiple comparisons were corrected by a Monte Carlo simulation with a p-value set at <0.05; and separate general linear model analyses were performed for the right and left hemispheres [51, 52].
Results
The flow of participants from the time of screening to study completion is presented in Figure 1. Recruitment was conducted from December 2010 to February 2015, and data collection was completed in November 2016. Of the randomized 73 cognitively normal older adults, 17 participants (23%) were excluded or withdrew from the study. The groups of participants who completed the study and those lost to attrition were similar in age, sex, baseline cognitive performance, and brain structural measures, except for the entorhinal thickness being thinner in the participants lost to attrition (data not shown).
Figure 1:
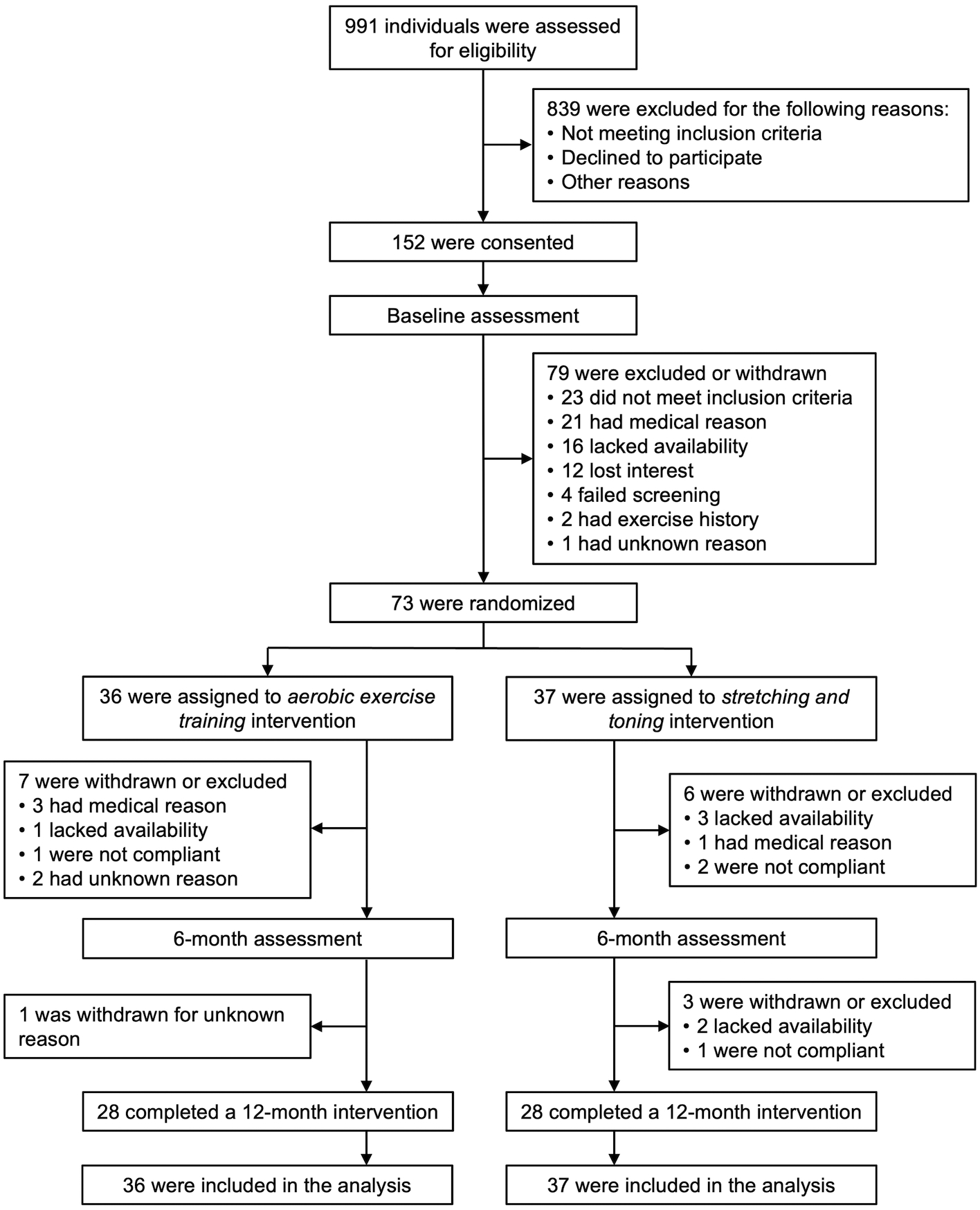
Flowchart for one-year exercise trial in cognitively normal older adults. Participants were randomized to a 12-month intervention of aerobic exercise training or stretching-and-toning program.
At baseline, aerobic and stretching groups were similar in age, sex, racial distributions, years of education, MMSE scores, height, body mass, BMI, and antihypertensive use (Table 1). Also, both groups had similar daily physical activity levels, peak VO2, and ambulatory blood pressure and heart rate at baseline. Cognitive composite scores (aerobic vs. stretching: 0.102±0.561 vs. −0.102±0.632, p=0.149) and brain structural measures were also similar at baseline, except for the pericalcarine thickness being greater in the stretching group compared with the aerobic (1.618±0.117 vs. 1.556±0.122 mm, p=0.032).
Table 1:
Baseline characteristics of the aerobic exercise and stretching groups
| Aerobic exercise | Stretching | p-values | |
|---|---|---|---|
| (n = 36) | (n = 37) | (t or χ2 test) | |
| Women [n (%)] | 27 (75) | 28 (76) | 0.947 |
| Age (years) | 69 ± 6 | 68 ± 5 | 0.419 |
| Race [n (%)] | 0.947 | ||
| White | 36 (100) | 34 (92) | |
| Black | 0 (0) | 3 (8) | |
| Education (years) | 17 ± 2 | 16 ± 2 | 0.160 |
| Mini-Mental State Exam | 29.2 ±0.9 | 29.2 ± 0.8 | 0.976 |
| Height (cm) | 165.9 ± 8.3 | 164.8 ± 8.6 | 0.559 |
| Body mass (kg) | 71.5 ± 15.6 | 74.0 ± 10.7 | 0.423 |
| Body mass index (kg/m2) | 25.8 ± 4.4 | 27.3 ± 3.6 | 0.120 |
| Hypertension [n (%)] | 8 (22) | 5 (14) | 0.331 |
| Physical activity and fitness measures | |||
| Peak oxygen uptake (ml·kg−1·min−1) | 22.8 ± 4.1 | 21.7 ± 3.2 | 0.196 |
| Physical activity (min/day) | |||
| Light (<4.0 METs) | 221 ± 81 | 240 ± 91 | 0.372 |
| Moderate (4.0–5.0 METs) | 4 ± 5 | 4 ± 4 | 0.938 |
| Vigorous (>5.0 METs) | 1 ± 3 | 2 ± 4 | 0.474 |
| Ambulatory blood pressure measures | |||
| 24-hour systolic blood pressure (mmHg) | 126 ± 10 | 125 ± 8 | 0.602 |
| 24-hour diastolic blood pressure (mmHg) | 71 ± 7 | 71 ± 5 | 0.871 |
| 24-hour heart rate (bpm) | 71 ± 8 | 72 ± 7 | 0.513 |
Values show means ± standard deviations or numbers (percentage). All hypertensive individuals had controlled blood pressure. Physical activity data were averaged for a week and available from 32 aerobic exercise and 35 stretching group participants. Ambulatory blood pressure data were missing from one aerobic exercise group participant. METs, metabolic equivalents
At baseline, cognitive composite score was correlated with age (r=−0.395, p<0.001) and years of education (r=0.336, p=0.004). Age was also related to total brain volume (r=−0.255, p=0.033), mean cortical thickness (r=−0.455, p<0.001), and peak VO2 (r=−0.279, p=0.017) at baseline. Compared with men, women had greater total brain volume (71.86±4.70 vs. 67.63±4.04 %ICV, p<0.001) and mean cortical thickness (2.476±0.068 vs. 2.444±0.068 mm, p=0.045) and lower peak VO2 (21.5±3.3 vs. 24.5±3.9 ml/kg/min, p<0.001). Conversely, these demographic covariates were not correlated with change in outcomes over time.
Intervention effect
Compared with stretching, aerobic exercise training significantly increased peak VO2 over one year, as shown by LMM adjusted for baseline age and sex (Figure 2A). In the aerobic group, peak VO2 increased by 2.12 ml·kg−1·min−1 (95% CI 1.17 to 3.07, p<0.001) over 12 months, with the most increase achieved during the first six months of training (2.10 ml·kg−1·min−1, 95% CI 1.12 to 3.07, p<0.001 vs. baseline). Conversely, peak VO2 did not change significantly over one year in the stretching group (0.69 ml·kg−1·min−1, 95% CI −0.26 to 1.63, p=0.241). The complete outcome analysis also showed a significant interaction of group by time (Figure 2B). Specifically, aerobic group exhibited a significantly greater increase in peak VO2 from the baseline to 12-month than the stretching group (10.2%, 95% CI 6.3 to 14.1 vs. 3.4%, 95% CI −0.4 to 7.1; p=0.013), with the large individual variability observed at each time point. In both groups, body mass and peak heart rate remained unchanged over 12 months (Table 2). Peak RER slightly decreased at 6 and 12 months, but they were higher than 1.1 at all time points. The average compliance to the aerobic exercise program was 81.3%, as calculated by the ratio of prescribed exercise sessions over the exercise sessions completed in which participants achieved their target heart rate. The average compliance to the stretching program was 70.2%. There was no group difference in the training compliances. In the aerobic group, the higher compliance rate was correlated with greater improvement in peak VO2 (r=0.450, p=0.016).
Figure 2:

A) Peak oxygen uptake (peak VO2) in the aerobic exercise and stretching groups at baseline and six- and 12-month follow-ups. Estimated marginal means (solid lines), 95% confidence intervals (dashed lines), and p-values were calculated from the linear mixed model based on the intent-to-treat analysis. B) Percent changes in peak VO2 compared between the aerobic and stretching groups at each time point. p-values were calculated from the linear mixed model based on complete outcome data. Both models were adjusted for baseline age and sex. *p<0.05 for post-hoc pairwise comparisons with the Bonferroni correction. Black lines represent mean values.
Table 2:
Peak oxygen uptake (VO2) measurements at baseline and 6- and 12-month aerobic exercise and stretching interventions
| Time | EMM | (95% CI) | EMM | (95% CI) | Group | Time | Group*Time | |
|---|---|---|---|---|---|---|---|---|
| 12-month | 1.74 | (1.66 to 1.83)*† | 1.61 | (1.53 to 1.70) | ||||
| 12-month | 25.0 | (23.9 to 26.1)*† | 22.2 | (21.1 to 23.3) | ||||
| 12-month | 70.7 | (66.2 to 75.1) | 73.5 | (69.1 to 77.9) | ||||
| 12-month | 160 | (156 to 165) | 161 | (157 to 165) | ||||
| 12-month | 1.14 | (1.11 to 1.17) | 1.13 | (1.10 to 1.16) |
Values are estimated marginal means (EMM), 95% confidence intervals (CI), and p-values calculated from the linear mixed model based on the intent-to-treat analysis. The models for peak oxygen uptake were adjusted for baseline age and sex. p<0.05 are bolded.
vs. baseline within the same group.
vs. stretching group at the same time point.
LMM adjusted for baseline age, sex, and years of education showed a significant increase in cognitive composite score in both the aerobic and stretching groups over one year, although no significant interaction effect was observed (Figure 3A). In the aerobic group, the composite score increased by 0.385 standardized z-scores (95% CI 0.236 to 0.534, p<0.001) over 12 months, including the increases of 0.212 (95% CI 0.067 to 0.357, p=0.002) for the first six months and 0.173 (95% CI 0.022 to 0.323, p=0.018) between six and 12 months. In the stretching group, the composite score increased by 0.341 standardized z-scores (95% CI 0.188 to 0.494, p<0.001) over 12 months, including the increases of 0.222 (95% CI 0.078 to 0.367, p=0.002) for the first six months and 0.119 (95% CI −0.036 to 0.274, p=0.192) between six and 12 months. The complete outcome analysis confirmed the results of intent-to-treat analysis with a significant time but interaction effect (Figure 3B). For individual neuropsychological test scores, there was no interaction effect while performance on the Raven’s Progressive Matrices accuracies, Logical Memory Immediate and Delayed recalls, Woodcock-Johnson Immediate and Delayed recalls, Operation Span, and Controlled Word Association FAS improved over time with repeated exposures to testing (Table S1).
Figure 3:
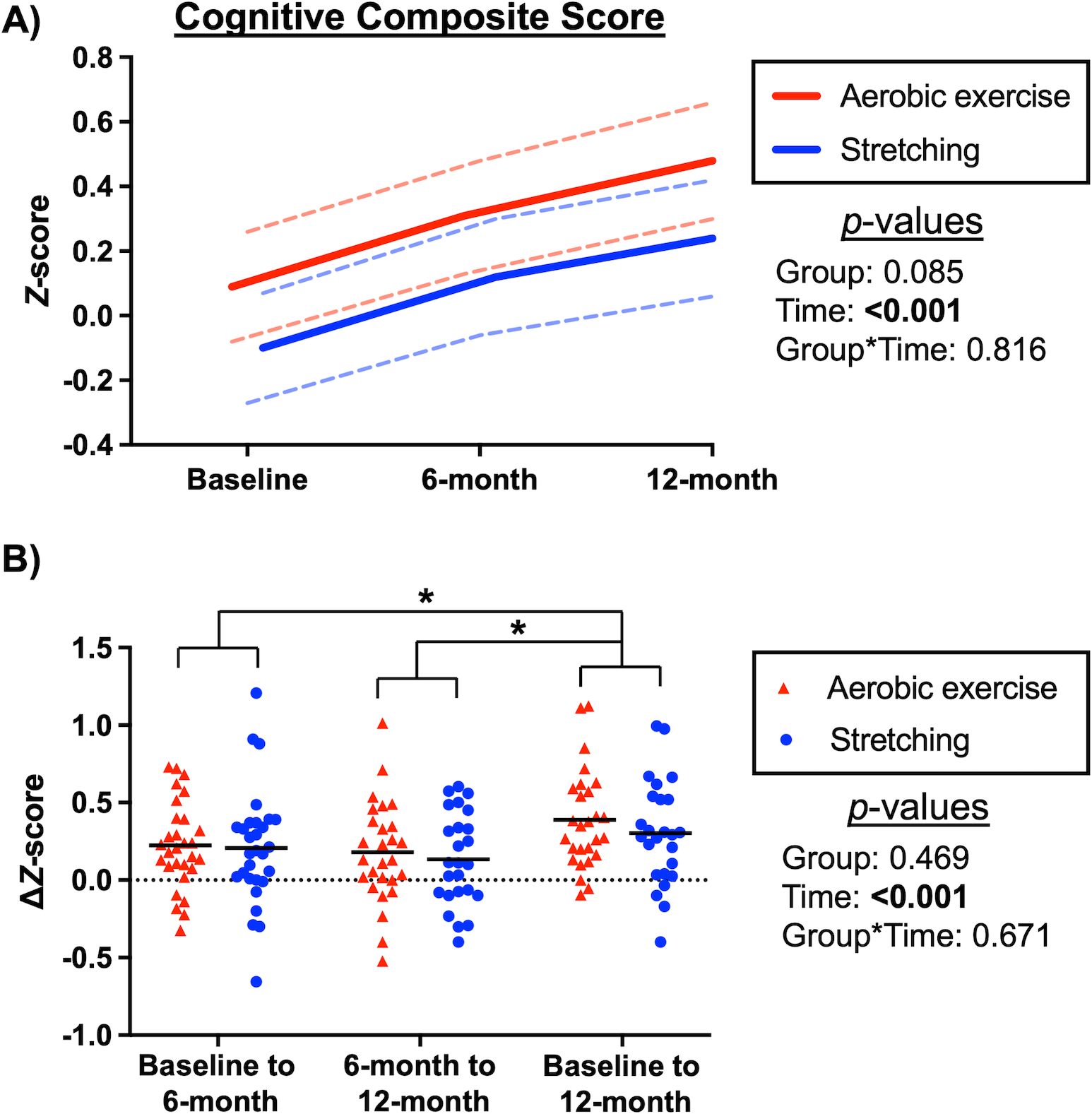
A) Cognitive composite scores in the aerobic exercise and stretching groups at baseline and six- and 12-month follow-ups. Estimated marginal means (solid lines), 95% confidence intervals (dashed lines), and p-values were calculated from the linear mixed model based on the intent-to-treat analysis. B) Changes in cognitive composite scores compared between the aerobic and stretching groups at each time point. P-values were calculated from the linear mixed model based on complete outcome data. Both models were adjusted for baseline age, sex, and years of education. *P<0.05 for post-hoc pairwise comparisons with the Bonferroni correction. Black lines represent mean values.
Table 3 shows change in brain volume and cortical thickness in the aerobic and stretching groups with adjustment for baseline age, sex, and years of education. In both groups, total brain volume (−0.611 %ICA, 95% CI −0.851 to −0.371) and mean cortical thickness (−0.020 mm, 95% CI −0.031 to −0.009) decreased over time. Regional cortical thickness in the prefrontal area (−0.017 mm, 95% CI −0.029 to −0.006), precuneus (−0.028 mm, 95% CI −0.044 to −0.012), and precentral gyrus (−0.037 mm, 95% CI −0.053 to −0.020) decreased over time. A significant interaction was observed for hippocampal volume, with reduced atrophy in the stretching (<0.001 %ICV, 95% CI −0.006 to 0.006, p=0.945) compared with the aerobic group (−0.009 %ICV, 95% CI −0.015 to −0.003, p=0.003). The complete outcome analysis also confirmed these results (Table S2). QDEC showed a significant increase in SPC of the right lingual cortical thickness in the stretching group (Figure 4A, cluster size=795.3 mm2, cluster-wise p=0.004).
Table 3:
Brain volume and cortical thickness at baseline and 12-month aerobic exercise and stretching interventions
| Time | EMM | (95% CI) | EMM | (95% CI) | Group | Time | Group*Time | |
|---|---|---|---|---|---|---|---|---|
| 12-month | 69.7 | (68.1 to 71.2) | 70.73 | (69.2 to 72.3) | ||||
| 12-month | 0.518 | (0.498 to 0.539)* | 0.521 | (0.501 to 0.541) | ||||
| 12-month | 2.444 | (2.421 to 2.466) | 2.452 | (2.429 to 2.474) | ||||
| 12-month | 2.566 | (2.540 to 2.593) | 2.575 | (2.548 to 2.601) | ||||
| 12-month | 3.391 | (3.319 to 3.463) | 3.352 | (3.280 to 3.423) | ||||
| 12-month | 2.778 | (2.695 to 2.860) | 2.718 | (2.636 to 2.799) | ||||
| 12-month | 2.356 | (2.323 to 2.388) | 2.356 | (2.324 to 2.389) | ||||
| 12-month | 2.423 | (2.387 to 2.460) | 2.428 | (2.392 to 2.465) | ||||
| 12-month | 2.497 | (2.455 to 2.539) | 2.499 | (2.458 to 2.541) | ||||
| 12-month | 1.551 | (1.510 to 1.591) | 1.623 | (1.583 to 1.664) |
Values are estimated marginal means (EMM), 95% confidence intervals (CI), and p-values calculated from the linear mixed model based on the intent-to-treat analysis. p<0.05 are bolded. All models were adjusted for baseline age, sex, and years of education.
vs. baseline within the same group.
ICV, intracranial volume
Figure 4:

Vertex-wise analysis of symmetrized percent change (SPC) in cortical thickness over one year. A) Group comparison of SPC between the aerobic exercise and stretching groups and B) simple correlation of SPC with change in peak oxygen uptake over one year. The analysis was performed with FreeSurfer’s QDEC (Query, Design, Estimate, Contrast) software.
Individual correlation analysis
In all participants, there was a weak but statistically significant positive correlation between increases in peak VO2 and cognitive composite score (r=0.282, p=0.042) (Figure 5). Conversely, change in cognitive composite score was negatively correlated with changes in the total brain volume (r=−0.402, p=0.004) and the prefrontal (r=−0.331, p=0.019), precuneus (r=−0.398, p=0.004), and posterior cingulate thickness (r=−0.393, p=0.005) (Figure S1). QDEC revealed a significant positive correlation between increases in peak VO2 and SPC of the right inferior parietal cortical thickness (Figure 4B, cluster size=634.7 mm2, cluster-wise p=0.016).
Figure 5:
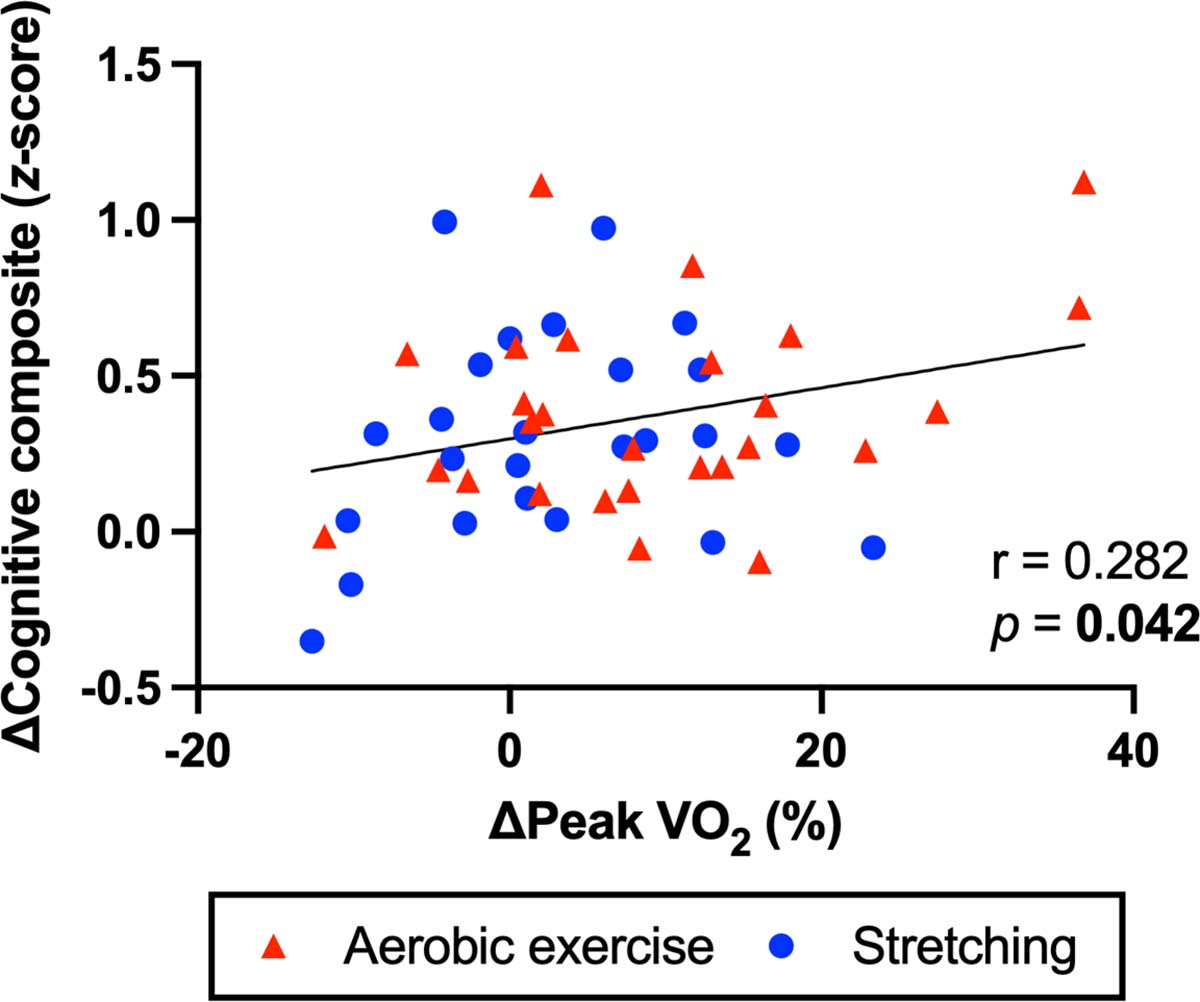
Simple correlation between changes in peak oxygen uptake (VO2) and cognitive composite score over one year. The correlation coefficient (r) and p-value were calculated by the Pearson product-moment correlation analysis.
Test-retest reliability assessment exhibited strong intraclass correlations for cognitive composite score (ICC=0.866, p<0.001), brain structural measures (ICC=0.871~0.989, all p<0.001), and peak VO2 (ICC=0.875~0.957, all p<0.001) over one year (Table S3).
Adverse events
Ten related or possibly related adverse events occurred during the study. Seven occurred in the aerobic group, and three in the stretching group. These events were mainly musculoskeletal pain or injury (i.e., four out of seven in the aerobic group, and one in the stretching group). No serious intervention-related adverse events were reported during the study.
Discussion
The primary findings from this exercise trial are as follows: First, cognitive composite scores increased in the aerobic and stretching groups over one year, although no group difference was observed. Second, brain volume and cortical thickness decreased in both groups over the same time, including the prefrontal, medial temporal, and parietal areas. In contrast to our hypothesis, stretching reduced hippocampal volume loss when compared with the aerobic group. Third, increased peak VO2 over one year was positively associated with improved cognitive performance and increased cortical thickness at the inferior parietal lobule in all participants. Collectively, our results showed that one-year aerobic exercise and stretching improved cognitive performance while not preventing age-related brain volume loss in healthy older adults. CRF gain was positively associated with cognitive performance and regional cortical thickness.
We suspect but cannot prove that increased cognitive composite scores in both the aerobic and stretching groups may reflect practice effects of repeated neuropsychological testing. Practice effects associated with repeated neuropsychological testing have been reported in the previous studies [53, 54]. A longitudinal study that assessed 1390 older adults four times at 15-month intervals showed significant practice effects for memory, attention, language, visual spatial reasoning, and global cognitive performance over three visits for ~30 months [53]. This study showed a particularly strong practice effect for memory, for which performance increased by ~0.23 standardized z-score points over the first 15 months in cognitively normal older adults [53]. Also, in a meta-analysis, the effect size of repeated neuropsychological testing was ~0.24 beta weights across cognitive domains over one year for a healthy middle-aged person [54]. Our results were consistent with these findings such that performance on memory (the Logical memory recalls and the Woodcock-Johnson recalls), inductive reasoning (Raven’s Progressive Matrices accuracies), and working memory (Operation Span) increased over one year when assessed at six-month intervals (Table S1). Across both groups, cognitive composite scores increased by 0.217 standardized z-score points for the first six months followed by an additional increase of 0.146 points between six and 12 months, with the overall increase of 0.363 points over one year. Consistent with our results, earlier exercise training studies reported improved cognitive performance in both the aerobic and stretching groups in non-demented older adults [28, 55]. Therefore, these findings collectively suggest that dissecting the practice effects of repeated neuropsychological testing from potential intervention effects is a challenge in exercise trials. Although there is evidence suggesting the attenuation of practice effects in older adults and/or with a clinical diagnosis of dementia, the use of alternate test forms may help eliminate practice effects and isolate the effect of exercise intervention in future studies [54].
Reductions in brain volume and cortical thickness in the aerobic and stretching groups followed a typical pattern of brain atrophy associated with normal aging [7, 44]. We observed that hippocampal volume and prefrontal and precuneus thickness decreased while pericalcarine thickness did not change over one year. These results are consistent with the findings from a one-year longitudinal study showing that the prefrontal, temporal, and parietal lobes are vulnerable to age while the occipital lobe is relatively insensitive in healthy older adults (a mean baseline age of 75.6 years) [44]. The annual rate of hippocampal atrophy we observed in the current study (i.e., 0.81%/year) was also consistent with the rates reported in the previous studies using automatic (0.84%/year) [44] and manual (0.79%/year) [56] tracing methods. Unexpectedly, we found negative correlations between reduced brain volume and increased cognitive composite scores over a year. While this observation contrasts with the dogma of “larger brain volume, better intelligence” [57, 58], they may reflect a reorganization of the brain’s neural network associated with exercise training and/or aging [59], or spurious correlations between the practice effects of neuropsychological testing and age-related changes in brain volume.
In contrast to our hypothesis, it was stretching rather than aerobic exercise that reduced hippocampal volume loss and regional cortical atrophy in the right lingual gyrus over one year. Participants in the stretching group performed full-body stretches and light-intensity exercise using resistance bands. It has been shown that resistance training is associated with increased hippocampal volume [60, 61] and altered neural activity in the right lingual gyrus in older adults [29]. Thus, resistance-band exercise and/or motor skill-learning associated with the stretching procedures may have influenced these brain structures [62]. Indeed, motor skill-learning has been linked with cortical reorganization of the visual network—including the right lingual gyrus—in athletes [63], and adult hippocampal neurogenesis was observed after physical skill training in an animal model study [64]. Nevertheless, the efficacies of exercise training on brain atrophy in older adults remains controversial with the recent meta-analyses showing that exercise interventions—regardless of exercise modalities—do not have a significant impact on brain volume, including the hippocampus [20–22]. Therefore, future studies using vigorous experimental design (e.g., consideration of a no-treatment control group, larger sample size, and training dose) are needed to determine whether exercise training alters brain volume in older adults.
In observational studies, higher CRF in older adults has been shown to associate with reduced cognitive decline [26, 65] and decreased incidence and mortality of dementia [66]. However, the results from exercise intervention studies have been inconsistent [16–18]. In this study, we observed large individual variability in the change of peak VO2 over one year (Figure 2B). Moreover, individual analysis revealed that change in peak VO2 was positively correlated with increases in cognitive composite score and cortical thickness in the right inferior parietal lobule. In support of our findings, the right inferior parietal lobule has shown altered neural activity and functional connectivity after physical exercise training [67, 68]. Thus, our results suggest a potential benefit of CRF gain for improving neurocognitive function, regardless of aerobic exercise or stretching. As for the possible mechanism, higher CRF is associated with improvements in white matter fiber integrity [69] and cerebral perfusion [70], which may contribute to improvement in cognitive function in older adults. Hence, our findings extend evidence in the literature by suggesting that improved cognitive function with exercise training may indeed require CRF gain which reflects physiological adaptations to exercise and improved cardiovascular health [71, 72]. Significant improvement and maintenance of a high level of CRF typically takes a long duration and/or a high intensity of aerobic exercise. Moreover, the benefits of increased CRF on the brain structure and function are likely to accumulate over years to prevent or reduce age-related cognitive decline or risk of dementia. Therefore, future exercise intervention studies may need a longer period of training, an increase in the intensity, and/or starting training at an earlier age (e.g., midlife) to achieve greater CRF gain and brain health.
This study has several strengths. First, cognitive composite score (primary outcome) was calculated from eight individual neuropsychological tests, with the main focus on fluid cognitive ability which is sensitive to age [73] and may improve with exercise training [16–18]. The use of composite score decreases the risk of Type 1 error while improving the sensitivity and statistical power to detect subtle longitudinal changes. This is because the effect of exercise training on cognitive function may be domain-specific and differ between individuals, and also because scores on a single neuropsychological test may fluctuate over time within each individual [74, 75]. Additionally, neuropsychological tests employed in this study are well-validated and have been used extensively in cognitive aging research [5, 6]. Second, a longitudinal processing pipeline implemented in the FreeSurfer software [40] exhibited the excellent test-retest reliability of brain volume and cortical thickness measurements over one year (Table S3). This may have increased the sensitivity in detecting subtle brain structural changes associated with exercise training and aging. Third, peak VO2—the gold-standard index of CRF—provided an objective measure of longitudinal changes in physical fitness and cardiovascular health [76]. Moreover, a one-week monitoring of physical activity level using an accelerometer provided an objective confirmation that our participants had a sedentary lifestyle prior to study enrollment. Fourth, a progressive training program was used in the aerobic exercise training group, which maintained a high level of cardiovascular stimulus over one year and contributed to a significant improvement of CRF. Fifth, participants were vigorously screened for potential cardiovascular and neurological risk factors which could confound the effect of exercise training on cognitive function [77–79].
This study has several limitations. First, sample size was relatively small, as estimated from a relatively large effect size (~0.6 SD) reported from a previous meta-analysis [49]. However, recent evidence suggests that the effect size of aerobic exercise on cognitive function may be smaller than previously reported [16–18]. Additionally, our sample included more women than men, and more than 90% of participants were well-educated Caucasians, which reduces the generalizability of our findings. Given the reported benefits of resistance exercise on neurocognitive function [29, 80], the stretching-and-toning program—including low-intensity TheraBand resistance exercise—may not have been an ideal control group to detect exercise training effects on neurocognitive function. While the active control group improved participants’ motivation in the study and provided similar investigator contact and attention to participants in both groups, it may have diminished the group differences we could potentially observe after training. Thus, future studies should consider adding a third, no-treatment passive control group [17]. Also, our participants were only supervised for the first several weeks of intervention programs until they could comfortably exercise by themselves. Although an exercise log and heart rate monitor were used to record participants’ adherence to the prescribed exercise programs monthly, differences in the exercise training compliance, dose, and change in leisure physical activity may have resulted in a large individual variability of peak VO2 over one year in both groups (Figure 2B). The one-year trial duration may still be too short to reveal the cumulative effect of aerobic exercise on brain structure and function given that these effects are likely to be accumulated over years for a significant impact. Finally, the use of alternate neuropsychological test forms could have reduced the practice effects during follow-up assessments and help to more selectively reveal the effect of exercise training on cognitive function.
Conclusions
In cognitively normal older adults who previously led a sedentary lifestyle, one-year aerobic exercise increased peak VO2 relative to the stretching group. Cognitive performance improved in both groups, which may reflect practice effects of repeated neuropsychological assessments. Brain volume and cortical thickness decreased with a typical pattern of age-related atrophy, while hippocampal volume loss was prevented in the stretching group. Individual improvements in peak VO2 across all participants were positively associated with increases in cognitive composite score and regional cortical thickness. These findings collectively suggest that CRF gains with exercise training may provide beneficial effects on neurocognitive function in older adults.
Supplementary Material
Acknowledgements
This study was supported by the National Institutes of Health (R01-HL-102457). We thank each of the study participants for their effort and time contributing to the study.
Footnotes
Conflict of interest
None
References
- 1.Brookmeyer R, Abdalla N, Kawas CH, Corrada MM. Forecasting the prevalence of preclinical and clinical Alzheimer’s disease in the United States. Alzheimers Dement 2018; 14: 121–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amanatkar HR, Papagiannopoulos B, Grossberg GT. Analysis of recent failures of disease modifying therapies in Alzheimer’s disease suggesting a new methodology for future studies. Expert Rev Neurother 2017; 17: 7–16. [DOI] [PubMed] [Google Scholar]
- 3.Shatenstein B, Barberger-Gateau P, Mecocci P. Prevention of Age-Related Cognitive Decline: Which Strategies, When, and for Whom? J Alzheimers Dis 2015; 48: 35–53. [DOI] [PubMed] [Google Scholar]
- 4.Norton S, Matthews FE, Barnes DE, Yaffe K, Brayne C. Potential for primary prevention of Alzheimer’s disease: an analysis of population-based data. Lancet Neurol 2014; 13: 788–94. [DOI] [PubMed] [Google Scholar]
- 5.Park DC, Reuter-Lorenz P. The adaptive brain: aging and neurocognitive scaffolding. Annu Rev Psychol 2009; 60: 173–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Park DC, Lautenschlager G, Hedden T, Davidson NS, Smith AD, Smith PK. Models of visuospatial and verbal memory across the adult life span. Psychol Aging 2002; 17: 299–320. [PubMed] [Google Scholar]
- 7.Fjell AM, McEvoy L, Holland D, Dale AM, Walhovd KB, Alzheimer’s Disease Neuroimaging I. What is normal in normal aging? Effects of aging, amyloid and Alzheimer’s disease on the cerebral cortex and the hippocampus. Prog Neurobiol 2014; 117: 20–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.den Heijer T, van der Lijn F, Koudstaal PJ, et al. A 10-year follow-up of hippocampal volume on magnetic resonance imaging in early dementia and cognitive decline. Brain 2010; 133: 1163–72. [DOI] [PubMed] [Google Scholar]
- 9.Engeroff T, Ingmann T, Banzer W. Physical Activity Throughout the Adult Life Span and Domain-Specific Cognitive Function in Old Age: A Systematic Review of Cross-Sectional and Longitudinal Data. Sports Med 2018; 48: 1405–36. [DOI] [PubMed] [Google Scholar]
- 10.Brown BM, Peiffer JJ, Sohrabi HR, et al. Intense physical activity is associated with cognitive performance in the elderly. Transl Psychiatry 2012; 2: e191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sofi F, Valecchi D, Bacci D, Abbate R, Gensini GF, Casini A, Macchi C. Physical activity and risk of cognitive decline: a meta-analysis of prospective studies. J Intern Med 2011; 269: 107–17. [DOI] [PubMed] [Google Scholar]
- 12.Barnes DE, Blackwell T, Stone KL, Goldman SE, Hillier T, Yaffe K, Study of Osteoporotic F. Cognition in older women: the importance of daytime movement. J Am Geriatr Soc 2008; 56: 1658–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hamer M, Chida Y. Physical activity and risk of neurodegenerative disease: a systematic review of prospective evidence. Psychol Med 2009; 39: 3–11. [DOI] [PubMed] [Google Scholar]
- 14.Middleton LE, Barnes DE, Lui LY, Yaffe K. Physical activity over the life course and its association with cognitive performance and impairment in old age. J Am Geriatr Soc 2010; 58: 1322–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gates N, Fiatarone Singh MA, Sachdev PS, Valenzuela M. The effect of exercise training on cognitive function in older adults with mild cognitive impairment: a meta-analysis of randomized controlled trials. Am J Geriatr Psychiatry 2013; 21: 1086–97. [DOI] [PubMed] [Google Scholar]
- 16.Kelly ME, Loughrey D, Lawlor BA, Robertson IH, Walsh C, Brennan S. The impact of exercise on the cognitive functioning of healthy older adults: a systematic review and meta-analysis. Ageing Res Rev 2014; 16: 12–31. [DOI] [PubMed] [Google Scholar]
- 17.Northey JM, Cherbuin N, Pumpa KL, Smee DJ, Rattray B. Exercise interventions for cognitive function in adults older than 50: a systematic review with meta-analysis. Br J Sports Med 2018; 52: 154–60. [DOI] [PubMed] [Google Scholar]
- 18.Chen FT, Etnier JL, Chan KH, Chiu PK, Hung TM, Chang YK. Effects of Exercise Training Interventions on Executive Function in Older Adults: A Systematic Review and Meta-Analysis. Sports Med 2020; 50: 1451–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wilckens KA, Stillman CM, Waiwood AM, et al. Exercise interventions preserve hippocampal volume: A meta-analysis. Hippocampus 2021; 31: 335–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Firth J, Stubbs B, Vancampfort D, Schuch F, Lagopoulos J, Rosenbaum S, Ward PB. Effect of aerobic exercise on hippocampal volume in humans: A systematic review and meta-analysis. Neuroimage 2018; 166: 230–8. [DOI] [PubMed] [Google Scholar]
- 21.Gogniat MA, Robinson TL, Miller LS. Exercise interventions do not impact brain volume change in older adults: a systematic review and meta-analysis. Neurobiol Aging 2021; 101: 230–46. [DOI] [PubMed] [Google Scholar]
- 22.Hvid LG, Harwood DL, Eskildsen SF, Dalgas U. A Critical Systematic Review of Current Evidence on the Effects of Physical Exercise on Whole/Regional Grey Matter Brain Volume in Populations at Risk of Neurodegeneration. Sports Med 2021. [DOI] [PubMed] [Google Scholar]
- 23.Hayes SM, Hayes JP, Cadden M, Verfaellie M. A review of cardiorespiratory fitness-related neuroplasticity in the aging brain. Front Aging Neurosci 2013; 5: 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bonafiglia JT, Rotundo MP, Whittall JP, Scribbans TD, Graham RB, Gurd BJ. Inter-Individual Variability in the Adaptive Responses to Endurance and Sprint Interval Training: A Randomized Crossover Study. PLoS One 2016; 11: e0167790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tanaka H Exercise Nonresponders: Genetic Curse, Poor Compliance, or Improper Prescription? Exerc Sport Sci Rev 2018; 46: 137. [DOI] [PubMed] [Google Scholar]
- 26.Pentikainen H, Savonen K, Ngandu T, et al. Cardiorespiratory Fitness and Cognition: Longitudinal Associations in the FINGER Study. J Alzheimers Dis 2019; 68: 961–8. [DOI] [PubMed] [Google Scholar]
- 27.Pentikainen H, Ngandu T, Liu Y, et al. Cardiorespiratory fitness and brain volumes in men and women in the FINGER study. Age Ageing 2017; 46: 310–3. [DOI] [PubMed] [Google Scholar]
- 28.Erickson KI, Voss MW, Prakash RS, et al. Exercise training increases size of hippocampus and improves memory. Proc Natl Acad Sci U S A 2011; 108: 3017–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nagamatsu LS, Handy TC, Hsu CL, Voss M, Liu-Ambrose T. Resistance training promotes cognitive and functional brain plasticity in seniors with probable mild cognitive impairment. Arch Intern Med 2012; 172: 666–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Albinet CT, Abou-Dest A, Andre N, Audiffren M. Executive functions improvement following a 5-month aquaerobics program in older adults: Role of cardiac vagal control in inhibition performance. Biol Psychol 2016; 115: 69–77. [DOI] [PubMed] [Google Scholar]
- 31.Liguori G, Medicine ACoS. ACSM’s guidelines for exercise testing and prescription. Lippincott Williams & Wilkins. 2020. [Google Scholar]
- 32.Tarumi T, Rossetti H, Thomas BP, et al. Exercise Training in Amnestic Mild Cognitive Impairment: A One-Year Randomized Controlled Trial. J Alzheimers Dis 2019; 71: 421–33. [DOI] [PubMed] [Google Scholar]
- 33.Ekstrom RB, Harman HH. Manual for kit of factor-referenced cognitive tests, 1976. Educational testing service. 1976. [Google Scholar]
- 34.Raven JC, Court JH. Raven’s progressive matrices and vocabulary scales. Oxford pyschologists Press Oxford, England. 1998. [Google Scholar]
- 35.Woodcock RW. Woodcock-Johnson-Revised (WJ-R) Tests of Cognitive Ability. Riverside Publishing Company. 1989. [Google Scholar]
- 36.Salthouse TA, Babcock RL. Decomposing adult age differences in working memory. Developmental psychology 1991; 27: 763. [Google Scholar]
- 37.Wechsler D Technical manual for the WAIS-III and WMS-III. The Psychological Corporation: San Antonio: 1997. [Google Scholar]
- 38.Turner ML, Engle RW. Is working memory capacity task dependent? Journal of memory and language 1989; 28: 127–54. [Google Scholar]
- 39.Bechtoldt HP, Benton AL, Fogel ML. An application of factor analysis in neuropsychology. The Psychological Record 1962; 12: 147. [Google Scholar]
- 40.Reuter M, Schmansky NJ, Rosas HD, Fischl B. Within-subject template estimation for unbiased longitudinal image analysis. Neuroimage 2012; 61: 1402–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Reuter M, Fischl B. Avoiding asymmetry-induced bias in longitudinal image processing. Neuroimage 2011; 57: 19–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Reuter M, Rosas HD, Fischl B. Highly accurate inverse consistent registration: a robust approach. Neuroimage 2010; 53: 1181–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Buckner RL, Snyder AZ, Shannon BJ, et al. Molecular, structural, and functional characterization of Alzheimer’s disease: evidence for a relationship between default activity, amyloid, and memory. The Journal of Neuroscience 2005; 25: 7709–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fjell AM, Walhovd KB, Fennema-Notestine C, et al. One-year brain atrophy evident in healthy aging. J Neurosci 2009; 29: 15223–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tseng BY, Uh J, Rossetti HC, et al. Masters athletes exhibit larger regional brain volume and better cognitive performance than sedentary older adults. J Magn Reson Imaging 2013; 38: 1169–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tarumi T, Tomoto T, Repshas J, et al. Midlife aerobic exercise and brain structural integrity: Associations with age and cardiorespiratory fitness. Neuroimage 2021; 225: 117512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Balke B, Nagle FJ, Daniels J. Altitude and maximum performance in work and sports activity. JAMA 1965; 194: 646–9. [PubMed] [Google Scholar]
- 48.Douglas CG. A method for determining the total respiratory exchange in man. Journal of Physiology 1911; 42: 17–8. [Google Scholar]
- 49.Colcombe S, Kramer AF. Fitness effects on the cognitive function of older adults: a meta-analytic study. Psychol Sci 2003; 14: 125–30. [DOI] [PubMed] [Google Scholar]
- 50.Koo TK, Li MY. A Guideline of Selecting and Reporting Intraclass Correlation Coefficients for Reliability Research. J Chiropr Med 2016; 15: 155–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hayasaka S, Nichols TE. Validating cluster size inference: random field and permutation methods. Neuroimage 2003; 20: 2343–56. [DOI] [PubMed] [Google Scholar]
- 52.Hagler DJ Jr., Saygin AP, Sereno MI. Smoothing and cluster thresholding for cortical surface-based group analysis of fMRI data. Neuroimage 2006; 33: 1093–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Machulda MM, Pankratz VS, Christianson TJ, et al. Practice effects and longitudinal cognitive change in normal aging vs. incident mild cognitive impairment and dementia in the Mayo Clinic Study of Aging. Clin Neuropsychol 2013; 27: 1247–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Calamia M, Markon K, Tranel D. Scoring higher the second time around: meta-analyses of practice effects in neuropsychological assessment. Clin Neuropsychol 2012; 26: 543–70. [DOI] [PubMed] [Google Scholar]
- 55.Barnes DE, Santos-Modesitt W, Poelke G, Kramer AF, Castro C, Middleton LE, Yaffe K. The Mental Activity and eXercise (MAX) trial: a randomized controlled trial to enhance cognitive function in older adults. JAMA Intern Med 2013; 173: 797–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Raz N, Lindenberger U, Rodrigue KM, et al. Regional brain changes in aging healthy adults: general trends, individual differences and modifiers. Cereb Cortex 2005; 15: 1676–89. [DOI] [PubMed] [Google Scholar]
- 57.Nave G, Jung WH, Karlsson Linner R, Kable JW, Koellinger PD. Are Bigger Brains Smarter? Evidence From a Large-Scale Preregistered Study. Psychol Sci 2019; 30: 43–54. [DOI] [PubMed] [Google Scholar]
- 58.Pietschnig J, Penke L, Wicherts JM, Zeiler M, Voracek M. Meta-analysis of associations between human brain volume and intelligence differences: How strong are they and what do they mean? Neurosci Biobehav Rev 2015; 57: 411–32. [DOI] [PubMed] [Google Scholar]
- 59.Meunier D, Stamatakis EA, Tyler LK. Age-related functional reorganization, structural changes, and preserved cognition. Neurobiol Aging 2014; 35: 42–54. [DOI] [PubMed] [Google Scholar]
- 60.Broadhouse KM, Singh MF, Suo C, et al. Hippocampal plasticity underpins long-term cognitive gains from resistance exercise in MCI. Neuroimage Clin 2020; 25: 102182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Furlano J, Nagamatsu L. Resistance exercise improves cognitive and brain health in overweight older adults: Neuropsychology/Neuropsychological correlates of physiologic markers of cognitive decline/Dementia. Alzheimer’s & Dementia 2020; 16: e046471. [Google Scholar]
- 62.Dayan E, Cohen LG. Neuroplasticity subserving motor skill learning. Neuron 2011; 72: 443–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pi YL, Wu XH, Wang FJ, Liu K, Wu Y, Zhu H, Zhang J. Motor skill learning induces brain network plasticity: A diffusion-tensor imaging study. PLoS One 2019; 14: e0210015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Curlik DM 2nd, Maeng LY, Agarwal PR, Shors TJ. Physical skill training increases the number of surviving new cells in the adult hippocampus. PLoS One 2013; 8: e55850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Barnes DE, Yaffe K, Satariano WA, Tager IB. A longitudinal study of cardiorespiratory fitness and cognitive function in healthy older adults. J Am Geriatr Soc 2003; 51: 459–65. [DOI] [PubMed] [Google Scholar]
- 66.Tari AR, Nauman J, Zisko N, et al. Temporal changes in cardiorespiratory fitness and risk of dementia incidence and mortality: a population-based prospective cohort study. Lancet Public Health 2019; 4: e565–e74. [DOI] [PubMed] [Google Scholar]
- 67.Huang L, Huang G, Ding Q, et al. Amplitude of low-frequency fluctuation (ALFF) alterations in adults with subthreshold depression after physical exercise: A resting-state fMRI study. J Affect Disord 2021; 295: 1057–65. [DOI] [PubMed] [Google Scholar]
- 68.Chirles TJ, Reiter K, Weiss LR, Alfini AJ, Nielson KA, Smith JC. Exercise Training and Functional Connectivity Changes in Mild Cognitive Impairment and Healthy Elders. J Alzheimers Dis 2017; 57: 845–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tarumi T, Thomas BP, Tseng BY, et al. Cerebral White Matter Integrity in Amnestic Mild Cognitive Impairment: A 1-Year Randomized Controlled Trial of Aerobic Exercise Training. J Alzheimers Dis 2020; 73: 489–501. [DOI] [PubMed] [Google Scholar]
- 70.Thomas BP, Tarumi T, Sheng M, et al. Brain Perfusion Change in Patients with Mild Cognitive Impairment After 12 Months of Aerobic Exercise Training. J Alzheimers Dis 2020; 75: 617–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Herold F, Muller P, Gronwald T, Muller NG. Dose-Response Matters! - A Perspective on the Exercise Prescription in Exercise-Cognition Research. Front Psychol 2019; 10: 2338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Barnes JN, Corkery AT. Exercise Improves Vascular Function, but does this Translate to the Brain? Brain Plast 2018; 4: 65–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Salthouse TA. Trajectories of normal cognitive aging. Psychol Aging 2019; 34: 17–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jonaitis EM, Koscik RL, Clark LR, et al. Measuring longitudinal cognition: Individual tests versus composites. Alzheimers Dement (Amst) 2019; 11: 74–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jacobs DM, Thomas RG, Salmon DP, et al. Development of a novel cognitive composite outcome to assess therapeutic effects of exercise in the EXERT trial for adults with MCI: The ADAS-Cog-Exec. Alzheimers Dement (N Y) 2020; 6: e12059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Myers J, Prakash M, Froelicher V, Do D, Partington S, Atwood JE. Exercise capacity and mortality among men referred for exercise testing. N Engl J Med 2002; 346: 793–801. [DOI] [PubMed] [Google Scholar]
- 77.Knopman D, Boland LL, Mosley T, et al. Cardiovascular risk factors and cognitive decline in middle-aged adults. Neurology 2001; 56: 42–8. [DOI] [PubMed] [Google Scholar]
- 78.Gardner RC, Burke JF, Nettiksimmons J, Kaup A, Barnes DE, Yaffe K. Dementia risk after traumatic brain injury vs nonbrain trauma: the role of age and severity. JAMA Neurol 2014; 71: 1490–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sen A, Capelli V, Husain M. Cognition and dementia in older patients with epilepsy. Brain 2018; 141: 1592–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Liu-Ambrose T, Nagamatsu LS, Graf P, Beattie BL, Ashe MC, Handy TC. Resistance training and executive functions: a 12-month randomized controlled trial. Arch Intern Med 2010; 170: 170–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


