Prefilled syringes (PFS) of aflibercept, an anti-vascular endothelial growth factor (anti-VEGF) agent, have several theoretical advantages, including reduced total preparation and injection time, improved precision in the volume and dose administered, and increased ease of use.1 Retina specialists within our institutions reported an increased incidence of transient vision loss associated with aflibercept PFS injections.2 Similar observations have been reported in Europe.3 Thus, we collected intravitreal injection experiences from ophthalmologists to better understand vision and intraocular pressure (IOP) outcomes following aflibercept PFS use.
This study followed the tenets of the Declaration of Helsinki. Institutional review board approval was obtained at Oregon Health & Science University (Protocol #00022596) prior to study initiation. Qualtrics software (Qualtrics, Provo, UT, USA) was used to create a 22-question cross-sectional anonymous online survey to query residency-trained ophthalmologists practicing within the U.S. Informed consent was obtained prior to the start of the survey.
There were 118 total survey respondents. Vitreoretinal surgeons were the most common sub-group, consisting of 41.7% of respondents (N=48), followed by comprehensive ophthalmologists (N=32, 27.8%), and medical retina specialists (N=29, 25.2%). Most respondents were attending ophthalmologists (N=103). The survey ended early if participants had never used aflibercept PFS. Seventy-eight participants (66.1%) met the screening criteria, with 68/78 respondents (87.2%) completing all 22 questions. Respondents reported performing a monthly average of 126 intravitreal anti-VEGF injections (95% CI 103–149), including an average of 68 monthly vial or PFS aflibercept injections (95% CI 55–81). Participants estimated that 84% of their aflibercept injections were performed using aflibercept PFS. Of those using aflibercept PFS, the majority reported improved efficiency of use (73/78, 94%), safer delivery (38/78, 49%), and ease of use (40/78, 51%) with the aflibercept PFS compared to the vial preparation.
Though the perception towards PFS was favorable in our study, approximately one-third of PFS users (24/77, 31%) noted a perceived increase in the frequency of IOP spikes with aflibercept PFS compared to other drugs and/or preparations (Figure 1A). Only one respondent noted a perceived decrease in frequency of IOP spikes with PFS. Fifty-one out of 76 respondents (67%) had experienced at least one episode of an acute IOP spike after aflibercept PFS use compared to 36% (27/76) after using the vial preparation (Figure 2; p<0.0001). Of these respondents, 11 ophthalmologists noted having at least 6 patients experience significant transient vision loss following aflibercept PFS use five-fold more than traditional vial preparation. Although the vision loss after aflibercept PFS often resolved without intervention, 32% of PFS users (25/78) reported performing an anterior chamber paracentesis to normalize IOP and/or to return vision following aflibercept PFS injection.
Figure 1:
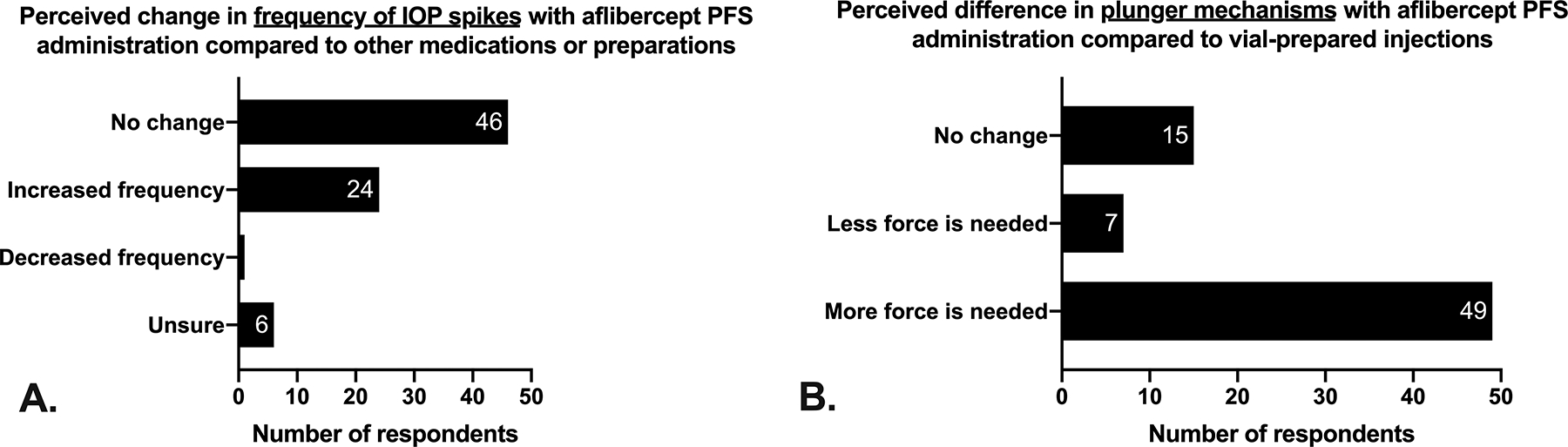
Perceived differences with aflibercept PFS when compared to other medications/preparations. (A) Nearly one third of respondents (31%) perceived an increased frequency in IOP spikes immediately following aflibercept PFS use when compared to other medications/preparations. (B) Most respondents (49/72, 68%) believed that more force was needed with aflibercept PFS when compared to vial-prepared injections.
Figure 2:
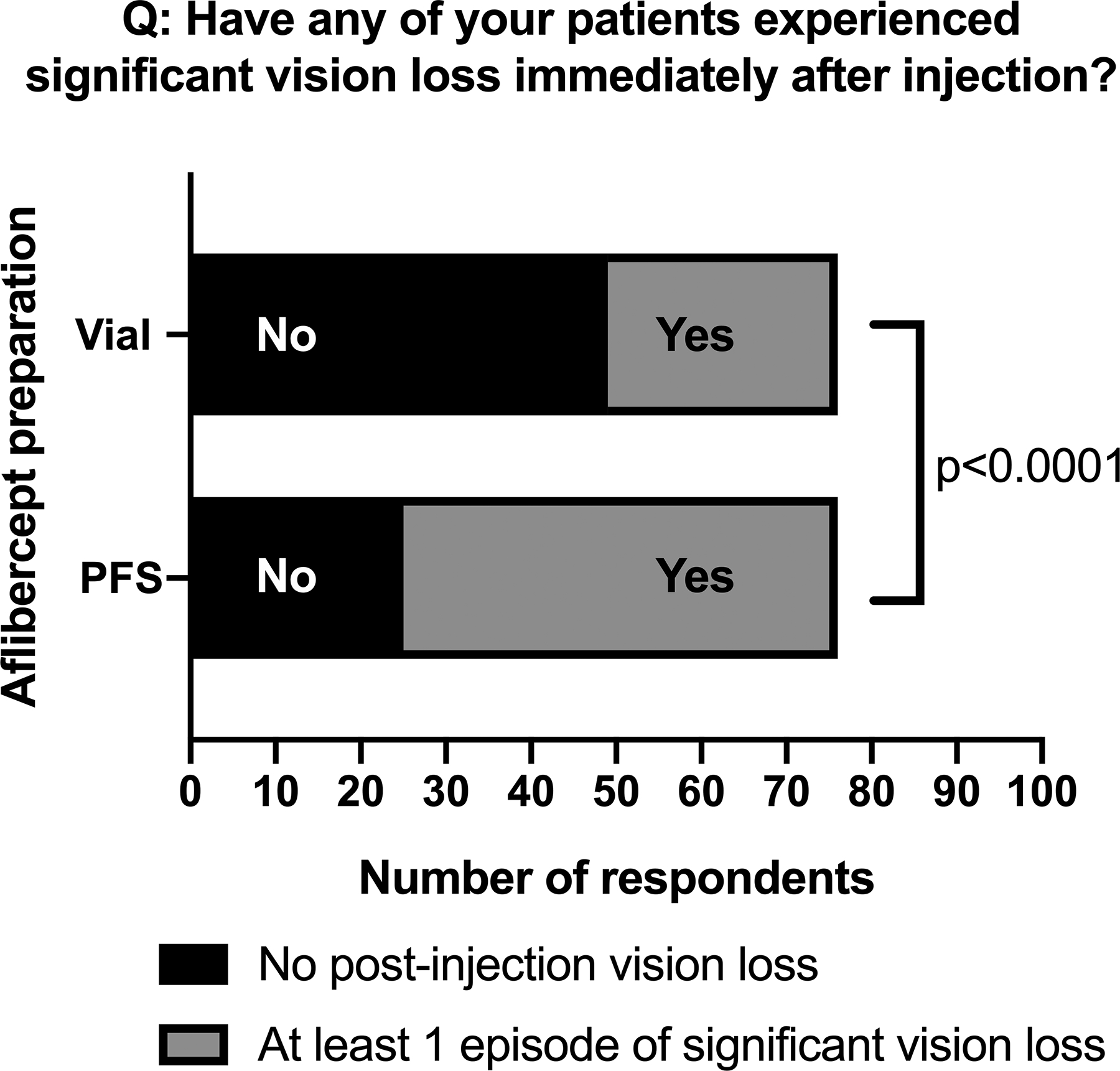
Reporting of significant vision loss following intravitreal aflibercept injection. Almost twice as many ophthalmologists reported at least 1 episode of significant vision loss with aflibercept PFS use (51/76, 67%) when compared to the vial preparation (27/76, 35%).
Many ophthalmologists believed that syringe design played a contributing factor to the IOP spikes observed after aflibercept PFS use. Commonly cited comments were 1) that there was little tactile feedback when pushing down on the PFS plunger and 2) that priming the syringe to deliver the correct volume can be difficult due to ambiguity in determining where to align the plunger tip with the marked line on the syringe. Two thirds of respondents (49/72, 68%) felt that more force was required to use the aflibercept PFS plunger compared to traditional 1mL syringes (Figure 1B). Gallagher, et al. were the first to investigate syringe design as a contributor to vision loss following aflibercept PFS use.3 They showed that the internal area for the aflibercept PFS lumen was twice that of the 1mL syringe; thus, any unit error in plunger alignment by the user could result in a two-fold greater error in volume delivered with the PFS. Additional training may be beneficial in instructing the correct handling and use of aflibercept PFS by ophthalmologists.
Our group also recently demonstrated that volumetric differences due to plunger misalignment may not sufficiently explain the entire story.2 In our lab-controlled experiment, we showed that, even if the plunger is painstakingly aligned with a telecentric lens optical system to the correct starting point, more force is still required to fully depress and inject with the aflibercept PFS when compared to other anti-VEGF options; this may be attributable to the wider barrel diameter of the aflibercept PFS.2 Modification in syringe design should be considered.
Though our study may be subject to various biases which could limit the generalizability of our survey, we believe that our findings are sufficiently concerning to warrant future prospective investigation into aflibercept PFS design and clinical outcomes following aflibercept PFS use.
Funding sources:
This work was supported in part by the Unrestricted Grant from Research to Prevent Blindness, Inc. to the Oregon Health & Science University Casey Eye Institute and by NIH/NEI core grant (P30EY010572).
Footnotes
Conflict of interest: None
REFERENCES
- 1.Sassalos TM, Paulus YM. Prefilled syringes for intravitreal drug delivery. Clin Ophthalmol 2019; 13: 701–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee DJ, Scruggs BA, Sánchez E, Thomas M, Faridi A. Transient vision loss associated with pre-filled aflibercept syringes: a case series and analysis of injection force. Ophthalmol Sci 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gallagher K, Raghuram AR, Williams GS, Davies N. Pre-filled aflibercept syringes-variability in expressed fluid volumes and a case series of transient central retinal artery occlusions. Eye (Lond) 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]


