Abstract
Latin America has notably elevated rates of adolescent fertility and obesity in women. Although numerous studies document associations between adolescent fertility and obesity across the life course, the pathways explaining their association are insufficiently theorized, especially regarding the factors in Latin America that may underpin both. Additionally, much of the existing research is from high income countries where fertility and obesity are trending down. In this paper, we review various complex pathways linking adolescent fertility and obesity, highlighting research gaps and priorities, with a particular focus on Latin American populations. We carefully consider pregnancy’s distinct impact on growth trajectories during the critical period of adolescence, as well as the cumulative effect that adolescent fertility may have over the life course. We also articulate a pathway through obesity as it may contribute to early puberty and thus, to adolescent fertility. If obesity is a cause of adolescent fertility, not a result of it, or if it is a mediator of early-life exposures to adulthood obesity, these are critical distinctions for policy aiming to prevent both obesity and early fertility. Research to better understand these pathways is essential for prevention efforts against obesity and undesired adolescent fertility in Latin America.
Keywords: Adolescence, life course epidemiology, obesity, maternal and child health, global health
INTRODUCTION
High levels of adolescent fertility, typically defined as any pregnancy-related experience before age 20, including live birth, abortion, stillbirth, or miscariage,1 is still a public health concern worldwide. Some middle-income settings, such as Latin America, appear resistant to the reductions in adolescent fertility associated with economic development elsewhere, with rates remaining relatively stable despite declines in other age groups.2 For instance, while the fertility rates among those aged 25–29 years in Latin America dropped almost by half, from 198.5 births per 1,000 women in 1980–1985, to 101.9 in 2015–2020, a slower downward trend was observed among those aged 15–19 years, from 89.5 to 63.0 in the same period3. Data show that Latin American countries have presented the slowest decline in adolescent fertility rates for those aged 15–19 years compared to all regions of the world and it is the only region with an increasing trend in fertility among those younger than 15 years.2 Indeed, an estimated 15% of all pregnancies in Latin America occur among girls 19 years old or younger.2 These statistics are possibly a consequence of the high social and gender inequality found in these settings that interplay with known drivers of adolescent fertility at individual, relational, community, and societal level.2 These include: lack of knowledge about sexuality and reproduction, early or forced sexual initiation and union, lack of supportive and empowering cultural and gender norms and values, tolerance for or practice of sexual violence, social norms and policies that do not acknowledge adolescents’ sexuality and need for sexual health education and reproductive health services, including contraceptives, limited educational and employment opportunities for young people/girls, unequal gender norms and values, and tolerance and acceptance of gender-based violence.2
Obesity has also become a major health challenge in Latin America. While it once was a problem for wealthy populations and concentrated in urban areas, data show that these trends are shifting.4,5 In some Latin American countries, obesity rates are increasing among low-income settings and in rural communities, creating further health disparities among these populations.5 For instance, in Argentina, Venezuela, and Mexico, obesity rates are concentrated among low wealth and education groups.5 Furthermore, although obesity rates are still higher among urban populations, most Latin American countries present larger increases in obesity among rural settings.5 The prevalence of overweight and obesity among children and adolescents in Latin America is also remarkably high (around 20–25%) and has statistically increased over time;6 although, this increase has been identified mainly among females.7
Numerous studies associate adolescent fertility with obesity at different stages in a woman’s life course.8–10 Among youth, there is evidence that pregnant adolescents gain and retain more weight during pregnancy than adults,11 and that they develop more central adiposity that,12 in turn, leads to deleterious effects on long-term weight, contributing to obesity in adulthood.11 Among middle-aged women, researchers have reported associations between higher obesity prevalence among those who gave birth during adolescence,8 and these findings have been replicated among cohorts of older women.9 Overall, a notable body of research suggests that adolescent fertility contributes to a greater weight gain over the life course than adult fertility;13 although, less is known about how and why these associations exist. Alternatively, there is also research, mostly from high-income countries, associating obesity in childhood to adolescent fertility due to early initiation of puberty.14 Therefore, it is possible that adolescent fertility is a consequence, not a cause of obesity.
The pathways explaining the association between adolescent fertility and obesity are insufficiently theorized, especially with regard to the factors in Latin America that may underpin both. Here, we present several pathways to explain how giving birth in adolescence may be related to obesity. We frame these pathways using adaptations of two well-accepted theoretical frameworks in life course epidemiology: the critical period approach and the cumulative risk model.15 The critical period approach posits that events occurring during salient periods of development may permanently alter health trajectories.15,16 The cumulative risk model implies that exposures accumulate over the life course and this accumulation is increasingly important over time. These frameworks are not mutually exclusive and cumulative social, environmental, and/or behavioral exposures may alter the risk of disease in combination with any critical period events. Some early life exposures are known risk factors for obesity in adulthood and adolescent fertility may be in this pathway. A careful examination of these pathways provides theoretical clarity, identifies research gaps, and offers hypotheses-testing opportunities for future research.
Pathway #1: Critical Period Approach Factors
Adolescence is a critical period and second only to fetal and infant life with regard to the rapidity of growth and pervasiveness of change across body systems.14,16 Puberty results in very rapid somatic growth, brain development, sexual maturation of multiple organ systems, major central nervous system changes, and dramatic psychological changes.16 Because childbearing can act as a medical stress test for women through dramatic alterations in physiology and metabolism,17 it may permanently alter biochemical pathways during the critical period of adolescence. These alterations may affect weight gain and growth trajectories, which may predispose adolescents to obesity as described below. Figure 1 presents a visual representation of the hypothesized causal model under this theoretical framework.
Figure 1.
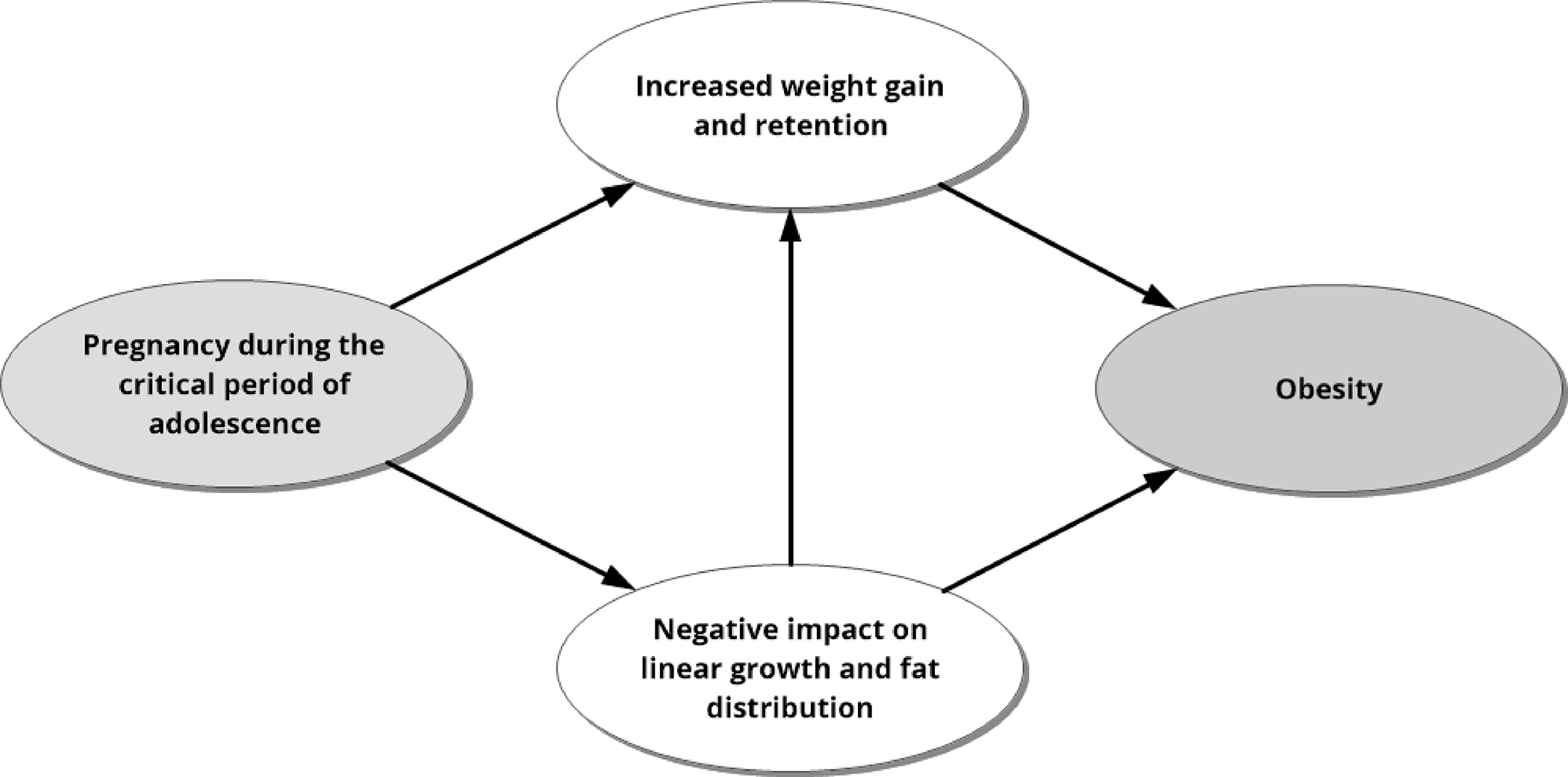
Adolescence is a critical period in growth and development; pregnancy during this period may alter growth patterns and predispose a person to obesity. Critical Period Approach factors, such as alterations in physiology and metabolism during adolescent pregnancy, may explain the connection between pregnancy during adolescence and obesity.
Adolescent pregnancy may affect weight gain trajectories and favor weight gain and retention
Longitudinal studies have established the significant potential for growth after menarche, both in terms of height and the accumulation of fat.12,18–20 Thus, excessive weight gains during adolescence, such as those associated with pregnancy, could exacerbate normal maturation-related processes of fat deposition.12
It is hypothesized that, because they are still growing, weight-gain trajectories differ for pregnant adolescents compared to pregnant adult women.21 While there is evidence from the United States (US) that the shape of the weight gain curve for adolescents is similar to adults, the median gain and rates of gain for adolescents are higher than adults.12 Further, differently from what is observed among adults, adolescents seem to continue accumulating fat rather than mobilizing fat stores after 28 weeks of gestation; this is despite similar average daily energy intakes and percent contributions of protein, carbohydrates, and dietary fat to total intake.12,22 Finally, in the postpartum period, adolescents retained significantly more weight than adult controls.12 This may be a consequence of the earlier exposure of biologically immature organs to a high dose of estrogen from the pregnancy that induces subtle, deleterious changes in glucose metabolism,23 contributing to persistent insulin resistance and weight gain. Thus, adolescent fertility may cause unique lifelong health risks and/or it may accentuate otherwise subtle health risks associated with pregnancy and childbirth (e.g. effect modification).
Adolescent pregnancy may limit maternal growth and impact fat distribution
Existing research is conflicting as to whether or not adolescents continue linear growth during pregnancy or the pregnancy limits linear growth.24 Fetal competition for nutrients is a factor in growth retardation or interruption in adolescents.25 This may prevent adolescent mothers from achieving their expected height and favor an increased body mass index (BMI).26 Moreover, the adolescent’s age may also determine whether or not her body invests in growth. The pubertal growth spurt among healthy non-pregnant female adolescents normally begins at 9–10 years old and lasts around 2.5 years, but there is a considerable variation among individuals and contexts27. This suggests that pregnancy during the first years of adolescence may interfere more with linear growth compared to late adolescence. Additionally, there are data to suggest that the physiology of younger adolescents invests in growth while for older adolescents, their bodies privilege reproductively valuable reproductive tissue.24 However, evidence is still conflicting28,29 and studies targeting Latin American teenagers are particularly absent. Further studies are needed to understand this mechanism.
The higher incidence of adolescent fertility among Latin American populations may be one of the many contributors to the shorter stature and greater BMI that they achieve during adolescence compared to populations from Europe or North America, even when presenting about the same height at age 5 years.30 Similarly, it has been described that median body weight among adolescents is low in many low-to-middle income countries (LMIC), and that the observed high BMI may be a consequence, at least in part, of stunting and suboptimal linear growth.26 However, studies are needed to investigate the impact of adolescent fertility on linear growth among the Latin American population.
Pathway #2: Cumulative Risk Factors from Adolescent Fertility leading to Obesity
Cumulative adversity, the occurrence of multiple, cascading adverse events across a lifetime, may be initiated by early childbirth. Different aspects associated with adolescent pregnancy and childbirth may act as cumulative factors and impact health over the life course. Socioeconomic disadvantages and behaviors following adolescent pregnancy can accumulate over the years and lead to obesity as described below (Figure 2).
Figure 2.
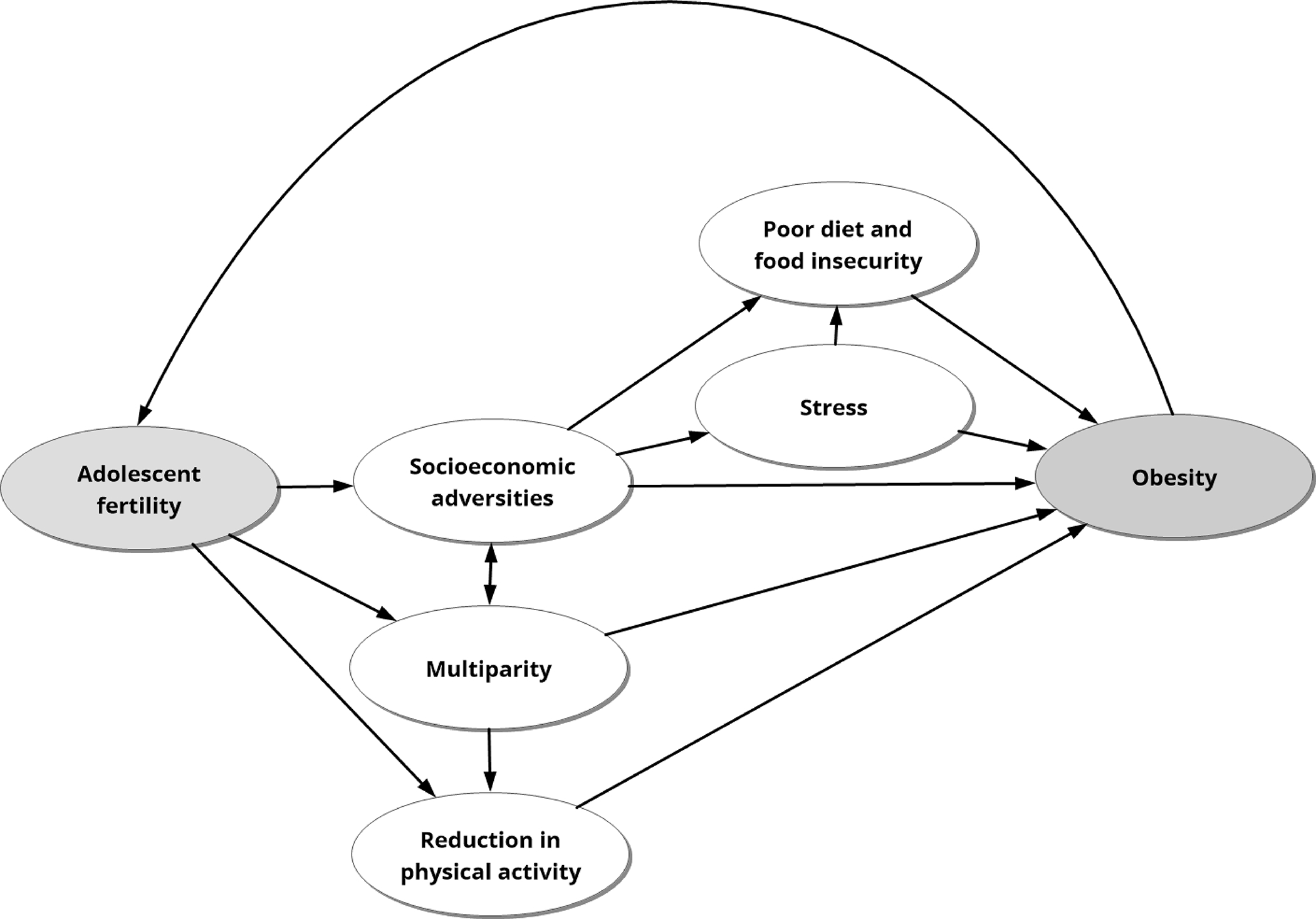
Adolescent fertility may result in adverse life events, such as reduction in physical activity and food insecurity, that accumulate over time and increase the risk for obesity later in life.
Adolescent childbirth may initiate a sequence of socioeconomic adversities that contribute to obesity
Adolescent childbirth can cause youths to drop out of school,31 earn less over their lifetimes,32 and experience stress.33 These can be particularly important when adolescent fertility is associated with economic vulnerability and in cultural contexts that discourage girls from returning to school after giving birth.31 A systematic review of educational attainment and obesity across the globe indicates that in high (e.g., the United States.) and upper-middle income countries (e.g. Brazil), education is inversely associated with obesity, and the association is stronger for women than men.34 Greater educational attainment may be associated with lower levels of obesity, because of higher health literacy and healthier behaviors, as well as a greater sense of control and empowerment.35,36 Higher levels of education are also associated with higher lifetime earnings and higher status jobs; in developed countries like the US, both higher incomes and higher status occupations are associated with lower obesity prevalence.37
The increased stress experienced by adolescent mothers because of social adversities may also contribute to obesity. Chronic stress has been associated with 6-month longitudinal weight gain in US adults.38 Additionally, British adolescents reporting higher stress levels had higher overall adiposity than their peers reporting lower stress when followed over 5 years.35 Interestingly, the study of adolescents did not observe differences in the rates of adiposity by stress levels during adolescence and hypothesized that early life stress may set adiposity trajectories before adolescence.35 Studies investigating the long-term impact of adolescent fertility on social adversities and stress are needed to better understand these pathways in the Latin American context and provide insights for prevention efforts.
Multiparity may be in the pathway between adolescent fertility and obesity
It is possible that adolescent fertility leads to obesity because of its association with multiparity. Because they start childbearing at younger ages, adolescent mothers tend to have more children during their lifetimes.8 As women have more children, their likelihood of post-pregnancy weight retention increases,39 which puts women on a trajectory of obesity throughout the life course. Nevertheless, the association between multiparity and obesity seems to be dependent on the inter-pregnancy intervals. Multiparous women with short inter-pregnancy intervals have a higher risk of obesity after childbirth compared to multiparous women with longer inter-pregnancy intervals.40
A systematic review of studies conducted in LMIC, including Latin America,41 reported significant associations between a short birth interval and a younger age of the mother. Moreover, current prevailing social norms in Latin America associate a female’s status with fertility which indicates that a proportion of adolescent pregnancies are wanted.42 This could potentially result in higher adolescent fertility and multiparity rates as prevailing social norms may influence adolescents’ decisions in starting families earlier and emphasize motherhood as a desired status. Accordingly, a pilot study on adolescent pregnancy in Brazil determined that over 56% of adolescent pregnancies were planned.43 Furthermore, socioeconomic factors such as education level, poverty status, and rural location may result in adolescents wanting and planning their pregnancies due to lack of alternative opportunities to motherhood.44 Finally, the socioeconomic adversities following early pregnancy described in the previous topic may be even more evident when adolescent fertility is followed by multiple childbirths over the life course.
Reduction in physical activity during and following pregnancy may contribute to weight gain and retention
Physical activity is a component of health and is known to be associated with fewer morbidities and improved quality of life.45 On a population level, women are less physically active than men and it is well-established that women decrease physical activity even more during pregnancy.46,47 Although we do not have data for pregnant adolescents, research from a study conducted in 26 Latin American and Caribbean countries found that, among 11–18 year-olds, only 15% meet the physical activity recommendations.48 The same study also found that in 18 of the studied countries, girls are more inactive and accumulate more sedentary behavior compared to boys. 48 Also, there is evidence from other regions that minorities, such as Black girls, are less likely to be active, compared to whites,49 and pregnancy is one of the factors leading to that outcome.
Despite many positive effects of physical activity during pregnancy,50 many women give up regular exercise entirely when they become pregnant and do not resume it soon after birth, with evidence that some women only return to exercise up to four years after birth.51 Similar to what is observed among adults, pregnancy during adolescence also leads to a reduction in physical activity and an increase in sedentary behaviors, such as TV viewing.52
Besides all the negative effects of physical inactivity, especially during pregnancy and puerperium, one that is easily observed is weight gain, as active women are more likely to present healthy gains throughout pregnancy compared to those who are inactive.53 Weight gained during pregnancy is not only a major problem because it increases the chances of acute conditions such as gestational diabetes, eclampsia, and macrosomia,54 but also excessive weight gains combined with physical inactivity make it harder for some women return to their pre-pregnancy weight. Weight retention following pregnancies is one of the main causes for adult obesity among women.55
Food insecurity and poor diet associated with adolescent fertility may contribute to obesity later in life
Pregnancy during adolescence might include a greater demand on nutrient requirements considering the growth of the mother-child dyad. In a life course approach, nutrition in adolescence and young adulthood is important for lifelong health with benefits to human capital, nutrition, and health in the next generation.13 In Latin America countries, adolescent girls are poorly nourished, with a disbalance in terms of both macro- and micronutrients in their diet.56 Diets rich in sugar and fat, insufficient vitamin and mineral density and poor bioavailability, and increased body requirements due to growth or infections are frequently observed.56 Food insecurity is also highly prevalent in Latin America, with rates of moderate or severe food insecurity presenting an upward trend in the past few years (22.6% in 2014 to 31.7% in 2019).57 Additionally, food insecurity has been associated with obesity among adults; women are more likely to be obese and to have food insecurity.58 In a systematic review including 26 studies, food insecurity increased the risk of pre-pregnancy obesity and weight gain.59 Inadequate and excessive weight gain during pregnancy were also associated with food insecurity in the meta-analysis.59 The authors found that social inequities, such as representing a racial minority, participation in social programs and low education level have increased food insecurity in pregnant women.59 Additionally, in a single-center observational study,60 although adolescent pregnancy was not considered, food insecurity was more likely among younger adult pregnant women and associated with additional social determinants related to adolescent pregnancy, such as being unmarried, unemployed, having less prenatal visits, and initiating prenatal care after the first trimester.60
The stress associated with adolescent fertility may also influence diet profile. Stress influences eating behaviors and food choices and may disrupt certain hormonal responses that regulate appetite and weight.61,62 Chronic stress promotes the seeking and intake of high-fat and energy-dense foods.61,63 It may also affect the secretion of cortisol and ghrelin, hormones associated with weight gain and food cravings, respectively.38
Pathway #3: Cumulative risk factors from childhood leading to adolescent fertility and obesity
Adolescent fertility may be a consequence of early life exposures, including disadvantageous economic conditions and obesity during childhood. These factors are also known risk factors for adulthood obesity. Adolescent fertility consequences may be cumulative with early life risk factors and lead to obesity (Figure 3).
Figure 3.
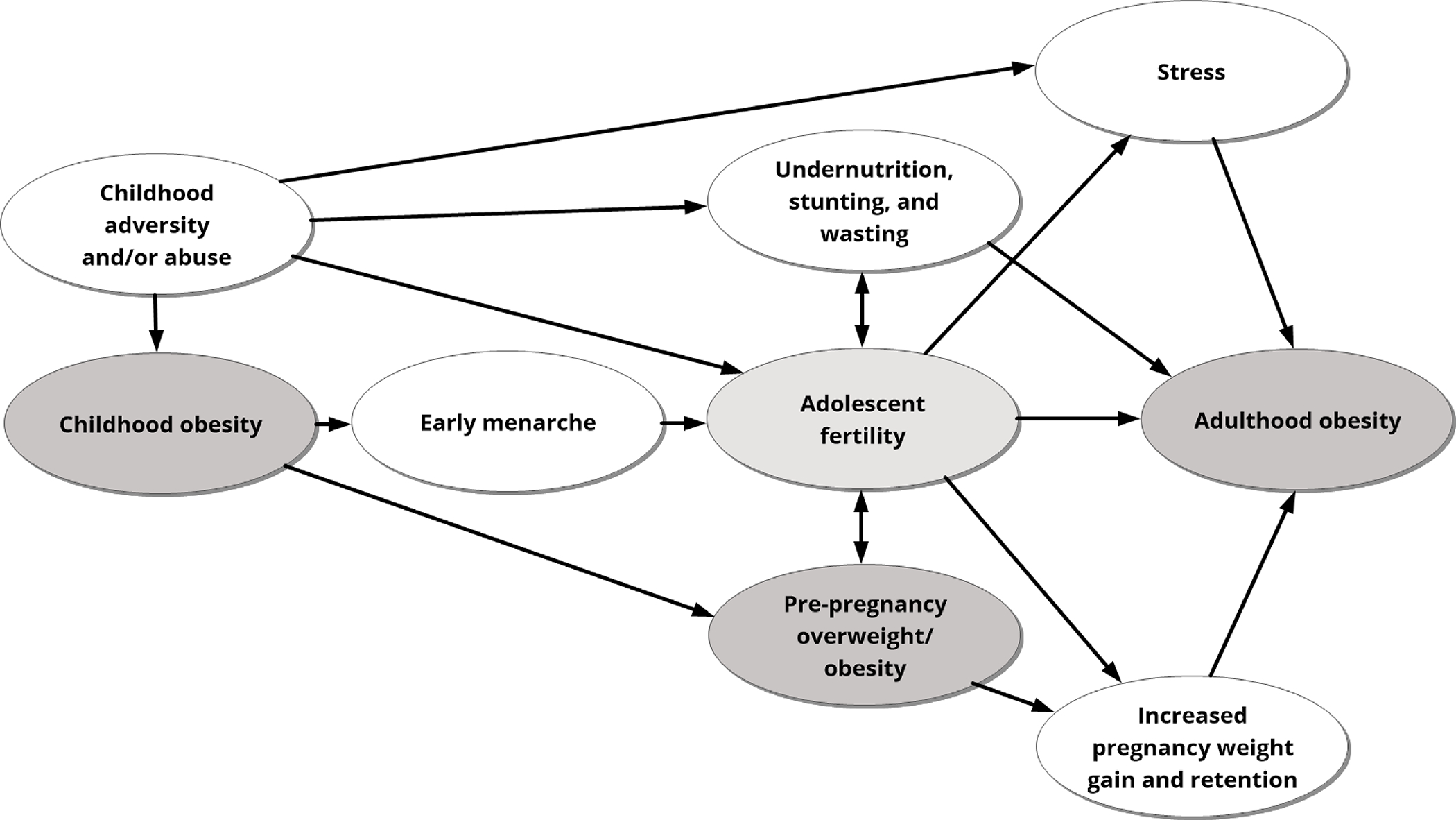
Risk factors for obesity may start in childhood and accumulate over time, which can produce multiple pathways that set a person on a trajectory toward adolescent fertility and obesity in adulthood.
Childhood obesity may contribute to early pregnancy through early menarche.
Research, mostly from high-income countries, demonstrates that obesity in childhood contributes to early puberty in girls.14 Research from Latin America also found cross-sectional associations between earlier ages at menarche and higher BMI.64 Obesity is one of the numerous factors that may influence the onset and timing of puberty among girls by affecting their hormonal profile. It has been reported that obesity during childhood may alter secretion and sensitivity of hormones and accumulated adipose tissue may therefore contribute to the orchestrated controls for pubertal development.65 Early age at menarche, in turn, increases the time in which a girl can become pregnant, which explains the association between early menarche and adolescent fertility reported by previous research.66,67 Thus, the association between adolescent fertility and obesity may have this opposite pathway, with obesity contributing to adolescent fertility. Nevertheless, studies investigating these pathways are all but absent in the literature.
Adolescent pregnancy may increase the effect of childhood nutrition and childhood obesity on adulthood obesity
It has been reported that obese children are at higher risk of being obese as adults and adolescent fertility may increase this effect.68 Previous research has demonstrated that being overweight or obese before pregnancy is a strong predictor of excessive gestational weight gain and weight retention among adolescents.69,70 Pre-pregnancy BMI is also related to BMI increase as these adolescents become adult women.69 Thus, overweight or obese children, when entering adolescence with excessive weight and becoming adolescent mothers, are at higher risk of gaining and retaining more weight from pregnancy and becoming obese adults. Additionally, the effect of pre-pregnancy BMI on long-term weight retention seems to be particularly harmful among younger mothers, ages 12–17 compared to those 18–19 years.69
Nutritional stunting, which is an indicator of chronic undernutrition and especially prevalent in LMIC,71 may also promote later life obesity.72 For example, in Brazil, one study from the 1990s in São Paulo provided mechanistic evidence that nutritionally stunted children ages 7–11 had impaired fat oxidation compared to non-stunted children.73 The fetal competition for nutrients during pregnancy among the nutritionally stunted youngest adolescents may increase these effects. When fat is not oxidized, it must be stored, which is one mechanism by which an adverse childhood nutritional environment may contribute to lifetime obesity.
Children from impoverished backgrounds are more likely to be in poor nutritional status,57,74 and they are also more likely to experience an early pregnancy.75,76 Pregnancy then might accentuate health issues and health may deteriorate more rapidly over time than would be expected based on either risk alone. Although there are increased trends of pregnancy among younger adolescents,2,77 as well as childhood obesity and malnutrition in Latin America,6 the interaction between them has not been the focus of previous research.
Socioeconomic adversities from childhood may interact with adolescent fertility and lead to obesity
Pregnancy during adolescence may be one event in a cascade of cumulative socioeconomic adversities that women with disadvantageous childhoods face.32 Cumulative socioeconomic adversities are a contributor to chronic stress, which, in turn, contributes to increased weight gain and visceral adiposity and obesity in adulthood.78,79 Sexual and physical abuse are additional adverse childhood experiences that may contribute to obesity and adolescent fertility in Latin America. Research from nine Latin American countries show that approximately 58% of children experienced physical, sexual, or emotional abuse in the past twelve months.80 Sexual violence produces serious health consequences for adolescents including increasing rates of adolescent pregnancy, encouraging unsafe abortion tactics, and generating high-risk pregnancies.81 Studies have also shown that childhood sexual and physical abuse are positively associated with obesity in adulthood.82 Therefore, there may also be an interaction effect between early childhood adversity and adolescent childbearing. Research specifically examining if adolescent childbirth is a mediator on the pathway between adversity in childhood and obesity is notably absent.
Pathway #4: Cumulative adversities and intergenerational consequences
There is evidence of the intergenerational transmission of adolescent fertility with adolescent mothers bearing future adolescent mothers.83 Many of the factors described above, especially social influences on diet and physical activity, may contribute to the intergenerational transmission of obesity among adolescent mothers. It is also reported that parental obesity influences up to two generations of the offspring.84 Disadvantageous early-life conditions are consistently related to poorer later-life health.85,86 Factors that contribute to lifetime obesity begin at preconception and during fetal development. The well-known “developmental origins of health and disease” hypothesis posits that there are windows of opportunity to promote health or alternatively, increase disease risk.86 Maternal diet and nutritional status during pregnancy are well-documented to influence fetal growth.87 For example, one study in Brazil reported that pre-pregnant individuals consuming a highly-processed diet, based on refined grains, high-fat foods, and low in fiber, were at increased risk of delivering a small-for-gestational age baby.88 Similar findings have been reported elsewhere and in controlled trials testing nutritional supplements.86 Critically, indicators of inadequate fetal growth, such as small-for-gestational age, low birth weight, etc. have been associated in numerous studies with lifetime obesity.89 What is less clear is whether these indicators are associated with childhood obesity, especially during critical periods such as the first year of life and the prepubertal period, when there is rapid adipose tissue deposition.90 It is plausible, indeed likely, that maternal nutrition and behaviors during pregnancy contribute to childhood obesity, which if associated with pubertal timing, may form a chain of causal factors that ultimately increase the risk of adolescent fertility. Figure 4 shows the depiction of this hypothesized causal model.
Figure 4.
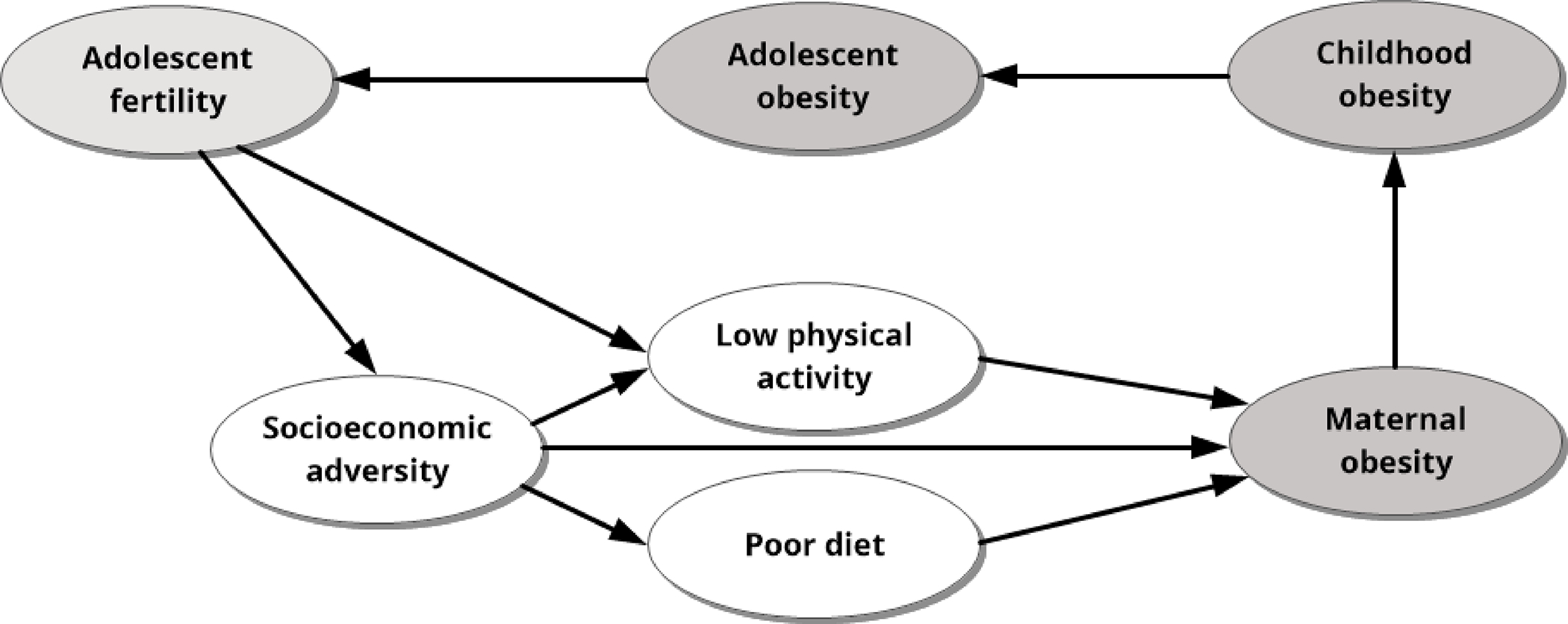
Adolescent fertility may catalyze adversities which accumulate and can result in intergenerational cycles of obesity and adolescent fertility.
CONCLUSION
This paper presented hypotheses that may explain the interactions between adolescent fertility and obesity, with a special focus on Latin American populations. Given the complexity of this issue, it is critical to clearly articulate evidence, pathways, suppositions, and extrapolations from the evidence. Our goal is to better direct future research and interventions by improving the theoretical underpinnings of investigations and providing hypotheses-testing opportunities on this subject. It is unlikely that there is a single causal pathway and likely, multiple pathways contribute to obesity as it relates to adolescent fertility. For example, adverse childhood nutrition, economic, and social factors may contribute to earlier pubertal timing, which in a context where fertility, especially among poorer Latin American women, is highly socially valued, promotes adolescent fertility. Adolescent fertility, in turn, negatively affects nutritional, physical activity, and other health behaviors that protect against overweight and obesity status across the life course. The present state of the evidence suggests multiple causal pathways but is still too nascent to provide “evidence-based” recommendations to health professionals and/or policy-makers. Research focused on these issues must consider the complexity among the interactions between adolescent fertility and obesity, as well as context-specific aspects that may contribute to both conditions, so that effective health policies can be developed.
Acknowledgments
This work was supported by the Fogarty International Center of the National Institutes of Health (Grant number R21 TW010466) and the U.S. Civilian Research & Development Foundation (CRDF Global). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or CRDF Global. We also would like to thank Julia Finn for helping us to create the figures.
Footnotes
Competing interests
None declared.
REFERENCES
- 1.Sentell T, da Câmara S, Ylli A, Velez MP, Domingues MR, Bassani DG et al. 2019. Data gaps in adolescent fertility surveillance in middle-income countries in Latin America and South Eastern Europe: Barriers to evidence-based health promotion. South East Eur J Public Health. 11: 214. [PMC free article] [PubMed] [Google Scholar]
- 2.PAHO 2016 - Accelerating progress toward the reduction of adolescent pregnancy in Latin America and the Caribbean. Report of a technical consultation (Washington, D.C., USA, 2016). ISBN: 978-92-75-11976-1 [Google Scholar]
- 3.United Nations Population Division. World Population Prospects 2019. File FERT/7: Age-specific fertility rates by region, subregion and country, 1950–2100 (births per 1,000). Available at https://population.un.org/wpp/Download/Standard/Fertility/
- 4.Malik VS, Willet WC, & Hu FB 2020. Nearly a decade on — trends, risk factors and policy implications in global obesity. Nat Rev Endocrinol. 16(11):615–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jiwani SS, Carrillo-Larco RM, Hernández-Vásquez A, Barrientos-Gutiérrez T, Basto-Abreu A, Gutierrez L, et al. 2019. The shift of obesity burden by socioeconomic status between 1998 and 2017 in Latin America and the Caribbean: a cross-sectional series study. The Lancet Global Health. 7(12):e1644–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rivera JÁ, de Cossío TG, Pedraza LS, Aburto TC, Sánchez TG, & Martorell R 2014. Childhood and adolescent overweight and obesity in Latin America: a systematic review. The Lancet Diabetes & Endocrinology. 2(4):321–32. [DOI] [PubMed] [Google Scholar]
- 7.Casagrande D, Waib PH, & Sgarbi JA 2017. Increase in the prevalence of abdominal obesity in Brazilian school children (2000–2015). International Journal of Pediatrics and Adolescent Medicine.4(4):133–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Câmara SM, Pirkle C, Moreira MA, Vieira MC, Vafaei A, & Maciel ÁC 2015. Early maternal age and multiparity are associated to poor physical performance in middle-aged women from Northeast Brazil: a cross-sectional community based study. BMC Women’s Health. 15(1):56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.We JS, Han K, Kwon HS, & Kil K 2016. Effect of Maternal Age at Childbirth on Obesity in Postmenopausal Women: A Nationwide Population-Based Study in Korea. Medicine. 95(19):e3584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Patchen L, Leoutsakos JM, & Astone NM 2017. Early Parturition: Is Young Maternal Age at First Birth Associated with Obesity? Journal of Pediatric and Adolescent Gynecology. 30(5):553–9 [DOI] [PubMed] [Google Scholar]
- 11.Chang T, Choi H, Richardson CR, & Davis MM 2013. Implications of teen birth for overweight and obesity in adulthood. American Journal of Obstetrics and Gynecology. 209(2):110.e1–110.e7. [DOI] [PubMed] [Google Scholar]
- 12.Hediger ML, Scholl TO, & Schall JI 1997. Implications of the Camden Study of Adolescent Pregnancy: Interactions Among Maternal Growth, Nutritional Status, and Body Composition. Ann NY Acad Sci. 817(1 Adolescent Nu):281–91. [DOI] [PubMed] [Google Scholar]
- 13.Hanson MA, Bardsley A, De-Regil LM, Moore SE, Oken E, Poston L, et al. 2015. The International Federation of Gynecology and Obstetrics (FIGO) recommendations on adolescent, preconception, and maternal nutrition: “Think Nutrition First” #. International Journal of Gynecology & Obstetrics. 131:S213–53. [DOI] [PubMed] [Google Scholar]
- 14.Steinberg L 2015. Age of Opportunity: Lessons from the New Science of Adolescence. Boston, MA: Houghton Mifflin Harcourt. [Google Scholar]
- 15.Ben-Shlomo Y, & Kuh D 2002. A life course approach to chronic disease epidemiology: conceptual models, empirical challenges and interdisciplinary perspectives. Int J Epidemiol. 31(2):285–93. [PubMed] [Google Scholar]
- 16.Viner RM, Ross D, Hardy R, Kuh D, Power C, Johnson A, et al. 2015. Life course epidemiology: recognising the importance of adolescence. J Epidemiol Community Health.69(8):719–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaaja RJ, & Greer IA 2005. Manifestations of Chronic Disease During Pregnancy. JAMA. 294(21):2751. [DOI] [PubMed] [Google Scholar]
- 18.Rao S, Joshi S, & Kanade A 1998. Height velocity, body fat and menarcheal age of Indian girls. Indian Pediatr. 35(7):619–28. [PubMed] [Google Scholar]
- 19.Chang SH, Tzeng SJ, Cheng JY, & Chie WC 2000. Height and Weight Change Across Menarche of Schoolgirls With Early Menarche. Arch Pediatr Adolesc Med. 154(9):880. [DOI] [PubMed] [Google Scholar]
- 20.Castilho SD, Saito MI, Barros FA 2005. Crescimento pós-menarca em uma coorte de meninas brasileiras. Arq Bras Endocrinol Metab.49(6):971–7. [DOI] [PubMed] [Google Scholar]
- 21.Gigante DP, Rasmussen KM, & Victora CG 2005. Pregnancy Increases BMI in Adolescents of a Population-Based Birth Cohort. The Journal of Nutrition. 135(1):74–80. [DOI] [PubMed] [Google Scholar]
- 22.Scholl TO, Hediger ML, Schall JI, Khoo CS, & Fischer RL 1994. Maternal growth during pregnancy and the competition for nutrients. The American Journal of Clinical Nutrition.60(2):183–8. [DOI] [PubMed] [Google Scholar]
- 23.Kim JH, Jung Y, Kim SY, & Bae HY 2014. Impact of Age at First Childbirth on Glucose Tolerance Status in Postmenopausal Women: The 2008–2011 Korean National Health and Nutrition Examination Survey. Dia Care. 37(3):671–7. [DOI] [PubMed] [Google Scholar]
- 24.Das JK, Salam RA, Thornburg KL, Prentice AM, Campisi S, Lassi ZS et al. Nutrition in adolescents: physiology, metabolism, and nutritional needs: Adolescents: physiology, metabolism, and nutrition. Ann NY Acad Sci. 2017;1393(1):21–33. [DOI] [PubMed] [Google Scholar]
- 25.Casanueva E, Roselló-Soberón ME, De-Regil LM, Argüelles M, & Céspedes MI 2006. Adolescents with Adequate Birth Weight Newborns Diminish Energy Expenditure and Cease Growth. The Journal of Nutrition. 136(10):2498–501. [DOI] [PubMed] [Google Scholar]
- 26.Christian P, & Smith ER 2018. Adolescent Undernutrition: Global Burden, Physiology, and Nutritional Risks. Ann Nutr Metab. 72(4):316–28. [DOI] [PubMed] [Google Scholar]
- 27.Soliman A, De Sanctis V, Elalaily R, et al. 2014. Advances in pubertal growth and factors influencing it: Can we increase pubertal growth? Indian J Endocr Metab 18: 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rah JH, Christian P, Shamim AA, Arju UT, Labrique AB, & Rashid M 2008. Pregnancy and Lactation Hinder Growth and Nutritional Status of Adolescent Girls in Rural Bangladesh. The Journal of Nutrition. 138(8):1505–11. [DOI] [PubMed] [Google Scholar]
- 29.Lundeen EA, Norris SA, Martorell R, Suchdev PS, Mehta NK, Richter LM, et al.2016. Adolescent Pregnancy and Attained Height among Black South African Girls: Matched-Pair Prospective Study. Baker JL, editor. PLoS ONE. 11(1):e0147861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.NCD Risk Factor Collaboration (NCD-RisC). 2020. Height and body-mass index trajectories of school-aged children and adolescents from 1985 to 2019 in 200 countries and territories: a pooled analysis of 2181 population-based studies with 65 million participants. The Lancet. 396(10261):1511–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cruz E, Cozman FG, Souza W, & Takiuti A 2021. The impact of teenage pregnancy on school dropout in Brazil: a Bayesian network approach. BMC Public Health. 21(1):1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Coley RL, & Chase-Lansdale PL 1998.Adolescent pregnancy and parenthood. Recent evidence and future directions. Am Psychol. 53(2):152–66. [DOI] [PubMed] [Google Scholar]
- 33.Grundy E, & Kravdal O 2010. Fertility history and cause-specific mortality: a register-based analysis of complete cohorts of Norwegian women and men. Soc Sci Med. 70(11):1847–57. [DOI] [PubMed] [Google Scholar]
- 34.Cohen AK, Rai M, Rehkopf DH, & Abrams B 2013. Educational attainment and obesity: a systematic review: Educational attainment and obesity. Obes Rev. 14(12):989–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van Jaarsveld CH, Fidler JA, Steptoe A, Boniface D, & Wardle J 2009. Perceived Stress and Weight Gain in Adolescence: A Longitudinal Analysis. Obesity. 17(12):2155–61. [DOI] [PubMed] [Google Scholar]
- 36.Chandola T, Clarke P, Morris JN and Blane D 2006. Pathways between education and health: a causal modelling approach. J Royal Statistical Soc A 169: 337–359. [Google Scholar]
- 37.Ball K, & Crawford D 2005. Socioeconomic status and weight change in adults: a review. Social Science & Medicine. 60(9):1987–2010. [DOI] [PubMed] [Google Scholar]
- 38.Chao AM, Jastreboff AM, White MA, Grilo CM, & Sinha R 2017. Stress, cortisol, and other appetite-related hormones: Prospective prediction of 6-month changes in food cravings and weight: Stress, Cravings, and Weight. Obesity. 25(4):713–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zanotti J, Capp E, & Wender MC 2015. Factors associated with postpartum weight retention in a Brazilian cohort. Rev Bras Ginecol Obstet. 37(4):164–71. [DOI] [PubMed] [Google Scholar]
- 40.Davis EM, Babineau DC, Wang X, Zyzanski S, Abrams B, Bodnar LM, et al. 2014. Short Inter-pregnancy Intervals, Parity, Excessive Pregnancy Weight Gain and Risk of Maternal Obesity. Matern Child Health J. 18(3):554–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pimentel J, Ansari U, Omer K, Gidado Y, Baba MC, Andersson N, et al. 2020. Factors associated with short birth interval in low- and middle-income countries: a systematic review. BMC Pregnancy Childbirth. 20(1):156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Córdova Pozo K, Chandra-Mouli V, Decat P, Nelson E, De Meyer S, Jaruseviciene L, et al. 2015.Improving adolescent sexual and reproductive health in Latin America: reflections from an International Congress. Reprod Health. 12(1):11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Barbosa de Andrade R, Pirkle CM, Sentell T, Bassani D, Rodrigues Domingues M, & Câmara S 2020. Adequacy of Prenatal Care in Northeast Brazil: Pilot Data Comparing Attainment of Standard Care Criteria for First-Time Adolescent and Adult Pregnant Women. IJWH. 12:1023–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kagawa R, Deardorff J, Domínguez Esponda R, Craig D, & Fernald L 2017. The experience of adolescent motherhood: An exploratory mixed methods study. J Adv Nurs. 73(11):2566–76 [DOI] [PubMed] [Google Scholar]
- 45.Anderson E & Durstine JL 2019. Physical activity, exercise, and chronic diseases: A brief review. Sports Medicine and Health Science 1: 3–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.The Lancet Public Health. 2019. Time to tackle the physical activity gender gap. The Lancet Public Health 4: e360. [DOI] [PubMed] [Google Scholar]
- 47.Coll CV, Domingues MR, Hallal PC, da Silva IC, Bassani DG, Matijasevich A, et al. 2017. Changes in leisure-time physical activity among Brazilian pregnant women: comparison between two birth cohort studies (2004 – 2015). BMC Public Health. 17(1):119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Aguilar-Farias N, Martino-Fuentealba P, Carcamo-Oyarzun J, Cortinez-O’Ryan A, Cristi-Montero C, Von Oetinger A, et al. 2018. A regional vision of physical activity, sedentary behaviour and physical education in adolescents from Latin America and the Caribbean: results from 26 countries. International Journal of Epidemiology. 47(3):976–86. [DOI] [PubMed] [Google Scholar]
- 49.Kimm SY, Glynn NW, Kriska AM, Barton BA, Kronsberg SS, Daniels SR, et al. 2002. Decline in Physical Activity in Black Girls and White Girls during Adolescence. N Engl J Med. 347(10):709–15. [DOI] [PubMed] [Google Scholar]
- 50.Activity Physical and Exercise During Pregnancy and the Postpartum Period: ACOG Committee Opinion, Number 804. 2020. Obstet Gynecol. 135(4):e178–88. [DOI] [PubMed] [Google Scholar]
- 51.Mielke GI, Crochemore-Silva I, Domingues MR, Silveira MF, Bertoldi AD, & Brown WJ 2021. Physical Activity and Sitting Time From 16 to 24 Weeks of Pregnancy to 12, 24, and 48 Months Postpartum: Findings From the 2015 Pelotas (Brazil) Birth Cohort Study. Journal of Physical Activity and Health. 18(5):587–93. [DOI] [PubMed] [Google Scholar]
- 52.Gamble A, Beech BM, Blackshear C, Herring SJ, Welsch MA, & Moore JB 2021. Changes in Physical Activity and Television Viewing From Pre-pregnancy Through Postpartum Among a Socioeconomically Disadvantaged Perinatal Adolescent Population. Journal of Pediatric and Adolescent Gynecology. 34(6):832–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.The International Weight Management in Pregnancy (i-WIP) Collaborative Group. 2017. Effect of diet and physical activity based interventions in pregnancy on gestational weight gain and pregnancy outcomes: meta-analysis of individual participant data from randomized trials. BMJ. 358:j3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Santos S, Voerman E, Amiano P, Barros H, Beilin LJ, Bergström A, et al. 2019. Impact of maternal body mass index and gestational weight gain on pregnancy complications: an individual participant data meta-analysis of European, North American, and Australian cohorts. BJOG: Int J Obstet Gy 1471–0528.15661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Catalano PM, & Shankar K 2017. Obesity and pregnancy: mechanisms of short term and long term adverse consequences for mother and child. BMJ. 356:j1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jones AD, Mundo-Rosas V, Cantoral A, & Levy TS 2017. Household food insecurity in Mexico is associated with the co-occurrence of overweight and anemia among women of reproductive age, but not female adolescents. Matern Child Nutr. 13(4):e12396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.FAO, PAHO, WFP, UNICEF and IFAD. 2021. Regional Overview of Food Security and Nutrition in Latin America and the Caribbean 2020 – Food security and nutrition for lagged territories – In brief. Santiago. [Google Scholar]
- 58.FAO, IFAD, UNICEF, WFP and WHO. 2017. The State of Food Security and Nutrition in the World 2017. Building resilience for peace and food security. Rome, FAO. [Google Scholar]
- 59.Demétrio F, Teles C, Santos D, & Pereira M 2020. Food insecurity in pregnant women is associated with social determinants and nutritional outcomes: a systematic review and meta-analysis. Ciênc saúde coletiva. 25(7):2663–76. [DOI] [PubMed] [Google Scholar]
- 60.Cheu LA, Yee LM, & Kominiarek MA 2020. Food insecurity during pregnancy and gestational weight gain. American Journal of Obstetrics & Gynecology MFM. 2(1):100068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Adam TC, & Epel ES 2007. Stress, eating and the reward system. Physiology & Behavior. 91(4):449–58. [DOI] [PubMed] [Google Scholar]
- 62.Wardle J, Chida Y, Gibson EL, Whitaker KL, & Steptoe A 2011.Stress and Adiposity: A Meta-Analysis of Longitudinal Studies. Obesity. 19(4):771–8. [DOI] [PubMed] [Google Scholar]
- 63.Torres SJ, & Nowson CA 2007. Relationship between stress, eating behavior, and obesity. Nutrition.23(11–12):887–94. [DOI] [PubMed] [Google Scholar]
- 64.Barros BS, Kuschnir M, Bloch KV, & Silva T 2019. ERICA: age at menarche and its association with nutritional status. Jornal de Pediatria. 95(1):106–11. [DOI] [PubMed] [Google Scholar]
- 65.Shalitin S & Gat-Yablonski G 2021. Associations of Obesity with Linear Growth and Puberty. Horm Res Paediatr 279–285. [DOI] [PubMed] [Google Scholar]
- 66.Dunbar J, Sheeder J, Lezotte D, Dabelea D, & Stevens-Simon C 2008. Age at Menarche and First Pregnancy Among Psychosocially At-Risk Adolescents. Am J Public Health. 98(10):1822–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ibitoye M, Choi C, Tai H, Lee G, & Sommer M 2017. Early menarche: A systematic review of its effect on sexual and reproductive health in low- and middle-income countries. Sear R, editor. PLoS ONE. 12(6):e0178884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Llewellyn A, Simmonds M, Owen CG, & Woolacott N 2016. Childhood obesity as a predictor of morbidity in adulthood: a systematic review and meta-analysis: Childhood obesity and adult morbidity. Obesity Reviews. 17(1):56–67. [DOI] [PubMed] [Google Scholar]
- 69.Groth SW 2008. The long-term impact of adolescent gestational weight gain. Res Nurs Health.31(2):108–18. [DOI] [PubMed] [Google Scholar]
- 70.Groth SW, Holland ML, Kitzman H, & Meng Y 2013. Gestational Weight Gain of Pregnant African American Adolescents Affects Body Mass Index 18 Years Later. Journal of Obstetric, Gynecologic & Neonatal Nursing. 42(5):541–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Black RE, Allen LH, Bhutta ZA, Caulfield LE, de Onis M, Ezzati M, et al. 2008. Maternal and child undernutrition: global and regional exposures and health consequences. The Lancet. 371(9608):243–60. [DOI] [PubMed] [Google Scholar]
- 72.Sawaya AL, & Roberts S 2003. Stunting and future risk of obesity: principal physiological mechanisms. Cad Saúde Pública.19(suppl 1):S21–8. [DOI] [PubMed] [Google Scholar]
- 73.Hoffman DJ, Sawaya AL, Verreschi I, Tucker KL, & Roberts SB 2000. Why are nutritionally stunted children at increased risk of obesity? Studies of metabolic rate and fat oxidation in shantytown children from São Paulo, Brazil. The American Journal of Clinical Nutrition. 72(3):702–7. [DOI] [PubMed] [Google Scholar]
- 74.UNICEF (ed.). 2019. “Children, food and nutrition.” New York, NY: UNICEF. [Google Scholar]
- 75.Almeida M de da C.C. & Aquino EML 2009. The Role of Education Level in the Intergenerational Pattern of Adolescent Pregnancy in Brazil. IPSRH 35: 139–146. [DOI] [PubMed] [Google Scholar]
- 76.Munakampe MN, Fwemba I, Zulu JM, & Michelo C 2021. Association between socioeconomic status and fertility among adolescents aged 15 to 19: an analysis of the 2013/2014 Zambia Demographic Health Survey (ZDHS). Reprod Health. 18(1):182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Heilborn ML, & Cabral CS 2011. A New Look at Teenage Pregnancy in Brazil. ISRN Obstetrics and Gynecology. 2011:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Scott KA, Melhorn SJ, & Sakai RR 2012. Effects of Chronic Social Stress on Obesity. Curr Obes Rep.1(1):16–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wiss DA, & Brewerton TD 2020. Adverse Childhood Experiences and Adult Obesity: A Systematic Review of Plausible Mechanisms and Meta-Analysis of Cross-Sectional Studies. Physiology & Behavior. 223:112964. [DOI] [PubMed] [Google Scholar]
- 80.Hillis S, Mercy J, Amobi A, et al. 2016. Global Prevalence of Past-year Violence Against Children: A Systematic Review and Minimum Estimates. Pediatrics 137: e20154079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Follow-up Mechanism of the Belém Do Pará Convention (MESECVI), Declaration on Violence against Women, Girls and Adolescents and their Sexual and Reproductive Rights, OEA/Ser.L/II.7.10 MESECVI/CEVI/DEC.4/14, 19 September 2014, p. 7, [Google Scholar]
- 82.Midei AJ & Matthews KA 2011. Interpersonal violence in childhood as a risk factor for obesity: a systematic review of the literature and proposed pathways: Childhood interpersonal violence and obesity. Obesity Reviews 12: e159–e172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wildsmith E, Manlove J, Jekielek S, Moore KA, & Mincieli L 2012. Teenage Childbearing Among Youth Born to Teenage Mothers. Youth & Society. 44(2):258–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Davis MM, McGonagle K, Schoeni RF, & Stafford F 2008. Grandparental and Parental Obesity Influences on Childhood Overweight: Implications for Primary Care Practice. The Journal of the American Board of Family Medicine. 21(6):549–54. [DOI] [PubMed] [Google Scholar]
- 85.Hoffman DJ, Reynolds RM, & Hardy DB 2017. Developmental origins of health and disease: current knowledge and potential mechanisms. Nutrition Reviews. 75(12):951–70. [DOI] [PubMed] [Google Scholar]
- 86.Hoffman DJ, Powell TL, Barrett ES, & Hardy DB 2021. Developmental origins of metabolic diseases. Physiological Reviews. 101(3):739–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wu G, Imhoff-Kunsch B, & Girard AW 2012. Biological Mechanisms for Nutritional Regulation of Maternal Health and Fetal Development: Maternal nutrition and healthy pregnancy. Paediatric and Perinatal Epidemiology. 26:4–26. [DOI] [PubMed] [Google Scholar]
- 88.Teixeira JA, Hoffman DJ, Castro TG, Saldiva S, Francisco R, Vieira SE, et al. 2021. Pre-pregnancy dietary pattern is associated with newborn size: results from ProcriAr study. Br J Nutr. 126(6):903–12. [DOI] [PubMed] [Google Scholar]
- 89.Nam HK, & Lee KH 2018. Small for gestational age and obesity: epidemiology and general risks. Ann Pediatr Endocrinol Metab. 23(1):9–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Orsso CE, Colin-Ramirez E, Field CJ, Madsen KL, Prado CM, & Haqq AM 2020. Adipose Tissue Development and Expansion from the Womb to Adolescence: An Overview. Nutrients. 12(9):2735. [DOI] [PMC free article] [PubMed] [Google Scholar]


