Abstract
Objective:
Patients with gynecologic malignancies commonly experience distressing symptoms during chemotherapy. This study sought to evaluate whether symptoms accumulated over the course of several chemotherapy cycles, which could provide essential information for planning supportive interventions.
Methods:
Patients with gynecologic malignancies completed questionnaires about fatigue, depressive symptoms, sleep, and physical activity one week before and after chemotherapy cycles 1, 3, and 6. Multilevel models examined the effects of time (pre-, post-chemotherapy), treatment cycle (1, 3, 6), and their interaction on symptoms. Logistic regression models examined the effects of time, treatment cycle, and their interaction on the proportion of participants exceeding thresholds for clinically meaningful symptomatology.
Results:
Most participants (N=140; Mage=60.8 years, SD=10.4) had ovarian cancer (49%) and stage III disease (55%). Participants reported worse fatigue, depressive symptoms, sleep disturbance, and sleep efficiency from pre- to post-treatment at each cycle (ps<0.001). With each successive cycle, participants reported worse pre-treatment fatigue (p<0.001) and depressive symptoms (p<0.01) but better sleep efficiency (p=0.02). Fatigue increases attenuated across cycles (p=0.04). There were no changes in physical activity. Across timepoints, at least half of participants met clinical thresholds for fatigue, sleep disturbance, and low sleep efficiency, and were minimally physically active. Post-chemotherapy cycle 6, 23% of participants reported clinically meaningful depressive symptoms.
Conclusions:
Patients with gynecologic malignancies have high rates of clinically meaningful symptomatology during chemotherapy. Patients may experience a cumulative burden of symptomatology as treatment progresses, which could have therapeutic implications. Early implementation of supportive interventions should be considered to prevent or mitigate cumulative treatment burden.
Keywords: antineoplastic agents, drug-related side effects and adverse reactions, genital neoplasms female
Patients with cancer commonly report distressing symptoms during chemotherapy, including fatigue, depressive symptoms, and disrupted sleep and physical activity (Chen & Tseng, 2006; Given et al., 2007). In many cases, these symptoms cluster together (Ho et al., 2015; Jim et al., 2011) and are highly prevalent and severe among patients with gynecologic malignancies (Beesley et al.; Kirchheiner et al., 2015; Stevinson et al., 2007), due in part to the common use of cytotoxic chemotherapies. Recent studies estimate that up to 78% of patients with gynecologic malignancies treated with chemotherapy report fatigue (Beesley et al., 2020; Cheong et al., 2019; Kirchheiner et al., 2015; Webber et al., 2019; Westin et al., 2016), up to 48% report mood disturbance or depressive symptoms (Beesley et al., 2020; Clevenger et al., 2013; Webber et al., 2019; Westin et al., 2016), and up to 71% report sleep disturbance (Clevenger et al., 2013; Kirchheiner et al., 2015; Webber et al., 2019; Westin et al., 2016). Moreover, 40% of patients with gynecologic malignancies report decreased physical activity (Beesley et al., 2011), with a systematic review finding that most gynecologic cancer survivors do not meet physical activity guidelines at two years (91%) or three years (58%) post-diagnosis (Lin et al., 2019). These highly prevalent symptoms contribute to elevated distress and worse quality of life (Beesley et al., 2011; Nho et al., 2017; Stevinson et al., 2007; Wen et al., 2017).
Understanding the natural course of symptomatology is critical for determining when and how to best support patients during treatment. For example, identifying the symptoms that emerge or worsen first during treatment may inform which symptoms should be prioritized for early symptom management interventions. In turn, early and effective symptom management may prevent worsening of symptomatology over time and improve downstream outcomes. However, few studies have prospectively evaluated symptomatology among patients with gynecologic malignancies during active treatment. As an exception, our team conducted a longitudinal study of 78 patients with gynecologic malignancies during treatment with platinum-based chemotherapy (Jim et al., 2011; Jim et al., 2013). Findings revealed that symptoms occurred in a cascade pattern during treatment such that disrupted sleep contributed to increased fatigue, and fatigue contributed to increased depressive symptoms (Jim et al., 2011; Jim et al., 2013). Another study evaluated symptoms during chemotherapy, radiation therapy, and brachytherapy among 56 patients with locally advanced cervical cancer (Kirchheiner et al., 2015). The prevalence of moderate to severe fatigue more than doubled from pre-treatment (36%) to active treatment (78%) and continued to affect three-quarters of patients immediately post-treatment (74%). In addition, prevalence of moderate to severe sleep disturbance increased from pre-treatment (22%) to active treatment (32%) and doubled immediately post-treatment (44%) (Kirchheiner et al., 2015). These studies confirm that symptoms are highly prevalent among patients with gynecologic malignancies during treatment, and they suggest that the severity of symptomatology changes over time. However, past studies were limited by relatively small sample sizes and/or simple pre-post treatment designs.
To address these limitations, we built on our prior work and examined symptomatology (i.e., fatigue, depressive symptoms, disturbed sleep, and physical activity) during active chemotherapy among a sample of 140 patients with gynecologic malignancies. The goal of this study was to examine whether symptoms accumulated over the course of several chemotherapy cycles. To our knowledge, no other studies have directly examined this possibility. Importantly, we did not seek to make causal attributions between specific treatment regimens and symptomatology. Patients reported symptoms one week before and after three cycles of chemotherapy: cycle 1 (beginning of treatment), cycle 3 (middle of treatment), and cycle 6 (end of treatment). This design allowed us to evaluate changes in symptomatology within individual treatment cycles as well as over the course of multiple cycles. We hypothesized that symptoms would be highly prevalent among participants, and subsequent cycles of chemotherapy would be associated with more severe symptomatology.
Methods
Participants and Procedures
This study was approved by the University of South Florida Institutional Review Board. Patients with gynecologic malignancies who were scheduled to start a new regimen of chemotherapy were recruited as part of a larger prospective study. Inclusion criteria included: 1) 18–89 years old; 2) able to speak and read English; 3) diagnosed with a gynecologic malignancy (e.g., ovarian, fallopian tube, peritoneal, endometrial, uterine, cervical, or vulvar); 4) scheduled to start intravenous or intraperitoneal chemotherapy at a National Cancer Institute-designated Comprehensive Cancer Center in the Southeastern United States; 5) no immune-related diseases (e.g., HIV, systemic lupus erythematosus, and rheumatoid arthritis); 6) no documented psychiatric, sleep, or neurological disorders that could interfere with the study participation (e.g., psychosis, dementia, sleep apnea); 7) have not undergone chemotherapy or radiotherapy in the month prior to enrollment; 8) not pregnant; and 9) able to provide informed consent. Exclusion criteria included: 1) no diagnosis of a gynecologic malignancy; 2) received treatment in the past month; 3) a documented psychiatric or neurologic disorder that could interfere with study participation; and 4) cannot speak and read English. Patients who had undergone previous lines of therapy were not excluded if they met all other eligibility criteria. Participants were recruited between August 2013 and July 2018.
Potentially eligible patients were identified by physician referral and by screening physicians’ schedules, and they were contacted by a trained research coordinator to determine eligibility and interest in participation. Eligible and interested patients provided informed consent during an outpatient clinic visit prior to starting chemotherapy. Participants completed self-report questionnaires assessing fatigue, depressive symptoms, sleep, and physical activity at six time-points: one week before and one week after chemotherapy cycle 1 (beginning of treatment), cycle 3 (middle of treatment), and cycle 6 (end of treatment). Participants were compensated with $25 for completing each questionnaire.
Measures
Demographic and Clinical Data
At the first assessment only, participants reported their demographic characteristics (e.g., age, education, race/ethnicity, marital status, income). Clinical data were extracted from medical records (e.g., cancer type, stage, recurrence status, prior chemotherapy).
Symptoms
Fatigue.
The 4-item fatigue severity subscale of the Fatigue Symptom Inventory (FSI) assessed patients’ most, least, and average fatigue in the past week as well as current fatigue on an 11-point scale from 0 (not at all fatigued) to 10 (as fatigued as I could be) (Hann et al., 2000; Stein et al., 1998). Scores of 3 and above indicated clinically meaningful fatigue (Donovan et al., 2008). A change in fatigue of 1.14 points indicated clinically meaningful change, as this reflected a change of 0.5 standard deviations in the observed data (Norman et al., 2003).
Depressive Symptoms.
The 7-item depression subscale of the Hospital Anxiety and Depression Scale (HADS) was designed to detect depressive symptoms in medically ill patients, including people with cancer (Moorey et al.; Zigmond & Snaith, 1983). Participants rated items on a 4-point scale from 0 (absence) to 3 (extreme presence). All items were summed with possible scores ranging from 0–21, and scores ≥8 indicated clinically meaningful depressive symptoms (Hipkins et al., 2004). A minimally important difference (MID) of 1.4 points indicated a clinically meaningful change in depressive symptoms (Puhan et al., 2008).
Sleep.
The 19-item Pittsburgh Sleep Quality Index (PSQI) assessed types and frequency of sleep disturbance experienced over the last month (Buysse et al., 1989). A global score was obtained by summing seven component scores (range 0–21) that captured subjective sleep quality, sleep latency, habitual sleep efficiency, nighttime disturbance, sleep duration, use of sleep medications, and daytime dysfunction. A global score ≥5 indicated clinically meaningful sleep disturbance (Buysse et al., 1989), and a MID of 3 points indicated clinically meaningful change (Hughes et al., 2009). Sleep efficiency was calculated as the proportion of total time in bed spent asleep, with scores <85% indicating clinically low sleep efficiency (Lichstein et al., 2003). The PSQI is psychometrically sound among cancer patients (Beck et al., 2004).
Physical Activity.
The 7-item International Physical Activity Questionnaire-Short Form (IPAQ-SF) was used to assess the frequency (days/week) and duration (minutes/day) of three intensities of physical activity (i.e., walking, moderate, vigorous) and time spent sitting (i.e., indicator of sedentary time) in the last seven days (Lee et al., 2011). Values at each intensity level were weighted by its energy requirements, which were defined by metabolic equivalents (METs) as follows: walking=3.3 METs/minute, moderate physical activity=4.0 METs/minute, vigorous physical activity=8.0 METs/minute (Forde). Total physical activity was calculated as the sum of METs/week for each type of physical activity. Patients were also categorized as minimally, moderately, or highly physically active according to published guidelines (Forde).
Statistical Analyses
All analyses were performed using SAS Version 9.4. Descriptive statistics were used to characterize participants by demographic and clinical variables at baseline. Multilevel models using Proc Mixed were used to examine changes in symptomatology from pre- to post-chemotherapy across treatment cycles 1, 3, and 6. Multilevel modeling accounts for correlations between repeated assessments and individual trajectories of scores over time (Kwok et al.). In addition, multilevel modeling uses all available data, as opposed to listwise deletion, so that participants were included at each timepoint at which they provided data. Missing data were not imputed. Each model assessed the effects of time (pre-, post-chemotherapy), treatment cycle (1, 3, 6), and the interaction of time and treatment cycle on individual symptoms (i.e., fatigue, depressive symptoms, disturbed sleep, and physical activity). The main effect of time indicated whether pre- vs. post-chemotherapy symptoms changed, regardless of treatment cycle. The main effect of treatment cycle examined change in pre-treatment symptoms at each cycle. Interactions between time and treatment cycle examined how symptoms changed from pre- to post-treatment across cycles (i.e., whether the slope of change from pre- to post-treatment increased or decreased). Statistical significance was indicated by p<0.05, and partial eta squared effect sizes were calculated for significant effects (0.01=small, 0.09=medium, 0.25=large). Models did not include covariates due to model complexity. However, post-hoc t-tests were used to explore the role of disease stage (stage I-II, stage III-IV) and history of previous treatment (pre-treated, treatment-naïve) on symptomatology at each time point.
Clinically meaningful changes in symptomatology were explored in two ways. First, differences in mean scores were calculated from pre-treatment cycle 1 to post-treatment cycle 6 for fatigue, depressive symptoms, and sleep disturbance. Differences in mean scores that exceeded MIDs were considered clinically meaningful. Second, logistic regression models using Proc Glimmix were used to examine changes in the proportion of participants who met clinical thresholds for fatigue, depressive symptoms, and sleep efficiency, and each physical activity category. Each logistic regression model assessed the effects of time (pre-, post-chemotherapy), treatment cycle (1, 3, 6), and their interaction on the proportion of participants in each category. The main effect of time indicated whether the proportion of participants with clinically meaningful symptomatology changed from pre- to post-chemotherapy, regardless of treatment cycle. The main effect of treatment cycle examined change in the proportion of participants with clinically meaningful symptomatology at the pre-treatment timepoint for each cycle. Interactions between time and treatment cycle examined how the proportion of participants with clinically meaningful symptomatology changed from pre- to post-treatment across cycles (i.e., whether the slope of change from pre- to post-treatment increased or decreased). Statistical significance was indicated by p<0.05, and odds ratios were calculated for significant effects (1.68=small, 3.47=medium, 6.71=large effect) (Chen et al., 2010).
Results
Sample Characteristics
Figure 1 shows the flow of study participants. Of 6,935 patients screened, 5,492 were ineligible for reasons including no suspected gynecologic malignancy (n=1,922) or not receiving chemotherapy at the study site (n=1,929). Smaller proportions of patients were ineligible for reasons such as having a documented psychiatric, sleep, or neurological disorder that could interfere with study participation (n=632), having received chemotherapy in the last month (n=257), and being unable to speak and read English (n=245). A total of 1,179 eligible patients were not approached due to staff availability. For example, many eligible patients were added to physicians’ schedules at the last minute, which precluded staff’s ability to enroll them and complete the baseline evaluations before the patient started chemotherapy. Of 264 patients approached, 150 consented to participate (57% recruitment rate). Ten participants did not have usable data and were excluded from analyses. Thus, the sample reported here included 140 participants. Sample characteristics are presented in Table 1. Briefly, participants were patients with gynecologic malignancies an average of 60.8 years old (SD=10.4). Most participants were White (94%) and non-Hispanic/Latina (96%). Approximately one third had graduated from college (35%). Table 2 presents descriptive statistics for symptomatology at each timepoint.
Figure 1.
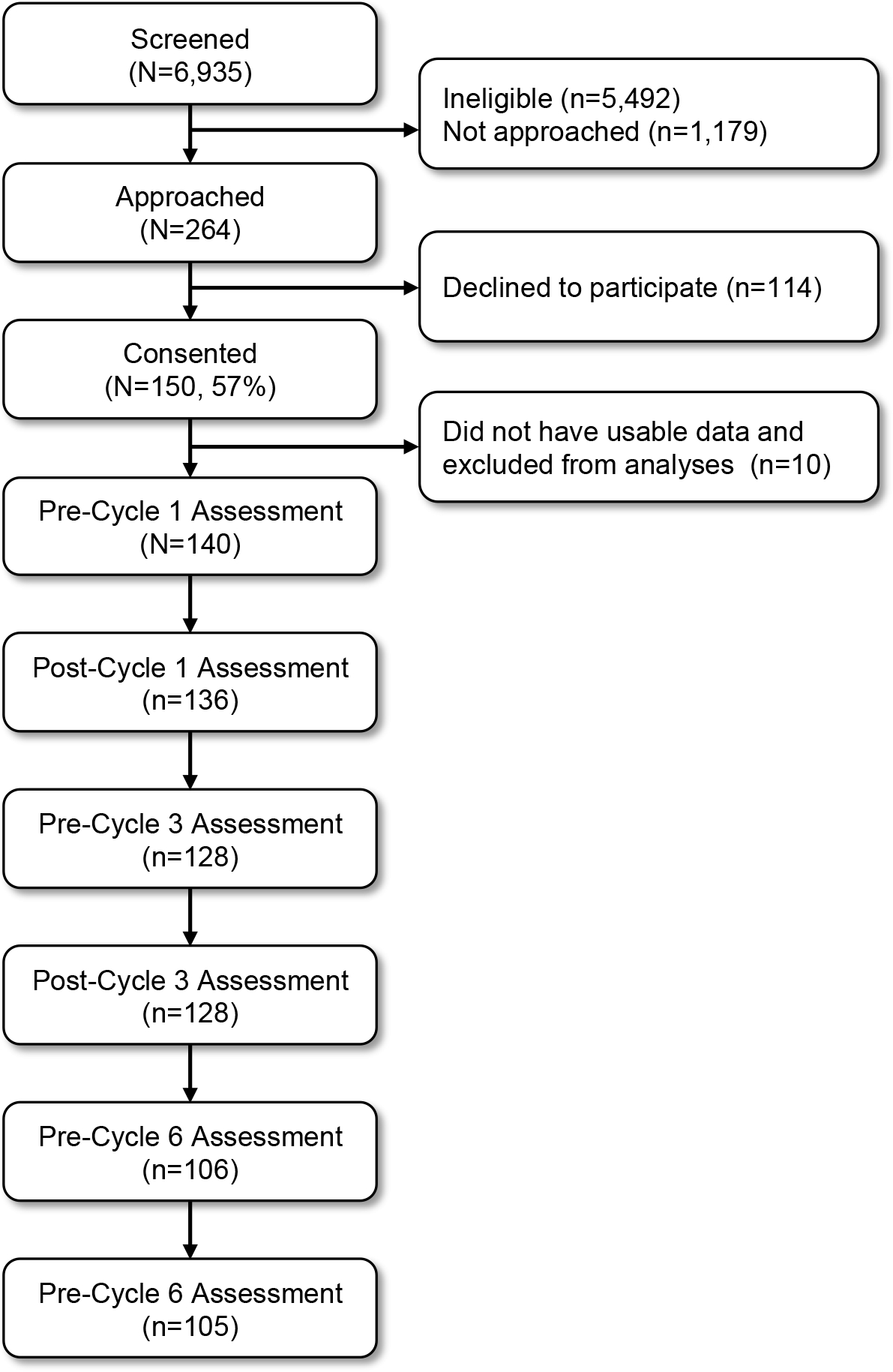
Diagram of participant flow throughout the study.
Table 1.
Demographic and Clinical Characteristics of the Sample (N=140)
| Characteristic | Statistic |
|---|---|
|
| |
| Age, M (SD) | 60.8 (10.4) |
| White, n (%) | 130 (94) |
| Non-Hispanic/Latina, n (%) | 132 (96) |
| College graduate, n (%) | 49 (35) |
| Annual income >$40,000, n (%) | 73 (68) |
| Stage of disease, n (%) | |
| I | 24 (19) |
| II | 12 (10) |
| III | 70 (55) |
| IV | 21 (17) |
| Gynecologic cancer diagnosis, n (%) | |
| Ovarian | 69 (49) |
| Endometrial | 33 (24) |
| Uterine | 15 (11) |
| Peritoneal | 9 (7) |
| Fallopian tube | 6 (4) |
| Cervical | 5 (4) |
| Vulvar | 1 (1) |
| Other | 2 (2) |
| Chemotherapy regimen, n (%) | |
| Intravenous | 126 (90) |
| Intraperitoneal | 14 (10) |
| Previous surgeries, n (%) | |
| 0 | 29 (21) |
| 1 | 86 (62) |
| 2 | 15 (11) |
| 3+ | 8 (6) |
| Previous lines of chemotherapy, n (%) | |
| 0 | 86 (61) |
| 1 | 28 (20) |
| 2 | 12 (9) |
| 3+ | 14 (10) |
| Previously received radiation therapy, n (%) | 7 (5) |
Table 2.
Descriptive Statistics of Symptomatology Across Chemotherapy Cycles (N=140)
| Side Effect | Chemotherapy Cycle 1 | Chemotherapy Cycle 3 | Chemotherapy Cycle 6 | |||
|---|---|---|---|---|---|---|
|
| ||||||
| Pre; M (SD) | Post; M (SD) | Pre; M (SD) | Post; M (SD) | Pre; M (SD) | Post; M (SD) | |
|
| ||||||
| Fatigue | 3.31 (1.95) | 4.37 (1.82) | 3.70 (1.84) | 4.41 (1.76) | 4.22 (2.27) | 4.80 (1.99) |
| Depressive symptoms | 4.53 (3.54) | 5.81 (4.02) | 4.96 (3.82) | 5.87 (4.07) | 5.52 (3.98) | 6.64 (4.34) |
| Sleep | ||||||
| Disturbance | 7.07 (3.45) | 8.55 (4.05) | 7.26 (3.94) | 8.41 (3.89) | 6.69 (3.38) | 7.68 (3.58) |
| Efficiency | 78.74 (16.67) | 73.17 (20.14) | 79.85 (16.77) | 76.99 (16.20) | 82.64 (14.18) | 78.83 (16.00) |
| Physical activity | ||||||
| Total METs | 1687.05 (2201.91) | 1521.12 (2142.28) | 1343.81 (1717.22) | 1479.11 (1913.58) | 1289.46 (2244.34) | 1448.52 (2757.32) |
| Sedentary time | 441.64 (301.35) | 451.88 (215.63) | 452.84 (380.11) | 449.95 (239.65) | 462.79 (215.30) | 439.37 (196.61) |
Fatigue
Fatigue scores increased from pre- to post-treatment at each chemotherapy cycle (main effect of time; B=1.05, SE=0.16, p<0.001, partial η2=0.08) and pre-treatment fatigue was worse with each successive cycle (main effect of treatment cycle; B=0.50, SE=0.09, p<0.001, partial η2=0.05; Figure 2A). Fatigue increases attenuated across cycles (interaction of time and treatment cycle; B=−0.25, SE=0.13, p=0.04, partial η2=0.01). The average fatigue score increased by 1.49 points from pre-treatment cycle 1 to post-treatment cycle 6, exceeding 0.5 standard deviations and indicating a clinically meaningful increase. At each timepoint, more than 55% of participants reported clinically meaningful fatigue (Figure 2B). The proportion of participants reporting clinically meaningful fatigue increased from pre- to post-treatment at each treatment cycle (main effect of time; B=1.16, SE=0.28, p<0.001, OR=2.75, 95% CI=1.87–4.04) and increased with each successive cycle (main effect of treatment cycle; B=0.43, SE=0.15, p=0.06, OR=1.43, 95% CI=1.13–1.81). The interaction of time and treatment cycle was not significant (B=−0.15, SE=0.24, p=0.54).
Figure 2.
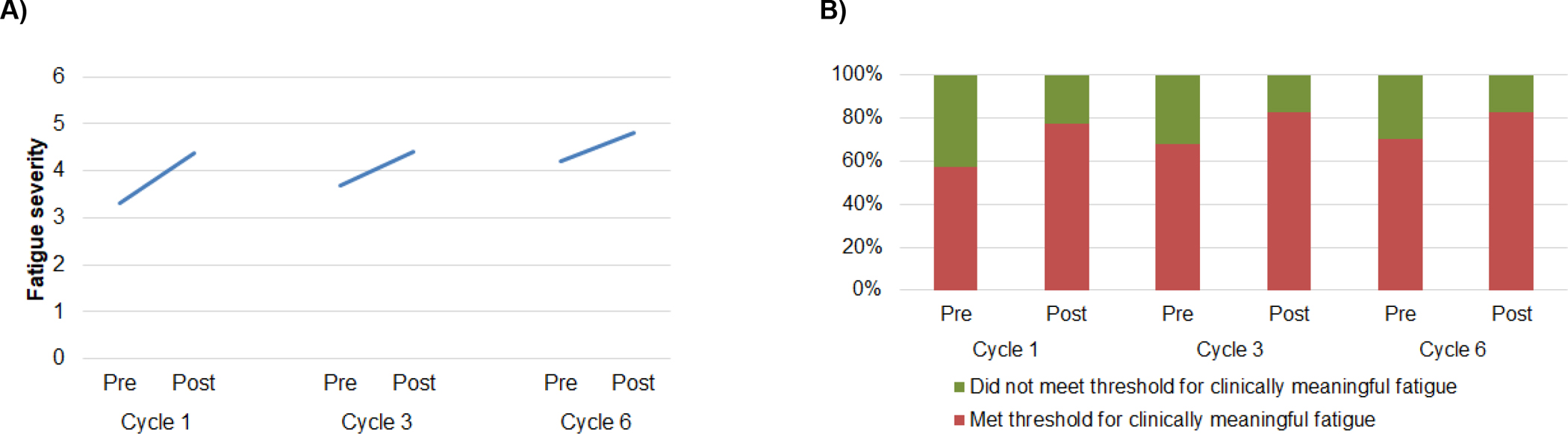
A) Fatigue increased from pre- to post-treatment at each cycle, and pre-treatment fatigue increased with each successive treatment cycle. Increases in fatigue were attenuated across treatment cycles. B) The proportion of participants reporting clinically meaningful fatigue (FSI fatigue severity ≥3) increased from pre- to post-treatment at each cycle and with each successive treatment cycle.
Depressive Symptoms
Average scores for depressive symptoms increased from pre- to post-treatment at each chemotherapy cycle (main effect of time; B=1.29, SE=0.27, p<0.001, partial η2=0.04) and pre-treatment depressive symptoms were higher with each successive cycle (main effect of treatment cycle; B=0.52, SE=0.16, p<0.01, partial η2=0.02; Figure 3A). Depressive symptoms were not related to the interaction of time and treatment cycle (B=−0.18, SE=0.22, p=0.41). Average scores for depressive symptoms increased by 2.11 points from pre-treatment cycle 1 to post-treatment cycle 6, exceeding the MID and indicating a clinically meaningful increase. Clinically meaningful depressive symptoms were most prevalent at the post-chemotherapy cycle 6 time point, with 23% of participants reporting clinically meaningful depressive symptoms (Figure 3B). The proportion of participants reporting clinically meaningful depressive symptoms was not related to time (B=0.49, SE=0.41, p=0.22), treatment cycle (B=0.30, SE=0.24, p=0.21), nor the interaction of time and treatment cycle (B=0.14, SE=0.31, p=0.66).
Figure 3.
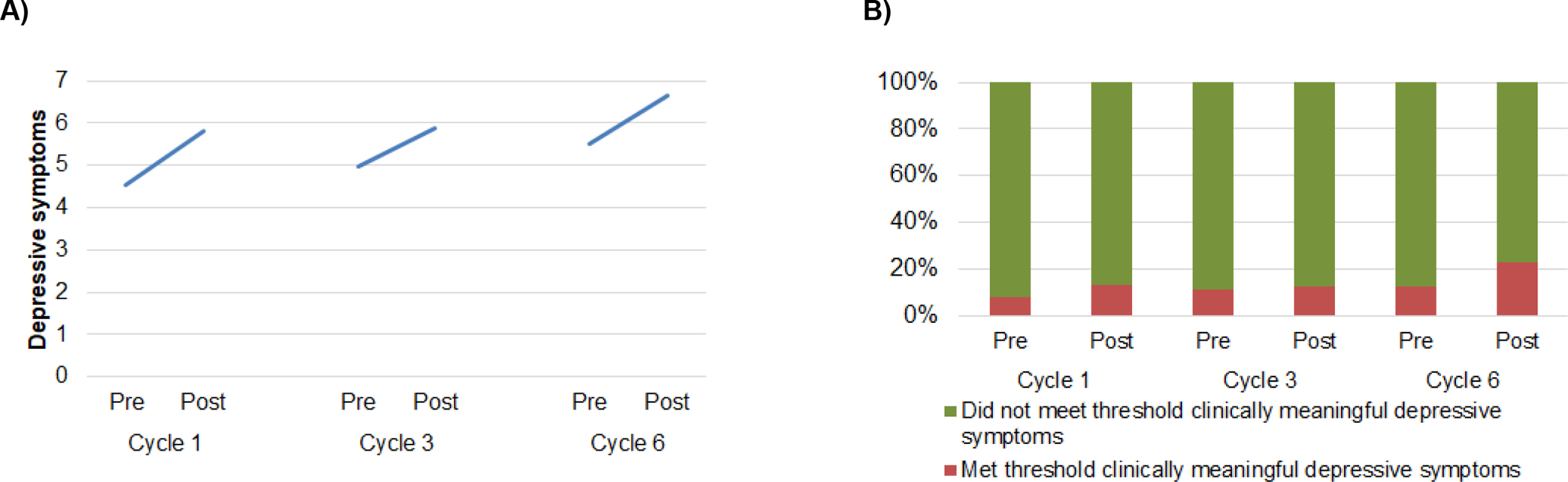
A) Depressive symptoms increased from pre- to post-treatment at each cycle, and pre-treatment depressive symptoms increased with each successive treatment cycle. B) The proportion of participants reporting clinically meaningful depressive symptoms (HADS ≥8) did not change within or across treatment cycles.
Sleep
Sleep disturbance scores increased from pre- to post-treatment at each chemotherapy cycle (main effect of time; B=1.43, SE=0.27, p<0.001, partial η2=0.05 Figure 4A). Sleep disturbance was not related to treatment cycle (B=−0.25, SE=0.15, p=0.11) nor the interaction of time and treatment cycle (B=−0.22, SE=0.22, p=0.32). Change in average sleep disturbance scores from pre-treatment cycle 1 to post-treatment cycle 6 did not exceed the MID. At each time point, at least 58% of participants reported clinically meaningful sleep disturbance (Figure 4B). The proportion of participants reporting clinically meaningful sleep disturbance increased from pre- to post-treatment at each chemotherapy cycle (main effect of time; B=0.92, SE=0.30, p<0.01, OR=2.31, 95% CI=1.57–3.40), but was not related to treatment cycle (B=−0.09, SE=0.16, p=0.56) nor the interaction of time and treatment cycle (B=−0.09, SE=0.24, p=0.72).
Figure 4.
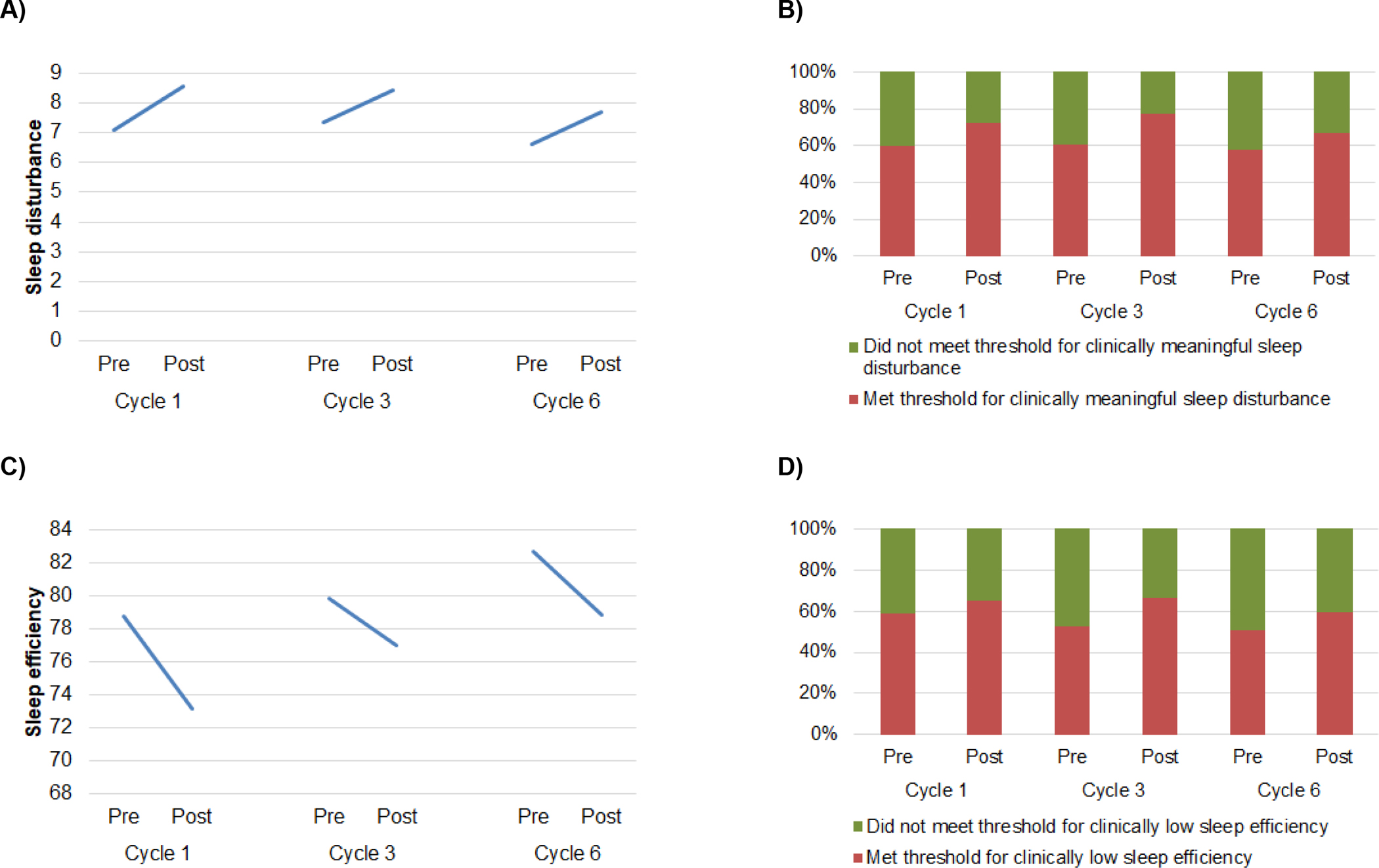
A) Sleep disturbance increased from pre- to post-treatment at each treatment cycle. B) The proportion of participants reporting clinically meaningful sleep disturbance (PSQI global ≥5) increased from pre- to post-treatment at each cycle. C) Sleep efficiency decreased from pre- to post-treatment at each chemotherapy cycle, and pre-treatment sleep efficiency increased with each successive cycle. D) The proportion of participants with clinically low sleep efficiency (<85%) did not change within or across treatment cycles.
Sleep efficiency decreased from pre- to post-treatment at each chemotherapy cycle (main effect of time; B=−5.17, SE=1.41, p<0.001, partial η2=0.02) and pre-treatment sleep efficiency was higher with each successive cycle (main effect of treatment cycle; B=1.92, SE=0.82, p=0.02, partial η2=0.01; Figure 4C). Sleep efficiency was not related to the interaction of time and treatment cycle (B=1.05, SE=1.15, p=0.36). At each time point, at least 52% of participants reported clinically low sleep efficiency (Figure 4D). The proportion of participants reporting clinically low sleep efficiency was not related to time (B=0.52, SE=0.28, p=0.07), treatment cycle (B=−0.24, SE=0.16, p=0.13) nor the interaction of time and treatment cycle (B=0.08, SE=0.23, p=0.72).
Physical Activity
Total METs were not related to time (B=−123.40, SE=193.64, p=0.52), treatment cycle (B=−209.46, SE=113.05, p=0.06), nor the interaction of time and treatment cycle (B=170.46, SE=158.69, p=0.28; Figure 5A). Sedentary time was also not related to time (B=6.77, SE=27.38, p=0.80), treatment cycle (B=5.56, SE=16.04, p=0.71), nor the interaction of time and treatment cycle (B=−8.44, SE=22.65, p=0.71; Figure 5B). At each time point, at least 49% of participants were minimally physically active (Figure 5C). The proportion of participants in each activity category was not related to time (B=0.26, SE=0.23, p=0.25), treatment cycle (B=0.25, SE=0.13, p=0.07), nor the interaction of time and treatment cycle (B=−0.08, SE=0.19, p=0.66).
Figure 5.
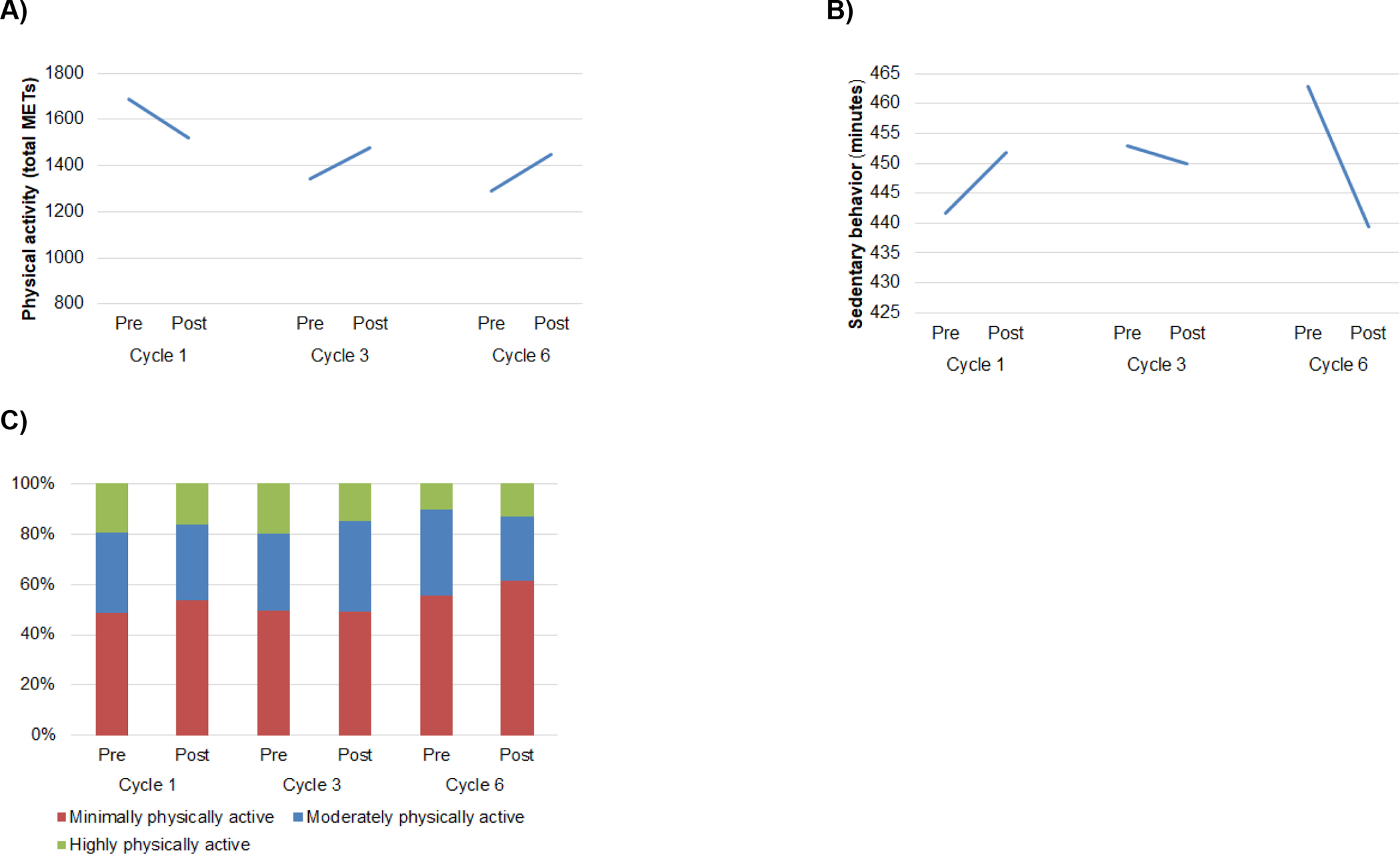
A) Physical activity (total METs) did not change within or across treatment cycles. B) Physical inactivity (sedentary time) did not change within or across treatment cycles. C) The proportion of participants categorized as highly, moderately, and minimally physically active according to the IPAQ-SF did not change within or across treatment cycles.
Post-Hoc Comparisons
At the pre-chemotherapy cycle 3 time point, participants with a history of prior treatment had worse fatigue (M=4.16, SD=1.56) than participants undergoing their first line of treatment (M=3.42, SD=1.95) (t(126)=−2.25, p=0.026). There were no other differences in symptomatology at any time point by participants’ history of previous treatment (previous treatment vs. treatment-naïve) or disease stage (stage I-II vs. stage III-IV) (ps>0.05).
Discussion
This study is among the first to prospectively examine fatigue, depressive symptoms, sleep disturbance, and physical activity during active chemotherapy for gynecologic malignancies. In a sample of 140 patients, we assessed symptomatology immediately before and after patients’ first, third, and sixth cycles of chemotherapy. We examined the individual and cumulative impact of treatment cycles on symptom severity and the proportion of participants with clinically meaningful symptomatology. Findings indicated that chemotherapy may have a cumulative behavioral impact on patients with gynecologic malignancies, which has implications for cancer treatment, clinical supportive care interventions, and future research.
This study shows that from pre- to post-treatment at each chemotherapy cycle, the severity of fatigue, depressive symptoms, and sleep disturbance increased, and sleep efficiency decreased. Moreover, with each successive cycle of chemotherapy, the severity of pre-treatment fatigue and depressive symptoms increased, whereas sleep efficiency improved. Prior work has consistently found increases in fatigue, depressive symptoms, and sleep disturbance during chemotherapy among patients with various cancers (Beesley et al., 2020; Kirchheiner et al., 2015; Prue et al., 2010; Wen et al., 2017; Wright et al., 2015; Wright et al., 2019). However, the present findings suggest a cumulative effect of chemotherapy on fatigue and depressive symptoms and improvement in sleep efficiency over time that is disrupted by treatment cycles. Sleep efficiency may have improved with each subsequent treatment cycle because patients’ acute symptoms from the prior cycle had resolved at least partially (e.g., nausea, vomiting), resulting in more consolidated sleep. However, patients’ sleep efficiency worsened from pre- to post-treatment within each treatment cycle, which may be due to increases in acute symptoms following treatment. These findings warrant more research to clarify the relationships between changes in sleep, fatigue, and depressive symptoms during treatment.
Findings from this study indicate that increases in fatigue from pre- to post-treatment attenuated across subsequent chemotherapy cycles. It is notable that, despite the observed attenuated increases, more than half of participants reported clinically meaningful levels of fatigue at each time point. Thus, fatigue was a notable and continuous burden for the majority of participants. Attenuated increases in fatigue might be explained by biopsychosocial adjustment to the effects of chemotherapy. For example, patients may adopt coping strategies after experiencing fatigue during the first cycle of chemotherapy (e.g., activity pacing, daytime napping, light physical activity) and apply these strategies during subsequent cycles (Fitch et al., 2008). Since increases in depressive symptoms and sleep disturbance were not attenuated with multiple cycles, it is possible that these side effects may not be as amenable to adjustment. These possibilities can be explored in future work. Researchers and clinicians may also consider monitoring patients’ fatigue, depressive symptoms, and sleep disturbance during treatment and implementing early intervention to address these symptoms.
Physical activity did not adhere to a consistent pattern of change over time or by treatment cycle. Moreover, although not statistically significant, it appeared that physical activity slightly increased and sedentary behavior decreased from pre- to post-treatment at cycle 3 and cycle 6, which is contrary to expectations. However, at each timepoint, at least half of participants fell within the minimal physical activity category. Prior research has shown that physical activity tends to decrease in the first year after chemotherapy for gynecologic malignancies (Beesley et al., 2011). By contrast, our research group previously found that physical activity increased before chemotherapy infusions and decreased after (Jim et al., 2011). Discrepant findings may be due to differences in study intervals (i.e., first three chemotherapy cycles in our prior study vs. first, third, and sixth cycles in the current study) or methods of assessment (i.e., accelerometry in our prior study vs. participant self-report in the current study). Future work should explore these options.
Notably, at least half of participants in this sample reported clinically meaningful fatigue and sleep disturbance, clinically low sleep efficiency, and minimal physical activity even before treatment cycle 1. This is consistent with past studies that also found elevated pre-treatment symptomatology among patients with breast cancer (Ancoli-Israel et al., 2014; Liu et al., 2009). Thus, symptomatology reported in this study was not necessarily attributable to chemotherapy alone and could also have been caused by cancer itself, and/or other factors such as previous treatments. Nonetheless, regardless of etiology, this study provides a close examination of how symptomatology changed over the course of active chemotherapy among a large and heterogeneous sample of patients with gynecologic malignancies.
This study extends prior work by evaluating symptoms during treatment for gynecologic malignancies across a wide span of treatment cycles. Moreover, this study evaluated a sample of patients with gynecologic malignancies that is roughly double the size of samples in prior work. Our consideration of statistical and clinical significance is a strength, and we documented a high prevalence of clinically meaningful symptomatology in this sample. Limitations of this work include the racial and ethnic homogeneity of the sample, as participants were mostly non-Hispanic/Latina and White. Research is needed in more diverse patient samples. In addition, self-reported physical activity is susceptible to social desirability bias and inaccurate recall. Future studies may consider using objective assessments of physical activity (e.g., accelerometry). We acknowledge that patients may experience other symptoms beyond those reported here (e.g., nausea, pain) and other factors not evaluated here may impact patients’ symptomatology during treatment (e.g., stage of disease, prior cancer treatments, specific chemotherapy regimens, concurrent treatment with radiation therapy). Moreover, symptoms may affect one another (e.g., symptom clusters). These possibilities can be explored in larger studies. Future work will also benefit from considering the role of biopsychosocial mechanisms associated with chemotherapy and patient-reported symptomatology (e.g., inflammation) (Demaria et al., 2017; Reinertsen et al., 2017; Yang et al., 2019).
Findings from this study may inform the development of supportive interventions to improve quality of life during gynecologic cancer treatment. Findings suggest that patients with gynecologic malignancies may experience a cumulative burden of fatigue and depressive symptoms as treatment progresses; thus, early implementation of supportive interventions to manage fatigue and depressive symptoms should be considered to prevent or mitigate this cumulative treatment burden.
Acknowledgments
This study was supported by National Cancer Institute grants R01CA164109 (PI: Jim), P30CA076292 (PI: Cleveland), and T32CA090314 (MPIs: Brandon/Vadaparampil). Dr. Gonzalez discloses that he is a paid consultant for SureMed Compliance Equity and Elly Health, Inc. Dr. Jim discloses that she is a paid consultant for RedHill BioPharma, Janssen Scientific Affairs, and Merck. The other authors have no conflicts of interest to disclose.
References
- Ancoli-Israel S, Liu L, Rissling M, Natarajan L, Neikrug AB, Palmer BW, Mills PJ, Parker BA, Sadler GR, & Maglione J (2014). Sleep, fatigue, depression, and circadian activity rhythms in women with breast cancer before and after treatment: a 1-year longitudinal study. Support Care Cancer, 22(9), 2535–2545. 10.1007/s00520-014-2204-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck SL, Schwartz AL, Towsley G, Dudley W, & Barsevick A (2004). Psychometric evaluation of the Pittsburgh Sleep Quality Index in cancer patients. J Pain Symptom Manage, 27(2), 140–148. 10.1016/j.jpainsymman.2003.12.002 [DOI] [PubMed] [Google Scholar]
- Beesley VL, Price MA, Butow PN, Green AC, Olsen CM, Australian Ovarian Cancer Study, G., Australian Ovarian Cancer Study - Quality of Life Study, I., & Webb, P. M. (2011). Physical activity in women with ovarian cancer and its association with decreased distress and improved quality of life. Psychooncology, 20(11), 1161–1169. 10.1002/pon.1834 [DOI] [PubMed] [Google Scholar]
- Beesley VL, Webber K, Nagle CM, DeFazio A, Obermair A, Williams M, Friedlander M, Webb PM, & Group OS (2020). When will I feel normal again? Trajectories and predictors of persistent symptoms and poor wellbeing after primary chemotherapy for ovarian cancer. Gynecol Oncol, 159(1), 179–186. 10.1016/j.ygyno.2020.07.029 [DOI] [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CF 3rd, Monk TH, Berman SR, & Kupfer DJ (1989). The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res, 28(2), 193–213. [DOI] [PubMed] [Google Scholar]
- Chen H, Cohen P, & Chen S (2010). How big is a big odds ratio? Interpreting the magnitudes of odds ratios in epidemiological studies. Communications in Statistics—Simulation and Computation®, 39(4), 860–864. [Google Scholar]
- Chen ML, & Tseng HC (2006). Symptom clusters in cancer patients. Support Care Cancer, 14(8), 825–830. 10.1007/s00520-006-0019-8 [DOI] [PubMed] [Google Scholar]
- Cheong IY, Yoo JS, Chung SH, Park SY, Song HJ, Lee JW, & Hwang JH (2019). Functional loss in daily activity in ovarian cancer patients undergoing chemotherapy. Arch Gynecol Obstet, 299(4), 1063–1069. 10.1007/s00404-018-4996-x [DOI] [PubMed] [Google Scholar]
- Clevenger L, Schrepf A, Degeest K, Bender D, Goodheart M, Ahmed A, Dahmoush L, Penedo F, Lucci J 3rd, Thaker PH, Mendez L, Sood AK, Slavich GM, & Lutgendorf SK (2013). Sleep disturbance, distress, and quality of life in ovarian cancer patients during the first year after diagnosis. Cancer, 119(17), 3234–3241. 10.1002/cncr.28188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demaria M, O’Leary MN, Chang J, Shao L, Liu S, Alimirah F, Koenig K, Le C, Mitin N, Deal AM, Alston S, Academia EC, Kilmarx S, Valdovinos A, Wang B, de Bruin A, Kennedy BK, Melov S, Zhou D, Sharpless NE, Muss H, & Campisi J (2017). Cellular Senescence Promotes Adverse Effects of Chemotherapy and Cancer Relapse. Cancer Discov, 7(2), 165–176. 10.1158/2159-8290.CD-16-0241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donovan KA, Jacobsen PB, Small BJ, Munster PN, & Andrykowski MA (2008). Identifying clinically meaningful fatigue with the Fatigue Symptom Inventory. J Pain Symptom Manage, 36(5), 480–487. 10.1016/j.jpainsymman.2007.11.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitch MI, Mings D, & Lee A (2008). Exploring patient experiences and self-initiated strategies for living with cancer-related fatigue. Can Oncol Nurs J, 18(3), 124–140. 10.5737/1181912x184124131 [DOI] [PubMed] [Google Scholar]
- Forde C Scoring the International Physical Activity Questionnaire (IPAQ). Trinity College Dublin. Retrieved April 30 from https://ugc.futurelearn.com/uploads/files/bc/c5/bcc53b14-ec1e-4d90-88e3-1568682f32ae/IPAQ_PDF.pdf [Google Scholar]
- Given BA, Given CW, Sikorskii A, & Hadar N (2007). Symptom clusters and physical function for patients receiving chemotherapy. Semin Oncol Nurs, 23(2), 121–126. 10.1016/j.soncn.2007.01.005 [DOI] [PubMed] [Google Scholar]
- Hann DM, Denniston MM, & Baker F (2000). Measurement of fatigue in cancer patients: further validation of the Fatigue Symptom Inventory. Quality of Life Research, 9(7), 847–854. 10.1023/a:1008900413113 [DOI] [PubMed] [Google Scholar]
- Hipkins J, Whitworth M, Tarrier N, & Jayson G (2004). Social support, anxiety and depression after chemotherapy for ovarian cancer: a prospective study. Br J Health Psychol, 9(Pt 4), 569–581. 10.1348/1359107042304542 [DOI] [PubMed] [Google Scholar]
- Ho SY, Rohan KJ, Parent J, Tager FA, & McKinley PS (2015). A longitudinal study of depression, fatigue, and sleep disturbances as a symptom cluster in women with breast cancer. J Pain Symptom Manage, 49(4), 707–715. 10.1016/j.jpainsymman.2014.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes CM, McCullough CA, Bradbury I, Boyde C, Hume D, Yuan J, Quinn F, & McDonough SM (2009). Acupuncture and reflexology for insomnia: a feasibility study. Acupunct Med, 27(4), 163–168. 10.1136/aim.2009.000760 [DOI] [PubMed] [Google Scholar]
- Jim HS, Small B, Faul LA, Franzen J, Apte S, & Jacobsen PB (2011). Fatigue, depression, sleep, and activity during chemotherapy: daily and intraday variation and relationships among symptom changes. Ann Behav Med, 42(3), 321–333. 10.1007/s12160-011-9294-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jim HSL, Jacobsen PB, Phillips KM, Wenham RM, Roberts W, & Small BJ (2013). Lagged Relationships Among Sleep Disturbance, Fatigue, and Depressed Mood During Chemotherapy. Health Psychology, 32(7), 768–774. 10.1037/a0031322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchheiner K, Nout RA, Czajka-Pepl A, Ponocny-Seliger E, Sturdza AE, Dimopoulos JC, Dorr W, & Potter R (2015). Health related quality of life and patient reported symptoms before and during definitive radio(chemo)therapy using image-guided adaptive brachytherapy for locally advanced cervical cancer and early recovery - a mono-institutional prospective study. Gynecol Oncol, 136(3), 415–423. 10.1016/j.ygyno.2014.10.031 [DOI] [PubMed] [Google Scholar]
- Kwok OM, Underhill AT, Berry JW, Luo W, Elliott TR, & Yoon M (2008). Analyzing Longitudinal Data with Multilevel Models: An Example with Individuals Living with Lower Extremity Intra-articular Fractures. Rehabil Psychol, 53(3), 370–386. 10.1037/a0012765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee PH, Macfarlane DJ, Lam TH, & Stewart SM (2011). Validity of the International Physical Activity Questionnaire Short Form (IPAQ-SF): a systematic review. Int J Behav Nutr Phys Act, 8, 115. 10.1186/1479-5868-8-115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichstein KL, Durrence HH, Taylor DJ, Bush AJ, & Riedel BW (2003). Quantitative criteria for insomnia. Behav Res Ther, 41(4), 427–445. 10.1016/s0005-7967(02)00023-2 [DOI] [PubMed] [Google Scholar]
- Lin KY, Edbrooke L, Granger CL, Denehy L, & Frawley HC (2019). The impact of gynaecological cancer treatment on physical activity levels: a systematic review of observational studies. Braz J Phys Ther, 23(2), 79–92. 10.1016/j.bjpt.2018.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Fiorentino L, Natarajan L, Parker BA, Mills PJ, Sadler GR, Dimsdale JE, Rissling M, He F, & Ancoli-Israel S (2009). Pre-treatment symptom cluster in breast cancer patients is associated with worse sleep, fatigue and depression during chemotherapy. Psychooncology, 18(2), 187–194. 10.1002/pon.1412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moorey S, Greer S, Watson M, Gorman C, Rowden L, Tunmore R, Robertson B, & Bliss J (1991). The Factor Structure and Factor Stability of the Hospital Anxiety and Depression Scale in Patients with Cancer. British Journal of Psychiatry, 158, 255–259. 10.1192/bjp.158.2.255 [DOI] [PubMed] [Google Scholar]
- Nho JH, Reul Kim S, & Nam JH (2017). Symptom clustering and quality of life in patients with ovarian cancer undergoing chemotherapy. Eur J Oncol Nurs, 30, 8–14. 10.1016/j.ejon.2017.07.007 [DOI] [PubMed] [Google Scholar]
- Norman GR, Sloan JA, & Wyrwich KW (2003). Interpretation of changes in health-related quality of life: the remarkable universality of half a standard deviation. Med Care, 41(5), 582–592. 10.1097/01.MLR.0000062554.74615.4C [DOI] [PubMed] [Google Scholar]
- Prue G, Allen J, Gracey J, Rankin J, & Cramp F (2010). Fatigue in gynecological cancer patients during and after anticancer treatment. J Pain Symptom Manage, 39(2), 197–210. 10.1016/j.jpainsymman.2009.06.011 [DOI] [PubMed] [Google Scholar]
- Puhan MA, Frey M, Buchi S, & Schunemann HJ (2008). The minimal important difference of the hospital anxiety and depression scale in patients with chronic obstructive pulmonary disease. Health Qual Life Outcomes, 6, 46. 10.1186/1477-7525-6-46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinertsen KV, Engebraaten O, Loge JH, Cvancarova M, Naume B, Wist E, Edvardsen H, Wille E, Bjoro T, & Kiserud CE (2017). Fatigue During and After Breast Cancer Therapy-A Prospective Study. J Pain Symptom Manage, 53(3), 551–560. 10.1016/j.jpainsymman.2016.09.011 [DOI] [PubMed] [Google Scholar]
- Stein KD, Martin SC, Hann DM, & Jacobsen PB (1998). A multidimensional measure of fatigue for use with cancer patients. Cancer Pract, 6(3), 143–152. 10.1046/j.1523-5394.1998.006003143.x [DOI] [PubMed] [Google Scholar]
- Stevinson C, Faught W, Steed H, Tonkin K, Ladha AB, Vallance JK, Capstick V, Schepansky A, & Courneya KS (2007). Associations between physical activity and quality of life in ovarian cancer survivors. Gynecologic Oncology, 106(1), 244–250. 10.1016/j.ygyno.2007.03.033 [DOI] [PubMed] [Google Scholar]
- Webber K, Carolus E, Mileshkin L, Sommeijer D, McAlpine J, Bladgen S, Coleman RL, Herzog TJ, Sehouli J, Nasser S, Inci G, & Friedlander M (2019). OVQUEST - Life after the diagnosis and treatment of ovarian cancer - An international survey of symptoms and concerns in ovarian cancer survivors. Gynecol Oncol, 155(1), 126–134. 10.1016/j.ygyno.2019.08.009 [DOI] [PubMed] [Google Scholar]
- Wen Q, Shao Z, Zhang P, Zhu T, Li D, & Wang S (2017). Mental distress, quality of life and social support in recurrent ovarian cancer patients during active chemotherapy. Eur J Obstet Gynecol Reprod Biol, 216, 85–91. 10.1016/j.ejogrb.2017.07.004 [DOI] [PubMed] [Google Scholar]
- Westin SN, Sun CC, Tung CS, Lacour RA, Meyer LA, Urbauer DL, Frumovitz MM, Lu KH, & Bodurka DC (2016). Survivors of gynecologic malignancies: impact of treatment on health and well-being. J Cancer Surviv, 10(2), 261–270. 10.1007/s11764-015-0472-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright F, D’Eramo Melkus G, Hammer M, Schmidt BL, Knobf MT, Paul SM, Cartwright F, Mastick J, Cooper BA, Chen LM, Melisko M, Levine JD, Kober K, Aouizerat BE, & Miaskowski C (2015). Predictors and Trajectories of Morning Fatigue Are Distinct From Evening Fatigue. J Pain Symptom Manage, 50(2), 176–189. 10.1016/j.jpainsymman.2015.02.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright F, Dunn LB, Paul SM, Conley YP, Levine JD, Hammer MJ, Cooper BA, Miaskowski C, & Kober KM (2019). Morning Fatigue Severity Profiles in Oncology Outpatients Receiving Chemotherapy. Cancer Nurs, 42(5), 355–364. 10.1097/NCC.0000000000000626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S, Chu S, Gao Y, Ai Q, Liu Y, Li X, & Chen N (2019). A Narrative Review of Cancer-Related Fatigue (CRF) and Its Possible Pathogenesis. Cells, 8(7). 10.3390/cells8070738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zigmond AS, & Snaith RP (1983). The hospital anxiety and depression scale. Acta Psychiatr Scand, 67(6), 361–370. 10.1111/j.1600-0447.1983.tb09716.x [DOI] [PubMed] [Google Scholar]


