Abstract
Chronic inflammation is highly prevalent among patients receiving maintenance hemodialysis and is associated with morbidity and mortality. Inhibiting inflammation with anti-cytokine therapy has been proposed but not well studied in this population. Therefore, we conducted the ACTION trial, a pilot, multicenter, randomized, placebo-controlled trial of an IL-1 receptor antagonist, anakinra, to evaluate safety, tolerability, and feasibility, and explore efficacy. Eighty hemodialysis patients with plasma concentrations of high sensitivity C-reactive protein (hsCRP) 2 mg/L and above were randomized 1:1 to placebo or anakinra 100 mg, three times per week via the hemodialysis circuit for 24 weeks, with an additional 24 weeks of post-treatment safety monitoring. Efficacy outcomes included change in hsCRP (primary), cytokines, and patient-reported outcomes. Rates of serious adverse events and deaths were similar with anakinra and placebo (serious adverse events: 2.71 vs 2.74 events/patient-year; deaths: 0.12 vs 0.22 events/patient-year). The rate of adverse events of interest (including infections and cytopenias) was significantly lower with anakinra than placebo (0.48 vs 1.40 events/patient-year). Feasibility was demonstrated by attaining the enrollment target, a retention rate of 80%, and administration of 72% of doses. The median decrease in hsCRP from baseline to Week 24 was 41% in the anakinra group and 6% in the placebo group, a between-group difference that was not statistically significant. For IL-6, the median decreases were significant; 25% and 0% in the anakinra and placebo groups, respectively. An effect of anakinra on patient-reported outcomes was not evident. Thus, anakinra was well tolerated and did not increase infections or cytopenias. The promising safety data and potential efficacy on CRP and IL-6 provide support for conducting definitive trials of IL-1 inhibition to improve outcomes in hemodialysis patients.
Keywords: end-stage kidney disease, inflammation, C-reactive protein, IL-1, IL-I receptor antagonist, IL-6
Graphical Abstract
Introduction
The mortality rate for patients undergoing maintenance hemodialysis is unacceptably high with most of the deaths due to cardiovascular disease, infection, or protein-energy wasting1. Randomized controlled trials targeting traditional and non-traditional cardiovascular risk factors have had minimal impact on survival in this population. Inflammatory biomarkers are predictors of cardiovascular events and mortality the hemodilaysis population2–4, and inflammation has been implicated in the pathogenesis of atherosclerosis and protein-energy wasting5–8.
Suppressing inflammation with anti-cytokine therapy has been proposed to reduce inflammation-associated morbidity but there are few studies using this strategy for patients receiving maintenance hemodialysis. High levels of interleukin 1 beta (IL-1β), a highly active pro-inflammatory cytokine and its naturally occurring receptor antagonist (IL-1ra) have been well documented in end-stage kidney disease2, 9, 10. Treatment with IL-1 receptor antagonists is an established approach for rheumatoid arthritis, auto-inflammatory conditions, and gout11–14, but neither the safety nor efficacy of targeting IL-1 for chronic inflammation in the setting of hemodiaysis has been established.
We performed a pilot, double-blind, randomized, placebo-controlled trial to evaluate the safety and tolerability of anakinra, an IL-1 receptor antagonist, for patients receiving maintenance hemodialysis, and to explore its efficacy on biochemical and patient-reported outcomes. An important goal of this early phase study was to assess the feasibility of a subsequent trial powered to assess efficacy on clinical outcomes.
Methods
Design, Oversight, and Population
ACTION was a parallel group, double-blind, randomized, placebo-controlled pilot trial to evaluate safety, tolerability, and feasibility, and explore efficacy of anakinra among hemodialysis patients (NCT03141983). (NCT03141983) The full protocol is available in the Supplementary Materials. Participants were enrolled from dialysis units affiliated with Brigham and Women’s Hospital, Boston, MA; George Washington University, Washington, DC; University of Washington, Seattle, WA; and Vanderbilt University Medical Center, Nashville, TN. An external Data and Safety Monitoring Board (DSMB), appointed by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), approved the protocol and reviewed progress, quality, and safety during trial conduct. The Institutional Review Boards affiliated with the enrolling centers and with the Data Coordinating Center (University of Pennsylvania) approved the protocol. All participants provided written informed consent.
The inclusion criteria included age 18–85 years, treatment with hemodialysis for ≥6 months, and negative testing for human immunodeficiency virus antibody, hepatitis B surface antigen, hepatitis C, and tuberculosis using an interferon gamma release assay. High-sensitivity C-reactive protein (hsCRP) concentration had to be ≥2.0 mg/L at two screening visits, with the second occurring ≤10 days before randomization. In the original protocol the eligibility threshold for hsCRP was ≥3.0 mg/L with a stipulation that the threshold could be lowered to ≥2.0 mg/L if the hsCRP threshold was an important barrier to enrollment. This pre-specified change to the hsCRP eligibility threshold was implemented on May 23, 2018 after 20 participants had been randomized. Women of child-bearing potential were required to have a negative serum pregnancy test and use an approved method of birth control until 4 weeks after treatment with the study drug.
The major exclusion criteria included current or anticipated use of a hemodialysis central venous catheter, acute bacterial infection within 60 days before screening unless treated with antibiotics and resolved, chronic bacterial infection, hospitalization within 30 days unless for a vascular access procedure, malignancy within 5 years other than basal or squamous cell carcinoma of the skin, cirrhosis, use of an immunosuppressive drug within the past 3 months, receipt of a live vaccine within the past 3 months, absolute neutrophil count <2,500 cells/mm3, and platelet count <100,000 cells/mm3. The neutrophil and platelet counts were measured from blood obtained at a screening visit.
Randomization and Intervention
Participants were randomized to placebo or anakinra in a 1:1 distribution using random permuted blocks of 4 or 6, with stratification by presence or absence of diabetes and enrolling center. Randomization was performed via a web-based system and participants and all research personnel were masked to treatment assignment. Study drug (anakinra or placebo) was administered via the hemodialysis blood circuit over 1 minute during the last 30 minutes of the dialysis session, 3 times per week for 24 weeks. This dosing approach was based on a pharmacokinetic study indicating that in hemodialysis the same dose can be used for intravenous and subcutaneous administration routes15. After the study drug treatment period participants were followed for an additional 24 weeks for further assessment of safety. Participants who discontinued study drug early were followed for the full 48 weeks.
Temporary discontinuation of study drug was required for non-serious infection, neutrophil count 500 cells/mm3 to <1000 cells/mm3, platelet count 25,000 to <75,000 cells/mm3, and use of a central venous dialysis catheter. Permanent discontinuation of study drug was required for serious infection, neutrophil count <500 cells/mm3, platelet count <25,000 cells/mm3, allergy to study drug, new malignancy, or initiation of immunosuppressive therapy.
Outcomes
Safety was assessed through monitoring of adverse events throughout the 24-week treatment period and the 24-week post-treatment follow-up period, with particular attention to pre-specified adverse events of interest that included infections, cytopenias, and hypersensitivity reactions. Tolerability was assessed by the need for study drug discontinuation due to a drug-related indication. Feasibility of a subsequent larger trial was assessed by recruitment, retention, and adherence to the study drug administration schedule. The primary measure of efficacy was the change in the concentration of hsCRP, a pharmacodynamic marker of IL-1 inhibition, over the 24-week treatment period. Secondary efficacy outcomes included additional circulating markers of inflammation, patient reported outcomes, and hand-grip strength. Patient reported outcomes were ascertained centrally at the University of New Mexico using computer-assisted telephone interviewing (Supplementary Table S1).
Laboratory Measurements
Blood was collected pre-dialysis at two screening visits, the baseline visit, and follow-up visits every 4 weeks. Serum and EDTA-treated plasma were stored at −80° C in 500 μl aliquots for batched measurements. For eligibility determination the two hsCRP measurements were performed in real time at the local laboratories affiliated with each enrolling site. Aliquots of plasma from the screening visits, as well as the baseline and follow-up visits, were stored for subsequent batched measurements of hsCRP performed at the University of Colorado-Denver using a nephelometric assay (Siemens). For outcome determination, the baseline CRP value was derived from the average of the central laboratory measurements from the two screening samples and the baseline sample. The central laboratory determined plasma concentrations of Interleukin-6, Interleukin-10, Interleukin 1β, and TNFα using multiplex enzyme-linked immunoassay assays (Quanterix) performed in duplicate, and serum concentrations of albumin using an enzymatic assay (Beckman Coulter). All measurements on batched samples were performed in duplicate. White blood cells and platelets were quantified at local clinical laboratories in real time for safety monitoring.
Eight participants at the Vanderbilt site participated in a pharmacokinetic study in which blood was collected at the following times:1) start of dialysis on Day 1 of study drug administration, 2) end of dialysis on Day 1 of study drug administration, 3) approximately 24 hours after sample 1, 4) start of dialysis on Day 2 of study drug administration (approximately 48 hours after sample 1), and 5) end of dialysis on Day 2 of study drug administration. The same sampling schedule was implemented at Week 4. EDTA-treated plasma was stored at −80° C in 500 μl aliquots for measurement of anakinra concentrations that was performed at Vanderbilt University Medical Center from batched samples using an enzyme-linked immunoassay assay (R&D Systems).
Statistical Analysis and Sample Size Determination
The primary analyses were based on intention to treat populations with all participants analyzed based on the randomized group assignment. On treatment analyses that included participants who had received study drug (anakinra or placebo) within 3 days prior to blood collection were performed as sensitivity analyses for efficacy outcomes. Study drug exposure was determined from records documenting each administration of study drug. Outcomes are presented as mean and standard deviation for normally distributed values, median and 25th-75th percentile for non-normally distributed values, number and percent, or event rate. P values for comparisons between the anakinra and placebo groups on efficacy endpoints measured repeatedly were determined using linear mixed effects models with adjustment for fixed effects of the stratification factors (diabetes and enrolling center) and random intercepts of participants. Generalized linear models, with adjustment for fixed effects of the stratification factors, were used with Poisson distribution for events that can occur repeatedly, binomial distribution for binary and incidence measures, and Gaussian distribution for continuous measures. Given the pilot nature of the study with a focus on safety, no corrections were made for multiple comparisons. All analyses were performed using SAS version 9.4 (SAS Institute Inc.) and lme4 packages in R version 4.0.2 (https://www.r-project.org). From a prior preliminary study, we assumed a mean hsCRP at baseline of 9.5 ± 4 mg/L and log-transformed mean hsCRP at baseline of 2.14 ± 0.55 log(mg/L)16.
We selected a sample size of 80 participants (40 patient per arm) with the expectation that it would provide 80% power to detect an effect size of 0.6SD (equivalent to 0.33 log(mg/L)) difference between treatment arms in the change from baseline to Week 24 in log-transformed hsCRP assuming a correlation of 0.6 between pre- and post-treatment hsCRP in the placebo group, and a withdrawal rate of 10% by the end of the 24 week treatment period. The assumption for the hsCRP correlation was based on a study of 50 healthy men and on data from a cohort of 128 hemodialysis patients with monthly hsCRP measurements17, 18.
Results
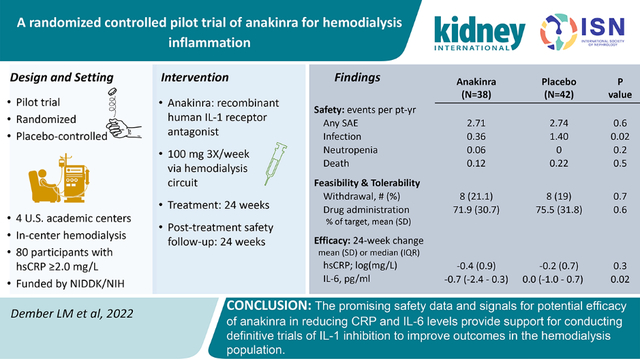
Participants
Between December 6, 2017 and October 1, 2019, 176 patients provided informed consent for the trial and underwent eligibility screening (Figure 1). Eighty patients met the eligibility criteria and were randomized to anakinra (N=38) or placebo (N=42). The most frequent reasons for ineligibility were an absolute neutrophil count <2500 cells/mm3, or at least one of the two screening hsCRP values below the required threshold. The baseline characteristics were similar across randomized groups except for a slightly higher age among participants randomized to anakinra (59.6 ± 10 versus 54.1 ± 13.5 years) (Table 1). The majority of participants (58.8%) were Black and the median duration of treatment with dialysis was 4.4 years (25th-95th percentile 1.9 – 7.2 years). Due to the emerging COVID-19 pandemic, a decision was made to stop administering study drug on March 13, 2020 at which time 3 participants had 1–3 doses of study drug that had not yet been administered; all other participants had completed the 24-week treatment period. Follow-up for the trial ended on September 8, 2020. All randomized participants were included in the safety analyses. For the primary analyses exploring efficacy, 1 participant randomized to placebo was excluded because there were no follow-up hsCRP measurements.
Figure 1.
Participant enrollment and follow-up
Table 1.
Baseline Characteristics
| All (N=80) | Anakinra (N=38) | Placebo (N=42) | |
|---|---|---|---|
|
| |||
| Age, year | 56.7 (12.2) | 59.6 (10.0) | 54.1 (13.5) |
|
| |||
| Black, No. (%) | 47 (58.8) | 23 (60.5) | 24 (57.1) |
| White, No. (%) | 24 (30.0) | 10 (26.3) | 14 (33.3) |
| Asian, No. (%) | 3 (3.8) | 2 (5.3) | 1 (2.4) |
|
| |||
| Hispanic/Latino, No. (%) | 8 (10.0) | 5 (13.2) | 3 (7.1) |
|
| |||
| BMI, kg/m2 | 33.2 (8.1) | 33.7 (8.6) | 32.8 (7.7) |
|
| |||
| Pre-dialysis systolic BP, mm Hg | 145.0 (23.6) | 145.1 (23.4) | 144.9 (24.1) |
|
| |||
| Pre-dialysis diastolic BP, mm Hg | 77.8 (17.6) | 77.9 (15.6) | 77.7 (19.4) |
|
| |||
| Hypertension, No. (%) | 72 (90.0) | 34 (89.5) | 38 (90.5) |
|
| |||
| Diabetes mellitus, No. (%) | 45 (56.2) | 22 (57.9) | 23 (54.8) |
|
| |||
| Coronary artery disease, No. (%) | 24 (30.0) | 11 (28.9) | 13 (31.0) |
|
| |||
| Congestive heart failure, No. (%) | 20 (25.0) | 9 (23.7) | 11 (26.2) |
|
| |||
| Hyperlipidemia, No. (%) | 37 (46.2) | 20 (52.6) | 17 (40.5) |
|
| |||
| Current tobacco use, No. (%) | 6 (7.5) | 4 (10.5) | 2 (4.8) |
|
| |||
| AV fistula, No. (%) | 67 (83.8) | 31 (81.6) | 36 (85.7) |
| AV graft, No. (%) | 13 (16.2) | 7 (18.4) | 6 (14.3) |
|
| |||
| Dialysis vintage, year | 4.43 (1.85 – 7.23) | 4.32 (1.74 – 6.50) | 4.56 (2.10 – 7.67) |
|
| |||
| Dialysis <1 year, No. (%) | 14 (17.5%) | 6 (15.8%) | 8 (19.0) |
|
| |||
| Single pool Kt/V | 1.5 (0.2) | 1.6 (0.2) | 1.5 (0.2) |
|
| |||
| Statin use, No. (%) | 33 (41.2) | 17 (44.7) | 16 (38.1) |
|
| |||
| Anti-platelet agent use, No. (%) | 40 (50.0) | 22 (57.9) | 18 (42.9) |
|
| |||
| Hemoglobin, g/dL | 11.0 (10.5 – 11.8) | 11.3 (10.5 – 12.2) | 10.7 (10.3 – 11.6) |
|
| |||
| White blood cells × 109 per L | 6.9 (6.2 – 8.0) | 6.8 (6.3 – 7.5) | 6.9 (6.1 – 8.4) |
|
| |||
| Neutrophils × 109 per L | 4.45 (3.52 – 5.45) | 4.41 (3.66 – 5.12) | 4.55 (3.51 – 5.59) |
|
| |||
| Platelets, × 109 per L | 224.5 (187.9 – 281.5) | 225.5 (199.2 – 261.4) | 222.8 (180.0 – 282.5) |
|
| |||
| Creatinine, mg/dL | 9.3 (7.7 – 11.6) | 9.3 (7.5 – 10.9) | 9.4 (8.0 – 11.7) |
|
| |||
| Albumin, g/dL | 3.9 (0.3) | 3.9 (0.3) | 3.9 (0.2) |
|
| |||
| hsCRP, mg/L1 | 7.2 (5.4 – 16.0) | 7.2 (5.4 – 15.8)2 | 7.2 (5.8 – 16.0)3 |
Values shown are mean (SD), or median (25th- 75th percentile) unless otherwise indicated
Abbreviations: No., number; BMI, body mass index; AV, arteriovenous; hsCRP, high-sensitivity C-reactive protein
hsCRP value is the average of values from the Screening 1, Screening 2, and Baseline visits from the batched measurements performed by the central laboratory. If all three samples (Screening 1, Screening 2, and Baseline) were not available for the centralized measurements, the average of the available samples was used.
For 35 participants, the average was based on 3 measurements, for 2 participants the average was based on 2 measurements, and for 1 participant the average was based on 1 measurement.
For 33 participants, the average was based on 3 measurements, for 5 participants the average was based on 2 measurements, and for 4 participants the average was based on 1 measurement.
Feasibility and Tolerability
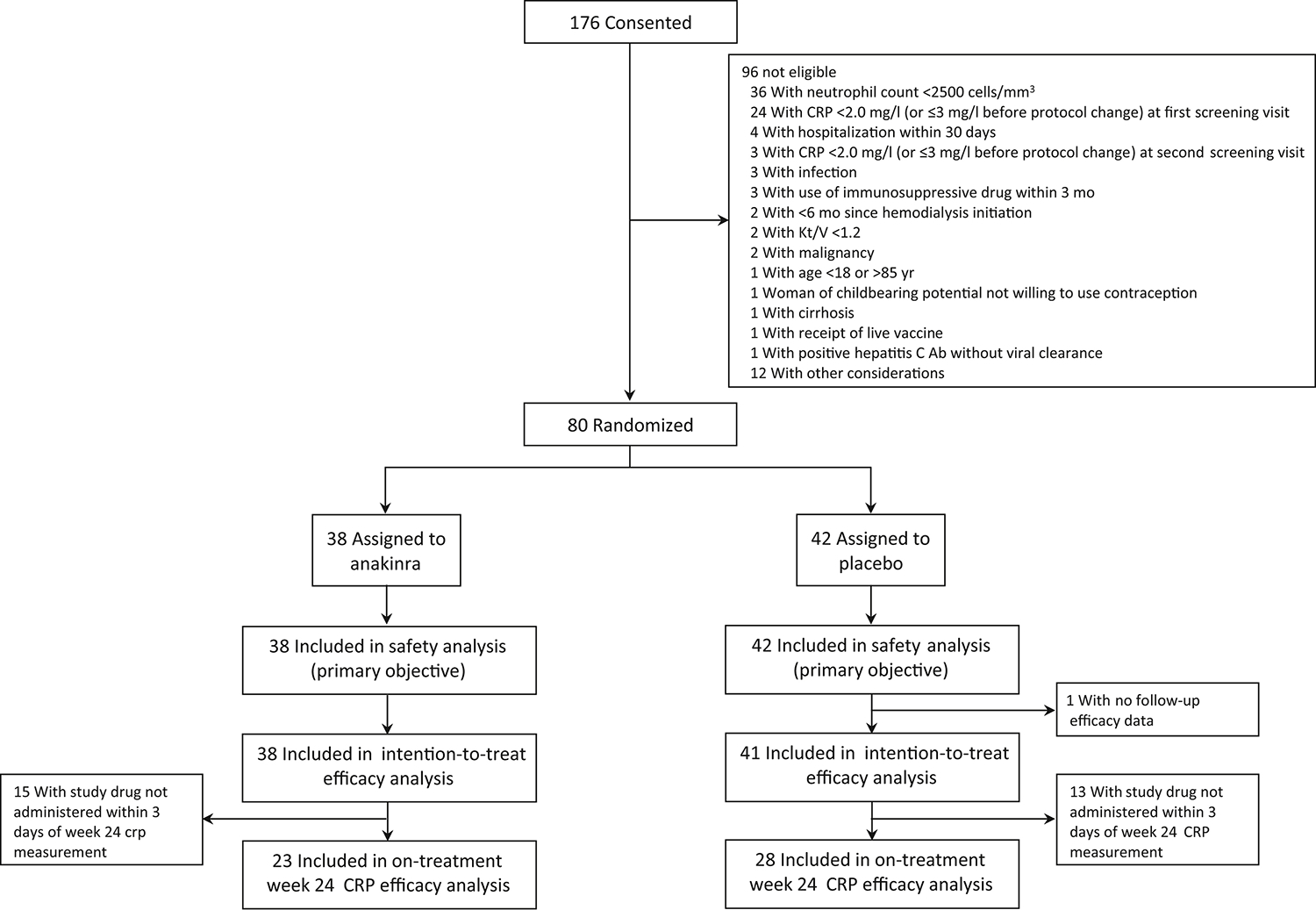
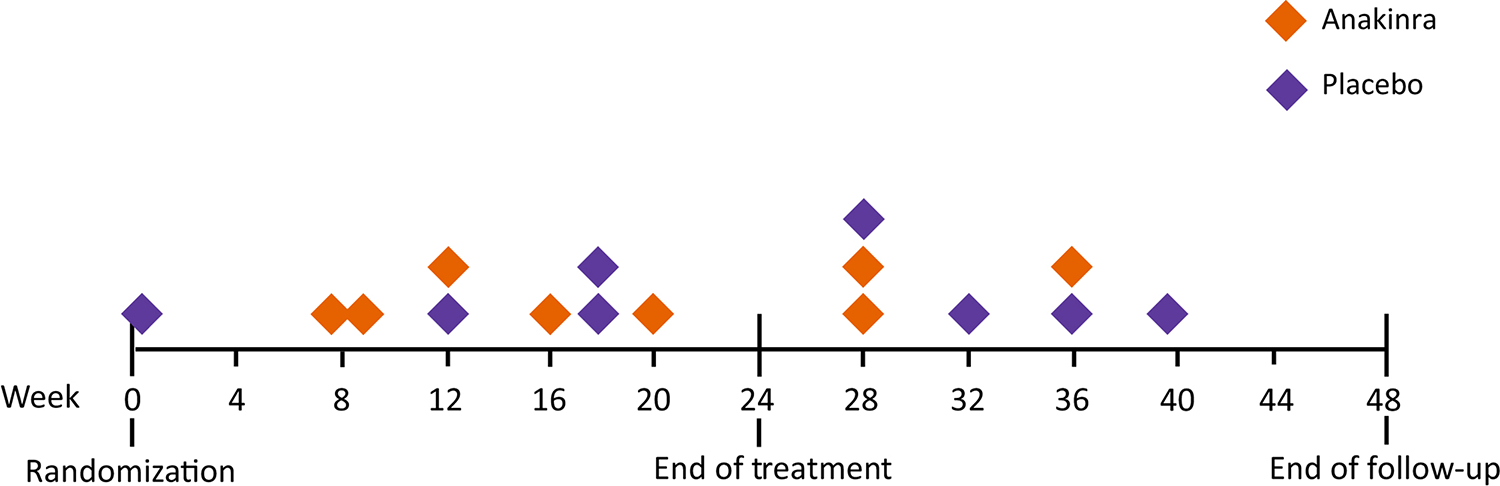
Participant retention and study drug exposure are shown in Table 2. Eight participants (21.1%) in the anakinra group and 8 (19.0%) in the placebo group withdrew from the trial; 5 of the withdrawals in the anakinra group and 4 of the withdrawals in the placebo group occurred during the 24-week treatment period (Figure 2). The most common reasons for withdrawal were death (N=6) and kidney transplantation (N=6). Study drug was permanently discontinued for 12 participants (31.6%) in the anakinra group and 11 participants (26.2%) in the placebo group (Table 2 and Figure 3). The most frequent reason for study drug discontinuation was a decision by the participant (N=5 in the anakinra group; N=3 in the placebo group) without a medical indication for stopping study drug. Study drug was permanently discontinued for a medical reason (infection, cytopenia, use of a prohibited medication, placement of a central venous catheter, or kidney transplantation) by 4 participants in the anakinra group and 5 participants in the placebo group. Study drug exposure was 71.9% of target and 75.5% of target in the anakinra and placebo groups, respectively, with the target defined as administration three times per week for the full 24 weeks (Table 2). Administration of expected doses, defined as those administrations that were intended, with administrations during period of temporary or permanent study drug discontinuation excluded from the denominator, was 94.6% and 94.9% in the anakinra and placebo groups, respectively.
Table 2.
Withdrawal and Study Drug Discontinuation
| Anakinra (N=38) | Placebo (N=42) | P-value | |
|---|---|---|---|
|
| |||
| Withdrawal from trial, No. (%) | 8 (21.1) | 8 (19.0) | 0.7 |
| Kidney transplantation | 3 | 3 | |
| Transfer to non-participating dialysis unit | 1 | 0 | |
| Participant decision | 1 | 0 | |
| Investigator decision | 0 | 1 | |
| Death | 2 | 4 | |
| Other1 | 1 | 0 | |
|
| |||
| Study drug permanent discontinuation, No. (%) | 12 (31.6) | 11 (26.2) | 0.6 |
| Infection | 1 | 1 | |
| Central venous dialysis catheter | 0 | 1 | |
| Allergy | 1 | 0 | |
| Cytopenia | 1 | 0 | |
| Kidney transplantation | 1 | 2 | |
| Initiation of prohibited medication | 0 | 1 | |
| Transfer to non-participating dialysis unit | 2 | 0 | |
| Participant decision | 5 | 3 | |
| Investigator decision | 1 | 1 | |
| Other | 0 | 2 | |
|
| |||
| Study drug temporary discontinuation, No (%) | 13 (34.2) | 12 (28.6) | 0.6 |
|
| |||
| % Study drug administration, mean (SD) | |||
| % of target dose2 | 71.9 (30.7) | 75.5 (31.8) | 0.6 |
| % of expected dose3 | 94.6 (10.8) | 94.9 (8.4) | 0.9 |
One participant was withdrawn at Week 4 of previously unrecognized pre-randomization neutrophil count <2,500 cells/mm3
Target dose is the total dose that would be administered if a participant remained on study drug for the full 24 weeks and is an indication of exposure to study drug
Expected dose is the total dose that should be administered while a participant is being followed and while study drug has not been temporarily or permanently discontinued and is an indication of protocol fidelity
Figure 2.
Timing of study withdrawal by treatment group
Figure 3.
Timing of permanent discontinuation of study drug by treatment group
Safety
Adverse events are summarized in Table 3. The total number of adverse events was 96 in the anakinra group and 129 in the placebo group. For serious adverse events, both the proportion of participants with one or more events, and the event rates were similar in the anakinra and placebo groups. Six participants died during the trial, 2 in the anakinra group and 4 in placebo group. None of the deaths in either group was thought by the site investigator to be related to study drug. The two deaths among participants randomized to anakinra were due to cardiac arrest during a cardiac catheterization procedure at Week 8 of anakinra administration, and COVID-19 at 25 weeks after discontinuation of study drug.
Table 3.
Adverse Events During Either the Study Drug or Post-Study Drug Administration Period
| Anakinra (N=38) | Placebo (N=42) | P-value for # pts w/event | P-value for event rate | ||||
|---|---|---|---|---|---|---|---|
| Pts w/event No. (%) | Events per pt-year | Pts w/event No. (%) | Events per pt-year | ||||
| Any Serious Adverse Event | 17 (44.7) | 2.71 | 23 (54.8) | 2.74 | 0.3 | 0.6 | |
| Deaths | 2 (5.3) | 0.12 | 4 (9.5) | 0.22 | 0.5 | 0.5 | |
| AEs of Interest | Any AE of Interest | 7 (18.4) | 0.48 | 11 (26.2) | 1.40 | 0.4 | 0.01 |
| Infection1 | 5 (13.2) | 0.36 | 11 (26.2) | 1.40 | 0.1 | 0.002 | |
| Non-serious infection2 | 3 (7.9) | 0.18 | 6 (14.3) | 0.43 | 0.3 | 0.1 | |
| Pneumonia | 0 (0) | 0 | 4 (9.5) | 0.38 | 0.1 | 0.003 | |
| Bacteremia | 1 (2.6) | 0.06 | 0 (0) | 0 | 0.5 | 0.2 | |
| Systemic fungal infection | 0 (0) | 0 | 0 (0) | 0 | -- | -- | |
| CNS infection | 0 (0) | 0 | 0 (0) | 0 | -- | -- | |
| Infection-associated sepsis | 0 (0) | 0 | 2 (4.8) | 0.11 | 0.5 | 0.1 | |
| Infection-associated death | 1 (2.6) | 0.06 | 1 (2.4) | 0.05 | 0.9 | 0.9 | |
| Other serious infection | 2 (5.3) | 0.12 | 5 (11.9) | 0.38 | 0.2 | 0.1 | |
| Neutropenia3, 4 | 1 (2.6) | 0.06 | 0 (0) | 0 | 0.5 | 0.2 | |
| Thrombocytopenia5 | 0 (0) | 0 | 0 (0) | 0 | -- | -- | |
| Hypersensitivity reaction | 1 (2.6) | 0.06 | 0 (0) | 0 | 0.5 | 0.2 | |
A single infection adverse event may be categorized under more than one of type of infection
Infection that did not require hospitalization for >2 days
Defined as absolute neutrophil count <1000/mm3
Occurred in participant who was found at Week 4 to have been ineligible based on pre-randomization neutrophil count
Defined as platelet count <75,000/mm3
The pre-specified adverse events of interest are shown in Table 3. The rate of adverse events of interest was lower in the anakinra group (0.48 per patient-year) than in the placebo group (1.40 per patient-year; p=0.01). Most of the adverse events of interest were infections. Fewer infections occurred in the anakinra group than the placebo group (6 events among 5 participants vs 26 events among 11 participants, yielding rates of 0.36 in the anakinra group vs 1.40 events per patient-year in the placebo group; p=0.002). There was one hypersensitivity reaction in the anakinra group that manifested as urticaria during Week 5 of drug administration. The participant was treated with an antihistamine medication and study drug was permanently discontinued. There was one neutropenia event in the anakinra group that was identified at Week 4 from the complete blood count performed for safety monitoring. On further review of pre-trial laboratory studies, it became evident that the participant had a neutrophil count below the lower limit required for eligibility. The participant was withdrawn from further participation because of ineligibility that had been previously unrecognized. The neutrophil count for the participant increased to >1000 cells/mm3 within 1 week after study drug discontinuation. The monitoring of complete blood counts every 4 weeks revealed 9 participants, 7 in the anakinra group and 2 in the placebo group, with a neutrophil count on at least 1 occasion between 1000 and 2000 cells/m3, values above the neutropenia threshold and thus not requiring study drug discontinuation. None of these participants with neutrophil counts between 1000 and 2000 cells/m3 subsequently developed neutropenia. There were no thrombocytopenia events. Among 6 participants, 2 in the anakinra group and 4 in the placebo group, with platelet counts between 75,000 and 120,000 on at least one weekly measurement during the study drug administration period, none developed thrombocytopenia, defined as <50,000 cells/mm3, despite continuing study drug.
Efficacy
The efficacy outcomes were considered exploratory with a goal of detecting signals rather than definitive evidence. As shown in Table 4, the mean decrease from Baseline to Week 24 in log-transformed hsCRP concentration was 0.4 in the anakinra group and 0.2 in the placebo group (p=0.3) or, using the original data, 2.8 mg/L in the anakinra group compared with 0.4 mg/L in the placebo group (p=0.3) (see also Supplementary Figure S1). The change from Baseline at each time point for the intention to treat and the on treatment populations is shown in Table 5, and at the individual participant level in Supplementary Figure S2. In the intention to treat populations, there were statistically significant differences favoring anakinra at Weeks 4 and 12, and non-statistically significant signals of efficacy at Weeks 8, 16, 20, and 24. The differences between anakinra and placebo groups were numerically greater for the on treatment compared with the intention to treat populations at most of the time points, and a statistically significant effect of anakinra was evident at Weeks 4, 8, 12, and 16. Among the other cytokines measured, IL-6 decreased over the 24-week treatment period by 0.7 pg/ml in the anakinra group and was unchanged in the placebo group (p=0.02) (Table 4). There were not signals for efficacy of anakinra on the patient reported outcomes or hand grip strength. Differences in efficacy were not apparent between participants who met the original eligibility criteria of both screening hsCRP values >3 mg/L (N=68) and those (N=12) who only met the revised criteria (both screening hsCRP values ≥2 mg/L) (data not shown). Anakinra concentrations obtained from the pharmacokinetic study are shown in Supplementary Figure S3.
Table 4.
Efficacy Outcomes
| Anakinra (N=38) | Placebo (N=42) | P-value | |||||
|---|---|---|---|---|---|---|---|
| Baseline | Week 24 | Change1 | Baseline | Week 24 | Change1 | ||
| Primary Measure | |||||||
| hsCRP, log(mg/L) | 2.2 (0.9) | 1.8 (1.3) | −0.4 (0.9) | 2.1 (0.7) | 1.9 (0.9) | −0.2 (0.7) | 0.3 |
| hsCRP, mg/L | 6.8 (4.7 – 16.6) | 6.8 (2.1 – 13.5) | −2.8 (−5.4 – 2.3) | 7.2 (5.4 – 15.9) | 7.5 (4.0 – 11.8) | −0.4 (−3.3 – 2.1) | 0.3 |
| Secondary Measures | |||||||
| IL-1β pg/ml | 0.0 (0.0 – 0.1) | 0.1 (0.0 – 0.2) | 0.0 (0.0 – 0.1) | 0.0 (0.0 – 0.1) | 0.0 (0.0 – 0.1) | 0.0 (0.0 – 0.0) | 0.1 |
| IL-6, pg/mL | 2.8 (1.1 –5.5) | 1.9 (1.1 –3.0) | −0.7 (−2.4 –−0.3) | 2.0 (1.0 – 4.4) | 2.1 (0.8 – 3.9) | 0.0 (−1.0 – 0.7) | 0.02 |
| IL-10, pg/mL | 0.5 (0.3 – 0.7) | 0.5 (0.3 – 0.6) | 0.0 (−0.1 – 0.1) | 0.4 (0.2 – 0.6) | 0.4 (0.2 – 0.7) | 0.0 (−0.1 – 0.1) | 0.2 |
| TNFα, pg/ml | 3.4 (1.8 – 5.5) | 3.2 (1.8 – 4.7) | −0.2 (−1.0 – 0.1) | 2.6 (2.1 – 4.9) | 2.8 (1.5 – 4.8) | 0.0 (−0.3 – 0.7) | 0.1 |
| Albumin, g/dL | 4.0 (3.6 – 4.2) | 3.9 (3.7 – 4.1) | 0.0 (−0.2 – 0.1) | 3.9 (3.7 – 4.1) | 3.9 (3.8 – 4.1) | 0.0 (−0.2 – 0.2) | 0.4 |
| Beck Depression Index-II | 11.0 (8.9) | 10.1 (9.6) | −0.9 (5.7) | 11.6 (8.0) | 11.5 (8.6) | −0.1 (7.5) | 0.8 |
| FACIT-Fatigue | 34.5 (11.7) | 29.6 (15.6) | −4.9 (12.1) | 32.4 (11.1) | 32.0 (12.7) | −0.4 (8.7) | 0.1 |
| KDQOL-SF36 Physical Functioning | 65.6 (24.3) | 62.1 (25.6) | −3.5 (21.5) | 64.5 (30.5) | 61.2 (28.0) | −3.3 (14.2) | 0.8 |
| Dialysis Symptom Index, Burden | 10.4 (6.8) | 10.6 (6.9) | 0.2 (5.6) | 11.1 (6.1) | 11.4 (6.2) | 0.3 (3.8) | 0.8 |
| Dialysis Symptom Index, Severity | 37.6 (31.4) | 33.7 (22.7) | −4.0 (24.4) | 35.0 (20.1) | 34.7 (19.2) | −0.3 (15.1) | 0.6 |
| Hand grip strength2 | 23.4 (9.4) | 22.9 (10.0) | −0.5 (4.1) | 25.4 (12.1) | 25.0 (11.9) | −0.4 (3.9) | 0.6 |
Values shown are mean (SD) or median (25th – 75th percentile) unless otherwise indicated. Baseline value for CRP differs from value in Table 1 because only participants with values at both Baseline and Week 24 are included in the analyses of change.
References for the patient-reported outcome instruments are provided with Supplementary Table S1.
The change between Baseline and Week 24 was calculated for each participant and displayed as either the mean (SD) of the change or the median (25th-75th percentile) of the change. Because the median values at 1) Baseline, 2) Week 24, and 3) change from Baseline to Week 24 are not necessarily from the same participant, for changes reported as medians, the direction and/or magnitude of the change may not be the same as the difference between the median values at the two time points.
Measured using the dominant hand
Abbreviations: hsCRP, high-sensitivity C-reactive protein; IL-1β, Interleukin 1 beta; IL-6, Interleukin 6; IL-10, Interleukin 10; TNFα, tumor necrosis factor alpha; KDQOL-SF 36, Kidney Disease Quality of Life Short Form 36.
Table 5.
hsCRP by Week: Intention to Treat and On Treatment Populations
| Intention to Treat1 | On Treatment2 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Anakinra | Placebo | P Value | Anakinra | Placebo | P Value | |||||
| N | Mean Change from Baseline Log(mg/L) | N | Mean Change from Baseline Log(mg/L) | N | Mean Change from Baseline Log(mg/L) | N | Mean Change from Baseline Log(mg/L) | |||
| Week 4 | 38 | −0.5 (0.8) | 40 | −0.0 (1.0) | 0.02 | 33 | −0.6 (0.8) | 35 | −0.1 (0.9) | 0.01 |
| Week 8 | 37 | −0.7 (0.8) | 41 | −0.3 (0.9) | 0.2 | 32 | −0.8 (0.7) | 34 | −0.3 (0.9) | 0.04 |
| Week 12 | 35 | −0.8 (0.8) | 39 | −0.3 (0.9) | 0.02 | 26 | −0.9 (0.8) | 32 | −0.2 (0.9) | 0.005 |
| Week 16 | 32 | −0.6 (0.7) | 39 | −0.3 (0.9) | 0.3 | 22 | −0.9 (0.6) | 28 | −0.4 (0.7) | 0.01 |
| Week 20 | 30 | −0.5 (1.0) | 36 | −0.3 (0.7) | 0.3 | 23 | −0.7 (1.0) | 28 | −0.3 (0.6) | 0.1 |
| Week 24 (end of treatment) | 30 | −0.4 (0.9) | 37 | −0.2 (0.7) | 0.3 | 22 | −0.6 (0.9) | 26 | −0.1 (0.7) | 0.1 |
| Week 28 (post treatment) | 30 | 0.1 (0.9) | 36 | −0.3 (1.0) | 0.1 | -- | -- | -- | -- | |
Abbreviations: hsCRP, high-sensitivity C-reactive protein
hsCRP value included regardless of whether participant was receiving study drug
hsCRP values included only if participant had received study drug within the prior 3 days
Discussion
In this randomized, placebo-controlled pilot study, we evaluated the safety, tolerability, feasibility, and efficacy of IL-1 inhibition as a strategy for reducing inflammation among patients treated with maintenance hemodialysis. We randomized 80 patients to treatment with anakinra or placebo for 24 weeks followed by an additional 24-week post-treatment safety monitoring period. We found that anakinra was well tolerated and that neither overall serious adverse events nor pre-specified adverse events of interest occurred with greater frequency with anakinra than with placebo. The intention to treat analysis of the change in hsCRP showed signals for efficacy of anakinra on this pharmacodynamic marker. The impact of temporary discontinuations of anakinra on the hsCRP outcome, as illustrated by the nominally greater reductions in hsCRP in the on treatment compared with the intention to treat populations, is not surprising given the short half-life of the drug and its known pharmacodynamic properties.
While definitive conclusions about safety cannot be drawn from a small trial, the safety findings are reassuring and suggest that proceeding with studying anakinra further in a larger, clinical outcomes trial would be reasonable from a safety standpoint. The overall rate of infections, the adverse event that was of greatest concern before conducting the trial, was higher in the placebo group than in the anakinra group, and there was not a difference between groups in serious infections. There were no thrombocytopenia events, and the single neutropenia event occurred in a participant who had a neutrophil count that was low before exposure to anakinra. One hypersensitivity reaction, which manifested as urticaria, occurred after 4 weeks of treatment with anakinra and required discontinuation of the drug.
Feasibility was assessed based on eligibility rates, retention, and study drug exposure. Among patients who consented to the trial, 45% met the eligibility criteria ascertained at the screening and baseline visits and proceeded to randomization. The most frequent reason for ineligibility, a screening neutrophil count <2500 cells/mm3, occurred in 28 (15.9%) of consented patients. The finding that neutropenia did not occur during anakinra exposure among any of the participants who met the neutrophil count eligibility criterion, even among those who had a reduction in neutrophil count to ≤1000 cells/ mm3, raises the question of whether our threshold for eligibility was overly stringent. The finding of no thrombocytopenia events with anakinra raises a similar question, although thrombocytopenia was not a major barrier to enrollment. The decision to implement the pre-specified change to the hsCRP threshold for eligibility from ≥3.0 mg/L to ≥2.0 mg/L is informative for future trials. Throughout the full duration of the trial, 27 of 176 patients (15.3%) who consented to participate did not proceed to randomization because the hsCRP concentration did not meet the threshold at both screening visits, and among the 60 participants who met the eligibility criteria after the change to the CRP criterion was made, 12 (20%) of those randomized after the change had a screening hsCRP value between 2 and 3 mg/L rather than >3 mg/L. Retention in the trial was consistent with the assumptions used for the sample size determination, and 12 of the 16 withdrawals (75%) were due to kidney transplantation or death. Only 1 withdrawal was due to participant preference suggesting that the trial was not overly burdensome to patients. The study drug discontinuations were, for the most part, not related to adverse effects of anakinra, suggesting that the drug was well tolerated.
While we saw signals for efficacy of anakinra on hsCRP and IL-6 levels we did not observe effects on the patient-reported outcomes or hand grip strength. We were interested in these outcomes because of known associations between inflammation and fatigue, depression, and muscle wasting19. The impact that temporary discontinuations of study drug had on the hsCRP outcome raises the question of whether IL-1 inhibition with a longer acting agent such as canakinumab, a monoclonal antibody that selectively targets IL-1β, would be preferable for future studies.
In the one previous trial of IL-1 inhibition for patients treated with maintenance hemodialysis, Hung and colleagues randomized 14 patients to anakinra, 100 mg administered subcutaneously 3 times per week, or placebo and found reductions in hsCRP and IL-6, after a 4-week administration period, that were similar in magnitude to those we observed16. In the prior study two of the 7 participants in the anakinra group had injection site reactions requiring study drug discontinuation at day 14. Injection-associated pain related to the drug vehicle and injection site reactions are known complications of anakinra. Anticipating the impact on tolerability and safety of subcutaneous administration we administered anakinra via the hemodialysis extracorporeal circuit, an approach that likely improved the adverse effect profile.
IL-1 inhibition has also been evaluated in the non-dialysis CKD population. Nowak and colleagues randomized 47 patients with stage 3 or 4 CKD to treatment with rilonacept, a soluble IL-1 decoy receptor, or placebo for 12 weeks and found reductions in CRP and improvements in brachial artery flow-mediated dilation, a marker of endothelial function20. Inhibition of IL-6, an inflammatory cytokine stimulated by IL-1β, has recently been evaluated in non-dialysis CKD. A phase 2 trial of multiple dosages of ziltivekimab, a monoclonal antibody directed at IL-6, was studied among 242 individuals with stage 3–5 CKD and a CRP concentration ≥2 mg/L21. Dose-dependent reductions in CRP and other inflammatory markers were observed during a 24-week treatment period, and the drug was well tolerated. Ziltivekimab was also found to substantially reduce CRP in a phase 1/2 dose-finding trial of 61 maintenance hemodialysis patients that was primarily designed to evaluate safety22. Neither our trial, nor the previous studies of anti-cytokine therapy in dialysis or pre-dialysis CKD evaluated clinical outcomes, and the relative advantages of IL-1 versus IL-6 inhibition in the setting of maintenance hemodialysis are not known.
Limitations of our study include a modest imbalance in age between the groups and lack of generalizability of the safety data to patients with central venous catheters. Our study has important strengths including its multicenter design, rigorous assessment of safety outcomes using standardized criteria, use of centralized, batched measurements of the hsCRP and cytokine efficacy indicators, centralized ascertainment of the patient-reported outcomes by individuals who were not members of the local research teams, and a substantial post-treatment follow-up period to identify late safety concerns.
In conclusion, we found that among hemodialysis patients with low-level inflammation the IL-1 receptor antagonist, anakinra, was well tolerated and without increased risks of infection or cytopenias compared with placebo. The promising safety data and the signals for efficacy on CRP and IL-6 provide support for moving forward with larger, more definitive clinical trials of IL-1 inhibition in this patient population.
Supplementary Material
1. Supplementary Table S1. Trial Outcomes
2. Supplementary Figure S1. Distribution of Change in hsCRP from Baseline to Week 24
3. Supplementary Figure S2. Percentage Change in hsCRP from Baseline for Each Participant at Each Timepoint
4. Supplementary Figure S3. Anakinra Concentrations
5. Appendix S1. Members of the Hemodialysis Novel Therapies Consortium
6. Appendix S2. Members of the Data and Safety Monitoring Board
7. Protocol
Acknowledgements
Anakinra (Kineret®) and matching placebo were provided by Swedish Orphan Biovitrum AB (publ) (Sobi). The authors would like to thank the participating patients, dialysis unit personnel, and DaVita, Dialysis Clinic Inc., and Fresenius Medical Care, for their important contributions to this work. Mark Unruh, MD, MS oversaw the ascertainment of patient reported outcomes at the University of New Mexico. Feng Sha, MS, Vanderbilt University Medical Center performed the assays to determine the anakinra concentrations. This trial was funded by the following cooperative agreements from the National Institute of Diabetes and Digestive and Kidney Diseases: U01 DK096189, U01 DK099923, U01 DK099914, and U01 DK099919. Adriana M. Hung was supported by U.S. Department of Veterans Affairs under Award Number CX001897. Dominic S. Raj was supported in part by R01 DK073665–01A1 and R01 5RO1DK125256–02 from the National Institutes of Health. T. Alp Ikizler was supported in part by 1UL-TR002243 and P30-DK114809 from the National Institutes of Health, and U.S. Department of Veterans Affairs under Award Number CX001755. Anakinra and matching placebo was provided by Sobi. Project officers from the National Institute of Diabetes and Digestive and Kidney Diseases worked collaboratively with the investigators in designing the study, monitoring the study performance, interpreting data, and preparing the manuscript. Sobi was not involved in designing or conducting the study, analyzing or interpreting the data, or preparing the article. The opinions expressed herein do not necessarily reflect those of the the National Institute of Diabetes and Digestive and Kidney Diseases, the National Institutes of Health, the Department of Health and Human Services or the government of the United States.
Footnotes
Disclosures
Laura M. Dember received compensation from the National Kidney Foundation for her role as Deputy Editor of American Journal of Kidney Diseases, and consulting fees from Merck, AstraZeneca, and Cara Therapeutics. Adriana Hung reports compensation from Vertex. Rajnish Mehrotra is supported in part by funds from the American Society of Nephrology as the Editor-in-Chief of the Clinical Journal of the American Society of Nephrology, and has served as a consultant for Light Line Medical, Inc. Jesse Y. Hsu received compensation from the National Kidney Foundation for his role as Statistics/Methods Editor of American Journal of Kidney Diseases, and from the Public Library of Science for his role as Statistical Advisor of PLOS ONE. Dominic S. Raj received compensation for consulting from Corvidia Therapeutics and Novo Nordics. David Charytan reports consulting fees from Eli Lilly/Boehringer Ingelheim, Janssen (steering committee), PLC medical (clinical events committee), Astra Zeneca, Allena Pharmaceuticals (DSMB), Fresenius, Amgen, Gilead, Novo Nordisk, GSK, Medtronic, Merck, Amgen, CSL Behring, Zogenix, Renalytix, Corvidia; research funding from Medtronic-clinical trial support, Bioporto-clinical trial support, Gilead, NovoNordisk, Amgen, and compensation for an advisory or leadership role from Clinical Journal of the American Society of Nephrology; and expert witness fees related to proton pump inhibitors. Finnian R. Mc Causland reports funding from NIDDK grants U01DK096189, R03DK122240, and K23DK102511 and research grants paid directly to his institution from Advanced Instruments and Fifth Eye. Paul L. Kimmel received royalties from Elsevier as the Co-Editor of Chronic Renal Disease and Psychosocial Aspects of Chronic Kidney Disease. Alan S. Kliger received compensation from the American Society of Nephrology for his role as Chair, Nephrologists Transforming Dialysis Safety and Co-Chair COVID-19 Response Team. T. Alp Ikizler received compensation from the International Society of Nephrology for his role as Associate Editor of Kidney International, and consulting fees from Fresenius Kabi, LaRenon, Nestle and Abbott Renal Care.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Laura M. Dember, Renal, Electrolyte and Hypertension Division, Department of Medicine, Department of Biostatistics, Epidemiology and Informatics, and Center for Clinical Epidemiology and Biostatistics, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA.
Adriana Hung, Division of Nephrology and Hypertension, Department of Medicine, and Vanderbilt Center for Kidney Disease, Vanderbilt University Medical Center and VA Tennessee Valley Healthcare System, Nashville, TN.
Rajnish Mehrotra, Kidney Research Institute and Harborview Medical Center, Division of Nephrology, Department of Medicine, University of Washington, Seattle, WA.
Jesse Y. Hsu, Department of Biostatistics, Epidemiology and Informatics, and Center for Clinical Epidemiology and Biostatistics, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA.
Dominic S. Raj, Division of Renal Diseases and Hypertension, George Washington University School of Medicine, Washington, DC.
David M. Charytan, Department of Medicine, Brigham and Women’s Hospital, Boston, MA.
Finnian R. Mc Causland, Renal Division, Department of Medicine, Harvard Medical School and Brigham and Women’s Hospital, Boston, MA
Renu Regunathan-Shenk, Division of Renal Diseases and Hypertension, George Washington University School of Medicine, Washington, DC.
J. Richard Landis, Department of Biostatistics, Epidemiology and Informatics, and Center for Clinical Epidemiology and Biostatistics, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA, Philadelphia, PA.
Paul L. Kimmel, National Institute of Diabetes Digestive and Kidney Diseases Bethesda, MD.
Alan S. Kliger, Department of Medicine, Yale School of Medicine and Yale New Haven Health System, New Haven, CT.
Jonathan Himmelfarb, Kidney Research Institute, Division of Nephrology, Department of Medicine, University of Washington, Seattle, WA.
T. Alp Ikizler, Division of Nephrology and Hypertension, Department of Medicine, and Vanderbilt Center for Kidney Disease, Vanderbilt University Medical Center, Nashville, TN.
Data Sharing Statement
Data from this clinical trial will be made available through the National Institute of Diabetes and Digestive and Kidney Diseases Central Repository.
References
- 1.Himmelfarb J, Ikizler TA. Hemodialysis. N Engl J Med 2010; 363: 1833–1845. [DOI] [PubMed] [Google Scholar]
- 2.Kimmel PL, Phillips TM, Simmens SJ, et al. Immunologic function and survival in hemodialysis patients. Kidney Int 1998; 54: 236–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stenvinkel P, Barany P, Heimburger O, et al. Mortality, malnutrition, and atherosclerosis in ESRD: what is the role of interleukin-6? Kidney Int Suppl 2002: 103–108. [DOI] [PubMed] [Google Scholar]
- 4.Stenvinkel P, Heimburger O, Jogestrand T. Elevated interleukin-6 predicts progressive carotid artery atherosclerosis in dialysis patients: association with Chlamydia pneumoniae seropositivity. Am J Kidney Dis 2002; 39: 274–282. [DOI] [PubMed] [Google Scholar]
- 5.Ross R Atherosclerosis--an inflammatory disease. N Engl J Med 1999; 340: 115–126. [DOI] [PubMed] [Google Scholar]
- 6.Ikizler TA. Nutrition, inflammation and chronic kidney disease. Curr Opin Nephrol Hypertens 2008; 17: 162–167. [DOI] [PubMed] [Google Scholar]
- 7.Amdur RL, Feldman HI, Dominic EA, et al. Use of Measures of Inflammation and Kidney Function for Prediction of Atherosclerotic Vascular Disease Events and Death in Patients With CKD: Findings From the CRIC Study. Am J Kidney Dis 2019; 73: 344–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ikizler TA, Burrowes JD, Byham-Gray LD, et al. KDOQI Clinical Practice Guideline for Nutrition in CKD: 2020 Update. Am J Kidney Dis 2020; 76: S1–S107. [DOI] [PubMed] [Google Scholar]
- 9.Descamps-Latscha B, Herbelin A, Nguyen AT, et al. Balance between IL-1 beta, TNF-alpha, and their specific inhibitors in chronic renal failure and maintenance dialysis. Relationships with activation markers of T cells, B cells, and monocytes. J Immunol 1995; 154: 882–892. [PubMed] [Google Scholar]
- 10.Pereira BJ, Shapiro L, King AJ, et al. Plasma levels of IL-1 beta, TNF alpha and their specific inhibitors in undialyzed chronic renal failure, CAPD and hemodialysis patients. Kidney Int 1994; 45: 890–896. [DOI] [PubMed] [Google Scholar]
- 11.Bresnihan B, Alvaro-Gracia JM, Cobby M, et al. Treatment of rheumatoid arthritis with recombinant human interleukin-1 receptor antagonist. Arthritis Rheum 1998; 41: 2196–2204. [DOI] [PubMed] [Google Scholar]
- 12.Hoffman HM, Rosengren S, Boyle DL, et al. Prevention of cold-associated acute inflammation in familial cold autoinflammatory syndrome by interleukin-1 receptor antagonist. Lancet 2004; 364: 1779–1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hawkins PN, Lachmann HJ, McDermott MF. Interleukin-1-receptor antagonist in the Muckle-Wells syndrome. N Engl J Med 2003; 348: 2583–2584. [DOI] [PubMed] [Google Scholar]
- 14.Saag KG, Khanna PP, Keenan RT, et al. A Randomized, Phase II Study Evaluating the Efficacy and Safety of Anakinra in the Treatment of Gout Flares. Arthritis Rheumatol 2021; 73: 1533–1542. [DOI] [PubMed] [Google Scholar]
- 15.Yang BB, Baughman S, Sullivan JT. Pharmacokinetics of anakinra in subjects with different levels of renal function. Clin Pharmacol Ther 2003; 74: 85–94. [DOI] [PubMed] [Google Scholar]
- 16.Hung AM, Ellis CD, Shintani A, et al. IL-1beta receptor antagonist reduces inflammation in hemodialysis patients. J Am Soc Nephrol 2011; 22: 437–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Platz EA, Sutcliffe S, De Marzo AM, et al. Intra-individual variation in serum C-reactive protein over 4 years: an implication for epidemiologic studies. Cancer Causes Control 2010; 21: 847–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hung A, Pupim L, Yu C, et al. Determinants of C-reactive protein in chronic hemodialysis patients: relevance of dialysis catheter utilization. Hemodial Int 2008; 12: 236–243. [DOI] [PubMed] [Google Scholar]
- 19.Jhamb M, Weisbord SD, Steel JL, et al. Fatigue in patients receiving maintenance dialysis: a review of definitions, measures, and contributing factors. Am J Kidney Dis 2008; 52: 353–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nowak KL, Chonchol M, Ikizler TA, et al. IL-1 Inhibition and Vascular Function in CKD. J Am Soc Nephrol 2017; 28: 971–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ridker PM, Devalaraja M, Baeres FMM, et al. IL-6 inhibition with ziltivekimab in patients at high atherosclerotic risk (RESCUE): a double-blind, randomised, placebo-controlled, phase 2 trial. Lancet 2021; 397: 2060–2069. [DOI] [PubMed] [Google Scholar]
- 22.Pergola PE, Devalaraja M, Fishbane S, et al. Ziltivekimab for Treatment of Anemia of Inflammation in Patients on Hemodialysis: Results from a Phase 1/2 Multicenter, Randomized, Double-Blind, Placebo-Controlled Trial. J Am Soc Nephrol 2021; 32: 211–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
1. Supplementary Table S1. Trial Outcomes
2. Supplementary Figure S1. Distribution of Change in hsCRP from Baseline to Week 24
3. Supplementary Figure S2. Percentage Change in hsCRP from Baseline for Each Participant at Each Timepoint
4. Supplementary Figure S3. Anakinra Concentrations
5. Appendix S1. Members of the Hemodialysis Novel Therapies Consortium
6. Appendix S2. Members of the Data and Safety Monitoring Board
7. Protocol
Data Availability Statement
Data from this clinical trial will be made available through the National Institute of Diabetes and Digestive and Kidney Diseases Central Repository.


