Abstract
Study Objective.
We conducted a randomized study to compare the efficacy and adverse event profile of 1000mg of IV acetaminophen to 0.5 mg of IV hydromorphone among patients ≥65 years with acute pain of sufficient severity to warrant IV opioids.
Methods.
This randomized comparative effectiveness study with 162 participants was conducted in two urban emergency departments. The primary outcome was improvement in the 0–10 pain scale between baseline and 60 minutes later. Secondary outcomes included need for additional analgesic medication and adverse events attributable to investigational medication. The minimum clinically important difference was an improvement of 1.3 on the 0–10 pain scale.
Results.
Median baseline pain scores were 10 (IQR: 8–10) in both groups. By 60 minutes, the acetaminophen patients improved by 3.6 (SD 2.9) on the 0–10 pain scale while hydromorphone patients improved by 4.6 (SD 3.3) (95%CI for difference of 1.0=0.1, 2.0). Additional analgesic medications were required for 37/81 (46%) acetaminophen patients and 31/81 (38%) hydromorphone patients (95%CI for the rounded difference of 7%: −8, 23%). Adverse events were reported by 6/81 (7%) acetaminophen patients and 10/81 (12%) hydromorphone patients (95%CI for difference of 5%: −4, 14%) and included dizziness, drowsiness, headache, and nausea.
Conclusion.
While 0.5mg of IV hydromorphone was statistically superior to 1000mg of IV acetaminophen for older patients in the ED with acute severe pain, this difference was not clinically meaningful. Regardless of medication received, many participants experienced minimal or incomplete pain relief.
Introduction
Older patients present to emergency departments with acute severe pain frequently, yet there are few randomized studies of pain management strategies for these patients.1–3 Older adults are often excluded from participation in acute pain studies.4 Older patients are at a high risk for under-treatment of pain—they are less likely to receive pain medication because of fear of medication induced side effects and medication interactions and commonly experience longer delays to treatment.5,6 Thus, data delineating efficacy and safety of various pain management strategies for older patients are urgently needed.
Intravenous opioids are the mainstays of treatment of acute, severe pain in the ED setting.7 Among older patients, IV hydromorphone is effective and safe using both weight-based dosing (0.0075 mg/kg) and fixed 0.5 mg doses.8,9 However, because of fear of adverse medication reactions such as respiratory depression or hypotension, medication interactions, and long-term sequelae such as opioid use disorder and chronic pain syndromes, some have called for minimizing the use of opioids in the ED. Among older post-operative patients, IV acetaminophen decreases pain without causing a meaningful increase in the rate of medication related adverse events.10 It may be that IV acetaminophen is sufficiently effective and that IV opioids are not warranted.
To help emergency physicians choose an appropriate first-line analgesic therapy for older patients with acute severe pain, we conducted a randomized study to compare the efficacy and adverse event profile of 1000mg of IV acetaminophen to 0.5 mg of IV hydromorphone among patients 65 years and older with acute pain of sufficient severity to warrant IV opioids per the clinical attending physician.
Methods
Study design and setting
This was a double-blind, parallel group, randomized trial comparing the analgesic efficacy of 1000mg IV acetaminophen versus 0.5 mg IV of hydromorphone for the treatment of acute severe pain in older Emergency Department patients. This study was performed in two EDs of Montefiore Medical Center in the Bronx, NY with a combined annual census of 180,000 adult visits. The Albert Einstein College of Medicine Institutional Review Board reviewed and approved the protocol and provided continuing oversight. It was registered online at http://www.clinicaltrials.gov (NCT03521102). Data were collected by salaried, bilingual (English and Spanish) research associates, who staffed the EDs 24 hours/day, 7 days/week during the study period.
Subject selection
Patients aged 65 years of age or older with acute pain, defined as onset within 7 days of the ED visit, were referred for participation by the clinical attending physician. To participate, patients had to have severe pain, which we defined operationally as the attending physician’s plan to use intravenous opioids. Our IRB asked that we include patients only if they could provide consent in either English or Spanish. Exclusion criteria included use of opioids or tramadol within the past 7 days, use of acetaminophen or non-steroidal anti-inflammatory medication within the previous 8 hours, prior adverse reaction to opioids or acetaminophen, or any type of daily or frequently recurrent pain that had lasted for 3 months or more. We excluded these latter patients because of concern that patients with chronic pain may be dissimilar in their experience of pain than patients with only acute pain. We also excluded patients if they had chronic liver or kidney disease, if they used MAO inhibitors, or for systolic blood pressure (SBP) of less than 100 mm Hg, a heart rate less than 60 beats per minute, or a baseline oxygen saturation < 95% on room air. We screened all patients for dementia using a validated instrument and included only those in whom it was excluded because we were concerned about obtaining adequate consent among patients with dementia.11 Lastly, patients were only eligible to be enrolled in the study once.
Intervention
This study included two treatment arms. In the acetaminophen arm, participants received 1000mg of IV acetaminophen in solution with 100ml of normal saline, administered as an intravenous drip over 10 minutes, and 2ml of normal saline, administered as a slow intravenous push. In the hydromorphone arm, participants received 100ml of normal saline, administered as an intravenous drip over 10 minutes followed by 0.5mg of IV hydromorphone in solution with 2ml of normal saline, administered as a slow intravenous push.
Assignment was concealed and the medications masked using the following mechanism: The research pharmacist, in a secure location removed from the ED, generated a sequence using an online random number generator and used this sequence to prepare study packets. Each packet contained a 100 ml vial containing either IV acetaminophen 1000mg or normal saline and a 2ml vial containing either 0.5 mg IV hydromorphone or normal saline. The acetaminophen, hydromorphone, and normal saline all appeared as colorless solutions to the naked eye. The research pharmacist then stored the research packets in a locked medication cabinet in the ED. These packets were used in sequential order by the clinical nurse.
Measures
We measured pain using a verbal zero to ten pain scale on which zero signified no pain and ten signified the worst pain imaginable. We measured these pain scores at baseline and 15, 30, 45, 60, 90, 120 and 180 minutes later.
We assessed side effects by asking participants if any new symptoms emerged after receipt of the investigational medication and followed an affirmative response with an open-ended question eliciting details.
We also assessed for clinically important side effects by asking the attending physician caring for the patient if the study medication negatively impacted the study participant’s clinical course. Finally, we determined whether naloxone was administered to any patient.
Outcomes
The primary outcome was the improvement in pain score, as measured on the 0–10 pain scale, between baseline and 60 minutes later. Secondary outcomes included the use of additional medication for treatment of pain at any time during the ED course and presence of side effects. Study participants did not receive any additional medication prior to assessment of the primary outcome. We also report the percentage of patients who failed to achieve a minimum clinically important improvement in pain (defined as an improvement of 1.3 points on the 0–10 scale), the percentage of patients who failed to achieve a 50% improvement in pain, and absolute pain scores at each assessed time point.12 We report the frequency of use of naloxone and the frequency with which the investigational medications negatively impacted the patient’s clinical course.
Data analysis
We report baseline characteristics using mean with standard deviation, number and percent, or median with interquartile range, as appropriate. We calculated the improvement in pain scores as the baseline 0–10 pain score minus the 60 minute pain score. We compared the mean and distribution in each arm and the 95%CI for the between-group difference. We report all dichotomous secondary outcomes as a number with percentage and the 95%CI for between-group differences. Absolute pain scores at each time point are presented graphically with 95%CI. For missing pain score data, we averaged the two temporally surrounding values, or if there was only a preceding value, we carried that one forward.
We based the sample size calculation on the following parameters: an alpha of 0.05, a beta of 0.20, a standard deviation of 2.8 units on the 0–10 scale, and a minimum clinically important difference of 1.3 units on the 0–10 scale. We calculated the need for 148 research subjects and decided to enroll an additional 14 (about 10%) to account for protocol violations and missing data.
Results
Enrollment began in September 2018, paused between March and June 2020, and concluded in October 2021. 2363 patients were screened for participation and 162 were enrolled (Figure 1). Baseline characteristics are reported in Table 1. Nearly two-thirds of participants were women. Median baseline pain scores were 10 (out of 10) in both groups. There was no important difference between the groups with regard to baseline characteristics.
Figure 1.
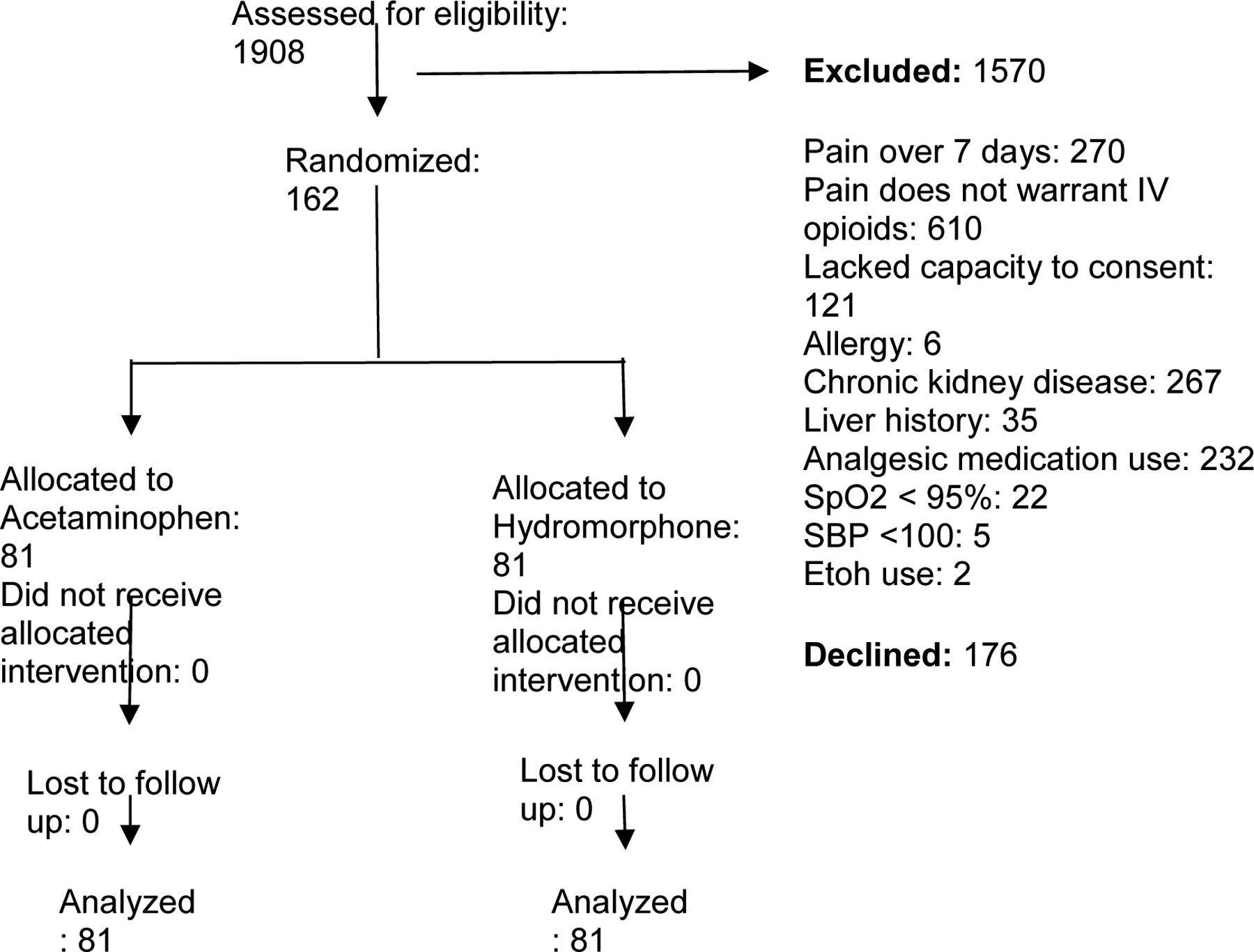
CONSORT flow diagram
Table 1.
Baseline characteristics
| Variable | Acetaminophen (n=81) | Hydromorphone (n=81) |
|---|---|---|
| Age in years, mean (SD) | 75 (8) | 74 (6) |
| Age in years, deciles | ||
| 60s | 23 (28%) | 22 (27%) |
| 70s | 40 (49%) | 45 (56%) |
| 80s | 12 (15%) | 14 (17%) |
| 90s | 6 (7%) | 0 (0%) |
| Sex | ||
| Female | 56 (69%) | 51 (63%) |
| Male | 25 (31`%) | 30 (37%) |
| Duration of pain in days, median (IQR) | 2 (1, 4) | 2 (1, 3) |
| Baseline 0–10 pain score, median (IQR) | 10 (8, 10) | 10 (8, 10) |
| Location of pain | ||
| Abdomen/flank/pelvis | 52 (64%) | 55 (68%) |
| Back/neck | 6 (7%) | 7 (9%) |
| Chest | 1 (1%) | 6 (7%) |
| Extremity | 21 (26%) | 11 (14%) |
| Head | 0 (0%) | 2 (2%) |
| Widespread | 1 (1%) | 0 (0%) |
By 60 minutes, the acetaminophen patients improved by 3.6 (SD 2.9) on the 0–10 pain scale while hydromorphone patients improved by 4.6 (SD 3.3) (95%CI for difference of 1.0= 0.1, 2.0). Dichotomous outcomes are presented in Table 2. Pain scores at all time points are presented in Figure 2. A graphical depiction of participant-level pain scores at baseline and 60 minutes is shown in Figure 3.
Table 2.
Outcomes
| Outcomes | Acetaminophen | Hydromorphone | Difference |
|---|---|---|---|
| Required additional analgesic medication in the ED | 37/81 (46%) | 31/81 (38%) | 7%* (−8, 23%) |
| Achieved minimum clinically important improvement in pain by one hour** | 62/81 (77%) | 63/81 (78%) | 1% (−12, 14%) |
| Improved ≥ 50% by one hour | 30/81 (37%) | 43/81 (53%) | 16% (1, 31%) |
| Reported adverse event related to medication during the ED visit | 6/81 (7%) | 10/81 (12%) | 5% (−4, 14%) |
rounded
A reduction in pain score of ≥1.3
Figure 2.
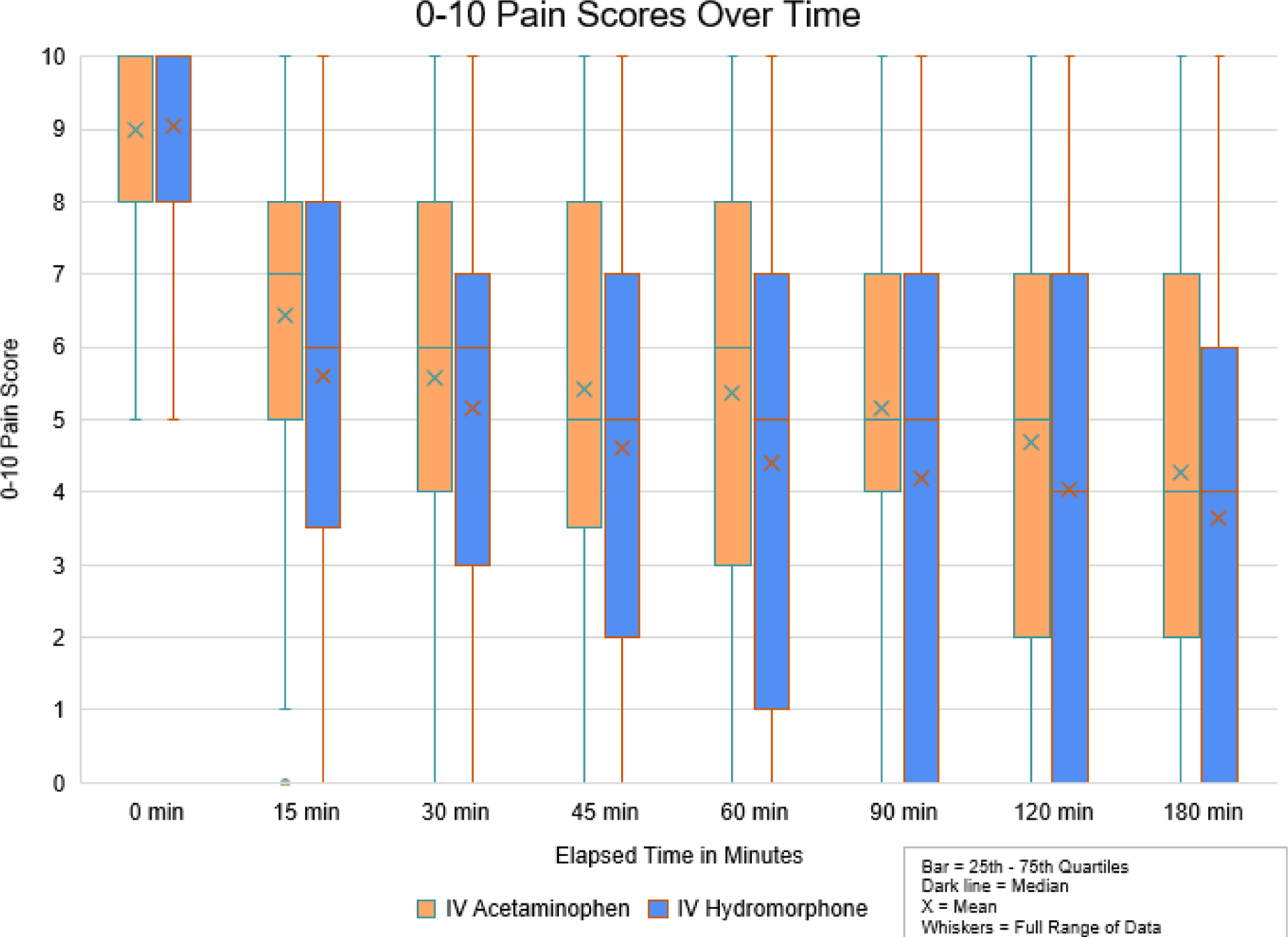
0–10 pain scores over time
Figure 3.
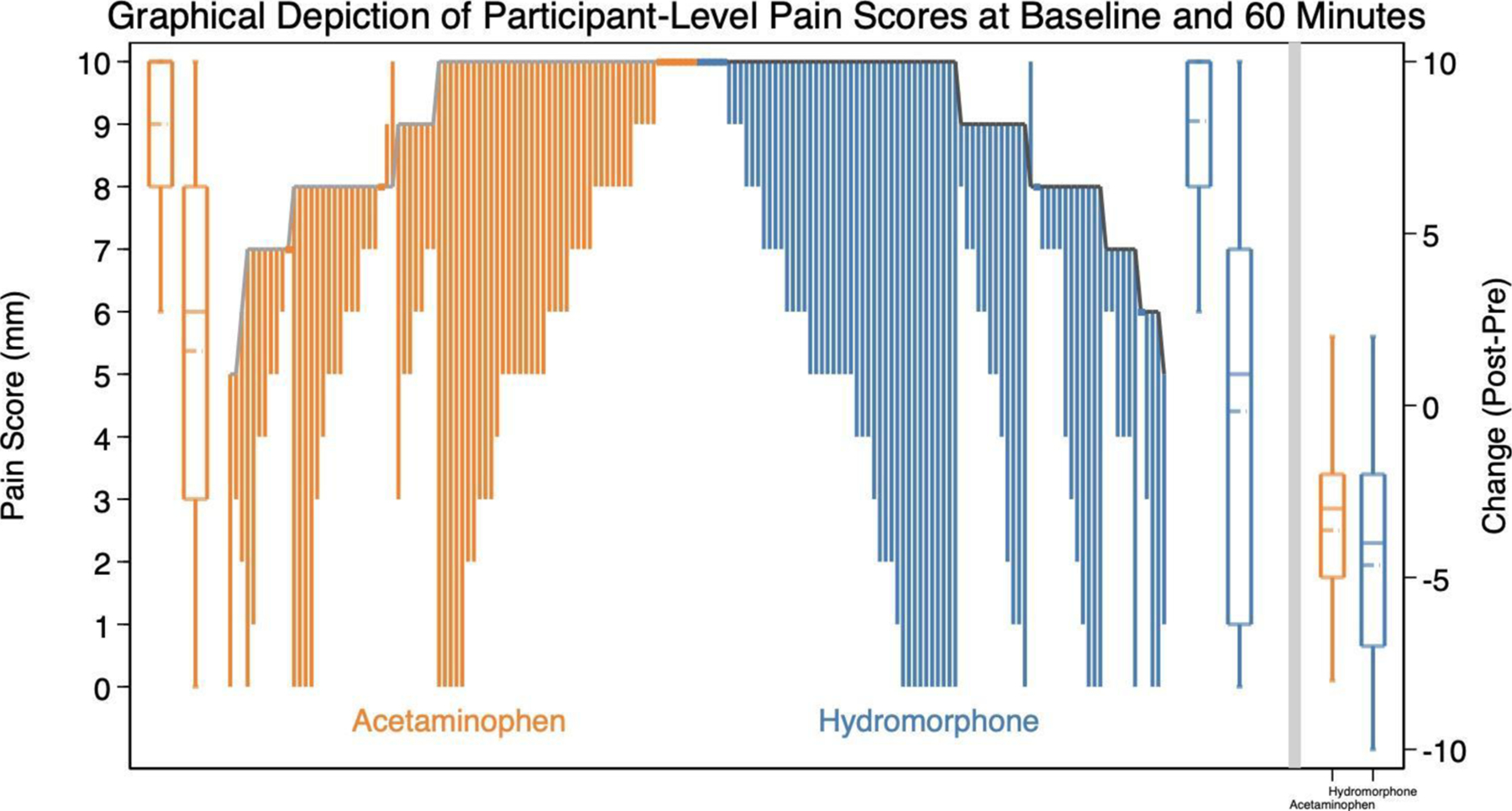
Graphical depiction of participant-level pain scores at baseline and 60 minutes
Overall rates of medication-related adverse events were comparable between the study arms (Table 2). Four participants who received hydromorphone reported dizziness, two participants who received acetaminophen reported drowsiness, two participants who received acetaminophen and one who received hydromorphone reported headache, and four participants who received hydromorphone and one who received acetaminophen reported nausea. No other side effects were reported by more than one participant and none were serious. When asked about the clinical course, the attending physicians determined that in no case did the study medication negatively impact the patient’s clinical course. Naloxone was not needed during this study.
Limitations
Limitations of this study include the following. First, this research was conducted in just two urban emergency departments and a large number of patients screened for the study were excluded. Thus, these results may not be generalizable to all older patients with acute severe pain. Second, we did not conduct dose-finding studies to determine the optimal dose of hydromorphone. It may be that larger doses would have demonstrated greater efficacy versus acetaminophen, though presumably this would have come at the cost of more side effects. Similarly, a two gram dose of IV acetaminophen may have resulted in improved outcomes in that arm.13 Third, we chose a value of 1.3 as the minimum clinically important improvement on the zero to ten scale. There is some uncertainty as to the correct numerical value of the minimum clinically important difference in the older population, but it does seem certain that the between-group difference we reported in this study was never above this threshold.12 Fourth, we relied on the clinical attending physician’s judgment of whether opioids were indicated. Thus, it is not clear how widely these results may be generalizable if local practice regarding opioids differs from our own. Fifth, we only assessed adverse events in the ED. Late developing adverse events such as constipation may have been missed. Sixth, this study cannot definitively assess the potential tradeoffs of each of the drugs in specific situations.
Discussion.
In this ED-based study of older patients with acute severe pain, there were no clinically important differences in pain outcomes among participants who received 1000mg of IV acetaminophen versus those who received 0.5mg of IV hydromorphone. Though the improvement in pain scores among those who received hydromorphone was statistically superior to the improvement in pain among those who received acetaminophen, the results did not surpass our threshold for a clinically important difference. This is further reflected in the frequency with which study participants failed to achieve a minimum clinically important improvement in pain between baseline and one hour–this occurred in about one-quarter of participants in both study arms--and that a comparable number of participants in both groups required additional medication for pain (46% in the acetaminophen arm and 38% in the hydromorphone arm). Finally, as can be seen in Figure 2, the between-group difference in pain scores is maximum at 60 minutes, the time point we chose a priori as our primary endpoint. For all other time points measured in the study, the between-group difference in pain improvement was even smaller.
Several other studies looking at acute pain in the emergency department have shown little difference in pain outcomes between IV opioids and IV acetaminophen in addition to IV opioids.14 Similarly, little difference between these medications was reported in the prehospital setting.15 A meta analysis of patients with acute musculoskeletal pain found an improved benefit-harm ratio when using acetaminophen compared to opioids.16
Perhaps the most remarkable finding from this study was the relatively modest reduction in pain afforded by both medications. One quarter of patients in both arms failed to achieve any clinically noticeable improvement in pain. Nearly 2/3rds of the acetaminophen arm and almost half of the hydromorphone arm did not experience a 50% reduction in pain. The very modest benefit of one dose of IV opioids for older patients has been demonstrated in other ED-based studies as well.8,9 Unfortunately, it is not clear what further pain management strategies emergency physicians should pursue for older patients with severe pain. While the concept of multimodal analgesia is intuitively appealing, combining acetaminophen with hydromorphone does not seem to be of benefit for older adult patients in the ED.14 Procedural based analgesic techniques may be useful for select patients but require expertise that is not widely present in emergency medicine.17,18 Successive doses of IV opioids results in high levels of adequate analgesia in younger adults and may be an appropriate strategy for older adult patients as well.9,19 If IV hydromorphone is titrated to the perceived pain level and hemodynamics of each patient, it probably can achieve satisfactory pain relief in this vulnerable population.9 The approved dosage for IV acetaminophen for patients over 50 kg is 1000 mg every 6 hours, not to exceed 4g/day, though larger doses may in fact confer benefit without harm.13
Both medications were very well tolerated, with few treatment emergent adverse events occurring in the ED. Naloxone was not required for any patient and the clinical teams reported no negative impact of the medications on clinical course. This is consistent with prior reports of 0.5mg doses of hydromorphone for older patients in the ED with acute severe pain that have demonstrated few adverse events at this dose.8,9
In conclusion, while 0.5mg of IV hydromorphone was statistically superior compared to 1000mg of IV acetaminophen for older patients in the ED with acute severe pain, this difference was not clinically important. Regardless of medication received, a large number of participants experienced minimal or incomplete relief of pain. These results may not generalize well outside of the population studied.
Figure 4.
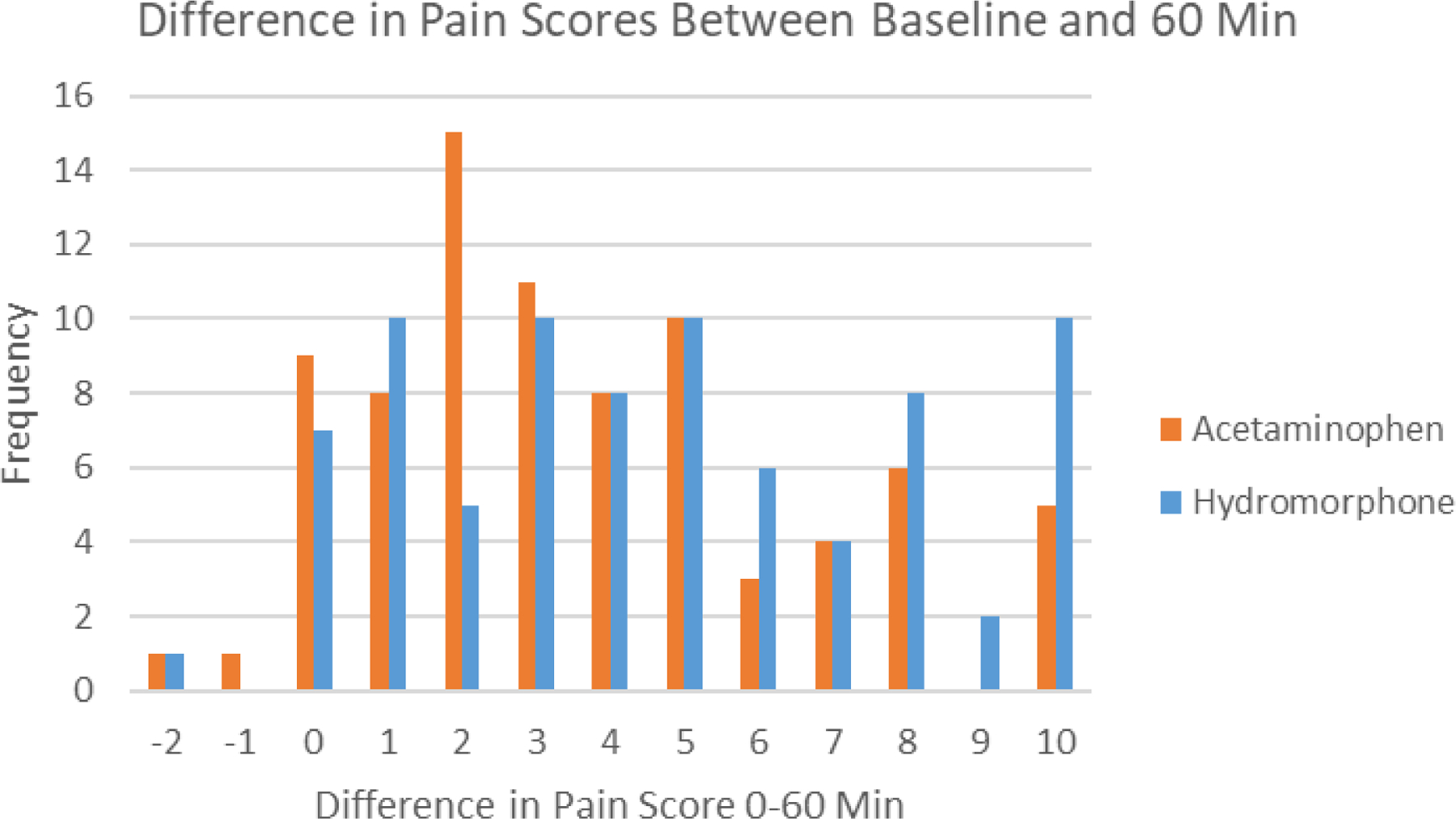
Histogram of change in pain scores between baseline and 60 minutes
Grant:
Supported by Harold and Muriel Block Institute for Clinical and Translational Research at Einstein and Montefiore (UL1TR002556)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Meetings: Will be presented at SAEM, New Orleans, LA, May, 2022
ClinicalTrials.gov Identifier: NCT03521102
Conflicts of Interest: none
References
- 1.Platts-Mills TF, Hunold KM, Esserman DA, Sloane PD, McLean SA. Motor vehicle collision-related emergency department visits by older adults in the united states. Academic emergency medicine. 2012;19(7):821–827. https://api.istex.fr/ark:/67375/WNG-GFXJLRRF-P/fulltext.pdf. doi: 10.1111/j.1553-2712.2012.01383.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hwang Ula, MD, MPH, Platts-Mills TF, MD. Acute pain management in older adults in the emergency department. Clinics in geriatric medicine. 2013;29(1):151–164. https://www.clinicalkey.es/playcontent/1-s2.0-S0749069012000924. doi: 10.1016/j.cger.2012.10.006. [DOI] [PubMed] [Google Scholar]
- 3.Gleason Lauren J., MD, MPH, Escue ED, MD, Hogan TM, MD. Older adult emergency department pain management strategies. Clinics in geriatric medicine. 2018;34(3):491–504. https://www.clinicalkey.es/playcontent/1-s2.0-S0749069018309819. doi: 10.1016/j.cger.2018.04.009. [DOI] [PubMed] [Google Scholar]
- 4.Hunold KM, Goldberg EM, Caterino JM, et al. Inclusion of older adults in emergency department clinical research: Strategies to achieve a critical goal. Academic emergency medicine. 2022;29(3):376–383. https://onlinelibrary.wiley.com/doi/abs/10.1111/acem.14386. doi: 10.1111/acem.14386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hwang U, Richardson LD, Harris B, Morrison RS. The quality of emergency department pain care for older adult patients. Journal of the American Geriatrics Society (JAGS). 2010;58(11):2122–2128. https://api.istex.fr/ark:/67375/WNG-07SHVSD7-V/fulltext.pdf. doi: 10.1111/j.1532-5415.2010.03152.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Platts-Mills TF, MD, Esserman DA, PhD, Brown DL, BA, Bortsov Andrey V., MD, PhD, Sloane Philip D., MD, MPH, McLean Samuel A., MD, MPH. Older US emergency department patients are less likely to receive pain medication than younger patients: Results from a national survey. Annals of emergency medicine. 2011;60(2):199–206. https://www.clinicalkey.es/playcontent/1-s2.0-S0196064411016052. doi: 10.1016/j.annemergmed.2011.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Walls R, Hockberger R, Gausche-Hill M, Erickson TB, Wilcox SR, eds. Rosen’s emergency medicine: Concepts and clinical practice . 10th edition ed. Philadelphia, PA: Mosby Elsevier; 2022. [Google Scholar]
- 8.Chang Andrew K., MD, MS, Bijur PE, PhD, Baccelieri A, MD, Gallagher EJ, MD. Efficacy and safety profile of a single dose of hydromorphone compared with morphine in older adults with acute, severe pain: A prospective, randomized, double-blind clinical trial. The American journal of geriatric pharmacotherapy. 2009;7(1):1–10. https://www.clinicalkey.es/playcontent/1-s2.0-S154359460900004X. doi: 10.1016/j.amjopharm.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 9.Chang AK, Bijur PE, Davitt M, Gallagher EJ. Randomized clinical trial of an intravenous hydromorphone titration protocol versus usual care for management of acute pain in older emergency department patients. Drugs Aging. 2013;30(9):747–754. https://link.springer.com/article/10.1007/s40266-013-0103-y. doi: 10.1007/s40266-013-0103-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jahr JS, Breitmeyer JB, Pan C, Royal MA, Ang RY. Safety and efficacy of intravenous acetaminophen in the elderly after major orthopedic surgery: Subset data analysis from 3, randomized, placebo-controlled trials. American journal of therapeutics. 2012;19(2):66–75. https://www.ncbi.nlm.nih.gov/pubmed/22354127. doi: 10.1097/MJT.0b013e3182456810. [DOI] [PubMed] [Google Scholar]
- 11.Callahan C, Unverzagt F, Hui S, Perkins A, Hendrie H. Six-item screener to identify cognitive impairment among potential subjects for clinical research. Medical care. 2002;40(9):771–781. https://www.jstor.org/stable/3768143. doi: 10.1097/00005650-200209000-00007. [DOI] [PubMed] [Google Scholar]
- 12.Bijur Polly E., PhD, MPH, Chang Andrew K., MD, MS, Esses D, MD, Gallagher EJ, MD. Identifying the minimum clinically significant difference in acute pain in the elderly. Annals of emergency medicine. 2009;56(5):517–521.e1. https://www.clinicalkey.es/playcontent/1-s2.0-S0196064410001204. doi: 10.1016/j.annemergmed.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 13.Juhl GI, Norholt SE, Tonnesen E, Hiesse-Provost O, Jensen TS. Analgesic efficacy and safety of intravenous paracetamol (acetaminophen) administered as a 2 g starting dose following third molar surgery. European journal of pain. 2006;10(4):371. 10.1016/j.ejpain.2005.06.004. doi: 10.1016/j.ejpain.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 14.Chang AK, Bijur PE, Ata A, et al. Randomized clinical trial of intravenous acetaminophen as an analgesic adjunct for older adults with acute severe pain. Academic emergency medicine. 2019;26(4):402–409. https://onlinelibrary.wiley.com/doi/abs/10.1111/acem.13556. doi: 10.1111/acem.13556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sobieraj DM, Martinez BK, Miao B, et al. Comparative effectiveness of analgesics to reduce acute pain in the prehospital setting. Prehospital emergency care. 2020;24(2):163–174. https://www.tandfonline.com/doi/abs/10.1080/10903127.2019.1657213. doi: 10.1080/10903127.2019.1657213. [DOI] [PubMed] [Google Scholar]
- 16.Busse JW, Sadeghirad B, Oparin Y, et al. Management of acute pain from non-low back, musculoskeletal injuries : A systematic review and network meta-analysis of randomized trials. Annals of internal medicine. 2020;173(9):730–738. https://www.ncbi.nlm.nih.gov/pubmed/32805127. doi: 10.7326/M19-3601. [DOI] [PubMed] [Google Scholar]
- 17.Luftig J, Mantuani D, Herring AA, Dixon B, Clattenburg E, Nagdev A. Successful emergency pain control for posterior rib fractures with ultrasound-guided erector spinae plane block. The American journal of emergency medicine. 2018;36(8):1391–1396. 10.1016/j.ajem.2017.12.060. doi: 10.1016/j.ajem.2017.12.060. [DOI] [PubMed] [Google Scholar]
- 18.Wan H, Li S, Ji W, Yu B, Jiang N. Fascia iliaca compartment block for perioperative pain management of geriatric patients with hip fractures: A systematic review of randomized controlled trials. Pain research & management. 2020;2020:8503963. 10.1155/2020/8503963. doi: 10.1155/2020/8503963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.CHANG AK, BIJUR PE, CAMPBELL CM, MURPHY MK, GALLAGHER EJ. Safety and efficacy of rapid titration using 1 mg doses of intravenous hydromorphone in emergency department patients with acute severe pain: The “1 + 1” protocol. Annals of emergency medicine. 2009;54(2):221–225. [DOI] [PubMed] [Google Scholar]


