Summary
A defining feature of early infancy is the immense neural plasticity that enables animals to develop a brain that is functionally integrated with a growing body. Early infancy is also defined as a period dominated by sleep. Here, we describe three conceptual frameworks that vary as to whether and how they incorporate sleep as a factor in the activity-dependent development of sensory and sensorimotor systems. The most widely accepted framework is exemplified by the visual system where retinal waves seemingly occur independent of sleep-wake states. An alternative framework is exemplified by the sensorimotor system where sensory feedback from sleep-specific movements activates the brain. We prefer a third framework that encompasses the first two but also captures the diverse ways that sleep modulates activity-dependent development throughout the nervous system. Appreciation of the third framework will spur progress toward a more comprehensive and cohesive understanding of both typical and atypical neurodevelopment.
Keywords: development, plasticity, REM sleep, non-REM sleep, ocular dominance, myoclonic twitch, rapid eye movements
In Brief
Infant animals spend the majority of each day asleep, but researchers are only beginning to delineate the contributions of sleep to sensory neurodevelopment. In this Perspective, Blumberg et al. provide a conceptual framework to guide future progress.
Introduction
An undisputed fact about early development is that it is a time of growth and qualitative change. As a newborn’s body weight doubles every few months (in humans) or days (in rats), the shape and relative proportions of the body change as well. At the same time, the infant brain also rapidly changes. Neurons are born and die, synaptic connections are formed and eliminated, and increasingly complex neural computations emerge. Against this background of physical and neural transformation, the brain becomes functionally integrated with the body, thus illustrating a second undisputed fact about early development—that it is a period of enhanced neural plasticity.
The functional integration of body and brain is all-the-more remarkable when considering the extraordinary diversity of animal sizes, shapes, and appendages. From the agile trunk of an elephant to the dexterous whiskers of a rat, animals of all kinds achieve exquisite integration of body and brain using a topographically organized system that maps neurons, muscles, and peripheral sensors (e.g., muscle spindles, mechanoreceptors, retina). Given the complexity of such species-typical systems, it is tempting to assume that evolution guarantees functionality by “hardwiring” it into the brain. However, developmental plasticity obviates the need for such guarantees, as demonstrated by individuals born with atypical bodies (Blumberg, 2009). For example, people born without arms—a condition called amelia—develop the ability to use their feet and toes like hands and fingers to type on a keyboard, play the piano, or shuffle a deck of cards. Such novel behavioral capacities are reflected in the development of novel topographic maps in somatosensory cortex (Dempsey-Jones et al., 2019). Similarly, people born blind develop enhanced auditory and somatosensory capacities (Rauschecker et al., 1992; Roder et al., 1999) along with novel cortical organization (Sadato et al., 1996). Such examples of developmental plasticity do not reflect an atypical developmental process. Rather, the process is inherently the same regardless of whether the animal is typically or atypically formed (Blumberg, 2009).
In young and adult animals, the neural plasticity that makes learning and memory possible depends on sleep (Rasch and Born, 2013). Might sleep also contribute to the developmental plasticity that enables functional integration of brain and body? Indeed, for decades, researchers addressed similar developmental questions by noting that early postnatal life is the time when animals—vertebrates and invertebrates alike—sleep the most (Blumberg and Rattenborg, 2017; Kayser and Biron, 2016). For example, in humans and rats, REM and non-REM sleep (or active and quiet sleep, respectively) exhibit distinct developmental profiles, with REM sleep being more prevalent in early life than non-REM sleep (Jouvet-Mounier et al., 1970; Roffwarg et al., 1966) (Figure 1). In humans, REM sleep is even more prominent during the prenatal period (Knoop et al., 2021).
Figure 1.
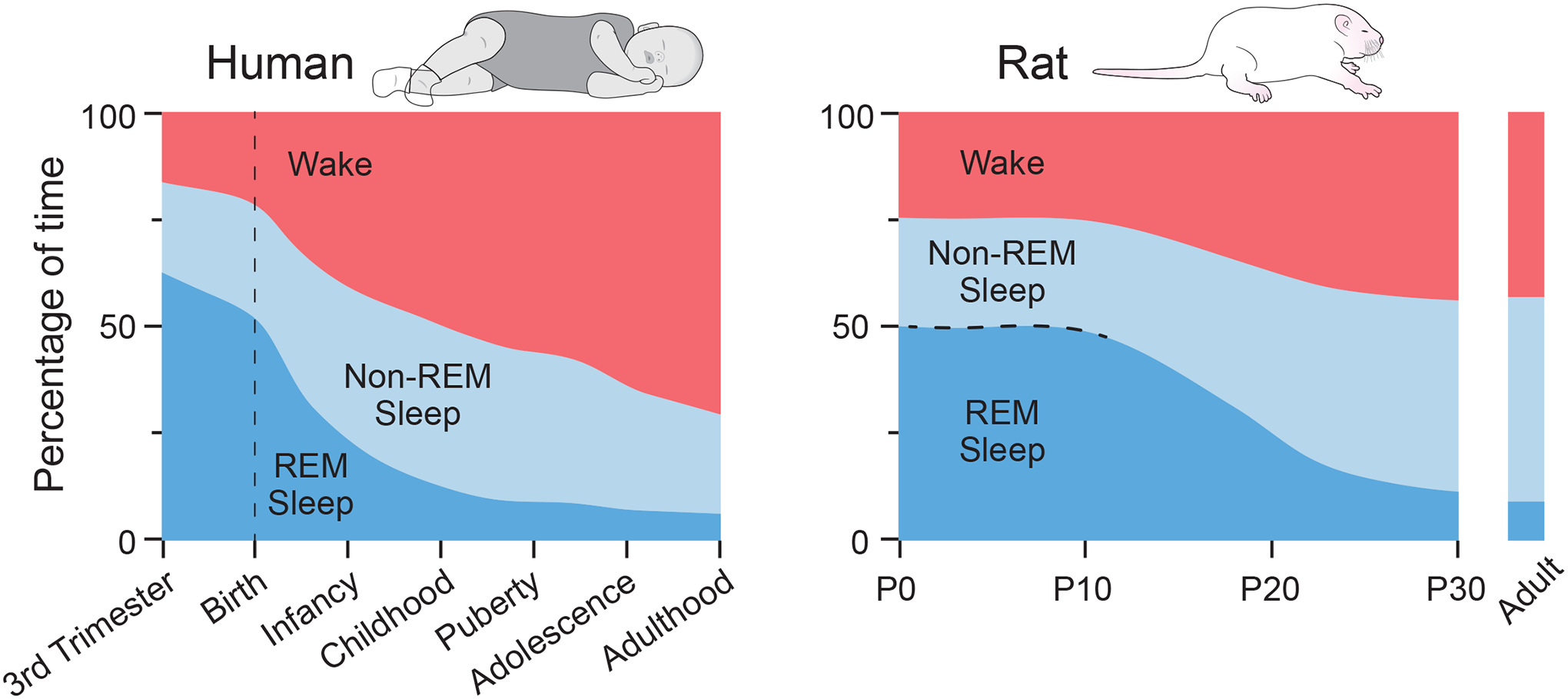
Sleep Permeates Early Life
Developmental changes in the percentage of REM sleep (blue) in humans (left) and rats (right) in relation to non-REM sleep (light blue) and wake (red). The dotted line for the rat data indicates uncertainty about the quantity of non-REM sleep before the emergence of cortical delta activity around P11. Adapted from Knoop et al. (2021) and Jouvet-Mounier et al. (1970).
The relative abundance of REM sleep in early life inspired the ontogenetic hypothesis, which posited functional links between REM sleep and development (Roffwarg et al., 1966). Consistent with the ontogenetic hypothesis, a formal model using available human data of sleep times, brain and body size, and brain and body metabolic rate from birth to adulthood showed that REM sleep specifically contributes to neural plasticity in early development (Cao et al., 2020). Nonetheless, many otherwise excellent recent reviews of sensory neurodevelopment largely ignore the role of sleep (e.g., Briscoe and Marín, 2020; Martini et al., 2021; Molnar et al., 2020; Sitko and Goodrich, 2021). Thus, here we aim to show how considerations of sleep enrich our understanding of neurodevelopment and help to identify paths to further progress. Toward this aim, we describe three conceptual frameworks that differ as to whether and how sleep contributes to the neurodevelopment of sensory and sensorimotor systems (Figure 2).
Figure 2.
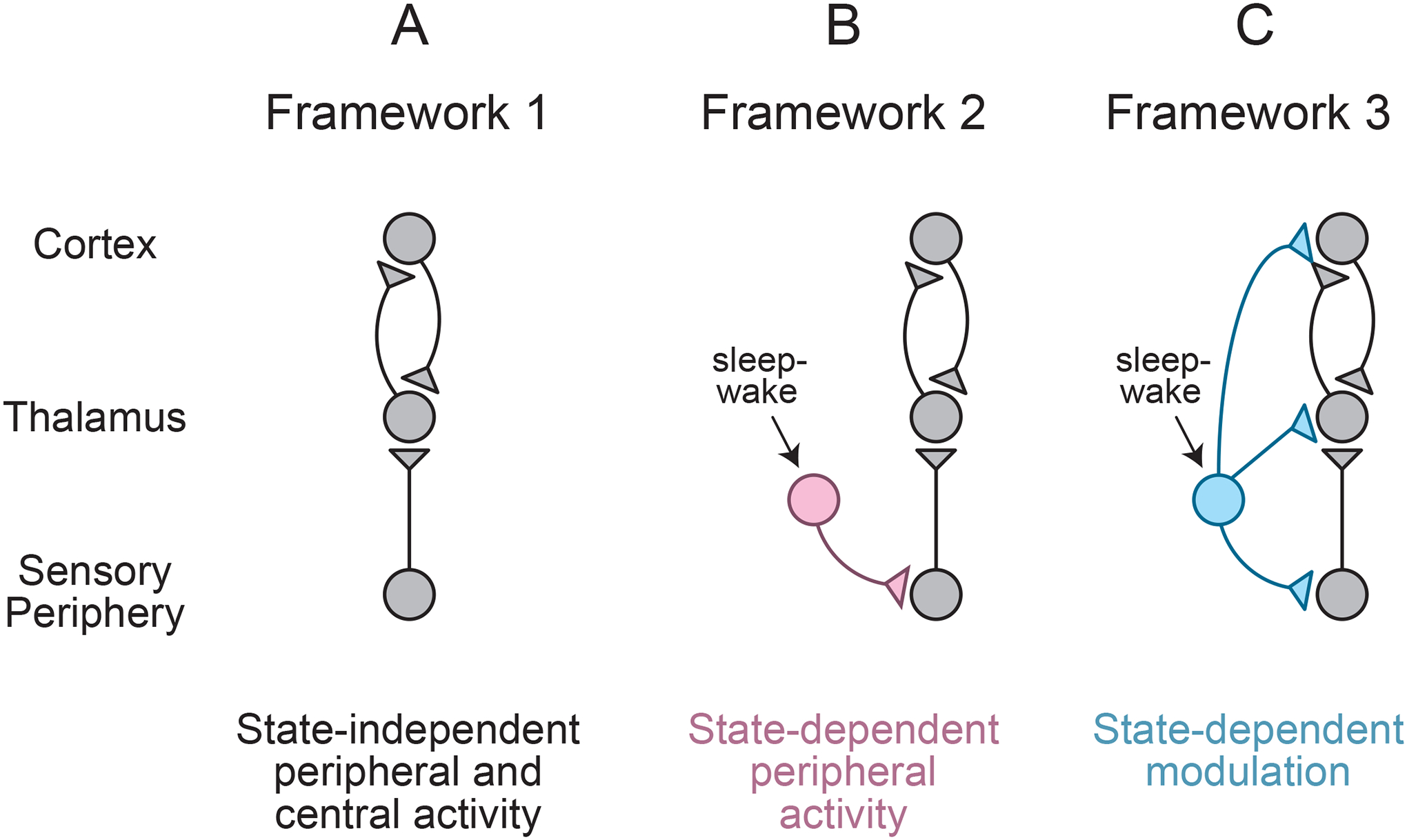
Frameworks Describing the Contributions of Peripheral and Central Neural Activity to Sensory Neurodevelopment
(A) State-independent spontaneous activity in the sensory periphery, thalamus, and/or cortex.
(B) State-dependent modulation (pink) of the sensory periphery triggers downstream activation of thalamus and cortex.
(C) State-dependent modulation at multiple levels of the sensory system (blue).
Framework 1: State-independent spontaneous activity in the sensory periphery and central neural circuits
The most common framework for understanding sensory neurodevelopment largely ignores a possible contribution of behavioral state (Figure 2A). This framework focuses on spontaneous, intrinsic activity in the sensory periphery or downstream neural structures (Martini et al., 2021). For example, spontaneous bursts of activity in the cochlea are thought to contribute to the development of auditory cortex (Meng et al., 2021), and spontaneous bursts of activity in the olfactory bulbs are thought to play a similar role in olfactory cortex (Zhang et al., 2020). However, it is the visual system that provides the prototypical example of this framework.
Retinal waves and intrinsic brain activity in the infant visual system
Before rod and cone photoreceptors are sensitive to light, cells within the retina spontaneously depolarize and generate waves of activity that propagate across the retina and are conveyed to downstream visual-system structures (Ackman et al., 2012; Gribizis et al., 2019; Hanganu et al., 2006). In rodents, retinal waves begin before birth and continue until the end of the second postnatal week, around the time when the eyes open. The circuits responsible for generating and propagating retinal waves change across development. In mice, retinal waves are mediated by cholinergic circuits during the first postnatal week and glutamatergic circuits during the second postnatal week (Ford and Feller, 2012). Cholinergic waves are spontaneous and exhibit a rhythmic pattern of generation that changes with age (Maccione et al., 2014). In contrast, glutamatergic waves occur spontaneously but can also be triggered by light (Ge et al., 2021; Tiriac et al., 2018), exhibiting more varied rhythmicity than cholinergic waves (Maccione et al., 2014).
As demonstrated in vivo in infant mice, retinal waves are faithfully transferred to primary visual cortex (V1) and the superior colliculus in the midbrain (Ackman et al., 2012). Indeed, retinal waves appear to be the primary source of activity for those structures. It is not until the second postnatal week, just before eye opening, that V1 (though not the superior colliculus) gains some independence from retinal input (Gribizis et al., 2019). Overall, the faithful transmission of sensory activity points to a critical role for the periphery in driving and structuring input to the developing brain, as must be the case if brain and body are to form an integrated functional system.
Retinal waves play various roles in the development of visual circuits. For example, in knockout mice in which cholinergic waves are reduced by 80%, the refinement and eye-specific segregation of downstream visual-system structures are disrupted (Burbridge et al., 2014; Rossi et al., 2001). The knockouts also exhibit reduced direction selectivity in the retina (Tiriac et al., 2022) and superior colliculus (Wang et al., 2009), and a perturbed optokinetic reflex to horizontal motion (Wang et al., 2009). In addition to such demonstrations that the amount of retinal activity matters, disruption of the spatiotemporal patterns of retinal waves interferes with normal binocular segregation of retinal axons (Xu et al., 2011) and direction selectivity after eye opening (Ge et al., 2021).
Intrinsic activity in V1 and the thalamic lateral geniculate nucleus (LGN), and interactions between the two structures, may also contribute to development of the visual system. Indeed, a decade after the discovery of retinal waves, researchers showed that activity in the LGN and V1 occurs in the absence of input from the retina (Weliky and Katz, 1999). Using ferrets ten days before eye opening, neural activity in the LGN was recorded before and after transection of the optic nerves. Before transection, LGN neurons exhibit bursts of synchronized neural activity. After transection, LGN neurons continue to burst spontaneously; moreover, this spontaneous bursting depends on cortical feedback because it is abolished when ipsilateral V1 was ablated. Similarly, spontaneous V1 activity occurs after LGN blockade (Chiu and Weliky, 2001; Smith et al., 2018). Because LGN activity persists after transection of the optic nerve, these findings suggest a developmental role for intrinsic LGN activity.
However, these findings also reinforce the notion that the sensory periphery exerts a powerful influence on the quantity and patterning of downstream neural activity. Specifically, the immediate effect of optic-nerve transection in infant ferrets is the loss of all LGN activity for 50 minutes, with activity levels only gradually returning over the next six hours (Chiu and Weliky, 2001; Weliky and Katz, 1999). Also, the recovered LGN activity does not revert to its original spatiotemporal pattern, but instead reorganizes as a novel pattern. The recovery of LGN activity after optic-nerve transection may reflect a process of homeostatic plasticity, as occurs in other systems when neural input is experimentally altered (Turrigiano and Nelson, 2004). Finally, similar to the downstream effect of optic-nerve transection on LGN activity, LGN inactivation causes a 90% reduction in downstream V1 activity (Smith et al., 2018).
Thalamic calcium waves in the somatosensory system of fetal mice
Beyond the visual system, experiments in the whisker somatosensory system reveal the importance of thalamic activity in the development of cortical somatotopy. In the whisker system, the mechanoreceptors that transduce whisker movement convey somatosensory input through the brainstem to the thalamic ventral posteromedial nucleus (VPM) that, in turn, conveys input to the whisker representation in primary somatosensory cortex (S1; barrel cortex). In adult mice, the layout of the whiskers on the snout is represented by anatomical and functional maps in VPM and S1, where distinct clusters of neurons in each structure—called barreloids in VPM and barrels in S1—represent individual whiskers. The cortical barrel structure, evident by postnatal day (P) 4, emerges from patterned VPM input present in fetal mice (Antón-Bolaños et al., 2019). First, using brain slices from mice at embryonic day 17.5, electrical stimulation of VPM evoked calcium-mediated “thalamic waves” and also triggered downstream S1 activity. Next, knockout mice that did not exhibit propagating calcium waves in VPM did not develop somatotopic maps in barrel S1, perhaps due to an inability to refine thalamocortical axons and control cortical excitability. The authors concluded that the early development of mouse barrel cortex requires patterned input (in the form of calcium waves) arriving from the fetal VPM.
In summary, developmental studies in the visual and somatosensory systems that conform with the first framework indicate an interdependence of activity in the sensory periphery, thalamus, and cortex. Changes to the patterning of activity at any of the three levels can lead to aberrant developmental outcomes. However, these studies implicitly assume that neural activity in these systems occurs independent of behavioral state. In the next section, we provide an example of sleep-dependent sensory activity that profoundly activates the infant brain.
Framework 2: State-dependent activity in the sensory periphery
The second framework builds on the first by adding a mechanism that allows for state-dependent modulation of peripheral sensors (Figure 2B). As we will see, this framework is best illustrated by the sensorimotor system, but is not necessarily restricted to this system. For example, with regard to the visual system, state-dependent modulation of retinal waves has not been seriously considered. Although the optic nerve is almost entirely a sensory nerve, it does contain axons that travel from the brain to the retina: Such retinopetal axons are few in number, but their branches within the retina are extensive (Gastinger et al., 2006). Moreover, there are two types of retinopetal axons: one contains histamine arising from the hypothalamic tuberomammillary nucleus (TMN), and the other contains serotonin arising from the brainstem dorsal raphe (DRN). In adult mice, increasing histamine in the retina alters ganglion-cell activity (Warwick et al., 2022), and both the TMN and DRN are strongly modulated by sleep-wake states (Jones, 2020). But whether retinal waves are modulated by either structure via retinopetal projections is unknown.
Myoclonic twitches are sleep-dependent drivers of activity in the sensorimotor system
For our discussion of the second framework and the sensorimotor system, we focus on myoclonic twitching, a unique behavior that occurs during REM sleep and that, like retinal waves, triggers downstream neural activity. Why would REM sleep have its own unique behavior? The answer may lie in the specific requirements of the developing sensorimotor system. In newborn rats and mice, retinal waves occur before eye opening when the visual system has only one primary function—that is, to develop. In contrast, the neonatal sensorimotor system must develop while also producing functional wake behaviors. The sensorimotor system achieves this end using a time-sharing approach that entails state-dependent modulation of the structures that produce movement. The use of time-sharing in the sensorimotor system reflects its unique demands and constraints; in contrast, because retinal waves occur before eye opening (at P15 in rats) and thus before the visual system is used to guide behavior, that system is freed from the need to time-share (Figure 3).
Figure 3.
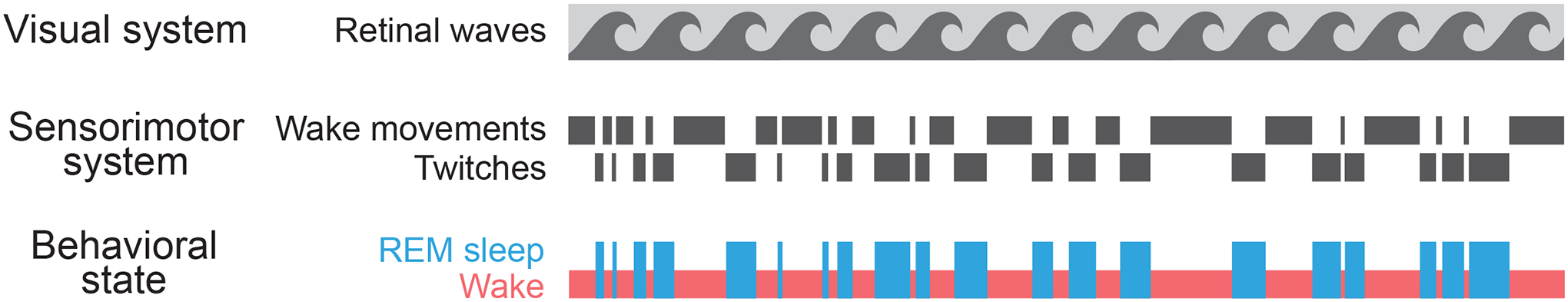
State-Dependent Time-Sharing in the Developing Sensorimotor System, But Not the Visual System
Bottom row: A 20-minute segment of representative data from a P5 rat showing transitions between REM sleep (blue) and wake (red). Middle row: In the sensorimotor system, the limb movements characteristic of REM sleep (i.e., twitches) are qualitatively different from those during wake, made possible by state-dependent time-sharing of the system. Top row: No such time-sharing appears to occur in the visual system; as currently understood, retinal waves are triggered independent of sleep-wake state.
Alternations between twitches and wake movements require state-dependent modulation of the brainstem motor structures responsible for neonatal movement (Mukherjee et al., 2018; Rio-Bermudez et al., 2015). This modulation produces two forms of movement with their own distinct features (Blumberg et al., 2020a). Specifically, whereas twitches are brief and discrete, wake movements are continuous and coordinated. And whereas twitches are produced against a background of muscle atonia (a second component of REM sleep), wake movements are produced against a background of high muscle tone. Wake movements also differ from twitches in that they are sufficiently organized to effect such functional outcomes as locomotion and suckling.
Twitches may not produce locomotion or achieve any self-evident behavioral goal, but the sensory feedback arising from tens of thousands of daily twitches does activate the neonatal nervous system (Blumberg et al., 2020a) (Figure 4). Briefly, when a twitch occurs, proprioceptors within the limb (and tactile receptors if the limb contacts a surface) are stimulated and reafference is triggered. Sensory signals activate neural circuits in the spinal cord (Inácio et al., 2016), contributing to the self-organization of spinal circuits (Blumberg et al., 2015; Granmo et al., 2008; Petersson et al., 2003). Beyond the spinal cord, reafference from twitches triggers a cascade of spiking activity throughout the sensorimotor system—from the medulla to the midbrain, cerebellum, thalamus, cortex, and hippocampus (Blumberg et al., 2020a). All this discrete twitch-related brain activity may be particularly important for developing and refining sensorimotor circuits and somatotopic maps, an idea that has received additional support from computational studies using computer simulations and robots (Blumberg et al., 2013; Dujany et al., 2020; Marques et al., 2014).
Figure 4.
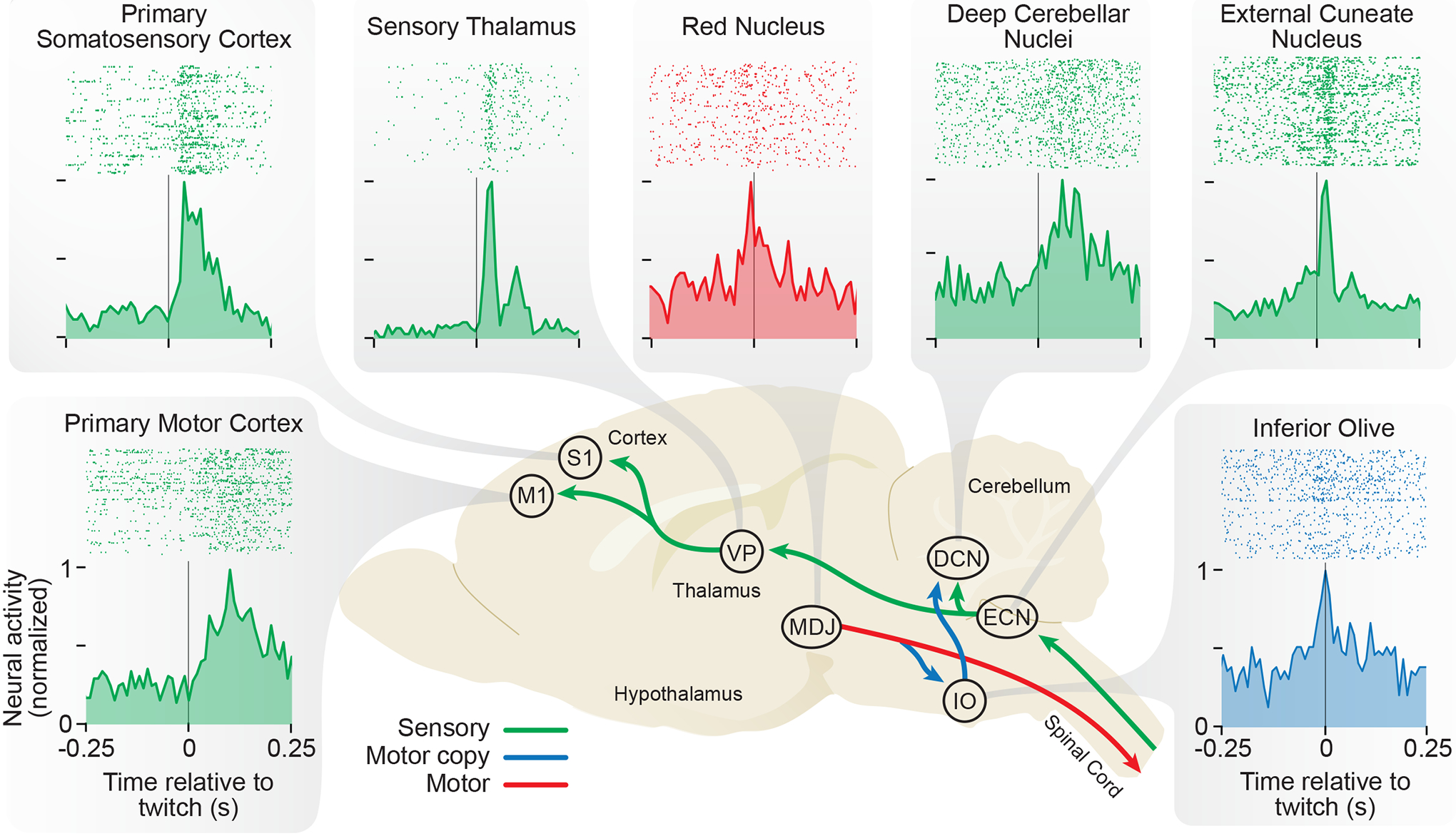
Diversity of Twitch-Related Neural Activity In the Infant Rat Brain
Representative perievent histograms depicting neural activity recorded from P8 rats in relation to forelimb twitches. The background image depicts a sagittal section of the infant brain. The production of a forelimb twitch begins in the brainstem, including the red nucleus and other motor structures within the mesodiencephalic junction (MDJ). The forelimb movement activates proprioceptors and triggers sensory feedback (reafference) that flows to the external cuneate nucleus (ECN) in the medulla, the ventral posterior nucleus (VP) in the thalamus, and primary somatosensory (S1) and primary motor (M1) cortex. The RN and adjacent motor neurons also convey motor copies (corollary discharge) to the inferior olive (IO) that, in turn, projects to the deep cerebellar nuclei (DCN). For clarity, not all structures known to exhibit twitch-related activity are shown. Arrows and histograms are color-coded based on whether they reflect motor commands (red), motor copies (blue), or sensory feedback (green). See text for references.
Much more is currently known about the phenomenology of twitching than about its functions. However, given the predominance of sleep in neonates and the abundance of twitch-related neural activity, it is reasonable to expect twitches to play outsized roles in the activity-dependent development, refinement, and maintenance of neural circuits throughout the sensorimotor system. For example, neural activity is implicated in the regulation of apoptosis (natural cell death) in cerebral cortex. Apoptosis exhibits two surges in mice: one around embryonic day 14 and the other in the first postnatal week (Nikolić et al., 2013). During the second surge, more than 30% of cortical neurons die. In S1 and primary motor cortex (M1), region-specific levels of apoptosis are moderated by cortical activity, with increased activity associated with decreased apoptosis (Blanquie et al., 2017). Given the quantity of twitch-dependent reafference to S1 and M1 over in the first postnatal week, it is likely that the refinement of cortical circuits via apoptosis is at least partly modulated by sleep.
The quantity of twitching matters, as does its patterning. For example, in P8 rats, each individual forelimb twitch triggers activity in a substantial percentage of neurons in the forelimb region of M1 (Dooley and Blumberg, 2018; Glanz et al., 2021). This pattern of activity, which becomes sparser by P12, reflects amplification of sensory input and dense coding, features that may be particularly effective for enhancing synaptic activity and somatotopic circuit formation in early development (Colonnese and Phillips, 2018).
Are rapid eye movements twitches of the extraocular muscles?
Eye movements, like limb movements, are controlled by striated muscles. Specifically, six extraocular muscles, controlled by several brainstem motor nuclei, enable each eye’s full range of motion. The brainstem nuclei are controlled by a complex sensorimotor system that, like the system that controls limb twitches, is modulated in a state-dependent manner. During wake, this system establishes the resting position of the eyes, produces saccadic eye movements, compensates for head and body movements, and integrates corollary discharge signals to track self-produced eye movements (Tehovnik et al., 2021). During REM sleep, this same system produces rapid eye movements.
One longstanding, popular view of rapid eye movements in human adults is that they reflect the visual scanning of dreams (Leclair-Visonneau et al., 2010); there are reasons to question this hypothesis (Blumberg and Plumeau, 2016). Moreover, from a developmental perspective, rapid eye movements are most parsimoniously interpreted as twitches of the extraocular muscles (Seelke et al., 2005). In infant rats as early as P3, twitches of the extraocular muscles occur during periods of limb twitching. However, because the eyes are not yet able to move freely within the socket, these extraocular muscle twitches are not yet able to produce eye movements. As eye mobility increases with age, extraocular muscle twitches are increasingly able to produce rapid eye movements.
We are not aware of evidence that rapid eye movements trigger sensory feedback—a seemingly necessary condition for their playing a role in the functional development of the neural circuits that support this system. However, in awake adult humans and rhesus macaques, oculomotor proprioception conveys information about eye position to S1 (Sun and Goldberg, 2016). Also, in humans during REM sleep, neural activity associated with rapid eye movements occurs not only in the visual system, but also in motor cortex and other non-visual structures (Hong et al., 2009). These findings suggest that rapid eye movements play a role in developing and refining the visual system’s sensorimotor circuitry, analogous to their role in calibrating the adult system (Herman and Roffwarg, 1983).
In summary, twitching provides the prototypical example of the second framework. However, as we will see in the next section, sleep-dependent activity in the sensory periphery represents only a fraction of the ways in which sleep shapes activity in the developing brain.
Framework 3: State-dependent modulation of sensory circuits
A third framework, depicted in Figure 2C, is necessary because the first two are incomplete. For example, the first framework (Figure 2A) is unable to account for the modulation of downstream brain activity that occurs after a retinal wave is triggered. Specifically, in P5–6 rats, retinal waves trigger thalamocortically generated spindle bursts in V1, and the rate of spindle bursts depends in part on cholinergic input from the basal forebrain (Hanganu et al., 2007). Although sleep was not monitored in that study, cholinergic neurons in the basal forebrain of adult rats are most active during REM sleep (Jones, 2020). Moreover, two recent studies provide additional evidence of state-dependent modulation of downstream visual-system structures. In P9 and P12 rats, rhythmic bursts of V1 activity occur 30–60 seconds apart, consistent with input from retinal waves (Mukherjee et al., 2017). However, when pups wake up and move their limbs—regardless of whether the awakening occurs spontaneously or is experimentally evoked—V1 bursts are suppressed. This wake-movement-related inhibition of spiking activity in V1 (and LGN) is developmentally transient: Whereas neural activity is inhibited by movement at P11, two days later it is enhanced by movement (Murata and Colonnese, 2018).
The second framework, depicted in Figure 2B, also cannot account for a number of observed phenomena in the sensorimotor system. For example, because sleep is typically considered a period of sensory isolation, it is surprising that twitch-related reafference so powerfully activates the sleeping infant brain. Even more surprising, however, is the consistent failure to find—in otherwise identical conditions—similar reafferent activation of the brain during wake movements. In neonatal rats, this state-dependent gating of wake-related reafferent processing occurs in the limbs and whiskers (Dooley et al., 2020; Tiriac et al., 2014). For the forelimbs, at least, the gating is transient, as it disappears by P11 (Dooley and Blumberg, 2018). Thus, before P11, twitch-related reafference is not only abundant—it is preferred. This preference of the pre-P11 brain for twitch-related over wake-related reafference requires state-dependent modulation of the processing of neural activity after it has been triggered in the sensory periphery.
The third framework incorporates the phenomena encompassed by the first two frameworks, while also capturing the diversity of ways that sleep modulates sensory processing and activity-dependent development throughout the nervous system. Before describing the research that illustrates this diversity, we first delve more deeply into the organization and functional significance of sleep-wake states, especially across early development.
Sleep comprises components and contexts
In adult mammals, REM sleep is composed of a suite of components: In addition to rapid eye movements, twitches, and muscle atonia, there is irregular respiration, activated EEG, and hippocampal theta. In contrast, non-REM sleep is characterized by high-amplitude slow waves and/or sleep spindles in the cortical EEG, regular respiration, and behavioral quiescence. Although the components of REM and non-REM sleep are tightly integrated in adults, that is not the case in early development (Blumberg et al., 2014, 2020b). For example, soon after birth in rats, REM sleep is identified primarily on the basis of muscle atonia accompanied by limb twitches. Over the first two postnatal weeks, additional REM-sleep components are added, including hippocampal theta, rapid eye movements, and an activated cortical EEG. Similarly, one of the core features of non-REM sleep—cortical slow waves—emerges only after P11. But, even as these sleep-related components emerge over development, it is easy to distinguish a pup that is asleep or awake based on its behavior alone.
A focus on the components of sleep is essential for appreciating the development and functions of sleep. But each sleep state is more than just the sum of its components: Sleep is also a context. Specifically, sleep entails the coalescence and regulation of its components in time, involving sleep-specific activation of a neural network that spans the neuraxis. Sleep-dependent increases or decreases in activity occur in neural structures, such as the locus coeruleus, raphe nuclei, and basal forebrain, that release such plasticity-promoting neuromodulators as norepinephrine, serotonin, and acetylcholine, respectively (Brzosko et al., 2019; Jones, 2020). Sleep also entails the differential allocation of energy to maximize vital processes such as growth, tissue maintenance and repair, and neural network reorganization (Schmidt, 2014). These modes of energy allocation are also intimately tied to how animals differentially regulate gene expression across the sleep-wake cycle to meet functional needs and energy demands. Thus, beyond their local effects on particular sensory systems, sleep-dependent neuromodulation and energy allocation produce global effects on nervous system function.
It is important to keep in mind the components and contexts of sleep when considering the ways in which researchers have sought to assess its functions. Specifically, when researchers deprive an animal of sleep, they are manipulating sleep as a context and thus affecting all features of sleep at once: each individual sleep component, the sleep-generating neural network, the system of plasticity-promoting neuromodulators, energy allocation, and gene expression. It is also possible, however, to perform more targeted manipulations of sleep, such as depriving an animal of an individual sleep component (Boyce et al., 2016).
Empirical support for the third framework derives in large part from studies in which total or selective sleep deprivation is used to assess sleep’s effects on neurodevelopment. In contrast, it is still rare to find developmental studies that use targeted manipulations of sleep components or subsystems. Instead, researchers have provided evidence for sleep’s developmental functions by demonstrating that sleep provides a unique opportunity for the expression of certain behavioral or neurophysiological phenomena (e.g., Dooley et al., 2021).
The representation of the third framework in Figure 2C is highly simplified and symbolic of the ways that sleep-wake modulation promotes plasticity in sensory neural circuits throughout the developing brain. In the sections that follow, we review specific examples of research findings across early development that fit within this framework. Some examples highlight the more global or contextual effects of sleep, including effects on plasticity in the visual and sensorimotor systems. Other examples highlight more specific ways that sleep-related neural activity contributes to plasticity in early life, including the development of internal models of movement in rats and sensorimotor learning in songbirds. Even as these examples reveal the diversity of sleep’s influences on early development, they also expose the need for a more systematic understanding of those influences at the behavioral, neural, cellular, and molecular levels.
Sleep modulates ocular dominance plasticity in the visual system
One effective strategy for investigating the global contributions of sleep to development is to deprive young animals of sleep during periods of enhanced neural plasticity—when a system is in the midst of rapid development change. For example, in cats (>P40) during a sensitive period of visual-system development, experimental occlusion of one eye (monocular deprivation) for two weeks reduces activation to downstream neurons in the LGN, causing cells in that structure to be smaller than normal; in contrast, LGN neurons that receive input from the unpatched eye are larger than normal. When kittens are selectively deprived of REM sleep for one week in the midst of monocular deprivation, the disparity in LGN cell sizes is even greater, suggesting that REM sleep provides intrinsic activation that complements the extrinsic activation from light (Shaffery et al., 1998). Indeed, researchers subsequently demonstrated that it wasn’t deprivation of REM sleep per se that produces the observed effects, but rather the loss of one component of REM sleep—namely, the ponto-geniculo-occipital (PGO) waves that activate the LGN (and V1) during REM sleep (Shaffery et al., 1999).
Researchers made further advances in understanding how sleep contributes to development of the visual system by targeting the period of peak ocular dominance plasticity (Frank et al., 2001). Using cats at ~P30, occlusion of one eye for as little as 6 hours is sufficient to trigger a plasticity event in V1, as detected using microelectrode recordings and optical imaging. Whereas a subsequent 6-hour period of sleep enhances the cortical plasticity induced by monocular deprivation, sleep deprivation blocks that enhancement. In cats that are allowed to sleep after 6 hours of monocular deprivation, neurons in V1 significantly increase their firing rates during periods of REM and non-REM sleep, but not wake, suggesting that both sleep states contribute to the synaptic strengthening underlying consolidation of cortical plasticity using this paradigm (Aton et al., 2009)
Because a bout of non-REM sleep typically precedes a bout of REM sleep, it is not possible to deprive animals of non-REM sleep selectively because REM sleep will necessarily also be affected. Instead, the contributions of non-REM sleep to ocular dominance plasticity after eye occlusion in cats were inferred from sleep-dependent changes in the temporal relations among fast-spiking interneurons and principle neurons in V1 (Aton et al., 2013). Moreover, the magnitude of the plasticity response was related to the magnitude of the change in firing rate of principle neurons—specifically during the slow waves of non-REM sleep.
The specific contribution of REM sleep to ocular dominance plasticity has also been investigated (Bridi et al., 2015). After occlusion of one eye for 6 hours, researchers assessed whether one hour of REM sleep deprivation alters the phosphorylation in V1 of two kinases (CaMKII and ERK) that are important for neural plasticity, including synaptic long-term potentiation (LTP). REM-sleep deprivation after eye occlusion specifically inhibits ERK phosphorylation, which was previously shown to be necessary for ocular dominance plasticity (Dumoulin et al., 2015). A subsequent study further demonstrated that after ocular dominance plasticity is triggered in cats, neurons are activated in V1 specifically during REM sleep (Renouard et al., 2018). Moreover, at the same time that V1 neurons are activated during REM sleep, several proteins involved in LTP—including mTOR and CREB—are expressed in visual cortex in a layer-specific manner; depriving cats of REM sleep prevents this layer-specific protein expression. In sum, these studies point to specific roles for both REM and non-REM sleep in a canonical form of developmental plasticity.
REM sleep modulates synaptic pruning
The pruning of synaptic connections is a foundational, activity-dependent process in early neural development. Here, too, REM sleep is implicated as a contributing contextual factor (Li et al., 2017). In P21 mice, researchers used two-photon microscopy to quantify the rate of dendritic spine formation on pyramidal neurons in M1. Compared with P30 mice, P21 mice exhibit significantly more REM sleep and higher rates of new dendrite formation; when P21 mice are allowed to sleep, newly formed dendrites are pruned, but this pruning is significantly reduced when mice are selectively deprived of REM sleep. The researchers found a similar relation between REM sleep and dendritic pruning in M1 using P30 mice previously trained on a motor task. Also, calcium transients on the dendrites of M1 pyramidal cells are more likely to occur during REM sleep; when the calcium transients are pharmacologically blocked, the pruning and subsequent strengthening of new spines are reduced as well—mimicking the effect of REM sleep deprivation. These findings were subsequently extended to two other cortical areas and experimental paradigms (i.e., ocular dominance plasticity in V1; auditory-cued fear condition in frontal associated cortex) in one-month-old mice (Zhou et al., 2020).
These studies are the first to directly implicate REM sleep in synaptic pruning. However, important questions remain regarding this phenomenon. For example, it is not known whether REM sleep modulates dendritic pruning at earlier ages (although sleep more generally has been implicated in the regulation of synaptic ultrastructure in two-week-old mice; Nagai et al., 2021). Also, the specific mechanisms by which REM sleep promotes pruning have not been identified: For example, does REM sleep exert its effects by altering the quantity or patterning of neural activity at the synapse or via the release of plasticity-promoting neuromodulators? Further, are the cellular and molecular mechanisms that guide REM-sleep-dependent synaptic pruning similar to those involved in ocular dominance plasticity? Answering such questions will help to establish the necessity of REM sleep for synaptic plasticity.
REM sleep promotes functional connectivity across distant sensorimotor structures
When neural rhythms in two distant brain structures oscillate synchronously, we infer that they are functionally connected. In developing rats, such coherent oscillations are particularly evident in sensorimotor structures during REM sleep, suggesting that REM sleep provides an important context for the early expression of these rhythms. For example, the hippocampal theta rhythm is a low-frequency (4–8 Hz) oscillation that, in adults, is closely associated with neural plasticity (Boyce et al., 2016; Puentes-Mestril et al., 2019). In the hippocampus of P8 rats, theta first emerges as brief bursts in response to twitches (Mohns and Blumberg, 2008); by P12, hippocampal theta oscillations occur continuously during periods of REM sleep. A similar developmental pattern occurs in the red nucleus: Twitch-triggered theta bursts at P8 are followed by continuous theta activity during REM sleep at P12 (Rio-Bermudez et al., 2017). Moreover, at P12, theta activity occurs synchronously in the hippocampus and red nucleus specifically during REM sleep. Thus, REM sleep provides the initial context for the expression of theta.
In addition to theta, twitches also trigger spindle bursts in S1 of infant rats (Khazipov et al., 2004) and in premature human infants (Milh et al., 2007). Spindle bursts are brief (<1 s) thalamocortical oscillations with a dominant frequency of ~15 Hz. Since their initial discovery in S1, spindle bursts were detected in M1 in response to twitches (Tiriac et al., 2014) and in V1 in response to retinal waves (Hanganu et al., 2006). Spindle bursts, which are typically called delta brushes in human infants, are now established as reliable features of early cortical development in mammals (Murata and Colonnese, 2019).
The period of prominent spindle-burst production overlaps with a period when somatotopic whisker maps are being refined (Mitrukhina et al., 2015). Moreover, during this same period, whisker twitches are a prominent trigger for spindle bursts in barrel cortex (Dooley et al., 2020). Although spindle bursts have a dominant frequency of ~15 Hz, they actually comprise a range of frequencies, even extending into the beta2 (20–30 Hz) and slow gamma (30–50 Hz) bands. To determine whether one or more of these spindle-burst frequency bands in barrel cortex are transmitted to the dorsal hippocampus, dual recordings were performed in the two structures in P8 rats (Rio-Bermudez et al., 2020). It was found that cortico-hippocampal coherence increases specifically in the beta2 band during REM sleep, especially in the immediate period after a twitch. Moreover, when sensory feedback from the whiskers is blocked, coherent beta2 activity disappears. Thus, as with the theta rhythm, REM sleep provides a context that enables long-distance communication in the infant brain.
Twitch-related corollary discharge during REM sleep and the emergence of internal models of movement
In addition to depicting the motor structures that produce twitches and the sensory structures that receive twitch-related reafference, Figure 4 also depicts the conveyance of twitch-related motor copies (or corollary discharges) from brainstem motor structures to precerebellar nuclei (Mukherjee et al., 2018). In effect, corollary discharge alters how movement-related sensory signals are interpreted, enabling the distinction between self (reafference) and other (exafference) (Crapse and Sommer, 2008). Also, in adults, the comparison of corollary discharge and reafferent signals over many individual movements enables animals to compute representations—or internal models—in the cerebellum that allow for accurate predictions of the sensory consequences of self-generated movements (Wolpert et al., 1998).
When researchers contemplated the developmental origins of internal models 30 years ago (Miall et al., 1993), they surmised that internal models develop through a learning process that entails the production of self-generated movements followed by comparisons of actual and estimated sensory feedback. They further surmised that discrete movements would be ideal for such a learning process. However, their suggestion was made without reference to any particular behavior—let alone one that occurs during sleep. But as we now know, discrete twitches and associated neural activity are abundant in early development, thus raising the possibility that twitches contribute to the development of internal models.
This possibility was tested by recording from the ventral lateral thalamic nucleus (VL) during sleep and wake in P12, P16, and P20 rats (Dooley et al., 2021). VL receives the major ascending output from the cerebellum and conveys that information to M1. For VL to reflect the output of an internal model, its activity should precisely mimic the kinematic properties of a twitch—occurring neither before nor after the movement, but with the movement. By P20, that is exactly what was found. In contrast to VL, the adjacent ventral posterior thalamic nucleus (VP) receives only sensory input, and its neural activity reliably follows the limb movement during a twitch. Then, when cerebellar output is inactivated and VL no longer has access to the internal model, neurons in VL and VP exhibit similar sensory responses. Finally, in contrast with twitches, it was difficult to unambiguously assess the relation between wake movements and VL activity, suggesting that twitches are more effective than wake movements for building internal models in the developing brain. To demonstrate that this is the case, future research will need to determine whether state-dependent neuromodulation enables the development and maintenance of internal models.
Non-REM sleep may promote sensorimotor plasticity in human infants
We described above how sensory input from retinal waves and twitches trigger spindle bursts in V1, S1, and M1. Although spindle bursts are a prominent feature of early cortical development, their prominence is short-lived. In human infants, spindle bursts are no longer detected by the end of the first postnatal month (Whitehead et al., 2018), as is also the case for rat pups by the end of the second postnatal week (Colonnese and Phillips, 2018). After spindle bursts fade away in human newborns, a second thalamocortical oscillation with a similar dominant frequency (12–15 Hz) but a specific affiliation with non-REM sleep—called sleep spindles—emerges around one month of age, becoming more frequent, robust, and clock-like over the next several months (Sokoloff et al., 2021; Wakai and Lutter, 2016). Sleep spindles are strongly implicated in neural plasticity, including sensorimotor plasticity, in early development and across the lifespan in both humans and rodents (Fernandez and Lüthi, 2020).
Surprisingly, limb twitches in human infants are not exclusive to REM sleep; beginning around three months of age, they also occur during non-REM sleep (Sokoloff et al., 2021). Moreover, also beginning around three months of age, non-REM sleep is increasingly replete with sleep spindles that are expressed most prominently in sensorimotor cortex. Finally, the twitches of the arms and legs during non-REM sleep are synchronized with sleep spindles. In other words, in addition to all that is known about twitching during REM sleep, twitching also occurs in concert with a plasticity-promoting EEG component of non-REM sleep. This discovery introduces a new and unexpected form of sleep-dependent sensorimotor plasticity.
Important questions remain. For example, we do not yet know whether twitching during non-REM sleep persists beyond 6 months of age, or whether it is unique to humans or primates. Answering such questions will be important for determining the functional significance of this unique form of twitching and how it differs from its counterpart during REM sleep.
Sleep promotes sensorimotor learning in young songbirds and visual imprinting in chicks
Although we have focused thus far on mammals, we conclude by discussing the links between sleep and neurodevelopment in juvenile songbirds and newly hatched chicks. Songbirds, which comprise about half of all avian species, learn their species-typical songs through a complex process of memorization and sensorimotor learning. Young birds begin their foray into singing by producing highly variable vocalizations. Next, they memorize a song template provided by an adult “tutor” and then gradually improve their singing performance by comparing auditory feedback against the memorized template. Throughout this period of sensorimotor learning, and also after this period when songs must be maintained, neural activity during sleep plays an important role (for review, see Giret, 2019).
In zebra finches, male birds first begin to sing at 30 days post-hatching and refine their song over the next 60 days. By continuously recording song production from days 43 to 90, researchers found that song quality (as measured against the adult template) deteriorates overnight as the birds sleep (Deregnaucourt et al., 2005). Overnight deterioration of song quality is most pronounced in the younger birds and disappears with age as sound quality reaches adult levels. Surprisingly, the birds that exhibit the most pronounced overnight deterioration exhibit more accurate songs by 90 days of age. Additional experiments suggested that sleep-related unstructuring of song opens opportunities for plasticity and further improvements in song quality. Thus, in young birds, competition between plasticity and consolidation may lead to the observed day-to-day variation in song quality.
To investigate the neural substrates of songbird learning in juvenile zebra finches, researchers recorded from the robust nucleus of the arcopallium (RA), a premotor structure that innervates the motor nucleus that controls the syrinx (the avian singing apparatus) (Shank and Margoliash, 2009). On the first night after daytime exposure to an adult tutor song, spontaneous RA bursting increases dramatically. Importantly, the firing-rate properties of RA neurons are shaped by the specific tutor song to which the birds are exposed. And when birds are blocked from hearing their songs during the day, RA activity at night is reduced. Thus, RA appears to mediate some of the sleep-dependent plasticity that occurs during the practice phase. An additional contributing structure to this plasticity may be the HVC, a forebrain nucleus immediately upstream to RA that merges song-related sensory and motor information: Song-related auditory input to HVC in juvenile birds is modulated by sleep-wake state (Nick and Konishi, 2005). Also, as measured by changes in the strength and stability of dendritic spines, neural plasticity appears to occur in HVC (Roberts et al., 2010).
Sleep also appears to promote the process by which newly hatched chicks learn about—and imprint on—the visual features of biologically relevant stimuli (Jackson et al., 2008). The imprinting process occurs over several hours post-hatching, when increasing numbers of neurons in the medial mesopallium (a likely homologue of mammalian cortex) are selectively responsive to the imprinting stimulus. The size of this population of responsive mesopallium neurons decreases when chicks’ sleep is experimentally disturbed. Because non-REM sleep is the predominant sleep state of undisturbed chicks, the findings suggested a specific role for non-REM sleep in the consolidation of the imprinted memory.
Thus, in both songbirds and chicks, sleep provides an important context for sensorimotor and visual-system plasticity, respectively. However, even more so than with mammals, the specific mechanisms by which sleep enables this plasticity are largely unknown. Moving forward, comparative investigations in mammals and birds will broaden our understanding of the mechanisms, functions, and evolutionary history of sleep-dependent neural plasticity in early life.
Concluding comments and future directions
Young animals change more rapidly than they do at any other time of life. Against this backdrop of rapid change, sleep is a consistent presence that profoundly shapes behavior and brain activity. At the neural level, REM sleep provides the impetus and context for synchronized spiking activity and coherent rhythmic activity, thus enabling its contributions to developmental plasticity.
The three frameworks described here share the recognition that the sensory periphery provides structured input to enable the integration of body and brain. Whereas both the second and third frameworks acknowledge a role for sleep in sensory development, only the third framework is able to capture all the ways that sleep shapes the development of neural circuits across the neuraxis. Accordingly, we argue that the third framework should guide future research in this domain.
At this time, the specific mechanisms by which sleep exerts its effects on sensory neurodevelopment are only beginning to be explored. These mechanisms include sleep-dependent molecular signaling (Abel et al., 2013) and the release of plasticity-promoting neuromodulators (Jones, 2020), two domains that offer enormous opportunities for future developmental research. Framework 3 is meant to capture these and all other mechanisms by which sleep exerts its global and specific effects. That said, we do not expect these mechanisms to apply identically across sensory modalities. For example, we highlighted one constraint—time-sharing—that differentiates the developing visual and sensorimotor systems (Figure 3); other constraints in other modalities (e.g., audition, olfaction, gustation) may similarly determine whether and how sleep contributes to their structural and functional development. Finally, although our focus here has been on sleep’s contributions to sensory neurodevelopment, aspects of Framework 3 apply to neurodevelopment more broadly.
Beyond mammals and birds, investigations in flies, worms, and zebrafish show that they also sleep most in early development. Accordingly, these animals offer additional and often unique opportunities for testing hypotheses about the developmental functions of sleep (Kayser and Biron, 2016). For example, using larval flies, researchers identified a sleep state that, when experimentally disrupted, significantly decreases neurogenesis—thus directly implicating sleep in neural stem cell proliferation (Szuperak et al., 2018). In addition, sensory feedback from twitches in larval flies is necessary for typical neural circuit development and behavior (Carreira-Rosario et al., 2021; Zeng et al., 2021). Thus, invertebrates expand the toolkit for investigating how functional neural circuits are established in early development. They may also prove better suited to resolving such fundamental mysteries as why young animals sleep more than older ones (Dilley et al., 2020).
The functional contributions of sleep are expected to change as new sleep components emerge across development, as bouts of sleep and wake consolidate, as circadian regulation of sleep-wake states emerges, and as the increasing complexity of waking experience through adolescence and adulthood introduces new functional challenges. Thus, although some of the functions of sleep may be consistent across the lifespan, others may emerge, transform, and even disappear depending on an animal’s life-history, ecological circumstances, and behavioral and cognitive demands.
Finally, independent of sleep, there is accelerating interest in understanding the origins and trajectories of neurodevelopmental disorders (Briscoe and Marín, 2020; Molnar et al., 2020). At the same time, dysregulation of sleep is increasingly garnering attention for its high prevalence in these disorders (e.g., Kamara and Beauchaine, 2019; MacDuffie et al., 2020). Thus, opportunities abound for integrating sleep with developmental neuroscience so as to provide a more comprehensive understanding of typical and atypical development.
Acknowledgments
We thank Karen Adolph, Marcos Frank, and Matthew Kayser for many helpful comments. Preparation of this paper was made possible by a grant from the National Institutes of Health (R37-HD081168) to M.S.B.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
The authors declare no competing interests.
References
- Abel T, Havekes R, Saletin JM, and Walker MP (2013). Sleep, plasticity and memory from molecules to whole-brain networks. Curr Biol 23, R774–R788. 10.1016/j.cub.2013.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ackman JB, Burbridge TJ, and Crair MC (2012). Retinal waves coordinate patterned activity throughout the developing visual system. Nature 490, 219–225. 10.1038/nature11529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antón-Bolaños N, Sempere-Ferràndez A, Guillaḿon-Vivancos T, Martini FJ, Pérez-Saiz L, Gezelius H, Filipchuk A, Valdeolmillos M, and López-Bendito G (2019). Prenatal activity from thalamic neurons governs the emergence of functional cortical maps in mice. Science 364, eaav7617–9. 10.1126/science.aav7617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aton SJ, Seibt J, Dumoulin M, Jha SK, Steinmetz N, Coleman T, Naidoo N, and Frank MG (2009). Mechanisms of sleep-dependent consolidation of cortical plasticity. Neuron 61, 454–466. 10.1016/j.neuron.2009.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aton SJ, Broussard C, Dumoulin M, Seibt J, Watson A, Coleman T, and Frank MG (2013). Visual experience and subsequent sleep induce sequential plastic changes in putative inhibitory and excitatory cortical neurons. Proc National Acad Sci 110, 3101–3106. 10.1073/pnas.1208093110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanquie O, Yang J-W, Kilb W, Sharopov S, Sinning A, and Luhmann HJ (2017). Electrical activity controls area-specific expression of neuronal apoptosis in the mouse developing cerebral cortex. ELife 6, e27696. 10.7554/elife.27696.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumberg MS (2009). Freaks of nature: What anomalies tell us about development and evolution (New York: Oxford University Press; ). [Google Scholar]
- Blumberg MS, and Plumeau AM (2016). A new view of “dream enactment” in REM sleep behavior disorder. Sleep Med Rev 39, 34–42. 10.1016/j.smrv.2015.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumberg MS, and Rattenborg NC (2017). Decomposing the evolution of sleep: Comparative and developmental approaches. In Evolution of Nervous Systems, Kaas JH, ed. (Oxford: Elsevier; ), pp. 523–545. [Google Scholar]
- Blumberg MS, Marques HG, and Iida F (2013). Twitching in sensorimotor development from sleeping rats to robots. Curr Biol 23, R532–R537. 10.1016/j.cub.2013.04.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumberg MS, Gall AJ, and Todd WD (2014). The development of sleep–wake rhythms and the search for elemental circuits in the infant brain. Behav Neurosci 128, 250–263. 10.1037/a0035891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumberg MS, Coleman CM, Sokoloff G, Weiner JA, Fritzsch B, and McMurray B (2015). Development of twitching in sleeping infant mice depends on sensory experience. Curr Biol 25, 656–662. 10.1016/j.cub.2015.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumberg MS, Dooley JC, and Sokoloff G (2020a). The developing brain revealed during sleep. Curr Opin Physiol 15, 14–22. 10.1016/j.cophys.2019.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumberg MS, Lesku JA, Libourel P-A, Schmidt MH, and Rattenborg NC (2020b). What Is REM Sleep? Curr Biol 30, R38–R49. 10.1016/j.cub.2019.11.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyce R, Glasgow SD, Williams S, and Adamantidis A (2016). Causal evidence for the role of REM sleep theta rhythm in contextual memory consolidation. Science 352, 812–816. 10.1126/science.aad5252. [DOI] [PubMed] [Google Scholar]
- Bridi MCD, Aton SJ, Seibt J, Renouard L, Coleman T, and Frank MG (2015). Rapid eye movement sleep promotes cortical plasticity in the developing brain. Sci Adv 1, e1500105–e1500105. 10.1126/sciadv.1500105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briscoe J, and Marín O (2020). Looking at neurodevelopment through a big data lens. Science 369, eaaz8627–10. 10.1126/science.aaz8627. [DOI] [PubMed] [Google Scholar]
- Brzosko Z, Mierau SB, and Paulsen O (2019). Neuromodulation of spike-timing-dependent plasticity: Past, present, and future. Neuron 103, 563–581. 10.1016/j.neuron.2019.05.041. [DOI] [PubMed] [Google Scholar]
- Burbridge TJ, Xu H-P, Ackman JB, Ge X, Zhang Y, Ye M-J, Zhou ZJ, Xu J, Contractor A, and Crair MC (2014). Visual circuit development requires patterned activity mediated by retinal acetylcholine receptors. Neuron 84, 1049–1064. 10.1016/j.neuron.2014.10.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao J, Herman AB, West GB, Poe G, and Savage VM (2020). Unraveling why we sleep: Quantitative analysis reveals abrupt transition from neural reorganization to repair in early development. Sci Adv 6, eaba0398. 10.1126/sciadv.aba0398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carreira-Rosario A, York RA, Choi M, Doe CQ, and Clandinin TR (2021). Mechanosensory input during circuit formation shapes Drosophila motor behavior through patterned spontaneous network activity. Curr Biol 31, 5341–5349. 10.1016/j.cub.2021.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu C, and Weliky M (2001). Spontaneous activity in developing ferret visual cortex in vivo. J Neurosci 21, 8906–8914.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colonnese MT, and Phillips MA (2018). Thalamocortical function in developing sensory circuits. Curr Opin Neurobiol 52, 72–79. 10.1016/j.conb.2018.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crapse TB, and Sommer MA (2008). Corollary discharge across the animal kingdom. Nat Rev Neurosci 9, 587–600. 10.1038/nrn2457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dempsey-Jones H, Wesselink DB, Friedman J, and Makin TR (2019). Organized toe maps in extreme foot users. Cell Reports 28, 2748–2756.e4. 10.1016/j.celrep.2019.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deregnaucourt S, Mitra P, Feher O, Pytte C, and Tchernichovski O (2005). How sleep affects the developmental learning of bird song. Nature 433, 710–716. 10.1038/nature03275. [DOI] [PubMed] [Google Scholar]
- Dilley LC, Szuperak M, Gong NN, Williams CE, Saldana RL, Garbe DS, Syed MH, Jain R, and Kayser MS (2020). Identification of a molecular basis for the juvenile sleep state. ELife 9, e52676. 10.7554/elife.52676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dooley JC, and Blumberg MS (2018). Developmental “awakening” of primary motor cortex to the sensory consequences of movement. ELife 7, e41841. 10.7554/elife.41841.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dooley JC, Glanz RM, Sokoloff G, and Blumberg MS (2020). Self-generated whisker movements drive state-dependent sensory Input to developing barrel cortex. Curr Biol 30, 2404–2410.e4. 10.1016/j.cub.2020.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dooley JC, Sokoloff G, and Blumberg MS (2021). Movements during sleep reveal the developmental emergence of a cerebellar-dependent internal model in motor thalamus. Curr Biol 31, 5501–5511. 10.1016/j.cub.2021.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dujany M, Hauser S, Mutlu M, van der Sar M, Arreguit J, Kano T, Ishiguro A, and Ijspeert A (2020). Emergent adaptive gait generation through Hebbian sensor-motor maps by morphological probing. In 2020 IEEE/RSJ International Conference on Intelligent Robots and Systems (IROS; ), pp. 7866–7873. [Google Scholar]
- Dumoulin MC, Aton SJ, Watson AJ, Renouard L, Coleman T, and Frank MG (2015). Extracellular signal-regulated kinase (ERK) activity during sleep consolidates cortical plasticity in vivo. Cereb Cortex 25, 507–515. 10.1093/cercor/bht250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez LMJ, and Lüthi A (2020). Sleep spindles: Mechanisms and functions. Physiol. Rev 100, 805–868. 10.1152/physrev.00042.2018. [DOI] [PubMed] [Google Scholar]
- Ford KJ, and Feller MB (2012). Assembly and disassembly of a retinal cholinergic network. Visual Neurosci 29, 61–71. 10.1017/s0952523811000216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank MG, Issa NP, and Stryker MP (2001). Sleep enhances plasticity in the developing visual cortex. Neuron 30, 275–287. 10.1016/s0896-6273(01)00279-3. [DOI] [PubMed] [Google Scholar]
- Gastinger MJ, Tian N, Horvath T, and Marshak DW (2006). Retinopetal axons in mammals: Emphasis on histamine and serotonin. Curr Eye Res 31, 655–667. 10.1080/02713680600776119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge X, Zhang K, Gribizis A, Hamodi AS, Sabino AM, and Crair MC (2021). Retinal waves prime visual motion detection by simulating future optic flow. Science 373, eabd0830. 10.1126/science.abd0830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giret N (2019). The role of sleep in song learning processes in songbird. Handb Behav Neurosci 30, 395–410. 10.1016/b978-0-12-813743-7.00026-8. [DOI] [Google Scholar]
- Glanz RM, Dooley JC, Sokoloff G, and Blumberg MS (2021). Sensory coding of limb kinematics in motor cortex across a key developmental transition. J Neurosci 41, 6905–6918. 10.1523/jneurosci.0921-21.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granmo M, Petersson P, and Schouenborg J (2008). Action-based body maps in the spinal cord emerge from a transitory floating organization. J Neurosci 28, 5494–5503. 10.1523/jneurosci.0651-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gribizis A, Ge X, Daigle TL, Ackman JB, Zeng H, Lee D, and Crair MC (2019). Visual cortex gains independence from peripheral drive before eye opening. Neuron 104, 711–723.e3. 10.1016/j.neuron.2019.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanganu IL, Ben-Ari Y, and Khazipov R (2006). Retinal waves trigger spindle bursts in the neonatal rat visual cortex. J Neurosci 26, 6728–6736. 10.1523/jneurosci.0752-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanganu IL, Staiger J, Ben-Ari Y, and Khazipov R (2007). Cholinergic modulation of spindle bursts in the neonatal rat visual cortex in vivo. J Neurosci 27, 5694–5705. 10.1523/jneurosci.5233-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman J, and Roffwarg H (1983). Modifying oculomotor activity in awake subjects increases the amplitude of eye movements during REM sleep. Science 220, 1074–1076.. [DOI] [PubMed] [Google Scholar]
- Hong CC-H, Harris JC, Pearlson GD, Kim J-S, Calhoun VD, Fallon JH, Golay X, Gillen JS, Simmonds DJ, van Zijl PCM, et al. (2009). fMRI evidence for multisensory recruitment associated with rapid eye movements during sleep. Hum Brain Mapp 30, 1705–1722. 10.1002/hbm.20635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inácio AR, Nasretdinov A, Lebedeva J, and Khazipov R (2016). Sensory feedback synchronizes motor and sensory neuronal networks in the neonatal rat spinal cord. Nat Commun 7, 13060. 10.1038/ncomms13060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson C, McCabe BJ, Nicol AU, Grout AS, Brown MW, and Horn G (2008). Dynamics of a memory trace: effects of sleep on consolidation. Curr Biol 18, 393–400. 10.1016/j.cub.2008.01.062. [DOI] [PubMed] [Google Scholar]
- Jones BE (2020). Arousal and sleep circuits. Neuropsychopharmacol 45, 6–20. 10.1038/s41386-019-0444-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jouvet-Mounier D, Astic L, and Lacote D (1970). Ontogenesis of the states of sleep in rat, cat, and guinea pig during the first postnatal month. Dev Psychobiol 2, 216–239. 10.1002/dev.420020407. [DOI] [PubMed] [Google Scholar]
- Kamara D, and Beauchaine TP (2019). A review of sleep disturbances among infants and children with neurodevelopmental disorders. Rev J Autism Dev Disord 7, 278–294. 10.1007/s40489-019-00193-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayser MS, and Biron D (2016). Sleep and development in genetically tractable model organisms. Genetics 203, 21–33. 10.1534/genetics.116.189589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khazipov R, Sirota A, Leinekugel X, Holmes GL, Ben-Ari Y, and Buzsáki G (2004). Early motor activity drives spindle bursts in the developing somatosensory cortex. Nature 432, 758–761.. [DOI] [PubMed] [Google Scholar]
- Knoop MS, de Groot ER, and Dudink J (2021). Current ideas about the roles of rapid eye movement and non–rapid eye movement sleep in brain development. Acta Paediatr Oslo Nor 1992 110, 36–44. 10.1111/apa.15485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leclair-Visonneau L, Oudiette D, Gaymard B, Leu-Semenescu S, and Arnulf I (2010). Do the eyes scan dream images during rapid eye movement sleep? Evidence from the rapid eye movement sleep behaviour disorder model. Brain 133, 1737–1746. 10.1093/brain/awq110. [DOI] [PubMed] [Google Scholar]
- Li W, Ma L, Yang G, and Gan W-B (2017). REM sleep selectively prunes and maintains new synapses in development and learning. Nat Neurosci 20, 427–437. 10.1038/nn.4479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maccione A, Hennig MH, Gandolfo M, Muthmann O, van Coppenhagen J, Eglen SJ, Berdondini, and Sernagor E (2014). Following the ontogeny of retinal waves: pan-retinal recordings of population dynamics in the neonatal mouse. J Physiol 592, 1545–1563. 10.1113/jphysiol.2013.262840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDuffie KE, Munson J, Greenson J, Ward TM, Rogers SJ, Dawson G, and Estes A (2020). Sleep problems and trajectories of restricted and repetitive behaviors in children with neurodevelopmental disabilities. J Autism Dev Disord 50, 3844–3856. 10.1007/s10803-020-04438-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marques HG, Bharadwaj A, and Iida F (2014). From spontaneous motor activity to coordinated behaviour: A developmental model. Plos Comput Biol 10, e1003653. 10.1371/journal.pcbi.1003653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martini FJ, Guillamón-Vivancos T, Moreno-Juan V, Valdeolmillos M, and López-Bendito G (2021). Spontaneous activity in developing thalamic and cortical sensory networks. Neuron 109, 2519–2534. 10.1016/j.neuron.2021.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng X, Mukherjee D, Kao JPY, and Kanold PO (2021). Early peripheral activity alters nascent subplate circuits in the auditory cortex. Sci Adv 7, eabc9155. 10.1126/sciadv.abc9155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miall RC, Weir DJ, Wolpert DM, and Stein JF (1993). Is the cerebellum a Smith Predictor? J Motor Behav 25, 203–216. 10.1080/00222895.1993.9942050. [DOI] [PubMed] [Google Scholar]
- Mitrukhina O, Suchkov D, Khazipov R, and Minlebaev M (2015). Imprecise whisker map in the neonatal rat barrel cortex. Cereb Cortex 25, 3458–3467. 10.1093/cercor/bhu169. [DOI] [PubMed] [Google Scholar]
- Mohns EJ, and Blumberg MS (2008). Synchronous bursts of neuronal activity in the developing hippocampus: Modulation by active sleep and association with emerging gamma and theta rhythms. J Neurosci 28, 10134–10144.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molnar Z, Luhmann HJ, and Kanold PO (2020). Transient cortical circuits match spontaneous and sensory-driven activity during development. Science 370, 1–11. 10.1126/science.abb2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee D, Yonk AJ, Sokoloff G, and Blumberg MS (2017). Wakefulness suppresses retinal wave-related neural activity in visual cortex. J Neurophysiol 118, 1190–1197. 10.1152/jn.00264.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee D, Sokoloff G, and Blumberg MS (2018). Corollary discharge in precerebellar nuclei of sleeping infant rats. ELife 7, e38213. 10.7554/elife.38213.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata Y, and Colonnese MT (2018). Thalamus controls development and expression of arousal states in visual cortex. J Neurosci 38, 8772–8786. 10.1523/jneurosci.1519-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata Y, and Colonnese MT (2019). Thalamic inhibitory circuits and network activity development. Brain Res 1706, 13–23. 10.1016/j.brainres.2018.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai H, de Vivo L, Marshall W, Tononi G, and Cirelli C (2021). Effects of severe sleep disruption on the synaptic ultrastructure of young mice. Eneuro 8, ENEURO.0077–21.2021. 10.1523/eneuro.0077-21.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nick TA, and Konishi M (2005). Neural song preference during vocal learning in the zebra finch depends on age and state. J Neurobiol 62, 231–242. 10.1002/neu.20087. [DOI] [PubMed] [Google Scholar]
- Nikolić M, Gardner HAR, and Tucker KL (2013). Postnatal neuronal apoptosis in the cerebral cortex: physiological and pathophysiological mechanisms. Neuroscience 254, 369–378. 10.1016/j.neuroscience.2013.09.035. [DOI] [PubMed] [Google Scholar]
- Petersson P, Waldenström A, Fåhraeus C, and Schouenborg J (2003). Spontaneous muscle twitches during sleep guide spinal self-organization. Nature 424, 72–75.. [DOI] [PubMed] [Google Scholar]
- Puentes-Mestril C, Roach J, Niethard N, Zochowski M, and Aton SJ (2019). How rhythms of the sleeping brain tune memory and synaptic plasticity. Sleep 42. 10.1093/sleep/zsz095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasch B, and Born J (2013). About sleep’s role in memory. Physiol Rev 93, 681–766. 10.1152/physrev.00032.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauschecker JP, Tian B, Korte M, and Egert U (1992). Crossmodal changes in the somatosensory vibrissa/barrel system of visually deprived animals. Proc. Natl. Acad. Sci. U.S.A 89, 5063–5067. 10.1073/pnas.89.11.5063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renouard L, Bridi MCD, Coleman T, Arckens L, and Frank MG (2018). Anatomical correlates of rapid eye movement sleep-dependent plasticity in the developing cortex. Sleep 41, 59–11. 10.1093/sleep/zsy124. [DOI] [PubMed] [Google Scholar]
- Rio-Bermudez CD, Sokoloff G, and Blumberg MS (2015). Sensorimotor processing in the newborn rat red nucleus during active sleep. J Neurosci 35, 8322–8332. 10.1523/jneurosci.0564-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rio-Bermudez CD, Kim J, Sokoloff G, and Blumberg MS (2017). Theta oscillations during active sleep synchronize the developing rubro-hippocampal sensorimotor network. Curr Biol 27, 1413–1424. 10.1016/j.cub.2017.03.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rio-Bermudez CD, Kim J, Sokoloff G, and Blumberg MS (2020). Active sleep promotes coherent oscillatory activity in the cortico-hippocampal system of infant rats. Cereb Cortex 30, 2070–2082. 10.1093/cercor/bhz223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts TF, Tschida KA, Klein ME, and Mooney R (2010). Rapid spine stabilization and synaptic enhancement at the onset of behavioural learning. Nature 463, 1–6. 10.1038/nature08759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roder B, Teder-Salejarvi W, Sterr A, Rosler F, Hillyard S, and Neville H (1999). Improved auditory spatial tuning in blind humans. Nature 400, 162–166.. [DOI] [PubMed] [Google Scholar]
- Roffwarg HP, Muzio JN, and Dement WC (1966). Ontogenetic development of the human sleep-dream cycle. Science 152, 604–619.. [DOI] [PubMed] [Google Scholar]
- Rossi FM, Pizzorusso T, Porciatti V, Marubio LM, Maffei L, and Changeux J-P (2001). Requirement of the nicotinic acetylcholine receptor β2 subunit for the anatomical and functional development of the visual system. Proc National Acad Sci 98, 6453–6458. 10.1073/pnas.101120998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadato N, PascualLeone A, Grafman J, Ibanez V, Deiber MP, Dold G, and Hallett M (1996). Activation of the primary visual cortex by Braille reading in blind subjects. Nature 380, 526–528. 10.1038/380526a0. [DOI] [PubMed] [Google Scholar]
- Schmidt MH (2014). The energy allocation function of sleep: a unifying theory of sleep, torpor, and continuous wakefulness. Neurosci Biobehav Rev 47, 122–153. 10.1016/j.neubiorev.2014.08.001. [DOI] [PubMed] [Google Scholar]
- Seelke AMH, Karlsson KÆ, Gall AJ, and Blumberg MS (2005). Extraocular muscle activity, rapid eye movements and the development of active and quiet sleep. Eur J Neurosci 22, 911–920.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaffery J, Oksenberg A, Marks G, Speciale S, Mihailoff G, and Roffwarg H (1998). REM sleep deprivation in monocularly occluded kittens reduces the size of cells in LGN monocular segment. Sleep 21, 837–845. 10.1093/sleep/21.8.837. [DOI] [PubMed] [Google Scholar]
- Shaffery J, Roffwarg H, Speciale S, and Marks G (1999). Ponto-geniculo-occipital-wave suppression amplifies lateral geniculate nucleus cell-size changes in monocularly deprived kittens. Dev Brain Res 114, 109–119. 10.1016/s0165-3806(99)00027-9. [DOI] [PubMed] [Google Scholar]
- Shank SS, and Margoliash D (2009). Sleep and sensorimotor integration during early vocal learning in a songbird. Nature 457, 73–77. 10.1038/nature07615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitko AA, and Goodrich LV (2021). Making sense of neural development by comparing wiring strategies for seeing and hearing. Science 371, eaaz6317–9. 10.1126/science.aaz6317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith GB, Hein B, Whitney DE, Fitzpatrick D, and Kaschube M (2018). Distributed network interactions and their emergence in developing neocortex. Nat Neurosci 21, 1600–1608. 10.1038/s41593-018-0247-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokoloff G, Dooley JC, Glanz RM, Wen RY, Hickerson MM, Evans LG, Laughlin HM, Apfelbaum KS, and Blumberg MS (2021). Twitches emerge postnatally during quiet sleep in human infants and are synchronized with sleep spindles. Curr Biol 1–12. 10.1016/j.cub.2021.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun LD, and Goldberg ME (2016). Corollary discharge and oculomotor proprioception: Cortical mechanisms for spatially accurate vision. Annu Rev Vis Sci 2, 61–84. 10.1146/annurev-vision-082114-035407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szuperak M, Churgin MA, Borja AJ, Raizen DM, Fang-Yen C, and Kayser MS (2018). A sleep state in Drosophila larvae required for neural stem cell proliferation. ELife 7, e33220. 10.7554/elife.33220.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tehovnik EJ, Froudarakis E, Scala F, Smirnakis SM, Patel SS, and Tolias AT (2021). Visuomotor control in mice and primates. Neurosci Biobehav Rev 130, 185–200. 10.1016/j.neubiorev.2021.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiriac A, Rio-Bermudez CD, and Blumberg MS (2014). Self-generated movements with “unexpected” sensory consequences. Curr Biol 24, 2136–2141. 10.1016/j.cub.2014.07.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiriac A, Smith BE, and Feller MB (2018). Light prior to eye opening promotes retinal waves and eye-specific segregation. Neuron 100, 1059–1065.e4. 10.1016/j.neuron.2018.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiriac A, Bistrong K, Pitcher MN, Tworig JM, and Feller MB (2022). The influence of spontaneous and visual activity on the development of direction selectivity maps in mouse retina. Cell Reports 38, 110225. 10.1016/j.celrep.2021.110225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakai RT, and Lutter WJ (2016). Slow rhythms and sleep spindles in early infancy. Neurosci Lett 630, 164–168. 10.1016/j.neulet.2016.07.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Rangarajan KV, Lawhn-Heath CA, Sarnaik R, Wang B-S, Liu X, and Cang J (2009). Direction-specific disruption of subcortical visual behavior and receptive fields in mice lacking the beta2 subunit of nicotinic acetylcholine receptor. J Neurosci 29, 12909–12918. 10.1523/jneurosci.2128-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warwick RA, Riccitelli S, Heukamp AS, Yaakov H, Ankri L, Mayzel J, Gilead N, Parness-Yossifon R, and Rivlin-Etzion M (2022). Top-down modulation of the retinal code via histaminergic neurons of the hypothalamus. BioRxiv 10.1101/2022.04.26.489509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weliky M, and Katz LC (1999). Correlational structure of spontaneous neuronal activity in the developing lateral geniculate nucleus in vivo. Science 285, 599–604. 10.1126/science.285.5427.599. [DOI] [PubMed] [Google Scholar]
- Whitehead K, Meek J, and Fabrizi L (2018). Developmental trajectory of movement-related cortical oscillations during active sleep in a cross-sectional cohort of pre-term and full-term human infants. Sci Rep 8, 111–118. 10.1038/s41598-018-35850-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolpert DM, Miall CR, and Kawato M (1998). Internal models in the cerebellum. Trends Cog Sci 2, 338–347.. [DOI] [PubMed] [Google Scholar]
- Xu H, Furman M, Mineur YS, Chen H, King SL, Zenisek D, Zhou ZJ, Butts DA, Tian N, Picciotto MR, et al. (2011). An instructive role for patterned spontaneous retinal activity in mouse visual map development. Neuron 70, 1115–1127. 10.1016/j.neuron.2011.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng X, Komanome Y, Kawasaki T, Inada K, Jonaitis J, Pulver SR, Kazama H, and Nose A (2021). An electrically coupled pioneer circuit enables motor development via proprioceptive feedback in Drosophila embryos. Curr Biol 10.1016/j.cub.2021.10.005. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Collins DC, and Maier JX (2020). Network dynamics in the developing piriform cortex of unanesthetized rats. Cereb Cortex 490, 219–13. 10.1093/cercor/bhaa300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Lai CSW, Bai Y, Li W, Zhao R, Yang G, Frank MG, and Gan W-B (2020). REM sleep promotes experience-dependent dendritic spine elimination in the mouse cortex. Nat Commun 11, 1–12. 10.1038/s41467-020-18592-5. [DOI] [PMC free article] [PubMed] [Google Scholar]


