Abstract
Objectives
Chronic joint pain is common in patients with osteoarthritis (OA). Non-steroidal anti-inflammatory drugs and opioids are used to relieve OA pain, but they are often inadequately effective. Dorsal root ganglion field stimulation (GFS) is a clinically used neuromodulation approach, although it is not commonly employed for patients with OA pain. GFS showed analgesic effectiveness in our previous study using the monosodium iodoacetate (MIA) - induced OA rat pain model. This study was to evaluate the mechanism of GFS analgesia in this model.
Methods
After osteoarthritis was induced by intra-articular injection of MIA, pain behavioral tests were performed. Effects of GFS on the spontaneous activity (SA) were tested with in vivo single-unit recordings from teased fiber saphenous nerve, sural nerve, and dorsal root.
Results
Two weeks after intra-articular MIA injection, rats developed pain-like behaviors. In vivo single unit recordings from bundles teased from the saphenous nerve and third lumbar (L3) dorsal root of MIA-OA rats showed a higher incidence of SA than those from saline-injected control rats. GFS at the L3 level blocked L3 dorsal root SA. MIA-OA reduced the punctate mechanical force threshold for inducing AP firing in bundles teased from the L4 dorsal root, which reversed to normal with GFS. After MIA-OA, there was increased retrograde SA (dorsal root reflex), which can be blocked by GFS.
Conclusions
These results indicate that GFS produces analgesia in MIA-OA rats at least in part by producing blockade of afferent inputs, possibly also by blocking efferent activity from the dorsal horn.
Keywords: Dorsal root ganglion, Monosodium iodoacetate, Osteoarthritis pain, Dorsal root reflex, Stimulation
Introduction:
Persistent pain in the joint is the common symptom and the leading reason patients with osteoarthritis (OA) pursue medical care. Knee joints are densely innervated by sensory endings of afferent fibers, which in rats travel in the saphenous nerve and enter the spinal cord at third lumbar (L3) and L4 levels1–3. Nociceptors in OA-affected joints become hypersensitive to stimuli such as joint loading or movement, and result in increased nociceptive input from knee joint, producing pain directly as well as sensitizing central pain processing4. Joint pain in OA is worsened by movement and relieved by rest. Treatment options for OA-pain are limited, and relief from chronic OA joint pain is often refractory to currently available pharmacological interventions including non-steroidal anti-inflammatory drugs and opioids5, while progression of joint damage leads to loss of joint function and disability, and often joint replacement surgery.
Dorsal root ganglion (DRG) field stimulation (GFS) is a recently established pain management modality, mostly performed in patients who have failed other treatment approaches. Animal studies have demonstrated that GFS is effective in treatment of neuropathic pain6, 7, collagen-induced arthritis pain8, and MIA-induced OA pain7. Compared with traditional pharmacological treatment, GFS exhibits several advantages including high efficacy, sustained pain relief, improvements in quality of life and psychological disposition, and minimal side effects9, 10. In a preclinical rat neuropathic pain model, we have shown that GFS segmentally interrupts action potential (AP) traffic going through the T-junction of sensory neurons in the stimulated DRG11, and blocks spontaneous activity (SA) from injured neurons12. It is not clear whether GFS provides analgesic effects with a similar mechanism in OA pain.
Elevated excitability of peripheral sensory neurons, including at their terminals in the dorsal horn (DH), is a key element contributing to the maintenance of chronic pain12. It has long been known that depolarization of sensory neuron central terminals in the DH can trigger efferent (retrograde, antidromic) AP trains in that neuron and other nearby sensory neurons13. Termed “dorsal root reflexes,” these AP trains can cause release of neuropeptides and other transmitters from affected sensory neurons’ distal terminals in tissues such as skin and knee joint, as well as from the in the DRG, resulting in neurogenic inflammation and hyperexcitability of neurons innervating these structures13–16. As these processes may contribute to both joint inflammation and mechanical hypersensitivity of the joint and plantar skin after knee injection of monosodium iodoacetate (MIA), we tested if GFS might also have analgesic effects by blocking this efferent activity.
Methods
Animals
Male Sprague Dawley rats weighing 200–250g were obtained from the Taconic Farms Biosciences (Rensselaer, NY), and were maintained and used according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals. As GFS was found to have comparable analgesic effects on male and female rats with neuropathic pain in our previous report7, only male rats were used in this study. All animal experiments were performed according to protocols approved by the Institutional Animal Care and Use Committee of the Medical College of Wisconsin (animal protocol AUA0454). Animals were housed in a pathogen-free facility, 2 animals per ventilated cage, in a room maintained at 25±1 °C at 35 to 45% humidity, with a 12/12-hour day/night cycle. Animals had free access to food and water. At the termination of the study, euthanasia was performed by decapitation during deep isoflurane anesthesia. Simple randomization was used in this study to allocate animals to different groups.
Monosodium iodoacetate-induced osteoarthritis pain model
Monosodium iodoacetate-induced osteoarthritis (MIA-OA) was performed as described in a previous report7. Animals were randomly assigned to two groups either with MIA or saline injection. Animals were anesthetized with 2% isoflurane/O2 mixture, during which a single intra-articular injection of MIA (Catalogue # I2512, Sigma-Aldrich, St Louis, MO) or saline through the infrapatellar ligament of the right knee was performed. MIA was dissolved in 0.9% sterile saline to final concentration 80mg/ml and administered in a volume of 25μl (containing 2mg MIA), using a 29-gauge needle. Sham OA control animals were given a single intra-articular injection of 25μl saline into the right knee. Evaluation of pain behaviors was performed 14d after the intra-articular injection of MIA or saline. After that, rats were randomly used for in vivo electrophysiological recording.
Behavioral tests
Sensory testing of the plantar skin included evoking reflexive behaviors at threshold intensity by punctate mechanical stimulation (von Frey test), by noxious mechanical stimulation (pin), by dynamic non-noxious mechanical stimulation (brush), and by cold stimulation (acetone). Use-dependent pain was assessed by measured by static weight bearing asymmetry (“incapacitance” test). Induced knee joint pain was tested by pressure application measurement. For animals with multiple behavioral tests on the same day, the presentation sequence was designed according to the potential stress of the test, specifically in the order of von Frey, brush, acetone, and pin. Incapacitance, and pressure application measurement were performed in separate days from other behavioral tests. The investigator performing behavior tests was blinded to the treatments. Group size was set by the test with the least power (greatest variance), i.e. von Frey threshold test. Specifically, our pilot data determined von Frey threshold for control [Mean±SD: 1.39±0.0log(g)] and MIA [Mean±SD: 0.58±0.51log(g)]. Thus, groups of minimum 8 animals will be used for behavioral tests, which was calculated with SigmaPlot 12.5 (Systat Software Inc, San Jose, CA).
Threshold punctate mechanical stimulation (von Frey)
The von Frey test was performed using calibrated monofilaments (Patterson Medical, Bolingbrook, IL). Briefly, beginning with the 2.8-gram filament, the tip of filament was applied perpendicularly to the glabrous skin on the lateral third of the plantar aspect of the hind paw for 1s, with just enough force to bend the fiber. If a paw withdrawal response was observed, then the next weaker filament was applied, and if no response was observed, then the next stiffer fiber was applied, until a reversal occurred. After a reversal event, four more stimulations were performed following the same pattern. Each application an interval of at least 10s after the prior application. The forces of the filaments before and after the reversal, and the 4 filaments applied after the reversal, were used to calculate the 50% withdrawal threshold17. Rats not responding to any filament were assigned a score of 25g. Because the magnitude of mechanical sense is perceived in a logarithmic scale, as described by the Weber law, von Frey paw withdrawal threshold data were log transformed12. In some experiments, we used von Frey fibers that were modified by attaching standardized tips of 100μm diameter tungsten with precisely squared ends.
Noxious punctate mechanical stimulation (pin test)
Pin test was performed using the point of a 22-gauge spinal anesthesia needle that was applied to the lateral third of the hind paw with enough force to indent the skin but not puncture it. This was repeated for 5 applications, with intervals of at least 10s between applications, and this set of applications was repeated after 1min, making a total of 10 touches. Each application induced a behavior that was categorized as 1 of 2 types. The response typical of uninjured rats consisted of a very brief (<1s) withdrawal, with immediate return of the foot to the cage floor. An alternate behavior that we termed a hyperalgesic response consisted of a complex event with sustained elevation of at least 1s, variably combined with grooming that included licking and chewing of the paw, and with shaking of the limb. This hyperalgesic behavior is specifically associated with place avoidance18, indicating that it represents an aversive experience. Hyperalgesia was quantified by tabulating the number of hyperalgesic responses as a percentage of total 10 touches.
Cold stimulation (acetone test)
Sensitivity to cold was assessed using application of acetone, which was expelled through tubing to form a convex meniscus on the end of the tubing and was touched to the lateral plantar skin without contact of the tubing with the skin. The response was scored as positive if the paw was removed, and 3 repetitions were spaced at least 1min apart.
Dynamic mechanical stimulation (brush test)
A camel hair brush (4mm wide) was applied to the lateral plantar skin of the hind paw by light stroking in the direction from heel to toe over the span of 2s. The response was scored as either positive if the paw was removed or none in the absence of movement. The test was applied three times to each paw, separated by intervals of at least 10s.
Weight-bearing (incapacitance test)
An incapacitance device (Columbus Instruments, Columbus, OH) with a dual-channel weighing design was used to measure asymmetry of weight-bearing as an indication of use-dependent pain7. Animals were trained for 2–3 days and 5min each day to be comfortable with being gently restrained in a chamber with two hind paws placed on the separate force transducers. On the test day, the weight borne on each hind limb was measured. The force of each hind limb measured in grams was calculated over a 3-s period, and the measurement was repeated 3 times, and the mean value was calculated. The incapacitance ratio was calculated by dividing the weight (g) bearing of ipsilateral hind paw with the sum of weight bearing from both ipsilateral and contralateral hind paws. A value of less than 50% indicates a reduction in weight borne on the ipsilateral hindlimb.
Knee pressure application test
A pressure application measurement device (Pressure application measurement, Ugo Basile, Varese, Italy) was used to give pressure stimuli directly to the knee7. A calibrated force sensor was worn on the thumb of the experimenter. With the animal gently restrained, the pressure application measurement device was pressed against the medial and lateral aspects of the knee joint and the peak force at which the animal showed sign of pain or discomfort, including freezing of whisker movement, wriggling, audible vocalizations, or attempt to withdraw from the device was recorded and designated as the limb withdrawal threshold. The measurement was repeated 3 times with an interval between applications of 1min, and the mean value was calculated.
In vivo electrophysiological recording
Animal preparation:
Anesthesia was induced with 2% inhalant isoflurane followed by urethane (100mg/kg, Subcutaneous injection, S.C). Thirty minutes later, isoflurane was reduced to 1.25–1.5%. During electrophysiological recording, the isoflurane was discontinued or run at levels of ≤0.2% such that the rats remained immobile but metabolically and hemodynamically stable, confirmed by arterial blood analysis with a blood gas analyzer (ABL800FLEX, Radiometer, Copenhagen, Denmark), and continuous blood pressure was monitored via a carotid artery PE-10 cannula. A heating pad was used to maintain body temperature at 37 °C. The sample size for electrophysiology experiments was based on our previous experience with this design12. No statistical power calculation was conducted before the study.
DRG electrode implantation and GFS:
In some electrophysiological recordings, acute effects of 120s-20Hz-GFS were observed. Bipolar GFS electrodes (P1Technologies, Roanoke, VA, USA) were prepared from 2 20cm-long platinum-iridium wires (0.127 millimeter and 0.254 millimeter), such that two conducting surfaces 1mm apart beside the DRG. Their other ends were secured in a standard plastic connection hub, which was secured to the skull with dental cement and screws, as described in our previous report6, where their implantation is also described. Briefly, during isoflurane anesthesia, a dorsal paramedian incision was made to expose the external aspect of the intervertebral foramen at the required level for GFS, which included L3 or L4. This requires removal of the small, overhanging accessory process. A blunt probe with 0.4-mm diameter was inserted into the intervertebral foramen dorsolateral to the DRG, to create a passage into which the electrode was inserted in juxtaposition to the DRG at that level. The electrode was secured to the surrounding muscle tissues with a ligature and then the motor threshold was determined as the current at which any further increase resulted in perceptible hind limb movement for GFS during stimulation at 2Hz with a pulse width of 200μs. After that, the wound was closed and we started other surgeries for the teased fiber recordings. We used stimulation of 120s-20Hz-GFS with intensity of 80% of the motor threshold in the experiments. A stimulator and an isolator (Master-9 and Iso-Flex, A.M.P.I., Jerusalem, Israel) were used for this purpose.
Saphenous nerve and sural nerve recording:
For recordings from saphenous nerve, the rats were placed supine, and 25mm of the right side saphenous nerve was exposed by incision at the medial thigh. The skin flaps of the incision were sutured to a metal ring to form a basin that was filled with warm mineral oil (36°C) to prevent nerve from drying. The nerve was transected either proximally at the inguinal region or distally near the knee joint. A bipolar platinum wire stimulating electrode was placed under the nerve at its distal end and the electrode was isolated from surrounding tissue by a piece of plastic film. For teased sural nerve fiber recording, the rats were placed prone and the right sciatic nerve at the trifurcation was exposed. The skin flaps of the incision were sutured to a metal ring to form a basin that was filled with warm mineral oil to prevent nerve from dry. Similarly, for recordings from the sural nerve, it was released from connective tissues for 15mm and cut at the sciatic trifurcation, and stimulating needles were inserted into the peripheral receptive field (RF). For recordings, nerve endings were desheathed, fine filaments were dissected from the nerve with sharp jeweler’s forceps, and placed on a platinum recording electrode. Recorded units were classified by calculating their conduction velocity (CV) as the distance between stimulation and recording sites divided by the latency of the evoked response with electrical stimulation.
Dorsal root teased fiber recording:
The spinal cord and dorsal roots were exposed by a laminectomy at the level of vertebrae T13 to L3 and covered with warm mineral oil (36°C) to prevent drying. Bone wax was used to stop bleeding from the bone, and the dura was removed. The vertebral column was stabilized by clamps applied to the spinal process rostral and caudal to the exposure. Skin edges of the midline incision were sutured to a metal ring (3×4 cm) to form a basin that was filled with warm mineral oil. The L3 or L4 dorsal root was gently released from connective tissues and transected near their entry into the spinal cord and then placed on a glass platform, upon which the distal portion of the dorsal root was repeatedly teased to create fine bundles from which single-unit activity could be recorded by a platinum/iridium recording electrode. A reference electrode was attached to the adjacent muscle tissue. Initially, SA was observed for 3 min and recorded if present. The motor threshold for DRG stimulation was then determined by identifying the lowest current that could trigger a leg muscle twitch.
Knee joint RFs of saphenous nerve and dorsal root were identified by searching with a glass probe (with 1mm round tip), and knee bending. Evoked firing was then measured at a calibrated force (measured as approximately 400gram) by applying a serrated forceps placed medially-laterally around the knee joint, and by bending the knee joint completely. These were repeated 3 times with an interval of 1min, and the averaged value of 3 repeated measurements was used for analysis. The RF of units in the sural nerve and dorsal root were identified in the hind paw by stimulation with a glass probe (1 mm tip) and a von Frey monofilament (29g). The skin with the RF was gently pinched with forceps to confirm that the RF was cutaneous. To characterize a unit’s firing properties, the mechanical threshold was defined as the minimal force of von Frey fiber that evoked firing. Then the threshold force to induce firing and the firing rate were determined in response to noxious stimulation with a von Frey filament (29g force) and a modified von Frey (a 100μm tungsten tip is attached to a fiber with 16g force), applied to the RF for 10s.
Electrophysiological signals from teased fiber recordings were collected with an Axoclamp 900A microelectrode amplifier (Molecular Devices, San Jose, CA), filtered at 2 kHz, and sampled at 10 kHz using a digitizer (DigiData 1440A, Molecular Devices). Individual APs were isolated and amplified by means of a window discriminator (Clampfit, Molecular Devices) or by template matching using Spike2 (Cambridge Electronic Design Limited, Cambridge, UK).
Conduction velocity measurement:
CV was calculated as the distance from the point of stimulation to the teased fiber recording electrode (measured after the final recording in each rat), divided by the time latency between stimulation and initiation of the AP (measured directly from the recorded firing in pClamp). We used the following CV standards to categorize different fiber types as we reported before11; for recordings from sural nerve and saphenous nerve, units with CV>20m/s are Aβ, 3<CV<20m/s for Aδ, CV<3m/s for C-type. This was similar to other reports with recordings from either sciatic nerve or somata of DRG neurons by stimulating sciatic nerve19. For recordings from dorsal root, units with CV>6m/s are Aβ, 1<CV<6m/s for Aδ, CV<1m/s for C-type. These definitions are consistent with other reports performing similar dorsal root recordings20, 21.
Statistical analysis
Significance testing was performed with Prism 9 (GraphPad Software, La Jolla, CA). Parametric and nonparametric analyses where appropriate were used for data analysis. X2 test was used for the comparison of bundle number with/without SA. Acute effects of GFS on electrophysiological recordings of units in teased bundles were compared with 1-way repeated measure of analysis of variance (ANOVA) followed by Dunn’s post hoc test, or 2-way repeated measure of ANOVA followed by Sidak’s post hoc test, where appropriate. Results are reported in the figures. P<0.05 was considered statistically significant. An analysis plan was not registered before the study. For all experiments, detailed statistical data are presented in Supplementary Table.
Results
MIA-OA produces pain behaviors
MIA produces its peak sensory behavioral effects by 14d after knee injection22, at which time rats in our study showed weight bearing avoidance on the side of the injected knee (Supplementary Fig. 1A), and secondary plantar hypersensitivity to pin (Supplementary Fig. 1B), von Frey (Supplementary Fig. 1C), brush (Supplementary Fig. 1D), and acetone (Supplementary Fig. 1E) tests. Additionally, MIA decreased the threshold force to cause limb withdrawal during the application of compressive force to the MIA-injected knee (Supplementary Fig. 1F). In our previous study, MIA also effectively induced spontaneous pain behavior.7 As all rats with MIA injection developed positive responses in these behavioral tests, no rat was excluded from the following electrophysiological recordings. Detailed statistical data are presented in Supplementary Table 1A.
MIA-OA induces increased afferent SA from affected knee joint
Knee joints are densely innervated by sensory endings of sensory neurons that have somata in the L3 and L4 DRG neurons1, 2. To investigate whether afferent inputs of L3 and L4 are affected by MIA-induced OA, we first examined the SA in units recorded from bundles teased from L3 and L4 dorsal roots with confirmed RF in knee. In MIA-injected rats, more bundles of L3 dorsal roots showed spontaneous activity than sham control (Fig. 1 A, B). As there are multiple axons in one teased nerve bundle11, we also counted the number of units with SA in each bundle, which was higher in rats with MIA (Fig. 1C). Also, the firing rate of SA was higher in teased L3 bundles from MIA-injected rats (Fig. 1D). In recordings from L4 dorsal roots with RF from knee (Fig. 1E), there were no changes in the incidence and firing rate of SA, and the average unit number for each bundle in MIA-injected rats (Fig. 1F–H). These results suggested that MIA-induced OA preferentially affected L3 afferent input. To confirm that this enhanced L3 SA is driven by afferent input from the knee joint, we examined SA more distally at the level of the saphenous nerve (Fig. 1I). This showed that the incidence of SA was increased in MIA-injected rats (Fig. 1J), although no difference was observed in the firing rate between MIA-injected rats and their age-matched controls (Fig. 1K, L). No data points were excluded from the analysis. Detailed statistical data are presented in Supplementary Table 1B.
Figure 1.
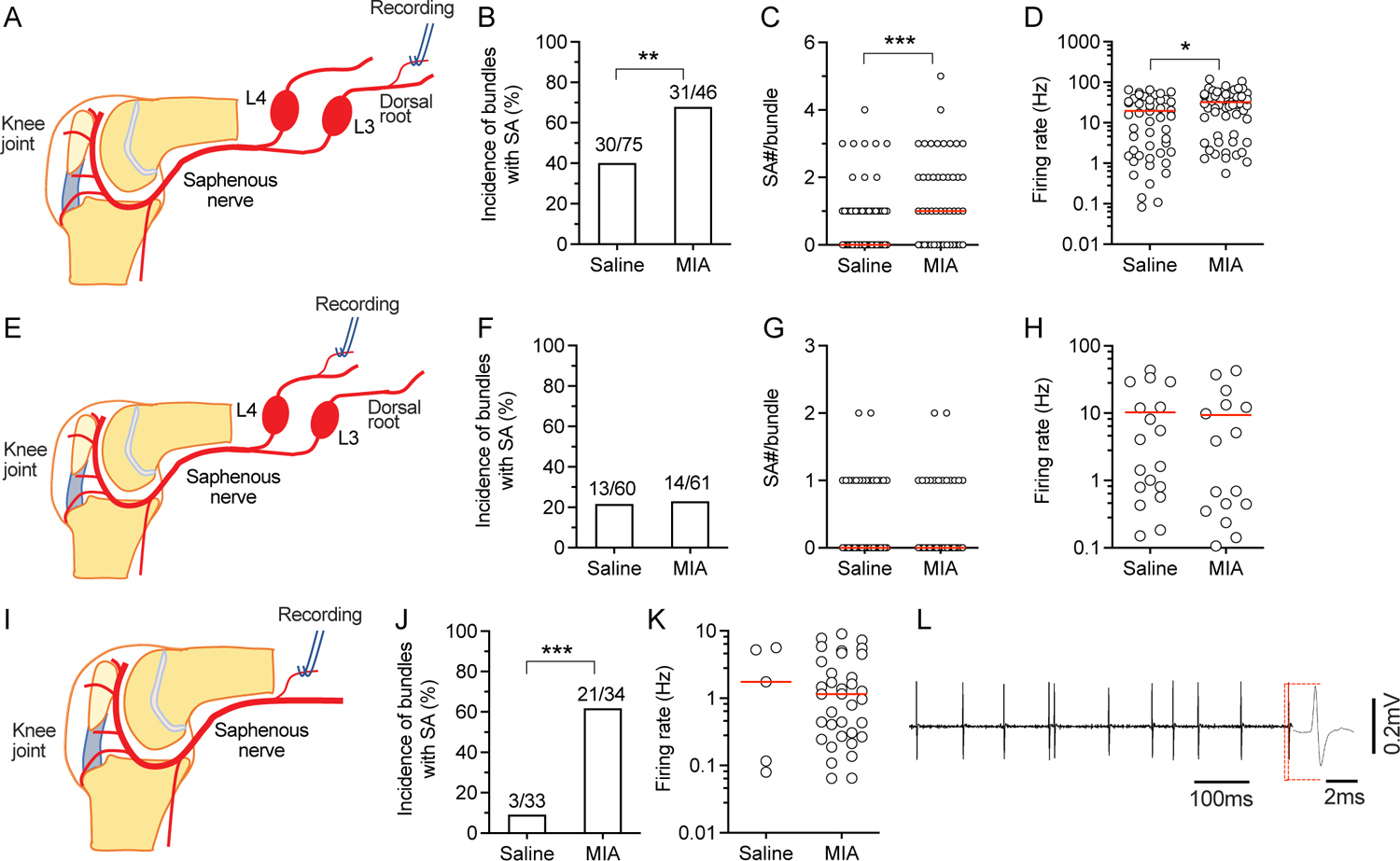
SA in afferent units innervating the affected knee joint after MIA-OA. (A) Preparation design; the L3 dorsal root is teased and resulting bundles are used for recording. (B) Recordings from L3 teased bundles of units with RFs in the knee show increased frequency of SA MIA-OA animals compared to saline-injected controls. (C) MIA-OA animals showed an increased number of units with SA per recorded bundle. (D) MIA-OA increased SA firing rates. (E) Preparation design for L4 dorsal root recording. (F) MIA-OA increases the frequency of SA in L4 dorsal root bundles with knee RFs. (G) No difference in units per recorded bundle with SA was observed in recordings from L4 dorsal root. (H) MIA-OA does not increase SA firing rates in recordings from the L4 dorsal root. (I) Preparation design for recording from the saphenous nerve. (J) MIA-OA increases frequency of SA in recorded bundles. (K) MIA-OA has no effect on SA firing rates in saphenous nerve recordings. (L) Sample trace of recordings from saphenous nerve. Red lines in C, G, K represent median and represent means in D and H. Data are from n=5 rats with saline injection and n=5 rats with injection of MIA for each type of recordings from L3, L4, and saphenous nerve. * P<0.05, ** P<0.01, *** P<0.001 by x2 test in B, F, and J, and t-test in all other comparison.
GFS inhibits SA afferent input from the affected knee joint
In our previous reports, we found that GFS relieves neuropathic pain by inhibiting SA from injured nerve11, 12, which is a key element generating chronic pain12. GFS can also relieve evoked and spontaneous pain in rats with MIA-OA7. Here, we tested whether GFS could block MIA-induced SA, and found that SA in C-type units from the L3 dorsal root was eliminated during 120s-20Hz GFS recorded (Fig. 2). No data points were excluded from the analysis. No data points were excluded from the analysis. Detailed statistical data are presented in Supplementary Table 1C.
Figure 2.
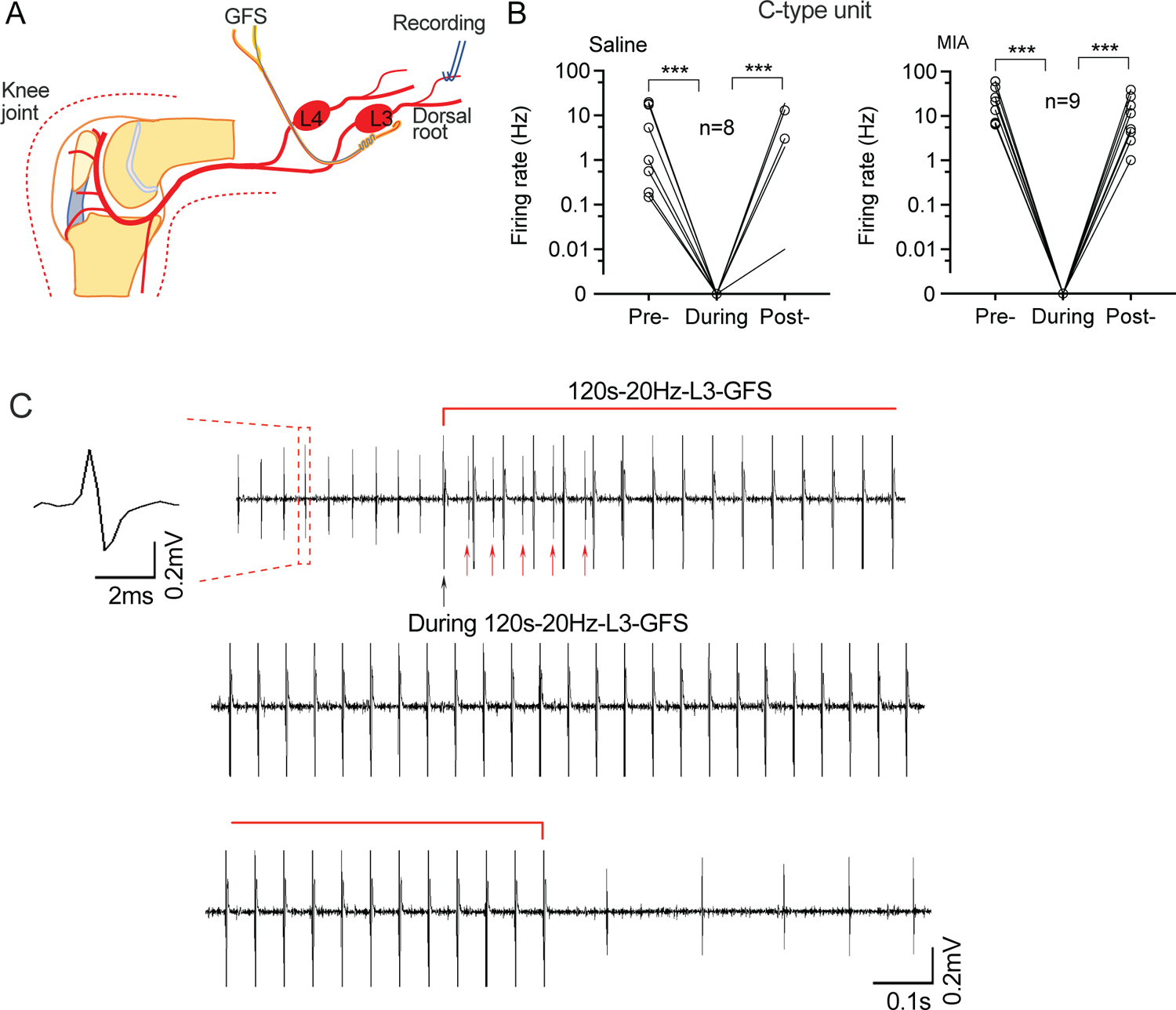
Effects of 120s-20Hz GFS on SA in L3 dorsal root units. (A) Preparation design. (B) SA recorded from C-type units in the L3 dorsal root is reduced during (the first 10s following) L3 GFS. (C) Representative trace from a C-type unit showing blocked SA soon after the start of GFS (top), during (middle), and after GFS (bottom). Black arrow points to the stimulation artifact of the first stimulation of GFS. Red arrows point to SA during the first several stimulations of 20Hz GFS. Data are from n=4 rats with saline injection and n=4 rats with injection of MIA. *** P<0.001 with nonparametric 1-way repeated measure ANOVA (Friedman test) followed by comparison of rank.
GFS reduces AP firing induced by mechanical knee joint stimulation
Pain during knee movement is an important symptom of OA and the affected joint is painful during palpation, indicating hypersensitivity of knee joint tissues to mechanical stimulation in OA. We have previously observed that AP trains evoked in L4/L5 sensory neurons by peripheral mechanical stimulation can be inhibited by GFS12. This suggests that GFS may similarly block transmission of evoked AP trains in the setting of MIA-OA. Accordingly, we examined the effects of GFS on AP firing by mechanical stimulation to knee, delivered by applying a calibrated force with a serrated forceps placed medially-laterally across the knee joint, or by bending the knee joint completely (Fig. 3A). L3 120s-20Hz GFS inhibited the firing rate of evoked by pinching (Fig. 3B–E) in C-type units in teased L3 dorsal root bundles in both saline and MIA-injected rats, and the firing induced by knee bending (Fig. 3F) in MIA-injected rats. L4 GFS significantly decreased the firing rate of both pinching (Fig. 3G) and bending (Fig. 3H) induced responses in C-type units of teased L4 dorsal root bundles in both saline and MIA-injected rats. No data points were excluded from the analysis. Detailed statistical data are presented in Supplementary Table 1D.
Figure 3.
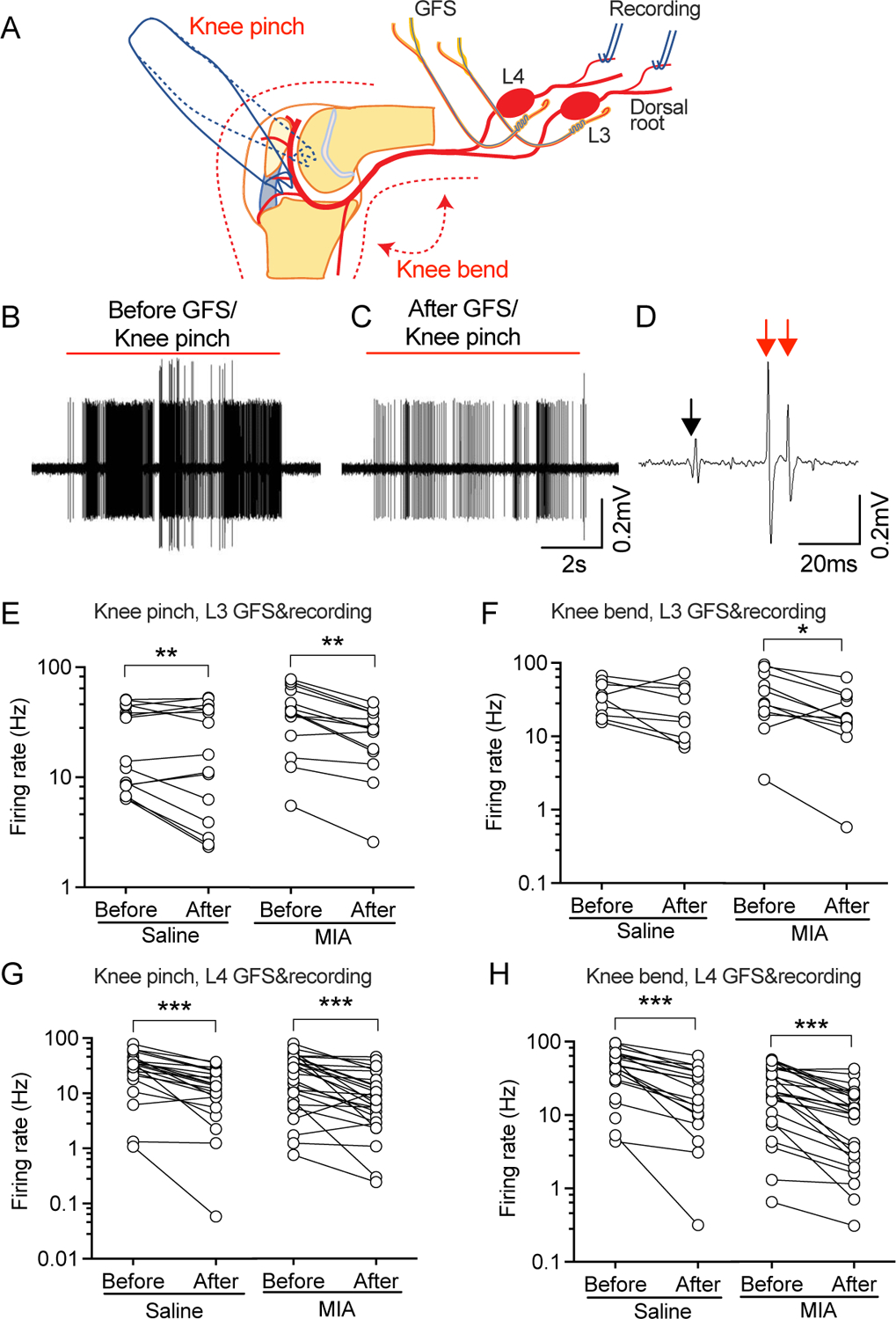
Effect of GFS on bending/pinching knee joint-evoked responses. (A) Preparation design. Knee joint is clamped by applying a calibrated force with a serrated forceps placed medially-laterally around the knee joint, or is fully bent to evoke the responses. (B) Representative traces showing pinching knee joint-evoked AP firing in a recorded unit before and after GFS. In this bundle, knee-pinching evoked two C-type units pointed by red arrows (CV of 0.95m/s for the big one and 0.74m/s for the small one), which were both suppressed by GFS. Black arrow points to the start of stimulation. (E, F) Effects of 20Hz GFS on AP firing of recorded units in teased L3 dorsal root bundles by pinching (E) and bending (F) the knee joint. (G, H) Effects of 120s-20Hz GFS on AP firing of L4 dorsal root units induced by pinching (G) and bending (H) the knee joint. Data are from n=5 rats with saline injection and n=5 rats with MIA injection. *P<0.05, ** P<0.01, *** P<0.001 by 2-way repeated measure ANOVA followed by Sidak’s post hoc test.
MIA decreases the threshold for mechanical stimulation of afferent AP trains
Referred pain, defined as pain felt at a site other than where the cause is situated, is a phenomenon often observed in MIA-induced OA in animals23, 24 as well as in OA patients25. This has been documented in OA animals in a number of previous studies by observing increased mechanical sensitivity of the plantar skin of the limb with OA26, 27, as we have also observed previously7 and here (Supplementary Fig. 1). Since GFS can also relieve this referred pain7, we next asked whether GFS can block the AP firing originated from the plantar surface of the hind paw in this rat model of MIA-OA. First, we did recordings from teased sural nerve, as sural nerve contains only somatosensory neurons and extends its innervation territory into the hind limbs without overlapping the saphenous nerve RF28. We found that neither the incidence nor the firing rate of SA in sural nerve units was changed in rats with MIA-OA (Fig. 4A, B, C). However, the threshold for mechanical stimulation-induced firing in sural nerve units was significantly lower in MIA-injected rats than control (Fig. 4D), which may underlie the referred pain found in MIA-induced OA23, 24. Second, we recorded SA in units of teased L4 dorsal root bundles with RFs in the plantar surface of the hind paw (Fig. 4E). Similar to that with afferent input from knee (Fig. 1, the SA of L4 dorsal root units with the RF in the plantar surface of the hind paw did not show differences in either the incidence or firing rate of SA between saline- and MIA-injected rats (Fig. 4F, G). No data points were excluded from the analysis. Detailed statistical data are presented in Supplementary Table 1E.
Figure 4.
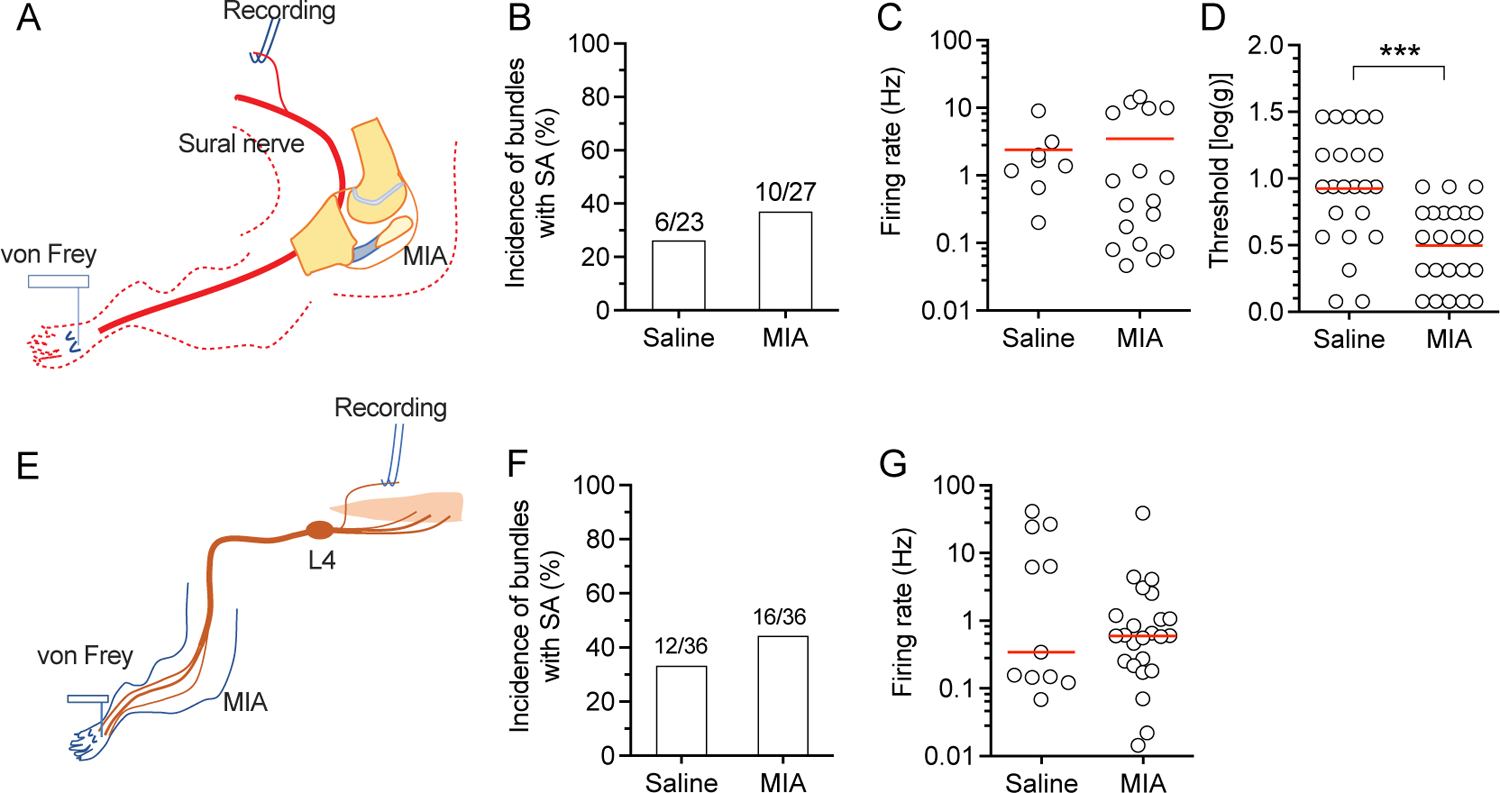
SA in sural nerve and L4 dorsal root units with the RFs in the plantar skin. (A) Preparation design for recording from the sural nerve. (B-D) Graphs show the frequency of SA in bundles (B), SA firing rate (C), and the threshold of mechanical stimulation of the RF in the hind paw for inducing firing of units in sural nerve teased bundles (D). (E) Preparation design for recording from the L4 dorsal root. Graphs show the frequency of bundles with SA (F) and firing rate (C). Red lines represent means. Data are from n=5 rats with saline injection and n=5 rats with injection of MIA for each type of recordings from sural nerve, and L4. *** P<0.001 by t-test.
As there were lowered thresholds for both evoked behavior by mechanical stimulation (Supplementary Fig. 1) and for mechanical stimulation-induced firing in sural fiber units after MIA injection (Fig. 4), we tested whether GFS can reverse these phenomena, as suggested by its efficacy in providing analgesia7. We found that the threshold of AP firing in teased L4 dorsal root bundles evoked by punctate mechanical stimulation of RF in the glabrous plantar skin was lowered in MIA-OA rats (Fig. 5A–C). 120s-20Hz GFS increased the threshold of firing from the baseline in both saline- and MIA-injected rats (Fig. 5B), with a greater effect in MIA rats than control (Fig. 5C). No data points were excluded from the analysis. Detailed statistical data are presented in Supplementary Table 1F.
Figure 5.
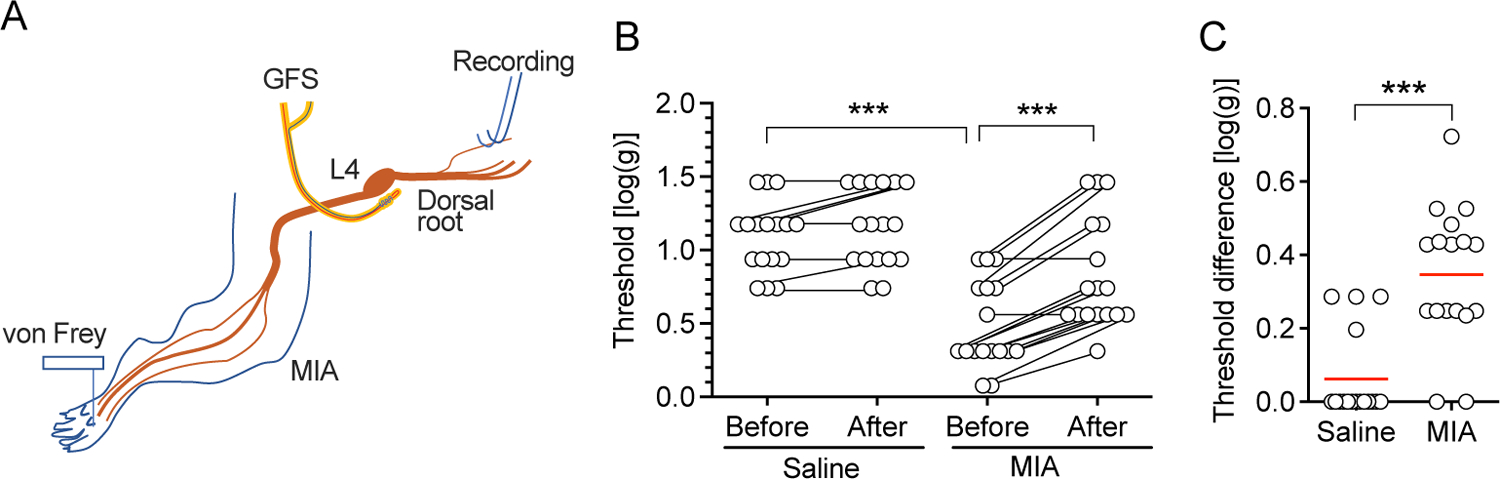
GFS normalizes the reduced mechanical stimulation threshold for firing in MIA-OA animals. (A) Preparation design for recording from the L4 dorsal root during application of von Frey filaments to the RF in the plantar skin. (B) Effect of 120s-20Hz GFS on the mechanical threshold for firing. *** P<0.001 by 2-way repeated measure ANOVA followed by Sidak’s post hoc test. (C) Effect size of GFS in MIA-OA and control animals. *** P<0.001 by t-test. Data are from n=5 rats with saline injection and n=5 rats with injection of MIA.
Next, we tested whether GFS could block afferent impulses evoked by punctate mechanical plantar stimulation at threshold and noxious intensities for 10s with a series of standard von Frey filaments plus a 16g von Frey filament modified with a 100μm diameter tungsten tip to produce noxious stimulation. Evoked APs were recorded from teased L4 dorsal root bundles (Fig. 6A, B). Consistent with our previous reports11, GFS inhibited C-type AP firing not only in MIA-injected rats but also in saline-injected control (Fig. 6C–F). No data points were excluded from the analysis. Detailed statistical data are presented in Supplementary Table 1G.
Figure 6.
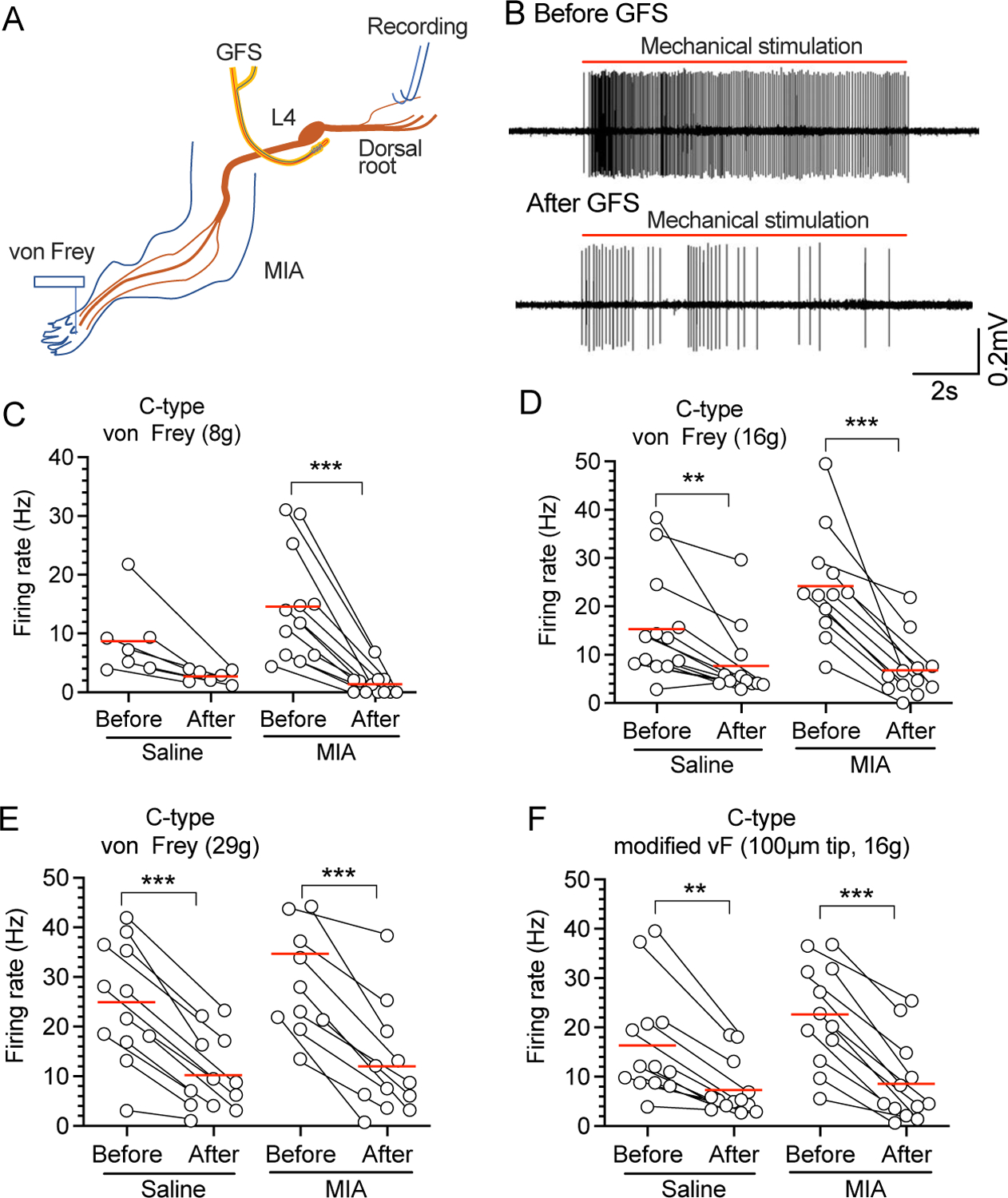
Effects of 120s-20-Hz GFS on AP firing of teased L4 dorsal root fibers by mechanical/noxious stimulation of RF in the plantar skin. (A) Preparation design for recording the GFS electrode was implanted besides L4 DRG, and the L4 dorsal root was teased and recorded. (B) Representative traces showing AP firing before and after GFS in rats with MIA injection in the ipsilateral knee joint. Effects of 120s-20Hz L4 GFS on AP firing in evoked by 8g (C), 16g (D), 29g (E), and sharp (F) punctate mechanical stimulation. These recordings were from 5 rats with saline injection and n=5 rats with injection of MIA. ** P<0.01, *** P<0.01 by 2-way repeated measure ANOVA followed by Sidak’s post hoc test. Each symbol is one unit.
MIA-OA induces dorsal root reflex retrograde impulses that are blocked by GFS
As the MIA-induced pain originates from an injury to the knee, it is important to consider what the possible mechanisms are for generating mechanical hypersensitivity in the plantar skin. It has been reported that injury induced effects at the levels of DRG and DH contribute to this process29, 30. As there is reduced mechanical threshold in recordings from sural nerve (Fig. 4), it is possible that this could be the result of dorsal root reflex efferent activity, and that GFS might also have analgesic effects by blocking this retrograde activity. To test this hypothesis, we examined whether there is increased retrograde firing after MIA-OA by recording from the proximal stump of the transected dorsal root (Fig. 7A). We first tested whether evoked retrograde responses could be recorded from the L4 dorsal root while stimulating L3 DRG, using the GFS electrode to deliver test pulses (0.2 Hz). This demonstrated that efferent activity could be induced by stimulation of adjacent afferent units, and was blocked by local application of tetrodotoxin (TTX) at the entry zone of DH of the efferent (L4) limb (Fig. 7B, C), confirming the presence of an inducible dorsal root reflex pathway by which afferent impulses can trigger efferent activity within the dorsal horn. We next recorded from rats with MIA-OA in the absence of electrical stimulation and identified retrograde SA, which was blocked by local application of TTX at the entry zone of DH of the efferent limb (Fig. 7D), confirming a DH origin of the activity. This activity was common in MIA-OA rats (20 of 46 bundles) whereas it was rare in controls (1 of 38; Fig. 7E). These results suggest that retrograde firing from DH in the pathological setting of MIA-OA could contribute to generating a hypersensitive state in plantar skin after MIA-OA. No data points were excluded from the analysis. Detailed statistical data are presented in Supplementary Table 1H.
Figure 7.
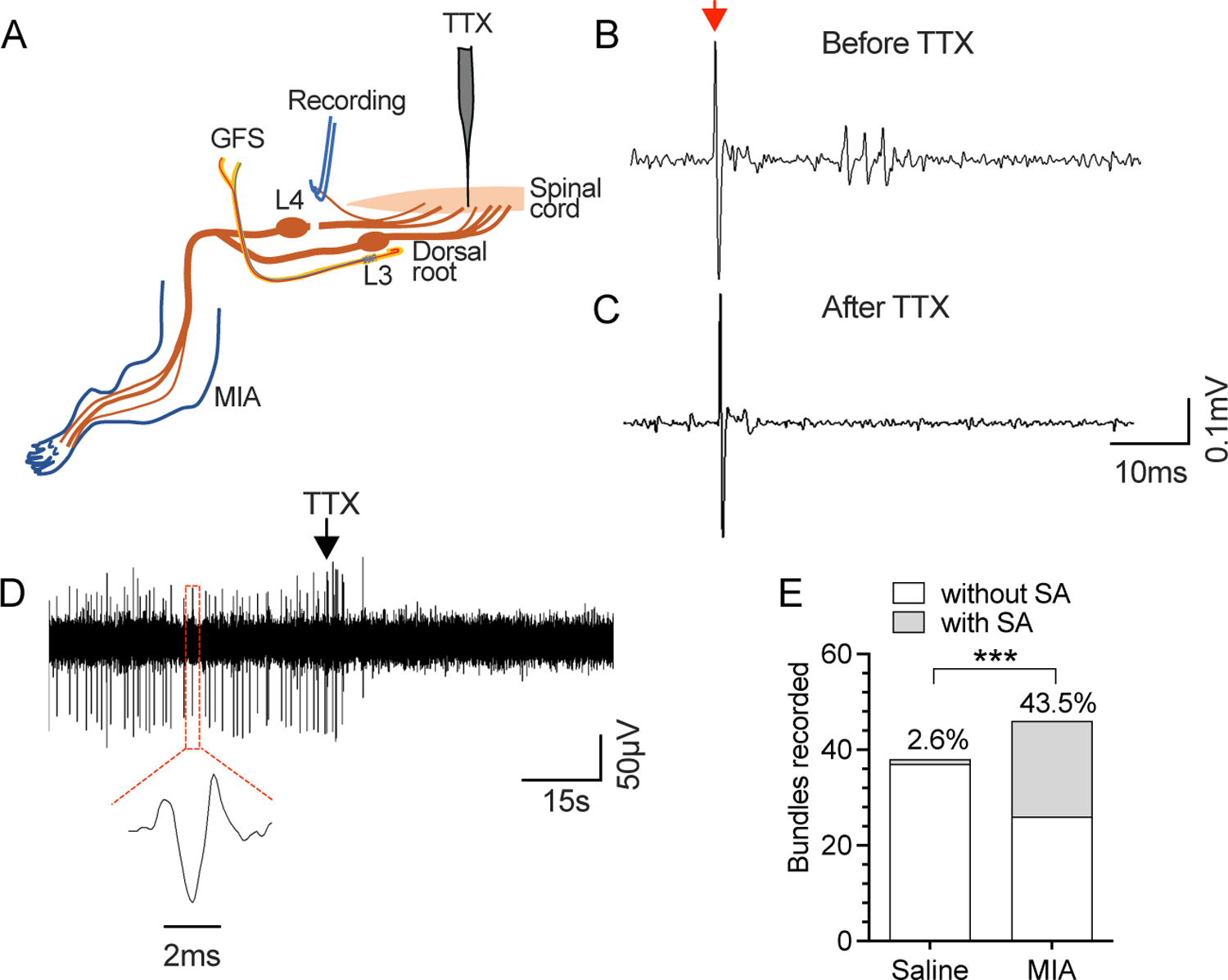
Retrograde SA recorded from the dorsal root in rats with MIA-OA. (A) Preparation design for recording efferent activity from the L4 dorsal triggered by afferent activity induced by L3 DRG stimulation using the GFS electrode. (B) Sample traces show evoked retrograde firing in response to single afferent impulse (0.4mA, 02ms, 0.2Hz, red arrow, which is blocked by TTX at the dorsal root entry zone (C), confirming it origin from the dorsal horn. SA was also recorded, which was similarly sensitive to TTX (D). MIA-OA increased the incidence of retrograde SA (E). These recordings were from 5 rats with saline injection and n=5 rats with injection of MIA. ***P<0.001 by Chi-square comparisons.
We then tested whether GFS could block efferent SA by recording single units from the sural nerve at the level of the knee (Fig. 8A), which is purely sensory at the level of the recording. Single units in teased bundles were identified by stimulation with individual impulses (0.2 Hz) delivered at the L4 DRG by the GFS electrode (Fig. 8B), which can be fully eliminated by lidocaine injected into the proximal sciatic nerve, confirming its origin from DRG neurons. SA was then measured in the absence of any stimulation, after which GFS was applied. This showed that retrograde SA was rare in control animals but common in MIA-OA rats (Fig. 8C), and that this retrograde SA activity was eliminated or substantially reduced by GFS at the L4 DRG (Fig. 8D, E). No data points were excluded from the analysis. Detailed statistical data are presented in Supplementary Table 1I.
Figure 8.
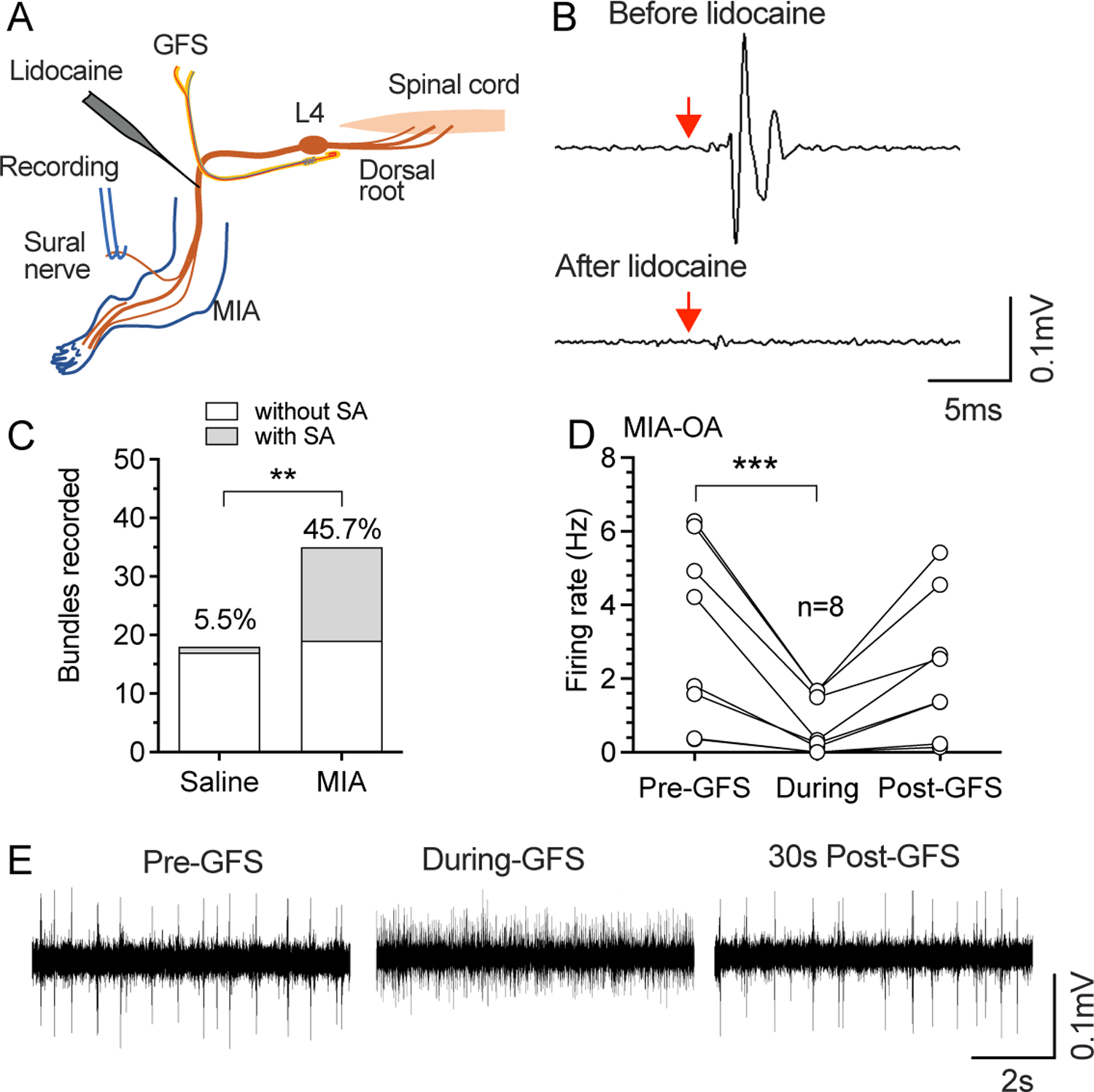
Retrograde SA recorded from the sural nerve. (A) Preparation design for recording efferent activity from the sural nerve. (B) Sample traces show retrograde firing evoked by single stimuli from the GFS electrode before (top) and after (bottom) application of TTX to sciatic nerve, which was used to identify recordable single units, and thereafter measure their SA. Red arrows point to the start of stimulation. (C) MIA-OA increased the frequency of retrograde SA recorded from sural nerve. ** P<0.01 by Chi-square comparisons. (D) 120s-20Hz GFS blocked the incidence of retrograde SA recorded from sural nerve after MIA-OA. ***P<0.001 nonparametric 1-way repeated measure ANOVA (Friedman test) followed by comparison of rank. (E) Sample traces shows effects of GFS on retrograde SA. These recordings were from 5 rats with saline injection and n=5 rats with injection of MIA.
Discussion
In this study, we found that GFS might produce analgesic effects in MIA-OA rats by causing use-dependent blockade of afferent activity induced both by knee manipulation and by punctate mechanical stimulation of the paw. Additionally, we observed the presence in MIA-OA animals of efferent activity originating from the DH, which also was ablated by GFS.
Even though both peripheral and central mechanisms contribute to OA pain, clinical evidence suggests that OA pain and sensitization are largely driven by peripheral input4. Consistent with this clinical observation, neurons in the saphenous nerve of rats, which innervates the knee joint, developed SA in MIA-induced OA in (Fig. 1) as has also been observed by others31. This reflects the activation of nociceptors from injured knee tissues, involving enhanced sensitization and expression of ion channels and receptors32–36 and upregulated expression of endogenous neuropeptides such as calcitonin-gene-related peptide37, 38 in articular nociceptors of the knee joint of OA, making nociceptors responsive to innocuous stimuli such as joint loading or movement31, 39.
Knee joints are densely innervated by sensory endings of the medial articular nerve which usually joins the saphenous nerve and merges into the spinal cord at L3 and L4 levels1, 2, 40. Distinguishing the contribution of each DRG in innervating knee joint in OA pain is helpful to develop accurate therapeutic manipulation with DRG stimulation. Even though previous reports detecting molecular and biochemical changes in DRGs after MIA-OA pain with pooled L3 and L4 DRGs provided valuable insight into the role of DRGs in OA pain, those studies provide little information about the possible different contributions of L3 and L4 to the OA pain in knee joints41–43, given that innervation of the knee joint from each DRG is not the same equal1–3. Our current study with teased fiber recording indicates that L3, not L4, afferent input is activated from the affected knee in rats with MIA-induced OA pain (Fig. 1). A recent study also shows different involvement of the L3 and L4 DRGs, in which activity of p38 mitogen-activated protein kinases and tyrosine hydroxylase immunostaining were elevated in the L3 but not L4 DRGs in both MIA and medial meniscal tear models of OA pain44. The role of articular cartilage degradation and subchondral bone erosion after MIA-OA might account for this different activity of L3 and L4 since in the rat knee. The majority of subchondral bone afferents are located in L3 DRG and fewer in L4 and L5, while other parts of the joint are innervated equally by joint afferents from both in L3 and L42. In the MIA model, the early inflammatory tissues may cause sensitization selectively of neurons that innervate the joint capsule, while pain in late MIA-induced OA involves the additional recruitment of neurons innervating the underlying subchondral bone due to the damage to the articular cartilage45. Histological studies have demonstrated that structural pathology of the knee joint, especially articular cartilage degradation and subchondral bone erosion, develop 2 weeks after MIA injection46, 47, a time point at which our electrophysiological recordings were done. Thus, enhanced L3 rather than L4 afferent input in MIA-induced OA rats observed in our study may result from recruitment of additional afferents that innervate the subchondral bone due to the damage to the articular cartilage and the subchondral bone45.
Referred pain is a phenomenon often observed in OA patients25, and has been noted as well in MIA-induced OA in animals23, 24, as shown by plantar von Frey testing26, 27. We also observed referred hyperalgesia and allodynia in the hind paw of the affected limb in MIA-OA rats (Supplementary Fig. 1). This could result from both peripheral and central mechanisms29, 30. We found that the threshold of mechanical stimulation for inducing firing is lower in MIA-injected rats than control in units recorded both in the hind paw-innervating sural fibers and in L4 dorsal root units with their RFs in the plantar skin (Fig. 4), which supports the participation of a peripheral mechanism. The referred hyperalgesia and allodynia in the hind paw may also result from central sensitization, since both neurons and microglia in the DH become highly activated after aberrant activation of knee nociceptors and dysfunction of descending pain modulatory system in the brain in OA31, 34, 42, 43, 48–50.
In addition to peripheral sensory neuron and DH mechanisms that contribute to the hypersensitivity of hind paw to mechanical stimulation, we identified efferent (retrograde) impulse trains that might also contribute to pain pathogenesis. The dorsal root reflex processes by which retrograde impulse trains are generated in sensory neurons has been well delineated13. Increased afferent input originating from peripheral sites of injury or inflammation cause dorsal horn release of excitatory neurotransmitters and depolarization of sensory neuron central terminals (i.e., primary afferent depolarization), which in turn generates impulse trains firing antidromically back to the periphery (i.e., dorsal root reflexes), not only in the active afferent but also in neighboring units13. This triggers peripheral release of inflammatory peptides from C-type and Aδ sensory neuron peripheral terminals in the target tissues, such as skin, joints, meninges, and viscera13–15, and possibly from neuronal somata in the DRG16, which incites inflammatory cascades. For the first time, we confirmed this efferent activity in the MIA-OA model and proved the effectiveness of GFS in blocking this efferent activity. This predicts a beneficial role of GFS in treating OA patients by not only blocking afferent activity underlying painful sensations but also blocking efferent activity that may reduce tissue inflammation and neuronal activation.
Overall, our findings indicate knee pain in MIA-OA is at least in part due to SA from the knee, and that plantar skin hypersensitivity to mechanical stimulation may be the result of efferent activity triggered by dorsal root reflex activity that in turn causes neurogenic inflammation in the innervated RFs and DRG supplying distal sites. GFS can block both afferent impulses from the injured knee and efferent traffic, which makes GFS a suitable consideration for clinical treatment of OA pain. As GFS can block AP firing in C-type fibers from saline injected rats (Fig. 2&3), which is consistent with our previous report that GFS at multiple levels of DRGs decreases the response to noxious mechanical stimulation7, it is possible that GFS delivered to multiple levels of DRGs in patients might cause impaired nociception and potential injury, which should be evaluated in clinical studies.
Supplementary Material
Supplementary Figure 1. Rats with developed evoked and spontaneous pain behaviors. Rats with intra-articular injection of MIA developed weight-bearing asymmetry (A), and hypersensitivity to noxious mechanical stimuli (pin, B), threshold mechanical stimuli (von Frey, C), brush (D), and cold (E). (F) MIA-OA rats are hypersensitive to knee pressure. * P<0.05, *** P<0.001 by parametric t-test for A, C, F, and nonparametric Mann–Whitney test for B, D, E. Each symbol is one rat.
Funding sources
This work was supported by National Institute of Health Grants R01NS103812 to QHH, and R01NS112194 to BP. These sources did not participate in data collection, analysis, interpretation, or decision to submit this publication.
Competing interest statement
Bin Pan received research grant from Abbott Neuromodulation. The other authors declare no conflicts of interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Data Availability Statement:
All data are available upon request.
Reference:
- 1.Salo PT, Theriault E. Number, distribution and neuropeptide content of rat knee joint afferents. J Anat 1997; 190 (Pt 4): 515–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aso K, Ikeuchi M, Izumi M, Sugimura N, Kato T, Ushida T, et al. Nociceptive phenotype of dorsal root ganglia neurons innervating the subchondral bone in rat knee joints. Eur J Pain 2014; 18: 174–181. [DOI] [PubMed] [Google Scholar]
- 3.Edoff K, Grenegard M, Hildebrand C. Retrograde tracing and neuropeptide immunohistochemistry of sensory neurones projecting to the cartilaginous distal femoral epiphysis of young rats. Cell Tissue Res 2000; 299: 193–200. [DOI] [PubMed] [Google Scholar]
- 4.Syx D, Tran PB, Miller RE, Malfait AM. Peripheral Mechanisms Contributing to Osteoarthritis Pain. Curr Rheumatol Rep 2018; 20: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Sousa Valente J. The Pharmacology of Pain Associated With the Monoiodoacetate Model of Osteoarthritis. Front Pharmacol 2019; 10: 974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pan B, Yu H, Fischer GJ, Kramer JM, Hogan QH. Dorsal Root Ganglionic Field Stimulation Relieves Spontaneous and Induced Neuropathic Pain in Rats. J Pain 2016; 17: 1349–1358. [DOI] [PubMed] [Google Scholar]
- 7.Yu G, Segel I, Zhang Z, Hogan QH, Pan B. Dorsal Root Ganglion Stimulation Alleviates Pain-related Behaviors in Rats with Nerve Injury and Osteoarthritis. Anesthesiology 2020; 133: 408425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pan B, Zhang Z, Chao D, Hogan QH. Dorsal Root Ganglion Field Stimulation Prevents Inflammation and Joint Damage in a Rat Model of Rheumatoid Arthritis. Neuromodulation 2018; 21: 247–253. [DOI] [PubMed] [Google Scholar]
- 9.Deer TR, Levy RM, Kramer J, Poree L, Amirdelfan K, Grigsby E, et al. Dorsal root ganglion stimulation yielded higher treatment success rate for complex regional pain syndrome and causalgia at 3 and 12 months: a randomized comparative trial. Pain 2017; 158: 669–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Esposito MF, Malayil R, Hanes M, Deer T. Unique Characteristics of the Dorsal Root Ganglion as a Target for Neuromodulation. Pain Med 2019; 20: S23–S30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chao D, Zhang Z, Mecca CM, Hogan QH, Pan B. Analgesic dorsal root ganglionic field stimulation blocks conduction of afferent impulse trains selectively in nociceptive sensory afferents. Pain 2020; 161: 2872–2886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chao D, Mecca CM, Yu G, Segel I, Gold MS, Hogan QH, et al. Dorsal root ganglion stimulation of injured sensory neurons in rats rapidly eliminates their spontaneous activity and relieves spontaneous pain. Pain 2021; 162: 2917–2932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Willis WD Jr., Dorsal root potentials and dorsal root reflexes: a double-edged sword. Exp Brain Res 1999; 124: 395–421. [DOI] [PubMed] [Google Scholar]
- 14.Sorkin LS, Eddinger KA, Woller SA, Yaksh TL. Origins of antidromic activity in sensory afferent fibers and neurogenic inflammation. Semin Immunopathol 2018; 40: 237–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin Q, Wu J, Willis WD. Dorsal root reflexes and cutaneous neurogenic inflammation after intradermal injection of capsaicin in rats. J Neurophysiol 1999; 82: 2602–2611. [DOI] [PubMed] [Google Scholar]
- 16.Huang LY, Neher E. Ca(2+)-dependent exocytosis in the somata of dorsal root ganglion neurons. Neuron 1996; 17: 135–145. [DOI] [PubMed] [Google Scholar]
- 17.Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods 1994; 53: 55–63. [DOI] [PubMed] [Google Scholar]
- 18.Wu HE, Gemes G, Zoga V, Kawano T, Hogan QH. Learned avoidance from noxious mechanical simulation but not threshold semmes weinstein filament stimulation after nerve injury in rats. J Pain 2010; 11: 280–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Villiere V, McLachlan EM. Electrophysiological properties of neurons in intact rat dorsal root ganglia classified by conduction velocity and action potential duration. J Neurophysiol 1996; 76: 1924–1941. [DOI] [PubMed] [Google Scholar]
- 20.Lawson SN, Crepps BA, Perl ER. Relationship of substance P to afferent characteristics of dorsal root ganglion neurones in guinea-pig. J Physiol 1997; 505 (Pt 1): 177–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fang X, McMullan S, Lawson SN, Djouhri L. Electrophysiological differences between nociceptive and non-nociceptive dorsal root ganglion neurones in the rat in vivo. J Physiol 2005; 565: 927943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Combe R, Bramwell S, Field MJ. The monosodium iodoacetate model of osteoarthritis: a model of chronic nociceptive pain in rats? Neurosci Lett 2004; 370: 236–240. [DOI] [PubMed] [Google Scholar]
- 23.Bove GM, Ransil BJ, Lin HC, Leem JG. Inflammation induces ectopic mechanical sensitivity in axons of nociceptors innervating deep tissues. J Neurophysiol 2003; 90: 1949–1955. [DOI] [PubMed] [Google Scholar]
- 24.Barton NJ, Strickland IT, Bond SM, Brash HM, Bate ST, Wilson AW, et al. Pressure application measurement (PAM): a novel behavioural technique for measuring hypersensitivity in a rat model of joint pain. J Neurosci Methods 2007; 163: 67–75. [DOI] [PubMed] [Google Scholar]
- 25.Moss P, Knight E, Wright A. Subjects with Knee Osteoarthritis Exhibit Widespread Hyperalgesia to Pressure and Cold. PLoS One 2016; 11: e0147526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pitcher T, Sousa-Valente J, Malcangio M. The Monoiodoacetate Model of Osteoarthritis Pain in the Mouse. J Vis Exp 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Philpott HT, McDougall JJ. Combatting joint pain and inflammation by dual inhibition of monoacylglycerol lipase and cyclooxygenase-2 in a rat model of osteoarthritis. Arthritis Res Ther 2020; 22: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kambiz S, Baas M, Duraku LS, Kerver AL, Koning AH, Walbeehm ET, et al. Innervation mapping of the hind paw of the rat using Evans Blue extravasation, Optical Surface Mapping and CASAM. J Neurosci Methods 2014; 229: 15–27. [DOI] [PubMed] [Google Scholar]
- 29.Dimitroulas T, Duarte RV, Behura A, Kitas GD, Raphael JH. Neuropathic pain in osteoarthritis: a review of pathophysiological mechanisms and implications for treatment. Semin Arthritis Rheum 2014; 44: 145–154. [DOI] [PubMed] [Google Scholar]
- 30.Bourassa V, Deamond H, Yousefpour N, Fitzcharles MA, Ribeiro-da-Silva A. Pain-related behavior is associated with increased joint innervation, ipsilateral dorsal horn gliosis, and dorsal root ganglia activating transcription factor 3 expression in a rat ankle joint model of osteoarthritis. Pain Rep 2020; 5: e846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kwok CHT, Kohro Y, Mousseau M, O’Brien MS, Matyas JR, McDougall JJ, et al. Role of Primary Afferents in Arthritis Induced Spinal Microglial Reactivity. Front Immunol 2021; 12: 626884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kelly S, Dunham JP, Murray F, Read S, Donaldson LF, Lawson SN. Spontaneous firing in C-fibers and increased mechanical sensitivity in A-fibers of knee joint-associated mechanoreceptive primary afferent neurones during MIA-induced osteoarthritis in the rat. Osteoarthritis Cartilage 2012; 20: 305–313. [DOI] [PubMed] [Google Scholar]
- 33.Schuelert N, McDougall JJ. Involvement of Nav 1.8 sodium ion channels in the transduction of mechanical pain in a rodent model of osteoarthritis. Arthritis Res Ther 2012; 14: R5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.He BH, Christin M, Mouchbahani-Constance S, Davidova A, Sharif-Naeini R. Mechanosensitive ion channels in articular nociceptors drive mechanical allodynia in osteoarthritis. Osteoarthritis Cartilage 2017; 25: 2091–2099. [DOI] [PubMed] [Google Scholar]
- 35.Chakrabarti S, Pattison LA, Singhal K, Hockley JRF, Callejo G, Smith ESJ. Acute inflammation sensitizes knee-innervating sensory neurons and decreases mouse digging behavior in a TRPV1-dependent manner. Neuropharmacology 2018; 143: 49–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Miller RE, Tran PB, Das R, Ghoreishi-Haack N, Ren D, Miller RJ, et al. CCR2 chemokine receptor signaling mediates pain in experimental osteoarthritis. Proc Natl Acad Sci U S A 2012; 109: 20602–20607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Walsh DA, Mapp PI, Kelly S. Calcitonin gene-related peptide in the joint: contributions to pain and inflammation. Br J Clin Pharmacol 2015; 80: 965–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ferreira-Gomes J, Adaes S, Sarkander J, Castro-Lopes JM. Phenotypic alterations of neurons that innervate osteoarthritic joints in rats. Arthritis Rheum 2010; 62: 3677–3685. [DOI] [PubMed] [Google Scholar]
- 39.Okun A, Liu P, Davis P, Ren J, Remeniuk B, Brion T, et al. Afferent drive elicits ongoing pain in a model of advanced osteoarthritis. Pain 2012; 153: 924–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hildebrand C, Oqvist G, Brax L, Tuisku F. Anatomy of the rat knee joint and fibre composition of a major articular nerve. Anat Rec 1991; 229: 545–555. [DOI] [PubMed] [Google Scholar]
- 41.Rahman W, Bauer CS, Bannister K, Vonsy JL, Dolphin AC, Dickenson AH. Descending serotonergic facilitation and the antinociceptive effects of pregabalin in a rat model of osteoarthritic pain. Mol Pain 2009; 5: 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Im HJ, Kim JS, Li X, Kotwal N, Sumner DR, van Wijnen AJ, et al. Alteration of sensory neurons and spinal response to an experimental osteoarthritis pain model. Arthritis Rheum 2010; 62: 29953005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lockwood SM, Lopes DM, McMahon SB, Dickenson AH. Characterisation of peripheral and central components of the rat monoiodoacetate model of Osteoarthritis. Osteoarthritis Cartilage 2019; 27: 712–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brederson JD, Chu KL, Xu J, Nikkel AL, Markosyan S, Jarvis MF, et al. Characterization and comparison of rat monosodium iodoacetate and medial meniscal tear models of osteoarthritic pain. J Orthop Res 2018. [DOI] [PubMed] [Google Scholar]
- 45.Morgan M, Thai J, Nazemian V, Song R, Ivanusic JJ. Changes to the activity and sensitivity of nerves innervating subchondral bone contribute to pain in late-stage osteoarthritis. Pain 2022; 163: 390–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hoshino T, Tsuji K, Onuma H, Udo M, Ueki H, Akiyama M, et al. Persistent synovial inflammation plays important roles in persistent pain development in the rat knee before cartilage degradation reaches the subchondral bone. BMC Musculoskelet Disord 2018; 19: 291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.An JS, Tsuji K, Onuma H, Araya N, Isono M, Hoshino T, et al. Inhibition of fibrotic changes in infrapatellar fat pad alleviates persistent pain and articular cartilage degeneration in monoiodoacetic acid-induced rat arthritis model. Osteoarthritis Cartilage 2021; 29: 380–388. [DOI] [PubMed] [Google Scholar]
- 48.Thakur M, Rahman W, Hobbs C, Dickenson AH, Bennett DL. Characterisation of a peripheral neuropathic component of the rat monoiodoacetate model of osteoarthritis. PLoS One 2012; 7: e33730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tarrago M, Deitos A, Brietzke AP, Vercelino R, Torres ILS, Fregni F, et al. Descending Control of Nociceptive Processing in Knee Osteoarthritis Is Associated With Intracortical Disinhibition: An Exploratory Study. Medicine (Baltimore) 2016; 95: e3353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Drake RA, Leith JL, Almahasneh F, Martindale J, Wilson AW, Lumb B, et al. Periaqueductal Grey EP3 Receptors Facilitate Spinal Nociception in Arthritic Secondary Hypersensitivity. J Neurosci 2016; 36: 9026–9040. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Rats with developed evoked and spontaneous pain behaviors. Rats with intra-articular injection of MIA developed weight-bearing asymmetry (A), and hypersensitivity to noxious mechanical stimuli (pin, B), threshold mechanical stimuli (von Frey, C), brush (D), and cold (E). (F) MIA-OA rats are hypersensitive to knee pressure. * P<0.05, *** P<0.001 by parametric t-test for A, C, F, and nonparametric Mann–Whitney test for B, D, E. Each symbol is one rat.
Data Availability Statement
All data are available upon request.


