Abstract
An enzyme-linked immunosorbent assay (ELISA) was developed based on a recombinant major Theileria annulata merozoite surface antigen, Tams1. Four different recombinant proteins derived from two different Tams1 alleles, both in two different truncated forms, were tested for their performance in the ELISA. Furthermore, antigen concentration, various buffers, washing protocol, and the choice of anti-total-immunoglobulin G (IgG), anti-IgG1, or anti-IgG2 as second antibody were evaluated. The performance of the resulting ELISA was analyzed by measuring the coefficient of variation (CV). A total of 22 sera were analyzed over the measurement range, resulting in a CV of ca. 10%, whereas 30% variation is the maximum acceptable. The cutoff value was determined by the two-graph receiver operating characteristic (TG-ROC), using the indirect fluorescent antibody test (IFAT) as a reference. It was shown that up to 3 months postinfection (p.i.) IFAT is more sensitive and specific, whereas beyond 3 months p.i. ELISA performed as well as IFAT. The cutoff was determined at maximal sensitivity, based on the TG-ROC after 3 months p.i. Nine calves experimentally infected with four different T. annulata stocks remained positive in the ELISA for at least 1 year p.i. Finally, limited cross-reaction was found only with T. parva antisera, but not with any other Theileria or Babesia species. Since the T. parva endemic area hardly overlaps with T. annulata, the Tams1 ELISA has the potential to become a useful tool in the epidemiology of tropical theileriosis.
The protozoan parasite Theileria annulata is the causative agent of tropical theileriosis and is endemic in the area around the Mediterranean and the Middle East and reaches the Southern parts of Asia (4). Transmission of the parasite to cattle takes place during a bloodmeal of infected ticks of the genus Hyalomma. Currently, the disease is kept under control by acaricides preventing tick infestation and attenuated vaccines, which are used in several areas where the disease is endemic. The parasite can be detected in ticks and cattle with a species-specific PCR (6, 7), and serodiagnosis in cattle is performed by an indirect fluorescent antibody test (IFAT) (33). Both techniques are impractical for large-scale epidemiological surveys. Moreover, cross-reactions have been observed with IFAT among several Theileria species (27) and, like PCR, IFAT is laborious.
Enzyme-linked immunosorbent assay (ELISA) is easier to perform, inexpensive, and would be useful for monitoring cattle for exposure to tropical theileriosis and facilitate the study of the epidemiology of the disease. Previous efforts to develop an ELISA for T. annulata were based on purified schizont or piroplasm antigen. The first attempt did not result in a high sensitivity or high specificity (25), whereas the second ELISA performed well (26). It is difficult, however, to standardize antigen purified from crude parasite material, and there is also the requirement of experimental animals for parasite production. Therefore, two ELISAs based on recombinant proteins have been developed: one uses the merozoite rhoptry antigen (18), whereas the other is based on a sporozoite antigen, SPAG-1 (2). However, the sensitivity and specificity of both tests has not been determined.
The immunodominant merozoite surface antigen Tams1-1 has been expressed in Escherichia coli (8, 35) and also used as a candidate for an ELISA (23). We report here the further characterization of the Tams1 ELISA by examining four recombinant proteins: two different allelic variants (Tams1-1 and Tams1-2) in two different truncated forms. The use of two different Tams1 allelic variants is based on the observation that Tams1 is a highly variable protein, and evidence for incomplete serological cross-reaction between allelic variants has been reported (26). Therefore, it could be that T. annulata infections not containing the particular Tams1 allele used in the ELISA are misdiagnosed. In one truncated form the signal peptide is not expressed since it is functional in the parasite wherein it is cleaved off during the transport process to the membrane and thus probably not involved in the development of an immune response. The second form is also expressed without signal peptide but, in addition, the membrane anchor domain is deleted. This domain is very hydrophobic and therefore probably contains no B-cell epitope either. Furthermore, the antigen concentration, buffer, washing protocol, and immunoglobulin G (IgG) class of second antibodies were optimized. Variation of second antibody is based on the observation that some animals have a heritable high concentration of IgG2 in their blood (38) which might lead to false-positive reactions in the ELISA. Therefore, an ELISA based on IgG1 only is preferred, since IgG2 can also agglutinate particulate antigens, whereas IgG1 cannot (42). The cutoff value resulting in both maximal sensitivity and specificity was determined by two-graph receiver operating characteristic (TG-ROC) analysis, ROC plots, efficiency, Youden's index, and likelihood ratios (13, 14). The repeatability of the ELISA was determined by calculating the coefficient of variation (CV) (21). The intraclass correlation coefficient (Ricc) value was calculated to compare the signal of the same sample on different plates and different days (10).
MATERIALS AND METHODS
Antigen production.
Four different recombinant proteins based on two allelic variants of Tams1 were used as candidates for the ELISA, and two different truncated forms from both proteins were tested; Tams1-1 and Tams1-2 without signal peptide encoded by, respectively, pETams1-1+ and pETams1-2+ and also Tams1-1 and Tams1-2 without both signal peptide and hydrophobic domain encoded by, respectively, pETams1-1 and pETams1-2 (8). Truncated Tams1 proteins were expressed and purified as described elsewhere (8). Briefly, truncated Tams1 proteins were expressed as His6-tagged proteins in E. coli and purified on a Ni2+-nitriloacetic acid column (Qiagen, Hilden, Germany). Subsequently, the protein was transferred to phosphate-buffered saline (PBS) over a Sephadex G-25 PD10 column (Pharmacia Biotech, Uppsala, Sweden) according to the manufacturer's instructions. Traces of detergent were removed by the addition of 2.5 μg of Extraction Gel D (Pierce, Rockford, United Kingdom) per ml. Protein concentration was measured with the Bradford assay (Pierce). Endogenous E. coli proteins possibly contaminating the recombinant proteins were purified as described above from bacteria containing the same plasmid without insert.
Experimental infections and test sera.
All calves used in this study were female Friesian Holstein calves housed under tick-free conditions and approximately 6 months of age at the time of infection. Infections were monitored as described previously (7). Theileria infections were given subcutaneously using ground-up tick supernatant (GUTS) sporozoite stabilates or were initiated by feeding infected Hyalomma ticks on the animal. Babesia infections were given intravenously. Infection with Babesia bovis and Babesia bigemina were established by infection with blood stabilates. Details of all parasite stocks used in this study as well as the number of animals infected with each stock are listed in Table 1. Sera were collected weekly and stored at −20°C until further use. From calves infected with T. annulata, sera were collected for at least 12 weeks postinfection (p.i.) and up to 67 weeks p.i. Thirty-five calves were infected with a single T. annulata isolate, two calves were infected with two different T. annulata isolates, and eight calves were infected with three different isolates per animal with 2-month intervals between subsequent infections (Table 1). Sera from animals infected with other parasites were also collected weekly until 16 weeks p.i. A panel of 169 negative sera was derived from 169 female Friesian Holstein calves ranging in age from 3 to 6 months. This negative panel contained preinfection sera of the calves infected with the different Theileria and Babesia species.
TABLE 1.
Theileria and Babesia isolates used for ELISA validation
| Species | Isolate | No. of animals tested | Stabilate | Reference of stock |
|---|---|---|---|---|
| T. annulata | Ankara | 14 | GUTS | 34 |
| T. annulata | Bahrain | 3 | Ticks | 41 |
| T. annulata | Spain | 3 | Ticks | 6 |
| T. annulata | Hissar | 13 | GUTS | 12 |
| T. annulata | Mauritania | 2 | Ticks | 22 |
| T. annulata | Hissar/Gharb/Ankara | 8 | GUTS | 32 |
| T. annulata | Ankara/Hissar | 2 | GUTS | |
| T. velifera | Lagarni | 1 | Ticks | Unpublished |
| T. velifera | Dar es Salaam | 1 | Ticks | 39 |
| T. taurotragi | Idobogo | 1 | Ticks | 40 |
| T. buffeli | England | 1 | Ticks | 31 |
| T. buffeli | Japan | 1 | Ticks | 20 |
| T. buffeli | Australia | 1 | Ticks | 37 |
| T. parva | Pugu 2 | 1 | GUTS | 40 |
| T. parva | Boleni | 1 | GUTS | 29 |
| T. parva | Muguga | 3 | GUTS | 3 |
| T. parva | Marikebuni | 1 | GUTS | 19 |
| B. bovis | Mexico | 1 | Blood | 1 |
| B. bigemina | Muguga | 1 | Blood | Unpublished |
ELISA conditions.
The four different truncated Tams1 proteins were checkerboard titrated on microtiter plates (Greiner, Frickenhausen, Germany) using concentrations of 0.5, 1.0, 2.0, and 4.0 μg/ml in combination with serum dilutions of 1:50, 1:100, 1:200, and 1:400 and conjugate (1:250, 1:500, 1:1,000 and 1:2,000) in order to reach the maximum difference between negative and positive sera (17). All incubations were performed in 100-μl/well volumes. Further varied conditions included PBS as coating buffer (28) versus 50 mM carbonate buffer (pH 9.6), different blocking agents (0.1% gelatin versus 1% skimmed milk), and different Tween 20 concentrations (0.1, 0.2, 0.4, and 0.8%), as well as different NaCl concentrations (150, 300, 600, and 1200 mM) during blocking, serum, and conjugate incubations. Finally, different washing conditions (three or five times, with or without 0.05% Tween 20) were tried.
The optimized protocol was as follows. Tams1-1 antigen was used at a concentration of 1 μg/ml in 50 mM carbonate buffer (pH 9.6) for 1 h at 37°C. Blocking was performed with 500 mM NaCl containing incubation buffer (PBS containing 1% skimmed milk powder [Nutricia, Zoetermeer, The Netherlands], 0.1% Tween 20, and an end concentration of 500 mM NaCl) for 30 min at 37°C. Sera and conjugate (rabbit anti-bovine total immunoglobulin G [IgG] conjugate; Nordic, Tilburg, The Netherlands) were diluted 1:200 and 1:500, respectively, in 500 mM NaCl incubation buffer and incubated for 1 h at 37°C. Plates were washed three times with 0.05% Tween 20 after serum and conjugate incubation with a plate washer (Microplate Autowasher EL 404; Bio-Tek Instruments, Inc., Winooski, Wis.). Peroxidase-mediated color development was performed for 30 min at room temperature in 100 mM Na2HPO4–50 mM citric acid buffer (pH 5.0) containing 0.5 mg of ABTS [2,2′-azinobis(3-ethylbenzthiazolinesulfonic acid); Sigma, St. Louis, Mo.] per ml and 0.0075% hydrogen peroxide (Aldrich, Steinheim, Germany). The absorbance was read at 405 nm using a Ceres UV900 C ELISA reader (Bio-Tek Instruments, Inc.).
ELISA results are presented as the percent positivity (PP) of a quadruple measurement on each plate of a high positive control serum in order to facilitate comparison of plates which are always subjected to small variations. This sample was obtained from calf 343 at 9 weeks after infection with the Mauritanian T. annulata isolate. Each plate included a quadruple measurement of a standard negative serum, i.e., calf 596, reflecting the mean value of the group of 169 negative animals. Initially, the cutoff was determined by doubling the mean of the negative controls. Where specifically indicated, the complement system in the sera was inactivated by incubating 100 μl of undiluted serum at 56°C for 30 min. Again, where indicated, diluted sera were incubated prior to application on the ELISA plate with E. coli proteins purified from bacteria containing the empty plasmid in concentrations of either 7, 20, 70, or 200 μg/ml for 30 min at 37°C.
ELISA using sheep anti-bovine IgG1 and IgG2 and mouse anti-bovine IgG1.
Sheep anti-bovine IgG1 and IgG2 were purchased from ICN (Costa Mesa, Calif.). Horseradish peroxidase was purchased from Boehringer (Mannheim, Germany). All other reagents were from Sigma. Conjugation was performed according to the method of Harlow (16). Essentially, 2.5 mg of peroxidase was conjugated with 35 mg of immunoglobulin. The precipitated pellet containing the conjugated antibodies was resuspended in 100 μl of PBS and supplemented with 1.25 μl of a 10-mg/ml concentration of bovine serum albumin. Peroxidase-conjugated mouse anti-bovine IgG1 monoclonal antibody was purchased from Chemicon International (Temecula, Calif.). The ELISA conditions in this section were as described for the standardized procedure in the previous section, but a standard incubation buffer (PBS, 0.1% Tween 20, 5% skimmed milk) was used for blocking, serum, and conjugate dilutions. In case of mouse anti-bovine IgG1, an additional peroxidase-conjugated rabbit anti-mouse (Dako, Glostrup, Denmark) incubation was performed diluted 1:1,000 in incubation buffer.
IFAT.
T. annulata piroplasm antigen slides were prepared from blood with a parasitemia of 30% derived from calf 184 at 3 weeks after experimental infection with GUTS stabilate no. 53 of the Ankara (Turkey) isolate. Test sera were serially diluted twofold from 1:40 to 1:10,240 in PBS and incubated for 25 min at room temperature after application onto the slides. The slides were washed once quickly in PBS, followed by two washes of 5 and 10 min each on a rocking platform. Fluorescein isothiocyanate-conjugated rabbit anti-bovine total IgG (Nordic) was diluted 1:100 in PBS and incubated for 25 min at room temperature. The slides were washed again as described above, and a few drops of Vectashield (Vector Laboratories, Inc., Burlingame, Calif.) were applied to the slides and covered by a coverglass. Fluorescence was examined by using a BH-2 Olympus fluorescence microscope (Olympus, Tokyo, Japan).
Repeatability and two-graph receiver operating characteristic (TG-ROC).
To measure the repeatability of the ELISA, 22 sera spanning the measurement range, including both T. annulata-positive and -negative sera, were selected and measured in eight duplicates on the same plate on eight different days. The CV was calculated as described by Jacobson (21), combining both intra- and interplate variation. Essentially, measurements were expressed as PP values, and for each serum the mean and the standard deviation of the repeated measurements were calculated. The CV was calculated by dividing the standard deviation by the mean and multiplying by 100.
To measure the variation between plates on different days, the intraclass correlation coefficient, Ricc, was calculated according to the method of Fleiss (10) from the sample set. In essence, for each serum the multiple PP measurements generated for the CV calculation were subjected to analysis of variance (ANOVA) in order to calculate the mean sum of squares between plates (BSS) and the mean sum of squares within plates (WSS). The Ricc value for each of the 22 sera was calculated as follows: Ricc = (WSS − BSS)/[BSS + (k0 − 1)WSS]. The k0 is the number of replicated duplicate measurements (in this case, 8). An overall Ricc value was calculated as the mean of all individual Ricc values. The closer the result came to 100%, the better the test.
ROC plots (including area under the curve [AUC]), TG-ROC plots, efficiency index, Youden's index, and likelihood ratios (LRs) were calculated using the template in Microsoft-Excel (13) applied similarly as described by Mboloi et al. (30). The AUC in ROC plots provide an index of accuracy by demonstrating the limits of a test's ability to discriminate positive from negative values over the complete set of operating conditions (44). The efficiency index (11) and Youden's index (43) express the general performance of the test by estimating the false-positive or false-negative proportion. LRs express the predictive value of the test as follows: LR+ = Se/(1 − Sp) and LR− = (1 − Se)/Sp, where Se is the sensitivity and Sp is the specificity (36). Essentially, for each analysis the measurement range was divided into 250 intervals, and in each interval sensitivity, specificity, efficiency, Youden's index, or LRs were calculated. Cutoffs were determined at maximum specificity and sensitivity for each analysis by using a negative serum panel of 50 different animals and two positive serum panels. The first IFAT-positive panel consisted of sera collected in the first 3 months p.i. from 45 different animals, and the second panel consisted of sera collected after 3 months p.i. from 30 different animals. A serum sample from one time point in the indicated period was randomly selected for each animal.
RESULTS
Optimization of conditions.
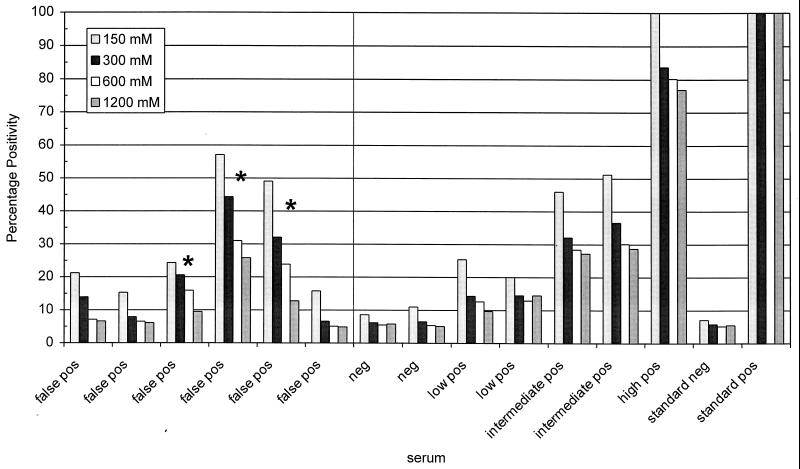
Antigen concentration and serum and conjugate dilutions were varied in order to reach maximum differences between optical density (OD) values of negative and positive sera in the ELISA. Under the optimized conditions, seroconversion could be measured in all of the T. annulata-infected animals listed in Table 1. Some minor variations in absolute OD values between the different recombinant proteins was observed, but the time until seroconversion values were identical. The negative panel of 169 animals resulted for all four proteins in six false-positive reactions using 2 times the mean of the negative panel as a cutoff. Since all four proteins reacted identically, pETams1-1, the Tams1-1 construct without both the N- and C-terminal domains, was selected because of its highest expression and the most efficient way it could be purified. In order to reduce the number of false-positive reactions, different concentrations of NaCl and Tween, as well as different washing procedures, were evaluated. Only the NaCl concentration influenced the ELISA performance (Fig. 1). The optimum was deduced at 500 mM NaCl so that the low-positive sera remained positive and only three false-positives remained. To rule out that false-positive reactions of naive animals were due to cross-reactivity with residual E. coli proteins in the recombinant antigen, diluted sera were preincubated with protein from E. coli, purified identically to the antigen. This did not reduce the number of false-positive reactions. The potential effect of complement in the sera was circumvented by inactivation of the complement system by heating the sera to 56°C prior to dilution. This also did not result in a reduction in the number of false-positive sera.
FIG. 1.
Influence of different NaCl concentrations on the specificity of the Tams1 ELISA. The NaCl concentration was varied in buffers used for blocking, serum incubation, and second antibody incubation. False-positive reacting sera were compared with negative, low-positive, moderate-positive, and high-positive control sera. ELISA results are expressed as the PP of the high-positive control serum for each different NaCl concentration. Asterisks indicate the three false-positive sera that could not be sufficiently reduced. The cutoffs were determined for each NaCl concentration by doubling the PP value of the standard negative serum.
Another attempt to improve ELISA performance was undertaken by measuring IgG isotype responses. Initially, total IgG was measured, but in animals with high innate IgG2 levels this can lead to a false-positive reaction. Sheep anti-bovine IgG1 and IgG2 were directly conjugated since secondary rabbit anti-sheep antibody cross-reacted with cattle immunoglobulins. Detected IgG2 levels did not relate very well with T. annulata infection, and low- and intermediately positive sera became negative. In general, a very high background was observed with anti-IgG2 antibody. Measuring IgG1 levels reduced the number of correctly diagnosed positive sera (data not shown). A monoclonal mouse anti-bovine IgG1 was tested as well but also resulted in values below the cutoff for low-positive sera. On the basis of these results the rabbit anti-bovine total IgG was selected for the Tams1 ELISA.
A negative serum (serum 596) with an OD value equal to the mean of the panel of negative sera was selected and included on all plates as an internal control for the correct determination of the cutoff (two times the level of the negative control) value for sera in individual plates. A high-T. annulata-positive serum (serum 343, 9 weeks p.i.) was also included to set measured ODs at 100% in order to compare different plates. Measured OD values are expressed as the PP of the positive control.
Repeatability.
Twenty-two sera covering the measurement range were used to evaluate ELISA performance on the same day in eight duplicates and on eight different days. From these repeated measurements the CV was calculated (Fig. 2). According to Jacobson (21), a CV of <30% is acceptable, and this is the case for all of the samples tested. From the same data set the mean Ricc was determined to be 45.4% using ANOVA analysis.
FIG. 2.
Precision profile plots of the Tams1 ELISA for 22 cattle sera expressed as the CV as a function of PP. Each point represents the mean of an individual serum measured 128 times on eight different plates.
TG-ROC analysis.
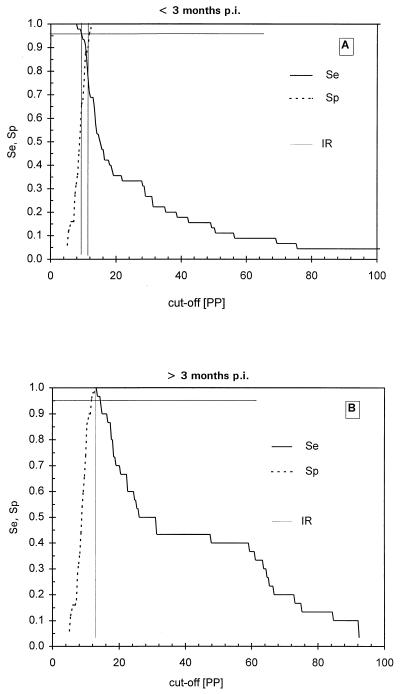
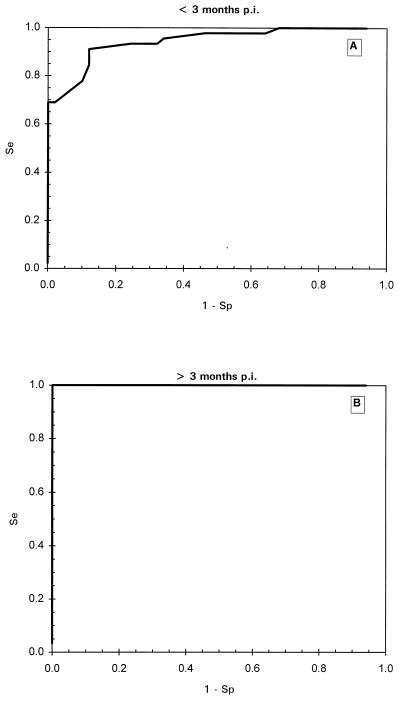
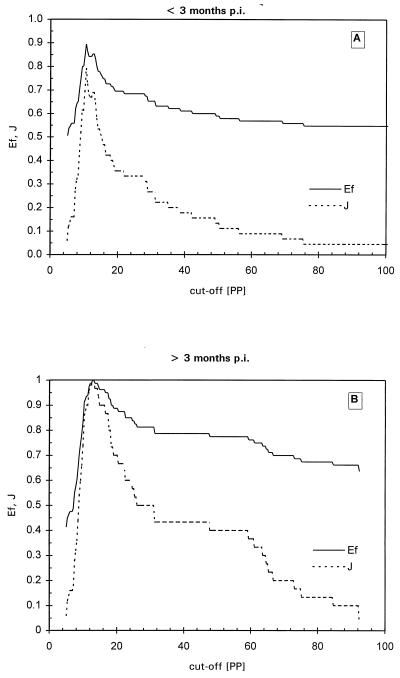
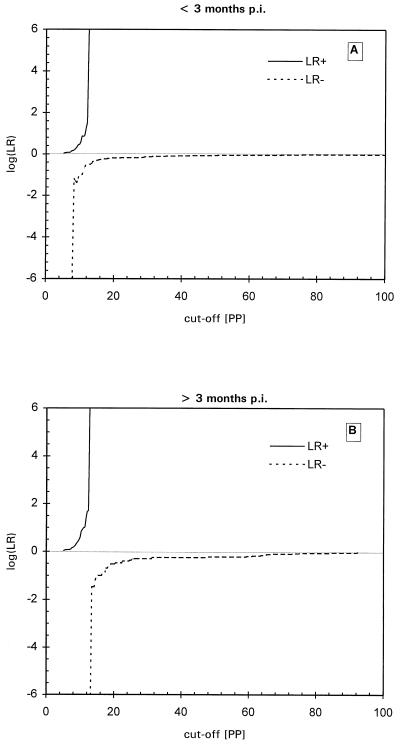
TG-ROC analysis was performed in order to determine the optimal cutoff value for the ELISA resulting in maximum specificity and sensitivity with the IFAT as a reference. When a set of 238 positive and negative samples was measured blindly, some false-negative results were found at under 3 months p.i. However, beyond 3 months p.i. all T. annulata-infected sera tested positive, using twice the mean of the negative sera panel as a cutoff value. Using these 238 sera in TG-ROC resulted in a cutoff at which low sensitivity and specificity was reached so that false-negative and false-positive results were generated. Therefore, it was decided to perform TG-ROC analysis on two groups of sera: IFAT-positive sera before 3 months p.i. and IFAT-positive sera after 3 months p.i. In the first period a group of 50 negative sera and 45 positive sera derived from different animals was analyzed. As seen in Fig. 3A, the best cutoff was at 10.6 PP, leading to a sensitivity and specificity of 87% with an intermediate range (IR) of between 10.2 and 11.8 PP using a 95% confidence interval. In the second group, the same animals as in group 1 from which sera were collected after 3 months p.i. (50 negative sera and 30 positive sera) were analyzed by TG-ROC (Fig. 3B), leading to 100% sensitivity and specificity at a cutoff of 12.7 PP. This cutoff value was identical to the previously used cutoff determined by the mean of the serum panel from the 169 uninfected calves. Further analysis using ROC plots (Fig. 4), Youden's index and efficiency ratios (Fig. 5), and LRs (Fig. 6) led to similar results for both periods. In the period under 3 months p.i., the AUC in the ROC plot was estimated to be 0.85, indicating a nonideal performance. The defined cutoffs as determined by Youden's index (0.79) and efficiency ratio (0.90) were 10.4 and 10.7 PP, respectively, neither of which is ideal. In the period after 3 months p.i., both the Youden's index and the efficiency ratio were 1.0 (the ideal value for a diagnostic test) at 12.5 PP. The AUC of the ROC plot (Fig. 4B) for the period over 3 months p.i. was 1.0, indicating 100% specificity and sensitivity. An ideal cutoff obtained from the LR plot (Fig. 6B) was 12.6 PP. Taking all of these values into account, a consensus cutoff was set at 12.6 PP, leading to no false-positive results over the entire infection period during which the animals were monitored. This cutoff was identical to twice the mean of the negative serum panel of 169 calves. In the panel of 169 negative animals, three false-positive results remained, leading to a specificity of 98%.
FIG. 3.
TG-ROC analysis of the Tams1 ELISA results for <3 months p.i. (A) and >3 months p.i. (B). The intermediate range (IR) is determined by the cutoff values at 95% sensitivity (Se) and 95% specificity (Sp).
FIG. 4.
ROC plots of Tams1 ELISA results for <3 months p.i. (A) and >3 p.i. (B). The graph in panel B falls precisely over the left y axis and the top x axis. Se, sensitivity; Sp, specificity.
FIG. 5.
Youden's index (J) and efficiency (Ef) of the Tams1 ELISA as a function of the selected cutoff value at <3 months p.i. (A) and >3 months p.i. (B).
FIG. 6.
Logarithm of negative (LR−) and positive (LR+) LRs as a function of the selected cutoff value at <3 months p.i. (A) and >3 months p.i. (B).
Species specificity.
Sera collected from experimentally infected calves with parasites other than T. annulata (Table 1) were analyzed at several time points p.i. with the Tams1 ELISA by using the optimized conditions described above. Antisera positive for T. velifera, T. taurotragi, T. buffeli, B. bovis, and B. bigemina resulted in values below the determined cutoff point. Measurement of sera collected from six calves infected with four different T. parva isolates resulted in OD values greater than the cutoff level in three calves. Cross-reacting sera were derived from calves infected by the Marikebuni and Pugu-2 T. parva isolates.
Comparison of IFAT and ELISA.
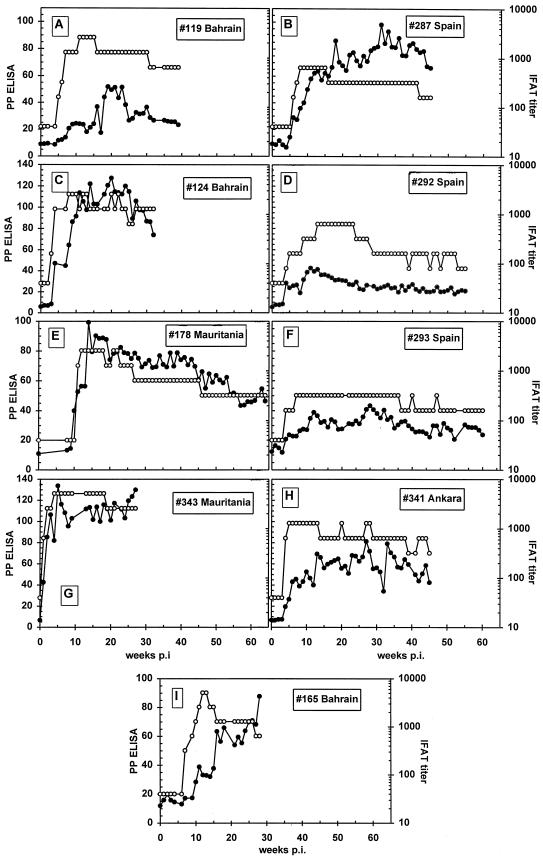
Four different T. annulata strains tested in nine different animals (one, Ankara; three, Bahrain; two, Mauritania; three, Spain) from which sera collected weekly for a prolonged period were analyzed by both IFAT and ELISA (Fig. 7). All animals became positive at between 1 and 8 weeks p.i. in both assays. In one case, calf 119 was positive in IFAT 1 week before the ELISA using 12.6% PP as the cutoff for the ELISA (Fig. 7A). ELISA resulted in a positive reaction 1 week earlier in calf 178 (Fig. 7E). In calves 165 and 293 (Fig. 7I and F, respectively), the ELISA reaction was above the cutoff starting at 1 week p.i., while the IFAT was still negative. In general, ELISA remained positive over the period studied, where the latest sample point was at 65 weeks p.i. for calf 178 (Fig. 7E). IFAT titer and ELISA results do not correlate with each other since some animals reacted more strongly in the IFAT than in the ELISA (Fig. 7A and D), whereas others reacted more strongly in the IFAT than in the ELISA (Fig. 7B). However, the observed trends in the rise and fall of antibody response in both assays were comparable.
FIG. 7.
Antibody profiles of four different T. annulata isolates in nine different animals as measured by Tams1 ELISA (●) and piroplasm IFAT (○). Panels: A, animal 119 infected with T. annulata from Bahrain; B, animal 287, Spain; C, animal 124, Bahrain; D, animal 292, Spain; E, animal 178, Mauritania; F, animal 293, Spain; G, animal 343, Mauritania; H, animal 341, Ankara; I, animal 165, Bahrain. The ELISA cutoff is 12.6 PP; the IFAT is considered positive at titers of >80.
DISCUSSION
The ELISA based on a truncated Tams1-1 antigen was shown to be a specific and sensitive assay for the detection of T. annulata antibodies. Although Tams1 is a highly variable protein since multiple alleles are found within (26) and between isolates (J. M. Gubbels, F. Katzer, G. Hide, F. Jongejan, and B. R. Shiels, unpublished results), this does not seem to interfere with the detection of specific antibodies. In conclusion, at least some epitopes must be conserved in all Tams1 variants enabling its detection by ELISA. Further evidence for conserved epitopes in at least the two allelic variants used here is provided by the observation that comparable ELISA results were obtained for both Tams1 allelic variants.
The results obtained using anti-IgG1 or anti-IgG2 instead of anti-total IgG show that the measured Tams1 antibody response is not strictly originating in either IgG1 or IgG2. Probably both isotypes are involved, but nothing is known about IgG isotype responses during T. annulata infection. Concerning specificity, the six false-positive reacting sera derived from uninfected and IFAT-negative calves could be reduced to three by inclusion of 500 mM NaCl during blocking, serum, and conjugate incubations. The nature of the repeatedly false-positive reacting calves remains, however, unresolved.
The performance of the ELISA was proven to be good by determining the CV value (Fig. 2). This was over the measurement range of the ELISA of <15%, whereas 30% is the maximum acceptable value for repeatability of a diagnostic test (21). The CV is higher and varies more in the lower-PP sera than in the higher-PP range, an unexplained phenomenon also observed in the MAP-1B Cowdria ruminantium ELISA using goat sera (M. Mboloi and C. Bekker, unpublished).
The determination of a proper cutoff was established using ROC plots, TG-ROC, Youden's index, efficiency ratios, and LRs (Fig. 3, 4, 5, and 6). For this ELISA the TG-ROC-defined cutoff was approximately twice the mean of a negative serum panel and is coincidental since there are no statistical grounds for the use of twice the mean as the cutoff. Although in the serum panel used for cutoff determination no false-positive or false-negative sera were present, some were observed with other sera, leading to 98% specificity in the negative serum panel from 169 animals. Between 3 months and 1 year p.i. no false-negative reactions were observed, so the overall sensitivity is 100% in this period, while the sensitivity and specificity before 3 months p.i. was determined to be 87% by TG-ROC. These results are comparable with the use of total piroplasm antigen for the ELISA (25), but recombinant antigens are preferred for obvious reasons. To reach optimal Tams1 ELISA performance, it is necessary to analyze sera collected 3 months or more after the start of the infection. It might be possible that monitoring of IgM levels against Tams1 will lead to positive signals. This was not analyzed since IgM levels wane fast and are therefore of no practical use in studying long-term epidemiology. When the described Tams1 ELISA is used for the study of the epidemiology in the field, it has to be kept in mind that the prevalence will be underestimated due to possible false-negative results from recently infected animals. When sampling is performed earlier than 3 months p.i., IFAT is more reliable. However, the Tams1 ELISA is less laborious than the IFAT for the analysis of large numbers of sera. Since the outbreak of tropical theileriosis correlates closely to the vector life cycle as was shown in Morocco (9), it is best to sample later than 3 months after the clinical outbreak or peak in tick infestation to obtain a realistic picture of the infection status. The titer to T. annulata remains high for at least 1 year p.i. and can thus be measured reliably in this period (Fig. 7). It may be possible to detect T. annulata early in the infection by using the ELISA based on SPAG-1, a sporozoite antigen (2). However, it is expected that SPAG-1 titers will fade fast after infection since there is only a short exposure time to the immune system. This will become clear when the SPAG-1 ELISA is studied in more detail. When SPAG-1 performs well early in the infection it might be possible to combine both antigens in one ELISA, resulting in a diagnostic tool that can detect T. annulata exposure reliably early in the infection up to at least 1 year p.i.
Some cross-reaction was observed with T. parva antisera but is not very problematic since T. parva does not occur sympatrically with T. annulata, with the only possible exception in southern Sudan. Cross-reaction of T. parva-positive sera were always lower than 20 PP, which is not far above the cutoff of 12.6 PP. This cross-reactivity can be explained by the high degree of similarity with Tams1 of the amino acid sequence of the homologous gene in T. parva (35). Because Tams1 is even more similar to the merozoite surface protein of T. lestoquardi (T. hirci) (26), some cross-reactions can be expected with this parasite as well. It has, however, been shown that T. lestoquardi is not able to infect cattle (5), and therefore potential cross-reactions with this parasite are unlikely.
In conclusion, the Tams1 ELISA could become a useful tool to study the epidemiology of tropical theileriosis. It has to be noted that the ELISA has been validated using defined animals under controlled conditions and does not represent the actual field situation. Influences of sex, age, or breed are some of the other factors to consider in diagnostic assays (15). The robustness of the Tams1 ELISA will thus only be fully revealed by testing larger numbers of animals kept under field conditions.
ACKNOWLEDGMENTS
This work was supported by a grant from the European Union, Directorate General XII, INCO-DC Program, contract no. IC18-CT95-0003 (entitled “Application of Recombinant DNA Technology to Vaccination, Diagnosis and Epidemiology of Tropical Theileriosis”). Additional support was provided by the ICTTD Concerted Action Project on Integrated Control of Ticks and Tick-borne Diseases, also supported by the INCO-DC Program of the European Union under contract no. IC18-CT95-0009.
We thank Duncan Brown and Suzanne Williamson of the Centre for Tropical Veterinary Medicine in Edinburgh for the kind supply of negative and positive test sera, Martin Mboloi for his help with the TG-ROC analysis, and Albert W. C. A. Cornelissen for his support throughout this study and for his critical review of the manuscript.
REFERENCES
- 1.Allred D R, Hines S A, Ahrens K P. Isolate-specific parasite antigens of the Babesia bovis-infected erythrocyte surface. Mol Biochem Parasitol. 1993;60:121–132. doi: 10.1016/0166-6851(93)90035-v. [DOI] [PubMed] [Google Scholar]
- 2.Boulter N R, Brown C G D, Kirvar E, Glass E, Campbell J, Morzaria S, Nene V, Musoke A, d'Oliveira C, Gubbels M-J, Jongejan F, Hall R. Different vaccine strategies used to protect against Theileria annulata. Ann N Y Acad Sci. 1998;849:234–246. doi: 10.1111/j.1749-6632.1998.tb11054.x. [DOI] [PubMed] [Google Scholar]
- 3.Brocklesby D W, Barnett S F, Scott G R. Morbidity and mortality rates in East Coast fever (Theileria parva infection) and their application to drug screening procedures. Br Vet J. 1961;117:529–531. [Google Scholar]
- 4.Brown C G D. Control of tropical theileriosis (Theileria annulata infection) of cattle. Parasitologia. 1990;32:23–31. [PubMed] [Google Scholar]
- 5.Brown C G D, Ilhan T, Kirvar E, Thomas M, Wilkie G, Leemans I, Hooshmand-Rad P. Theileria lestoquardi and T. annulata in cattle, sheep and goats. Ann N Y Acad Sci. 1998;849:44–51. doi: 10.1111/j.1749-6632.1998.tb11032.x. [DOI] [PubMed] [Google Scholar]
- 6.de Kok J B, d'Oliveira C, Jongejan F. Detection of the protozoan parasite Theileria annulata in Hyalomma ticks by the polymerase chain reaction. Exp Appl Acarol. 1993;17:839–846. doi: 10.1007/BF00225857. [DOI] [PubMed] [Google Scholar]
- 7.d'Oliveira C, van der Weide M, Habela M A, Jacquiet P, Jongejan F. Detection of Theileria annulata in blood samples of carrier cattle by PCR. J Clin Microbiol. 1995;13:2665–2669. doi: 10.1128/jcm.33.10.2665-2669.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.d'Oliveira C, Tijhaar E J, Shiels B R, van der Weide M, Jongejan F. Expression of genes encoding two major Theileria annulata merozoite antigens in Escherichia coli and Salmonella typhimurium aroA vaccine strain. Gene. 1996;172:33–39. doi: 10.1016/0378-1119(96)00194-1. [DOI] [PubMed] [Google Scholar]
- 9.Flach E J, Ouhelli H. The epidemiology of tropical theileriosis (Theileria annulata infection in cattle) in an endemic area of Morocco. Vet Parasitol. 1992;44:51–65. doi: 10.1016/0304-4017(92)90143-w. [DOI] [PubMed] [Google Scholar]
- 10.Fleiss J F. The design and analysis of clinical experiments. New York, N.Y: John Wiley & Sons, Inc.; 1986. The simple replication reliability study; pp. 8–14. [Google Scholar]
- 11.Galen R S. Use of predictive value theory in clinical immunology. In: Rose N R, Friedmann H, Fahey J L, editors. Manual of clinical laboratory immunology. 3rd ed. Washington, D.C.: American Society for Microbiology; 1986. pp. 966–970. [Google Scholar]
- 12.Gill B S, Bansal G C, Bhattacharyulu Y, Kaur D, Singh A. Immunological relationship between strains of Theileria annulata (Dschunkowsky and Luhs 1904) Res Vet Sci. 1980;29:93–97. [PubMed] [Google Scholar]
- 13.Greiner M. Two-graph receiver operating characteristic (TG-ROC): a Microsoft-EXCEL template for the selection of cut-off values in diagnostic tests. J Immunol Methods. 1995;185:145–146. doi: 10.1016/0022-1759(95)00078-o. [DOI] [PubMed] [Google Scholar]
- 14.Greiner M, Sohr D, Gobel P. A Modified ROC analysis for the selection of cut-off values and the definition of intermediate results of serodiagnostic tests. J Immunol Methods. 1995;185:123–132. doi: 10.1016/0022-1759(95)00121-p. [DOI] [PubMed] [Google Scholar]
- 15.Greiner M, Bhat T S, Patzelt R J, Kakaire D, Schares G, Dietz E, Bohning D, Zessin K H, Mehlitz D. Impact of biological factors on the interpretation of bovine trypanosomosis serology. Prev Vet Med. 1997;30:61–73. doi: 10.1016/s0167-5877(96)01088-4. [DOI] [PubMed] [Google Scholar]
- 16.Harlow E. Antibodies: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1988. pp. 347–348. [Google Scholar]
- 17.Ho H J. Development and optimization of an enzyme-linked immunosorbent assay employing two murine monoclonal antibodies for absolute quantitation of human β-glucoronidase. Biotech Appl Biochem. 1992;16:1–10. [PubMed] [Google Scholar]
- 18.Ilhan T, Williamson S, Kirvar E, Shiels B, Brown C G D. Theileria annulata: carrier state and immunity. Ann N Y Acad Sci. 1998;849:109–125. doi: 10.1111/j.1749-6632.1998.tb11040.x. [DOI] [PubMed] [Google Scholar]
- 19.Irvin A D, Dobbelaere D A E, Mwamachi D M, Minami M, Spooner P R, Ocama J G R. Immunisation against East Coast fever: correlation between monoclonal antibody profiles of Theileria parva stocks and cross-immunity in vivo. Res Vet Sci. 1983;35:341–346. [PubMed] [Google Scholar]
- 20.Ishihara T, Minami T. Theileriosis. In: Ishizaki T, Inoki S, Fujita J, editors. Protozoan diseases. Tokyo, Japan: Japanese-German Association of Protozoan Diseases; 1978. pp. 201–209. [Google Scholar]
- 21.Jacobson R. Office International des Epizooties Standards Commision (ed.), Manual of standards for diagnostic tests and vaccines. 3rd ed. Paris, France: Office International de Epizooties; 1996. Principles of validation of diagnostic assays for infectious diseases; pp. 8–15. [Google Scholar]
- 22.Jacquiet P, Dia M L, Perié N M, Jongejan F, Uilenberg G, Morel P C. Présence de Theileria annulata en Mauritanie. Rev Elev Med Vet Pays Trop. 1990;43:489–490. . (In French.) [PubMed] [Google Scholar]
- 23.Jongejan F, de Kok J B, van der Weide M, d'Oliveira C. Diagnosis of Theileria annulata infection in carrier cattle and Hyalomma ticks by PCR and development of an ELISA based on a recombinant 30 kDa merozoite surface antigen. In: Spooner R, Campbell J, editors. Proceedings of the European Union Third Coordination Meeting on Tropical Theileriosis. European Union; 1994. pp. 59–63. , Antalya, Turkey. Roslin Institute, Edinburgh, Scotland. [Google Scholar]
- 24.Kachani M, Spooner R L, Rae P, Bell-Sakyi L, Brown C G D. Stage-specific responses following infection with Theileria annulata as evaluated using ELISA. Parasitol Res. 1992;78:43–47. doi: 10.1007/BF00936180. [DOI] [PubMed] [Google Scholar]
- 25.Kachani M, Flach E J, Williamson S, Ouhelli H, El Hasnaoui M, Spooner R L. The use of an enzyme-linked immunosorbent assay for tropical theileriosis research in Morocco. Prev Vet Med. 1996;26:329–339. [Google Scholar]
- 26.Katzer F, McKellar S, Ben Miled L, d'Oliveira C, Shiels B. Selection for antigenic diversity of Tams1, the major merozoite antigen of Theileria annulata. Ann N Y Acad Sci. 1998;849:96–108. doi: 10.1111/j.1749-6632.1998.tb11039.x. [DOI] [PubMed] [Google Scholar]
- 27.Kiltz H H, Uilenberg G, Franssen F F J, Perié N M. Theileria orientalis occurs in Central Africa. Res Vet Sci. 1986;40:197–200. [PubMed] [Google Scholar]
- 28.Kuen L S, Ming C H, Fan Y S. Background noise in ELISA procedures. Influence of the pH of the coating buffer and correlations with serum IgM concentration. J Immunol Methods. 1993;163:277–278. doi: 10.1016/0022-1759(93)90133-r. [DOI] [PubMed] [Google Scholar]
- 29.Lawrence J A, Mackenzie P K I. Isolation of a non-pathogenic Theileria of cattle transmitted by Rhipicephalus appendiculatus. Zimbabwe Vet J. 1980;11:27–35. [Google Scholar]
- 30.Mboloi M M, Bekker C P J, Kruitwagen C, Greiner M, Jongejan F. Validation of the indirect MAP-1B enzyme-linked immunosorbent assay for diagnosis of experimental Cowdria ruminantium infection in small ruminants. Clin Diagn Lab Immunol. 1999;6:66–72. doi: 10.1128/cdli.6.1.66-72.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morzaria S P, Barnett S F, Brocklesby D W. Isolation of Theileria mutans from cattle in Essex. Vet Rec. 1974;94:256. doi: 10.1136/vr.94.12.256. [DOI] [PubMed] [Google Scholar]
- 32.Ouhelli H. Theileriosis bovine a Theileria annulata (Dschunkowsky and Luhs, 1904). Recherche sur la biologie des vecteurs (Hyalomma spp.) et sur les interactions hôte-parasite. Doctor of Science thesis. Toulouse, France: University of Toulouse; 1985. [Google Scholar]
- 33.Pipano E, Cahana M. Fluorescent antibody test for the serodiagnosis of Theileria annulata. J Parasitol. 1969;55:765. [PubMed] [Google Scholar]
- 34.Schein E, Büscher G, Friedhoff K T. Lichtmikroskopische Untersuchungen über die Entwicklung von Theileria annulata (Dschunkowsky und Luhs 1904) in Hyalomma anatolicum excavatum (Kock 1844): I. Die Entwicklung im Darm vollgesogener Nymphen. Zentbl Parasitenkd. 1975;48:123–136. doi: 10.1007/BF00389643. [DOI] [PubMed] [Google Scholar]
- 35.Shiels B R, d'Oliveira C, McKellar S, Ben-Miled L, Kawazu S, Hide G. Selection of diversity at the putative glycosylation sites in the immunodominant merozoite/piroplasm surface antigen of Theileria parasites. Mol Biochem Parasitol. 1995;72:149–162. doi: 10.1016/0166-6851(95)00074-b. [DOI] [PubMed] [Google Scholar]
- 36.Smith R D. Evaluation of diagnostic tests. In: Smith R D, editor. Veterinary clinical epidemiology. Stoneham, Mass: Butterworth-Heinemann; 1991. pp. 29–34. [Google Scholar]
- 37.Stewart N P, de Vos A J, Shiels I, McGregor W. The experimental transmission of Theileria buffeli of cattle in Australia by Haemaphysalis humerosa. Aust Vet J. 1987;64:81–83. doi: 10.1111/j.1751-0813.1987.tb09621.x. [DOI] [PubMed] [Google Scholar]
- 38.Tizard I, editor. Veterinary Immunology. Philadelphia, Pa: The W. B. Saunders Co.; 1987. pp. 43–46. [Google Scholar]
- 39.Uilenberg G, Schreuder B E C. Studies on Theileriidae (Sporozoa) in Tanzania I. Tick transmission of Haematoxenus veliferus. Tropenmed Parasitol. 1976;27:106–111. [PubMed] [Google Scholar]
- 40.Uilenberg G, Perié N M, Lawrence J A, de Vos A J, Paling R W, Spanjer A A M. Causal agents of bovine theileriosis in Southern Africa. Trop Anim Health Prod. 1982;14:127–140. doi: 10.1007/BF02242143. [DOI] [PubMed] [Google Scholar]
- 41.Uilenberg G, Franssen F F, Perié N M. Stage-specific antigenicity in Theileria annulata: a case report. Vet Q. 1986;8:73–75. doi: 10.1080/01652176.1986.9694021. [DOI] [PubMed] [Google Scholar]
- 42.Uzal F A, Carrasco A E, Nielsen K, Echaide S, Cabrera R F. An indirect ELISA using a monoclonal anti IgG1 enzyme conjugate for the diagnosis of bovine brucellosis. Vet Microbiol. 1996;52:72–180. doi: 10.1016/0378-1135(96)00026-0. [DOI] [PubMed] [Google Scholar]
- 43.Youden D. Index for rating diagnostic tests. Cancer. 1950;3:32–35. doi: 10.1002/1097-0142(1950)3:1<32::aid-cncr2820030106>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 44.Zweig M H, Campbell G. Receiver-operating characteristic (ROC) plots: a fundamental evaluation tool in clinical medicine. Clin Chem. 1993;39:561–577. [PubMed] [Google Scholar]