ACCUMULATING EVIDENCE SUPPORTS A COMPLEX interplay between diet, sleep, and various health outcomes. A large body of literature exists investigating independent influences of dietary quality1,2 and sleep3,4 on health outcomes, particularly chronic disease risk such as obesity,5,6 cardiovascular disease,1,3,4,7–10 diabetes,1,3,4,11,12 and cancer.1,4,13,14 However, emerging evidence suggests that one’s diet may impact the quality of subsequent sleep,15–18 and conversely sleep duration and quality may impact the amount and quality of foods and beverages consumed.17,19–21 Additionally, these behavioral patterns are circadian, occurring rhythmically within the context of a 24-hour day.22 The timing of when one eats and the timing of when one sleeps may impact one another.23,24 Meal timing patterns, including timing of first and last eating occasion, eating jetlag, and variability in temporal eating patterns, have been linked to a number of metabolic outcomes.24–27 Evidence would be strengthened by elucidating the impact of the intricate interplay between diet and sleep patterns on health and disease. Timing of dietary and sleep behaviors also may interact with other health-related behaviors, such as physical activity, and biological factors, such as circadian rhythms, chronotype, and metabolism, to impact long-term health outcomes.22–24 The collective area of research about the relationship between temporal eating patterns, circadian rhythms, and health and disease is often referred to as chrono-nutrition.22
Few tools exist to capture the multidimensional natures of diet (including meal timing, diet quality, and fasting windows) and sleep health (including sleep timing, efficiency, duration, quality, regularity, and alertness28), their day-to-day variability (including weekday vs weekend), and their interrelationship within the 24-hour day. Much of the observational evidence examining associations between dietary intake and sleep outcomes are cross-sectional and often use questionnaires that capture these behaviors over the span of a month or year (eg, via food frequency or sleep questionnaires asking about the past month). 16,17,29 This limits examination of direct, temporal associations between diet and sleep within a 24-hour day. Assessment tools that are designed to capture behavior soon after it occurs, such as 24-hour dietary recalls (referred to herein as recalls) and sleep diaries, and data in real time, such as with actigraphy, allow researchers to better integrate dietary and sleep measures into health research. These shorter-term tools are often used in randomized controlled trials investigating how dietary intake and sleep interact and influence chronic disease risk factors.17,18 However, diet and sleep are often not assessed concurrently, prohibiting researchers’ abilities to integrate data and estimate their joint associations with health outcomes. For those studies that may capture both behaviors within the same period, assessment tools (eg, recalls, diaries, wearables, and digital assessments) usually capture these two complex behaviors separately, increasing both participant and researcher burden. The objective was to thus update a widely used dietary assessment tool to additionally capture self-reported sleep health.
The Automated Self-Administered 24-Hour (ASA24) Dietary Assessment Tool is a fully automated, web-based, self-administered assessment tool that captures self-reported dietary intake over a 24-hour period.30 It was developed by the National Cancer Institute under contract with Westat and is publicly available at no cost to researchers, clinicians, and educators. It enables the collection of multiple recalls or food records via desktop computers and mobile devices. ASA24’s web-based system probes participants to recall foods, beverages, and dietary supplements consumed throughout the reporting period and the time of each meal; codes data; and provides detailed food-level and summary data for each day of dietary assessment captured. Additionally, a variety of novel modules can be added to capture information on food sources, meal locations, with whom meals are eaten, and electronic device use during meals. Such optional modules are periodically added to the ASA24 system based on research trends or user needs. The aim of this commentary is to thus describe the ASA24 Sleep module development process and module content to inform researchers interested in studying the relationship between diet quantity and quality, chrono-nutrition, sleep health, and health outcomes.
ASA24 SLEEP MODULE DEVELOPMENT PROCESS
The goal for the Sleep module was to capture measures of self-reported sleep health, including questions specific to timing, quantity, and quality. The team, which included researchers with diet and sleep expertise, addressed the following main decision points during the development of the Sleep module: (1) the optimal order of the ASA24 modules and the period of sleep data collection in relation to dietary data collection; (2) the potential respondent target population(s) the tool would be relevant for; (3) the aspects of sleep health most important and feasible to capture; and (4) the implementation and refinement of the tool as an electronic data capture module.
First, the optimal placement of the Sleep module in relation to dietary assessment was considered. Given dietary assessment is the main objective of ASA24, it was decided the Sleep module would be a subsequent module to be completed after dietary intake data collection is completed. Additional discussions addressed which sleep period(s) were best to capture in the module for implementation with recalls and records: the sleep period before the first meal vs after the last meal reported. For both assessment approaches (ie, recall and record), the team opted to assess the participant’s most recent sleep period to reduce recall bias and lower participant burden. To illustrate, a recall asks the respondent to report their dietary intake from the previous day; therefore, the accompanying Sleep module primarily focuses on the sleep period after the last meal (ie, the respondent’s most recent sleep period), rather than the sleep period preceding the first meal (ie, 2 nights before the day that the recall is completed) (Fig 1A). Conversely, a food record is completed in real time, and thus, the sleep period before the first meal (ie, the respondent’s most recent sleep period) is captured (Fig 1B).
Figure 1.
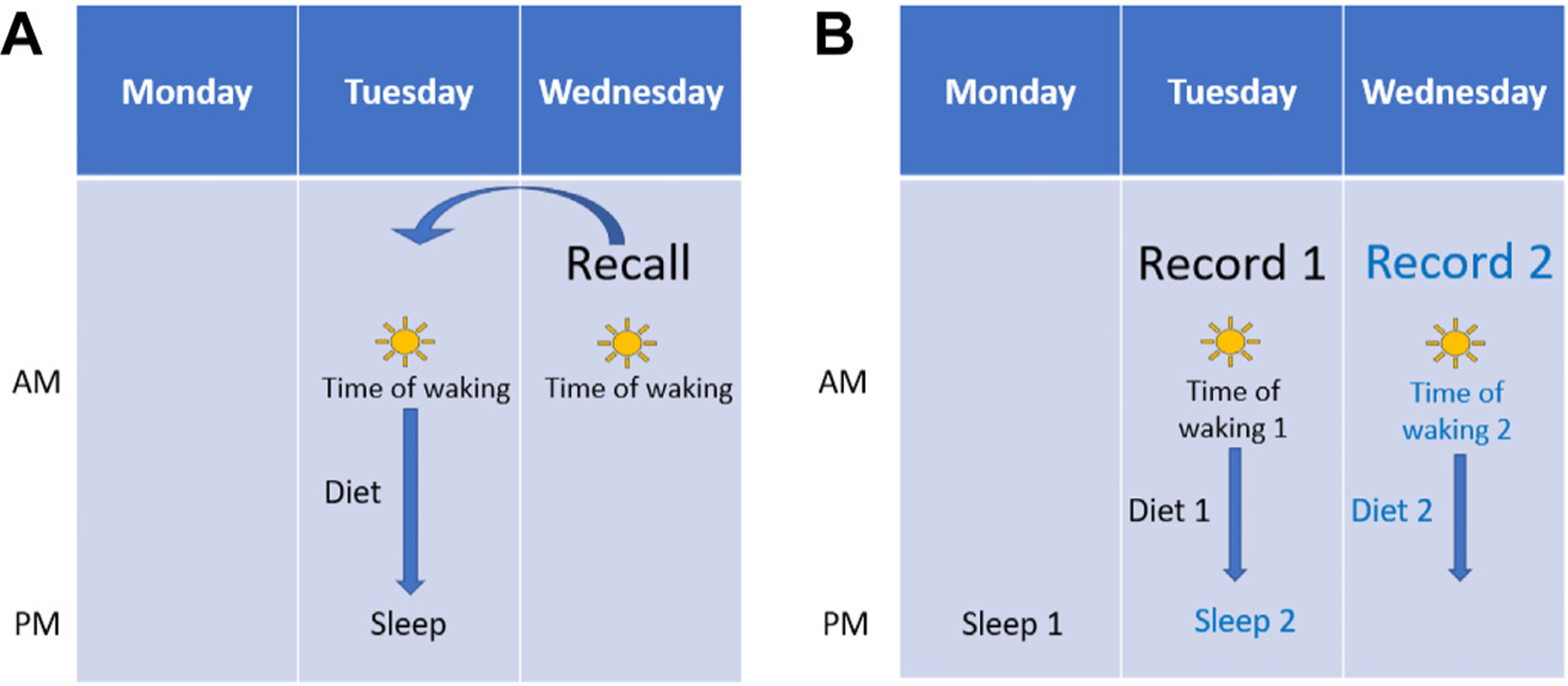
The sequence of activities captured in the Sleep module: recall vs record. (A) Recalls collected dietary information from the previous day. When respondents complete a dietary recall with the Sleep Module, information is gathered on the sleep periods immediately preceding and immediately following the dietary reporting period. First, two questions are asked about wake time and sleep quality for the sleep period immediately preceding the first reported meal of the recall day. The same two questions are asked again along with the other eight sleep questions for the sleep period immediately after the last meal of the recall day. (B) Records collect dietary data in real time. When respondents complete a food record with the Sleep module, all 10 sleep questions are asked about the sleep period before the first meal of the record day. The collection of multiple, consecutive records with the Sleep module allows researchers to assess consecutive nights of sleep.
Next, potential target populations for the Sleep module were discussed. Although ASA24 is used in various populations, the focus for developing the Sleep module was on the general adult population and nocturnal sleep period. Therefore, the language and questions included focused on nocturnal sleep rather than periodic sleep episodes (eg, daytime naps, biphasic sleepers) and were not necessarily tailored to those with sleep disorders or irregular sleeping schedules (eg, nightshift workers, caretakers with young infants, or those with purposeful night awakenings).
To determine module content, a thorough literature review was conducted to identify validated questionnaires currently being used to assess sleep in healthy populations. To develop a more comprehensive understanding of the tools and variety of sleep questionnaires being used by researchers, the search was expanded to include assessment tools for populations with sleep disorders. Of the multiple self-reported sleep assessment tools reviewed, the main tools considered included the National Sleep Foundation Sleep Diary,31 Munich Chronotype Questionnaire,32 Consensus Sleep Diary (CSD) Core,33 and expanded CSD, Morning administration (CSD-M).33 The search results were narrowed down based on multiple priorities: a focus on the nocturnal sleep period, interest in day-level data (ie, not asking about average sleep quantity or quality over multiple days), and the goal to apply minimal modifications to validated survey questions.
Additionally, all public-facing features of the ASA24 respondent website must comply with the Plain Writing Act of 201034 and Section 508 of the Rehabilitation Act of 197335 to ensure ASA24 uses language that can be easily understood by the public and is accessible to people with disabilities. Thus, any assessment tools that would require administering an image-heavy module were excluded. Module length was also strongly considered to limit participant burden. Given the average time to complete a recall using ASA24 is 24 minutes,36 the aim was to limit the Sleep module to approximately 5 additional minutes or less to keep the entire assessment under 30 minutes. With these criteria in mind, the CSD Core33 was determined to be the best fit for the Sleep module. Additional external researchers with expertise in meal timing and sleep health were consulted for feedback on module content and utility throughout the decision-making process; in these discussions, additional survey items were considered that may be needed to capture a fuller picture of sleep health.
Programmers at Westat with expertise in survey design were consulted throughout the module content development and review process and provided guidance for implementation. As previously mentioned, to ensure the Sleep module complied with writing and formatting requirements, CSD Core questions underwent plain language review, with a target of a fifth-grade reading level. The questions and responses were also formatted in a way that enables those with disabilities, particularly those who may use a screen reader and other assistive technology, to be able to read and respond to the questions. Finally, the data collection process within the ASA24 System was internally piloted, and output data files were checked for accuracy.
CONTENT AND UTILITY OF THE ASA24 SLEEP MODULE
ASA24 now contains an optional Sleep module that provides researchers with an opportunity to capture data on sleep health after completion of a dietary recall or food record. Therefore, ASA24 can now be used to assess self-reported dietary intake and sleep health among English- and Spanish-speaking adult populations with at least a fifth-grade reading level. Key summary points for the Sleep module are highlighted in Figure 2. As previously mentioned, the Sleep module focuses on nocturnal sleep rather than periodic sleep episodes (eg, daytime naps or biphasic sleepers) and is not tailored for respondents with sleep disorders or irregular sleeping schedules (eg, nightshift workers, caretakers with young infants, or those with purposeful night awakenings). At this time, irregular sleep schedules and nap periods are outside the scope of this module and would require researchers to administer a separate sleep survey or device-based measure if choosing to pair with ASA24 dietary data.
Figure 2.

Key takeaway points for the ASA24 Sleep module.
The ASA24 Sleep module contains 10 unique questions (Fig 3) that address the aforementioned dimensions of sleep health (including sleep timing, efficiency, duration, quality, regularity, and alertness28) and can be administered immediately after a dietary assessment (recall or record) if the researcher turns on the module within the researcher website. Eight CSD Core questions were adapted for inclusion, and two additional questions adapted from other assessment tools were added in the module to capture respondents’ perceptions of how refreshed they felt on awakening31,33 and comparability of the reported sleep night to usual nighttime sleep duration37 (Fig 3). In addition to the assessed variables, repeated assessment with the Sleep module can enable the examination of sleep regularity or variability, a component of sleep health that has been linked to several health outcomes.38–43
Figure 3.
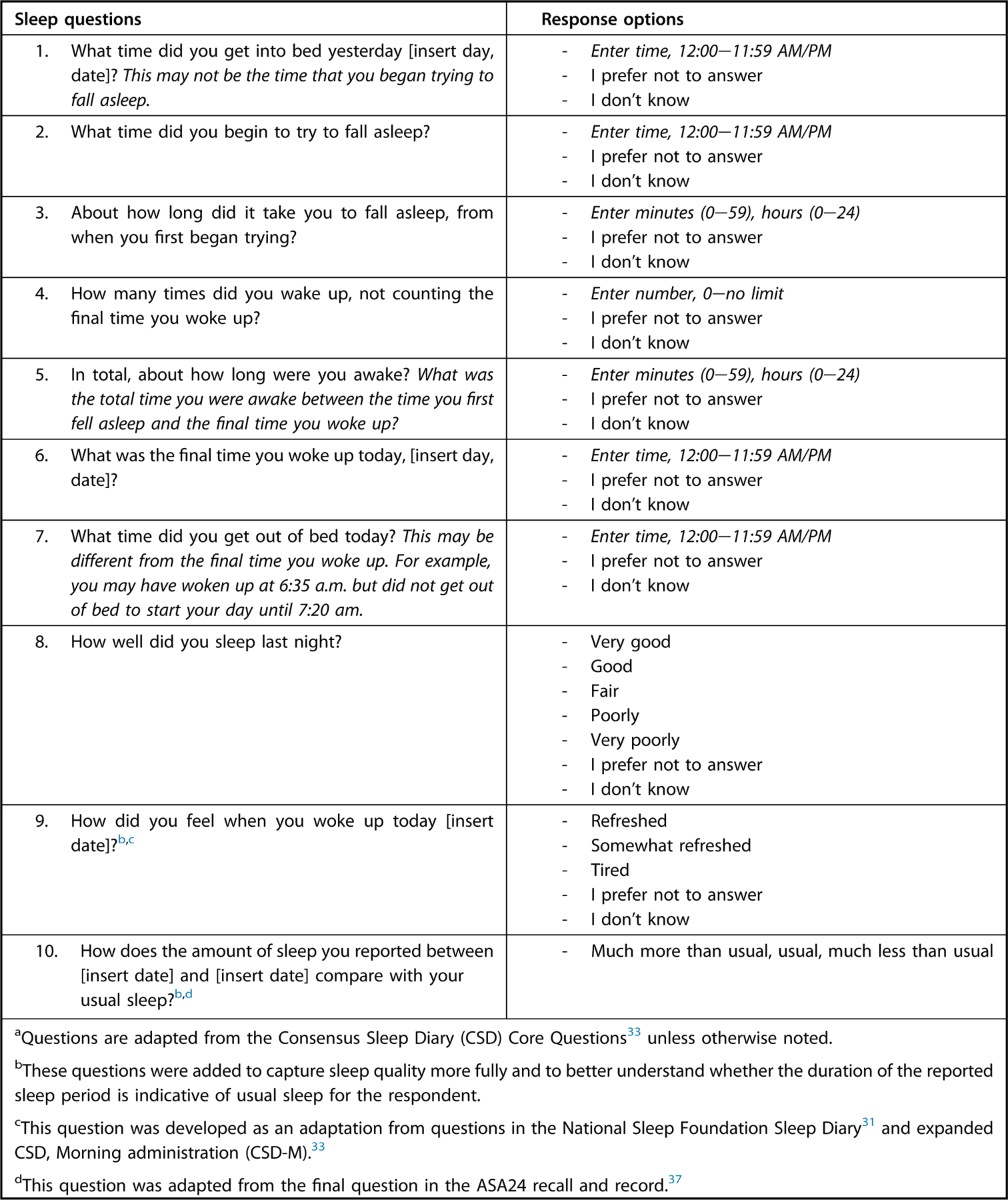
Questions included in the ASA24 Sleep Module.
The order of questions and sleep period captured by the Sleep module depends on the dietary assessment tool chosen by the researcher during study setup, as depicted in Figure 1. For studies in which respondents complete a recall(s), the module includes two questions on wake time and sleep quality for the sleep period immediately preceding the first reported meal (questions 6 and 9 from Fig 3). The same two questions are then asked again with the other sleep questions (ie, all 10 sleep questions in Fig 3) for the nocturnal sleep period immediately after the last meal (ie, 12 sleep questions total per dietary recall) (Fig 1A). A study that pairs a recall with the Sleep module allows researchers to assess the time between waking and first meal (eg, morning fast period) or associations between dietary intake with the following nocturnal sleep period.
In contrast, pairing a food record with the Sleep module focuses all 10 sleep questions from Figure 3 on the nocturnal sleep period before the first meal (Fig 1B). A single record can be used to assess how the previous night of sleep may relate to subsequent dietary intake. The collection of multiple, consecutive records with the Sleep module allows researchers to also assess how dietary intake may influence the following night of sleep. (Fig 1B).
Once study data are collected in ASA24, all sleep health variables will be included in Total Nutrients and Supplements analysis files and available for download on the Researcher Website under “Data and Results.”
CONSIDERATIONS AND FUTURE OPPORTUNITIES
A few points are important to note as researchers begin to use this new module. First, although the module is based on a validated survey, the wording of the CSD Core questions were modified, and two additional sleep questions were added. This survey is thus a beta version and has not been validated; future research efforts can compare this tool with more objective measures, for example, from polysomnography and actigraphy. ASA24 also relies on self-report of both dietary intake and sleep and the potential for recall bias should be considered.44 As previously mentioned, the Sleep module is currently recommended for use among adults without pre-existing sleep conditions or irregular sleep schedules and was thus not developed with these subpopulations in mind. Additionally, given this is the first launch of the ASA24 Sleep module, it has yet to undergo extensive user testing, and the utility of collecting dietary and sleep data together in this platform and its feasibility for administration is unknown. Indeed, the research community can help determine whether the module should be refined and, if so, can help improve the module by providing feedback through the ASA24 Help Desk45 for consideration in future ASA24 updates.
CONCLUSION
ASA24 is a publicly available resource that now includes a standardized approach to examine the associations between dietary intake and sleep health in the context of the 24-hour day, as well as their joint associations on health outcomes of interest. ASA24 can allow both behaviors to be captured temporally, and with minimal burden added, via a short Sleep module for use across study designs, including clinical studies, interventions, observational studies, or surveillance research. Additional support will soon be available on the ASA24 webpage to guide researchers, including variable definitions and guidance to calculate additional sleep (eg, total sleep duration) and chrono-nutrition (eg, interval between awakening and first eating occasion) variables of interest.
ACKNOWLEDGEMENTS
We thank Christie Kaefer, MBA (National Cancer Institute, Division of Cancer Control and Population Sciences), Amy Miller, MPH (Westat, Inc.), Beth Mittl, BA (Westat, Inc.), and Tom Nicholson, BS (Westat, Inc.) for their support in creating the ASA24 Sleep module. The authors also thank Charles Matthews, PhD (National Cancer Institute, Division of Cancer Epidemiology and Genetics), David Berrigan, PhD, MPH (National Cancer Institute, Division of Cancer Control and Population Sciences), Marie-Pierre St-Onge, PhD, MSc (Columbia University Irving Medical Center, Department of Medicine); and Faris Zuraikat, PhD (Columbia University Irving Medical Center, Department of Medicine) for sharing their scientific expertise during the development of the ASA24 Sleep module. Authors have received permission to acknowledge those named above.
FUNDING/SUPPORT
This project was funded in whole or in part with federal funds from the National Cancer Institute; National Heart, Lung, and Blood Institute; National Institute of Diabetes and Digestive and Kidney Diseases; and the Office of Dietary Supplements, Office of Disease Prevention, and Office of Behavioral and Social Science Research at the National Institutes of Health, under contract 75N91019D00023. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
Footnotes
STATEMENT OF POTENTIAL CONFLICT OF INTEREST
No potential conflict of interest was reported by the authors.
Contributor Information
Marissa M. Shams-White, National Cancer Institute, Bethesda, MD.
Lauren E. O’Connor, National Cancer Institute, National Institutes of Health, Bethesda, MD.
Sydney G. O’Connor, National Cancer Institute, National Institutes of Health, Bethesda, MD.
Kirsten A. Herrick, National Cancer Institute, National Institutes of Health, Bethesda, MD.
References
- 1.Morze J, Danielewicz A, Hoffmann G, Schwingshackl L. Diet quality as assessed by the Healthy Eating Index, Alternate Healthy Eating Index, Dietary Approaches to Stop Hypertension Score, and health outcomes: A second update of a systematic review and meta-analysis of cohort studies. J Acad Nutr Diet 2020;120(12):1998–2031. [DOI] [PubMed] [Google Scholar]
- 2.Schwingshackl L, Bogensberger B, Hoffmann G. Diet quality as assessed by the Healthy Eating Index, Alternate Healthy Eating Index, Dietary Approaches to Stop Hypertension Score, and health outcomes: an updated systematic review and meta-analysis of cohort studies. J Acad Nutr Diet 2018;118(1):74–100. [DOI] [PubMed] [Google Scholar]
- 3.Itani O, Jike M, Watanabe N, Kaneita Y. Short sleep duration and health outcomes: A systematic review, meta-analysis, and meta-regression. Sleep Med 2017;32:246–256. [DOI] [PubMed] [Google Scholar]
- 4.Li J, Cao D, Huang Y, et al. Sleep duration and health outcomes: An umbrella review [Published online ahead of print August 26, 2021]. Sleep Breath 2021. 10.1007/s11325-021-02458-1 [DOI] [PubMed] [Google Scholar]
- 5.Bacaro V, Ballesio A, Cerolini S, et al. Sleep duration and obesity in adulthood: An updated systematic review and meta-analysis. Obes Res Clin Pract 2020;14(4):301–309. [DOI] [PubMed] [Google Scholar]
- 6.Asghari G, Mirmiran P, Yuzbashian E, Azizi F. A systematic review of diet quality indices in relation to obesity. Br J Nutr 2017;117(8):1055–1065. [DOI] [PubMed] [Google Scholar]
- 7.Bechthold A, Boeing H, Schwedhelm C, et al. Food groups and risk of coronary heart disease, stroke and heart failure: A systematic review and dose-response meta-analysis of prospective studies. Crit Rev Food Sci Nutr 2019;59(7):1071–1090. [DOI] [PubMed] [Google Scholar]
- 8.Kwok CS, Kontopantelis E, Kuligowski G, et al. Self-reported sleep duration and quality and cardiovascular disease and mortality: A dose-response meta-analysis. J Am Heart Assoc 2018;7(15):e008552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yin J, Jin X, Shan Z, et al. Relationship of sleep duration with all-cause mortality and cardiovascular events: A systematic review and dose-response meta-analysis of prospective cohort studies. J Am Heart Assoc 2017;6(9):e005947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schwingshackl L, Schwedhelm C, Hoffmann G, et al. Food groups and risk of hypertension: A systematic review and dose-response meta-analysis of prospective studies. Adv Nutr 2017;8(6):793–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cappuccio FP, D’Elia L, Strazzullo P, Miller MA. Sleep duration and all-cause mortality: A systematic review and meta-analysis of pro-spective studies. Sleep 2010;33(5):585–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schwingshackl L, Hoffmann G, Lampousi AM, et al. Food groups and risk of type 2 diabetes mellitus: A systematic review and meta-analysis of prospective studies. Eur J Epidemiol 2017;32(5):363–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen Y, Tan F, Wei L, et al. Sleep duration and the risk of cancer: A systematic review and meta-analysis including dose-response relationship. BMC Cancer 2018;18(1):1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stone CR, Haig TR, Fiest KM, McNeil J, Brenner DR, Friedenreich CM. The association between sleep duration and cancer-specific mortality: A systematic review and meta-analysis. Cancer Causes Control 2019;30(5):501–525. [DOI] [PubMed] [Google Scholar]
- 15.St-Onge MP, Mikic A, Pietrolungo CE. Effects of diet on sleep quality. Adv Nutr 2016;7(5):938–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Godos J, Grosso G, Castellano S, Galvano F, Caraci F, Ferri R. Association between diet and sleep quality: A systematic review. Sleep Med Rev 2021;57:101430. [DOI] [PubMed] [Google Scholar]
- 17.Zuraikat FM, Wood RA, Barragán R, St-Onge MP. Sleep and diet: Mounting evidence of a cyclical relationship. Annu Rev Nutr 2021;41:309–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Binks H, G EV, Gupta C, Irwin C, Khalesi S. Effects of diet on sleep: A narrative review. Nutrients 2020;12(4):936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chaput J-P. Sleep patterns, diet quality and energy balance. Physiol Behav 2014;134:86–91. [DOI] [PubMed] [Google Scholar]
- 20.Al Khatib HK, Harding SV, Darzi J, Pot GK. The effects of partial sleep deprivation on energy balance: A systematic review and meta-analysis. Eur J Clin Nutr 2017;71(5):614–624. [DOI] [PubMed] [Google Scholar]
- 21.Zhu B, Shi C, Park CG, Zhao X, Reutrakul S. Effects of sleep restriction on metabolism-related parameters in healthy adults: A comprehensive review and meta-analysis of randomized controlled trials. Sleep Med Rev 2019;45:18–30. [DOI] [PubMed] [Google Scholar]
- 22.Flanagan A, Bechtold DA, Pot GK, Johnston JD. Chrono-nutrition: From molecular and neuronal mechanisms to human epidemiology and timed feeding patterns. J Neurochem 2021;157(1):53–72. [DOI] [PubMed] [Google Scholar]
- 23.Jansen EC, Dunietz GL, Tsimpanouli ME, et al. Sleep, diet, and cardiometabolic health investigations: A systematic review of analytic strategies. Curr Nutr Rep 2018;7(4):235–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xiao Q, Garaulet M, Scheer F. Meal timing and obesity: Interactions with macronutrient intake and chronotype. Int J Obes (Lond) 2019;43(9):1701–1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Makarem N, Sears DD, St-Onge MP, et al. Variability in daily eating patterns and eating jetlag are associated with worsened cardiometabolic risk profiles in the American Heart Association Go Red for Women strategically focused research network. J Am Heart Assoc 2021;10(18):e022024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Makarem N, Sears DD, St-Onge MP, et al. Habitual nightly fasting duration, eating timing, and eating frequency are associated with cardiometabolic risk in women. Nutrients 2020;12(10):3043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xiao Q, Bauer C, Layne T, Playdon M. The association between overnight fasting and body mass index in older adults: The interaction between duration and timing. Int J Obes (Lond) 2021;45(3):555–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Buysse DJ. Sleep health: Can we define it? Does it matter? Sleep 2014;37(1):9–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dashti HS, Scheer FA, Jacques PF, Lamon-Fava S, Ordovás JM. Short sleep duration and dietary intake: Epidemiologic evidence, mechanisms, and health implications. Adv Nutr 2015;6(6):648–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Subar AF, Kirkpatrick SI, Mittl B, et al. The automated self-administered 24-hour dietary recall (ASA24): A resource for researchers, clinicians, and educators from the National Cancer Institute. J Acad Nutr Diet 2012;112(8):1134–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.National Sleep Foundation. National Sleep Foundation Sleep Diary Accessed November 11, 2021. https://www.thensf.org/wp-content/uploads/2021/02/NSF-Sleep-Diary-Rev-2-2021.pdf
- 32.Roenneberg T, Wirz-Justice A, Merrow M. Life between clocks: Daily temporal patterns of human chronotypes. J Biol Rhythms 2003;18(1):80–90. [DOI] [PubMed] [Google Scholar]
- 33.Carney CE, Buysse DJ, Ancoli-Israel S, et al. The consensus sleep diary: Standardizing prospective sleep self-monitoring. Sleep 2012;35(2):287–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Plain Language Action and Information Network. Laws and requirements Accessed March 18, 2022. https://www.plainlanguage.gov/law/
- 35.General Services Administration. IT Accessibility Laws and Policies: Section 508 of the Rehabilitation Act of 1973 Accessed March 18, 2022. https://www.section508.gov/manage/laws-and-policies/
- 36.National Cancer Institute. ASA24® frequently asked questions (FAQs) Accessed December 15, 2021. https://epi.grants.cancer.gov/asa24/resources/faq.html
- 37.National Cancer Institute. ASA24® respondent website methodology Accessed December 15, 2021. https://epi.grants.cancer.gov/asa24/respondent/methodology.html#usual
- 38.Chaput J-P, Dutil C, Featherstone R, et al. Sleep timing, sleep consistency, and health in adults: A systematic review. Appli Physiol Nutr Metabol 2020;45(10 Suppl 2):S232–S247. [DOI] [PubMed] [Google Scholar]
- 39.Lunsford-Avery JR, Engelhard MM, Navar AM, Kollins SH. Validation of the Sleep Regularity Index in older adults and associations with cardiometabolic risk. Sci Rep 2018;8(1):14158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zuraikat FM, Makarem N, Redline S, Aggarwal B, Jelic S, St-Onge MP. Sleep regularity and cardiometabolic heath: Is variability in sleep patterns a risk factor for excess adiposity and glycemic dysregulation? Curr Diab Rep 2020;20(8):38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Makarem N, Zuraikat FM, Aggarwal B, Jelic S, St-Onge MP. Variability in sleep patterns: An emerging risk factor for hypertension. Curr Hypertens Rep 2020;22(2):19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huang T, Mariani S, Redline S. Sleep irregularity and risk of cardiovascular events: The multi-ethnic study of atherosclerosis. J Am Coll Cardiol 2020;75(9):991–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Huang T, Redline S. Cross-sectional and prospective associations of actigraphy-assessed sleep regularity with metabolic abnormalities: The multi-ethnic study of atherosclerosis. Diabetes Care 2019;42(8): 1422–1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Althubaiti A. Information bias in health research: Definition, pitfalls, and adjustment methods. J Multidiscip Healthc 2016;9:211–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.National Cancer Institute. Contact the ASA24 Support Team 2022. Accessed July 6, 2022. https://epi.grants.cancer.gov/asa24/support/contact.html


