Abstract
Cardiovascular diseases (CVD) remain one of the leading causes of mortality worldwide. Despite recent advances in diagnosis and interventions, there is still a crucial need for new multifaceted therapeutics that can address the complicated pathophysiological mechanisms driving CVD. Extracellular vesicles (EVs) are nanovesicles that are secreted by all types of cells to transport molecular cargo and regulate intracellular communication. EVs represent a growing field of nanotheranostics that can be leveraged as diagnostic biomarkers for the early detection of CVD and as targeted drug delivery vesicles to promote cardiovascular repair and recovery. Though a promising tool for CVD therapy, clinical application of EVs is limited by the inherent challenges in EV isolation, standardization, and delivery. Hence, this review will present the therapeutic potential of EVs and introduce bioengineering strategies that augment their natural functions in CVD.
Keywords: extracellular vesicles, cardiovascular disease, targeted delivery, cargo loading, nanotherapeutics
Graphical Abstract
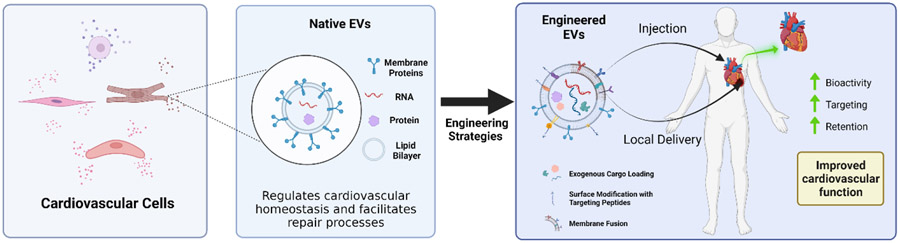
Extracellular vesicles are membrane-bound nanovesicles secreted by cells to mediate biological functions. In the cardiovascular system, EVs maintain homeostasis and promote repair mechanisms following CVD onset. However, poor standardization and targeting specificity challenges the clinical potential of EV therapy. New bioengineering strategies have been developed to augment the natural bioactive properties of EVs and enhance therapeutic efficacy.
1. Introduction
To date, cardiovascular disease (CVD) remains the leading cause of mortality worldwide and contributes to significant socioeconomic burden due to its prolonged and debilitating nature. [1,2] CVD comprises a spectrum of conditions and disorders that affect the heart and blood vessels. Majority of CVD is caused by the development of atherosclerosis, which is a progressive disease of the arteries notable by deposition of fatty plaques within the vessel wall. This atherosclerosis can then lead to the development of coronary artery disease (CAD) and peripheral arterial disease (PAD), which can further progress to heart failure, myocardial infarction, limb ischemia, and stroke.[1] Underlying contributing factors of these diseases are numerous, including genetics, diet, smoking, and lack of exercise.[1-3] With development of angiography, drug-eluting stents, balloon catheters, bypass surgery, and new disease-modifying drug therapies, there has been significant improvement in the care and treatment of patients with CVD.[4] Despite these innovations and progress, CVD is still the leading cause of death in the United States and worldwide. Furthermore, these treatment options bring their own set of complications, including intimal hyperplasia and thrombosis, as well as drug therapy side effects. Therefore, broader therapeutic interventions are still needed in order to ameliorate the devastating impacts of CVD.
Recently, a new class of therapeutics have emerged that are known as extracellular vesicles (EVs). EVs are membrane-bound vesicles released by different types of prokaryotic and eukaryotic cells, and are ubiquitously found in most body fluids such as blood, urine, breast milk, saliva and cerebrospinal fluid (CSF).[5] In general, EVs can be classified into three subclasses which are differentiated by their biogenesis mechanisms.[5,6] The first class of EVs are microvesicles, also known as ectosomes or microparticles. They are produced by the outward budding and fission of the plasma membrane and range from 50 nm to 1 micron in size.[7,8] The second EV subset are termed apoptotic bodies (50 nm to 5 microns) and are released when plasma membrane blebbing occurs during late apoptosis. The final EV subset is known as exosomes. Exosomes are the smallest type of EV (also often referred to as sEV), ranging between 50-150 nm, and originate from the inward budding of multivesicular bodies (MVB). Exosomes are released into the extracellular space upon fusion of MVBs with the plasma membrane, specifically at the lipid raft subdomains.[9,10] All subsets of vesicles contain bioactive cargo, including proteins, mRNAs, microRNAs (miRNAs, miRs), and lipids, that are efficiently delivered to recipient cells to regulate different biological processes. [6,8,11-13] Within the cardiovascular system, EVs play important roles in maintaining normal physiological function by facilitating cellular crosstalk.[8,11,14] Under disease or injury conditions, EV phenotypes are seen to shift in order to indicate cardiovascular dysfunction and to restore physiological balance. However, administration of EVs as therapeutics has been limited due to difficulties in isolation and standardization, ineffective targeting, and poor retention. [15,16] Advances in research and technology have addressed these challenges by engineering EVs to augment their therapeutic efficacy and developing new delivery mechanisms to improve retention (Figure 1). Several recent reviews have thoroughly detailed the therapeutic potential of EVs, espeically in the context of myocardial repair. [17-19] This review will continue the discussion by summarizing the role of native EVs as mediators of normal cardiovascular health and as diagnostic biomarkers and regenerative therapeutics across different CVD pathologies. This review will additionally delve into recently emerging bioengineering strategies that have been applied to improve the clinical potential of EVs.
Figure 1.
Summary of EV functions within the cardiovascular system and engineering strategies to improve therapeutic efficicacy for the the treatment of CVD.
2. Role of EVs in the Cardiovascular System
2.1. EVs in Cardiovascular System Homeostasis
Within the cardiovascular system, EVs are secreted by different cell types such as fibroblasts, smooth muscle cells (SMCs), endothelial cells (ECs), cardiomyocytes (CM), and inflammatory cells (e.g. leukocytes, platelets).[11,14,20] As primary messengers of intracellular communication, EVs play a critical role in maintaining homeostasis within the cardiovascular system. For example, EC-derived EVs function to regulate angiogenesis and maintain normal vascular physiology. In one study, Hergenreider et al. described the atheroprotective communication between ECs and vascular SMCs in a miR-143/145–dependent manner.[21] In this mechanism, EC-derived EVs transport miR-143/145 clusters to control SMC phenotype and prevent the formation of atherosclerotic lesions. Moreover, EC-derived EVs also transfer of miR-214 to adjacent ECs to stimulate migration and proliferation by suppressing cell-cycle arrest genes.[22] EC EVs also exhibit immunomodulatory properties, such as with the delivery of miR-10a and miR-222 to inhibit leukocyte recruitment and activation.[23,24] Cardiac cells secrete EVs to regulate heart function, especially in response to stress or injury.[25] CM EVs are known to contain an abundance of heat shock proteins (HSP20, HSP60, HSP70) which mediate CM growth and survival by directing CMs towards a pro-survival phenotype and by upregulating proliferative signaling pathways.[26-28] CMs are also seen to reduce cardiac fibrosis through EV-mediated delivery of HSP20 and miR-133a to cardiac fibroblasts (CF).[29,30] Other vascular cells, such as CFs and SMCs, also contribute significantly to maintaining normal vascular homeostasis via delivery of miRNAs and proteins to recipient cells.[31]
2.2. EVs in CVD Development and Application as Diagnostic and Prognostic Biomarkers
Cardiovascular dysfunction can be marked by EV phenotypic shifts. In fact, identification of abnormal EV cargo has been significantly correlated to the onset and development of several CVDs (Table 1). One study quantified 10 miRNAs in exosomes isolated from patients with stable CAD, and found that miR-126 and miR-199a were overexpressed in circulating EV subsets, thus demonstatining prognostic value in predicting cardiovascular events in patients.[32] In another study, upregulation of circulating miR-1, miR-133a, miR-133b, and miR-499-5p was seen in plasma EVs of patients with acute myocardial infarction (AMI), indicating cardiac damage.[33] Other molecules carried abundantly by EVs, such as ncRNAs and circRNAs, were also proposed as potential biomarkers.[34] It was reported that circRNA, specifically mitochondrial fission and apoptosis-related circRNA (MFACR), could represent a useful biomarker to predict cardiac cell death.[35] Abnormal EV cargo can also drive the progression of CVD. For example, EVs secreted from atherosclerotic plaques transport tumor necrosis factor (TNF)-α–converting enzyme (TACE/ADAM17) to promote inflammatory responses and drive atherogenesis. [36]
Table 1.
Abnormal cargo expressed in EVs that serve as biomarkers to signal the onset and progression of different CVD pathologies.
| Cargo | Disease Biomarker | Ref |
|---|---|---|
| Angiopoietin 1 | Acue Myocardial Infarction | [40] |
| C5a | Atherosclerosis, Acute Coronary Syndrome | [41] |
| CD14 | Ischemic Heart Disease, Heart Failure, Myocardial Ischemia | [42-44] |
| circRNA | Myocardial damage | [45] |
| Cystatin C | Systemic Hypertension, Heart Failure, Coronary Artery Disease, Myocardial Ischemia, Unstable Angina | [42-44,46,47] |
| miR-1 | Acute Myocardial Infarction | [33] |
| miR-133a | Acute Myocardial Infarction | [33] |
| miR-133b | Acute Myocardial Infarction | [33] |
| miR-199a | Coronary Artery Disease, Atherosclerosis | [48] |
| miR-499-5p | Acute Myocardial Infarction | [33] |
| Placental growth factor | Acute Myocardial Infarction | [40] |
| Plasminogen | Atherosclerosis, Myocardial Ischemia | [47,49] |
| Platelet-derived growth factor | Acute Myocardial Infarction | [40] |
| Polygenic immunoglobulin receptor (pIgR) | Acute Coronary Syndrome | [41] |
| Serpin F2 | Heart Failure, Myocardial Ischemia | [43,44] |
| Serpin G1 | Heart Failure, Myocardial Ischemia | [44] |
| SRC | Coronary Artery Disease | [40] |
| TACE/ADAM17 | Atherosclerosis | [36] |
| von Willebrand factor (VWF) | Atherosclerosis | [49] |
In addition to cargo, changes in EV membrane compositions can further signal the development of CVD. Endothelial injury is one of the initial hallmarks of CVD and phenotypes of different EV subtypes reflect the early pathological conditions. In one study, endothelial cells were found to secrete greater amounts of CD62e+ and CD144+ microparticles following arterial hypertension and endothelial injury.[37] EVs derived from activated ECs and monocytes express higher levels of cell adhesion markers (VCAM-1, ICAM-1) and procoagulants (tissue factors) that are positively correlated with early vascular dysfunction in low-risk menopausal women.[38] The lipidomic profile of EVs secreted from apoptotic ECs showed an enrichment of proinflammatory oxidized phospholipids, leading to monocyte adhesion to EC and initiating artherogenesis. [39] As CVD progresses, injured cells continue to secrete EVs that reflect cardiac damage. For example, comparative proteomic analysis of plasma-derived EVs from patients with AMI and healthy controls revealed a total of 11 dysregulated proteins in EVs from MI patients, including placental growth factor (PGF), platelet-derived growth factor (PDGF) and glycoproteins such as angiopoietin-1.[40]
The cargo and membrane compositional changes in EVs in response to pathological disease environments can not only be leveraged as diagnostic biomatrkers for early CVD detection but also inform and aid the development of disease-specific therapeutics. Abnormal EVs can be targeted with new drug agents to inhibit or reverse their ability to drive CVD progression.
2.3. EVs as Therapeutics for CVD
EVs have promising biological functions that allow them to be effective therapeutic agents for CVD. Their native biological properties allow EVs to bypass biological barriers and deliver biologically active molecular cargo to recipient cells to stimulate repair and recovery.[11,50] A plentitude of EV-shuttled cargo and associated targets have been identified in the context of CVD (Table 2). Furthermore, EVs are not limited by poor engraftment and do not display any known tumorigenicity, two concerns that currently hinder the clinical success of traditional cell-based therapies.[20] Unlike synthetic drug carriers, EVs are also able to evade immune system cells, allowing for greater bioavailability and reduced adverse immune system responses.[51]
Table 2.
Different types of EVs and their associated cargo that have been found to mediate biological functions and disease pathologies in CVD.
| EV Source | Cargo | Biological Functions | Disease target | Ref |
|---|---|---|---|---|
| Endothelial cell | miR-143/miR-145 | Mediate SMC phenotype | Atherosclerosis | [21] |
| miR-214 | Stimulates angiogenesis, suppress senescence | Ischemic cardiac diseases | [22] | |
| miR-10a | Inhibit leukocyte activation | Vascular inflammation, Thrombosis, Atherosclerosis | [23] | |
| miR-222 | Inhibit leukocyte recruitment | Atherosclerosis | [32] | |
| miR-199a | Regulate cell growth | Coronary Artery Disease, Atherosclerosis | [48] | |
| miR-126 | Promotes reendothelialization and CXCl12-mediated vascular repair | Endothelium damage, atherosclerosis | [60,61] | |
| Kruppel-like factor 2 (KLF2) | Immunomodulation | Atherosclerosis | [62] | |
| Angiopoietin-2 | Regulates vascular quiescence and inflammation | Vessel dysfunction and inflammation | [63] | |
| Mesenchymal stem/stromal cell | miR-182 | Regulates macrophage polarization and leukocyte infiltration | Ischemia reperfusion injury | [64] |
| miR-125b-5p | Decrease cardiomyocyte apoptosis | Acute Myocardial Infarction | [65] | |
| miR-210 | Increases vascular density, decrease cardiomyocyte apoptosis, reduce fibrosis | Acute Myocardial Infarction | [52] | |
| Cardiac progenitor cell | miR-21 | Decrease cardiomyocyte apoptosis | Ischemic Heart Disease | [55] |
| miR-210 | Decrease cardiomyocyte apoptosis | Ischemic Heart Disease | [55] | |
| miR-132 | Decrease cardiomyocyte apoptosis | Ischemic Heart Disease | [55] | |
| miR-146a-3p | Decrease cardiomyocyte apoptosis | Ischemic Heart Disease | [55] | |
| miR-451 | Decrease cardiomyocyte apoptosis | Ischemic Heart Disease | [56] | |
| Cardiomyocyte | miR-30a | Regulate autophagy responses | Ischemic Heart Disease | [66] |
| circHIPK3 | Regulates oxidative damage | Ischemia reperfusion injury | [67] | |
| miR-93-5p | Reduces proinflammatory cytokine levels and suppresses autophagy | Acute Myocardial Infarction | [68] | |
| miR-222 | Promotes angiogenesis and neovascularization | Acute Myocardial Infarction | [69] | |
| miR-143 | Promotes angiogenesis and neovascularization | Acute Myocardial Infarction | [69] | |
| MMP-2 | Extracellular matrix remodelling | Cardiac damage | [70] | |
| HSP60 | Regulates cardiomyocyte apoptosis | Ischemia reperfusion injury | [71] | |
| Smooth muscle cell | Tissue nonspecific alkaline phosphatase (TNAP) | Regulates vascular calcification | Coronary Artery Disease | [72] |
| miR-150 | Promotes angiogenesis and neovascularization | Ischemic diseases | [73] |
Multiple types of EVs have shown pro-regenerative capabilities in CVD. In CVD pathologies where there is significant hypoxic damage, cardiovascular cells are stimulated to secrete EVs to promote tissue repair.[34] For instance, in cases of AMI, MSCs secrete EVs enriched in miR-210 to repair ischemic damage while CD34+ hematopoietic stem cell-derived EVs facilitate recovery by delivering pro-angiogenic factors such as miR-126 to mediate EC proliferation and survival.[52-54] Cells of the cardiac lineage have also been found to contribute to repair mechanisms in CVD. Human cardiac progenitor cells (CPCs), which consist of generally quiescent cells that only differentiate into myocytes and vascular cells under stress conditions, secrete EVs to limit cardiomyocyte apoptosis and promote recovery of left ventricle ejection fraction after AMI.[55] CPC-derived exosomes also protect the myocardium and exert cardioprotective effects in acute ischemia/reperfusion (MI/R) injury by again inhibiting cardiomyocyte apoptosis.[56] The cardioprotective properties of CPC-derived EVs are thought to be mediated by miRNA-dependent mechanisms, such as miR-21, miR-210, miR-132, miR-146a-3p, and miR-451, all of which are enriched to protect ischemic cardiomyocytes from apoptosis, limit the degree of damage, and improve EC proliferation.[55-57] Cardiosphere-derived cells (CDCs) also secrete EVs to promote recovery of cardiac function.[58] In rat and pig models of coronary artery reperfusion injury, intramyocardial administration of CDC-derived exosomes can reduce infarct size and promote recovery of left ventricular ejection fraction.[59]
2.4. Current Challenges in EV Therapy
Though EVs present a promising approach for the treatment of CVD, EV heterogeneity and poor delivery methods prevent successful clinical translation. EV heterogeneity results from isolation procedures that are difficult to standardize and scale-up for mass production. Several excellent reviews have detailed the multiple methods that have been established for EV isolation, including differential ultracentrifugation, density gradient ultracentrifugation, size-exclusion chromatography, microfluidics, and precipitation with polyethylene glycol (PEG), organic solvents, or other chemicals.[16,74-76] However, due to overlapping size and density ranges, it is often a major challenge to obtain high yields of pure EV subsets that are free from other vesicles or non-vesicular components.[5] In an effort to standardize EV research and understand the heterogeneity of isolated samples, the International Society for Extracellular Vesicles (ISEV) has set forth criteria for minimum information required for EV characterization. Briefly, all EVs must be assessed for specific transmembrane and cytosolic protein markers, characterized for morphology, and quantified for amount isolated.[77] Any disparities in source parent cells and impurities in EV isolation will be reflected in EV membrane and cargo composition which consequently affects downstream therapeutic functionalities. EV therapeutics often face the challenge of nonspecific delivery, which results in reduced therapeutic efficacy.[1] When administered systemically, EVs lack homing mechanisms to target injured cardiovascular cells and are cleared from circulation quickly or face off-target accumulation [15,78]. Emergence of new engineering approaches can be used to improve EV bioactivity, targeting, and pharmacokinetics to augment and maximize the therapeutic efficacy of EVs for CVD.
3. Engineering EV Cargo
EVs are efficient drug carriers given their unique and intrinsic advantages. For example, the membrane structure of EVs can protect their bioactive contents from degradation in the extracellular environment.[51,79] The membrane surface is also decorated with specific lipids and proteins that allow the EV to target and fuse with specific recipient cells in order to release cargo.[80-82] Nevertheless, though EVs carry bioactive content, the abundance of actual therapeutic factors may be in low amounts per individual EV particle and necessitates the need for high EV doses. However, this limitation may be bypassed by engineering parent cells to overexpress endogenous cargo of interest in EVs or by exogenously loading cargo in EVs using different loading approaches. A complete summary of cargo loading techniques can be found in Table 3.
Table 3.
A summary of engineering strategies used to load EVs with bioactive cargo.
| Method | Description | Cargo Loaded | Ref |
|---|---|---|---|
| Endogenous Loading Methods | |||
| Hypoxic preconditioning | Cells are grown in hypoxic conditions to enrich or deplete naturally-occurring cargo in secreted EVs. | Nucleic acids | [83-86] |
| Cytokine preconditioning | Cells are incubated with signaling molecules to regulate cargo sorting into EVs. | Nucleic acids | [89,91,92] |
| Transfection/transduction | Plasmids or lentiviral vectors are used to genetically modify cells to express cargo-specific EVs. | Proteins | [96,98] |
| Nucleic acids | [99] | ||
| Coincubation | Parent cells are incubated with cargo that are then sequestered into secreted EVs. | Small molecules | [114] |
| Exogenous Loading Methods | |||
| Coincubation | Cargo is coincubated with EVs. Loading efficiency depends on cargo hydrophobicity and interaction with EV lipid membrane layer. | Small molecules | [100,115] |
| Proteins | [115,116] | ||
| Membrane Permeabilizers | Chemical membrane permeabilizers (e.g. saponin) create pores in the EV membrane that allow cargo to diffuse into the EV. | Proteins | [116-118] |
| Small molecule | [104] | ||
| Sonication | Ultrasonic frequencies create pores in the EV membrane to allow for cargo diffusion. | Nucleic acids | [106] |
| Small molecule | [108] | ||
| Proteins | [107,116] | ||
| Extrusion | A mixture of EVs and cargo is extruded through porous membranes to mechanically disrupt the EV membrane and allow cargo internalization. | Small molecule | [104] |
| Lipids | [119] | ||
| Electroporation | An electric field is applied to disturb the phospholipid membrane and create temporary pores through which cargo can diffuse into the EV. | Small molecules | [104,120] |
| Nucleic acids | [109-111,121] | ||
| Protein | [118] | ||
| pH gradient | EVs are protonated to render the EV interior acidic and positively charged. Basic or negatively charged cargo travel across the transmembrane pH gradient and into the EV. | Nucleic acids | [122] |
| Freeze/Thaw | Cargo is incubated with EVs and snap-frozen and thawed for multiple cycles. During the freeze/thaw process, the membrane of EVs is disrupted, allowing cargo to diffuse into the EVs. | Small molecules | [123] |
| Protein | [116] | ||
3.1. Genetic Engineering of Parent Cells
EVs can be bioengineered to preferentially express specific cargo by genetically modifying parent cells. In this methodology, cells are conditioned, either through environmental or chemical stimulus, to secrete EVs that are enriched or depleted of naturally occurring cargo using native biological machinery.
3.1.1. Cell Preconditioning
To date, hypoxia preconditioning is one of the most popular methods of cell conditioning for CVD therapies, possibly due to the major role that ischemia plays in CVD pathologies. With this technique, cells are grown in hypoxic (i.e. low oxygen concentration) conditions to induce secretion of EVs that contain higher amounts of regenerative cargo. Bone marrow MSCs, for example, release a large number of pro-angiogenic EVs following hypoxic stimulation. [82,83] Improved angiogenesis and cardioprotection was attributed to the hypoxia-induced enrichment of miR-210 and miR98-5p, which improved cell survival and reduced fibrosis via P13K/AKT and p53 signaling pathways.[83,84] CPCs have also been the subject of many hypoxic preconditioning studies to produce selectively therapeutic EVs. Hypoxic CPCs secrete lncRNA MALAT1-enriched EVs that improved vascularization by enhancing endothelial cell viability and reduced cardiomyocyte apoptosis compared to normoxic CPC-derived EVs.[85] Hypoxic preconditioning of CPCs also results in EVs that are enriched in pro-angiogenic and anti-fibrotic miRNAs (miR-15b, miR-17, miR-210, miR-103, miR-199a, miR-20a) and depleted in pro-apoptotic and pro-fibrotic miRNAs (miR-320, miR-222).[86]
Cells can also be preconditioned with proinflammatory mitogens to generate EVs with more immunomodulatory properties. Traditionally, inflammatory priming has been commonly used to increase the immunomodulatory properties of cells, especially MSCs, and current research has focused on the effect of such priming on the paracrine secretions of cells, such as their EVs.[87-89] In one study, bone marrow MSCs were treated with lipopolysaccharides to generate EVs that had greater efficacy in attenuating inflammation and driving macrophage polarization to a more anti-inflammatory M2 phenotype. When translated in a murine AMI model, EVs derived from LPS-conditioned MSCs greatly reduced post-infarction inflammation and promoted cardiomyocyte survival and recovery.[90] While the specific cargo enrichment was not investigated in this particular study, others have shown that LPS conditioning may lead to the preferential sorting and enrichment of let-7b, an miRNA that regulates multiple signaling pathways associated with macrophage plasticity and inflammatory responses.[91] Cytokines have also been used to induce cells to secrete EVs with more immunomodulatory cargo. IFNγ -primed MSCs secreted exosomes that carried greater levels of miR-12a and miR-125b, which repressed Th17 cell differentiation to decrease inflammatory responses.[92] When applied to a cardiac model, human cardiac-derived adherent proliferating (CardAP) cells were stimulated with IFNγ, TNFα, or IL-1β and secreted EVs with reduced ß1 expression that decreased T cell proliferation and pro-inflammatory cytokine levels.[93]
Cells can also be pretreated with small molecules that can influence the biological components packaged into EVs. For instance, MSCs treated with atorvastatin were found to secrete EVs that contained significantly higher levels of cardioprotective and angiogenic lncRNAs compared to EVs secreted from untreated MSCs.[94] In another study, MSCs preconditioned with thrombin produced EVs enriched in angiogenic protein content which demonstrated enhanced wound healing functions in in vivo studies.[95]
Cellular preconditioning allows for the synthesis of cargo-modified EVs using natural mechanisms of EV biogenesis without compromising EV stability or other biophysical parameters. However, environmental preconditioning is an imprecise method of cargo loading. Multiple biological components can be affected by preconditioning and there is little exogenous control over any preferential sequestration of cargo within EVs.
3.1.2. Cell Transduction/Transfection
Transfection and transduction methods have also been used to direct cells to produce EVs with specific cargo. In one model, myocardial and endothelial cells were transfected with SDF1 plasmids to generate EVs with high levels of SDF1, a chemokine that has been associated with myocardial repair and ventricular remodeling.[96,97] The SDF1-enriched exosomes successfully promoted myocardial cell survival and increased endothelial cell tube formation. Cell transduction has also been used to increase specific protein levels in secreted EVs. MSCs were transduced with lentiviral-TIMP2 to obtain exosomes with significant enrichment of TIMP2 cargo, which remained functionally active as shown by its ability to inhibit oxidative stress-induced myocardial apoptosis and hypertrophy following MI injury.[98] Lentivirus-mediated transduction can additionally be used to preferentially overexpress miRNAs in EVs. Wei et al. used lentivirus vectors to transduce MSCs to secrete exosomes with high levels of miRNA-181a cargo.[99] The engineered exosomes successfully delivered the miR-181a to injured cardiac sites in a murine I/R injury model to mediate Treg polarization and modulate expression of inflammatory cytokines to facilitate cardiac recovery. However, one challenge of this method is that not all cells can be efficiently modified with plasmids or lentiviruses. As such, particle standardization and homogeneity become a concern as secreted EVs may carry different levels of therapeutic cargo.
3.2. Exogenous Cargo Loading in EVs
3.2.1. Passive Loading
Exogenous cargo loading allows for flexibility in the type of bioactive molecules that may be loaded into EVs. Passive cargo loading relies on the natural diffusion of exogenous cargo into EV. One of the most popular methods of passive loading includes incubation, where the cargo of interest is simply mixed with EVs without the addition of any other active reagents. Here, the loading efficiency is dependent on the hydrophobicity of the drug molecules and its interaction with the lipid layers of vesicle membrane.[79,100] Though not yet used specifically for cardiovascular applications, Sun et al. demonstrated the feasibility of such technique by loading curcumin, an anti-inflammatory small molecule, into EL4 lymphoma tumor-derived EVs by coincubation at 22 °C for 5 minutes.[100] Curcumin-loaded EVs were able to successfully improve the bioavailability of curcumin and facilitate anti-inflammatory activity in a septic shock murine model. Although incubation is a relatively simple process, it can only be used effectively if a concentration gradient is established appropriately to allow for diffusion and if the cargo of interest has the optimal biophysical profile to interact with the lipid bilayers.
The freeze and thaw method also involves incubation of cargo with EVs at room temperature for a predetermined amount of time. The mixture is then rapidly frozen at −80°C or in liquid nitrogen before being thawed at room temperature. To ensure drug encapsulation, multiple cycles of freezing and thawing are required.[101] As a relatively uncommon method of cargo loading, this technique has thus far been mainly used for the loading of proteins for Parkinson’s treatment with some success. Despite a moderate drug loading efficiency, the potential aggregation of exosomes and degradation of membrane and cargo components tempers the potential advantages of this method.[101,102]
3.2.2. Active Loading
Many of the current approaches for active cargo loading rely on the transient permeabilization of EV membranes that allows for diffusion of compounds into the vesicle.[51] Both chemical and mechanical techniques are often used, the most common of which include saponin treatment, sonication, and electroporation.[79]
Incubation with membrane permeabilizers is an example of chemical-based active cargo loading methods. One such chemical treatment is saponin, which is a surfactant that forms complexes with cholesterol within the cell membrane to generate pores in the membrane.[103] This allows the cargo to travel through the pores and into the interior of the EV before the membrane reseals. Similar to the passive incubation method, saponin assists in loading hydrophilic molecules into exosomes but much more efficiently. One study comparing both methods found that saponin can increase hydrophilic drug loading into exosomes by 11-fold compared to passive incubation.[104] Nevertheless, one concern in this method is the concentration of saponin and its ability to permeabilize the cell membrane. Therefore, insufficient removal of saponin from the EV sample before administration can lead to hemolysis and cytotoxicity.[103,105]
With the sonication technique, EVs are mixed with the cargo molecules and a high frequency wave is applied to the mixture. The ultrasonic frequencies create micropores within the EV membranes that increase cargo permeability into the vesicle.[106] In one study using EVs derived from IC21 macrophages, sonication was found to improve loading efficiency by about 30% compared to saponin permeabilization.[107] The advantage of this method is that, though the membrane microviscosity decreases after sonication, EV protein and lipid structures are not significantly affected.[79] Nevertheless, one potential drawback of the method is that EV deformation can occur, thus affecting the drug release kinetics and EV membrane protein functions.[102,108]
Electroporation is another major active cargo loading technique and is widely used to load nucleic acids such as siRNA or miRNA.[79] This method utilizes electrical currents to create small, temporary pores on the EV membrane. Unlike small hydrophobic molecules which can spontaneously diffuse into EVs, nucleic acids are relatively large in size and hydrophilic, and thus requires active cargo loading.[79,101] Nucleic acids, such as DNA, siRNA, and miRNA, have been efficiently electroporated into EVs and successfully delivered into cells to exert therapeutic functions.[109-111] While electroporation is more effective in loading nucleic acids compared to other methods, loading efficiency is still low. As a result, new electroporation-based procedures, such as cell nanoporation, are under development to load EVs with higher amounts of nucleic acid cargo.[112]
A common concern for all exogenous cargo loading processes is preserving the functionality of the biomacromolecules and the overall stability of the EVs as exogenous compounds are introduced into the vesicle.[51] There is potential that these permeabilizing techniques may destabilize EVs and induce aggregation, and as such, appropriate stability tests must be conducted following cargo loading.[113]
4. Engineering EV Targeting and Delivery
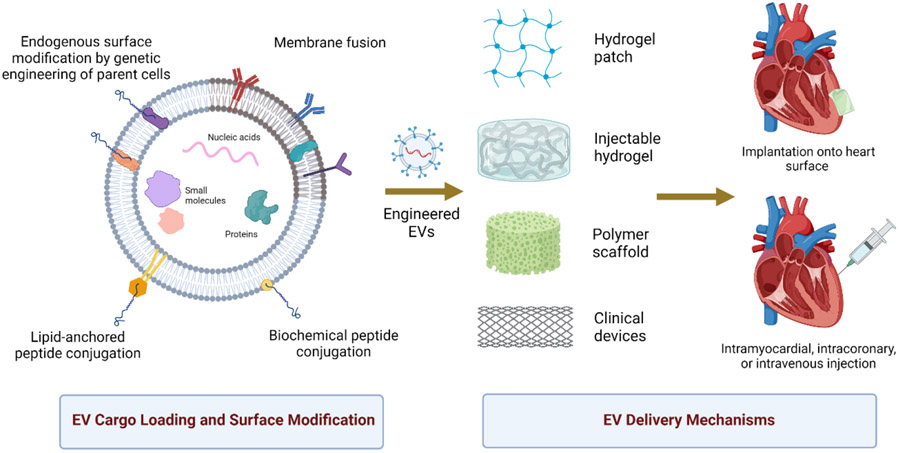
Though EVs are natural drug delivery vehicles, the half-life of EVs is generally shorter compared to their synthetic liposome counterparts, with a difference of 60 minutes and several hours.[124,125] Aside from their short half-life, it is also particularly difficult to deliver EV therapeutics to the heart with high efficiency and specificity.[126,127] EVs lack specific targeting properties that could home in on the injured myocardium or other diseased sites. As a result, EVs are often quickly cleared from blood circulation and accumulate in the lungs, GI tract, and spleen.[15,78] Therefore, a regimen of multiple high doses of EVs is often necessary to compensate for the rapid clearance.[128] Engineering strategies have been developed to overcome these challenges with the exploration of targeted EV delivery. Targeting specificity and cellular uptake can be facilitated by introducing homing molecules to the surface of the EV membrane while EV retention can be improved through the controlled release of EVs through biomaterial-mediated delivery (Figure 2).
Figure 2.
EVs can be engineered to 1) carry bioactive cargo, and 2) express targeting proteins on the membrane surface to improve homing and cellular uptake. Engineered EVs can be delivered using multimodal biomaterial-based, clinically relevant approaches.
4.1. Surface Modification of EVs for Improved Targeting
4.1.1. Peptide Conjugation
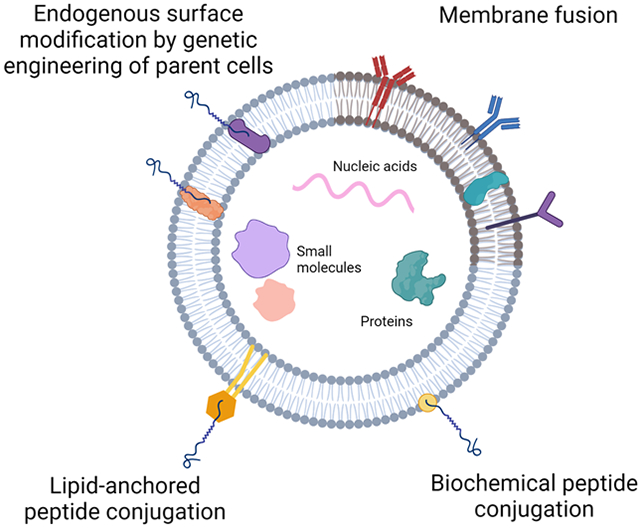
To improve targeting, EV surfaces can be modified with targeting peptides with specific homing to therapeutic sites. This modification can be engineered to occur endogenously within the cell or exogenously using biochemical conjugation methods.
Endogenous methods involve modifying the parent cells from which the EV is secreted. This technique fuses peptide ligands to transmembrane proteins present on EVs. Lysosomal associated membrane protein 2b (Lamp2b) has been commonly used due to its ubiquitous and abundant presence on most EV membranes. Here, peptides of interest are fused to the N-terminus of Lamp2b to be presented on the surface of EVs. For cardiovascular applications, EVs are typically modified with peptides with specific targeting ability to the heart, ischemic sites, or endothelial injury. Kim et al. fused cardiac-targeting peptide (CTP, APWHLSSQYSRT) to Lamp2b-encoding vectors and transfected HEK293 cells to generate targeted exosomes.[129] CTP-modified exosomes showed enhanced targeting to the heart, though off-target accumulation in the liver and spleen was still observed. CDCs underwent similar Lamp2 fusion to generate exosomes with cardiomyocyte-specific binding peptide (CMP, WLSEAGPVVTVRALRGTGSW) on the surface.[130] CMP-exosomes had enhanced and preferential uptake into cardiomyocytes in vitro and demonstrated cardiac tropism following intramyocardial injections in a murine model. Though both CTP and CMP peptides improved targeting to the heart, neither facilitated targeting to specifically injured cardiac sites. Thus, an ischemic myocardium-targeting peptide (IMTP, CSTSMLKAC) was fused with Lamp2b to modify bone marrow MSC-derived exosomes.[131] IMTP preferentially targets ischemic regions of the heart, thus focusing the EV therapeutic effects to injury sites.[132] In a murine AMI model, IMTP-exosomes showed greater accumulation in the myocardial infarction region compared to unmodified exosomes following tail vein injections. Improved targeting resulted in greater therapeutic efficacy as IMTP-exosomes significantly reduced proinflammatory cytokine levels and reduced macrophage polarization to an inflammatory phenotype. IMTP-exosomes also significantly improved cardiac recovery and function by promoting vascularization and reducing cardiomyocyte apoptosis. Other membrane proteins, such as platelet-derived growth factor receptor and lactadherin, have also shown promise for the coupling of targeting peptides, though they have yet to be used for cardiovascular applications.[133,134] While this endogenous method of peptide expression does not disrupt EV membrane stability as exogenous methods can, there is a possibility that some peptides that are fused to the membrane proteins may be cleaved during exosome biogenesis. Therefore, current approaches have evolved to incorporate a glycosylation motif to protect fused proteins from degradation before the exosomes are secreted.[129,135]
Alternatively, EVs can be modified with peptides post-isolation using biochemical conjugation methods. Bio-orthogonal click chemistry is used to exogenously conjugate peptides to EV surfaces through an alkyne-azide cycloaddition reaction. An alkyne linker, such as dibenzobicyclooctyne (DBCO), is initially added to modify the EV surface, and a peptide with an azide group is reacted with the DBCO to complete the conjugation. Click chemistry was used to conjugate cyclo Arg-Gly-Asp-D-Tyr-Lys ([c(RGDyK)]), a peptide with high affinity to integrin αvβ3, to the surface of bone marrow MSC-derived exosomes to improve targeting to cerebral vascular endothelial cells in ischemic conditions.[136] In a murine stroke model, c(RGDyK)-exosomes were intravenously injected and preferentially targeted the ischemic regions of the brain. Further histological analysis revealed that c(RGDyK)-exosomes were preferentially uptaken by cerebral endothelial cells, indicating strong targeting specificity. In another approach, peptides can first be synthesized with linkers and then covalently conjugated to EV surfaces. This method was validated in a study by Nakase et al. where arginine-rich cell-penetrating peptide (CPP) was first modified with Rn-EMCS (N-ε-malemidocaproyl-oxysuccinimide ester).[137] The resulting CPP-EMCS peptides were conjugated to EV through the covalent interaction of ECMS to amino groups of EV membrane proteins. Successfully modified CPP-exosomes showed improved cellular uptake via a micropinocytosis-mechanism.
The phospholipid composition of the EV membrane allows for the opportunity to directly modify the surface using lipid-based approaches. Initial approaches have involved the use of lipid-anchors that act as linkers between the EV and the exogenous targeting molecule. These lipid-anchors usually consist of phospholipid tails that self-insert into the lipid bilayer of the EV membrane while the head displays binding sites to which targeting molecules can subsequently be attached. Antes et al. reported that DMPE-PEG-streptavidin could be used to successfully modify CDC-derived EVs by using the DMPE as a phospholipid anchor and the PEG-streptavidin to couple biotinylated ischemia-targeting peptides.[138] Intravenous injections in a rat ischemia/reperfusion model showed that engineered CDC EVs had enhanced targeting to the heart compared to the unmodified EVs, suggesting that the peptide conjugation was successful in directing CDC EVs to infarcted heart tissue. A similar study was conducted to modify cardiac stem-cell derived exosomes with cardiac homing peptide to improve targeting to the infarcted heart. In this study, DOPE-NHS was used, where DOPE constituted the phospholipid anchor that was coupled with the cardiac homing peptide.[139] Intravenous administration in a rat ischemia/reperfusion model showed improved targeting to the infarcted heart compared to unmodified exosomes, and a greater recovery in cardiac repair and function.
4.1.2. Membrane Fusion
Targeting efficacy of EVs can also be improved via the fusion of EVs with lipid-based micelles or liposomes. With this methodology, the synthetic lipid vesicles fuse seamlessly with the lipid components of the EV membrane to insert exogenous functional lipids or peptides. The fusion can be facilitated by several different methodologies. One of the more commonly used techniques involves freeze-thaw method where EVs and liposomes are co-incubated and cyclically snap-frozen and thawed. This was first validated with EVs isolated from Raw264.7 and CMS7 cancer cells and fused with liposomes composed of functional lipids.[101] Fused EVs were found to have improved cellular uptake and presented as efficient transporter of exogenous hydrophobic lipids. Membrane-fused EVs were applied to a cardiac model when CPC EVs were fused with liposomes to improve uptake efficiency and delivery, especially at a higher ratio of EV components to liposomes.[140] The CPC EV-liposome hybrid particles improved wound healing and promoted Akt phosphorylation in a dose-dependent manner compared to liposomes alone, suggesting that the fusion process did not impact EV functional properties. In another model, liposomes were first modified with PEG to mediate lipid mixing and improve fusion efficiency as well as to introduce PEG molecules onto the EV surface in order to improve their circulation time.[141] Here, PEGylated liposomes were fused with EVs derived from MSCs or HUVECs using simple co-incubation methods. Maximum fusion efficiency was seen at higher EV to liposome ratios and with greater PEG8000 concentrations. Unlike in the previous studies, hybrid EVs were found to have reduced cellular uptake into macrophages. However, this is likely due to their PEGylated surface as PEG is known to assist in evading immune clearance by reducing opsonization and phagocytic internalization.[142] Liposome fusion with EVs can also introduce hydrophilic or lipophilic molecules to the interior of the EV to act as a method of cargo loading. Piffoux et al. found that mTHPC, a small antitumor photosensitizer loaded into liposomes, could be successfully transferred to the interior of EVs following membrane fusion while Evers et al. demonstrated the loading of siRNAs into CPC-EV hybrids. [140,141] Though liposome-mediated EV modification has shown some promise, standardization remains a challenge as fusion efficiency was found to be dependent on EV origin and liposome composition.[101,141] This can potentially be attributed to the interactions of different EV membrane proteins with the lipids in the synthetic liposomes that may mediate the degree of fusion and can be difficult to control. Furthermore, cellular uptake is also seen to be dependent on the target cell and the EV-liposome ratio, which can make this system of engineering somewhat unreliable and requires stringent characterization for every unique application.[140]
A relatively new membrane fusion technique involves the use of cell plasma membranes as a natural biomaterial alternative to synthetic liposomes. Cell membranes contain many native proteins that have innate targeting properties. The functional properties of cell membranes were realized with the modification of cardiac stem cells with platelet membrane nanovesicles which have natural targeting affinity to infarcted cardiac sites.[143] The CSCs, which have limited innate homing to injury sites, showed significantly improved targeting and retention in the infarcted heart after decoration with platelet nanovesicles using PEG-mediated fusion. Cell membrane fusion was applied to EVs in a separate study to improve MSC EV targeting to the injured myocardium in a murine myocardial ischemia-reperfusion injury model.[144] Bone-marrow MSC EVs were fused with monocyte membranes using serial co-extrusion. MSCs, though functionally angiogenic, had insufficient opportunities to exert their therapeutic effects due to their poor targeting properties. Monocytes, on the other hand, possess an abundance of adhesion proteins (e.g. α4β1, ALB2, P-selectin glycoprotein ligand I) that drive homing and retention to injured cardiac sites. The fusion of the MSC EVs and monocyte membranes resulted in a hybrid particle that demonstrated both improved targeting to injured myocardium and greater cardiac recovery by promoting endothelial maturation and modulating inflammatory responses.
4.2. Biomaterial-aided EV delivery
Biomaterial-aided approaches have emerged to allow for the local and sustained delivery of EVs for CVD treatment. Delivery and retention have been major challenges facing EV therapy as rapid systemic clearance reduces the ability of EVs to reach injury sites and exert their full therapeutic potential. For cardiovascular diseases in particular, local intramyocardial or intracoronary delivery can improve therapeutic effects but require invasive procedures and are limited to the area of injection.[145] Local and controlled release of EVs over a long duration is a more practical approach to maintain a clinically relevant dosage level in the body.
4.2.1. Hydrogel Patches
Hydrogels are crosslinked polymer networks with high water content and have been popularly used as controlled release systems for drug delivery due to their biocompatibility and tunable spatiotemporal properties.[146,147] Hydrogels have been successfully used for the encapsulation of EVs for CVD therapy. Importantly, EV delivery via hydrogels greatly improves the EV retention at cardiac injury and remodeling sites, and facilitates greater recovery compared to non-specific intravenous injections.[146] Hydrogel patches or meshes are one mechanism of delivery. In one study, a collagen gel-foam mesh was used to encapsulate EVs derived from induced pluripotent stem cell-derived cardiomyocytes (iCMs). Implantation of the mesh on the myocardium led to the controlled release of miRNA cargo from the EVs and stimulated cardioprotective processes including rhythm recovery, reduced cardiac dilation, and higher ejection fraction.[148] Encapsulation of MSC-derived EVs by a similar collagen matrix hydrogel demonstrated prolonged retention of EVs and further improved angiogenesis while also decreasing fibrosis and apoptosis.[149] However, these types of structures have larger pore size, which can result in a burst release of EVs rather than a sustained delivery.
4.2.2. Injectable Hydrogels
Implantation of hydrogel patches often requires an invasive surgical procedure in order to place the hydrogel directly onto the site of interest. Therefore, injectable hydrogels have been developed to allow for minimally invasive EV delivery.
Natural biomaterials have been popular choices for injectable hydrogel delivery of EVs. In one study, thermosensitive decellularized extracellular matrix hydrogel or a pre-crosslinked hyaluronic acid (HA) hydrogel was loaded with exosomes derived from MSCs or CPCs and delivered via an intrapericardial injection.[126] There, the hydrogels formed a cardiac patch-like structure in the cavity, and slowly released encapsulated EVs to promote cardiac recovery following AMI. In another study, shear thinning injectable hydrogels made of adamantane-modified HA (Ad-HA) and β-cyclodextrin-modified HA (CD-HA) remained in a liquid state in response to the shear stress when injected from the syringe but gelled at the target site.[150] Using this strategy, EPC EVs were delivered via intramyocardial injections to infarcted heart in rat models of AMI. Shear-thinning hydrogels regulated the steady, controlled release of EVs, thus allowing for greater retention and improved hemodynamics, angiogenesis, and cardiac function. Other natural hydrogel materials include chitosan and alginate. MSC-derived exosomes were mixed with chitosan hydrogel and intramuscularly injected into the ischemic hindlimb to enhance neovascularization and microvascular density while bone marrow MSC-EV-loaded alginate hydrogels were delivered via intramyocardial injection to prolong retention, promote angiogenesis, and reduce inflammation.[151,152] MSC-derived exosomes were also shown to be effectively loaded into silk fibroin hydrogel to improve retention and enhance blood perfusion in ischemic hindlimbs.[153]
More unique forms of injectable hydrogels include peptide-based self-assembly hydrogels. These types of hydrogels are advantageous in that they can be readily designed with smaller pore sizes. They are formed from amphiphilic peptides that couple to create porous structures of antiparallel β-sheet bilayers. [154] Han et al. used cardioprotective peptides to assemble hydrogels (termed PGN hydrogels) and loaded human umbilical cord MSC-derived exosomes.[155] Encapsulated exosomes were released steadily over time in vitro and demonstrated superior retention in the ischemic myocardium compared to intramyocardial injected exosomes. As a result, exosome loaded-PGN hydrogels promoted greatest cardiac function and endothelial recovery while reducing fibrosis and inflammation. Though PGN hydrogels were found to improve EV retention in comparison to intramyocardial injection alone, it is difficult to control degradation rate and regulate EV release kinetics from the PGN hydrogel.
While injectable hydrogels have been mainly delivered via intramyocardial injections, another method of delivery is the spraying technique. For example, in one study, gelatin methacryloyl (GelMA) precursors, EVs, and photoinitiators were sprayed onto infarcted heart tissue and crosslinked in situ to create a cardiac hydrogel patch that slowly released encapsulated EVs over time[156] Sprayed EV-loaded GelMA facilitated uptake and improved EV retention at the injury to promote significant recovery in ventricular function and fibrosis in a murine model of MI. However, this technology is very much nascent and more work remains to be done in order to improve in vivo degradation profiles and increase the specificity of the sprayed area with precision instruments.
4.2.3. Polymeric Materials for 3D Printing and Additive Manufacturing
Advent of three-dimensional (3D) printing technologies have further inspired the use of polymeric biomaterials to facilitate improved delivery of EVs. Different materials, including polylactic acid (PLA) and polycaprolactone (PCL), were used to incorporate MSC EVs onto 3D printed scaffolds in order to promote local bone tissue repair.[157,158] For instance, a 3D printed exosome scaffold was able to polarize synovial macrophage response to an M2 phenotype, enhance chondrocyte migration, and consequently facilitate cartilage regeneration.[159] To date, these have had limited applications for treatment of CVD, potentially because most 3D printed structures require surgical implantation and are not injectable, which has been the main focus for delivery methods for cardiac repair. However, 3D printing for CVD has potential as a future area of innovation, where patient-specific patches, stents, or vascular grafts can be designed and modified with EVs for improved biological efficacy.
5. EV-modified Vascular Devices for Potential Clinical Application
Due to the multiple factors (e.g. culture conditions, cell passage, cell density, and frequency of harvesting) that influence the production of EVs, especially in a large-scale manufacturing process, it is difficult to produce a clinically relevant dose of EVs that have guaranteed regenerative bioactivity.[51] Therefore, to date, there are still no clinical trials involving EV administration for CVD treatment, though there are some EV therapies for cancer and wound healing that are currently in Phase I-III trials.[160] One potential path to clinical translation involves modification of pre-existing clinical devices with EVs to augment their biological properties.
Synthetic vascular grafts are biomaterial conduits, usually made of polytetrafluoroethylene (PTFE), that are used to replace or bypass an injured or occluded vessel. Due to their foreign polymer source, many vascular grafts are limited by low patency rate as a result of the thrombosis and neointimal hyperplasia that often occurs after implantation.[161] EV-modified grafts are hypothesized to improve patency by regulating immune responses and facilitating reendothelialization. In one study that loaded human placenta MSC derived sEVs onto an electrospun poly (ε-caprolactone) (PCL) vascular graft in a rat model of hyperlipidemia, the EV-modified graft significantly improved patency rate up to 3 months post-grafting, increasing from 40% to 69%.[162] Importantly, the incorporation of sEVs also promoted vascular regeneration, as the pro-angiogenic contents of the EV, such as vascular endothelial growth factors (VEGF), miR-126, −145, stimulated higher capillary density on the graft. Furthermore, the EVs on the modified graft were also seen to demonstrate immunomodulatory properties by inducing macrophage polarization to an anti-inflammatory M2 phenotype. This resulted in decreased calcification and thrombosis of the modified vascular grafts, therefore addressing a major dysregulation concern in atherosclerosis.[162,163] In another study, human adipose-derived mesenchymal stem cell (hADMSC) EVs were vacuum seeded onto porous silk conduits and grafted in a rat aortic interposition model. Grafts modified with EVs demonstrated significantly higher patency rates (100% EVs vs 56% hADMSC cells vs 82% unmodified controls) with improved endothelium regrowth and SMC infiltration, and reduced macrophage adhesion.[164] Despite the biological advantages of EV modification onto vascular grafts, little research has been conducted to study the long-term impacts of EV modification on the overall biomechanical properties of the graft, such as tensile strength, following implantation. Furthermore, EV modification of autologous or cryopreserved cadaveric grafts to improve patency rates and clinical outcomes is yet to be investigated.
Stent placement is a widespread intervention that restores blood flow in occluded vessels. However, the metal composition of stents damages the endothelium, leading to neointimal hyperplasia, thrombosis, and eventually restenosis. While drug-eluting stents aid in reducing smooth muscle cell proliferation to prevent neointimal hyperplasia, they also inhibit re-endothelialization.[165] Modification of stents with EVs presents an opportunity to prevent neointimal hyperplasia while also accelerating re-endothelialization. In a proof-of-concept study, Hou et al. modified poly-dopamine coated stainless steel, a clinical stent material, with plasma exosomes via electrostatic interactions.[166] The modified surface improved endothelial cell adhesion and function while inhibiting macrophage adhesion and smooth muscle proliferation. Rather than simply remaining bound on the surface, exosomes can also be eluted from stents. MSC-derived exosomes were coated onto 1,2-distearoyl-sn-glycero-3-phosphoethanolamine (DSPE)-modified stents using a reactive oxidative species (ROS) linker and released upon ROS stimulation.[167] Modified stents promoted re-endothelialization and reduced platelet and monocyte adhesion. In rat models of renal or hindlimb ischemia, increased vascular regeneration was seen with modified stents along with reduced inflammation but did not prevent neointimal hyperplasia. Future stent modifications could also incorporate surface-specific coating, where the luminal surface can be coated with one type of EVs that can promote endothelial regeneration while the abluminal surface can be coated with a different type of EVs to prevent smooth muscle cell proliferation.
For all EV-modified surgical devices, rigorous testing in large animal models is necessary to confirm the long-term patency before translation into human clinical trials. Further testing of stability and mechanical properties are also required to assess the off-the-shelf, commercial prospects.
6. Conclusion
EVs have gained immense popularity over the last decade and emerged as promising agents for pathophysiological biomarkers and as regenerative therapeutics in the cardiovascular space. Recent scientific progress has shed significant light on the biological composition of EVs and their complex roles in facilitating critical intercellular communication. Clinical application, however, has not been straightforward and has been impeded by the multiple limitations brought forth by the innate EV heterogeneity, targeting, and retention. Bioengineering approaches offer a versatile solution to shaping EVs as more homogenous, standardized, and personalized therapeutics that can be readily applied for CVD. Shifts in EV phenotypes during CVD development can aid in designing disease-specific EV therapeutics using different engineering strategies. For example, advances in cargo loading technologies present an opportunity to load EVs with specific bioactive molecules to allow for more efficacious therapy while the advent of new delivery approaches ensures the sustained and targeted delivery of EVs to cardiac or endothelial injury sites. Though the field is quickly growing, current engineering approaches still result in poorly reproducible and heterogenous population of engineered EVs which often fall short of their expected therapeutic potential. More stringent standardization, validation, and safety assessments are still required before bioengineered EVs can reach the clinical translation stage. Nevertheless, the promise of personalized EV therapeutics holds strong and rapidly advancing research and technologies could allow for the development of breakthrough CVD treatments.
Acknowledgements
This study was partially supported by the Tobacco Related Disease Research Program High Impact Pilot Award (T31IP1530) to A.W. and Predoctoral Fellowship (T31DT1599) to L.R, the UC Davis Science Translation and Innovative Research (STAIR) grant to A.W., Postdoctoral Fellowship from NIH T32 Training Grant in Basic & Translational Cardiovascular Science (NIH T32 HL086350) to N.B, and Shriners Hospitals for Children Postdoctoral Fellowship (84705-NCA-19) to D.H.
All figures were created with BioRender.com.
Biographies

Lalithasri Ramasubramanian is a PhD candidate in Biomedical Engineering at the University of California, Davis with a Designated Emphasis in Biotechnology. Her research interests involve the development of new therapeutic strategies for regenerative medicine, with a specific focus on the cardiovascular system. Her current work centers around engineering extracellular vesicles and biomimetic nanoparticles to promote vascular repair and regeneration.

Shixian Du graduated from the University of California, Davis with a Bachelor of Science degree in Biomedical Engineering. She will start graduate school at University of California, San Francisco in Biomedical Imaging. Her research interest is to apply novel bioengineering approaches to investigate and treat cardiovascular diseases.

Siraj Gidda recently received his B.S. in Global Disease Biology from the University of California, Davis. He is currently interested in researching various biological mechanisms with the hope of leveraging that information to help create new therapeutics that can improve patient outcomes. Additionally, he plans on pursuing a career in medicine to help aid in improving patient care.

Nataliya Bahatyrevich, MD is a physician resident in integrative cardiothoracic surgery program at the University of California, Davis. She is the first resident in this program to undergo a dedicated research year and to receive T32 NHLBI Postdoctoral Fellowship award in cardiovascular research. Her research interests include improvement of revascularization therapies for cardiovascular disease, vessel bioengineering, organ bioengineering and modification for transplantation, and stem cell and other regenerative therapies for treatment of heart failure.

Dake Hao is a Postdoctoral Fellow in Biomedical Engineering at University of California, Davis. He received his Ph.D. from Shandong University, China. His research focuses on developing innovative technologies and products that combine molecular, stem cell, biomaterial, extracellular vesicle, and extracellular matrix engineering for tissue regeneration and treating clinical diseases, including ischemic vascular diseases, adult and fetal bone defects and neurological disorders.

Priyadarsini Kumar is a senior scientist with over 25 years of research experience. She obtained her PhD from University of California, Davis, and Master’s/Bachelor’s degrees from University of Madras, India. Her research focus is stem cell and stem cell derived extracellular vesicles for treatment of neurodegenerative diseases. She is currently leading the cell manufacturing team for the Phase1/2a spina bifida clinical trial. Her other research focus is in utilizing placenta-derived extracellular vesicles and developing a serum-free cell-free product for clinical applications like adult acquired spinal cord injury.

Aijun Wang is a Chancellor’s Fellow, Professor of Surgery and of Biomedical Engineering at the University of California, Davis (UC Davis). He is the vice chair for Translational Research, Innovation and Entrepreneurship, and codirector of the Center for Surgical Bioengineering at the Department of Surgery, UC Davis School of Medicine. Dr. Wang’s research focuses on developing tools and technologies that combine molecular, cellular, tissue, and biomaterial engineering to promote regeneration and restore function. The Wang Group engineers and develops stem cells, extracellular vesicles/nanomedicine and extracellular matrix/biomaterial scaffolds for the treatment of surgical conditions and diseases.
References
- [1].Kaptoge S, Pennells L, De Bacquer D, Cooney MT, Kavousi M, Stevens G, Riley LM, Savin S, Khan T, Altay S, Amouyel P, Assmann G, Bell S, Ben-Shlomo Y, Berkman L, Beulens JW, Björkelund C, Blaha M, Blazer DG, Bolton T, Bonita Beaglehole R, Brenner H, Brunner EJ, Casiglia E, Chamnan P, Choi Y-H, Chowdry R, Coady S, Crespo CJ, Cushman M, Dagenais GR, D’Agostino RB Sr, Daimon M, Davidson KW, Engström G, Ford I, Gallacher J, Gansevoort RT, Gaziano TA, Giampaoli S, Grandits G, Grimsgaard S, Grobbee DE, Gudnason V, Guo Q, Tolonen H, Humphries S, Iso H, Jukema JW, Kauhanen J, Kengne AP, Khalili D, Koenig W, Kromhout D, Krumholz H, Lam T, Laughlin G, Marín Ibañez A, Meade TW, Moons KGM, Nietert PJ, Ninomiya T, Nordestgaard BG, O’Donnell C, Palmieri L, Patel A, Perel P, Price JF, Providencia R, Ridker PM, Rodriguez B, Rosengren A, Roussel R, Sakurai M, Salomaa V, Sato S, Schöttker B, Shara N, Shaw JE, Shin H-C, Simons LA, Sofianopoulou E, Sundström J, Völzke H, Wallace RB, Wareham NJ, Willeit P, Wood D, Wood A, Zhao D, Woodward M, Danaei G, Roth G, Mendis S, Onuma O, Varghese C, Ezzati M, Graham I, Jackson R, Danesh J, Di Angelantonio E, The Lancet Global Health 2019, 7, e1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Roth GA, Johnson C, Abajobir A, Abd-Allah F, Abera SF, Abyu G, Ahmed M, Aksut B, Alam T, Alam K, Alla F, Alvis-Guzman N, Amrock S, Ansari H, Ärnlöv J, Asayesh H, Atey TM, Avila-Burgos L, Awasthi A, Banerjee A, Barac A, Bärnighausen T, Barregard L, Bedi N, Belay Ketema E, Bennett D, Berhe G, Bhutta Z, Bitew S, Carapetis J, Carrero JJ, Malta DC, Castañeda-Orjuela CA, Castillo-Rivas J, Catalá-López F, Choi J-Y, Christensen H, Cirillo M, Cooper L, Criqui M, Cundiff D, Damasceno A, Dandona L, Dandona R, Davletov K, Dharmaratne S, Dorairaj P, Dubey M, Ehrenkranz R, El Sayed Zaki M, Faraon EJA, Esteghamati A, Farid T, Farvid M, Feigin V, Ding EL, Fowkes G, Gebrehiwot T, Gillum R, Gold A, Gona P, Gupta R, Habtewold TD, Hafezi-Nejad N, Hailu T, Hailu GB, Hankey G, Hassen HY, Abate KH, Havmoeller R, Hay SI, Horino M, Hotez PJ, Jacobsen K, James S, Javanbakht M, Jeemon P, John D, Jonas J, Kalkonde Y, Karimkhani C, Kasaeian A, Khader Y, Khan A, Khang Y-H, Khera S, Khoja AT, Khubchandani J, Kim D, Kolte D, Kosen S, Krohn KJ, Kumar GA, Kwan GF, Lal DK, Larsson A, Linn S, Lopez A, Lotufo PA, El Razek HMA, Malekzadeh R, Mazidi M, Meier T, Meles KG, Mensah G, Meretoja A, Mezgebe H, Miller T, Mirrakhimov E, Mohammed S, Moran AE, Musa KI, Narula J, Neal B, Ngalesoni F, Nguyen G, Obermeyer CM, Owolabi M, Patton G, Pedro J, Qato D, Qorbani M, Rahimi K, Rai RK, Rawaf S, Ribeiro A, Safiri S, Salomon JA, Santos I, Santric Milicevic M, Sartorius B, Schutte A, Sepanlou S, Shaikh MA, Shin M-J, Shishehbor M, Shore H, Silva DAS, Sobngwi E, Stranges S, Swaminathan S, Tabarés-Seisdedos R, Tadele Atnafu N, Tesfay F, Thakur JS, Thrift A, Topor-Madry R, Truelsen T, Tyrovolas S, Ukwaja KN, Uthman O, Vasankari T, Vlassov V, Vollset SE, Wakayo T, Watkins D, Weintraub R, Werdecker A, Westerman R, Wiysonge CS, Wolfe C, Workicho A, Xu G, Yano Y, Yip P, Yonemoto N, Younis M, Yu C, Vos T, Naghavi M, Murray C, Journal of the American College of Cardiology 2017, 70, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Tsao CW, Aday AW, Almarzooq ZI, Alonso A, Beaton AZ, Bittencourt MS, Boehme AK, Buxton AE, Carson AP, Commodore-Mensah Y, Elkind MSV, Evenson KR, Eze-Nliam C, Ferguson JF, Generoso G, Ho JE, Kalani R, Khan SS, Kissela BM, Knutson KL, Levine DA, Lewis TT, Liu J, Loop MS, Ma J, Mussolino ME, Navaneethan SD, Perak AM, Poudel R, Rezk-Hanna M, Roth GA, Schroeder EB, Shah SH, Thacker EL, VanWagner LB, Virani SS, Voecks JH, Wang N-Y, Yaffe K, Martin SS, on behalf of the American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee, Circulation 2022, 145, DOI 10.1161/CIR.0000000000001052. [DOI] [PubMed] [Google Scholar]
- [4].Hajar R, Heart Views 2017, 18, 68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Lötvall J, Hill AF, Hochberg F, Buzás EI, Di Vizio D, Gardiner C, Gho YS, Kurochkin IV, Mathivanan S, Quesenberry P, Sahoo S, Tahara H, Wauben MH, Witwer KW, Théry C, Journal of Extracellular Vesicles 2014, 3, 26913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Zheng M, Huang M, Ma X, Chen H, Gao X, Bioconjugate Chem. 2019, 30, 994. [DOI] [PubMed] [Google Scholar]
- [7].Bank IE, Timmers L, Gijsberts CM, Zhang Y-N, Mosterd A, Wang J-W, Chan MY, De Hoog V, Lim SK, Sze SK, Lam CS, De Kleijn DP, Expert Review of Molecular Diagnostics 2015, 15, 1577. [DOI] [PubMed] [Google Scholar]
- [8].Kraus L, Mohsin S, Journal of Cardiovascular Pharmacology 2020, 76, 650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Skryabin GO, Komelkov AV, Savelyeva EE, Tchevkina EM, Biochemistry Moscow 2020, 85, 177. [DOI] [PubMed] [Google Scholar]
- [10].Tan SS, Yin Y, Lee T, Lai RC, Yeo RWY, Zhang B, Choo A, Lim SK, Journal of Extracellular Vesicles 2013, 2, 22614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Fu S, Zhang Y, Li Y, Luo L, Zhao Y, Yao Y, Cell Death Discov. 2020, 6, 68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Caruso S, Poon IKH, Front. Immunol 2018, 9, 1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Battistelli M, Falcieri E, Biology 2020, 9, 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Saludas L, Oliveira CC, Roncal C, Ruiz-Villalba A, Prósper F, Garbayo E, Blanco-Prieto MJ, Nanomaterials 2021, 11, 570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Kang M, Jordan V, Blenkiron C, Chamley LW, Journal of Extracellular Vesicles 2021, 10, DOI 10.1002/jev2.12085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Brennan K, Martin K, FitzGerald SP, O’Sullivan J, Wu Y, Blanco A, Richardson C, Mc Gee MM, Sci Rep 2020, 10, 1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Liu C, Bayado N, He D, Li J, Chen H, Li L, Li J, Long X, Du T, Tang J, Dang Y, Fan Z, Wang L, Yang PC, Front. Cardiovasc. Med 2021, 8, 758050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Huang C, Neupane YR, Lim XC, Shekhani R, Czarny B, Wacker MG, Pastorin G, Wang J-W, in Advances in Clinical Chemistry, Elsevier, 2021, pp. 47–95. [DOI] [PubMed] [Google Scholar]
- [19].Chong SY, Lee CK, Huang C, Ou YH, Charles CJ, Richards AM, Neupane YR, Pavon MV, Zharkova O, Pastorin G, Wang J-W, IJMS 2019, 20, 3272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].de Abreu RC, Fernandes H, da Costa Martins PA, Sahoo S, Emanueli C, Ferreira L, Nat Rev Cardiol 2020, 17, 685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Hergenreider E, Heydt S, Tréguer K, Boettger T, Horrevoets AJG, Zeiher AM, Scheffer MP, Frangakis AS, Yin X, Mayr M, Braun T, Urbich C, Boon RA, Dimmeler S, Nat Cell Biol 2012, 14, 249. [DOI] [PubMed] [Google Scholar]
- [22].van Balkom BWM, de Jong OG, Smits M, Brummelman J, den Ouden K, de Bree PM, van Eijndhoven MAJ, Pegtel DM, Stoorvogel W, Würdinger T, Verhaar MC, Blood 2013, 121, 3997. [DOI] [PubMed] [Google Scholar]
- [23].Njock M-S, Cheng HS, Dang LT, Nazari-Jahantigh M, Lau AC, Boudreau E, Roufaiel M, Cybulsky MI, Schober A, Fish JE, Blood 2015, 125, 3202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Rautou P-E, Leroyer AS, Ramkhelawon B, Devue C, Duflaut D, Vion A-C, Nalbone G, Castier Y, Leseche G, Lehoux S, Tedgui A, Boulanger CM, Circ Res 2011, 108, 335. [DOI] [PubMed] [Google Scholar]
- [25].Yu H, Wang Z, Front. Physiol 2019, 10, 1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Yu D-W, Ge P-P, Liu A-L, Yu X-Y, Liu T-T, European Review for Medical and Pharmacological Sciences 2019, 23, 4873. [DOI] [PubMed] [Google Scholar]
- [27].Gupta S, Knowlton AA, American Journal of Physiology-Heart and Circulatory Physiology 2007, 292, H3052. [DOI] [PubMed] [Google Scholar]
- [28].Feng Y, Huang W, Meng W, Jegga AG, Wang Y, Cai W, Kim HW, Pasha Z, Wen Z, Rao F, Modi RM, Yu X, Ashraf M, Stem Cells 2014, 32, 462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Wang X, Gu H, Huang W, Peng J, Li Y, Yang L, Qin D, Essandoh K, Wang Y, Peng T, Fan G-C, Diabetes 2016, 65, 3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Matkovich SJ, Wang W, Tu Y, Eschenbacher WH, Dorn LE, Condorelli G, Diwan A, Nerbonne JM, Dorn GW, Circulation Research 2010, 106, 166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Wagner KT, Radisic M, Adv NanoBio Res 2021, 1, 2100047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Jansen F, Yang X, Proebsting S, Hoelscher M, Przybilla D, Baumann K, Schmitz T, Dolf A, Endl E, Franklin BS, Sinning J, Vasa-Nicotera M, Nickenig G, Werner N, JAHA 2014, 3, DOI 10.1161/JAHA.114.001249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].D’Alessandra Y, Devanna P, Limana F, Straino S, Di Carlo A, Brambilla PG, Rubino M, Carena MC, Spazzafumo L, De Simone M, Micheli B, Biglioli P, Achilli F, Martelli F, Maggiolini S, Marenzi G, Pompilio G, Capogrossi MC, European Heart Journal 2010, 31, 2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Femminò S, Penna C, Margarita S, Comità S, Brizzi MF, Pagliaro P, Vascular Pharmacology 2020, 135, 106790. [DOI] [PubMed] [Google Scholar]
- [35].Milano G, Biemmi V, Lazzarini E, Balbi C, Ciullo A, Bolis S, Ameri P, Di Silvestre D, Mauri P, Barile L, Vassalli G, Cardiovascular Research 2019, cvz108. [DOI] [PubMed] [Google Scholar]
- [36].Canault M, Leroyer AS, Peiretti F, Lesèche G, Tedgui A, Bonardo B, Alessi M-C, Boulanger CM, Nalbone G, The American Journal of Pathology 2007, 171, 1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Sansone R, Baaken M, Horn P, Schuler D, Westenfeld R, Amabile N, Kelm M, Heiss C, Atherosclerosis 2018, 273, 67. [DOI] [PubMed] [Google Scholar]
- [38].Miller VM, Lahr BD, Bailey KR, Hodis HN, Mulvagh SL, Jayachandran M, Atherosclerosis 2016, 246, 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Huber J, Vales A, Mitulovic G, Blumer M, Schmid R, Witztum JL, Binder BR, Leitinger N, ATVB 2002, 22, 101. [DOI] [PubMed] [Google Scholar]
- [40].Gidlöf O, Evander M, Rezeli M, Marko-Varga G, Laurell T, Erlinge D, Sci Rep 2019, 9, 8991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].de Hoog VC, Timmers L, Schoneveld AH, Wang J-W, van de Weg SM, Sze SK, van Keulen JK, Hoes AW, den Ruijter HM, de Kleijn DP, Mosterd A, Eur Heart J Acute Cardiovasc Care 2013, 2, 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Verbree-Willemsen L, Zhang Y-N, Ibrahim I, Ooi SBS, Wang J-W, Mazlan MI, Kuan WS, Chan S-P, Peelen LM, Grobbee DE, Richards AM, Lam CSP, de Kleijn DPV, ESC Heart Fail 2020, 7, 2240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Kanhai DA, Visseren FLJ, van der Graaf Y, Schoneveld AH, Catanzariti LM, Timmers L, Kappelle LJ, Uiterwaal CSPM, Lim SK, Sze SK, Pasterkamp G, de Kleijn DPV, International Journal of Cardiology 2013, 168, 2358. [DOI] [PubMed] [Google Scholar]
- [44].Zhang Y-N, Vernooij F, Ibrahim I, Ooi S, Gijsberts CM, Schoneveld AH, Sen KW, den Ruijter HM, Timmers L, Richards AM, Jong CT, Mazlan I, Wang J-W, Lam CSP, de Kleijn DPV, PLoS One 2016, 11, e0148073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Sun R, Liu W, Zhao Y, Chen H, Wang Z, Zhang Y, Sun X, Cui X, Cancer Cell Int 2021, 21, 311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Kranendonk MEG, de Kleijn DPV, Kalkhoven E, Kanhai DA, Uiterwaal CSPM, van der Graaf Y, Pasterkamp G, Visseren FLJ, SMART Study Group, Cardiovasc Diabetol 2014, 13, 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Dekker M, Waissi F, van Bennekom J, Silvis MJM, Timmerman N, Schoneveld AH, Grobbee DE, de Winter RJ, Mosterd A, Timmers L, de Kleijn DPV, PLoS ONE 2020, 15, e0237036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Li L, Wang H, Zhang J, Chen X, Zhang Z, Li Q, Cell Death Discov. 2021, 7, 235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Verbree-Willemsen L, Zhang Y-N, Gijsberts CM, Schoneveld AH, Wang J-W, Lam CSP, Vernooij F, Bots ML, Peelen LM, Grobbee DE, Raichlen JS, de Kleijn DPV, International Journal of Cardiology 2018, 271, 247. [DOI] [PubMed] [Google Scholar]
- [50].Loyer X, Vion A-C, Tedgui A, Boulanger CM, Circ Res 2014, 114, 345. [DOI] [PubMed] [Google Scholar]
- [51].Herrmann IK, Wood MJA, Fuhrmann G, Nat. Nanotechnol 2021, 16, 748. [DOI] [PubMed] [Google Scholar]
- [52].Zhu J, Lu K, Zhang N, Zhao Y, Ma Q, Shen J, Lin Y, Xiang P, Tang Y, Hu X, Chen J, Zhu W, Webster KA, Wang J, Yu H, Artificial Cells, Nanomedicine, and Biotechnology 2017, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Sahoo S, Klychko E, Thorne T, Misener S, Schultz KM, Millay M, Ito A, Liu T, Kamide C, Agrawal H, Perlman H, Qin G, Kishore R, Losordo DW, Circ Res 2011, 109, 724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Mathiyalagan P, Liang Y, Kim D, Misener S, Thorne T, Kamide CE, Klyachko E, Losordo DW, Hajjar R, Sahoo S, Circ Res 2017, 120, 1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Barile L, Lionetti V, Cervio E, Matteucci M, Gherghiceanu M, Popescu LM, Torre T, Siclari F, Moccetti T, Vassalli G, Cardiovasc Res 2014, 103, 530. [DOI] [PubMed] [Google Scholar]
- [56].Chen L, Wang Y, Pan Y, Zhang L, Shen C, Qin G, Ashraf M, Weintraub N, Ma G, Tang Y, Biochemical and Biophysical Research Communications 2013, 431, 566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Xiao J, Pan Y, Li XH, Yang XY, Feng YL, Tan HH, Jiang L, Feng J, Yu XY, Cell Death & Disease 2016, 7, e2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Caccioppo A, Franchin L, Grosso A, Angelini F, D’Ascenzo F, Brizzi MF, IJMS 2019, 20, 5024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].de Couto G, Gallet R, Cambier L, Jaghatspanyan E, Makkar N, Dawkins JF, Berman BP, Marbán E, Circulation 2017, 136, 200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Jansen F, Yang X, Hoelscher M, Cattelan A, Schmitz T, Proebsting S, Wenzel D, Vosen S, Franklin BS, Fleischmann BK, Nickenig G, Werner N, Circulation 2013, 128, 2026. [DOI] [PubMed] [Google Scholar]
- [61].Zernecke A, Bidzhekov K, Noels H, Shagdarsuren E, Gan L, Denecke B, Hristov M, Köppel T, Jahantigh MN, Lutgens E, Wang S, Olson EN, Schober A, Weber C, Sci. Signal 2009, 2, DOI 10.1126/scisignal.2000610. [DOI] [PubMed] [Google Scholar]
- [62].He S, Wu C, Xiao J, Li D, Sun Z, Li M, Scand J Immunol 2018, 87, e12648. [DOI] [PubMed] [Google Scholar]
- [63].Ju R, Zhuang ZW, Zhang J, Lanahan AA, Kyriakides T, Sessa WC, Simons M, Journal of Biological Chemistry 2014, 289, 510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Zhao J, Li X, Hu J, Chen F, Qiao S, Sun X, Gao L, Xie J, Xu B, Cardiovascular Research 2019, 115, 1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Zhu L-P, Tian T, Wang J-Y, He J-N, Chen T, Pan M, Xu L, Zhang H, Qiu X-T, Li C-C, Wang K-K, Shen H, Zhang G-G, Bai Y-P, Theranostics 2018, 8, 6163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Yang Y, Li Y, Chen X, Cheng X, Liao Y, Yu X, J Mol Med 2016, 94, 711. [DOI] [PubMed] [Google Scholar]
- [67].Wang Y, Zhao R, Liu W, Wang Z, Rong J, Long X, Liu Z, Ge J, Shi B, Oxidative Medicine and Cellular Longevity 2019, 2019, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Liu J, Jiang M, Deng S, Lu J, Huang H, Zhang Y, Gong P, Shen X, Ruan H, Jin M, Wang H, Molecular Therapy - Nucleic Acids 2018, 11, 103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Ribeiro-Rodrigues TM, Laundos TL, Pereira-Carvalho R, Batista-Almeida D, Pereira R, Coelho-Santos V, Silva AP, Fernandes R, Zuzarte M, Enguita FJ, Costa MC, Pinto-do-Ó P, Pinto MT, Gouveia P, Ferreira L, Mason JC, Pereira P, Kwak BR, Nascimento DS, Girão H, Cardiovascular Research 2017, 113, 1338. [DOI] [PubMed] [Google Scholar]
- [70].Vrijsen KR, Sluijter JPG, Schuchardt MWL, Van Balkom BWM, Noort WA, Chamuleau SAJ, Doevendans PAFM, Journal of Cellular and Molecular Medicine 2010, no. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Malik ZA, Kott KS, Poe AJ, Kuo T, Chen L, Ferrara KW, Knowlton AA, American Journal of Physiology-Heart and Circulatory Physiology 2013, 304, H954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Goettsch C, Hutcheson JD, Aikawa M, Iwata H, Pham T, Nykjaer A, Kjolby M, Rogers M, Michel T, Shibasaki M, Hagita S, Kramann R, Rader DJ, Libby P, Singh SA, Aikawa E, Journal of Clinical Investigation 2016, 126, 1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Zhao Y, Li Y, Luo P, Gao Y, Yang J, Lao K-H, Wang G, Cockerill G, Hu Y, Xu Q, Li T, Zeng L, Sci Rep 2016, 6, 28627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Carnino JM, Lee H, Jin Y, Respir Res 2019, 20, 240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Dickhout A, Koenen RR, Front. Cardiovasc. Med 2018, 5, 113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Liga A, Vliegenthart ADB, Oosthuyzen W, Dear JW, Kersaudy-Kerhoas M, Lab Chip 2015, 15, 2388. [DOI] [PubMed] [Google Scholar]
- [77].Théry C, Witwer KW, Aikawa E, Alcaraz MJ, Anderson JD, Andriantsitohaina R, Antoniou A, Arab T, Archer F, Atkin-Smith GK, Ayre DC, Bach J-M, Bachurski D, Baharvand H, Balaj L, Baldacchino S, Bauer NN, Baxter AA, Bebawy M, Beckham C, Bedina Zavec A, Benmoussa A, Berardi AC, Bergese P, Bielska E, Blenkiron C, Bobis-Wozowicz S, Boilard E, Boireau W, Bongiovanni A, Borràs FE, Bosch S, Boulanger CM, Breakefield X, Breglio AM, Brennan MÁ, Brigstock DR, Brisson A, Broekman ML, Bromberg JF, Bryl-Górecka P, Buch S, Buck AH, Burger D, Busatto S, Buschmann D, Bussolati B, Buzás EI, Byrd JB, Camussi G, Carter DR, Caruso S, Chamley LW, Chang Y-T, Chen C, Chen S, Cheng L, Chin AR, Clayton A, Clerici SP, Cocks A, Cocucci E, Coffey RJ, Cordeiro-da-Silva A, Couch Y, Coumans FA, Coyle B, Crescitelli R, Criado MF, D’Souza-Schorey C, Das S, Datta Chaudhuri A, de Candia P, De Santana EF, De Wever O, del Portillo HA, Demaret T, Deville S, Devitt A, Dhondt B, Di Vizio D, Dieterich LC, Dolo V, Dominguez Rubio AP, Dominici M, Dourado MR, Driedonks TA, Duarte FV, Duncan HM, Eichenberger RM, Ekström K, EL Andaloussi S, Elie-Caille C, Erdbrügger U, Falcón-Pérez JM, Fatima F, Fish JE, Flores-Bellver M, Försönits A, Frelet-Barrand A, Fricke F, Fuhrmann G, Gabrielsson S, Gámez-Valero A, Gardiner C, Gärtner K, Gaudin R, Gho YS, Giebel B, Gilbert C, Gimona M, Giusti I, Goberdhan DC, Görgens A, Gorski SM, Greening DW, Gross JC, Gualerzi A, Gupta GN, Gustafson D, Handberg A, Haraszti RA, Harrison P, Hegyesi H, Hendrix A, Hill AF, Hochberg FH, Hoffmann KF, Holder B, Holthofer H, Hosseinkhani B, Hu G, Huang Y, Huber V, Hunt S, Ibrahim AG-E, Ikezu T, Inal JM, Isin M, Ivanova A, Jackson HK, Jacobsen S, Jay SM, Jayachandran M, Jenster G, Jiang L, Johnson SM, Jones JC, Jong A, Jovanovic-Talisman T, Jung S, Kalluri R, Kano S, Kaur S, Kawamura Y, Keller ET, Khamari D, Khomyakova E, Khvorova A, Kierulf P, Kim KP, Kislinger T, Klingeborn M, Klinke DJ, Kornek M, Kosanović MM, Kovács ÁF, Krämer-Albers E-M, Krasemann S, Krause M, Kurochkin IV, Kusuma GD, Kuypers S, Laitinen S, Langevin SM, Languino LR, Lannigan J, Lässer C, Laurent LC, Lavieu G, Lázaro-Ibáñez E, Le Lay S, Lee M-S, Lee YXF, Lemos DS, Lenassi M, Leszczynska A, Li IT, Liao K, Libregts SF, Ligeti E, Lim R, Lim SK, Linē A, Linnemannstöns K, Llorente A, Lombard CA, Lorenowicz MJ, Lörincz ÁM, Lötvall J, Lovett J, Lowry MC, Loyer X, Lu Q, Lukomska B, Lunavat TR, Maas SL, Malhi H, Marcilla A, Mariani J, Mariscal J, Martens-Uzunova ES, Martin-Jaular L, Martinez MC, Martins VR, Mathieu M, Mathivanan S, Maugeri M, McGinnis LK, McVey MJ, Meckes DG, Meehan KL, Mertens I, Minciacchi VR, Möller A, Møller Jørgensen M, Morales-Kastresana A, Morhayim J, Mullier F, Muraca M, Musante L, Mussack V, Muth DC, Myburgh KH, Najrana T, Nawaz M, Nazarenko I, Nejsum P, Neri C, Neri T, Nieuwland R, Nimrichter L, Nolan JP, Nolte-’t Hoen EN, Noren Hooten N, O’Driscoll L, O’Grady T, O’Loghlen A, Ochiya T, Olivier M, Ortiz A, Ortiz LA, Osteikoetxea X, Østergaard O, Ostrowski M, Park J, Pegtel DM, Peinado H, Perut F, Pfaffl MW, Phinney DG, Pieters BC, Pink RC, Pisetsky DS, Pogge von Strandmann E, Polakovicova I, Poon IK, Powell BH, Prada I, Pulliam L, Quesenberry P, Radeghieri A, Raffai RL, Raimondo S, Rak J, Ramirez MI, Raposo G, Rayyan MS, Regev-Rudzki N, Ricklefs FL, Robbins PD, Roberts DD, Rodrigues SC, Rohde E, Rome S, Rouschop KM, Rughetti A, Russell AE, Saá P, Sahoo S, Salas-Huenuleo E, Sánchez C, Saugstad JA, Saul MJ, Schiffelers RM, Schneider R, Schøyen TH, Scott A, Shahaj E, Sharma S, Shatnyeva O, Shekari F, Shelke GV, Shetty AK, Shiba K, Siljander PR-M, Silva AM, Skowronek A, Snyder OL, Soares RP, Sódar BW, Soekmadji C, Sotillo J, Stahl PD, Stoorvogel W, Stott SL, Strasser EF, Swift S, Tahara H, Tewari M, Timms K, Tiwari S, Tixeira R, Tkach M, Toh WS, Tomasini R, Torrecilhas AC, Tosar JP, Toxavidis V, Urbanelli L, Vader P, van Balkom BW, van der Grein SG, Van Deun J, van Herwijnen MJ, Van Keuren-Jensen K, van Niel G, van Royen ME, van Wijnen AJ, Vasconcelos MH, Vechetti IJ, Veit TD, Vella LJ, Velot É, Verweij FJ, Vestad B, Viñas JL, Visnovitz T, Vukman KV, Wahlgren J, Watson DC, Wauben MH, Weaver A, Webber JP, Weber V, Wehman AM, Weiss DJ, Welsh JA, Wendt S, Wheelock AM, Wiener Z, Witte L, Wolfram J, Xagorari A, Xander P, Xu J, Yan X, Yáñez-Mó M, Yin H, Yuana Y, Zappulli V, Zarubova J, Žėkas V, Zhang J, Zhao Z, Zheng L, Zheutlin AR, Zickler AM, Zimmermann P, Zivkovic AM, Zocco D, Zuba-Surma EK, Journal of Extracellular Vesicles 2018, 7, 1535750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Wiklander OPB, Nordin JZ, O’Loughlin A, Gustafsson Y, Corso G, Mäger I, Vader P, Lee Y, Sork H, Seow Y, Heldring N, Alvarez-Erviti L, Smith CE, Le Blanc K, Macchiarini P, Jungebluth P, Wood MJA, Andaloussi SE, Journal of Extracellular Vesicles 2015, 4, 26316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Luan X, Sansanaphongpricha K, Myers I, Chen H, Yuan H, Sun D, Acta Pharmacologica Sinica 2017, 38, 754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Mathieu M, Martin-Jaular L, Lavieu G, Théry C, Nat Cell Biol 2019, 21, 9. [DOI] [PubMed] [Google Scholar]
- [81].Pitt JM, Kroemer G, Zitvogel L, Journal of Clinical Investigation 2016, 126, 1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].El-Andaloussi S, Lee Y, Lakhal-Littleton S, Li J, Seow Y, Gardiner C, Alvarez-Erviti L, Sargent IL, Wood MJA, Nat Protoc 2012, 7, 2112. [DOI] [PubMed] [Google Scholar]
- [83].Cheng H, Chang S, Xu R, Chen L, Song X, Wu J, Qian J, Zou Y, Ma J, Stem Cell Res Ther 2020, 11, 224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Zhang L, Wei Q, Liu X, Zhang T, Wang S, Zhou L, Zou L, Fan F, Chi H, Sun J, Wang D, International Immunopharmacology 2021, 101, 107592. [DOI] [PubMed] [Google Scholar]
- [85].Wu Q, Wang J, Tan WLW, Jiang Y, Wang S, Li Q, Yu X, Tan J, Liu S, Zhang P, Tiang Z, Chen Z, Foo RS-Y, Yang H-T, Cell Death Dis 2020, 11, 354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Gray WD, French KM, Ghosh-Choudhary S, Maxwell JT, Brown ME, Platt MO, Searles CD, Davis ME, Circ Res 2015, 116, 255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].English K, Barry FP, Field-Corbett CP, Mahon BP, Immunology Letters 2007, 110, 91. [DOI] [PubMed] [Google Scholar]
- [88].Magne B, Dedier M, Nivet M, Coulomb B, Banzet S, Lataillade J-J, Trouillas M, Journal of Investigative Dermatology 2020, 140, 688. [DOI] [PubMed] [Google Scholar]
- [89].Zhang Q, Fu L, Liang Y, Guo Z, Wang L, Ma C, Wang H, J Cell Physiol 2018, 233, 6832. [DOI] [PubMed] [Google Scholar]
- [90].Xu R, Zhang F, Chai R, Zhou W, Hu M, Liu B, Chen X, Liu M, Xu Q, Liu N, Liu S, J Cell Mol Med 2019, 23, 7617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Ti D, Hao H, Tong C, Liu J, Dong L, Zheng J, Zhao Y, Liu H, Fu X, Han W, J Transl Med 2015, 13, 308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Yang R, Huang H, Cui S, Zhou Y, Zhang T, Zhou Y, Cell Death Dis 2020, 11, 603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Beez CM, Haag M, Klein O, Van Linthout S, Sittinger M, Seifert M, J Nanobiotechnol 2019, 17, 72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Huang P, Wang L, Li Q, Tian X, Xu J, Xu J, Xiong Y, Chen G, Qian H, Jin C, Yu Y, Cheng K, Qian L, Yang Y, Cardiovasc Res 2020, 116, 353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Sung DK, Chang YS, Sung SI, Ahn SY, Park WS, J Clin Med 2019, 8, E533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Gong X, Liu H, Wang S, Liang S, Wang G, J Cell Physiol 2019, 234, 13878. [DOI] [PubMed] [Google Scholar]
- [97].Penn MS, Pastore J, Miller T, Aras R, Gene Ther 2012, 19, 583. [DOI] [PubMed] [Google Scholar]
- [98].Ni J, Liu X, Yin Y, Zhang P, Xu Y-W, Liu Z, Oxidative Medicine and Cellular Longevity 2019, 2019, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Wei Z, Qiao S, Zhao J, Liu Y, Li Q, Wei Z, Dai Q, Kang L, Xu B, Life Sciences 2019, 232, 116632. [DOI] [PubMed] [Google Scholar]
- [100].Sun D, Zhuang X, Xiang X, Liu Y, Zhang S, Liu C, Barnes S, Grizzle W, Miller D, Zhang H-G, Molecular Therapy 2010, 18, 1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Sato YT, Umezaki K, Sawada S, Mukai S, Sasaki Y, Harada N, Shiku H, Akiyoshi K, Scientific Reports 2016, 6, 21933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Gangadaran P, Ahn B-C, Pharmaceutics 2020, 12, 442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Podolak I, Galanty A, Sobolewska D, Phytochem Rev 2010, 9, 425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Fuhrmann G, Serio A, Mazo M, Nair R, Stevens MM, J Control Release 2015, 205, 35. [DOI] [PubMed] [Google Scholar]
- [105].Ahmad W, Shetab Boushehri MA, Lamprecht A, Journal of Colloid and Interface Science 2021, 596, 500. [DOI] [PubMed] [Google Scholar]
- [106].Lamichhane TN, Jeyaram A, Patel DB, Parajuli B, Livingston NK, Arumugasaamy N, Schardt JS, Jay SM, Cell Mol Bioeng 2016, 9, 315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Haney MJ, Klyachko NL, Harrison EB, Zhao Y, Kabanov AV, Batrakova EV, Adv. Healthcare Mater 2019, 8, 1801271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Kim MS, Haney MJ, Zhao Y, Mahajan V, Deygen I, Klyachko NL, Inskoe E, Piroyan A, Sokolsky M, Okolie O, Hingtgen SD, Kabanov AV, Batrakova EV, Nanomedicine 2016, 12, 655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Lamichhane TN, Raiker RS, Jay SM, Mol. Pharmaceutics 2015, 12, 3650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Ma T, Chen Y, Chen Y, Meng Q, Sun J, Shao L, Yu Y, Huang H, Hu Y, Yang Z, Yang J, Shen Z, Stem Cells International 2018, 2018, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Wahlgren J, Karlson TDL, Brisslert M, Vaziri Sani F, Telemo E, Sunnerhagen P, Valadi H, Nucleic Acids Research 2012, 40, e130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Yang Z, Shi J, Xie J, Wang Y, Sun J, Liu T, Zhao Y, Zhao X, Wang X, Ma Y, Malkoc V, Chiang C, Deng W, Chen Y, Fu Y, Kwak KJ, Fan Y, Kang C, Yin C, Rhee J, Bertani P, Otero J, Lu W, Yun K, Lee AS, Jiang W, Teng L, Kim BYS, Lee LJ, Nat Biomed Eng 2020, 4, 69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Johnsen KB, Gudbergsson JM, Skov MN, Christiansen G, Gurevich L, Moos T, Duroux M, Cytotechnology 2016, 68, 2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Pascucci L, Coccè V, Bonomi A, Ami D, Ceccarelli P, Ciusani E, Viganò L, Locatelli A, Sisto F, Doglia SM, Parati E, Bernardo ME, Muraca M, Alessandri G, Bondiolotti G, Pessina A, Journal of Controlled Release 2014, 192, 262. [DOI] [PubMed] [Google Scholar]
- [115].Kalinec GM, Gao L, Cohn W, Whitelegge JP, Faull KF, Kalinec F, Front. Cell. Neurosci 2019, 13, 530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Haney MJ, Klyachko NL, Zhao Y, Gupta R, Plotnikova EG, He Z, Patel T, Piroyan A, Sokolsky M, Kabanov AV, Batrakova EV, J Control Release 2015, 207, 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Fuhrmann G, Chandrawati R, Parmar PA, Keane TJ, Maynard SA, Bertazzo S, Stevens MM, Adv. Mater 2018, 30, 1706616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Park O, Choi ES, Yu G, Kim JY, Kang YY, Jung H, Mok H, Macromol. Biosci 2018, 18, 1800301. [DOI] [PubMed] [Google Scholar]
- [119].Jhan Y-Y, Prasca-Chamorro D, Palou Zuniga G, Moore DM, Arun Kumar S, Gaharwar AK, Bishop CJ, International Journal of Pharmaceutics 2020, 573, 118802. [DOI] [PubMed] [Google Scholar]
- [120].Tian Y, Li S, Song J, Ji T, Zhu M, Anderson GJ, Wei J, Nie G, Biomaterials 2014, 35, 2383. [DOI] [PubMed] [Google Scholar]
- [121].Naseri Z, Kazemi Oskuee R, Jaafari MR, Forouzandeh M, IJN 2018, Volume 13, 7727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Jeyaram A, Lamichhane TN, Wang S, Zou L, Dahal E, Kronstadt SM, Levy D, Parajuli B, Knudsen DR, Chao W, Jay SM, Molecular Therapy 2020, 28, 975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Tran PHL, Wang T, Yin W, Tran TTD, Nguyen TNG, Lee B-J, Duan W, International Journal of Pharmaceutics 2019, 572, 118786. [DOI] [PubMed] [Google Scholar]
- [124].Murphy DE, de Jong OG, Evers MJW, Nurazizah M, Schiffelers RM, Vader P, Nano Lett. 2021, 21, 1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Kooijmans SAA, Fliervoet LAL, van der Meel R, Fens MHAM, Heijnen HFG, van Bergen en Henegouwen PMP, Vader P, Schiffelers RM, Journal of Controlled Release 2016, 224, 77. [DOI] [PubMed] [Google Scholar]
- [126].Zhu D, Li Z, Huang K, Caranasos TG, Rossi JS, Cheng K, Nat Commun 2021, 12, 1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Immordino ML, Dosio F, Cattel L, Int J Nanomedicine 2006, 1, 297. [PMC free article] [PubMed] [Google Scholar]
- [128].Riau AK, Ong HS, Yam GHF, Mehta JS, Front. Pharmacol 2019, 10, 1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Kim H, Yun N, Mun D, Kang J-Y, Lee S-H, Park H, Park H, Joung B, Biochemical and Biophysical Research Communications 2018, 499, 803. [DOI] [PubMed] [Google Scholar]
- [130].Mentkowski KI, Lang JK, Sci Rep 2019, 9, 10041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Wang X, Chen Y, Zhao Z, Meng Q, Yu Y, Sun J, Yang Z, Chen Y, Li J, Ma T, Liu H, Li Z, Yang J, Shen Z, JAHA 2018, 7, DOI 10.1161/JAHA.118.008737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Kanki S, Jaalouk DE, Lee S, Yu AYC, Gannon J, Lee RT, Journal of Molecular and Cellular Cardiology 2011, 50, 841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Ohno S, Takanashi M, Sudo K, Ueda S, Ishikawa A, Matsuyama N, Fujita K, Mizutani T, Ohgi T, Ochiya T, Gotoh N, Kuroda M, Molecular Therapy 2013, 21, 185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Zeelenberg IS, Ostrowski M, Krumeich S, Bobrie A, Jancic C, Boissonnas A, Delcayre A, Le Pecq J-B, Combadière B, Amigorena S, Théry C, Cancer Res 2008, 68, 1228. [DOI] [PubMed] [Google Scholar]
- [135].Hung ME, Leonard JN, J Biol Chem 2015, 290, 8166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Tian T, Zhang H-X, He C-P, Fan S, Zhu Y-L, Qi C, Huang N-P, Xiao Z-D, Lu Z-H, Tannous BA, Gao J, Biomaterials 2018, 150, 137. [DOI] [PubMed] [Google Scholar]
- [137].Nakase I, Noguchi K, Aoki A, Takatani-Nakase T, Fujii I, Futaki S, Sci Rep 2017, 7, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Antes TJ, Middleton RC, Luther KM, Ijichi T, Peck KA, Liu WJ, Valle J, Echavez AK, Marbán E, J Nanobiotechnol 2018, 16, 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Vandergriff A, Huang K, Shen D, Hu S, Hensley MT, Caranasos TG, Qian L, Cheng K, Theranostics 2018, 8, 1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Evers MJW, Wakker SI, Groot EM, Jong OG, Gitz-François JJJ, Seinen CS, Sluijter JPG, Schiffelers RM, Vader P, Adv Healthcare Materials 2022, 11, 2101202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Piffoux M, Silva AKA, Wilhelm C, Gazeau F, Tareste D, ACS Nano 2018, 12, 6830. [DOI] [PubMed] [Google Scholar]
- [142].Sanchez L, Yi Y, Yu Y, Nanoscale 2017, 9, 288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Tang J, Su T, Huang K, Dinh P-U, Wang Z, Vandergriff A, Hensley MT, Cores J, Allen T, Li T, Sproul E, Mihalko E, Lobo LJ, Ruterbories L, Lynch A, Brown A, Caranasos TG, Shen D, Stouffer GA, Gu Z, Zhang J, Cheng K, Nature Biomedical Engineering 2018, 2, 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Zhang N, Song Y, Huang Z, Chen J, Tan H, Yang H, Fan M, Li Q, Wang Q, Gao J, Pang Z, Qian J, Ge J, Biomaterials 2020, 255, 120168. [DOI] [PubMed] [Google Scholar]
- [145].Gallet R, Dawkins J, Valle J, Simsolo E, de Couto G, Middleton R, Tseliou E, Luthringer D, Kreke M, Smith RR, Marbán L, Ghaleh B, Marbán E, Eur Heart J 2016, ehw240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Chen P, Wang L, Fan X, Ning X, Yu B, Ou C, Chen M, Theranostics 2021, 11, 2263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Li J, Mooney DJ, Nat Rev Mater 2016, 1, 16071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Liu B, Lee BW, Nakanishi K, Villasante A, Williamson R, Metz J, Kim J, Kanai M, Bi L, Brown K, Di Paolo G, Homma S, Sims PA, Topkara VK, Vunjak-Novakovic G, Nature Biomedical Engineering 2018, 2, 293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Liu Y, Cui J, Wang H, Hezam K, Zhao X, Huang H, Chen S, Han Z, Han Z-C, Guo Z, Li Z, Stem Cell Res Ther 2020, 11, 161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Chen CW, Wang LL, Zaman S, Gordon J, Arisi MF, Venkataraman CM, Chung JJ, Hung G, Gaffey AC, Spruce LA, Fazelinia H, Gorman RC, Seeholzer SH, Burdick JA, Atluri P, Cardiovascular Research 2018, 114, 1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Lv K, Li Q, Zhang L, Wang Y, Zhong Z, Zhao J, Lin X, Wang J, Zhu K, Xiao C, Ke C, Zhong S, Wu X, Chen J, Yu H, Zhu W, Li X, Wang B, Tang R, Wang J, Huang J, Hu X, Theranostics 2019, 9, 7403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Zhang K, Zhao X, Chen X, Wei Y, Du W, Wang Y, Liu L, Zhao W, Han Z, Kong D, Zhao Q, Guo Z, Han Z, Liu N, Ma F, Li Z, ACS Appl. Mater. Interfaces 2018, 10, 30081. [DOI] [PubMed] [Google Scholar]
- [153].Han C, Zhou J, Liu B, Liang C, Pan X, Zhang Y, Zhang Y, Wang Y, Shao L, Zhu B, Wang J, Yin Q, Yu X-Y, Li Y, Materials Science and Engineering: C 2019, 99, 322. [DOI] [PubMed] [Google Scholar]
- [154].Fu K, Wu H, Su Z, Biotechnology Advances 2021, 49, 107752. [DOI] [PubMed] [Google Scholar]
- [155].Han C, Zhou J, Liang C, Liu B, Pan X, Zhang Y, Wang Y, Yan B, Xie W, Liu F, Yu X-Y, Li Y, Biomater. Sci 2019, 7, 2920. [DOI] [PubMed] [Google Scholar]
- [156].Tang J, Cui X, Zhang Z, Xu Y, Guo J, Soliman BG, Lu Y, Qin Z, Wang Q, Zhang H, Lim KS, Woodfield TBF, Zhang J, Adv Healthcare Materials 2022, 11, 2100312. [DOI] [PubMed] [Google Scholar]
- [157].Diomede F, Gugliandolo A, Cardelli P, Merciaro I, Ettorre V, Traini T, Bedini R, Scionti D, Bramanti A, Nanci A, Caputi S, Fontana A, Mazzon E, Trubiani O, Stem Cell Res Ther 2018, 9, 104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Bari E, Scocozza F, Perteghella S, Sorlini M, Auricchio F, Torre ML, Conti M, Pharmaceutics 2021, 13, 515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Chen P, Zheng L, Wang Y, Tao M, Xie Z, Xia C, Gu C, Chen J, Qiu P, Mei S, Ning L, Shi Y, Fang C, Fan S, Lin X, Theranostics 2019, 9, 2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Lener T, Gimona M, Aigner L, Börger V, Buzas E, Camussi G, Chaput N, Chatterjee D, Court FA, del Portillo HA, O’Driscoll L, Fais S, Falcon-Perez JM, Felderhoff-Mueser U, Fraile L, Gho YS, Görgens A, Gupta RC, Hendrix A, Hermann DM, Hill AF, Hochberg F, Horn PA, de Kleijn D, Kordelas L, Kramer BW, Krämer-Albers E-M, Laner-Plamberger S, Laitinen S, Leonardi T, Lorenowicz MJ, Lim SK, Lötvall J, Maguire CA, Marcilla A, Nazarenko I, Ochiya T, Patel T, Pedersen S, Pocsfalvi G, Pluchino S, Quesenberry P, Reischl IG, Rivera FJ, Sanzenbacher R, Schallmoser K, Slaper-Cortenbach I, Strunk D, Tonn T, Vader P, van Balkom BWM, Wauben M, Andaloussi SE, Théry C, Rohde E, Giebel B, J Extracell Vesicles 2015, 4, DOI 10.3402/jev.v4.30087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Pashneh-Tala S, MacNeil S, Claeyssens F, Tissue Engineering Part B: Reviews 2016, 22, 68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Wei Y, Wu Y, Zhao R, Zhang K, Midgley AC, Kong D, Li Z, Zhao Q, Biomaterials 2019, 204, 13. [DOI] [PubMed] [Google Scholar]
- [163].Johnson RC, Leopold JA, Loscalzo J, Circulation Research 2006, 99, 1044. [DOI] [PubMed] [Google Scholar]
- [164].Cunnane EM, Lorentz KL, Ramaswamy AK, Gupta P, Mandal BB, O’Brien FJ, Weinbaum JS, Vorp DA, ACS Appl. Mater. Interfaces 2020, 12, 26955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Joner M, Finn AV, Farb A, Mont EK, Kolodgie FD, Ladich E, Kutys R, Skorija K, Gold HK, Virmani R, J. Am. Coll. Cardiol 2006, 48, 193. [DOI] [PubMed] [Google Scholar]
- [166].Hou Y, Li J, Zhu S, Cao C, Tang J, Zhang J, Guan S, Colloids and Surfaces B: Biointerfaces 2020, 189, 110831. [DOI] [PubMed] [Google Scholar]
- [167].Hu S, Li Z, Shen D, Zhu D, Huang K, Su T, Dinh P-U, Cores J, Cheng K, Nat Biomed Eng 2021, 5, 1174. [DOI] [PMC free article] [PubMed] [Google Scholar]