Abstract
Sarcopenia has been established as a predictor of poor outcomes in various clinical settings. It is particularly prevalent in heart failure, a clinical syndrome that poses significant challenges to healthcare worldwide. Despite this, sarcopenia remains overlooked and undertreated in cardiology practice. Understanding the currently proposed diagnostic process is paramount for the early detection and treatment of sarcopenia to mitigate downstream adverse health outcomes.
Subject Terms: Sarcopenia, Heart Failure, Aging, Diagnostic Testing, Nutrition, Exercise
Introduction
Heart failure (HF) is a clinical syndrome that poses significant challenges to healthcare worldwide. The prevalence of HF increases dramatically with age, particularly with more effective therapeutics having augmented life expectancy in these patients.1 The World Health Organization estimated in 2019 that the number of people over the age of 60 will grow by 56% in 2030; additionally, they have identified muscle mass as a critical component of well-being in the elderly.2
Sarcopenia, originating from the Greek words Sarx and penia, translates to “loss of flesh” and refers to a reduction in muscle mass and strength.3 Primary sarcopenia refers to an age-related process without any evident secondary cause. Secondary sarcopenia, sometimes referred to as compound sarcopenia, is due to other causes with or without aging, and it is particularly prevalent in individuals with cardiovascular disease (31%) in addition to other age-associated diseases such as diabetes mellitus (31%), respiratory disease (27%), and dementia (26%).4 The prevalence of sarcopenia in HF has been shown to be 20% higher than in healthy subjects of the same age.5 Recent definitions further classify sarcopenia into acute (<6 months) and chronic (≥6 months), with acute sarcopenia resulting from acute illness or injury.6
Sarcopenia is associated with difficulties with simple daily activities such as walking or standing from a chair, leading to functional decline, physical disability, and subsequent morbidity and mortality in the elderly, thus making it a predictor of poor outcomes in different clinical settings.7,8 It also imposes high economic costs, doubling the odds of hospitalization in persons with sarcopenia, with the hospitalization costs having been estimated at $40 billion per year in the United States.9
Wasting Continuum in Heart Failure
Sarcopenia and cachexia are separate clinical entities with important distinctions despite their overlap. Sarcopenia, initially described as age-related muscle loss, is now recognized as a skeletal muscle disorder characterized by a loss of muscle strength with concomitant loss of muscle mass and function.8 Cachexia is a multifactorial syndrome characterized by an underlying disease causing the loss of various tissues (mostly fat and muscle), which leads to severe involuntary weight loss.1 They are both highly prevalent with a wide overlap, and the “wasting continuum in HF” suggests that sarcopenia precedes cachexia since skeletal muscle is typically lost before fat tissue (Figure 1).10 Malnutrition is a clinical syndrome of deficient or excess nutrient intake, imbalance of essential nutrients, or impaired nutrient utilization.11 It contributes to both sarcopenia and cachexia given the compensatory reduction in lean mass seen with protein-poor diets, further worsening outcomes in those suffering from a combination of these disorders.12 Frailty is a geriatric syndrome that has significant overlap with sarcopenia, but it goes beyond physical factors to encompass cognitive, psychological, and social dimensions.7
Figure 1.
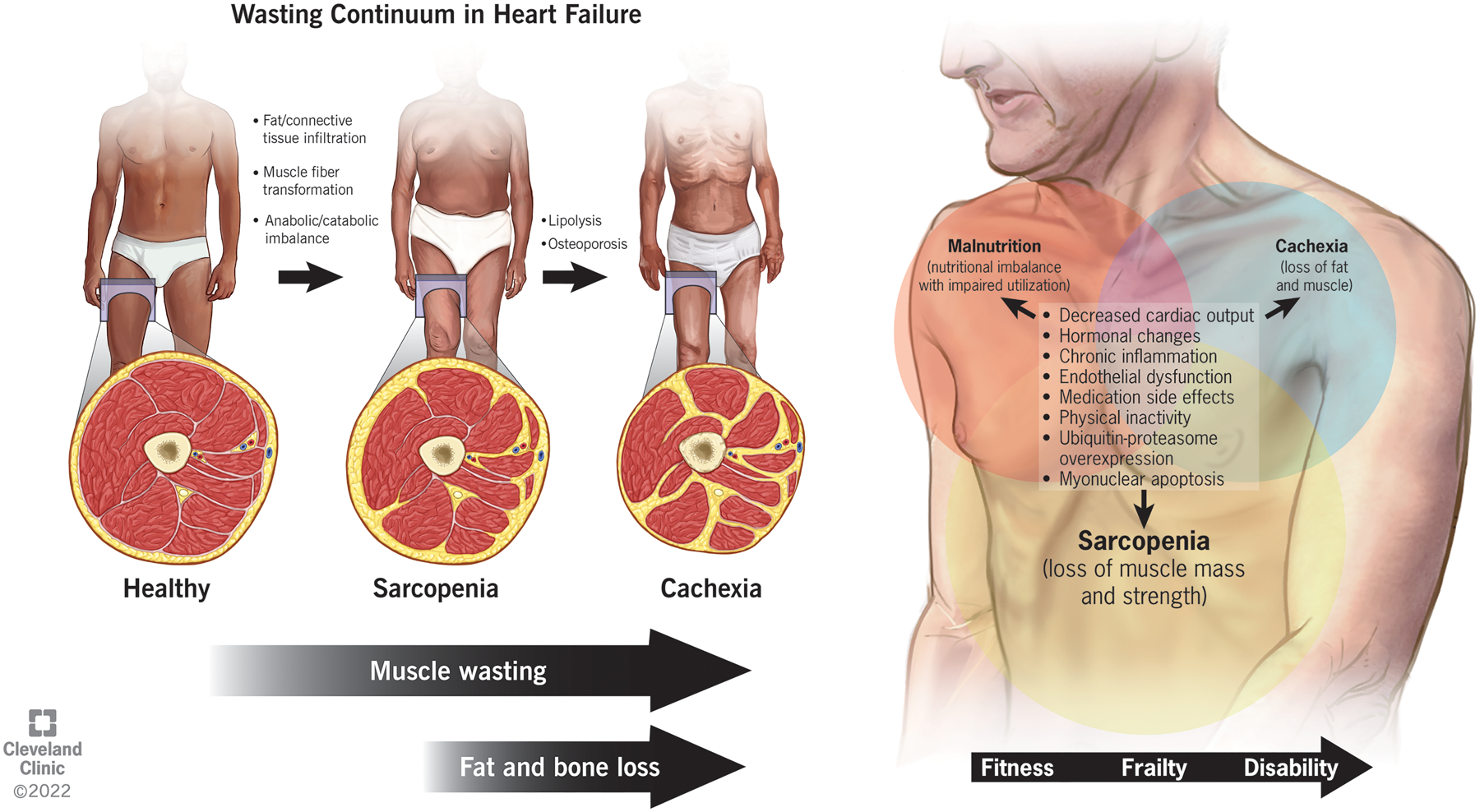
The wasting continuum in heart failure entails the loss of muscle quantity and quality preceding the loss of adipose tissue and bone mass. There is significant overlap between the pathogenesis of sarcopenia, cachexia, and malnutrition in heart failure with a gradual decline in functional status from fitness to frailty and finally disability without appropriate intervention.
The pathogenesis of sarcopenia and cachexia is multifactorial, with cachexia involving a hypermetabolic state and greater systemic inflammation than sarcopenia (Figure 1).13 The mechanism of HF-associated muscle wasting, a form of secondary sarcopenia, involves hormonal changes, malnutrition as a side effect of drugs, chronic low-level inflammation and oxidative stress, ubiquitin-proteasome system overexpression, and myonuclear apoptosis.14 The hemodynamic changes additionally lead to poor cardiorespiratory fitness leading to physical inactivity, low muscle blood flow from the decreased cardiac output, endothelial dysfunction, and malabsorption from gut edema.1 Fat loss occurs later in the course of HF with a higher prevalence in right than left ventricular dysfunction.15 The mechanism of HF-associated fat wasting has been less extensively studied, with speculations that it can be attributed to natriuretic peptides, proinflammatory cytokines, and catecholamines.10 Natriuretic peptides specifically induce lipolysis through the release of adiponectin, the adipose tissue-derived factor that promotes weight loss.
Of additional note, inverse to the effect of HF on sarcopenia, sarcopenia has been hypothesized to play a role in the severity of HF with preserved ejection fraction (HFpEF); exercise intolerance, a hallmark of HFpEF, has been improved with physical training, whereas drug trials have not shown such improvement.16,17 A Studies Investigating Comorbidities Aggravating Heart Failure (SICA-HF) sub-study also showed a strict relationship between HFpEF and sarcopenia given higher E/e1 values (>15), and thus higher left ventricular pressures, in these patients.18,19 Previous studies by Beyer et al. have shown an association between reduced skeletal muscle strength and increased ventricular mass.20
Initial Screening of Sarcopenia
Early sarcopenia diagnosis is essential for treatment and prevention of downstream adverse health outcomes; however, this is not routinely performed in clinical practice or endorsed by any HF guidelines to date. Unlike cachexia which can be diagnosed through clinical history and non-edematous weight loss of more than 6% in ≤12 months, the diagnosis of sarcopenia is more complex.7 Although prior definitions of sarcopenia focused on muscle mass only, the current consensus definition by the European Working Group on Sarcopenia in Older People 2 (EWGSOP2) requires the presence of both low muscle mass and function. Evaluation for sarcopenia begins when a patient reports symptoms of sarcopenia, such as weakness, slow ambulation, difficulty rising from a chair or climbing stairs, or falls (Figure 2).6 The definition by EWGSOP2 recommends initiating sarcopenia evaluation by screening using the Strength, Assistance with walking, Rising from chair, Climbing stairs, and Falls (SARC-F) questionnaire.6
Figure 2.
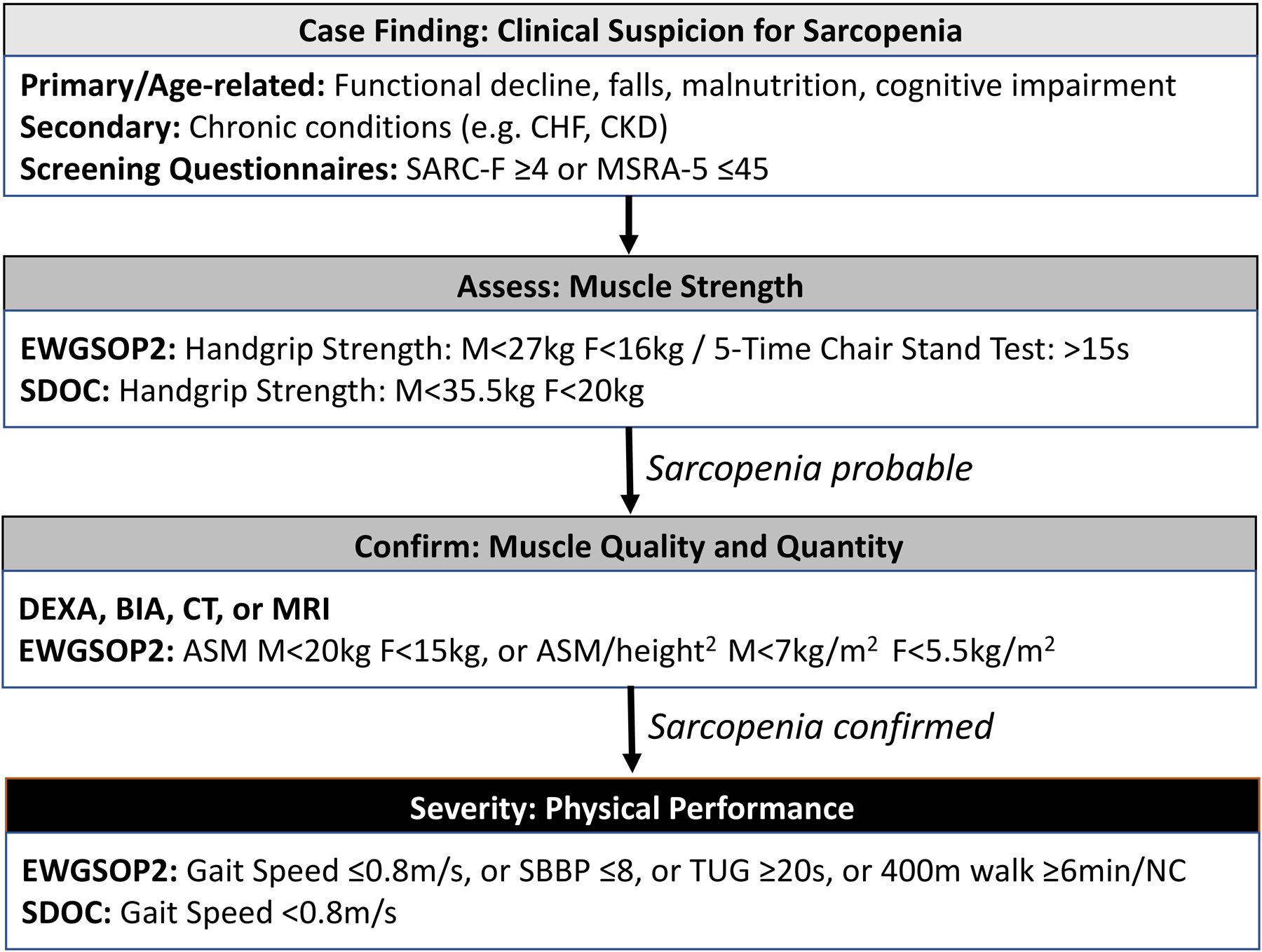
Algorithm for the diagnosis and grading of sarcopenia in clinical practice, adapted from the consensus definitions by the European Working Group on Sarcopenia in Older People 2 (EWGSOP2) in 2018, the Asian Working Group for Sarcopenia (AWGS) in 2019, and the Sarcopenia Definition and Outcomes Consortium (SDOC) in 2020. SARC-F, Strength, Assistance with walking, Rising from chair, Climbing stairs, and Falls; MSRA, Mini Sarcopenia Risk Assessment; CT, computed tomography; MRI, magnetic resonance imaging; DEXA, dual-energy x-ray absorptiometry; BIA, bioelectrical impedance analysis; US, ultrasound; SPPB, short physical performance battery; TUG, timed-up-and-go.
SARC-F examines the patient’s own perspective of the five domains comprising its name with a score of ≥4 indicating probable sarcopenia.21 A shorter version, SARC-F-3, has been proposed by Woo et al. and examines three domains (strength, stair climbing, and walking) with a score of ≥2 screening positive.22 Compared to the Asian Working Group for Sarcopenia (AWGS) criteria,23 the SARC-F has been shown to have low sensitivity (29.5%) with high specificity (98.1%); it outperforms the SARC-F-3 due to higher sensitivity (29.5% vs. 13.1%), but such low sensitivity overall indicates that primarily severe cases will be detected.24 To overcome such limitations, SARC-F with calf measurements using a measuring tape (SARC-CalF) has also been proposed to enhance sensitivity and allow for more diagnostic accuracy.25
The Mini Sarcopenia Risk Assessment (MSRA) has alternatively been used as a screening questionnaire. It was developed by Rossi et al. with the first version having 7-items (age, hospitalization in the preceding year, level of activity, regularity of meals, daily dairy consumption, daily calorie consumption, and weight loss ≥2 kg in the preceding year), and the second version having 5-items (omitting dairy and calorie consumption);26 a score of 30 and 45 or less on MSRA-7 and MSRA-5, respectively, indicates sarcopenia.25 Compared to the EWGSOP criteria, the MSRA-7 has a sensitivity and specificity of 80.4% and 50.5%, and the MSRA-5 has a sensitivity and specificity of 80.4% and 60.4%, respectively.25 Using AWGS criteria, the sensitivity and specificity of SARC-F, MSRA-7, and MSRA-5 have been compared and shown to be 29.5% and 98.1%, 86.9% and 39.6%, and 90.2% and 70.6%, respectively.25 Thus, MSRA-5 has better sensitivity, but SARC-F has better specificity. It should be noted that these questionnaires must be answered based on symptomatology in compensated HF, given the potential for symptom overlap of chronic sarcopenia and acute decompensated HF.
A more formal but more complex tool, the Ishii screening test, has also been developed which uses age, grip strength, and calf circumference to estimate the probability of sarcopenia with a sensitivity of 75.5–84.9% and specificity of 88.2–92.0% when validated against EWGSOP criteria.3,25,27 On the opposite spectrum, Yu et al. attempted to develop a prediction equation to estimate low muscle mass in those 65 or older using weight, BMI, and sex.28 This equation was compared to DEXA measurements with low sensitivity of 60% in men and 46% in women but high specificity above 85% in both sexes. It was then combined with grip strength to screen for sarcopenia, maintaining comparable sensitivity of 58% in men while improving sensitivity to 57% in women with specificity above 90% in both sexes;28 overall, this suggests their anthropometric prediction equation may be a good “rule-out” test.25
Evaluation of Muscle Strength
If sarcopenia is suspected on screening, the next recommended step is assessing muscle strength as the primary parameter of sarcopenia (Figure 2).6,29 Measuring grip strength, typically using the Jamar dynamometer, is advised for routine muscle strength measurement in the hospital and community settings due to its ease of use and low cost (Table 1).6 Low grip strength correlates moderately with weakness in other muscle compartments and is a powerful predictor of poor patient outcomes, including all-cause death, cardiovascular death, and cardiovascular disease, per the 2015 Prospective Urban Rural Epidemiology (PURE) study.6,30 For patients in whom measuring grip strength is not possible, such as those with advanced arthritis or stroke, alternate options exist, including isometric torque methods of lower limb strength and chair stand testing.6 The chair stand, or rise, test specifically checks the strength of the quadriceps muscle by timing the patient’s rise five times from a seated position without using the arms for assistance.6 Current definitions recommend initiation of intervention for sarcopenia once it is deemed probable based on strength testing alone.6
Table 1.
Currently recommended cutoff points for sarcopenia based on the consensus definitions by the European Working Group on Sarcopenia in Older People 2 (EWGSOP2) in 2018, the Asian Working Group for Sarcopenia (AWGS) in 2019, and the Sarcopenia Definition and Outcomes Consortium (SDOC) in 2020. The EWGSOP2 cutoffs are based on Western populations, whereas AWGS cutoffs come from Asian populations.
| Assessment | Test | Test description | EWGSOP2 cut-off points for men | EWGSOP2 cut-off points for women | AWGS cut-off points for men | AWGS cut-off points for women | SDOC cut-off points for men | SDOC cut-off points for women |
|---|---|---|---|---|---|---|---|---|
| Muscle Strength | Grip strength | Measuring grip strength using a calibrated handheld dynamometer, usually a Jamar dynamometer. | <27 kg | <16 kg | <28 kg | <18 kg | <35.5 kg | <20 kg |
| Chair stand/rise | Measuring time needed to rise five times from a seated position without using arms. | >15 s for five rises | ≥12 s | No recommendations | ||||
| Physical Performance | Gait speed | Measuring time needed to walk 3–10 meters at a comfortable pace. | ≤0.8 m/s (usually 4-meter walk) | <1.0 m/s (usually 6-meter walk) | <0.8 m/s | |||
| SPPB | Scoring based on balance while standing, gait speed test, and chair stand test. | ≤8 points | ≤9 points | No recommendations | ||||
| TUG | Measuring time needed to rise from chair, walk 3 meters, turn around, walk back to chair, and sit down again. | ≥20 s | No recommendations | No recommendations | ||||
| 400-meter walk | Measuring time needed to complete 20 laps of 20 meters as fast as possible with up to 2 rest stops as needed. | Non-completion or ≥6 min for completion | No recommendations | No recommendations | ||||
SPPB, short physical performance battery; TUG, timed-up-and-go; kg, kilograms; s, seconds; m, meters; min, minutes.
Evaluation of Muscle Quantity and Quality
If sarcopenia is probable based on muscle strength testing, the diagnosis can be confirmed by the presence of low muscle quantity and quality (Figure 2).6 Muscle quantity refers to its mass, typically measured through non-invasive imaging techniques using muscle size as a surrogate in place of true mass measurement (Table 2).8 The cross-sectional area (CSA) of muscles can be measured individually or in groups at various parts of the body, such as the axial skeleton or extremities.3 With muscle mass correlating with body size, the CSA can be reported as skeletal muscle index (SMI) with adjustment using either the height squared, weight, or body mass index (BMI); however, which method is superior remains an open question.31 EWGSOP2 recommends muscle quantity measurement using dual-energy x-ray absorptiometry (DEXA) and bioelectrical impedance analysis (BIA) in clinical settings and DEXA, magnetic resonance imaging (MRI), or computed tomography (CT) in research and specialty care settings for individuals at high risk for adverse outcomes.6 Muscle quality is a newer term referring to muscle architecture and composition. It can be studied via CT and MRI by assessing fat infiltration, BIA by measuring phase angle, or muscle function through muscle strength ratio to appendicular muscle mass.6 No universal consensus currently exists for routine clinical practice.6 EWGSOP2 recommendation for cutoff points for sarcopenia tests is 2 standard deviations below the sex-specific means of a young reference group, with 2.5 standard deviations being used for more conservative diagnosis.6
Table 2.
Summary of the commonly used imaging techniques for skeletal muscle evaluation.
| Imaging modality | Advantages | Limitations | Measurements | Heart failure specific concerns |
|---|---|---|---|---|
| Computed tomography | Gold standard. High accuracy. Measures muscle quantity and quality. Numerous indications allow opportunistic use. | Relatively expensive. Requires high space. Low availability. High radiation risk. No cutoff values. | Cross-sectional area of individual or group of muscles. Attenuation values. | Commonly used L3 level is of low opportunistic utility in cardiac conditions. Metal artifact from cardiac implantable electronic devices. |
| Magnetic resonance imaging | Gold standard. High accuracy. Best spatial resolution. Measures muscle quantity and quality. No radiation risk. | Expensive. Requires high space. Low availability. Contraindications. No cutoff values. Long acquisition time. | Cross-sectional area of individual or group of muscles. Fat content by Dixon imaging. Experimental advanced sequences. | Provider concern for safety with cardiac implantable electronic devices. |
| Dual-energy x-ray absorptiometry | Good accuracy. Cheap. Widely available. Low radiation risk. Cutoff values available. | Low uniformity between protocols. No muscle quality data. | Whole-body and appendicular lean mass. | Confounded by edema and obesity. |
| Bioelectrical impedance analysis | Cheap. Portable. Minimal maintenance. Immediate results. Measures muscle quantity and quality. | Less accurate than gold standard methods. No muscle quality data. | Fat mass and fat-free mass. | Confounded by edema. |
| Ultrasound | Cheap. Portable. Reliable. Measures muscle quantity and quality. | Operator dependent. Low standardization. Cutoffs are population and device specific. | Muscle thickness, cross-sectional area, and volume. Pennation angle. Fascicle length. Echo-intensity. Muscle stiffness. Contraction potential. Micro-circulation. | |
| Anthropometric measures | Cheap. Easy to perform. Minimal resources required. | Inaccurate. No muscle quality data. | Calf and mid-upper arm circumference. A Body Shape Index. | Confounded by edema. |
1. Computed Tomography and Magnetic Resonance Imaging
CT and MRI are the gold standards for the non-invasive assessment of muscle quantity and quality. However, their use is limited by cost and availability, particularly in rural areas or developing nations.32 Furthermore, no widely agreed-upon standardized imaging protocol exists to assess body fat and muscle mass on CT or MRI. Therefore, various techniques are being developed and tested for validity, reliability, and accuracy to estimate total body skeletal muscle mass (SMM) using specific landmarks and muscles as surrogates.3,6 With the growing interest surrounding early detection and intervention for sarcopenia, high-resolution imaging is expected to continue to grow and expand in use for research studies and clinical practice in the near future.6
As imaging assessment of SMM is often impractical, such evaluations are usually done opportunistically in clinical settings, relying on CT or MRI examinations acquired as part of the work-up for other disease states.33 CT is a common and effective imaging technique for sarcopenia evaluation. Within the United States, 88 million CT scans were obtained in 2018 as opposed to 39 million MRI scans.34 MRI is another imaging technique that allows adipose and lean tissue measurement without ionizing radiation, unlike CT, thus allowing appropriate use even in healthy volunteers and children, but at higher costs.35,36 The most common techniques for muscle measurements are MR “fat-water separated” imaging (e.g., Dixon imaging) methods.35,37 MR spectroscopy is also used to assess muscle composition, including 1H spectroscopic evaluation of lipid content in muscle34 and 31P MR spectroscopic evaluation of metabolite concentrations38 and mitochondrial function.39 Additional MRI techniques for muscle quality assessment include magnetization transfer imaging for assessment of protein content,40 T2* mapping for assessment of hydration,41 and diffusion MRI for assessment of muscle fiber structure.42
Although MRI enables the assessment of these various muscle properties, Park et al. have shown CT to be the more robust and reliable method for sarcopenia assessment based on inter-scan and inter-reader agreement of muscle quantity measurements (muscle quality was not evaluated).36 Additionally, dual-energy CT can enable material decomposition for skeletal muscle fat fraction quantification comparable to Dixon MRI, offering potential improvements beyond single-energy CT attenuation values.43 MRI studies may be particularly uncommon in the HF population given the prevalence of cardiac implantable electronic devices (CIED) and provider discomfort with performing such studies despite the growing literature on its safety.44 However, the presence of CIEDs and orthopedic hardware can cause metal artifacts in CT studies which impede assessment of adjacent structures; prior studies have addressed this limitation by making unilateral tissue measurements opposite the side of device implantation.45 Regardless, CT studies, particularly of the chest, are of the best opportunistic utility in HF given their high prevalence and the growing body of literature surrounding them.
1.1. Abdominal/Pelvic Measurements
A 2019 systematic review by Amini et al. looked at 388 studies that performed CT muscle measurements and found vast heterogeneity in the assessment metrics used.46 Overall, total SMM at L3 was preferred, although many studies used the mid-thigh muscles. A consensus for cutoff points was found for abdominal SMI (52–55 cm2/m2 for men, 39–41 cm2/m2 for women) with much less standardization outside the abdomen.46,47 Single-slice CT measurements of muscles at L3 have correlated well with whole-body muscle measurements performed by DEXA33,48 in addition to correlating with various poor outcome parameters in prior studies.49–53
The abdominal region from L3 to the iliac crests contains several muscles, including the psoas (major and minor), paraspinal muscles (erector spinae and quadratus lumborum), and abdominal muscles (transversus abdominis, external and internal obliques, and rectus abdominis) (Figure 3E–F).37 These muscles have the advantage of not being influenced by activity like the appendicular muscles. Among them, the psoas muscles have been frequently used alone, typically at L3 or L4,46 to predict whole-body SMM, morbidity, and mortality in cirrhosis,54 colorectal surgery,55 left ventricular assist devices (LVAD),56 and transcatheter aortic valve replacement (TAVR).57,58 Cutoff values have been proposed in consensus definitions, but most studies derive cutoff values from morbidity and mortality or sex-specific lowest tertile, quartile, or fifth percentile of subjects.37
Figure 3.
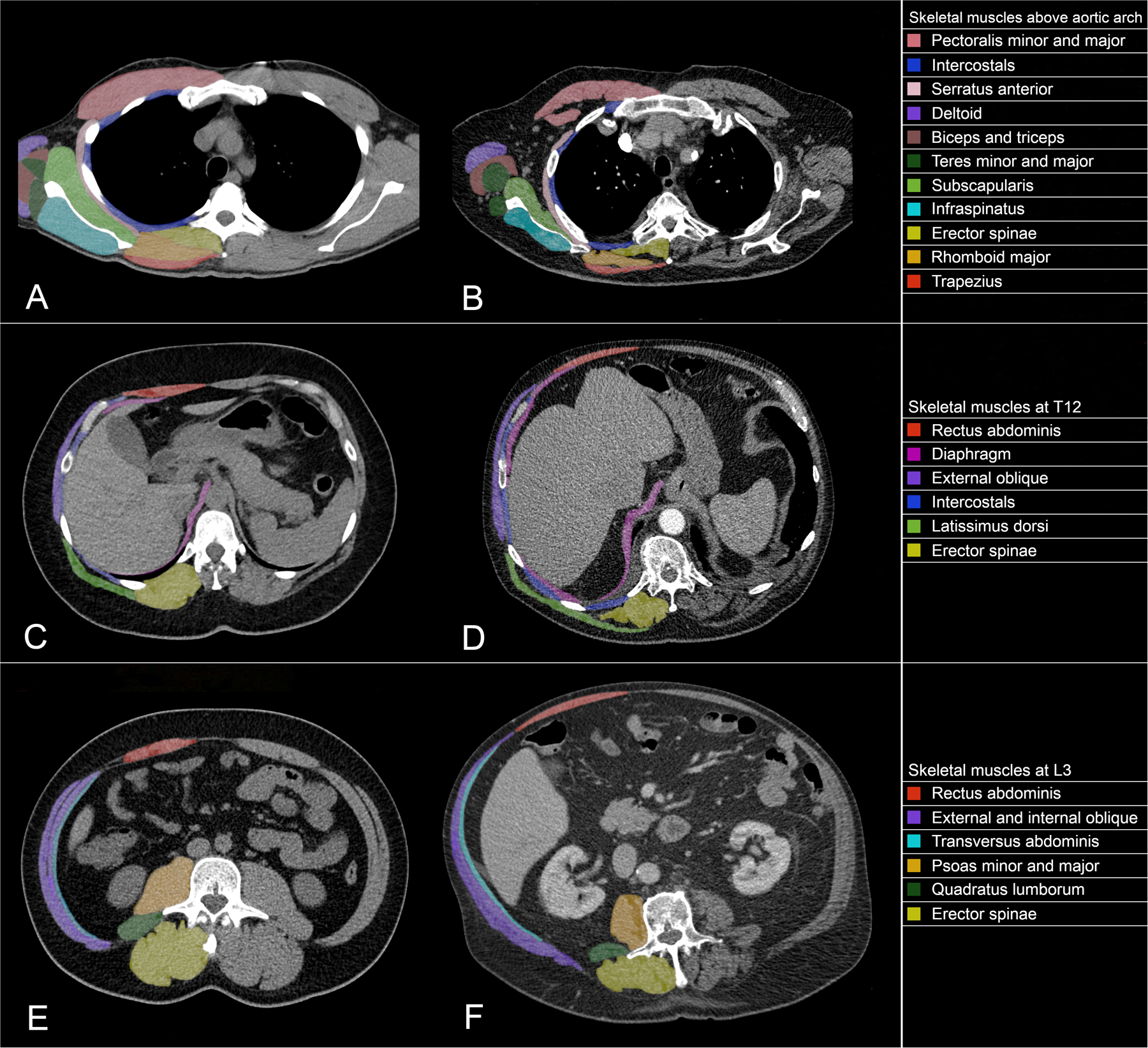
Computed tomography axial slices demonstrating the skeletal muscles found at the most commonly studied vertebral levels, including normal (2A) and low (2B) muscle mass above the aortic arch (about the third thoracic vertebra), normal (2C) and low (2D) muscle mass at the twelfth thoracic vertebra, and normal (2E) and low (2F) muscle mass at the third lumbar vertebra.
A 2019 study by Park et al. compared total abdominal muscle area to psoas muscle area alone at different levels from L2 to L4, measured by two abdominal radiologists.36 They found total abdominal muscles to be more reliable than the psoas muscle alone in terms of inter-scan and inter-reader agreement with more uniformity across the vertebral levels.36 Given this and the lack of evidence supporting the correlation of psoas muscle to whole body mass, the total single-slice muscle area at L3 may be the more accurate representation of whole-body SMM.37
1.2. Thoracic Measurements
Despite being extensively studied, abdominal landmarks such as L3 are limited in patients with thoracic diseases where abdominal CT is not routinely obtained.49 There currently exists a paucity in the literature regarding standard methods for thoracic CT measurement of sarcopenia.49 Various vertebral levels and muscles have been measured in patients with lung disease to study outcomes. For example, a 2020 systematic review by Rozenberg et al. reviewed 13 studies where CT muscle measurements in the chest and abdomen were made of lung transplant patients.59 Among them, the most common muscles were (in descending order) psoas, paraspinals, and pectoralis; vertebral levels included the carina, T7–9, T12, and L1–5, typically at a single slice (Figure 3A–D). Similarly, a 2019 meta-analysis by Nishimura et al. reviewed 9 studies with CT muscle measurements for patients undergoing lung cancer resection which showed single or total muscle measurements were made at different vertebral levels, including L3 in the majority of cases in addition to T5, T8, and T12.60 These reviews serve to further show the heterogeneity of muscle measurement, mainly when using thoracic imaging.
Kim et al. in 2016 provided cutoff values for diagnosis of sarcopenia at L1 in lung cancer, however, alterations in anatomy and respiration could result in L1 not being included in thoracic studies due to alterations in the position of the costophrenic sulci, making such measurement inconsistently available.61 A 2017 study by Nemec et al. compared total muscle area measurements at L3 (cutoff values of ≤52.4 cm2/m2 in men and ≤38.9 cm2/m2 in women per cancer cachexia criteria) to T12 and T7, normalized by height, in TAVR patients with preoperative contrast-enhanced CT examination of the entire aorta.49,62 The authors found a higher correlation between L3 and T12 with cutoff values of ≤42.6 cm2/m2 in men and ≤30.6 cm2/m2 in women, and SMI at these levels showed a significant relationship with prolonged hospital length of stay but no significant impact on outcomes, possibly due to the low number of sarcopenic patients in the study (53/157 patients).49
Like the psoas muscles on abdominal imaging, single muscle measurements have also been proposed for thoracic imaging, primarily with pectoralis muscles (major and minor). Teigen et al. have shown pectoralis muscle size and attenuation measurements on preoperative CT of patients who underwent LVAD implantation to be strong prognostic markers for mortality,63 similar to findings by Heberton et al. one year prior.56 These measurements performed better as predictors of adverse outcomes than pre-albumin, INTERMACS profile, BMI, and right atrial pressure.63 From this, Cogswell et al. created the Minnesota Pectoralis Risk Score, which incorporates pectoralis muscle mass and attenuation for post-LVAD mortality prediction.45 A prospective 2019 study by Kumar et al. measured major thoracic muscle groups on cardiac MRI in patients with and without heart failure.64 They found that higher muscle area was associated with lower mortality, and among the muscles studied (pectoralis minor and major, trapezius, latissimus dorsi, and paraspinal), pectoralis major was the most representative of overall thoracic muscle area and the most robust predictor of death.45 Thus, the pectoralis muscles have shown promising results thus far, but further studies are required.
A study by Derstine et al. in 2018 attempted to derive SMI cutoff values at different thoracic vertebral levels by using the 2010 EWGSOP2 recommendations of selecting diagnostic cutoffs for sarcopenia of two standard deviations below the mean reference values for a normative reference population.58,65 Their population of 735 healthy young kidney donor candidates (ages 18–40) gave rise to SMI and attenuation cutoffs from T10 to L5 in males and females.65 The study found L3 measurements to be significantly different from the other vertebral levels and recommended it as the preferred level for measurements; however, in cases where this level is not available on the specific CT imaging, their cutoffs may be helpful for available vertebral levels with the preference being (in descending order) L2, L4, L5, L1, T12, T11, and T10.65
1.3. Appendicular Measurements
Appendicular muscles are clinically relevant due to their importance for preserving mobility and functional independence in the elderly.2 Several consensus definitions with cutoff values have defined sarcopenia based on low appendicular SMM which is the sum of the muscle mass in the four extremities, typically measured by DEXA with BIA as an alternative, divided by height squared.7 Measurements on CT are usually made of the thigh muscle CSA divided by weight.66
2. Dual-energy X-ray Absorptiometry
DEXA allows two-dimensional imaging of body fat, muscle, and bone mineral density using two x-rays with different energies and thus different absorption.35 It has advantages in that it is widely available, low-cost, and fast; hence, the EWGSOP2 currently recommends it as the method of choice for evaluating muscle mass in clinical practice.2 The proposed cutoff values for appendicular SMI (adjusted for height) by EWGSOP2 are 7.0 kg/m2 in men and 5.5 kg/m2 in women.6 However, DEXA measurements are affected by fluid status which frequently fluctuates with HF and cirrhosis, making it less useful in these cases.37 It also cannot assess muscle quality (fat infiltration), and DEXA-measured appendicular SMI has shown only moderate correlation with CT-measured SMI, which is considered the gold standard.37
3. Bioelectrical impedance analysis
Unlike imaging techniques, BIA equipment is used to estimate body composition rather than direct measurements.67 It is based on whole-body electrical conductivity and uses a conversion equation calibrated with DEXA-measured lean mass in a reference population.6 The general principle is that current is well-conducted by water, blood, and muscle but poorly conducted by fat, air-filled spaces, and bone.3 Although these devices are widely available and affordable, muscle mass estimates can vary based on device brands and reference populations used. EWGSOP2 recommends using raw measures with the cross-validated Sergi equation for standardization, but it should be noted that discrepancies can arise between clinic patients and the Sergi equation which is based on an older European population;6 further studies are needed to validate prediction equations for specific populations. Additionally, like DEXA, measurements can be influenced by hydration which again poses limitations in HF and cirrhosis.
4. Ultrasound
US has been applied for sarcopenia due to its ability to assess muscles quantitatively (muscle thickness, CSA, and volume) and qualitatively (pennation angle, fascicle length, echo-intensity, muscle stiffness, contraction potential, and micro-circulation).68 US measurements have shown a positive correlation with CT, MRI, and DEXA measurements,69 and updated guidelines for the standardization of techniques have been proposed in a 2021 review by the SARCUS (SARCopenia through UltraSound) working group.68 Although this method is operator-dependent and only gives information regarding the muscles specifically studied, it is an option when more advanced imaging is not available given its portability, low cost, and lack of ionizing radiation.2 This is particularly true when utilizing texture analysis methods to assess muscle composition. US has the potential for increased utilization, particularly in the clinical setting, as more advanced techniques are introduced that address confounding factors such as equipment settings and adipose tissue thickness.70
5. Anthropometry
Although anthropometric measures are not preferred for measuring muscle mass, techniques such as calf and mid-upper arm circumference have been identified as proxy measures for SMM in the Geriatric outpatient setting, but their association with physical function was weak.71 It should be noted that EWGSOP2 has recommended using calf circumference as a diagnostic proxy for SMM in older adults in settings where other diagnostic methods are not available.6 This method has shown a positive correlation with appendicular SMM and SMI with suggested cutoff values of <34cm in men and <33cm in women of Japanese descent.72
A Body Shape Index (ABSI) is a newer anthropometric measurement using waist circumference, BMI, and height. This formula is applicable for the commonly missed sarcopenic obesity, a syndrome defined as higher fat mass relative to fat-free mass where catabolic adipokines released by visceral adipose tissue induce skeletal muscle protein catabolism. Biolo et al. previously demonstrated ABSI as a possible index of decreased muscle mass due to its negative association with muscle mass measured via BIA.73
Evaluation of Physical Performance
Finally, following diagnosis, the severity of sarcopenia can be categorized by assessment of physical performance. Physical performance is the objective measurement of whole-body function related to locomotion, a concept that involves the muscles, central nervous system, and peripheral nervous system (Table 1).6 Gait speed is widely used in clinical practice due to its simplicity, reliability, safety, and ability to predict adverse outcomes related to sarcopenia.74 The 4-meter gait speed test is a standard version with EWGSOP2 advising a value of ≤0.8 m/s as a single cutoff speed to indicate severe sarcopenia.6 The short physical performance battery consists of gait speed, a balance test, and a chair stand test. The timed-up-and-go test asks patients to stand from a seated position, walk 3 meters, turn around, walk back, and sit down again. The 400-meter walk test, also known as the long-distance corridor walk, assesses walking ability and endurance by having patients walk 20 laps of 20 meters as fast as possible with two optional rest stops.6
Biomarkers
Given the complex pathophysiology of sarcopenia, a single biomarker will likely not be found to diagnose and monitor these individuals.6 Instead, the focus should be placed on developing a panel of biomarkers, including markers of the neuromuscular junction, endocrine system, growth factors, muscle protein turnover, behavior-mediated pathways, and inflammation-mediated pathways.75 Although such panels do not currently exist for routine clinical use, several commonly measured blood tests have been suggested for muscle mass estimation. These measurements are best used as an adjunct to the above diagnostic process rather than a replacement.
Creatine is produced in the liver and kidneys in addition to being consumed in meat and fish. It is taken up by tissues with high energy demands, primarily the muscles (95% of body reserve), and converted to phosphocreatine as an energy reserve. A small portion of creatine in muscle is turned into creatinine each day which is excreted in the urine, and low baseline serum creatinine has been used as an indicator of low muscle mass. This concept can be used to estimate whole-body SMM via the creatine dilution test where labeled creatine is ingested by a fasting patient, and labeled creatinine is later measured in the urine.6 This method is currently limited to use for research, but it has correlated well with muscle mass on MRI and modestly on DEXA and BIA.76
Sarcopenia index (SI) is another method that utilizes creatinine along with Cystatin C, a small protein derived from all nucleated cells with less impact from SMM.77 Kashani et al. have reported SI (serum creatinine/cystatin C × 100) to be a fair measurement of SMM with modest prediction of in-hospital mortality in critically ill patients with normal kidney function.78,79 Similarly, Romeo et al. used it as a surrogate for SMM with good prediction of adverse outcomes in elderly patients undergoing TAVR.80 This test is low cost and easy to calculate, but recent studies such as that done by He et al. have shown that it may not lead to accurate sarcopenia diagnosis.81
Fat-free mass index (FFMI) is an alternative to BMI which takes into account the actual composition of excess body weight such as adipose tissue, muscle hypertrophy, or volume overload in the case of HF (when measured rather than calculated); therefore, FFMI has been used for the clinical diagnosis of sarcopenia.82 Fat-free mass can be estimated using the Forbes formula, which utilizes urinary creatinine that can be directly measured or calculated using the patient’s weight and height; this is then adjusted using the height squared to get FFMI.83 FFMI itself can more accurately be measured using BIA or DEXA.73
The Forbes formula for FFMI calculation does make several assumptions, including a constant relationship between urinary creatinine excretion and SMM, between SMM and lean muscle mass, and a constant hydration fraction of fat-free mass.84 Calculated FFMI via the Forbes formula has shown promising results by Narumi et al. to predict poor prognosis in chronic HF and Tsuchida et al. to detect more severe acute HF, but there has been limited validation against more accurate methods.83,85 Of note, alternate formulas for calculating body composition have been proposed by Kuch et al. and Boer et al. with similarly limited validation.86
Addressing Sarcopenia in Heart Failure
Early detection of sarcopenia, particularly as the focus on imaging evaluation of skeletal muscle grows in the field of HF, will allow for the identification of these vulnerable patients for early implementation of interventions. The best therapeutic plan to mitigate the progression of sarcopenia in HF targets the previously described disease-specific mechanisms. This includes regular and tolerable levels of physical activity, resistance exercise training, optimal nutrition to increase proteins and micronutrients, and early involvement of our geriatric colleagues.5,87 Exercise is the most effective therapy with sufficient clinical evidence for muscle wasting in HF by targeting many of the underlying mechanisms, including inflammation and hormonal changes.88 Cardiac rehabilitation specifically has shown improvement in physical and cognitive function in patients hospitalized for HF exacerbation, thus leading to a better quality of life and diminished physical frailty.89
Nutrition optimization will ensure anabolic-catabolic balance.90 Evidence shows that high-protein oral nutritional supplements containing beta-hydroxy-beta-methylbutyrate in malnourished, older adults hospitalized for HF can reduce readmission and mortality.91 The strong impact of nutrition on HF was further shown in the 2021 Effect of early nutrition support on Frailty, Functional Outcomes, and Recovery of malnourished medical inpatients (EFFORT) study, which showed a reduction in risk of mortality and major cardiovascular events with individualized nutritional support as opposed to standard hospital meals in HF patients at high nutritional risk.92
Standard HF medications may have muscle-protective properties by targeting the underlying cause, but further studies are needed to establish the effect of these medications on sarcopenia in HF; regardless, the prognostic indications for these medications are clear.1 There has also been great interest revolving around sarcopenia treatment with hormone replacement therapies such as testosterone, growth hormone, insulin-like growth factor, dehydroepiandrosterone, estrogen, and estradiol.88 The results of these studies have been mixed with no clear indications, particularly given the risk of adverse clinical outcomes with such therapies.88
Conclusion
Sarcopenia, a particularly prevalent disease in HF patients, has been extensively associated with worse clinical outcomes. Here, we discussed the currently proposed diagnostic process for sarcopenia evaluation; the Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols were not followed, given the narrative nature of the review. A low threshold for sarcopenia evaluation in high-risk patients is paramount for early detection and treatment of sarcopenia to avoid its negative impact on quality of life later in life. Evaluation for sarcopenia should be incorporated into the routine care of HF patients, whether in the outpatient or inpatient setting. Further investigation is required to standardize techniques for muscle mass quantification, particularly on thoracic CT imaging, where there currently exists a gap of knowledge. As the utilization of thoracic CT for sarcopenia becomes more standardized, prior imaging can be used opportunistically to diagnose and address this syndrome.
Sources of Funding:
Dr. Tang is partially supported by grants from the National Institutes of Health (R01HL146754). Dr. Eck is partially supported by grants from the National Institutes of Health (T32AR007505, K25AG070321) and the Cleveland Clinic Musculoskeletal Research Center/Program of Advanced Musculoskeletal Imaging Pilot Project Program. Dr. Mirzai is partially supported by the Cleveland Clinic Caregiver Catalyst Grant.
Footnotes
Disclosures: Dr. Tang is a consultant for Sequana Medical A.G., Owkin Inc, PreCARDIA Inc, Cardiol Therapeutics Inc, Genomics plc, and has received honorarium from Springer Nature for authorship/editorship and American Board of Internal Medicine for exam writing committee participation - all unrelated to the subject and contents of this paper. Dr. Estep is a consultant and medical advisor for Abbott and Medtronic Inc - unrelated to the subject and contents of this paper. All other authors have no relationships to disclose.
References
- 1.Curcio F, Testa G, Liguori I, et al. Sarcopenia and heart failure. Nutrients. 2020;12(1). doi: 10.3390/nu12010211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Giraudo C, Cavaliere A, Lupi A, Guglielmi G, Quaia E. Established paths and new avenues: A review of the main radiological techniques for investigating sarcopenia. Quant Imaging Med Surg. 2020;10(8):1602–1613. doi: 10.21037/QIMS.2019.12.15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hollingworth TW, Oke SM, Patel H, Smith TR. Getting to grips with sarcopenia: Recent advances and practical management for the gastroenterologist. Frontline Gastroenterol. 2021;12(1):53–61. doi: 10.1136/flgastro-2019-101348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pacifico J, Geerlings MAJ, Reijnierse EM, Phassouliotis C, Lim WK, Maier AB. Prevalence of sarcopenia as a comorbid disease: A systematic review and meta-analysis. Exp Gerontol. 2020;131(October 2019):110801. doi: 10.1016/j.exger.2019.110801 [DOI] [PubMed] [Google Scholar]
- 5.Springer J, Springer JI, Anker SD. Muscle wasting and sarcopenia in heart failure and beyond: update 2017. ESC Hear Fail. 2017;4(4):492–498. doi: 10.1002/ehf2.12237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cruz-Jentoft AJ, Bahat G, Bauer J, et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing. 2019;48(1):16–31. doi: 10.1093/ageing/afy169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bielecka-Dabrowa A, Ebner N, dos Santos MR, Ishida J, Hasenfuss G, von Haehling S. Cachexia, muscle wasting, and frailty in cardiovascular disease. Eur J Heart Fail. Published online 2020. doi: 10.1002/ejhf.2011 [DOI] [PubMed] [Google Scholar]
- 8.Marasco G, Sadalla S, Vara G, et al. Imaging Software-Based Sarcopenia Assessment in Gastroenterology: Evolution and Clinical Meaning. Can J Gastroenterol Hepatol. 2021;2021. doi: 10.1155/2021/6669480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goates S, Du K, Arensberg MB, Gaillard T, Guralnik J, Pereira SL. Economic Impact of Hospitalizations in US Adults with Sarcopenia. J frailty aging. 2019;8(2):93–99. doi: 10.14283/jfa.2019.10 [DOI] [PubMed] [Google Scholar]
- 10.Von Haehling S The wasting continuum in heart failure: From sarcopenia to cachexia. Proc Nutr Soc. 2015;74(4):367–377. doi: 10.1017/S0029665115002438 [DOI] [PubMed] [Google Scholar]
- 11.Lochs H, Allison SP, Meier R, et al. Introductory to the ESPEN Guidelines on Enteral Nutrition: Terminology, Definitions and General Topics. Clin Nutr. 2006;25(2):180–186. doi: 10.1016/j.clnu.2006.02.007 [DOI] [PubMed] [Google Scholar]
- 12.Lardiés-Sánchez B, Sanz-París A. Sarcopenia and Malnutrition in the Elderly. Frailty Sarcopenia - Onset, Dev Clin Challenges. Published online 2017. doi: 10.5772/intechopen.68426 [DOI] [Google Scholar]
- 13.Evans WJ, Morley JE, Argilés J, et al. Cachexia: A new definition. Clin Nutr. 2008;27(6):793–799. doi: 10.1016/j.clnu.2008.06.013 [DOI] [PubMed] [Google Scholar]
- 14.Sayer AA, Syddall H, Martin H, Patel H, Baylis D. Europe PMC Funders Group The developmental origins of sarcopenia. Nutr Heal Aging. 2009;12(7):427–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Melenovsky V, Kotrc M, Borlaug BA, et al. Relationships between right ventricular function, body composition, and prognosis in advanced heart failure. J Am Coll Cardiol. 2013;62(18):1660–1670. doi: 10.1016/j.jacc.2013.06.046 [DOI] [PubMed] [Google Scholar]
- 16.Haykowsky MJ, Tomczak CR, Scott JM, Paterson DI, Kitzman DW. Determinants of exercise intolerance in patients with heart failure and reduced or preserved ejection fraction. J Appl Physiol. 2015;119(6):739–744. doi: 10.1152/japplphysiol.00049.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Testa G, Cacciatore F, Galizia G, et al. Waist circumference but not body mass index predicts long-term mortality in elderly subjects with chronic heart failure. J Am Geriatr Soc. 2010;58(8):1433–1440. doi: 10.1111/j.1532-5415.2010.02979.x [DOI] [PubMed] [Google Scholar]
- 18.Bekfani T, Pellicori P, Morris DA, et al. Sarcopenia in patients with heart failure with preserved ejection fraction: Impact on muscle strength, exercise capacity and quality of life. Int J Cardiol. 2016;222:41–46. doi: 10.1016/j.ijcard.2016.07.135 [DOI] [PubMed] [Google Scholar]
- 19.Emami A, Saitoh M, Valentova M, et al. Comparison of sarcopenia and cachexia in men with chronic heart failure: results from the Studies Investigating Co-morbidities Aggravating Heart Failure (SICA-HF). Eur J Heart Fail. 2018;20(11):1580–1587. doi: 10.1002/ejhf.1304 [DOI] [PubMed] [Google Scholar]
- 20.Beyer SE, Sanghvi MM, Aung N, et al. Prospective association between handgrip strength and cardiac structure and function in UK adults. PLoS One. 2018;13(3):1–13. doi: 10.1371/journal.pone.0193124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Malmstrom TK, Morley JE. SARC-F: A simple questionnaire to rapidly diagnose sarcopenia. J Am Med Dir Assoc. 2013;14(8):531–532. doi: 10.1016/j.jamda.2013.05.018 [DOI] [PubMed] [Google Scholar]
- 22.Woo J, Yu R, Leung J. A 3-Item SARC-F. J Am Med Dir Assoc. 2018;19(3):223–228. doi: 10.1016/j.jamda.2017.09.006 [DOI] [PubMed] [Google Scholar]
- 23.Chen LK, Woo J, Assantachai P, et al. Asian Working Group for Sarcopenia: 2019 Consensus Update on Sarcopenia Diagnosis and Treatment. J Am Med Dir Assoc. 2020;21(3):300–307.e2. doi: 10.1016/j.jamda.2019.12.012 [DOI] [PubMed] [Google Scholar]
- 24.Yang M, Hu X, Xie L, et al. SARC-F for sarcopenia screening in community-dwelling older adults Are 3 items enough? Med (United States). 2018;97(30). doi: 10.1097/MD.0000000000011726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mohd Nawi SN, Khow KS, Shiong Lim W, Yu SC, Yu Ching Yeh S. Screening Tools for Sarcopenia in Community-Dwellers: A Scoping Review Screening Tools for Sarcopenia-Siti N Mohd Nawi et al et al. Ann Acad Med Singapore. 2019;48(7):201. [PubMed] [Google Scholar]
- 26.Rossi AP, Micciolo R, Rubele S, et al. Assessing the risk of sarcopenia in the elderly: The Mini Sarcopenia Risk Assessment (MSRA) questionnaire. J Nutr Heal Aging. 2017;21(6):743–749. doi: 10.1007/s12603-017-0921-4 [DOI] [PubMed] [Google Scholar]
- 27.Ishii S, Tanaka T, Shibasaki K, et al. Development of a simple screening test for sarcopenia in older adults. Geriatr Gerontol Int. 2014;14(SUPPL.1):93–101. doi: 10.1111/ggi.12197 [DOI] [PubMed] [Google Scholar]
- 28.Yu S, Appleton S, Chapman I, et al. An Anthropometric Prediction Equation for Appendicular Skeletal Muscle Mass in Combination With a Measure of Muscle Function to Screen for Sarcopenia in Primary and Aged Care. J Am Med Dir Assoc. 2015;16(1):25–30. doi: 10.1016/j.jamda.2014.06.018 [DOI] [PubMed] [Google Scholar]
- 29.Bhasin S, Travison TG, Manini TM, et al. Sarcopenia Definition: The Position Statements of the Sarcopenia Definition and Outcomes Consortium. J Am Geriatr Soc. 2020;68(7):1410–1418. doi: 10.1111/jgs.16372 [DOI] [PubMed] [Google Scholar]
- 30.Leong DP, Teo KK, Rangarajan S, et al. Prognostic value of grip strength: Findings from the Prospective Urban Rural Epidemiology (PURE) study. Lancet. 2015;386(9990):266–273. doi: 10.1016/S0140-6736(14)62000-6 [DOI] [PubMed] [Google Scholar]
- 31.Kim KM, Jang HC, Lim S. Differences among skeletal muscle mass indices derived from height-, weight-, and body mass index-adjusted models in assessing sarcopenia. Korean J Intern Med. 2016;31(4):643–650. doi: 10.3904/kjim.2016.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Everton KL, Mazal J, Mollura DJ. White paper report of the 2011 RAD-AID conference on international radiology for developing countries: Integrating multidisciplinary strategies for imaging services in the developing world. J Am Coll Radiol. 2012;9(7):488–494. doi: 10.1016/j.jacr.2012.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shen W, Punyanitya M, Wang ZM, et al. Total body skeletal muscle and adipose tissue volumes: Estimation from a single abdominal cross-sectional image. J Appl Physiol. 2004;97(6):2333–2338. doi: 10.1152/japplphysiol.00744.2004 [DOI] [PubMed] [Google Scholar]
- 34.Boutin RD, Lenchik L. Value-added opportunistic CT: Insights into osteoporosis and sarcopenia. Am J Roentgenol. 2020;215(3):582–594. doi: 10.2214/AJR.20.22874 [DOI] [PubMed] [Google Scholar]
- 35.Hemke R, Buckless C, Torriani M. Quantitative Imaging of Body Composition. Semin Musculoskelet Radiol. 2020;24(4):375–385. doi: 10.1055/s-0040-1708824 [DOI] [PubMed] [Google Scholar]
- 36.Park J, Gil JR, Shin Y, et al. Reliable and robust method for abdominal muscle mass quantification using CT/MRI: An explorative study in healthy subjects. PLoS One. 2019;14(9):1–14. doi: 10.1371/journal.pone.0222042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee CM, Kang BK, Kim M. Radiologic definition of sarcopenia in chronic liver disease. Life. 2021;11(2):1–16. doi: 10.3390/life11020086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hinkley JM, Cornnell HH, Standley RA, et al. Older adults with sarcopenia have distinct skeletal muscle phosphodiester, phosphocreatine, and phospholipid profiles. Aging Cell. 2020;19(6):1–11. doi: 10.1111/acel.13135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Andreux PA, Van Diemen MPJ, Heezen MR, et al. Mitochondrial function is impaired in the skeletal muscle of pre-frail elderly. Sci Rep. 2018;8(1):1–12. doi: 10.1038/s41598-018-26944-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Power GA, Allen MD, Booth WJ, Thompson RT, Marsh GD, Rice CL. The influence on sarcopenia of muscle quality and quantity derived from magnetic resonance imaging and neuromuscular properties. Age (Omaha). 2014;36(3):1377–1388. doi: 10.1007/s11357-014-9642-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sanz-Requena R, Martínez-Arnau FM, Pablos-Monzó A, et al. The role of imaging biomarkers in the assessment of sarcopenia. Diagnostics. 2020;10(8):1–14. doi: 10.3390/diagnostics10080534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Galbán CJ, Maderwald S, Stock F, Ladd ME. Age-related changes in skeletal muscle as detected by diffusion tensor magnetic resonance imaging. Journals Gerontol - Ser A Biol Sci Med Sci. 2007;62(4):453–458. doi: 10.1093/gerona/62.4.453 [DOI] [PubMed] [Google Scholar]
- 43.Molwitz I, Leiderer M, McDonough R, et al. Skeletal muscle fat quantification by dual-energy computed tomography in comparison with 3T MR imaging. Eur Radiol. Published online 2021. doi: 10.1007/s00330-021-07820-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Muthalaly RG, Nerlekar N, Ge Y, Kwong RY, Nasis A. MRI in patients with cardiac implantable electronic devices. Radiology. 2018;289(2):281–292. doi: 10.1148/radiol.2018180285 [DOI] [PubMed] [Google Scholar]
- 45.Cogswell R, Trachtenberg B, Murray T, et al. A Novel Model Incorporating Pectoralis Muscle Measures to Predict Mortality After Ventricular Assist Device Implantation: The Minnesota Pectoralis Risk Score. J Card Fail. 2020;26(4):308–315. doi: 10.1016/j.cardfail.2019.11.021 [DOI] [PubMed] [Google Scholar]
- 46.Amini B, Boyle SP, Boutin RD, Lenchik L. Approaches to assessment of muscle mass and myosteatosis on computed tomography: A systematic review. Journals Gerontol - Ser A Biol Sci Med Sci. 2019;74(10):1671–1678. doi: 10.1093/gerona/glz034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Daly LE, Prado CM, Ryan AM. A window beneath the skin: How computed tomography assessment of body composition can assist in the identification of hidden wasting conditions in oncology that profoundly impact outcomes. Proc Nutr Soc. 2018;77(2):135–151. doi: 10.1017/S0029665118000046 [DOI] [PubMed] [Google Scholar]
- 48.Mourtzakis M, Prado CMM, Lieffers JR, Reiman T, McCargar LJ, Baracos VE. A practical and precise approach to quantification of body composition in cancer patients using computed tomography images acquired during routine care. Appl Physiol Nutr Metab. 2008;33(5):997–1006. doi: 10.1139/H08-075 [DOI] [PubMed] [Google Scholar]
- 49.Nemec U, Heidinger B, Sokas C, Chu L, Eisenberg RL. Diagnosing Sarcopenia on Thoracic Computed Tomography: Quantitative Assessment of Skeletal Muscle Mass in Patients Undergoing Transcatheter Aortic Valve Replacement. Acad Radiol. 2017;24(9):1154–1161. doi: 10.1016/j.acra.2017.02.008 [DOI] [PubMed] [Google Scholar]
- 50.Kumar A, Moynagh MR, Multinu F, et al. Muscle composition measured by CT scan is a measurable predictor of overall survival in advanced ovarian cancer. Gynecol Oncol. 2016;142(2):311–316. doi: 10.1016/j.ygyno.2016.05.027 [DOI] [PubMed] [Google Scholar]
- 51.Lieffers JR, Bathe OF, Fassbender K, Winget M, Baracos VE. Sarcopenia is associated with postoperative infection and delayed recovery from colorectal cancer resection surgery. Br J Cancer. 2012;107(6):931–936. doi: 10.1038/bjc.2012.350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kuroki LM, Mangano M, Allsworth JE, et al. Pre-operative Assessment of Muscle Mass to Predict Surgical Complications and Prognosis in Patients With Endometrial Cancer. Ann Surg Oncol. 2015;22(3):972–979. doi: 10.1245/s10434-014-4040-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Higashi T, Hayashi H, Taki K, et al. Sarcopenia, but not visceral fat amount, is a risk factor of postoperative complications after major hepatectomy. Int J Clin Oncol. 2016;21(2):310–319. doi: 10.1007/s10147-015-0898-0 [DOI] [PubMed] [Google Scholar]
- 54.Gu DH, Kim MY, Seo YS, et al. Clinical usefulness of psoas muscle thickness for the diagnosis of sarcopenia in patients with liver cirrhosis. Clin Mol Hepatol. 2018;24(3):319–330. doi: 10.3350/cmh.2017.0077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hanaoka M, Yasuno M, Ishiguro M, et al. Morphologic change of the psoas muscle as a surrogate marker of sarcopenia and predictor of complications after colorectal cancer surgery. Int J Colorectal Dis. 2017;32(6):847–856. doi: 10.1007/s00384-017-2773-0 [DOI] [PubMed] [Google Scholar]
- 56.Heberton GA, Nassif M, Bierhals A, et al. Usefulness of Psoas Muscle Area Determined by Computed Tomography to Predict Mortality or Prolonged Length of Hospital Stay in Patients Undergoing Left Ventricular Assist Device Implantation. Am J Cardiol. 2016;118(9):1363–1367. doi: 10.1016/j.amjcard.2016.07.061 [DOI] [PubMed] [Google Scholar]
- 57.Soud M, Alahdab F, Ho G, et al. Usefulness of skeletal muscle area detected by computed tomography to predict mortality in patients undergoing transcatheter aortic valve replacement: a meta-analysis study. Int J Cardiovasc Imaging. 2019;35(6):1141–1147. doi: 10.1007/s10554-019-01582-0 [DOI] [PubMed] [Google Scholar]
- 58.Derstine BA, Holcombe SA, Goulson RL, et al. Quantifying Sarcopenia Reference Values Using Lumbar and Thoracic Muscle Areas in a Healthy Population. J Nutr Heal Aging. 2018;22(1):180–185. doi: 10.1007/s12603-017-0983-3 [DOI] [PubMed] [Google Scholar]
- 59.Rozenberg D, Orsso CE, Chohan K, et al. Clinical outcomes associated with computed tomography-based body composition measures in lung transplantation: a systematic review. Transpl Int. 2020;33(12):1610–1625. doi: 10.1111/tri.13749 [DOI] [PubMed] [Google Scholar]
- 60.Nishimura JM, Ansari AZ, D’Souza DM, Moffatt-Bruce SD, Merritt RE, Kneuertz PJ. Computed Tomography-Assessed Skeletal Muscle Mass as a Predictor of Outcomes in Lung Cancer Surgery. Ann Thorac Surg. 2019;108(5):1555–1564. doi: 10.1016/j.athoracsur.2019.04.090 [DOI] [PubMed] [Google Scholar]
- 61.Kim EY, Kim YS, Park I, et al. Evaluation of sarcopenia in small-cell lung cancer patients by routine chest CT. Support Care Cancer. 2016;24(11):4721–4726. doi: 10.1007/s00520-016-3321-0 [DOI] [PubMed] [Google Scholar]
- 62.Fearon K, Strasser F, Anker SD, et al. Definition and classification of cancer cachexia: An international consensus. Lancet Oncol. 2011;12(5):489–495. doi: 10.1016/S1470-2045(10)70218-7 [DOI] [PubMed] [Google Scholar]
- 63.Teigen LM, John R, Kuchnia AJ, et al. Preoperative pectoralis muscle quantity and attenuation by computed tomography are novel and powerful predictors of mortality after left ventricular assist device implantation. Circ Hear Fail. 2017;10(9):1–8. doi: 10.1161/CIRCHEARTFAILURE.117.004069 [DOI] [PubMed] [Google Scholar]
- 64.Kumar A, Ansari BA, Kim J, et al. Axial muscle size as a strong predictor of death in subjects with and without heart failure. J Am Heart Assoc. 2019;8(4):1–9. doi: 10.1161/JAHA.118.010554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Derstine BA, Holcombe SA, Ross BE, Wang NC, Su GL, Wang SC. Skeletal muscle cutoff values for sarcopenia diagnosis using T10 to L5 measurements in a healthy US population. Sci Rep. 2018;8(1):1–8. doi: 10.1038/s41598-018-29825-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ochi M, Tabara Y, Kido T, et al. Quadriceps sarcopenia and visceral obesity are risk factors for postural instability in the middle-aged to elderly population. Geriatr Gerontol Int. 2010;10(3):233–243. doi: 10.1111/j.1447-0594.2010.00610.x [DOI] [PubMed] [Google Scholar]
- 67.Aleixo GFP, Shachar SS, Nyrop KA, Muss HB, Battaglini CL, Williams GR. Bioelectrical Impedance Analysis for the Assessment of Sarcopenia in Patients with Cancer: A Systematic Review. Oncologist. 2020;25(2):170–182. doi: 10.1634/theoncologist.2019-0600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Perkisas S, Bastijns S, Baudry S, et al. Application of ultrasound for muscle assessment in sarcopenia: 2020 SARCUS update. Eur Geriatr Med. 2021;12(1):45–59. doi: 10.1007/s41999-020-00433-9 [DOI] [PubMed] [Google Scholar]
- 69.Nijholt W, Scafoglieri A, Jager-Wittenaar H, Hobbelen JSM, van der Schans CP. The reliability and validity of ultrasound to quantify muscles in older adults: a systematic review. J Cachexia Sarcopenia Muscle. 2017;8(5):702–712. doi: 10.1002/jcsm.12210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Paris MT, Mourtzakis M. Muscle Composition Analysis of Ultrasound Images: A Narrative Review of Texture Analysis. Ultrasound Med Biol. 2021;47(4):880–895. doi: 10.1016/j.ultrasmedbio.2020.12.012 [DOI] [PubMed] [Google Scholar]
- 71.Ling CHY, Meskers CGM, Maier AB. Can anthropometric measures be used as proxies for body composition and physical function in geriatric outpatients? Arch Gerontol Geriatr. 2021;94(February):104379. doi: 10.1016/j.archger.2021.104379 [DOI] [PubMed] [Google Scholar]
- 72.Kawakami R, Murakami H, Sanada K, et al. Calf circumference as a surrogate marker of muscle mass for diagnosing sarcopenia in Japanese men and women. Geriatr Gerontol Int. 2015;15(8):969–976. doi: 10.1111/ggi.12377 [DOI] [PubMed] [Google Scholar]
- 73.Biolo G, Di Girolamo FG, Breglia A, et al. Inverse relationship between “a body shape index” (ABSI) and fat-free mass in women and men: Insights into mechanisms of sarcopenic obesity. Clin Nutr. 2015;34(2):323–327. doi: 10.1016/j.clnu.2014.03.015 [DOI] [PubMed] [Google Scholar]
- 74.Peel NM, Kuys SS, Klein K. Gait speed as a measure in geriatric assessment in clinical settings: A systematic review. Journals Gerontol - Ser A Biol Sci Med Sci. 2013;68(1):39–46. doi: 10.1093/gerona/gls174 [DOI] [PubMed] [Google Scholar]
- 75.Curcio F, Ferro G, Basile C, et al. Biomarkers in sarcopenia: A multifactorial approach. Exp Gerontol. 2016;85:1–8. doi: 10.1016/j.exger.2016.09.007 [DOI] [PubMed] [Google Scholar]
- 76.Clark RV, Walker AC, Miller RR, O’Connor-Semmes RL, Ravussin E, Cefalu WT. Creatine (methyl-d3) dilution in urine for estimation of total body skeletal muscle mass: Accuracy and variability vs. MRI and DXA. J Appl Physiol. 2018;124(1):1–9. doi: 10.1152/japplphysiol.00455.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lopez-Ruiz A, Kashani K. Assessment of muscle mass in critically ill patients: role of the sarcopenia index and images studies. Curr Opin Clin Nutr Metab Care. 2020;23(5):302–311. doi: 10.1097/MCO.0000000000000673 [DOI] [PubMed] [Google Scholar]
- 78.Kashani KB, Frazee EN, Kukrálová L, et al. Evaluating Muscle Mass by Using Markers of Kidney Function: Development of the Sarcopenia Index. Crit Care Med. 2017;45(1):e23–e29. doi: 10.1097/CCM.0000000000002013 [DOI] [PubMed] [Google Scholar]
- 79.Tang T, Zhuo Y, Xie L, Wang H, Yang M. Sarcopenia index based on serum creatinine and cystatin C is associated with 3-year mortality in hospitalized older patients. Sci Rep. 2020;10(1):1–9. doi: 10.1038/s41598-020-58304-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Romeo FJ, Chiabrando JG, Seropian IM, et al. Sarcopenia index as a predictor of clinical outcomes in older patients undergoing transcatheter aortic valve replacement. Catheter Cardiovasc Interv. 2021;(January):1–8. doi: 10.1002/ccd.29799 [DOI] [PubMed] [Google Scholar]
- 81.He Q, Jiang J, Xie L, Zhang L, Yang M. A sarcopenia index based on serum creatinine and cystatin C cannot accurately detect either low muscle mass or sarcopenia in urban community-dwelling older people. Sci Rep. 2018;8(1):6–11. doi: 10.1038/s41598-018-29808-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Avesani CM, Draibe SA, Kamimura MA, et al. Assessment of body composition by dual energy X-ray absorptiometry, skinfold thickness and creatinine kinetics in chronic kidney disease patients. Nephrol Dial Transplant. 2004;19(9):2289–2295. doi: 10.1093/ndt/gfh381 [DOI] [PubMed] [Google Scholar]
- 83.Narumi T, Watanabe T, Kadowaki S, et al. Sarcopenia evaluated by fat-free mass index is an important prognostic factor in patients with chronic heart failure. Eur J Intern Med. 2015;26(2):118–122. doi: 10.1016/j.ejim.2015.01.008 [DOI] [PubMed] [Google Scholar]
- 84.Forbes GB. Lean Body Mass-Body Fat Interrelationships in Humans. Nutr Rev. 1987;45(10):225–231. doi: 10.1111/j.1753-4887.1987.tb02684.x [DOI] [PubMed] [Google Scholar]
- 85.Tsuchida K, Fujihara Y, Hiroki J, et al. Significance of sarcopenia evaluation in acute decompensated heart failure: Skeletal muscle mass index versus fat-free mass index. Int Heart J. 2018;59(1):143–148. doi: 10.1536/ihj.17-057 [DOI] [PubMed] [Google Scholar]
- 86.Gotsman I, Keren A, Amir O, Zwas DR. Increased estimated fat-free mass and fat mass associated with improved clinical outcome in heart failure. Eur J Clin Invest. 2021;(March):1–10. doi: 10.1111/eci.13655 [DOI] [PubMed] [Google Scholar]
- 87.Martone AM, Marzetti E, Calvani R, et al. Exercise and Protein Intake: A Synergistic Approach against Sarcopenia. Biomed Res Int. 2017;2017. doi: 10.1155/2017/2672435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Delmonico MJ, Beck DT. The current understanding of sarcopenia: Emerging tools and interventional possibilities. Am J Lifestyle Med. 2017;11(2):167–181. doi: 10.1177/1559827615594343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ijaz N, Buta B, Xue QL, et al. Interventions for Frailty Among Older Adults With Cardiovascular Disease: JACC State-of-the-Art Review. J Am Coll Cardiol. 2022;79(5):482–503. doi: 10.1016/j.jacc.2021.11.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yin J, Lu X, Qian Z, Xu W, Zhou X. New insights into the pathogenesis and treatment of sarcopenia in chronic heart failure. Theranostics. 2019;9(14):4019–4029. doi: 10.7150/thno.33000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Deutz NE, Matheson EM, Matarese LE, et al. Readmission and mortality in malnourished, older, hospitalized adults treated with a specialized oral nutritional supplement: A randomized clinical trial. Clin Nutr. 2016;35(1):18–26. doi: 10.1016/j.clnu.2015.12.010 [DOI] [PubMed] [Google Scholar]
- 92.Hersberger L, Dietz A, Bürgler H, et al. Individualized Nutritional Support for Hospitalized Patients With Chronic Heart Failure. J Am Coll Cardiol. 2021;77(18):2307–2319. doi: 10.1016/j.jacc.2021.03.232 [DOI] [PubMed] [Google Scholar]


