Abstract
Background & aims
Ketogenic medium-chain-fatty acids (MCFAs) with profound health benefits are commonly found in dairy products, palm kernel oil and coconut oil. We hypothesize that magnesium (Mg) supplementation leads to enhanced gut microbial production of MCFAs and, in turn, increased circulating MCFAs levels.
Methods
We tested this hypothesis in the Personalized Prevention of Colorectal Cancer Trial (PPCCT) (NCT01105169), a double-blind 2 × 2 factorial randomized controlled trial enrolling 240 participants. Six 24-hour dietary recalls were performed for all participants at the baseline and during the intervention period. Based on the baseline 24-hour dietary recalls, the Mg treatment used a personalized dose of Mg supplementation that would reduce the calcium (Ca): Mg intake ratio to around 2.3. We measured plasma MCFAs, sugars, ketone bodies and tricarboxylic acid cycle (TCA cycle) metabolites using the Metabolon’s global Precision Metabolomics™ LC-MS platform. Whole-genome shotgun metagenomics (WGS) sequencing was performed to assess microbiota in stool samples, rectal swabs, and rectal biopsies.
Results
Personalized Mg treatment (mean dose 216.5 mg/day with a range from 77.25 mg/day to 389.55 mg/day) significantly increased the plasma levels of C7:0, C8:0, and combined C7:0 and C8:0 by 18.45%, 25.28%, and 24.20%, respectively, compared to 14.15%, 10.12%, and 12.62% decreases in the placebo arm. The effects remain significant after adjusting for age, sex, race and baseline level (P=0.0126, P=0.0162, and P=0.0031, respectively) and FDR correction at 0.05 (q=0.0324 for both C7:0 and C8:0). Mg treatment significantly reduced the plasma level of sucrose compared to the placebo arm (P=0.0036 for multivariable-adjusted and P=0.0216 for additional FDR correction model) whereas alterations in daily intakes of sucrose, fructose, glucose, maltose and C8:0 from baseline to the end of trial did not differ between two arms. Mediation analysis showed that combined C7:0 and C8:0 partially mediated the effects of Mg treatment on total and individual ketone bodies (P for indirect effect =0.0045, 0.0043, 0.03, respectively). The changes in plasma levels of C7:0 and C8:0 were significantly and positively correlated with the alterations in stool microbiome α diversity (r=0.51, p=0.0023 and r=0.34, p=0.0497, respectively) as well as in stool abundance for the signatures of MCFAs-related microbiota with acyl-ACP thioesterase gene producing C7:0 (r=0.46, p=0.0067) and C8:0 (r=0.49, p=0.003), respectively, following Mg treatment.
Conclusions
Optimizing Ca:Mg intake ratios to around 2.3 through 12-week personalized Mg supplementation leads to increased circulating levels of MCFAs (i.e. C7:0 and C8:0), which is attributed to enhanced production from gut microbial fermentation and, maybe, sucrose consumption.
Keywords: Ca:Mg intake ratio, medium-chain fatty acids, sucrose consumption, microbial alpha diversity, ketone body, randomized controlled trial
Introduction
Unlike long-chain fatty acids (LCFAs), medium-chain fatty acids (MCFAs), a class of saturated fatty acids with 6 to 12 carbon atoms, are absorbed directly by intestinal enterocytes and rapidly transferred by the portal vein to the liver [1]. Further, MCFAs cross the blood-brain barrier and permeate the mitochondrial membrane independently of the carnitine transport system and undergo preferential β-oxidation, suggesting a high efficiency in absorption, transportation and utilization for energy production [1]. In the agriculture industry, MCFAs have emerged as a promising intervention to improve survival of baby animals due to their inhibitory effects against viral and bacterial pathogens and immunomodulatory activities [2]. Furthermore, evidence from human studies suggests that MCFAs have potentials to treat a wide range of neurological and metabolic disorders such as drug-resistant epilepsy [3,4], obesity, type 2 diabetes [5,6], nonalcoholic fatty liver disease (NAFLD) [7], cancer [8,9] and Alzheimer’s disease [10,11].
The natural sources of even-chain MCFAs are dairy products and tropical oils such as palm kernel and coconut oil [12]. Additionally, during industrial ethanolic fermentation, yeasts produce MCFA ethyl esters such as ethyl caproate (C6:0) and ethyl caprylate (C8:0) as important flavor-active compounds[13,14]. Furthermore, a variety of bacteria, mostly anaerobic bacteria, and plants are equipped with acyl-acyl carrier protein (ACP) thioesterases, which are essential chain-terminating enzymes during de novo synthesis of fatty acids, including MCFAs [15]. Anaerobic bacteria including Megasphaera elsdenii and Clostridium sp. BS-1 have been shown to produce MCFAs from sucrose or D-galactitol [16,17]. In vitro studies further indicate that metabolic engineered Escherichia coli resulted in substantially increased production of not only even-, but also odd-chain MCFAs like heptanoate (C7:0) [18], through endogenous expression of acyl-ACP thioesterases from Acinetobacter baylyi [19] and Umbellularia californica (BTE) [19,20]. However, no human or animal studies have been conducted to investigate whether gut microbiota can generate MCFAs.
Ionized calcium (Ca2+) and magnesium (Mg2+) play an important role in determining fermentation performance in yeasts [21,22]. Previous studies consistently found that optimizing the Ca:Mg ratio by supplementing Mg2+ in growth media enhanced the rate of sugar consumption and production yield in industrial yeast fermentation processes [22–24]. Furthermore, the thioesterase enzymes for MCFAs production are highly conserved across different species [25] and their activity is Mg-dependent [26]. Nevertheless, no studies have examined whether optimizing the Ca:Mg ratios by Mg supplementation affects microbial production of MCFAs in in vivo or human studies. In this regard, we hypothesized that optimized dietary Ca:Mg intake ratios could lead to increased circulating level of MCFAs (i.e. C6:0, C7:0 and C8:0) which can be attributed to enhanced production from gut microbial fermentation and sucrose consumption. We tested this hypothesis in the first precision-based randomized trial “Personalized Prevention of Colorectal Cancer Trial” (PPCCT) in which Ca:Mg ratios in diets were optimized to around 2.3 using personized supplementation of Mg [27,28].
Methods
Study Participants
The PPCCT (registered at clinicaltrials.gov as NCT01105169) is a double-blind 2×2 factorial randomized controlled trial conducted at Vanderbilt University Medical Center, Nashville, TN. The detailed study design has been reported previously [27]. In brief, eligible participants were those aged 40 to 85 years old who had a calcium (Ca) intake ≥ 700 mg/day and < 2000 mg/day and in whom the Ca:Mg intake ratio was ≥ 2.6.
Dietary intake assessment
Dietary intake data were collected and analyzed using Nutrition Data System for Research (NDSR) Software Food and Nutrient Database developed at University of Minnesota Nutrition Coordinating Center (NCC) that facilitates the collection of recalls in a standardized fashion (http://www.ncc.umn.edu/products/). The NDSR program automatically generates intake of nutrients per day. It also includes a dietary supplement assessment module so that nutrient intake from both food and supplemental sources can be captured and quantified. Finally, the NDSR software is commonly used in clinical settings and in nutrition research, and NDSR software has been demonstrated to have high inter-rater reliability and good construct validity for identifying individual dietary intake patterns [29].
Interventions and precision-based dosing strategy
Eligible participants were assigned to Mg treatment or placebo according to the randomization schedule. Two 24-h dietary recalls were performed for all participants at the baseline of the PPCCT to determine Ca and Mg intakes. Based on their baseline intakes of Ca and Mg as well as their Ca: Mg intake ratio, each participant was assigned to a customized dose of Mg supplementation that would reduce the Ca: Mg intake ratio to ~2.3, as suggested by several previous studies [30–33]. The mean recorded intake from two 24-h recalls was used to estimate the baseline intakes of Ca and Mg, and the Ca: Mg ratio. Mg glycinate and identical-appearing placebo (i.e. microcrystalline cellulose) were provided in capsules. Four additional 24-hour dietary recalls were conducted for all participants during the intervention period with two during weeks 1 to 6 and the other two during weeks 7 to 12.
Among 250 randomized participants who started treatments, 239 completed the trial and one had donated blood at baseline and at the end of the trial. Therefore, 240 participants had blood collected at baseline and at the end of the trial. The mean daily dose of personalized Mg supplementation was 205.52 mg, with a range from 77.25 to 389.55 mg. Compliance with the pill regimen were very high for both the treatment and placebo arms based on pill counts (96.70% ± 7.61% and 96.60% ± 6.87%, respectively; Pdifference= 0.91). The mean ± SD Ca:Mg ratios for the treatment and placebo arms after administering Mg and placebo supplementation were 2.27 ± 0.13 and 3.87 ± 1.46, respectively (Pdifference<0.0001), at baseline and remained stable at 2.13 ± 0.68 and 3.51 ± 1.32, respectively (Pdifference<0.0001), based on the four 24-hour dietary recalls conducted over the 12-week period of the trial. In this ancillary study, 68 participants were available for plasma metabolites measurement who were balanced on treatment (34 in the treatment and 34 in the placebo arm) and sex.
Measurement of Plasma Metabolites
For all participants, fast blood samples collected pre-intervention and at the end of the trial (12 weeks) will be assayed. Fasting blood samples were stored on ice immediately until processed within 6 hours. All samples were placed in long-term storage in −80°C freezers [27]. Plasma samples were organized in treatment-placebo sets (4 samples in each set: 2 from pre- and 2 from post-treatment). Samples were shipped overnight on dry ice to Metabolon Inc. To control for batch variability, samples from each set were analyzed in the same run. Lab staff were blinded to sample status. We measured plasma MCFAs, sugars, ketone bodies and tricarboxylic acid cycle (TCA cycle) metabolites using the Metabolon’s global Precision Metabolomics™, ultrahigh performance liquid chromatography (UHPLC)-mass spectrometry (MS) as reported in detail previously [28]. Lab staff were blinded to sample status. Sucrose consists of one fructose and one glucose and undergoes hydrolyzation by the enzyme sucrase present in the small intestine or spill over to the liver and to the colonic microbiota [34,35]. Recent controlled feeding studies found that the 24-hour urinary excretion of combined free fructose and the fructose from sucrose is significantly correlated with total sugar intakes, highlighting the importance of combined free fructose and the fructose from sucrose as objective biomarkers of total sugar intakes [34,36,37]. Thus, we calculated total fructose load using the sum of free fructose and the fructose from sucrose in circulation levels, and total glucose load using the sum of free glucose and the glucose from sucrose.
Microbiome Genome Sequencing and Shannon Diversity
The stool, rectal swab and mucosal tissue samples were collected at home or in-person visits at baseline and at the end of the trial. All the samples were frozen at −80 °C until use. For DNA isolation, samples were transferred to a 2 ml tube containing 200 mg of ≤106 μm glass beads (Sigma, St. Louis, MO, USA) and 0.3 ml of Qiagen ATL buffer (Qiagen, Valencia, CA, USA), supplemented with lysozyme (20 mg/ml) (Thermo Fisher Scientific, Grand Island, NY, USA). The suspension was incubated at 37°C for 1 h with occasional agitation and then supplemented with 600IU of proteinase K and incubated at 60°C for 1 h. Finally, 0.3 ml of Qiagen AL (Qiagen, Valencia, CA, USA) buffer were added and incubated at 70°C for 10 minutes, followed with 3 min bead beating in a Qiagen TissueLyser II (Qiagen, Valencia, CA, USA) at 30Hz. After a brief centrifugation, supernatants were transferred to a new tube containing 0.3 ml of ethanol. DNA was purified using a standard on-column purification method with Qiagen buffers AW1 and AW2 (Qiagen, Valencia, CA, USA) as washing agents and eluted in 10mM Tris (pH 8.0).
Whole-genome shotgun metagenomics (WGS) DNA sequencing was performed as previously described [38]. 1 ng of genomic DNA was processed using the Illumina Nextera XT DNA Sample Preparation Kit (Illumina, San Diego, CA, USA). Fragmented and tagged DNA was amplified using a limited-cycle PCR program. In this step, index 1(i7) and index 2(i5) were added between the downstream bPCR adaptor and the core sequencing library adaptor, as well primer sequences required for cluster formation. The DNA library was purified using Agencourt® AMPure® XP Reagent (Beckman Coulter, Brea, CA). The DNA library pool was loaded on the Illumina platform reagent cartridge and on the Illumina HiSeq instrument (Illumina, San Diego, CA, USA). A blank composed of only DNA isolation reagents was included in the DNA extraction process and again in the library preparation. ZymoBIOMICS Microbial Community DNA Standard (Zymo Research Corporation, Irvine, CA, USA, Cat#D6305) and a library blank composed of library preparation reagents alone were used as controls. Sequencing reads were demultiplexed using Illumina Bcl2Fastq 2.18.0.12. Quality of the demultiplexed sequences was verified with FastQC. KneadData was used to remove the human genome contamination from the shotgun metagenome sequencing reads. The taxonomic composition of the filtered reads was characterized with MetaPhlAn2 [39], while the functional genes were annotated with HUMAnN2 [40] following the developers’ instructions. The Shannon diversity of samples were calculated with function ‘diversity’ in R package ‘vegan’.
Microbial Signature of Medium Chain Fatty Acids (MCFAs)
HUMANn2 [40] was used to annotate the functional gene families in our shotgun metagenome sequencing dataset using the UniRef90 database. We identified the abundance of acyl-ACP thioesterase genes using the HUMAnN2 gene family annotations. The acyl-ACP thioesterase encoding microbiota that are potentially responsible for MCFAs production were identified based on the taxonomic stratification of acyl-ACP thioesterase at the species level in taxonomy stratified HUMAnN2 gene family annotations. We then selected all the identified acyl-ACP thioesterase encoding bacteria from the taxonomic count table generated with MetaPhlAn2 on the PPCCT shotgun metagenomics sequencing data.
Statistical Analyses
Continuous variables were summarized as mean ± standard deviations (SD) and between-group comparisons were made using Wilcoxon test; categorical variables were summarized with count and proportion and between-group comparisons were made using chi-square test. A logarithmic transformation was used to improve the normality of circulating MCFAs, sugars, ketone bodies and TCA cycle metabolites, MCFAs intake and sugars intake, bacterial abundance, and acyl-ACP thioesterase gene abundance distributions. Geometric means (coefficient of variation) of baseline MCFAs levels and % changes from baseline were calculated and compared between treatment and placebo arms. GLM models adjusting for age, sex, race and baseline levels were used to assess between-group differences on log-transformed data. To rule out the possibility that increased plasma MCFAs came from dietary and supplement intake, we examined whether the increase in plasma MCFAs were caused by the pre- to post-treatment changes in total intake of MCFAs (C7:0 not measurable due to negligible amounts). Pre-treatment intakes of C6:0, C8:0, sucrose, fructose, glucose, maltose, total fructose load, and total glucose load were averaged from two 24-hour dietary recalls at baseline, and post-treatment intakes were averaged from the last two 24-hour dietary recalls during weeks 7 to 12. In addition, we evaluated whether Mg treatment changed the plasma levels of sucrose, fructose, glucose, maltose, total fructose load, and total glucose load compared to the placebo arm. Mediation analyses [41] were conducted with plasma levels of combined C7:0 and C8:0 as a mediator. Pearson correlation was conducted to examine the correlations between changes in Shannon diversity index, abundance for the signatures of MCFAs-related microbiota with acyl-ACP thioesterase gene, acyl-ACP thioesterase gene abundance/copy number, and the alterations in plasma levels of MCFAs. Based on a positive correlation coefficient (r ≥0.2) following Mg treatment, a microbial signature of MCFAs, i.e. Mg-modifiable signature of MCFAs-related microbiota with acyl-ACP thioesterase gene was developed. All P values are two sided and statistical significance was determined using an alpha level of 0.05. The data analyses used software SAS Enterprise Guide 7.1. To account for multiple comparisons, we set false discovery rate (FDR) at 0.05 using the FDR-controlling procedures by Benjamini and Hochberg [42].
Results
Presented in Table 1 are the comparisons of baseline characteristics between the treatment and placebo arms for 68 participants included in the current study (Supplemental Figure S1). The treatment arm was not significantly different from the placebo arm for baseline demographic variables, including age, sex, race, educational achievement, smoking status, alcohol drinking status, physical activity status, daily intake of total energy, fat, total MCFAs, total Ca, total Mg, intake ratio of Ca to Mg and factors related to cardiometabolic events, including body mass index (BMI), blood pressure and eGFR (estimated glomerular filtration rate).
Table 1.
Characteristics of treatment vs. placebo arms at baseline, PPCCT
| Placebo | Treatment | P value | |
|---|---|---|---|
| n=34 | n=34 | ||
| Age, year | 63.24±7.65 | 61.35±9.00 | 0.32 |
| Sex - male (%) | 55.88 | 47.06 | 0.47 |
| Race - white (%) | 97.06 | 100 | 0.31 |
| Education under college (%) | 5.88 | 11.76 | 0.45 |
| Smoking status (%) | 0.61 | ||
| Never | 55.88 | 44.12 | |
| Ever | 38.24 | 47.06 | |
| Current | 5.88 | 8.82 | |
| Drinking status (%) | 0.15 | ||
| Never | 47.06 | 26.47 | |
| Ever | 14.71 | 29.41 | |
| Current | 38.24 | 44.12 | |
| Physically active in ≥ 2 days per week (%) | 82.35 | 64.71 | 0.10 |
| Body mass index (BMI), kg/m2 | 29.54±4.77 | 29.61±6.13 | 0.98 |
| Systolic blood pressure, mmHg | 127±14 | 127±14 | 0.95 |
| Diastolic blood pressure, mmHg | 73.39±7.68 | 71.97±7.79 | 0.37 |
| eGFR, mL/(min·1.73m2) | 77.41±14.74 | 82.71±15.32 | 0.10 |
| Daily nutrients intake | |||
| Total energy intake, kcal/day | 2060±535 | 2000±478 | 0.32 |
| Fat, g/day | 86.82±31.21 | 79.06±28.70 | 0.13 |
| Total MCFA intake (C6:0-C12:0), g/day | 2.67±1.92 | 2.35±1.72 | 0.40 |
| Caproate (C6:0), g/day | 0.45±0.30 | 0.34±0.25 | 0.07 |
| Caprylate (C8:0), g/day | 0.36±0.24 | 0.31±0.25 | 0.28 |
| Caprate (C10:0), g/day | 0.68±0.42 | 0.57±0.40 | 0.15 |
| Laurate (C12:0), g/day | 1.18±1.23 | 1.13±0.93 | 0.96 |
| Total Ca intake, mg/day | 1225±462 | 1223±404 | 0.84 |
| Total Mg intake, mg/day | 354±173 | 355±88 | 0.22 |
| Ca:Mg intake ratio | 3.64±0.97 | 3.46±0.91 | 0.39 |
Continuous variables were presented as mean±SD and P values were calculated using Wilcoxon test; categorical variables were presented as % and P values were calculated using Pearson chi-square test.
eGFR, estimated glomerular filtration rate; MCFA, medium chain fatty acid; PPCCT, Personalized Prevention of Colorectal Cancer Trial.
We found Mg treatment did not significantly affect C6:0 compared to the placebo arm. Mg treatment significantly increased plasma levels of C7:0 and C8:0 by 18.45% and 25.28%, respectively, compared to 14.15% and 10.12% decreases in the placebo arm (P=0.0093, P=0.0046, respectively) (Table 2). Thus, we examined the effect of Mg treatment on combined C7:0 and C8:0 and found Mg treatment significantly increased levels of combined C7:0 and C8:0 by 24.20% compared to a 12.62% reduction in the placebo arm (P=0.0005). The effects remained significant after adjusting for age, sex, race and baseline levels for C7:0, C8:0 and combined C7:0 and C8:0 (P=0.0126, P=0.0162, and P=0.0031, respectively) and additionally controlling FDR at 0.05 (P=0.0324 for both C7:0 and C8:0). In exploratory analysis, we found Mg treatment did not alter the levels of caprate (C10:0) and laurate (C12:0), other two MCFAs (data not shown). In additional analysis, the changes in intakes of C8:0 from both dietary and supplemental sources from before treatment to the end of the trial did not significantly differ by treatment status. We found Mg treatment significantly reduced the plasma levels of free sucrose, total fructose load, and total glucose load compared to the placebo arm (P=0.0216, 0.0444, 0.0444, respectively, for multivariable-adjusted model after additional FDR correction). On the other hand, we did not find the changes in intakes of sucrose, fructose, glucose and maltose from baseline to the end of trial differed between the treatment and placebo arms.
Table 2.
Mg treatment and changes in circulating levels and intakes of MCFAs and sugars, PPCCT
| Placebo arma | Treatment arma | P values | |||||
|---|---|---|---|---|---|---|---|
| Baseline level | % Change | Baseline level | % Change | P 1 | P 2 | P 3 | |
| n=34 | n=34 | ||||||
| Plasma MCFAs | |||||||
| Caproate (C6:0) | 0.81 (0.82) | 30.14% | 0.65 (0.66) | 14.69% | 0.54 | 0.16 | 0.16 |
| Heptanoate (C7:0) | 0.75 (0.54) | −14.15% | 0.70 (0.44) | 18.45% | 0.0093** | 0.0126* | 0.0324* |
| Caprylate (C8:0) | 1.19 (0.63) | −10.12% | 0.96 (0.35) | 25.28% | 0.0046** | 0.0162* | 0.0324* |
| C7:0 + C8:0 | 2.06 (0.51) | −12.62% | 1.70 (0.32) | 24.20% | 0.0005*** | 0.0031** | - |
| Plasma sugars | |||||||
| Free sucrose | 0.62 (2.17) | 36.46% | 0.72 (2.52) | −55.04% | 0.002** | 0.0036** | 0.0216* |
| Free fructose | 0.95 (0.33) | 2.11% | 1.10 (0.30) | −6.36% | 0.27 | 0.68 | 0.68 |
| Free glucose | 1.04 (0.15) | 0.56% | 1.00 (0.10) | −0.58% | 0.69 | 0.43 | 0.64 |
| Free maltose | 0.99 (0.98) | −17.41% | 1.18 (1.13) | −15.60% | 0.91 | 0.57 | 0.68 |
| Total fructose loadb | 1.94 (0.68) | 29.54% | 2.23 (0.72) | −28.38% | 0.0063** | 0.0222* | 0.0444* |
| Total glucose loadc | 2.01 (0.65) | 17.08% | 2.13 (0.71) | −25.24% | 0.0080** | 0.0150* | 0.0444* |
| Daily intaked | |||||||
| Caprylate (C8:0) | 0.29 (0.86) | −35.59% | 0.25 (0.75) | −19.14% | 0.31 | 0.42 | 0.88 |
| Sucrose | 34.83 (0.96) | −20.30% | 34.10 (1.17) | −15.16% | 0.92 | 0.78 | 0.88 |
| Fructose | 15.57 (0.75) | −25.88% | 19.15 (0.83) | −18.43% | 0.75 | 0.62 | 0.88 |
| Glucose | 18.28 (0.71) | −9.41% | 17.80 (0.77) | −9.27% | 0.93 | 0.88 | 0.88 |
| Maltose | 2.64 (0.74) | −29.22% | 2.15 (1.06) | −1.14% | 0.23 | 0.21 | 0.88 |
Data are presented in geometric mean (coefficient of variation) or percent change in geometric mean.
Total fructose load was defined as the sum of free fructose and free sucrose because sucrose consists of one fructose and one glucose.
Total glucose load was defined as the sum of free glucose and free sucrose.
Daily intake incorporated dietary intake from food and supplement use.
P1: P value for unadjusted GLM model using log-transformed data; P2: P value for GLM model adjusting for age, sex, race and baseline level using log-transformed data; P3: P value for GLM model adjusting for age, sex, race and baseline level after FDR correction using log-transformed data. Mg, magnesium; MCFA, medium chain fatty acid; PPCCT, Personalized Prevention of Colorectal Cancer Trial.
P<0.05,
P<0.01,
P<0.001.
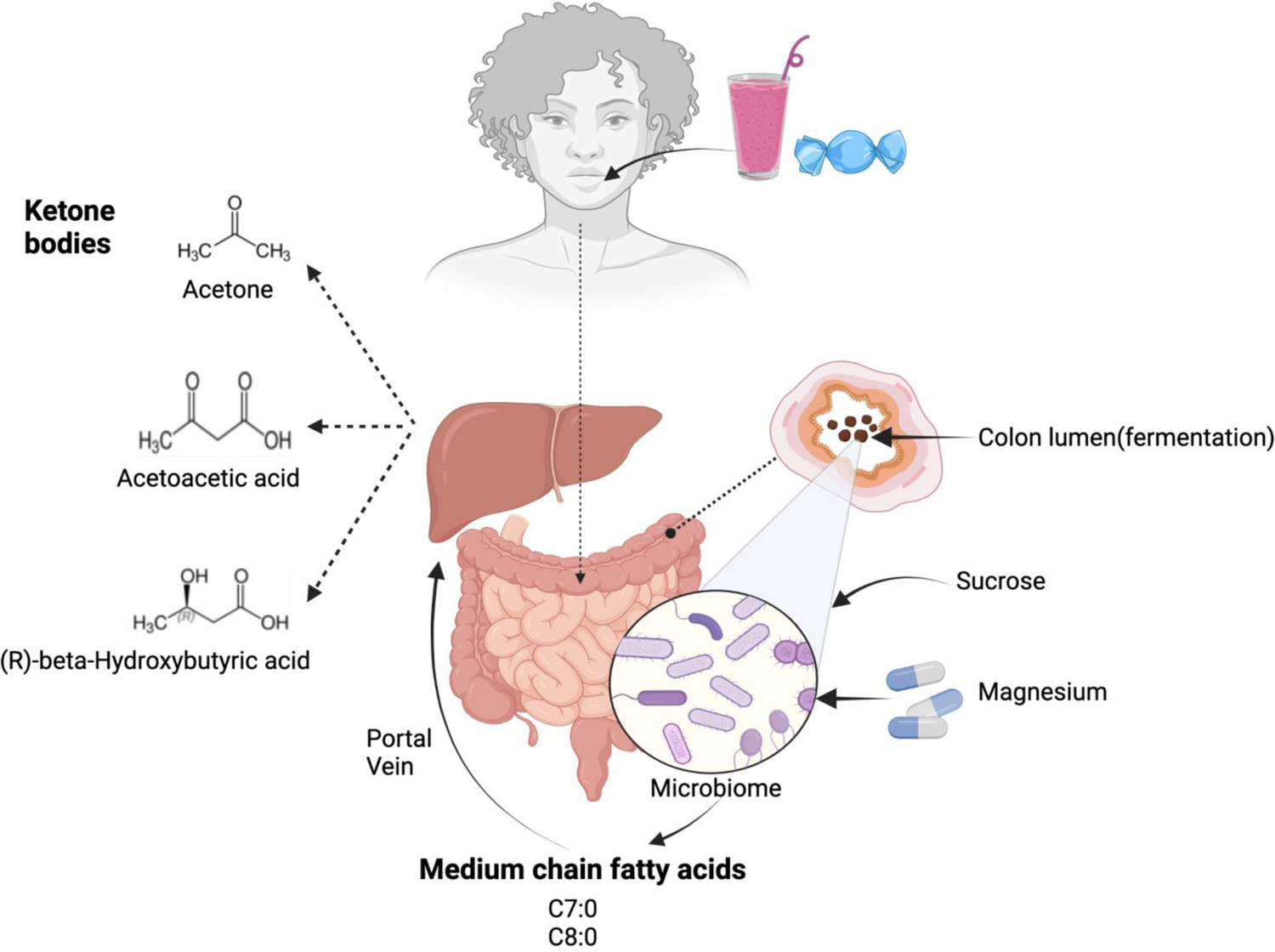
Since we found Mg treatment significantly increased plasma levels of MCFAs (i.e. C7:0, C8:0, and combined C7:0 and C8:0) which improve cellular energy production including ketone bodies [43], we next examined whether the increased MCFAs act as a mediator of Mg treatment on levels of total ketone bodies and TCA cycle metabolites. Table 3 shows results from the mediation analysis. We found that combined C7:0 and C8:0 significantly mediated the effects of Mg treatment on total and individual ketone bodies (P for indirect effect=0.0045, 0.0043 and 0.03, respectively). The indirect effect mediated by combined C7:0 and C8:0 on total ketone bodies accounted for 76.78% of the total effect. However, we did not find combined C7:0 and C8:0 significantly mediate the effect of Mg treatment on fumarate, malate and total TCA cycle metabolites (P for indirect effect =0.17, 0.23, 0.14, respectively). Based on these findings, Figure 1 schematically illustrates our proposed molecular mechanism for the effect of Mg treatment on MCFAs and ketone bodies (see our detailed interpretations in the Discussion section).
Table 3.
Total, direct and indirect effects of Mg treatment on ketone bodies and TCA cycle metabolites, PPCCT
| Total effect | Direct effect | Indirect effect | ||||
|---|---|---|---|---|---|---|
| Outcomes (mediator: combined C7:0 and C8:0)d | Point estimate (95% CI)c | P value | Point estimate (95% CI)c | P value | Point estimate (95% CI)c | P value |
| Total ketone bodies a | 0.53 (0.11, 1.00) | 0.02 | 0.12 (−0.28, 0.49) | 0.58 | 0.41 (0.14, 0.75) | 0.0045** |
| Acetoacetate | 0.38 (−0.08, 0.82) | 0.10 | −0.03 (−0.43, 0.35) | 0.88 | 0.42 (0.17, 0.77) | 0.0043** |
| 3-hydroxybutyrate (BHBA) | 0.51 (0.09, 1.00) | 0.03 | 0.25 (−0.16, 0.59) | 0.31 | 0.26 (0.02, 0.66) | 0.03* |
| Total TCA cycle metabolites b | 0.37 (−0.07, 0.83) | 0.12 | 0.21 (−0.30, 0.77) | 0.41 | 0.16 (−0.07, 0.44) | 0.17 |
| Fumarate | 0.42 (−0.03, 0.91) | 0.08 | 0.28 (−0.22, 0.84) | 0.26 | 0.13 (−0.07, 0.41) | 0.23 |
| Malate | 0.26 (−0.23, 0.74) | 0.28 | 0.09 (−0.48, 0.68) | 0.73 | 0.17 (−0.04, 0.44) | 0.14 |
Total ketone bodies=acetoacetate+3-hydroxybutyrate. All values are log transformed.
Total TCA cycle metabolites=fumarate+malate. All values are log transformed.
95%CIs are bootstrap bias corrected.
Mediation analyses were conducted with plasma levels of combined C7:0 and C8:0 as a mediator.
P<0.05,
P<0.01,
P<0.001.
Mg: magnesium. TCA cycle, tricarboxylic acid cycle; PPCCT, Personalized Prevention of Colorectal Cancer Trial.
Figure 1.
Proposed Molecular Mechanism
Table 4 shows the correlations between changes in gut microbial diversity measured by Shannon diversity index at genus level from stool samples, abundance for the signatures of MCFAs-related microbiota with acyl-ACP thioesterase gene, acyl-ACP thioesterase gene abundance/copy number in stool and plasma levels of MCFAs. In the treatment arm, the changes in plasma levels of combined C7:0 and C8:0 were significantly positively correlated with alterations in stool Shannon index at genus level (r=0.50, P=0.0026) whereas there were no significant correlations in the placebo arm in stool samples. There were no significant correlations in swab and tissue samples regardless of treatment arm (Data not shown). Based on these findings, we next identified what microbiota in stool samples contribute to the increased levels of C7:0, C8:0 and combined C7:0 and C8:0. Using the shotgun metagenomics sequencing data for stool samples in the PPCCT, we first identified three groups of MCFAs-related microbiota with acyl-ACP thioesterase gene (Supplemental Table S1) for C7:0, C8:0, or combined C7:0 and C8:0. The variations in abundance of bacteria in these three groups were positively correlated (correlation coefficient (r) ≥0.2) with the changes in plasma levels of C7:0, C8:0 and combined C7:0 and C8:0, respectively, in the treatment arm, but not in the placebo arm following Mg treatment. Subsequent, we formed three microbial signatures of MCFAs for C7:0, C8:0 and combined C7:0 and C8:0 by summing up the individual abundance in each group. The changes in the specific microbial signatures in the treatment arm were correlated with circulating C7:0 (r=0.46, p=0.0067), C8:0 (r=0.49, p=0.003) and combined C7:0 and C8:0 (r=0.56, p=0.0006), respectively. The variations in acyl-ACP thioesterase gene abundance/copy number in stool in these three groups were positively correlated with the changes in plasma levels of C7:0(r=0.30, p=0.08), C8:0 (r=0.23, p=0.20) and combined C7:0 and C8:0 (r=0.32, p=0.06), respectively, in the treatment arm, but not in the placebo arm. The correlations for C7:0 and combined C7:0 and C8:0 in the treatment arm were of borderline significance.
Table 4.
Changes in gut microbial α-diversity index, abundance for the signatures of MCFAs-related microbiota with acyl-ACP thioesterase gene, and acyl-ACP thioesterase gene abundance with plasma MCFAs by Mg treatment
| Plasma MCFAs | Mg treatment | Placebo | ||||
|---|---|---|---|---|---|---|
| N | r | P | N | r | P | |
| Shannon diversity index in stool | ||||||
| Combined C7:0 and C8:0 | 0.50 | 0.0026** | 0.003 | 0.99 | ||
| C7:0 | 34 | 0.51 | 0.0023** | 30 | 0.003 | 0.99 |
| C8:0 | 0.34 | 0.0497* | 0.03 | 0.87 | ||
| Abundance for the signatures of MCFAs-related microbiota with acyl-ACP thioesterase gene | ||||||
| Combined C7:0 and C8:0 | 0.56 | 0.0006*** | −0.05 | 0.79 | ||
| C7:0 | 0.46 | 0.0067** | 30 | 0.04 | 0.81 | |
| 34 | ||||||
| C8:0 | 0.49 | 0.003** | −0.08 | 0.69 | ||
| Acyl-ACP thioesterase gene abundance/copy number | ||||||
| Combined C7:0 and C8:0 | 0.32 | 0.06 | −0.21 | 0.26 | ||
| C7:0 | 34 | 0.30 | 0.08 | 30 | −0.22 | 0.24 |
| C8:0 | 0.23 | 0.20 | −0.19 | 0.31 | ||
P<0.05,
P<0.01,
P<0.001. Abundance for the signatures of MCFAs-related microbiota with acyl-ACP thioesterase gene, and acyl-ACP thioesterase gene abundance in stool were log-transformed. Microbial signature of MCFAs was the sum of the relative abundance of all related bacteria. Pearson correlation was used for detecting a correlation.
Mg, magnesium; MCFA, medium chain fatty acid; PPCCT, Personalized Prevention of Colorectal Cancer Trial.
Microbial signature of C7 related bacteria included Prevotella buccalis, Clostridium hylemonae, Ruminococcus obeum, Dorea formicigenerans, Catenibacterium mitsuokai, and Pyramidobacter piscolens.
Microbial signature of C8 related bacteria included Prevotella buccalis, Prevotella copri, Eubacterium ventriosum, and Pyramidobacter piscolens.
Microbial signature of combined C7 and C8 related bacteria included Prevotella buccalis, Prevotella copri, Ruminococcus obeum, Coprococcus catus, and Pyramidobacter piscolens.
Discussion
In this precision-based randomized trial conducted among participants who consumed high Ca:Mg ratio diets, reducing Ca:Mg ratios to around 2.3 through a 12-week personalized Mg treatment significantly increased plasma levels of C7:0 and C8:0 that were not explained by changes in intake levels of MCFAs. Likewise, the significant reduction in circulating levels of sucrose, total fructose load, and total glucose load that accompanied the increase in plasma levels of MCFAs cannot be explained by changes in intakes of sucrose, fructose, glucose, and maltose. Further, we found combined C7:0 and C8:0 partially mediated the effects of Mg treatment on total and individual ketone bodies. Finally, we found the increase in plasma levels of combined C7:0 and C8:0 were positively correlated with increased microbial α-diversity and abundance for the signatures of MCFAs-related microbiota with acyl-ACP thioesterase gene in stool samples following Mg treatment. Taken together, these novel findings indicate that improvement in Mg availability in the gut that occurs by optimizing Ca:Mg intake ratio leads to increased MCFAs entering the circulation, and in turn, elevated levels of ketone bodies, which is attributable to increased abundance in Mg-modifiable signatures of MCFAs-related microbiota and enhanced gut microbial fermentation and sucrose consumption.
Despite accumulating observations from in vitro studies [13,14,16,17,20,44–47], no in vivo study has been conducted to examine whether MCFAs can be microbially produced in animals or humans. Our finding is consistent with our hypothesis that optimized dietary Ca:Mg intake ratios could lead to increased circulating level of MCFAs which can be attributed to enhanced production from gut microbial fermentation and sucrose consumption. Previous animal studies found that a Mg-deficient diet leads to lower gut Bifidobacteria levels which are associated with increased intestinal permeability and inflammatory response [48] and Mg supplementation enhanced the diversity of gastrointestinal microbiota in animal models [49]. In the current study, we found the changes in MCFAs caused by Mg treatment were significantly and positively correlated with changes in both microbial α-diversity and abundance in Mg-modifiable signatures of MCFAs-related microbiota with acyl-ACP thioesterase gene in stool samples, supporting the involvement of the gut microbiota in the production of MCFAs. Meanwhile, we found there was a significant concomitant reduction in plasma levels of sucrose following the increases in plasma MCFAs induced by Mg treatment. This finding is biologically plausible because microbial fermentation has been suggested to rely on the irreversible conversion of sugar substrates including sucrose into distinct short chain fatty acid (SCFA) and MCFA products [16,17,44–47]. For example, one major member of signatures of MCFAs-related microbiota, Prevotella buccalis, characteristically can metabolize sucrose [50]. Sucrose, or table sugar, one common component in Western diet, has been consumed at an increasing trend over the past 100 years [51]. While sucrose is one type of dimeric sugar consisting of one glucose and one fructose, sucrose and free fructose are metabolically equivalent and have similar pathological effects [52]. The health hazards associated with excess intake of sugar (i.e., mainly sucrose and fructose in sugar-sweetened beverages) have been increasingly recognized, including NAFLD [53], metabolic diseases [54], cardiovascular disease [55], colorectal cancer [56], cognitive decline and Alzheimer’s disease [57], all of which are also associated with Mg deficiency and may benefit from treatment of MCFAs. Recent evidence shows that sucrose is capable of impeding gut colonization by beneficial microbes through specifically silencing a critical colonization factor called Roc protein [58]. Our findings of significant reduction in plasma levels of free sucrose, total fructose load and total glucose load following Mg treatment indicate that optimizing Ca:Mg ratio in diets may convert excessive detrimental sugars into microbially produced beneficial MCFAs and, in turn, provide potential benefits for ameliorating metabolic diseases linked to Mg deficiency and high sugar consumption. However, future studies are required to confirm this possibility.
An anaerobic environment is a requisite under which MCFAs are favorably produced relative to LCFAs. Saccharomyces cerevisiae, a wine fermentative yeast which naturally consumes sucrose as a substrate, produces fatty acid products with a high MCFA/LCFA ratio under anaerobic conditions, but a low ratio (<1) if an aeration strategy is used [59]. Previous studies found the colon has the steepest oxygen gradient in the body, with the oxygen decline from the intestinal mucosa towards the lumen [38,60]. Thus, anaerobic microorganisms which play a key role in fermentation and metabolism of luminal contents (e.g. nutrients or carcinogens) [61–63] are more abundant in luminal than mucosal environments [64]. This may explain why increased levels of MCFAs following Mg treatment are positively correlated with the rise in microbial α-diversity only in stool samples, but not rectal swab and rectal biopsies. Overall, the correlations between changes in acyl-ACP thioesterase gene abundance in stool and the alterations in plasma MCFA levels in the treatment group are consistent with those between abundance for the signatures of MCFAs-related microbiota with acyl-ACP thioesterase gene and plasma MCFA levels (Combined C7:0 & C8:0, C7:0, C8:0), respectively. However, the correlations were stronger for the abundance for the signatures of MCFAs-related microbiota than for acyl-ACP thioesterase gene abundance in the treatment arm. One possible cause for the difference is that acyl-ACP thioesterase gene is one necessary factor, but not a sufficient factor to generate C7:0 and/or C8:0. Unlike the abundance for the signatures of MCFAs-related microbiota with acyl-ACP thioesterase gene, which only include microbiota positively correlating circulating levels of C7:0 and/or C8:0, the gene abundance includes microbiota generating C7:0 and/or C8:0 and those not generating C7:0 and/or C8:0. As a result, the correlations for acyl-ACP thioesterase gene abundance are diluted.
Therapy using MCFAs has consistently been shown to improve cellular energy utilization both in animal models and humans [3–6,8–11], however, the mechanisms are not entirely clear. In the current study, we examined whether the increased MCFAs act as a mediator on levels of total and individual ketone bodies and TCA cycle metabolites that are involved in energy production. Results from our mediation analysis showed that Mg treatment led to increased levels of C7:0 and C8:0, and in turn, an increase in levels of individual and total ketone bodies. This finding further supports the biological mechanism from previous observations suggesting that a medium-chain triglyceride (MCT) ketogenic diet was beneficial for a wide range of neurological and metabolic disorders characterized as dysregulated cellular energy metabolism [3–11]. Our findings that C8:0, considering that C7:0 is in negligible amounts in natural sources, rather than any other MCFAs (i.e., C6:0, C10:0 and C12:0), mediated the Mg-induced effects on ketone bodies. C8:0 is the most potent ketogenic MCFA, which generating three times more ketones than C10:0 and about six times more than C12:0 [65]. Our findings also provide a novel mechanism through which optimal Mg status reduce risks of a variety of metabolic and neurodegenerative disorders [66–72].
One promising finding from the current study is that in addition to C8:0, we found Mg treatment also significantly increased plasma levels of C7:0, a MCFA with an odd number of carbons. C7:0 possesses unique beneficial features that even-numbered MCFAs (e.g. C8:0) do not [73–75]. Previous studies found C7:0 has anti-inflammatory effects and can be used for treating psoriasis, allergies and autoimmune diseases [75]. Regardless of their nutritional and pharmaceutical applications, the amounts of nature-originated odd-numbered MCFAs in dairy products and tropical oils are negligible [76]. Therefore, it is unlikely that the increase in plasma levels of C7:0 is caused by dietary intake or supplemental use of C7:0. In addition, dietary intakes of both C8:0 and sucrose did not change significantly from baseline to the end of the trial. Thus, our findings indicate that Mg treatment not only increases even-numbered MCFAs, but also increases the microbial production of odd-numbered MCFAs (i.e. C7:0). Collectively, our findings indicate the synergistic use of Mg and MCFAs, especially C8:0, as a potential therapy for the prevention and treatment of a number of neurodegenerative and metabolic diseases.
This study has several strengths, including the double-blinded randomized trial design. Furthermore, a precision-based design was used. Intakes of Mg and Ca from both diet and supplements were measured twice before and four times during the treatment and a personalized dosing strategy of Mg supplementation was administered to each participant. In addition, we had a high compliance with the pill regimen for both the treatment and placebo arms based on pill counts (96.70% ± 7.61% and 96.60% ± 6.87%, respectively; Pdifference= 0.91) and the dropout rate was very low (4%).
There are some limitations for our study. Although we identified Mg-modifiable signatures of MCFAs-related microbiota with acyl-ACP thioesterase gene, and observed its correlation with changes in plasma MCFAs, the microbiome associated MCFAs production in the human gut and underlying mechanisms are not well understood. Other microorganisms may also contribute to the MCFAs production process. Furthermore, we cannot rule out the possibility of impact of other metabolic pathways involved in MCFAs production and release. In particular, Mg may increase the enzyme activity of acyl-ACP thioesterase, but not the abundance of acyl-ACP thioesterase encoding bacteria. In these cases, the metagenomic approach used in the current study cannot detect these changes, neither can a transcriptomics approach. Future experiments using RT PCR or RNAseq are needed to further verify the findings that acyl-ACP thioesterase is expressed by members of the stool microbiota in the Mg supplemented arm compared to the others. Secondly, the plasma levels of MCFAs are influenced by homeostasis between synthesis and degradation, which may not accurately reflect the amount of MCFAs that were initially produced by microbiota in the human gut. Furthermore, the intestinal absorption of nutrients including sucrose is regulated by gut mucosal barrier function and intestinal permeability so that it is not clear whether the reduction in plasma levels of sucrose after Mg treatment is due to increased consumption of sucrose by gut microbiota or reduced absorption following decreased intestinal permeability. Lastly, the sample size is relatively small in our study. However, the effects of Mg on MCFAs are still highly significant even after the multiple comparison corrections indicating the effects of Mg treatment on MCFAs are robust. Furthermore, considering the results from pathway analyses including mediation analysis are highly consistent, the findings from our study are unlikely to be solely due to chance. In addition, it is possible that the non-significant effects of Mg treatment on TCA cycle metabolites may be due to a small sample size. Future studies are necessary to further address these important scientific inquiries.
In summary, optimizing Ca:Mg intake ratios to around 2.3 through 12-week personalized Mg supplementation leads to increased circulating levels of MCFAs (i.e. C7:0 and C8:0), which are attributable to enhanced production from gut microbial fermentation and, maybe, sucrose consumption. These findings, if confirmed, have significant implications for the development of novel and effective interventions for a large variety of human diseases, such as neurodegenerative, metabolic, and immunomodulatory disorders, and improved animal health, particularly survival in baby animals.
Supplementary Material
Funding Statement:
Part of the study was supported by R01 CA149633, R01 CA202936 and R01 DK110166 from the National Cancer Institute, Department of Health and Human Services as well as the Ingram Cancer Center Endowment Fund. Data collection, sample storage and processing for this study were partially conducted by the Survey and Biospecimen Shared Resource, which is supported in part by P30CA68485. Clinical visits to the Vanderbilt Clinical Research Center were supported in part by the Vanderbilt CTSA grant UL1 RR024975 from NCRR/NIH. The parent study data were stored in Research Electronic Data Capture (REDCap) and data analyses (VR12960) were supported in part by the Vanderbilt Institute for Clinical and Translational Research (UL1TR000445). The UNC Microbiome Core is funded in part by the Center for Gastrointestinal Biology and Disease (CGIBD P30 DK034987) and the UNC Nutrition Obesity Research Center (NORC P30 DK056350). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Ethics declarations: All authors have no conflicts of interest.
The Personalized Prevention of Colorectal Cancer Trial (PPCCT) was registered at clinicaltrials.gov as NCT01105169.
References
- [1].Schönfeld P, Wojtczak L. Short- and medium-chain fatty acids in energy metabolism: the cellular perspective. J Lipid Res 2016;57:943–54. 10.1194/jlr.R067629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Jackman JA, Boyd RD, Elrod CC. Medium-chain fatty acids and monoglycerides as feed additives for pig production: towards gut health improvement and feed pathogen mitigation. J Anim Sci Biotechnol 2020;11:44. 10.1186/s40104-020-00446-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Ye F, Li X-J, Jiang W-L, Sun H-B, Liu J. Efficacy of and patient compliance with a ketogenic diet in adults with intractable epilepsy: a meta-analysis. J Clin Neurol 2015;11:26–31. 10.3988/jcn.2015.11.1.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Martin K, Jackson CF, Levy RG, Cooper PN. Ketogenic diet and other dietary treatments for epilepsy. Cochrane Database Syst Rev 2016;2:CD001903. 10.1002/14651858.CD001903.pub3. [DOI] [PubMed] [Google Scholar]
- [5].Geng S, Zhu W, Xie C, Li X, Wu J, Liang Z, et al. Medium-chain triglyceride ameliorates insulin resistance and inflammation in high fat diet-induced obese mice. Eur J Nutr 2016;55:931–40. 10.1007/s00394-015-0907-0. [DOI] [PubMed] [Google Scholar]
- [6].Mumme K, Stonehouse W. Effects of medium-chain triglycerides on weight loss and body composition: a meta-analysis of randomized controlled trials. J Acad Nutr Diet 2015;115:249–63. 10.1016/j.jand.2014.10.022. [DOI] [PubMed] [Google Scholar]
- [7].Wang B, Li L, Fu J, Yu P, Gong D, Zeng C, et al. Effects of Long-Chain and Medium-Chain Fatty Acids on Apoptosis and Oxidative Stress in Human Liver Cells with Steatosis. J Food Sci 2016;81:H794–800. 10.1111/1750-3841.13210. [DOI] [PubMed] [Google Scholar]
- [8].Abdelwahab MG, Fenton KE, Preul MC, Rho JM, Lynch A, Stafford P, et al. The ketogenic diet is an effective adjuvant to radiation therapy for the treatment of malignant glioma. PLoS One 2012;7:e36197. 10.1371/journal.pone.0036197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Jansen N, Walach H. The development of tumours under a ketogenic diet in association with the novel tumour marker TKTL1: A case series in general practice. Oncol Lett 2016;11:584–92. 10.3892/ol.2015.3923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Chatterjee P, Fernando M, Fernando B, Dias CB, Shah T, Silva R, et al. Potential of coconut oil and medium chain triglycerides in the prevention and treatment of Alzheimer’s disease. Mech Ageing Dev 2020;186:111209. 10.1016/j.mad.2020.111209. [DOI] [PubMed] [Google Scholar]
- [11].Taylor MK, Swerdlow RH, Sullivan DK. Dietary Neuroketotherapeutics for Alzheimer’s Disease: An Evidence Update and the Potential Role for Diet Quality. Nutrients 2019;11. 10.3390/nu11081910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Marten B, Pfeuffer M, and Schrezenmeir J. Medium-chain triglycerides. Int. Dairy J 2006;16:1374–1382. [Google Scholar]
- [13].Viegas CA, Rosa MF, Sá-Correia I, Novais JM. Inhibition of Yeast Growth by Octanoic and Decanoic Acids Produced during Ethanolic Fermentation. Appl Environ Microbiol 1989;55:21–8. 10.1128/AEM.55.1.21-28.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Yin H, Liu L-P, Yang M, Ding X-T, Jia S-R, Dong J-J, et al. Enhancing Medium-Chain Fatty Acid Ethyl Ester Production During Beer Fermentation Through EEB1 and ETR1 Overexpression in Saccharomyces pastorianus. J Agric Food Chem 2019;67:5607–13. 10.1021/acs.jafc.9b00128. [DOI] [PubMed] [Google Scholar]
- [15].Beld J, Blatti JL, Behnke C, Mendez M, Burkart MD. Evolution of acyl-ACP-thioesterases and β-ketoacyl-ACP-synthases revealed by protein-protein interactions. J Appl Phycol 2014;26:1619–29. 10.1007/s10811-013-0203-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Choi K, Jeon BS, Kim B-C, Oh M-K, Um Y, Sang B-I. In situ biphasic extractive fermentation for hexanoic acid production from sucrose by Megasphaera elsdenii NCIMB 702410. Appl Biochem Biotechnol 2013;171:1094–107. 10.1007/s12010-013-0310-3. [DOI] [PubMed] [Google Scholar]
- [17].Jeon BS, Kim B-C, Um Y, Sang B-I. Production of hexanoic acid from D-galactitol by a newly isolated Clostridium sp. BS-1. Appl Microbiol Biotechnol 2010;88:1161–7. 10.1007/s00253-010-2827-5. [DOI] [PubMed] [Google Scholar]
- [18].Volker AR, Gogerty DS, Bartholomay C, Hennen-Bierwagen T, Zhu H, Bobik TA. Fermentative production of short-chain fatty acids in Escherichia coli. Microbiology (Reading) 2014;160:1513–22. 10.1099/mic.0.078329-0. [DOI] [PubMed] [Google Scholar]
- [19].Sherkhanov S, Korman TP, Bowie JU. Improving the tolerance of Escherichia coli to medium-chain fatty acid production. Metab Eng 2014;25:1–7. 10.1016/j.ymben.2014.06.003. [DOI] [PubMed] [Google Scholar]
- [20].Lennen RM, Braden DJ, West RA, Dumesic JA, Pfleger BF. A process for microbial hydrocarbon synthesis: Overproduction of fatty acids in Escherichia coli and catalytic conversion to alkanes. Biotechnol Bioeng 2010;106:193–202. 10.1002/bit.22660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Walker GM. Metals in yeast fermentation processes. Adv Appl Microbiol 2004;54:197–229. 10.1016/S0065-2164(04)54008-X. [DOI] [PubMed] [Google Scholar]
- [22].Walker GM, Birch RM, Chandrasena G, Maynard AI. Magnesium, calcium, and fermentative metabolism in industrial yeasts. Journal of the American Society of Brewing Chemists 1996;54:13–8. [Google Scholar]
- [23].Udeh HO, Kgatla TE, Jideani AIO. Effect of mineral ion addition on yeast performance during very high gravity wort fermentation. International Journal of Biological, Biomolecular, Agricultural, Food and Biotechnological Engineering 2014;8:1208–16. [Google Scholar]
- [24].Walker GM, Maynard AI, Johns CGW. The importance of magnesium ions in yeast biotechnology. Fermentation technologies: industrial applications. 1 ed. London: Elsevier Applied Science. 1990;233–240. [Google Scholar]
- [25].Cantu DC, Chen Y, Reilly PJ. Thioesterases: a new perspective based on their primary and tertiary structures. Protein Sci 2010;19:1281–95. 10.1002/pro.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Libertini LJ, Smith S. Purification and properties of a thioesterase from lactating rat mammary gland which modifies the product specificity of fatty acid synthetase. J Biol Chem 1978;253:1393–401. [PubMed] [Google Scholar]
- [27].Dai Q, Zhu X, Manson JE, Song Y, Li X, Franke AA, et al. Magnesium status and supplementation influence vitamin D status and metabolism: results from a randomized trial. Am J Clin Nutr 2018;108:1249–58. 10.1093/ajcn/nqy274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Fan L, Yu D, Zhu X, Huang X, Murff HJ, Azcarate-Peril MA, et al. Magnesium and imidazole propionate. Clin Nutr ESPEN 2021;41:436–8. 10.1016/j.clnesp.2020.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Sneed NM, Ukwuani S, Sommer E, Samuels L, Truesdale K, Matheson D, et al. Reliability and Validity of Assigning Ultra-Processed Food Categories to 24-Hour Dietary Recall Data Collected Using the Nutrition Data System for Research (NDS-R). Current Developments in Nutrition 2022;6:778–778. [Google Scholar]
- [30].Dai Q, Shrubsole MJ, Ness RM, Schlundt D, Cai Q, Smalley WE, et al. The relation of magnesium and calcium intakes and a genetic polymorphism in the magnesium transporter to colorectal neoplasia risk. Am J Clin Nutr 2007;86:743–51. 10.1093/ajcn/86.3.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Dai Q, Shu X-O, Deng X, Xiang Y-B, Li H, Yang G, et al. Modifying effect of calcium/magnesium intake ratio and mortality: a population-based cohort study. BMJ Open 2013;3. 10.1136/bmjopen-2012-002111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Dai Q, Sandler R, Barry E, Summers R, Grau M, Baron J. Calcium, magnesium, and colorectal cancer. Epidemiology 2012;23:504–5. 10.1097/EDE.0b013e31824deb09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Dai Q, Cantwell MM, Murray LJ, Zheng W, Anderson LA, Coleman HG, et al. Dietary magnesium, calcium:magnesium ratio and risk of reflux oesophagitis, Barrett’s oesophagus and oesophageal adenocarcinoma: a population-based case-control study. Br J Nutr 2016;115:342–50. 10.1017/S0007114515004444. [DOI] [PubMed] [Google Scholar]
- [34].Joosen AMCP, Kuhnle GGC, Runswick SA, Bingham SA. Urinary sucrose and fructose as biomarkers of sugar consumption: comparison of normal weight and obese volunteers. Int J Obes (Lond) 2008;32:1736–40. 10.1038/ijo.2008.145. [DOI] [PubMed] [Google Scholar]
- [35].Jang C, Hui S, Lu W, Cowan AJ, Morscher RJ, Lee G, et al. The Small Intestine Converts Dietary Fructose into Glucose and Organic Acids. Cell Metab 2018;27:351–361.e3. 10.1016/j.cmet.2017.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Tasevska N, Sagi-Kiss V, Palma-Duran SA, Barrett B, Chaloux M, Commins J, et al. Investigating the performance of 24-h urinary sucrose and fructose as a biomarker of total sugars intake in US participants - a controlled feeding study. Am J Clin Nutr 2021;114:721–30. 10.1093/ajcn/nqab158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Te Morenga L, Kruimer D, McLean R, Sabadel AJM, van Hale R, Tatin X, et al. Associations Between Sugars Intakes and Urinary Sugars Excretion and Carbon Stable Isotope Ratios in Red Blood Cells as Biomarkers of Sugars Intake in a Predominantly Māori Population. Front Nutr 2021;8:637267. 10.3389/fnut.2021.637267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Jones RB, Zhu X, Moan E, Murff HJ, Ness RM, Seidner DL, et al. Inter-niche and inter-individual variation in gut microbial community assessment using stool, rectal swab, and mucosal samples. Sci Rep 2018;8:4139. 10.1038/s41598-018-22408-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Wood DE, Lu J, Langmead B. Improved metagenomic analysis with Kraken 2. Genome Biol 2019;20:257. 10.1186/s13059-019-1891-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Franzosa EA, McIver LJ, Rahnavard G, Thompson LR, Schirmer M, Weingart G, et al. Species-level functional profiling of metagenomes and metatranscriptomes. Nat Methods 2018;15:962–8. 10.1038/s41592-018-0176-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].MacKinnon DP. Introduction to statistical mediation analysis. Routledge; 2012. [Google Scholar]
- [42].Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal Statistical Society: Series B (Methodological) 1995;57:289–300. [Google Scholar]
- [43].Augustin K, Khabbush A, Williams S, Eaton S, Orford M, Cross JH, et al. Mechanisms of action for the medium-chain triglyceride ketogenic diet in neurological and metabolic disorders. Lancet Neurol 2018;17:84–93. 10.1016/S1474-4422(17)30408-8. [DOI] [PubMed] [Google Scholar]
- [44].Jang Y-S, Woo HM, Im JA, Kim IH, Lee SY. Metabolic engineering of Clostridium acetobutylicum for enhanced production of butyric acid. Appl Microbiol Biotechnol 2013;97:9355–63. 10.1007/s00253-013-5161-x. [DOI] [PubMed] [Google Scholar]
- [45].Jiang L, Wang J, Liang S, Wang X, Cen P, Xu Z. Production of butyric acid from glucose and xylose with immobilized cells of Clostridium tyrobutyricum in a fibrous-bed bioreactor. Appl Biochem Biotechnol 2010;160:350–9. 10.1007/s12010-008-8305-1. [DOI] [PubMed] [Google Scholar]
- [46].Barker HA, Taha SM. Clostridium kluyverii, an Organism Concerned in the Formation of Caproic Acid from Ethyl Alcohol. J Bacteriol 1942;43:347–63. 10.1128/JB.43.3.347-363.1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Holdeman LV, Cato EP, Moore WEC (1977) Anaerobe laboratory manual, 4th edn. Virginia Polytechnic Institute and State University, Blacksburg. n.d. [Google Scholar]
- [48].Pachikian BD, Neyrinck AM, Deldicque L, De Backer FC, Catry E, Dewulf EM, et al. Changes in intestinal bifidobacteria levels are associated with the inflammatory response in magnesium-deficient mice. J Nutr 2010;140:509–14. 10.3945/jn.109.117374. [DOI] [PubMed] [Google Scholar]
- [49].Crowley EK, Long-Smith CM, Murphy A, Patterson E, Murphy K, O’Gorman DM, et al. Dietary Supplementation with a Magnesium-Rich Marine Mineral Blend Enhances the Diversity of Gastrointestinal Microbiota. Mar Drugs 2018;16:E216. 10.3390/md16060216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Yousefi-Mashouf R, Duerden BI. An identification scheme for oral non-pigmented Prevotella (Bacteroides) species. Microbial Ecology in Health and Disease 1992;5:31–41. [Google Scholar]
- [51].Guyenet S, Landen J. Sugar consumption in the US diet between 1822 and 2005. Online statistics education: a multimedia course of study [Internet]. Houston, TX: Rice University and Tufts University. Available from: http://onlinestatbook.com. n.d. [Google Scholar]
- [52].Tappy L, Lê K-A. Metabolic effects of fructose and the worldwide increase in obesity. Physiol Rev 2010;90:23–46. 10.1152/physrev.00019.2009. [DOI] [PubMed] [Google Scholar]
- [53].Jegatheesan P, De Bandt J-P. Fructose and NAFLD: The Multifaceted Aspects of Fructose Metabolism. Nutrients 2017;9:E230. 10.3390/nu9030230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Johnson RJ, Sánchez-Lozada LG, Andrews P, Lanaspa MA. Perspective: A Historical and Scientific Perspective of Sugar and Its Relation with Obesity and Diabetes. Adv Nutr 2017;8:412–22. 10.3945/an.116.014654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Khan TA, Sievenpiper JL. Controversies about sugars: results from systematic reviews and meta-analyses on obesity, cardiometabolic disease and diabetes. Eur J Nutr 2016;55:25–43. 10.1007/s00394-016-1345-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Goncalves MD, Lu C, Tutnauer J, Hartman TE, Hwang S-K, Murphy CJ, et al. High-fructose corn syrup enhances intestinal tumor growth in mice. Science 2019;363:1345–9. 10.1126/science.aat8515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Pase MP, Himali JJ, Jacques PF, DeCarli C, Satizabal CL, Aparicio H, et al. Sugary beverage intake and preclinical Alzheimer’s disease in the community. Alzheimers Dement 2017;13:955–64. 10.1016/j.jalz.2017.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Townsend GE, Han W, Schwalm ND, Raghavan V, Barry NA, Goodman AL, et al. Dietary sugar silences a colonization factor in a mammalian gut symbiont. Proc Natl Acad Sci U S A 2019;116:233–8. 10.1073/pnas.1813780115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Restrepo S, Espinoza L, Ceballos A, Urtubia A. Production of fatty acids during alcoholic wine fermentation under selected temperature and aeration conditions. American Journal of Enology and Viticulture 2019;70:169–76. [Google Scholar]
- [60].Sun S, Zhu X, Huang X, Murff HJ, Ness RM, Seidner DL, et al. On the robustness of inference of association with the gut microbiota in stool, rectal swab and mucosal tissue samples. Sci Rep 2021;11:14828. 10.1038/s41598-021-94205-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Espey MG. Role of oxygen gradients in shaping redox relationships between the human intestine and its microbiota. Free Radic Biol Med 2013;55:130–40. 10.1016/j.freeradbiomed.2012.10.554. [DOI] [PubMed] [Google Scholar]
- [62].Wu GD, Chen J, Hoffmann C, Bittinger K, Chen Y-Y, Keilbaugh SA, et al. Linking long-term dietary patterns with gut microbial enterotypes. Science 2011;334:105–8. 10.1126/science.1208344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Sonnenburg JL, Angenent LT, Gordon JI. Getting a grip on things: how do communities of bacterial symbionts become established in our intestine? Nat Immunol 2004;5:569–73. 10.1038/ni1079. [DOI] [PubMed] [Google Scholar]
- [64].Albenberg L, Esipova TV, Judge CP, Bittinger K, Chen J, Laughlin A, et al. Correlation between intraluminal oxygen gradient and radial partitioning of intestinal microbiota. Gastroenterology 2014;147:1055–1063.e8. 10.1053/j.gastro.2014.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].St-Pierre V, Vandenberghe C, Lowry C-M, Fortier M, Castellano C-A, Wagner R, et al. Plasma Ketone and Medium Chain Fatty Acid Response in Humans Consuming Different Medium Chain Triglycerides During a Metabolic Study Day. Front Nutr 2019;6:46. 10.3389/fnut.2019.00046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Chiuve SE, Sun Q, Curhan GC, Taylor EN, Spiegelman D, Willett WC, et al. Dietary and plasma magnesium and risk of coronary heart disease among women. J Am Heart Assoc 2013;2:e000114. 10.1161/JAHA.113.000114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Dong J-Y, Xun P, He K, Qin L-Q. Magnesium intake and risk of type 2 diabetes: meta-analysis of prospective cohort studies. Diabetes Care 2011;34:2116–22. 10.2337/dc11-0518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Li W, Zhu X, Song Y, Fan L, Wu L, Kabagambe EK, et al. Intakes of magnesium, calcium and risk of fatty liver disease and prediabetes. Public Health Nutr 2018;21:2088–95. 10.1017/S1368980018000642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Wu L, Zhu X, Fan L, Kabagambe EK, Song Y, Tao M, et al. Magnesium intake and mortality due to liver diseases: Results from the Third National Health and Nutrition Examination Survey Cohort. Sci Rep 2017;7:17913. 10.1038/s41598-017-18076-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Kieboom BCT, Licher S, Wolters FJ, Ikram MK, Hoorn EJ, Zietse R, et al. Serum magnesium is associated with the risk of dementia. Neurology 2017;89:1716–22. 10.1212/WNL.0000000000004517. [DOI] [PubMed] [Google Scholar]
- [71].Ozawa M, Ninomiya T, Ohara T, Hirakawa Y, Doi Y, Hata J, et al. Self-reported dietary intake of potassium, calcium, and magnesium and risk of dementia in the Japanese: the Hisayama Study. J Am Geriatr Soc 2012;60:1515–20. 10.1111/j.1532-5415.2012.04061.x. [DOI] [PubMed] [Google Scholar]
- [72].Zhu X, Borenstein AR, Zheng Y, Zhang W, Seidner DL, Ness R, et al. Ca:Mg Ratio, APOE Cytosine Modifications, and Cognitive Function: Results from a Randomized Trial. J Alzheimers Dis 2020;75:85–98. 10.3233/JAD-191223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Jenkins BJ, Seyssel K, Chiu S, Pan P-H, Lin S-Y, Stanley E, et al. Odd Chain Fatty Acids; New Insights of the Relationship Between the Gut Microbiota, Dietary Intake, Biosynthesis and Glucose Intolerance. Sci Rep 2017;7:44845. 10.1038/srep44845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Jenkins B, West JA, Koulman A. A review of odd-chain fatty acid metabolism and the role of pentadecanoic Acid (c15:0) and heptadecanoic Acid (c17:0) in health and disease. Molecules 2015;20:2425–44. 10.3390/molecules20022425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Park Y-K, Dulermo T, Ledesma-Amaro R, Nicaud J-M. Optimization of odd chain fatty acid production by Yarrowia lipolytica. Biotechnology for Biofuels 2018;11:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Marten B, Pfeuffer M, and Schrezenmeir J. 2006. Medium-chain triglycerides. Int. Dairy J 16: 1374–1382. n.d. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


