Abstract
Background
Carbon dioxide concentration trending is used in chronic management of children with invasive home mechanical ventilation (HMV) in clinical settings, but options for end‐tidal carbon dioxide (EtCO2) monitoring at home are limited. We hypothesized that a palm‐sized, portable endotracheal capnograph (PEC) that measures EtCO2 could be adapted for in‐home use in children with HMV.
Methods
We evaluated the internal consistency of the PEC by calculating an intraclass correlation coefficient of three back‐to‐back breaths by children (0–17 years) at baseline health in the clinic. Pearson's correlation was calculated for PEC EtCO2 values with concurrent mean values of in‐clinic EtCO2 and transcutaneous CO2 (TCM) capnometers. The Bland–Altman test determined their level of agreement. Qualitative interviews and surveys assessed usability and acceptability by family‐caregivers at home.
Results
CO2 values were collected in awake children in varied activity levels and positions (N = 30). The intraclass correlation coefficient for the PEC was 0.95 (p < 0.05). The correlation between the PEC and in‐clinic EtCO2 device was 0.85 with a mean difference of −3.8 mmHg and precision of ±1.1 mmHg. The correlation between the PEC and the clinic TCM device was 0.92 with a mean difference of 0.2 mmHg and precision of ±1.0. Family‐caregivers (N = 10) trialed the PEC at home; all were able to obtain measurements at home while children were awake and sometimes asleep.
Conclusions
A portable, noninvasive device for measuring EtCO2 was feasible and acceptable, with values that trend similarly to currently in‐practice, outpatient models. These devices may facilitate monitoring of EtCO2 at home in children with invasive HMV.
Keywords: carbon dioxide, children with medical complexity, home mechanical ventilation, long‐term mechanical ventilation, remote patient monitoring
Abbreviations
- BPD
bronchopulmonary dysplasia
- CLD
chronic lung disease
- CO2
carbon dioxide
- EtCO2
end‐tidal carbon dioxide
- FDA
Food and Drug Administration
- HMV
home mechanical ventilation
- PEC
portable endotracheal capnograph
- PvCO2
partial pressure of venous carbon dioxide
- REDCap
Research Electronic Data Capture
- TCM
transcutaneous CO2
1. INTRODUCTION
Children who require invasive home mechanical ventilation (HMV) via tracheostomy are medically vulnerable patients with high health care use and cost. 1 , 2 , 3 The number of pediatric patients benefiting from HMV has grown over the past decades, 4 , 5 , 6 , 7 , 8 and HMV allows affected children to live and grow at home despite the need for ongoing ventilator support. Periodically, the children reliant on HMV are evaluated for readiness to decrease or wean ventilatory support, either by decreasing settings or providing successively longer periods off the ventilator (“breaks”). While no standard process for HMV weaning exists, the published literature does recommend routine clinical monitoring to minimize morbidity and mortality during prolonged HMV use. 9 Developing approaches to conducting this clinical monitoring and weaning HMV remotely may allow for more efficient and individualized care in a patient‐family‐centered manner at home.
As an indicator of effective respiration, carbon dioxide (CO2) is one measurement that is often used by clinicians to monitor the effectiveness of ventilation, along with other data such as oxygenation, measured tidal volume, inspiratory pressure, and weight. Since the 1970s, noninvasive capnographs and capnometers that can measure either end‐tidal carbon dioxide (EtCO2) or transcutaneous CO2 (TCM) have become increasingly available in the outpatient clinic setting to assist in this objective assessment as an alternative to more invasive blood gas monitoring. 10 However, reporting of CO2 monitoring at home has been relatively limited in the literature. Previous work by Liptzin et al. showed that weaning children with HMV with close observation was safe in the context of a pediatric respiratory care unit, but reported that intermittent EtCO2 monitoring was not always feasible. 11 In addition to Liptzin's work, we are unaware of published studies that explore the use of CO2 data collection to support weaning in pediatric patients reliant on HMV. Current CO2 devices seem to require several minutes to calibrate, are sufficiently large to require them to be placed on a stand or table for use, and often are connected to the patient via a wire; characteristics which together limit their use to the clinic setting, thereby requiring patients to go to the clinic for weaning evaluations. Because of the challenges with transportation of children with HMV, we were interested in identifying whether there were any alternative portable CO2 devices that could be tested as a first step in a series of studies to build evidence‐based approaches to facilitate proactive HMV weaning at home.
Since Liptzin's published work, a palm‐sized, portable endotracheal capnograph (PEC) (EMMA™ capnograph; Masimo) has been designed to measure mainstream EtCO2 when attached in‐line directly to an artificial airway during transport or anesthesia. 12 Previous studies of PEC showed a strong correlation (0.87) between PEC EtCO2 values and partial pressure of venous carbon dioxide (PvCO2) in nine hospitalized infants with tracheostomy tubes. 13 The PEC EtCO2 values were also compared in anesthetized adults to partial pressure of arterial carbon dioxide (PaCO2) and capnometers connected side stream—via tubing connected to the side of the mainstem tubing—with comparative mean values of 29.1 ± 2.5 mmHg (PEC), 35.1 ± 3.8 mmHg (PaCO2), 31.3 ± 2.4 mmHg (side stream capnometer). 14 , 15 However, no research has demonstrated its use in awake children at their baseline state of health who rely on invasive HMV in an outpatient setting, whose age may range from infancy to young adulthood, and who may have a range of physical activity levels and medical conditions.
Given PEC's small size, we hypothesized it could be adapted for portable measurements of EtCO2 at home in lieu of measurement of EtCO2 or TCM capnometers in clinics. To test this, we performed a pilot study designed to evaluate: (1) the internal consistency of the PEC device EtCO2 measurement in back‐to‐back breaths; (2) the correlation and agreement between the PEC and standard clinic EtCO2 and TCM capnometers; and (3) the feasibility and usability of parents measuring CO2 with the PEC in their children reliant on invasive HMV at home.
2. METHODS
2.1. Settings and patient inclusion
Children receiving HMV were recruited from an independent children's hospital's Pulmonary Habilitation Program which provides longitudinal care for children with a tracheostomy tube receiving HMV. Parent–guardians of eligible patients were approached and provided informed consent. Inclusion criteria were children aged 0–17 years old currently using invasive HMV. Children who would be weaned completely from the ventilator within the next 2 months or were at the end of life were excluded. Given the objective of the project was to confirm the ability to feasibly measure end‐tidal EtCO2 in a broad population of children cared for in HMV programs, children were included regardless of their indication for HMV.
The Institutional Review Board approved all study procedures (IRB # 2020‐3600). Study data were collected and managed using REDCap (Research Electronic Data Capture) secure electronic data capture tools hosted at Northwestern University. 16
2.2. Devices
The PEC used was the EMMATM, a patented Food and Drug Administration (FDA)‐approved noninvasive mainstream CO2 monitor that uses a spectrometer and infrared detector to measure and then display a continuous quantitative EtCO2 value (0–99 mmHg) and capnogram waveform (0–53 mg per 0–7 kPa scale), as well as a respiratory rate (3–150 breaths/min). 17 The PEC device fits a disposable airway adapter (6 ml dead space) which can be attached to the end of a patient's endotracheal or tracheostomy tube, which then measures the EtCO2 from the expired respiratory gas. The device is ∼2″ × 1.5″ × 1.5" and weighs ∼2 oz with two standard AAA batteries that last 6–10 h; it turns off automatically after 2 min if not in use.
We compared the PEC to two clinic devices: (1) a device that measures EtCO2 and (2) a device that measures TCM, both considered standard‐of‐care approaches to in‐clinic evaluations. The standard clinic EtCO2 device was a mainstream CAPNOSTAT 5™ sensor and adapter which attaches to the tracheostomy tube and then connects via a cable to the Respironics NM3 device (Model 7900) that displays quantitative EtCO2 (0–150 mmHg) and other parameters, including respiratory rate. 18 The clinical TCM device was a Sentec Digital Monitoring System, designed for noninvasive ventilation and oxygenation monitoring, that measures transcutaneous CO2 tensions (0–200 mmHg) using a sensor applied to the skin surface. 19
2.3. Data collection to assess feasibility, consistency, and correlation with clinic models
First, we developed a brief protocol to use the PEC CO2 measurements in children requiring HMV presenting for routine follow‐up care in clinic. The goal was to establish a feasible, reproducible method for PEC EtCO2 data collection in awake children with HMV, defined as reproducible data collection using the device by any member of the health care team when a child was connected or disconnected from the ventilator. Participants were recruited and consented to participate until a protocol was established (n = 9) to ensure a consistent process before the larger analysis. The protocol involved turning the PEC on, waiting 15 s per manufacturer's instructions, then attaching the PEC adapter inline as close to the tracheostomy as the child's circuit as would allow, and watching for the end‐tidal breath waveforms to be displayed across the entire screen. If the child began moving due to the placement of the device, we would allow the child to reposition and acclimatize to the device before taking the reading to ensure its accuracy.
We then conducted CO2 measurements in additional consented participants to establish agreement and correlation between the PEC and standard EtCO2 and TCM devices. To assess internal consistency, each measurement was taken three times per device per patient.
2.4. Data collection to assess usability
We then evaluated the usability of the PEC in new family‐caregiver (parent) end‐users, through a user‐centered design approach. 20 , 21 After consenting to participate, parents were shown how to use the PEC during an outpatient in‐person visit, asked to perform it independently with their child, then asked their initial impressions of the device. Next, the PECs were sent home with parents with instructions to use it while the child was awake, asleep, and, if applicable, at the end of a ventilatory break. Parents met with the interview team remotely from home (Zoom Video Communications, Inc.) to provide qualitative feedback on the home use experience, including their perceptions of its acceptability and usability. Survey items were administered at the initial training session and after use at home using a Likert agreement scale (Strongly disagree [1] to Strongly agree [5]) to assess specific components of usability, including perceived learnability, memorability, and satisfaction including whether the device was acceptable and valued. 21 , 22
2.5. Analysis
Descriptive statistics were used to evaluate participant characteristics. Feasibility was assessed by the ability to use the PEC in patients with different activity levels, need to stop measurement due to patient discomfort, frequency of adapter malfunction, and ability to use the device both connected and disconnected to the ventilator.
Internal consistency of the three device readings was summarized using intraclass correlation coefficients. To assess correlation and agreement of the PEC EtCO2 values with the clinic models, first, a Pearson's correlation was used to compare the linear relationship between the mean values of the PEC with the mean clinic EtCO2 device and the mean clinic TCM model. The Bland–Altman approach was used to determine the level of paired agreement between devices. 23 , 24 Bias was defined as the mean difference in values obtained with two different methods of measurement. 24 The precision was assessed by the width of a 95% confidence interval (CI). 24
To assess parents' perceptions of usability, research professionals' written notes and taped recordings were reviewed in a real‐time manner as feedback was gathered, and user feedback was summarized using rapid qualitative analytic methods. 25 , 26 Parents' responses to the Likert scale questions also were analyzed using descriptive statistics.
3. RESULTS
3.1. Participating patient characteristics in in‐clinic comparison
Data were collected from patients (N = 30) with a mean age of 4.9 years (SD ± 4.4, range 1–17), of which 43% were female biological sex, and slightly more than half (N = 16, 53%) were of minority race/ethnicity (Table 1). Half (N = 15, 50%) were on HMV due to chronic lung disease (CLD) from prematurity, and participants had a range of tracheostomy tube sizes ranging from 3.5 to 6.0 mm.
Table 1.
Characteristics of patients participating in in‐clinic measurements
| Patient characteristics | n (%) |
|---|---|
| Age (years), mean (SD) | 4.9 (4.4) |
| Patient biological sex | |
| Male | 17 (57) |
| Female | 13 (43) |
| Patient race ethnicity | |
| Non‐Hispanic White | 14 (43) |
| Non‐Hispanic Black | 7 (23) |
| Hispanic/Latinx | 7 (23) |
| Asian | 3 (10) |
| Indication for home ventilation | |
| Chronic lung disease/bronchopulmonary dysplasia | 15 (50) |
| Central control | 8 (27) |
| Mixed indication | 5 (16) |
| Neuromuscular | 2 (7) |
| Anthropormorphic measurements, mean (SD) | |
| Patient weight (kg) | 19.5 (16.9) |
| Patient length (cm) | 91.0 (21.2) |
| Tracheostomy tube size (mm) | |
| 3.5 | 4 (13) |
| 4.0 | 11 (37) |
| 4.5 | 8 (27) |
| 5.0 | 4 (13) |
| 6.0 | 1 (3) |
| Ventilator type | |
| LTV | 25 (83) |
| Trilogy | 5 (17) |
| Number of hours ventilated out of 24 on typical day, mean (SD) | 19.6 (5.4) |
| Whether patient was using ventilator during measurement | |
| Yes, receiving positive pressure | 21 (70) |
| No, not receiving positive pressure | 9 (30) |
| Patient's activity level and position during measurement | |
| Awake calm, sitting | 16 (53) |
| Asleep, laying down | 4 (13) |
| Awake calm, laying | 4 (13) |
| Awake active, sitting | 3 (10) |
| Asleep, sitting | 1 (3) |
| Awake active, laying down | 1 (3) |
| Awake calm, laying down | 1 (3) |
| Tracheostomy tube inflated during measurement | |
| Yes, inflated cuffed tube | 16 (53) |
| No, not inflated or no cuff | 14 (47) |
Abbreviation: SD, standard deviation.
3.2. Feasibility
Once a protocol was established, EtCO2 measurements were obtained with N = 30 additional patients in a variety of activity levels and positions (Table 1). Measurements were successfully obtained in patients who were both ventilated at the time of measurement (N = 21, 70%) or on ventilator break (N = 9, 30%). In one case (<1%), the PEC adapter alert alarmed, likely due to secretions blocking the reading window. The adapter was exchanged and there were no further issues. No measurements had to be stopped due to patient discomfort or parental concerns. Figure 1 shows use of the PEC in two different patients, one who is also attached to the tracheostomy tube and ventilator tubing and one in which the PEC is only attached to the tracheostomy tube.
Figure 1.
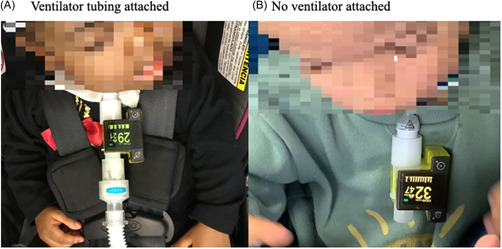
Portable end‐tidal capnograph use attached via pediatric tracheostomy tube (A). Ventilator tubing attached (B). No ventilator attached. [Color figure can be viewed at wileyonlinelibrary.com]
3.3. Internal consistency
The intraclass correlation coefficients that compared the three back‐to‐back breaths were 0.95 (p < 0.01) for the PEC, 0.98 (<0.01) for the clinic EtCO2 device, and 0.99 (p < 0.01) for the clinic TCM device.
3.4. Agreement and correlation with clinic models
The PEC EtCO2 values across all patients were a mean of 31.4 mmHg (SD 5.6, range 21–46), compared to clinic EtCO2 device mean values of 35.1 mmHg (SD 5.6, range 27–47) and clinic TCM device mean values of 31.5 mmHg (SD 6.5, range 22.7–53.2).
A scatterplot comparing the PEC EtCO2 values to the in‐clinic EtCO2 values is shown in Figure 2A; the correlation was 0.85. Agreement between the two devices is displayed in the Bland–Altman plot in Figure 2B. The mean difference between PEC EtCO2 and in‐clinic EtCO2 values were −3.8 mmHg (95% CI, −4.9 to −2.7), yielding a precision of ±1.1. One patient (ID#7) was 2 SD outside the limits of agreement with PEC CO2 values that ranged from 34 to 35 and clinic end‐tidal ranging from 28 to 30.
Figure 2.
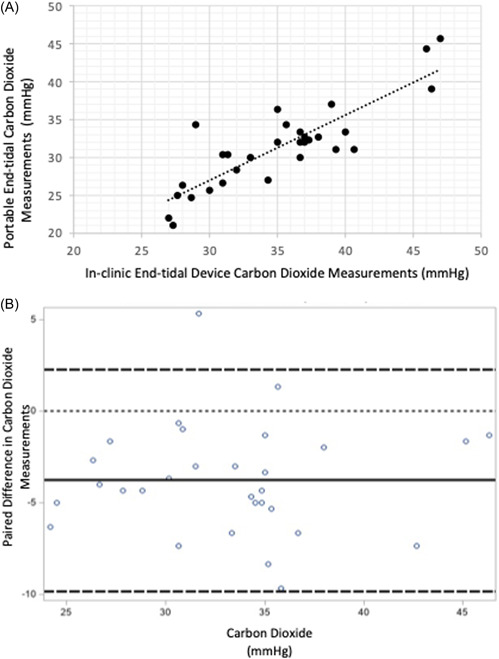
(A) Correlation between portable end‐tidal carbon dioxide measurements with in‐clinic end tidal device measurements graphs shows correlation of 0.85 (95% confidence interval, 0.70–0.93; p < 0.01) (B) Bland–Altman plot comparing portable end‐tidal carbon dioxide measurements with in‐clinic end tidal device measurements dotted line at 0 indicates ideal (no difference between device measurements). The solid line indicates the mean of the differences, indicating the EMMA tends to be lower, on average, compared to the in‐clinic end‐tidal model. Thick dashed lines indicate ±2 standard deviation. [Color figure can be viewed at wileyonlinelibrary.com]
A scatterplot comparing the PEC EtCO2 device to the clinical TCM device is shown in Figure 3A; the correlation was 0.92. The agreement between the PEC EtCO2 values and TCM device is displayed in the Bland–Altman plot in Figure 3B. The mean difference between PEC EtCO2 values and TCM values was 0.2 mmHg (95% CI, −1.2 to 0.8), yielding a precision of ±1.0. Two patients (ID#1 and ID#23) were 2 SD outside the limits of agreement; one patient's PEC CO2 values ranged from 35 to 48 and the TCM value of 30.2. The second patient's PEC CO2 values were 45–47 and TCM was 53.2.
Figure 3.
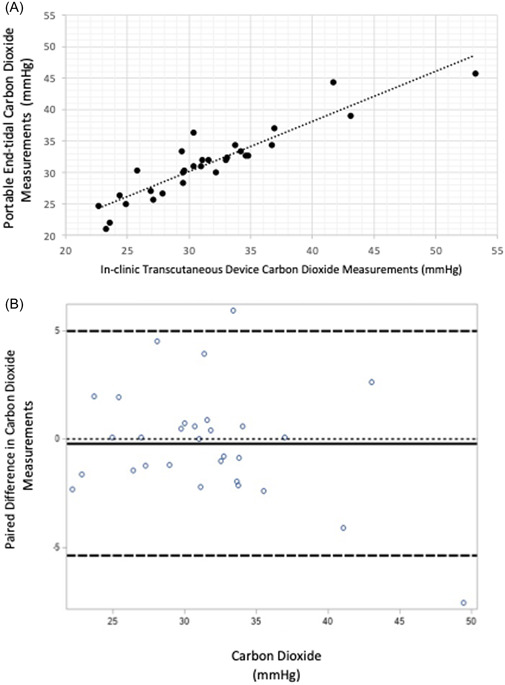
(A) Correlation between EMMA end‐tidal carbon dioxide measurements with clinical transcutaneous device measurements graphs shows a correlation of 0.92 (95% confidence interval, 0.83–0.96; p < 0.01). (B) Bland–Altman plot comparing portable end‐tidal carbon dioxide measurements with clinic transcutaneous device measurements dotted line at 0 indicates ideal (no difference between device measurements). The solid line indicates the mean of the differences, indicating the EMMA tends to be slightly lower, on average, compared to the in‐clinic transcutaneous model. Thick dashed lines indicate ±2 standard deviation. [Color figure can be viewed at wileyonlinelibrary.com]
3.5. Participating patient‐family characteristics in usability testing
A total of N = 10 additional new patient‐family dyads participated in the usability portion of the study, for which patients had a mean age of 3.18 years (SD ± 2.98). Their indication for home ventilation was most commonly CLD from prematurity (60%) followed by impaired central control of breathing (30%) and mixed indication (10%, CLD and central control of breathing disorder). Participating caregivers had a mean age of 34.4 years old (SD ± 4.25), all identified as female and included a diverse racial/ethnic family background (40% minority race/ethnicity, n = 2 Black and n = 2 Hispanic/Latinx) with a range of educational background (50% with less than a college degree).
Qualitative strengths of the device's design from the perspective of the majority of parent users (n = 9) were the small size of the device and its simplicity, “I loved that it was compact. It's very bright. It's easy to use and that the fonts of the numbers [are] very large,” (Parent 2) and “I like that it's really easy to use, really easy to put together” (Parent 9). In particular, parents explained that these features were important for the active child who may be sensitive to anything new placed on their tracheostomy, “[The PEC] is small and compact, which is helpful and [he] didn't mind it, which is probably the biggest obstacle…He's a very active kid. So having big, bulky things that you have to make him sit and then manage him and a big bulky piece of machinery is not ideal.…he already pulls off his vent circuit when he doesn't want it. So, something as small as the [PEC], if he didn't like it, we would never get a reading because he would just pull it off,” (Parent 3) and “I liked that it was really small and it's not heavy, so I don't think he really notices…” (Parent 4). Two children did need distraction techniques, including dangling a toy or showing of a phone screen during the initial measurements, to avoid the child grabbing at the device or vocalizing through the device, but measurements were able to be obtained once the child was calmed per the established protocol. While the study presented the goal of the PEC measurement for use in chronic weaning management, three parents identified the potential for its use during acute illness.
The main constructive feedback by three patient–parent dyads was that obtaining measurements during sleep could disrupt the child if they slept prone and/or lightly, for example, “The only trouble we had was during nap. Only because he's a light sleeper. And when I tried to do it while he was sleeping, he wasn't having it. Just woke up in between it. I was able to put him back to sleep…It was a little process” (Parent 3). Another parent explained trying to avoid waking the child by putting the PEC farther down the circuit during sleep to minimize disruption but worried the numbers were off, so readjusted it directly at the tracheostomy tube, explaining, “So I did go directly to his trach and I was getting a better number …[so] the challenging part is just because how he sleeps, he sleeps on his belly, so it was really hard for me to get it in because [the device] is pretty bulky” (Parent 7). However, in each case, the parents were able to obtain measurements.
Overall, the survey‐based feedback from the parent participants was positive with all respondents reporting they either agreed or strongly agreed that they could imagine using the device for their child's care and that it could help with their child's care (note: one parent did not complete the survey questions). Once home, almost all parents endorsed the PEC was usable by answering that they “strongly disagreed” that the PEC was “hard to use.” One respondent (Parent 3) was neutral (chose option “neither agree/disagree”); notably that parent was one of the parents who reported challenges with measurement during sleep. Finally, all responding parents either agreed and or strongly agreed that it was easy to remember how to use the device once home.
4. DISCUSSION
In this study, we found that a PEC device was a feasible, acceptable, and usable tool to measure EtCO2 values in‐clinic and at home in children with invasive HMV in a range of positions and activity levels, with some limitations during prone sleep. The PEC EtCO2 values were on average more closely aligned with the in‐clinic TCM device than the in‐clinic EtCO2 device, which ran about 4 mmHg higher. This finding is consistent with previous research which showed that the PEC device measurement was lower than the PvCO2 measured in infants 13 and lower than EtCO2 measured in anesthesia machines in adults, 27 which authors attributed to gas mixing and dead space at the tracheostomy tube. 13 While this study only correlated the PEC with in‐clinic models, sidestream EtCO2 measurement is already known to correlate with measurement of partial PaCO2 and is used in hospitalized children for clinical trending. 28 Together, this suggests that PEC may be a reasonably safe and accurate option for spot‐checking EtCO2 and trending while at home when a child with a tracheostomy tube is either connected or disconnected from the ventilator circuit. However, because EtCO2 is only one datum relevant for evaluating ventilation management and because the PEC EtCO2 values did not correlate perfectly with the in‐clinic models, clinical decision‐making regarding ventilation management should be done within the context of the patient to include their other vital signs and clinical appearance, as would be done in a clinic setting. Future research is needed to investigate whether routine longitudinal use of the PEC at home in addition to other remote data collection can provide improved clinical decision‐making protocols than in current practice.
In fact, at this time, American Thoracic Society Clinical Practice guidelines only recommend children with HMV be sent home with a pulse oximeter and other equipment to ensure monitoring for hypoxia, 29 primarily for detection of acute deterioration rather than use in chronic ventilatory management. With the expansion of telemedicine virtual visits since the start of the COVID‐19 pandemic in 2020, increasingly more provider face‐to‐face care is provided remotely. 30 , 31 However, patients may still travel to provider visits for routine evaluation of their HMV care, in part so that their providers can conduct an objective assessment of how well their HMV is supporting their growth and development and whether the child's positive pressure support can be decreased. Traveling with such children is frequently difficult, given the need to travel with two caregivers to ensure safety as well as transporting all the necessary equipment, which increases the already high burden of care for families of children receiving HMV. Developing healthcare delivery strategies that can safely support care in the home may help reduce some of this health care travel burden and allow for more proactive management between in‐office visits.
Notably, Liptzin et al. found that weaning with close observation in small groups of children with invasive HMV was safe, but found that intermittent EtCO2 data collection was not feasible about half of the time despite showing good correlation with blood testing. 11 Given the small size of the PEC device, we found a high rate of feasibility and usability in our test population, albeit small, especially while awake, which may provide a potential opportunity to expand this weaning model more broadly. However, future work is needed to evaluate the clinical utility and cost‐effectiveness of monitoring CO2 to support the weaning process, including developing methods for longitudinal tracking of CO2 in a manner that integrates with provider workflows. 9 , 32 This future research should investigate what data can be collected during what parts of a child's daily routine to best inform clinical decision‐making and how to best integrate that information into existing health platforms and workflows. 32
While the purpose of this study was focused on the feasibility of CO2 collection at home to inform chronic ventilatory management, parents shared their ideas about its potential use for assessment during an illness causing acute deterioration. For example, if a child has a viral illness that is affecting their respiratory clearance, including a home CO2 data point, they may provide potentially helpful data to providers to decide on the next steps in care. However, further work would ideally assess PEC performance during illness to assess its safety and use in that context.
4.1. Limitations
Given this evaluation was done in an outpatient setting in which tracheostomy tube leaks are not routinely measured, we were not able to report on whether the presence of a large leak impacted measurement correlation or agreement. Also, the Bland–Altman procedure assumes a linear relation between errors and measurements, which may not be the case in extreme measurements; however, we did collect data from a range of clinical values. Despite previous research correlating capnography with CO2 levels, this study also did not directly compare portable capnography with gold standard blood CO2 levels. Likewise, we did not assess for correlation between CO2 readings with patient size or HMV indications, which could potentially affect CO2 readings. We also did not repeat the comparison of the PEC with other modalities in the home setting as was done in the clinic setting.
We also note that parents who consented to participate may be more comfortable with technology, potentially introducing both selection bias and more positive feedback than may be seen in a broader population; nonetheless, all parents with children with HMV typically have baseline experience with equipment, given their children's condition.
Lastly, whether CO2 readings at home will benefit decision making surrounding weaning HMV support was not assessed in this study and should be the focus of future work before any widespread adoption.
5. CONCLUSIONS
A PEC is a noninvasive option for measuring EtCO2 in children with HMV both on and off the ventilator circuit, with high internal consistency with values that trend similarly to in‐clinic models. Family caregivers found the device acceptable and feasible for use at home, especially when awake, with the potential to facilitate management of chronic ventilation remotely and even clinical deterioration at home in children with a tracheostomy.
AUTHOR CONTRIBUTIONS
Carolyn C. Foster: Conceptualization; investigation; funding acquisition; writing – original draft; methodology; writing – review & editing; data curation; formal analysis; visualization; validation. Soyang Kwon: Formal analysis; methodology; writing – review & editing. Avani V. Shah: Writing – review & editing; conceptualization; project administration. Caroline A. Hodgson: Conceptualization; writing – review & editing. Lindsey P. Hird‐McCorry: Conceptualization; writing – review & editing. Angela Janus: Conceptualization; writing – review & editing. Aneta M. Jedraszko: Investigation; writing – review & editing; methodology; data curation. Philip Swanson: Conceptualization; writing – review & editing; data curation. Matthew M. Davis: Funding acquisition; writing – review & editing; supervision. Denise M. Goodman: Funding acquisition; writing – review & editing; methodology. Theresa A. Laguna: Conceptualization; funding acquisition; writing – review & editing; methodology; supervision.
CONFLICT OF INTEREST
Dr. Foster has received compensation for medical record consultation and/or expert witness testimony. The remaining authors declare no conflict of interest.
ACKNOWLEDGMENTS
Thank you to Masimo for providing the EMMA™ device and adapters free of cost for the purpose of this research study. This study, including Dr. Foster's time, was supported by the National Heart, Lung, and Blood Institute (NHLBI) under 1K23HL149829‐01A1 for research on the care of children with home mechanical ventilation. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NHLBI or National Institutes of Health. This work was also funded in part by the Children's Research Fund Junior Board, an Affiliated Organization of Ann & Robert H. Lurie Children's Hospital of Chicago. REDCap is supported at FSM by the Northwestern University Clinical and Translational Science (NUCATS) Institute, Research reported in this publication was supported, in part, by the National Institutes of Health's National Center for Advancing Translational Sciences, Grant Number UL1TR001422. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The EMMA™ capnograph device manufacturer (Masimo Corporation, Irvine, CA) provided the device and related adapters free of charge for the study. Masimo did not participate in study conceptualization, design, data analysis, or manuscript writing.
Foster CC, Kwon S, Shah AV, et al. At‐home end‐tidal carbon dioxide measurement in children with invasive home mechanical ventilation. Pediatric Pulmonology. 2022;57:2735‐2744. 10.1002/ppul.26092
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Sobotka SA, Gaur DS, Goodman DM, Agrawal RK, Berry JG, Graham RJ. Pediatric patients with home mechanical ventilation: the health services landscape. Pediatr Pulmonol. 2018;54(1): 40‐46. 10.1002/ppul.24196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Moore PE, Boyer D, O'connor MG, et al. Pediatric chronic home invasive ventilation. Ann Am Thorac Soc. 2016;13(7):1170‐1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Benneyworth BD, Gebremariam A, Clark SJ, Shanley TP, Davis MM. Inpatient health care utilization for children dependent on long‐term mechanical ventilation. Pediatrics. 2011;127(6):e1533‐e1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Amin R, Sayal P, Syed F, Chaves A, Moraes TJ, MacLusky I. Pediatric long‐term home mechanical ventilation: twenty years of follow‐up from one Canadian center. Pediatr Pulmonol. 2014;49(8):816‐824. [DOI] [PubMed] [Google Scholar]
- 5. McDougall CM, Adderley RJ, Wensley DF, Seear MD. Long‐term ventilation in children: longitudinal trends and outcomes. Arch Dis Child. 2013;98(9):660‐665. [DOI] [PubMed] [Google Scholar]
- 6. Wallis C, Paton JY, Beaton S, Jardine E. Children on long‐term ventilatory support: 10 years of progress. Arch Dis Child. 2011;96(11):998‐1002. [DOI] [PubMed] [Google Scholar]
- 7. Paulides FM, Plotz FB, Verweij‐van den Oudenrijn LP, van Gestel JPJ, Kampelmacher MJ. Thirty years of home mechanical ventilation in children: escalating need for pediatric intensive care beds. Intensive Care Med. 2012;38(5):847‐852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cristea AI, Carroll AE, Davis SD, Swigonski NL, Ackerman VL. Outcomes of children with severe bronchopulmonary dysplasia who were ventilator dependent at home. Pediatrics. 2013;132(3):e727‐e734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cristea AI, Baker CD. Ventilator weaning and tracheostomy decannulation in children: more than one way. Pediatr Pulmonol. 2016;51(8):773‐774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Harper CM. Capnography: clinical aspects. J R Soc Med. 2005;98(4):184‐185. [Google Scholar]
- 11. Liptzin DR, Connell EA, Marable J, Marks J, Thrasher J, Baker CD. Weaning nocturnal ventilation and decannulation in a pediatric ventilator care program. Pediatr Pulmonol. 2016;51(8):825‐829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Masimo Sweden AB . Emma (TM) canograph portable real‐time capnography. 6544/PLM‐10642A‐0417. Accessed November 8, 2021. https://www.masimo.com/siteassets/us/documents/pdf/plm-10642a_product_information_emma_capnograph_us.pdf
- 13. Hotta M, Hirata K, Nozaki M, Mochizuki N, Hirano S, Wada K. Availability of portable capnometers in children with tracheostomy. Pediatr Int. 2021;63(7):833‐837. [DOI] [PubMed] [Google Scholar]
- 14. Kameyama M, Uehara K, Takatori M, Tada K. Clinical usefulness of EMMA for monitoring end‐tidal carbon dioxide. Masui. 2013;62(4):477‐480. [PubMed] [Google Scholar]
- 15. Kim KW, Choi HR, Bang SR, Lee JW. Comparison of end‐tidal CO2 measured by transportable capnometer (EMMA™ capnograph) and arterial pCO2 in general anesthesia. J Clin Monit Comput. 2016;30(5):737‐741. [DOI] [PubMed] [Google Scholar]
- 16. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata‐driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377‐381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Masimo Sweden AB . EMMA (TM) emergency capnograph user's manual. Article no: 0000‐8114. Accessed November 8, 2021.
- 18. Koninklijke Phillips Electronics N.V. Simple answers to complex questions: Philips respironics NM3 respiratory profile monitor specifications. Netherlands. 2010. Accessed January 10, 2021. www.philips.com/healthcare
- 19. Sentec AG . Sentec Digital Monitoring System Instruction Manual. Sentec AG. 2021. [Google Scholar]
- 20. Kinzie MB, Cohn WF, Julian MF, Knaus WA. A user‐centered model for web site design: needs assessment, user interface design, and rapid prototyping. J Am Med Inform Assoc. 2002;9(4):320‐330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lyon AR, Koerner K. User‐Centered design for psychosocial intervention development and implementation. Clin Psychol (New York). 2016;23(2):180‐200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zhang D, Adipat B. Challenges, methodologies, and issues in the usability testing of mobile applications. J Hum–Comput Interact. 2005;18(3):293‐308. [Google Scholar]
- 23. Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1(8476):307‐310. [PubMed] [Google Scholar]
- 24. Hanneman SK. Design, analysis, and interpretation of method‐comparison studies. AACN Adv Crit Care. 2008;19(2):223‐234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gale RC, Wu J, Erhardt T, et al. Comparison of rapid vs in‐depth qualitative analytic methods from a process evaluation of academic detailing in the Veterans Health Administration. Implement sci. 2019;14(1):11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Vindrola‐Padros C, Johnson GA. Rapid techniques in qualitative research: a critical review of the literature. Qual Health Res. 2020;30(10):1596‐1604. [DOI] [PubMed] [Google Scholar]
- 27. Hildebrandt T, Espelund M, Olsen KS. Evaluation of a transportable capnometer for monitoring end‐tidal carbon dioxide. Anaesthesia. 2010;65(10):1017‐1021. [DOI] [PubMed] [Google Scholar]
- 28. Coates BM, Chaize R, Goodman DM, Rozenfeld RA. Performance of capnometry in non‐intubated infants in the pediatric intensive care unit. BMC Pediatr. 2014;14:163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sterni LM, Collaco JM, Baker CD, et al. An Official American Thoracic Society Clinical Practice Guideline: pediatric chronic home invasive ventilation. Am J Respir Crit Care Med. 2016;193(8):e16‐e35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mann DM, Chen J, Chunara R, Testa PA, Nov O. COVID‐19 transforms health care through telemedicine: evidence from the field. J Am Med Inform Assoc. 2020;27(7):1132‐1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kichloo A, Albosta M, Dettloff K, et al. Telemedicine, the current COVID‐19 pandemic and the future: a narrative review and perspectives moving forward in the USA. Fam Med Community Health. 2020;8(3):e000530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Foster C, Schinasi D, Kan K, Macy M, Wheeler D, Curfman A. Remote monitoring of patient‐ and family‐generated health data in pediatrics. Pediatrics. 2022;149(2):e2021054137. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


