Abstract
We review perioperative ocular complications and implications of ocular diseases during non-ocular surgeries. Exposure keratopathy, the most common perioperative eye injury, is preventable. Ischemic optic neuropathy (ION), the leading cause of perioperative blindness, has well defined risk factors. The incidence of ION after spine fusion, but not cardiac surgery, has been decreasing. Central retinal artery occlusion during spine fusion surgery can be prevented by protecting eyes from compression. Perioperative acute angle closure glaucoma is a vision-threatening emergency which can be successfully treated by rapid reduction of elevated intraocular pressure. Differential diagnoses of visual dysfunction in the perioperative period, and treatments are detailed. Although glaucoma is increasingly prevalent and often questions arise concerning perioperative anesthetic management, evidence-based recommendations to guide safe anesthesia care in patients with glaucoma are currently lacking. Patients with low vision present challenges to the anesthesia provider that are becoming more common as the population ages.
Keywords: Blindness, cornea, exposure keratopathy, eye diseases, glaucoma, idiopathic intracranial hypertension, ischemic optic neuropathy, retina
Summary statement:
We review the essential material that anesthesia providers need to be aware of for safe conduct of anesthesia in patients with eye diseases or at risk to develop complications affecting the eye during non-ocular surgeries.
Perioperative injury to the eye ranges from corneal injuries producing pain and reversible blurred vision, to serious disorders of the retina or optic nerve causing permanent blindness. The eye is the most important sensory organ, with up to 50% of cerebral cortical neurons serving visual function.1 Low vision or blindness are major disabilities accompanied by significant emotional suffering and high cost to the health care system.2 Accordingly, prevention of vision loss is of paramount concern, and it is critically important that anesthesia providers be competent in prevention, diagnosis, and treatment of vision-impacting complications of non-ocular surgery and the important considerations in delivering anesthesia to patients with chronic ophthalmic disease.
Corneal injury from exposure keratopathy is the most common perioperative eye injury, presenting with pain, foreign body sensation in the eye, blurry vision, and photophobia. Its incidence has been dramatically reduced by educating providers in best preventive measures, from 1.20/1,000 to 0.09/1,000 in one quality improvement study.3 A full-thickness corneal transplant is associated with a risk for one year of incision dehiscence, leading to catastrophic loss of eye contents.4 The anesthesia provider needs to protect the eye of such individuals undergoing anesthesia and surgery from even minor trauma or compression.
The leading cause of perioperative blindness is ischemic optic neuropathy. There have been significant advances in determining the perioperative risk factors, publication of multi-specialty driven evidence-based advisories, and encouragingly, a dramatic decrease in its incidence in spinal fusion surgery,5,6 where the estimated national US incidence was 1.63/10,000 spine fusion surgeries in 1998-2000 and 0.6/10,000 in 2010-2012.5,7 While some patients have regained vision spontaneously or via various treatment modalities, a major remaining challenge is the lack of any proven treatment. Central retinal artery occlusion and cerebral blindness can produce overlapping signs and symptoms, hence accurate diagnosis of these entities is essential to drive appropriate therapies.
Idiopathic intracranial hypertension is an elevation in cerebrospinal fluid pressure caused by decreased cerebrospinal fluid absorption or elevated cerebral venous sinus pressures.8,9 Its main permanent morbidity is to the visual system, characterized by papilledema associated with vision loss, and sixth nerve palsy causing diplopia.10 These patients often present for labor analgesia, and commonly questioned is the safety of placing epidural catheters.11 There is, however, presently no evidence to justify the withholding of epidural or spinal analgesia.
Less certain are the anesthetic implications of patients with primary open angle glaucoma.12 To date there are only limited studies on the impact of elevated intraocular pressure from patient positioning for surgery on the functioning of the optic nerve and outcomes. On the other hand, acute angle closure glaucoma in the post-operative period is a medical emergency usually triggered by specific drugs in susceptible individuals that requires immediate reduction of intraocular pressure to prevent permanent damage to the optic nerve.13
With the increasingly aging population and rising prevalence of chronic degenerative diseases including cataract, glaucoma, diabetic retinopathy, and age-related macular degeneration, the anesthesia provider is likely to encounter patients with existing vision impairment. Low vision or blindness currently affects about 2.5% of the population of the United States.2 Circadian rhythm disorders, altered alertness, and mood changes may be present.14 The lack of visual cues may complicate communication, including difficulties in discussing the anesthetic plan and the obtaining of informed consent.15
This review provides a best evidence-based approach to the anesthetic implications of diseases of the eye, and the prevention of peri-operative eye complications. The review is organized anatomically, beginning with diseases of the outer covering of the eye, the cornea, followed by the posterior eye, the retina and optic nerve.
Cornea
Exposure Keratopathy
Epidemiology
Exposure keratopathy is a defect in the corneal surface epithelium and the most common cause of postoperative ocular complaints. The incidence is 0.9-3.3/1,000 general anesthetics.16,17 The majority completely heal, however, in the American Society of Anesthesiologists Closed Claims Study of cases from 1974 to 1987, 16% resulted in permanent ocular damage, although the authors did not report the nature of the residual injury.18 Since the corneal epithelium is heavily innervated by the trigeminal nerve, eye pain, foreign body sensation, photophobia, and erythema are common presenting complaints. Blurry vision can also occur. 19 Insufficient closure of the eyelid and/or insufficient tear production, inadvertent contact with the eye by the patient or provider (e.g., a finger upon awakening, or hanging name tag, respectively), are the most common causes.16,17 Pain control and follow up with an eye provider for monitoring of proper healing and prevention of infection are the focus of treatment.20
Perioperative Evaluation of Patients at Risk of Exposure Keratopathy
Several independent risk factors are associated with perioperative exposure keratopathy.16,21–23 These include patient-specific characteristics, and factors related to surgery and anesthesia. Impaired tear production during general anesthesia decreases the lubrication and proper nourishment of the corneal surface.24 Zernii et al demonstrated in a rabbit model that general anesthesia decreased tear film stability. The tear break-up time test demonstrated short-term tear film destabilization. Total antioxidant activity of tears declined for up to 1 hour after 3 hours of general anesthesia.25 The resulting increase in reactive oxygen species was responsible for the damage to the corneal epithelium.26 Thyroid eye disease is associated with proptosis, and lagophthalmos, which can prevent full closure of the eye, increasing the likelihood of exposure keratopathy.27,28 Prone, lateral, or Trendelenburg positioning of the patient for surgery may result in the cornea contacting items or surfaces.16 Extreme head down positioning, e.g., for robotic surgery, is another risk factor, possibly because of periorbital swelling and partial opening of the eyelids during anesthesia.29
Prevention
Prevention is centered on proper taping of the eyes, and verifying that eyelids are completely closed soon after induction of general anesthesia, preferably even before airway management.30 Taping alone provides protection equivalent or superior to ointment, goggles, hydrophilic contact lenses, Geliperm dressings, and bio-occlusive dressings.31 Disadvantages of these adjuvants should be considered, e.g., flammability of petroleum gel, sloughing of the corneal epithelium and conjunctival hyperemia from preservative-based ointments, foreign body sensation and rubbing of the eyes upon awakening from ointments and gels, and risk of compression of the eye from improperly placed goggles. 32–35
Some patients present with elaborate and expensive glued false eyelashes or eyelash extensions, and they may be reluctant or even unable to easily remove them. False eyelashes are best removed if possible before anesthesia and surgery.36 We find that the best strategy is sensitivity to the patient’s concerns by counseling them that the eyelashes will be carefully protected, although we do warn them of the heightened risk of corneal injury from contact of the false lashes with the corneal surface. We suggest gentle closure of the eyes with cotton eye pads, then covering the pads with a layer of bio-occlusive dressing. This effectively covers the eyes and prevents the tape or bio-occlusive dressing from pulling out the eyelashes. The anesthesia provider should frequently examine the face during surgery to verify that there is no pressure on the eyes and that the eyes are protected from exposure.
Successful Quality Improvement Decreased Anesthesia-related Exposure Keratopathy
Large scale quality improvement programs have significantly reduced the incidence of perioperative exposure keratopathy. Vetter et al utilized a “Plan-Do-Check-Act” cycle and a standardized eye protection protocol consisting of eye lubrication with an aqueous-based gel and clear occlusive dressings to cover the eyelids and surrounding skin.3 The intraoperative injury rate decreased significantly from 1.20/1,000 to 0.09/1,000 after implementation, and was sustained for the entire 45-month follow-up period. This study again demonstrated that most perioperative incidents of exposure keratopathy are preventable and is an excellent example of the success of provider education in reducing an anesthesia morbidity affecting the eye.
Corneal Transplant (Penetrating Keratoplasty)
The anesthesia provider is likely to encounter a patient with a recent penetrating keratoplasty.37 Approximately 48,000 penetrating keratoplasties, also known as full-thickness corneal transplants, were performed annually in the United States in 2021. Traumatic globe rupture or wound dehiscence after even minimal trauma is an important concern in a patient for non-ocular surgery after penetrating keratoplasty. This devastating complication (Fig 1) can result in choroidal hemorrhage (bleeding from the choroidal circulation that supplies blood to the outer retina), and loss of vitreous and retina through the wound opening followed by profound vision loss.38 The highest period of traumatic rupture is in the month following corneal surgery because wound strength is almost entirely dependent upon sutures. It takes 6-12 months after suture removal for the corneal wound strength to typically reach its maximum.38,39 To minimize the risk of rupture within this time period a conservative and do-no-harm recommendation is to apply an eye shield over the taped eyelids, providing two layers of protection from direct pressure or unintended trauma.40 Anesthesia providers should be particularly cautious to avoid pressure at any time on an eye with a penetrating keratoplasty when positioning the patient in the prone or lateral positions for surgery.4,41,42
Figure 1: Penetrating Keratoplasty:
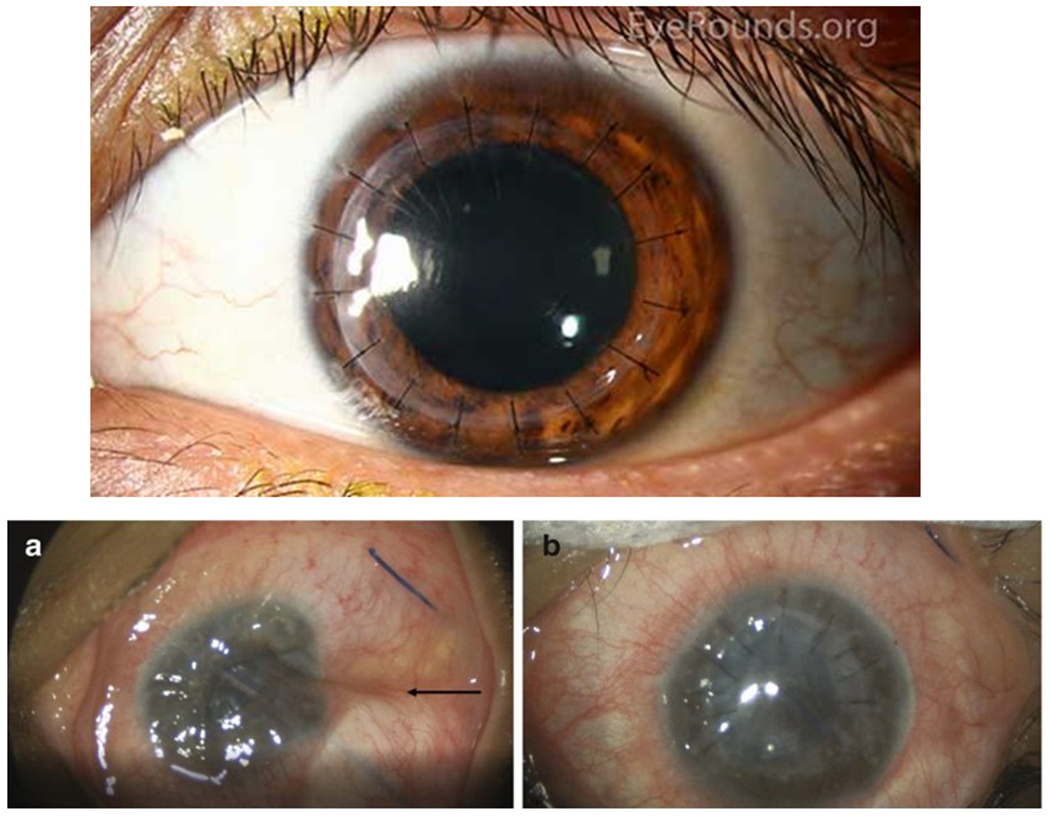
Top, grafts for penetrating keratoplasty with 16 interrupted sutures. Reproduced with permission from the Department of Ophthalmology at the University of Iowa, and available at EyeRounds.org. Accessed 24March22. Below are intra-operative photographs from a different case of dehisced penetrating keratoplasty. (A) Note the deflated globe with indentation of the sclera (black arrow) and corneal edema. (B) After repair, the return of the globe contour and wound closure with ten interrupted sutures can be seen. Reproduced with permission from the publisher from Davies E., Yonekawa Y. (2018) Case 6: Dehiscence of Penetrating Keratoplasty from Blunt Trauma. In: Grob S., Kloek C. (eds) Management of Open Globe Injuries. Springer. https://doi.org/10.1007/978-3-319-72410-2_11
Open Globe Injury
It may be necessary to anesthetize a patient with an open globe injury who must first undergo a more urgent surgical procedure. The major risk is loss of eye contents through the wound from increased intraocular pressure. Use of succinylcholine in open globe injury is a long-standing controversy, the history of which, and reasons for the common recommendations not to use succinylcholine, have been well considered elsewhere.43 Many of the recommendations not to use succinylcholine are based upon anecdotal reports. In a feline open globe injury model, succinylcholine did not result in loss of intraocular components, significant because the cat eye closely resembles that of humans.44 A sensible strategy in addition to protecting the eye from further trauma as explained above, is to use alternative non-depolarizing neuromuscular blocking agents, in conjunction with induction and other agents that lower intraocular pressure, but where indicated appropriately, succinylcholine may be used safely.43
Contact Lenses
Contact lenses are in widespread use, and a common question is whether they should be removed before surgery and anesthesia. Contact lenses have been associated with conjunctival epithelial defects, defects of the lid wiper epithelium, and diminished tear film breakup time.45 Contact lens wearers have an increased risk of corneal injuries compared to those who do not wear contact lenses.46 Although there are no studies examining intraoperative contact lens use as an independent risk factor for corneal injury, we recommend a conservative and do-no-harm approach of removing them before induction of anesthesia.
Diseases of the Retina
Patients who have undergone vitrectomy
Vitrectomy is a common eye surgical procedure performed for proliferative diabetic eye disease, retinal detachment, and retinal tumors.47 Tamponade agents provide surface tension in the vitreous across retinal breaks, and prevent fluid flow into the subretinal space until the surgical repair becomes complete. Gases may be used,48 including sulfur hexafluoride (SF6), which dissipates in 10-14 days, and perfluoropropane (C3F8), which lasts 55-65 days.49 Most centers provide a brightly colored wrist band indicating the type of gas used.50 Anesthesia providers must be aware of the presence and type of vitreous gas bubble to prevent inadvertent vision-threatening increases in intraocular pressure which can lead directly to central retinal artery occlusion in patients with recent retinal detachment surgeries or vitrectomies.
The major risk is from the administration of nitrous oxide, which diffuses into the gas bubble faster than it can exit,51 dramatically increasing intraocular pressures. Case reports documented devastating vision loss from the sudden onset of profoundly increased intraocular pressure and resulting central retinal artery occlusion in patients undergoing surgery after recent vitrectomy with use of nitrous oxide for anesthesia.52–57 Accordingly, nitrous oxide should be avoided for at least two weeks in a patient with SF6, and two months in a patient with the longer lasting C3F8. 58
Retinal Artery Occlusion
Retinal artery occlusion can be central, or branch retinal artery occlusion. Perioperatively, it most often occurs after cardiac surgery, or in spinal fusion surgery with the patient positioned prone.59 It also may occur with a vascular injury in sinus or nasal surgery, or from emboli during interventional radiological procedures involving the head and face.60
Signs and symptoms (Table 1) are a sudden onset of painless visual loss, abnormal pupils, retinal whitening, and narrowed retinal arterioles.61 Pallor of the ischemic retina renders the underlying choroidal circulation visible, causing the classic cherry-red spot seen on ocular fundus examination. Perioperative central retinal artery occlusion in the setting of spine surgery is usually due to external compression of the eye, and in cardiac surgery, it is usually due to emboli.62
Table 1.
Typical eye examination findings and differential diagnosis in perioperative cornea, retinal, optic nerve, or visual cortex injury.
| Exposure keratopathy | Acute angle closure glaucoma | Anterior ischemic optic neuropathy | Posterior ischemic optic neuropathy | Cerebral blindness | Central retinal artery occlusion | |
|---|---|---|---|---|---|---|
| Visual acuity | May be blurry | Cloudy or blurry; excessive tearing; redness; seeing halos | Altitudinal defect; scotoma; less commonly, may have no light perception | Variable, ranging from visual field loss to complete blindness in affected eye(s) | Hemianopia | Varies from visual field loss to no light perception |
| Pain | May be severe. May have photophobia | May be severe | None | None | None | May be present in cases of external compression of the eye |
| Other non eye symptoms | Headache, nausea, vomiting | Signs and symptoms of stroke | ||||
| Intraocular pressure | Normal | Very high (> 60 mm Hg) | Normal | Normal | Normal | Normal |
| Optic disk | Normal | Optic nerve head edema | Optic nerve head edema, later optic atrophy | Normal, later optic atrophy | Normal | Normal, later optic atrophy |
| Retina | Normal | Normal | May have attenuated retinal arterioles | May have attenuated retinal arterioles | Normal | Normal in the hyperacute and chronic stages. In acute stage, cherry red spot (macula), pallor, narrowed retinal arteries |
| Pupillary Light reflex | Normal | Dilated pupil | Absent or APD | Absent or APD | Normal | Absent or APD |
| Ocular muscle function | Normal | Normal | Normal | Normal | Normal | May be impaired in if the eye has been externally compressed during surgery |
| Gonioscopy | Narrow angle, corneal edema |
APD = afferent pupil defect
Retinal Artery Occlusion in Spine surgery
Square or circular foam headrests or devices with mirrors to view the eyes, or securing the head into a frame using pins, should prevent ocular compression in the patient positioned prone for spinal fusion surgery.63 Anesthesia providers should check the head and the eyes intermittently to ensure there is no compression of the eye which could lead to increased intraocular pressure and retinal damage. The eyes should also be checked after any movement of the body, where the head position may have become altered. As human studies are obviously unethical or impractical no “safe” or accepted period of time for these eye inspections has been established. Rodents have similar ocular circulation and retinal structure to humans. Their retinal ganglion cells were damaged within a minimum of 20-30 min of elevated intraocular pressure. In monkeys, occluding the central retinal artery occlusion at its site of entry into the dural sheath of the optic nerve could be tolerated for a longer period, requiring at least 105 min before damage was detectable.64 However, the difference is that in monkeys, the central retinal artery was specifically occluded without increasing intraocular pressure. In the rodents, the increased intraocular pressure produced by elevating pressure in the anterior chamber of the eye resulted in an occlusion of both the central retinal artery to the inner retina and the choroidal circulation to the outer retina.65 The latter is more comparable experimentally to external compression of the human eye. Therefore, besides proper precautions to prevent external compression, a conservative and do-no-harm recommendation is to check the eyes for compression every 20 min in the patient positioned prone for surgery.
Retinal Artery Occlusion in Cardiac Surgery
In the United States Nationwide Inpatient Sample there were 5.8 million cardiac operative discharges from 1998-2013, and 4,564 cases of retinal artery occlusion, or 7.8/10,000. Systemic risk factors were a history of giant cell arteritis, transient ischemic attack, carotid artery stenosis, embolic stroke, hypercoagulability, myxoma, diabetes mellitus with ophthalmic complications, and aortic insufficiency. Operative risk factors were bleeding, and aortic, mitral valve, and septal surgery.62 Conditions that increased the risk of embolization to the retinal circulation, namely, carotid disease, opening the heart, and a pre-existing abnormal retina were predictors of the complication in cardiac surgery. Although an exceptionally large study, it is limited by its retrospective nature, reliance upon and inability to confirm diagnostic codes, and not distinguishing between branch and central retinal artery occlusion.
Prevention of retinal artery occlusion in cardiac surgery is a poorly studied area in need of more research. There are some recommendations for prevention of stroke that appear relevant. Calcific emboli in the central retinal artery and its branches with accompanying visual field deficits from opening of the left ventricle, cardiopulmonary bypass, and manipulating a calcified aorta, have been described after coronary artery bypass grafting.66 Little can be done about opening the left ventricle and cardiopulmonary bypass as they are necessary accompaniments to the surgery, but epiaortic ultrasound can detect atheromatous regions and guide the location of aortic cannulation to decrease emboli.67–69 Its efficacy to decrease retinal microemboli in particular has not yet been documented but it is currently recommended for coronary artery bypass grafting as class IIA evidence to decrease stroke by the American College of Cardiology and the American Heart Association.70
Treatment
Central retinal artery occlusion is a medical emergency with only a limited time window before retinal neurons die and severe vision loss occurs.71 Early recognition (Table 1) is essential in order to enable treatment within the time window and the opportunity to achieve at least some recovery of vision. Thrombolysis with tissue plasminogen activator is the most commonly offered treatment when a stroke center is accessible within 4-5 hours of suspected central retinal artery occlusion. The efficacy of thrombolysis has not yet been demonstrated in randomized clinical trials, but non-randomized studies and meta-analyses strongly suggest it improves visual outcome.72–74 Some groups have also reported success with thrombolysis combined with other therapies.75–77 An important concern in the post-operative patient is the risk of bleeding from thrombolytic agents.71 Due to these risks, where there is a delayed diagnosis, or where rapid access to a stroke center is not available, how to proceed must be decided on a case-by-case basis in consultation with a neuro-ophthalmologist. Without thrombolysis, inhaling carbogen (95% oxygen, 5% CO2), anterior chamber paracentesis, hyperbaric oxygen delivery, and acetazolamide have been used with some reported successes.77–82
Optic Nerve
Idiopathic Intracranial Hypertension and Vision
Definition and Pathophysiology
Idiopathic intracranial hypertension is elevated intracranial pressure caused by decreased cerebrospinal fluid absorption or elevated cerebral venous sinus pressures.8,9 Its major permanent morbidity is vision loss due to optic neuropathy.8,10 Visual signs and symptoms are optic nerve swelling (papilledema), diplopia (“double vision”), and vision loss.10 The modified Dandy criteria that are used for diagnosis include: (1) signs and symptoms of increased intracranial pressure, (2) absence of localizing or focal neurologic signs other than cranial nerve VI paresis, (3) elevated cerebrospinal fluid pressures, and (4) no etiology for increased intracranial pressure on neuroimaging or analysis of cerebrospinal fluid.8,10 Incidence is 0.9 per 100,000 per year, higher in obese women of childbearing age.10 Anesthesiologists are most likely to encounter idiopathic intracranial hypertension in pregnant women requesting labor analgesia.
Preoperative Evaluation
Medications to lower intracranial pressure should not be stopped. A neuro-ophthalmologist should be consulted if there any concerns about intracranial pressure control or evidence of worsening visual function. Acetazolamide, commonly used to treat idiopathic intracranial hypertension, inhibits carbonic anhydrase, increasing renal bicarbonate excretion, which may result in metabolic acidosis. Caution is recommended in the anesthetized patient who also has chronic obstructive pulmonary disease, as they may be unable to increase ventilation in response to the metabolic acidosis, leading to prolonged postoperative mechanical ventilation.83
Anesthetic Management
The most common perioperative concern is the safety of neuraxial anesthesia or analgesia. Spinal or epidural anesthesia has been used effectively and uneventfully for labor analgesia and Cesarean delivery, including in those with cerebrospinal fluid diversion devices.84 Since there is no obstruction to cerebrospinal fluid flow in idiopathic intracranial hypertension, a rapid decline in cerebrospinal fluid pressure from a lumbar puncture is quickly balanced by caudal cerebrospinal fluid flow and does not result in uncal herniation or visual changes. However, there are some controversies and concerns about the use of epidural catheters. Some have postulated that large volumes of epidural local anesthetics may further increase intracranial pressures. For example, Hilt et al reported an exaggerated increase in intracranial pressure in a patient with idiopathic intracranial hypertension following a lumbar epidural injection of bupivacaine.85 A review examined four case reports of epidural analgesia for labor in patients with idiopathic intracranial hypertension. One was complicated by an inadvertent dural puncture headache. There are four additional case reports concerning five patients, of which three received a combined epidural-spinal, or a spinal catheter, with no complications reported.86–89 Although the literature is sparse, there does not seem to be enough justification to withhold or alter the normal procedures for labor analgesia in patients with idiopathic intracranial hypertension.
Ischemic Optic Neuropathy
Epidemiology
Ischemic optic neuropathy (ION) is the leading cause of sudden vision loss in those older than 50 years of age, typically occurring spontaneously and without prior warning signs. Anterior ischemic optic neuropathy affects the optic nerve head and always causes optic nerve swelling in the acute setting. Posterior ischemic optic neuropathy impacts the portion of the optic nerve behind the eye that is not visible by examination of the ocular fundus. Optic disc swelling upon symptom onset clinically differentiates ischemic optic neuropathy into, respectively, anterior ION, and posterior ION (Table 1).90 ION is also classified as non-arteritic or arteritic, the latter associated with vasculitis. Non-arteritic anterior ION has an estimated annual incidence of 2.3-10.2 per 100,000 in the United States.91 Arteritic anterior ION, caused by mechanical vascular occlusion of the short posterior ciliary arteries from giant cell or temporal arteritis, generally occurs in those older than 60 years of age, has a female predilection, and responds to steroids.92 Non-arteritic posterior ION is rare outside of the perioperative setting. In contrast, posterior ION is a common presentation of arteritic ION.93 ION is an important consideration in anesthesia planning because peri-operative ION is a devastating complication seen most commonly in association with spine or cardiac surgery.
Risk factors
Although the exact cause of non-arteritic anterior ION remains elusive, most affected patients have a small optic cup-to-disc ratio (Fig 2A).94 As a result, axons subjected to ischemia are compressed at the lamina cribrosa, the exit point of the optic nerve from the eye (Fig 2B).95 Other risk factors for anterior ION are hypertension, diabetes, smoking, hyperlipidemia, hyper-coagulable states, obstructive sleep apnea, and migraine.96,97
Figure 2A: The components of the cup and disc in the fundus image of the eye.
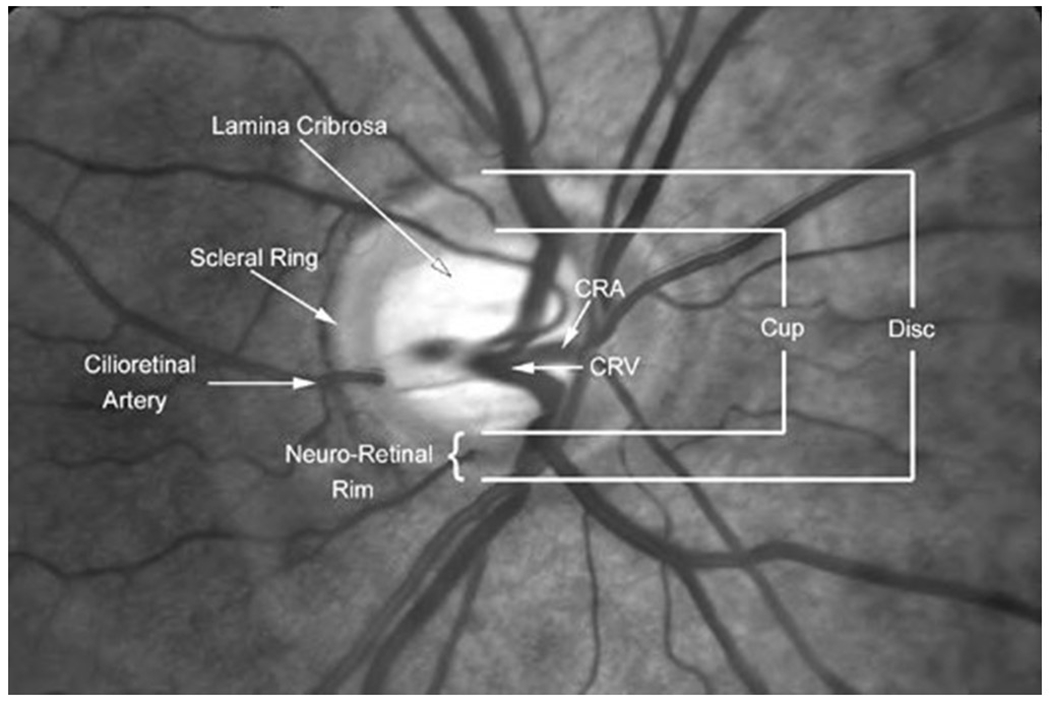
In a low cup-to-disc ratio, the axons that constitute the optic nerve are at risk of compression as they exit the eye in the lamina cribrosa. In a high cup-to-disc ratio, there is a higher risk of glaucoma. Reproduced with permission, from novel.utah.edu and Dr. Kathleen Digre, the copyright holder. A link to the figure is available at https://collections.lib.utah.edu/ark:/87278/s6d24vxw. Accessed 24March22
Figure 2B: Cross section of the eye illustrating locations of retina, optic nerve, and the lamina cribrosa.
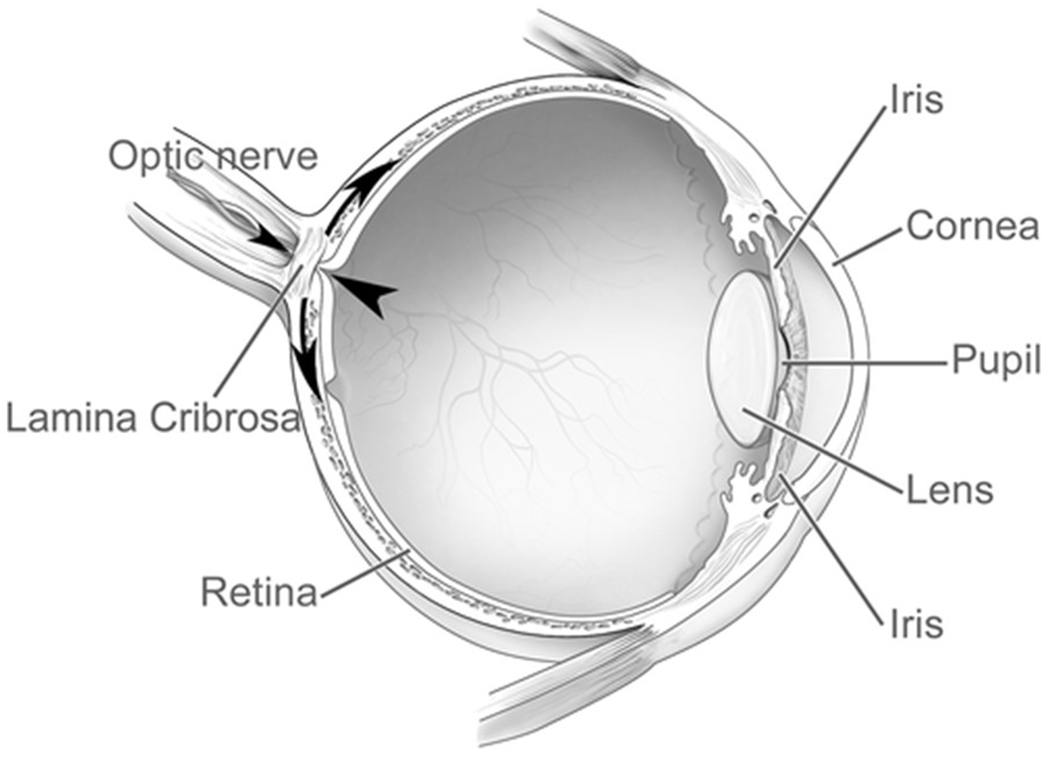
Large arrowhead shows the pressure exerted by the intraocular pressure, and the small arrowhead the retrobulbar cerebrospinal fluid pressure. The intraocular pressure also generates a pressure load to the inner surface of the eye wall, as shown by the curved arrows. Reprinted with permission from the publisher from Imaging of the lamina cribrosa and its role in glaucoma: a review. Ching-Yu Cheng, Michaël JA Girard, Victor Koh, et al, Clinical & Experimental Ophthalmology, John Wiley and Sons, Jan 10, 2018
Pathophysiology
It has been hypothesized that non-arteritic anterior ION is the result of vascular dysregulation, as well as to anatomical and physiological variations in the blood supply to the optic nerve.97 In a small study, 20% of normal subjects demonstrated abnormal autoregulation of blood flow in the optic nerve head. 98 Due to the constricted space of the lamina cribrosa, edema resulting from axonal ischemia leads to a vicious circle of vascular compression and ischemia of the optic nerve axons.99 Rodent models have demonstrated the presence of a significant neuro-inflammatory component with high levels of pro-inflammatory prostaglandins and cytokines.100 In later stages, there is death of the axons in the optic nerve and apoptotic death of the retinal ganglion cells.101 The latter results in the optic disc pallor or atrophy that is visible in the ocular fundus in the late stages of ION. People who have spontaneous non-arteritic ION in one eye are at increased risk of non-arteritic ION in the fellow eye. To date, treatment of sleep apnea is the only intervention that has been shown to be associated with reduced risk of fellow eye involvement.102
In contrast, posterior ION affects the part of the optic nerve between the lamina cribrosa and the optic canal, a region with a blood supply (Fig 3) that results in watershed zones. A peripheral centripetal vascular system is from recurrent branches of the peripapillary choroid and the circle of Zinn, which are anastomoses of branches of the posterior ciliary arteries. Pial branches from the central retinal artery and ophthalmic arteries, and posterior ciliary arteries are added contributors. An axial centrifugal vascular system formed by branches from the intraneural central retinal artery contributes but is not present in every eye. These differences in blood supply in the posterior optic nerve may render some individuals more susceptible to posterior ION, however there is no current method for detecting such differences in humans.103,104
Figure 3: The circulation of the optic nerve head and posterior portion of the optic nerve.
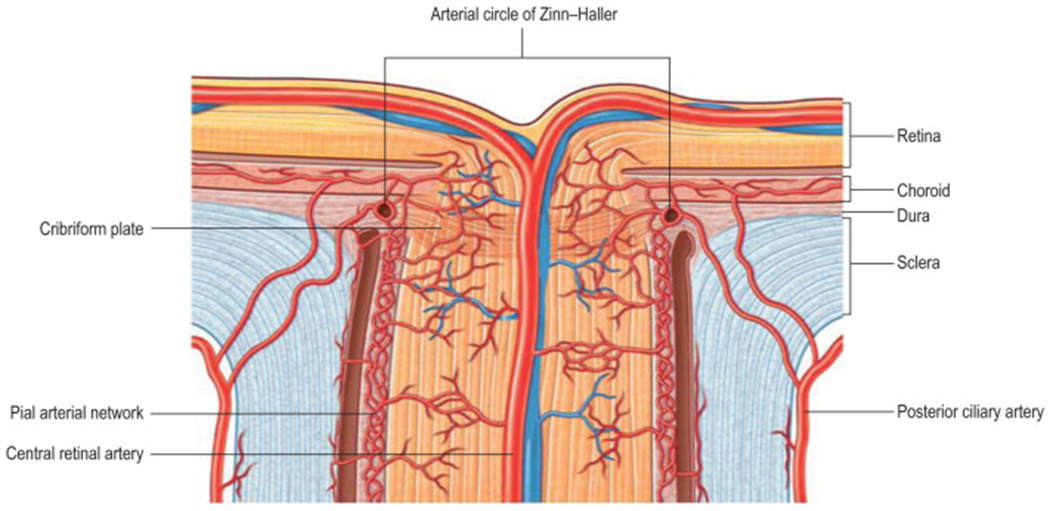
The blood supply of the optic nerve head (top) is derived primarily from the arteriolar anastomotic circle of Zinn–Haller, which is supplied by the posterior ciliary arteries, the pial arteriole plexus, and the peripapillary choroid. The posterior optic nerve is supplied by pial branches from the central retinal and ophthalmic arteries, and posterior ciliary arteries. An axial centrifugal vascular system formed by branches from the intraneural central retinal artery contributes but is not present in every eye. Reprinted with permission from the publisher from Visual Loss: Optic Neuropathies: Stacy L. Pineles and Laura J. Balcer in Liu, Volpe, and Galetta’s Neuro-Ophthalmology, 5, 101-196, 1996, Elsevier
Patients at Increased Risk for Perioperative ION
Epidemiology
ION is the most common cause of perioperative vision loss. Anterior ION occurs more frequently in patients after cardiac surgery and with a history of coronary artery disease.66 Posterior ION is more common in those undergoing spine fusion surgery, especially on the lumbar or sacral spine, and is usually bilateral.105 Posterior ION is also associated with intraoperative blood loss, and more likely to cause complete blindness compared to anterior ION.106
The estimated incidence of perioperative ION is 1 in 60,000 to 125,000 anesthetics,16,107 mostly in spine, cardiac, vascular, orthopedic joint replacement, and head and neck surgery.59,108,109 In the United States Nationwide Inpatient Sample, spine and cardiac surgery had the highest rates of perioperative vision loss, at 3.09 per 10,000 and 8.64 per 10,000 respectively.59 There has been little change in incidence of ION after cardiac surgery. An encouraging trend is that the incidence of ION after spinal fusion surgery has been declining, from an estimated 1.63/10,000 spine fusion surgeries in 1998-2000 to 0.6/10,000 in 2010-2012.5,7
Clinical Presentation and Treatment
The onset of peri-operative ION is usually within 24 to 48 hours after surgery; often the patient recognizes a change in vision upon awakening. There is an afferent pupil defect or nonreactive pupil(s), and vision loss ranging from visual field deficits to no light perception. Color vision is often diminished.105 An altitudinal deficit, i.e., loss of visual sensation in the horizontal half of the visual field, is more typical of anterior than posterior ION.97 Cases that follow spinal fusion surgery are more commonly posterior ION, and are bilateral > 50% of the time.105 When a patient complains of loss of vision following surgery or ION is suspected, expeditious ophthalmological consultation is paramount for diagnosis110 and rapid initiation of treatment (Table 1).
Unfortunately, there are no evidence-based treatments for perioperative ION.111–115 The disease often produces vision loss that will profoundly and unexpectedly change the patient’s life, therefore, any non-harmful treatment that has some chance of restoring vision should be considered. Case reports have documented some improvement using the following treatments: 1) Restoring hemoglobin and systemic blood pressures to or close to baseline.116,117 2) Elevating the head to decrease the presumed increase in venous pressure if there is significant facial edema.118 3) High dose steroids. 93,119,120 4) Hyperbaric oxygen.111 The treatment should be chosen in consultation with a neuro-ophthalmologist.
ION and Spinal Fusion Surgery
Prone positioning, large estimated blood losses, lower colloid compared to crystalloid fluid administration, lengthier anesthesia duration, Wilson frame use, obesity, and male sex were significantly and independently associated with ION after spinal fusion surgery.105 These factors reduce oxygen delivery to the optic nerve (Fig 4), leading to axonal degeneration, which is evident as optic nerve pallor or atrophy within weeks to months after the event.121–123 Although not proven, if fluids administered perioperatively are comprised of a lower percentage of colloid vs crystalloid solution, there may be accumulation of fluid within the optic nerve. There have been no studies of differences with specific types of colloids. Prone position can increase venous pressure in the optic nerve, an effect potentiated by use of the Wilson surgical frame (where the head is placed lower than the heart) and obesity.90 Increased interstitial fluid accumulation, and decreased perfusion pressures may contribute to the pathophysiology of ION.
Figure 4: Possible factors contributing to perioperative ischemic optic neuropathy.
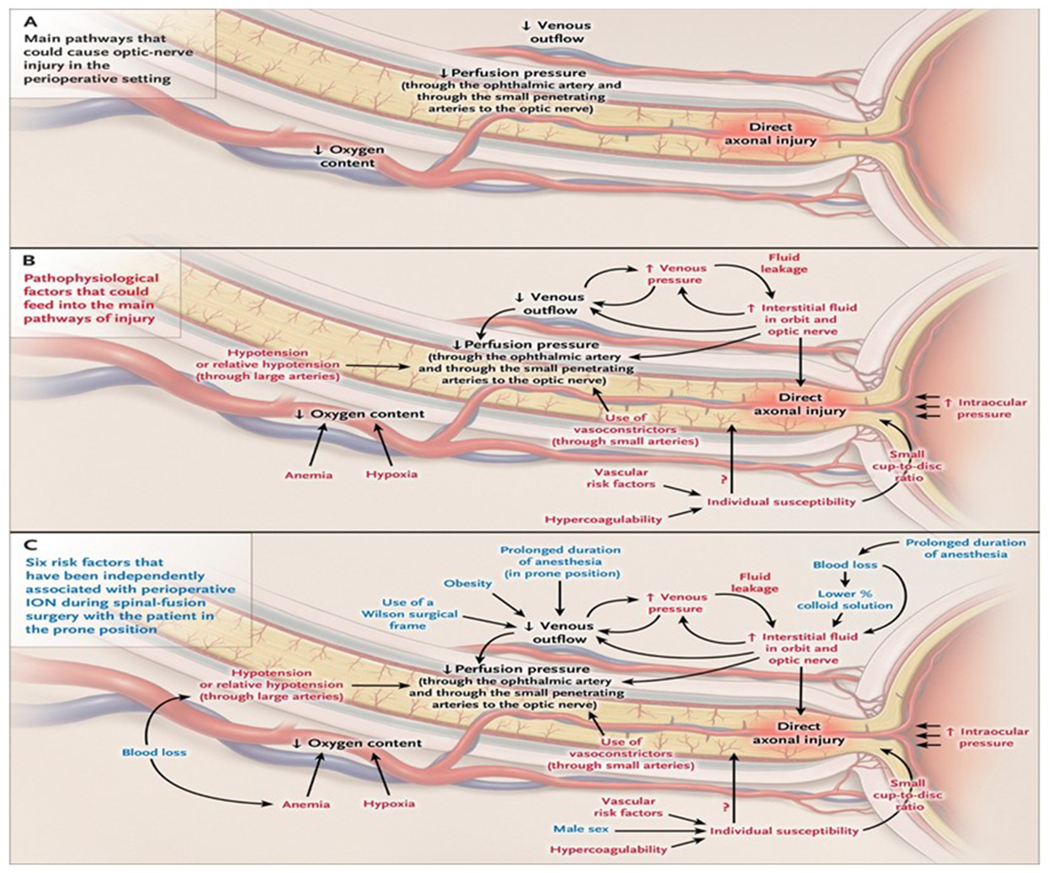
(A) Shows decreased perfusion pressure, decreased oxygen delivery, increased venous pressure, and direct axonal injury as components of axonal damage. (B) Shows the multiple proposed mechanisms of perioperative ION. (C) Shows in blue the impact of the six independent risk factors for ischemic optic neuropathy after spinal-fusion surgery with the patient positioned prone for surgery: male sex, obesity, Wilson frame, longer anesthesia, greater estimated blood loss, and lower percent colloid solution. Reprinted with permission from the publisher from Biousse, V, and Newman, NJ, Ischemic Optic Neuropathies, NEJM 372, 2428-36, 2015.
Animal models were developed to try to determine the mechanisms of perioperative ION. Lee et al showed that a hematocrit decreased to 15%, jugular venous occlusion, and hypotension (50 mmHg) combined in adult pigs significantly decreased blood flow to the optic nerve. But limitations were lack of histology of the optic nerve or optic nerve function, and that the pig eye circulation differs significantly from human.123 Roth et al found that hemodilution and 70 degree head down tilt in rats significantly altered visual evoked potentials, electrical activity specifically originating in retinal ganglion cells (scotopic threshold response), and increased glial reactivity in the optic nerve.122 However, this study lacked histological measurements of axonal damage in the optic nerve to correlate with the functional changes. Updated animal models may be useful in determining mechanisms of perioperative ION and new treatment strategies. Regeneration of the optic nerve and connection to the visual cortex remain ongoing challenges.124 There are exciting new experimental studies in rodents suggesting that regeneration of the optic nerve may be possible using cell-based therapies, but still few clinical trials in humans.125
ION and Cardiac Surgery
There are only a few studies on perioperative ION in cardiac surgery.66,126 Two single institution studies retrospectively examined risk factors in cardiac surgery. In a study of 600 patients, factors differing in those with ION vs unaffected were longer cardiopulmonary bypass time, lower minimum hematocrit, greater mean 24-hour postoperative weight gain, and greater vasoactive drug requirements to maintain adequate blood pressures.127 In 28,000 cardiac surgeries, ION was in 0.06%. Low minimum postoperative hemoglobin, severe vascular disease, preoperative coronary angiography within 48 hours before surgery, longer cardiopulmonary bypass, and blood transfusions were associated with ION.128 This study had no correction for multiple comparisons. Rubin et al studied 5,559,395 discharges after cardiac surgery in the United States Nationwide Inpatient Sample. Carotid artery stenosis, stroke, diabetic or hypertensive retinopathy, macular degeneration, glaucoma, and cataract were associated with increased risk of ION.7 This was the first study demonstrating a correlation between pre-existing eye disease and perioperative ION, but further studies are necessary to assess the significance of this novel finding. Findings are limited by inability to confirm discharge diagnoses, and possible reporting bias.129 Treatment considerations for ION after cardiac surgery are similar to those after spine surgery.
Anesthetic Management in Patients at Elevated Risk for Perioperative ION
In 2019 the American Society of Anesthesiologists Task Force on Peri-operative Visual Loss published a practice advisory specific to ION in lumbar and sacral spinal fusion surgery.130 The Task Force recommended that patients in whom prolonged procedures, substantial blood loss, or both are anticipated should be informed of the increased risk of visual loss. These recommendations were based upon evidence from the literature, and consensus of a group of expert neuro-anesthesiologists, spine surgeons, and neuro-ophthalmologists. The Advisory recommended maintaining arterial pressure at appropriately elevated levels in hypertensive patients, avoiding deliberate hypotension in high-risk patients unless the anesthesiologist and surgeon agree its use is essential, and treating prolonged significant decreases in blood pressure. Monitoring hemoglobin or hematocrit values was recommended in high-risk patients with substantial blood loss and keeping the head level with or higher than the rest of the body when possible. Consideration to stage complex procedures on a case-by-case basis was recommended. In theory, staging should result in less blood loss and fluid administration, but its impact on perioperative outcomes including on ION has still not been well described.131
Perioperative ION, Malpractice litigation, and Informed Consent
Considering the serious nature of perioperative vision loss, malpractice litigation and large monetary payments are likely. In nine cases of perioperative vision loss in one US insurer’s database (Controlled Risk Insurance Company, Boston, MA), the mean indemnity payment was $906,000.132 An informed consent discussion that includes a rare but life-changing complication like ION can be challenging for the anesthesia provider. Over 80% of patients preferred full disclosure of risk of vision loss in spine surgery by the surgeon before the day of surgery.133 Informing the patient about the heightened risk of ION in advance allows more time for discussion and can decrease the considerable anxiety and possible delay of surgery.
About half of US states apply the “reasonable patient standard,” where the physician is expected to disclose all information which would influence any reasonable person to decide whether or not to undergo a procedure.134 Vision loss, while rare, is life-changing and serious enough to constitute a material risk to a reasonable patient.135 Although controversial, it has been suggested that the reasonable patient standard be applied uniformly.134 Even in states where such informed consent is not required, the anesthesia provider should consider informing the patient of the risk of vision loss as such informed consent is consistent with modern concepts of patient autonomy.136
Cerebral visual loss
Epidemiology
Cerebral vision loss is a heterogeneous disorder encompassing multiple different causes of brain parenchymal dysfunction leading to visual impairment. There are few studies of peri-operative cerebral visual loss. Understanding the differential diagnoses is critical because this entity may be confused with posterior ischemic optic neuropathy and retinal artery occlusion since both have normal retinal and optic nerve exams at the time of presentation (Table 1). Among 808 coronary artery bypass grafting operations, there were 10 cases, but the responsible cerebral injury was confirmed by computerized tomography of the brain in just five.137 In 700 coronary artery bypass grafting operations and valve replacements by a single surgeon, there were two unilateral occipital cortex infarctions.138 Shaw et al found a 5% incidence of cerebral vision loss in 312 patients after coronary bypass grafting in a prospective study.139
Cerebral vision loss has also been reported after spine fusion surgery, but as with cardiac, there are mostly case series reporting very few cases and little information to discern cause. 140–142 Shen et al examined > 5.6 million discharges in the Nationwide Inpatient Sample with a procedure code for spine, cardiac, orthopedic, or abdominal (as control) surgery. A cerebral visual loss ICD code was in 0.38 per 10,000 discharges.59 The highest risk to develop cerebral visual loss was in cardiac and spine surgery. A surprising finding was that those < 18 years of age were the highest risk age group. Subsequently, this was confirmed in the Nationwide Inpatient Sample for discharges specifically < 18 years of age between 2002 and 2011 with a discharge diagnosis of scoliosis and a procedure code for spinal fusion surgery. Post-operative visual loss was in 0.16%; all were due to cerebral vision loss. Fusion of > eight spinal levels was associated with an odds ratio of 2.4 for developing cerebral vision loss.143 The cause of this increased risk of cerebral vision loss in spinal fusion in those < 18 years of age is still unexplained.
Clinical Features
Symptoms differ depending upon the location of cerebral dysfunction ranging from isolated homonymous (same in both eyes) unilateral visual field defects from unilateral occipital lobe pathology, to homonymous visual field loss with other neurological symptoms due to unilateral parietal or temporal lobe pathology, to complete blindness with denial of symptoms due to bilateral occipital lobe pathology, and to visual processing disturbances with intact visual acuity and visual fields due to bilateral parietal lobe pathology. Perioperative stroke involving the internal carotid, or middle, or posterior cerebral arteries territories can result in cerebral vision loss. Bilateral occipital cortex (area V1) damage produces binocular bilateral visual loss, and unilaterial damage presents as binocular unilateral visual loss in the form of homonymous hemianopia. If central vision is present and only V1 is infarcted, responses to threat and optico-kinetic nystagmus are intact. Pupil reaction, eye movements, and ocular fundus are normal. Agnosia (lack of awareness of deficit) and confabulation may be present in complete blindness due to cerebral injury, which is known as Anton Syndrome (Table 1). 144
Mechanism and Pathophysiology
Cerebral vision loss is generally caused by emboli to the posterior cerebral arteries supplying area V1, or a watershed infarct in the parieto-occipital area that may be associated with hypotension.145 The occipital pole, with its foveal representation in the retina, receives a dual blood supply from the middle cerebral and posterior cerebral arteries, therefore central vision is often spared in posterior cerebral artery infarction.146 The pathophysiology of cerebral vision loss after spine fusion and coronary artery bypass grafting remains incompletely understood. Embolism as fat and atheroma or microemboli of lipid and fibrin-platelet aggregates occurs during open heart sugery;147 those with aortic atherosclerosis appear to be at increased risk of embolic phenomena.148 The watershed zone between the middle and posterior cerebral arteries, worsened by existing cerebrovascular disease, can increase the susceptibility of a patient to decreased cerebral perfusion such as with systemic hypotension.149
Prevention and Treatment of Cerebral Vision Loss
Generally there is incomplete resolution of visual field defects. There are no specific treatments apart from prioritizing prevention of the progression of acute stroke. A stroke specialist should be consulted. If eligible for reperfusion therapy, treatment must be initiated rapidly, but thrombolysis may be contraindicated in postoperative patients. Mechanical thrombectomy without anti-coagulation may be an option.150 There are no randomized studies on prevention of cerebral vision loss in cardiac surgery but adequate removal of air and particulate matter from the heart may decrease the risk of embolism.151,152 Maintenance of adequate systemic perfusion pressure may prevent episodes of hypoperfusion in patients with known cerebrovascular disease, but no controlled studies have associated visual loss and perfusion pressure in open heart surgery, therefore it is not possible to provide any evidence-based recommendations to prevent cerebral vision loss in this setting.
Glaucoma
Epidemiology
Glaucoma is a group of progressive age-related and intraocular pressure-dependent optic neuropathies causing slow, progressive retinal ganglion cell degeneration, eventually resulting in irreversible visual field loss.153–155 It is the second leading cause of blindness in the world, affecting > 3 million in the United States, > 70 million worldwide, expected to reach 112 million by 2040.156 The elderly are disproportionately affected.157,158 With an increasingly aging population, anesthesia providers are very likely to encounter patients with glaucoma.
There are three main types of glaucoma: 1) High tension glaucoma, characterized by increased resistance to, or blockage of aqueous humor drainage, accounts for > 80% of glaucoma in the United States.12 Patients are usually asymptomatic during the early stages. Risk factors are a high degree of near-sightedness, family history, Black race, increasing age, cup-to-disc ratio ≥ 0.7 (Fig 2A), optic disc asymmetry, and intraocular pressure ≥ 22 mm Hg. 159 2) Normal tension glaucoma is glaucoma occurring despite normal intraocular pressure.160 Hypotheses concerning its origin include retinal ganglion cell or axonal hypersensitivity, restricted glymphatic flow,161 activated microglia,162 vasculopathy, or intermittent hypotension.163 Alternatively, low intracranial pressure causes a pressure gradient across the lamina cribosa.164,165 The lamina cribosa displaces posteriorly (Fig 2B), deforming its pores and compressing the axonal fibers and blood vessels of the optic nerve.166 3) Angle-closure glaucoma is an emergent form of glaucoma characterized by acute reduction in the angle between the iris and cornea, narrowing the trabecular meshwork, and rapidly elevating the intraocular pressure (Fig 5).
Figure 5:
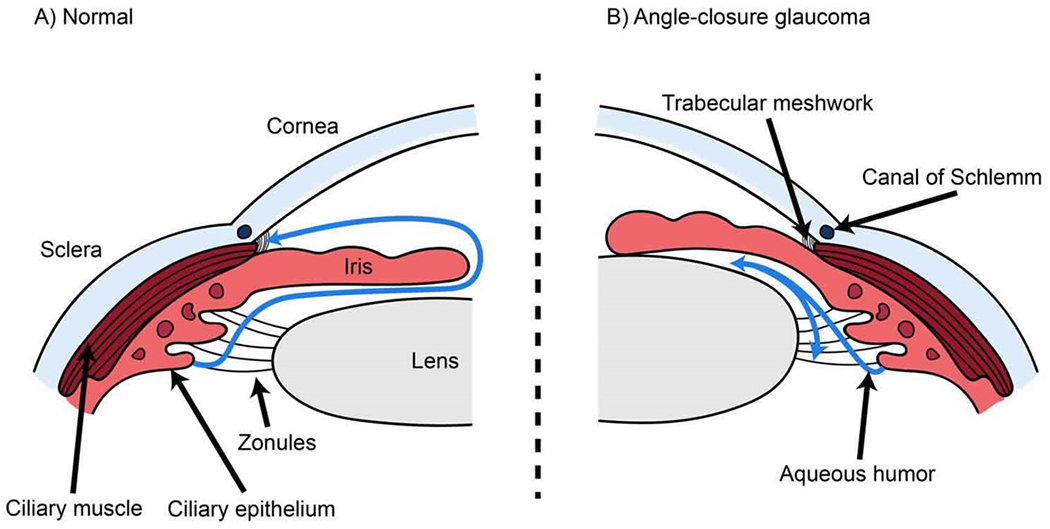
(A) In a normal eye, aqueous humor flows out of the eye into draining veins through the Canal of Schlemm as shown with a blue curving line on the left. (B) In acute angle closure glaucoma, forward bowing of the iris obstructs the normal aqueous humor flow around the lens and iris and there is no access to the Canal of Schlemm (blue curving lines), resulting in an acute increase in intraocular pressure. Reproduced by permission from healthjade.com. Accessed 24March22
Pharmacological and Surgical Treatment
Reducing intraocular pressure is the only proven method to treat glaucoma.167 Beta-adrenergic receptor antagonism or carbonic anhydrase inhibition decrease aqueous humor production. Prostaglandin analogues increase aqueous humor outflow via the uveoscleral pathway. Alpha-adrenergic agonists decrease aqueous humor production and increase uveoscleral outflow.168 Surgical interventions include minimally invasive surgery, trabeculectomy, and drainage devices.169
Preoperative Evaluation and Anesthetic Care
The main perioperative concern in glaucoma is a degradation in visual acuity or worsening of visual field deficits due to increased intraocular pressure, however their occurrence has, to date, not been well documented. Many factors in the perioperative period are associated with increased intraocular pressure, including but not limited to, head down, prone, and lateral positioning, insufflation of gas into the abdomen for laparoscopic and robotic surgery, endotracheal intubation, coughing, succinylcholine, and volume of intravenous fluids.170,171 Conversely, inhalation anesthetics and induction agents including propofol, lower intraocular pressure.172 Most of these alterations are short-lived with the exception of the more prolonged increases from surgical positioning, particularly with the patient positioned head-down or prone. The impact of prolonged prone or head down patient positioning on visual outcomes in those with glaucoma has not been well described. Most studies are small and limited, measuring intraocular pressure mostly in non-glaucomatous subjects.173–180
1). Head down positioning in subjects with glaucoma; Non-surgical
Moderate head down tilting can be used as a provocative test to identify patients who progress to development of glaucoma. However, these findings are derived from small studies. After 8 minutes of 10-degree head down tilt, there were greater changes in the pattern electroretinogram, a specific test of retinal ganglion cell function, in those previously identified with early glaucoma. 181,182
2). Prone or head down positioning and the surgical patient with glaucoma
Numerous studies documented increased IOP in patients positioned prone for surgery, mostly related to alterations in chest wall mechanisms and intrathoracic pressure.183–191 Head up tilting, and eye drops lowered intraocular pressure.186,187 The degree and duration of IOP increases that can adversely affect vision, and the specific impact upon glaucoma patients, of prone positioning have not been determined. Robotic-assisted laparoscopic prostatectomy or hysterectomy with steep Trendelenburg positioning (25-45 degree head down), increases IOP due to increased episcleral venous pressure,192 predictable from the Goldmann equation,193 IOP = (F/C) + P, where F represents aqueous flow rate, C aqueous outflow, and P is episcleral venous pressure. Most studies of steep Trendelenburg positioning and robotic-assisted prostatectomy or hysterectomy have small sample sizes and are in patients without glaucoma.179,194–197 The biggest study included 51 non-glaucomatous subjects who underwent robotic-assisted laparoscopic prostatectomy or hysterectomy whilst positioned in steep Trendelenburg. No changes were found at 3 months in retinal nerve fiber layer or ganglion cell complex thickness, foveal threshold, mean deviation, or pattern standard deviation.194 One small study examined visual outcomes in 10 glaucoma patients who underwent robotic-assisted laparoscopic prostatectomy in steep Trendelenburg. Two subjects developed progressive thinning of the retinal nerve fiber layer two months after surgery. The visual fields were not described.175
Conclusions on anesthesia for patients with glaucoma or at risk for glaucoma
Most studies have focused solely on intraoperative changes in IOP. There have been very few studies of patients with glaucoma having anesthesia and surgery; clearly there is a need for further research with measurement of visual outcomes. The authors are each typically asked a few times a year what should be done with the glaucoma patient who requires surgery while placed in the prone or head down position. There is currently no evidence-based answer to this question. Rather than intraocular pressure, the more meaningful parameter in surgical patients positioned head down or prone is likely mean ocular perfusion pressure. This can be inferred from long-term follow up studies of glaucoma patients, where ocular perfusion pressure variability appears to a risk factor for progression of visual field deficits. The 24-hour mean ocular perfusion pressure variability correlated with faster paracentral visual field loss progression.198,199 But these findings were in patients studied over a much longer time than would be experienced by a surgical patient. Since ocular perfusion pressure is approximately the difference between mean arterial blood pressure and intraocular pressure, a reasonable do-no-harm recommendation based upon this known physiology is that systemic blood pressures should be maintained close to preoperative baseline in subjects with glaucoma when positioned prone or head down for surgeries. Intraocular pressure-lowering drugs should be continued in patients with glaucoma until the time of surgery.
Perioperative Acute Angle Closure Glaucoma
Acute angle-closure glaucoma is a rare, vision-altering perioperative complication, first reported in 1957. 200 This ophthalmological emergency must be recognized and treated promptly to prevent optic nerve damage. The signs and symptoms are abrupt and severe eye pain, conjunctival redness, blurry vision, visual halos, a fixed mid-dilated pupil, corneal edema, headaches, and nausea or vomiting (Table 1). Risk factors include a history of glaucoma, genetic predisposition, female sex, increased age, far-sightedness, shallow anterior chamber depth, increased lens thickness, small corneal diameter, and Asian race.201 Most of what is known about perioperative acute angle-closure glaucoma is derived from case reports, e.g. 190,191,202–205
Drugs used by anesthesia providers that may provoke acute angle-closure glaucoma in susceptible individuals include para-sympatholytic or sympathomimetic drugs, such as atropine, scopolamine, ephedrine, or epinephrine, which cause pupil dilation. Psychological stress causing sympathetic nervous system activation and mydriasis may also contribute.206 It is imperative that a postoperative patient complaining of symptoms suggesting acute angle-closure glaucoma have a timely consultation with an ophthalmologist for immediate reduction of intraocular pressure and prevention of blindness. The diagnosis is confirmed by the findings of closed angles using gonioscopy. Smart phone and other portable devices enable these measurements to be conducted at the bedside without losing critical time transporting to the eye office or clinic.207,208 A diagnosis can also be made rapidly using a penlight; with narrow angles, a shadow is visible in the nasal side of the iris.209 Vision can be successfully preserved using medications to rapidly lower intraocular pressure, as well as via definitive therapy by a peripheral iridectomy, or a lens extraction.210
Patients with low vision
Anesthesia providers will likely encounter patients with low vision or blindness, present in approximately 2.5% of the population of the United States,2 and expected to increase due to rising prevalence of diabetic retinopathy, age related macular degeneration, glaucoma, and cataracts.211
Light perception via retinal photoreceptors, and a class of specialized intrinsically-photosensitive retinal ganglion cells expressing melanopsin, is a major influence upon circadian rhythms and mood that is lacking in blind people.212–214 Disordered sleep and alertness, and mood changes are common.14 Non-24 hour sleep-wake disorder is present in some blind subjects due to inability to perform the normal circadian pacemaker reset. Tasimelteon, a novel melatonin receptor 1 and 2 agonist, is currently the only FDA approved treatment.215 There have not been any human studies on this drug or on the impact of low vision or blindness on outcomes after anesthesia and surgery. In rodents, isoflurane-induced cognitive impairment was decreased by circadian rhythm resynchronization with melatonin, suggesting the importance of attempting to adjust circadian rhythm for higher brain function. The anesthesia provider should consider the possibility of delayed awakening or heightened emergence delirium in these subjects.216
The lack of visual cues that humans rely upon for routine interpersonal interactions complicates communication with the low vision patient and may render it challenging to convey the anesthesia plan and to obtain informed consent.15 Sensitivity to the special needs of low vision subjects is an essential part of compassionate and appropriate care and our obligation to patients both legally and ethically. Appropriate accommodations for low vision patients undergoing care in health care organizations are mandated in the US by the Americans with Disability Act, 217 and by similar laws in other countries.218 A suggestion for informed consent is a recorded audio consent.219 However, this could be difficult to implement and may not be compatible with all electronic medical record systems. It may instead be necessary for the anesthesia provider to read the entire consent form to the patient, receive acknowledgement of understanding, allow the opportunity for clarifications and answering questions, and then the patient can make “a mark” in place of the signature on the written or electronic consent form.
Summary
Ocular diseases have a profound impact on the quality of life of patients, and comprehensive, conscientious care of these patients is instrumental in mitigating the risk of preventable complications, ranging from corneal injury to blindness. Some of these injuries are easily preventable and treatable, such as exposure keratopathy, while others such as ischemic optic neuropathy presently have no recognized effective treatment, and the emphasis should be upon prevention. Table 2 is a summary of risk factors for ocular complications (Table 2A) and commonly encountered ocular conditions (Table 2B), as well as anesthesia management recommendations.
Table 2:
(A) Ocular complications in non-ocular surgery, risk factors, prevention, and treatment. (B) Commonly encountered ocular conditions, pre- and intra-operative concerns, what is known/not known in relation to anesthesia, and management recommendations. See text for more details and references. Due to space constraints, not all conditions are shown.
| A | Exposure keratopathy | Loss of eye contents | Acute angle closure glaucoma | Ischemic optic neuropathy | Central retinal artery occlusion |
|---|---|---|---|---|---|
| Risk Factors | *General anesthesia *Decreased tear production *Patient positioning *Abnormal eyelid closure |
*Corneal transplant *Open globe injury |
*History of glaucoma *Genetics *Female *Elderly *Anatomical factors *Asian race |
*Spine fusion *Cardiac surgery *Head and neck *For spine surgery: Prone positioning, large estimated blood losses, lower colloid compared to crystalloid fluid administration, lengthier anesthesia duration, Wilson frame use, obesity, and male sex |
*Spine fusion *Cardiac surgery *Head and neck *Use of N2O with recent vitrectomy and gas bubble *For spine surgery: compression of the eye *For cardiac surgery: emoblism from heart or aorta |
| Prevention | *Completely cover the eyes after induction of anesthesia *Use of ointments in some cases *Remove contact lenses *Protect eye from false eyelashes |
*Protect eyes from trauma/increased IOP *Eye shield, caution during positioning of patient |
*Medications to avoid in susceptible individuals (see text): para-sympatholytic or sympathomimetic drugs | No established strategy but see text for ASA Task Force on Perioperative Visual Loss recommendations | *Headrests and care to prevent eye compression in spine surgery with patient prone *Decrease embolic phenomena in heart surgery *Avoid N2O when vitreous gas bubble present (see text) |
| Treatment | *Analgesia (local anesthetic eye drops not advisable) *Antibiotics/eye cover in some cases *Treatment/followup by an ophthalmologist |
*None. Prevention is paramount to avoid profound visual loss | * Immediate reduction of IOP by medications AND *Peripheral iridectomy *Treatment/followup by an ophthalmologist |
*Increase blood pressure and hemoglobin levels *Elevate the head *Steroids *Hyperbaric oxygen None of these are proven effective. In all cases, consultation on treatment with neuro-ophthalmologist |
*Thrombolysis, but may be risky in post-operative patient. *Consultation on treatment with neuro-ophthalmologist |
| B | Idiopathic intracranial hypertension | Glaucoma | Low vision |
|---|---|---|---|
| Pre-op eye concerns | *Optic nerve disease: double vision, loss of vision, optic nerve swelling | *Visual field loss, low vision, increased intraocular pressure | *Difficulty with consent *Communication issues *Altered mood, alertness |
| Intra-operative concerns | *Spinal and epidural analgesia *Caution in COPD patients on acetazolamide |
*Head down or prone positioning and visual outcomes | *None |
| What is known | *Both spinal and epidural may be used safely *Continue pre-operative medications for the disease |
*Intraocular pressure increased by positioning prone or head down | *Many causes including glaucoma, diabetic retinopathy, macular degeneration, congenital |
| What is not known/Perioperative management recommendations | *Impact of further increases in intraocular pressure on vision after surgery/positioning prone or head down is not known *Advisable to keep systemic blood pressure at or near patient’s baseline, as systemic blood pressure is a major influence on ocular perfusion pressure; evidence base still lacking. *Continue the patient’s pre-operative eye drops up until time of surgery |
*The impact of low vision with or without altered alertness on awakening after anesthesia is not known. *Anticipate the need for more time to obtain a pre-operative anesthesia consent. |
Abbreviations: ASA = American Society of Anesthesiologists. COPD = chronic obstructive pulmonary disease. IOP = intraocular pressure. N2O = nitrous oxide
Acknowledgements:
Dr. Roth dedicates this review in the memory of his parents, Yitzhak Yaakov, and Miriam Hinda Roth.
Funding Statement:
This work was supported by National Institutes of Health (Bethesda, MD) grants EY010343 (Roth), EY027447 (Roth), EY028690 (Roth), EY024345 (Moss), EY022949 (Vajaranant), EY026877 (Department of Ophthalmology at Stanford University), EY001792 (Department of Ophthalmology at the University of Illinois at Chicago), and a Research to Prevent Blindness unrestricted grant (Department of Ophthalmology at Stanford University).
Footnotes
Clinical Trial Registration: Not applicable
Prior presentation: None
Conflicts of Interest:
Dr. Roth has received compensation for expert witness evaluation and testimony in cases of perioperative visual loss on behalf of patients, hospitals, and health care providers. Dr. Roth is Chair of the American Society of Anesthesiologists Task Force on Peri-operative Visual Loss. The views expressed are those of the authors only and not of the American Society of Anesthesiologists.
Contributor Information
Steven Roth, Michael Reese Professor of Anesthesiology, Professor of Ophthalmology, Vice Head for Research and Faculty Development, Department of Anesthesiology, University of Illinois at Chicago, College of Medicine, Chicago, IL.
Heather E. Moss, Associate Professor, Departments of Ophthalmology and Neurology & Neurological Sciences, Stanford University, Palo Alto, CA.
Thasarat Sutabutr Vajaranant, Professor of Ophthalmology, Director, Glaucoma Service, Vice Head for Strategic Initiatives, Department of Ophthalmology and Visual Science, University of Illinois at Chicago, College of Medicine, Chicago, IL.
Bobbie Jean Sweitzer, Professor of Medical Education, University of Virginia, Charlottesville VA; Systems Director, Perioperative Medicine, Inova Health System, Falls Church, VA.
References
- 1.Sells SB, Fixott RS: Evaluation of Research on Effects of Visual Training on Visual Functions*. American Journal of Ophthalmology 1957; 44: 230–236 [DOI] [PubMed] [Google Scholar]
- 2.Rein DB, Wittenborn JS, Zhang P, Sublett F, Lamuda PA, Lundeen EA, Saaddine J: The Economic Burden of Vision Loss and Blindness in the United States. Ophthalmology 2022; 129: 369–378 [DOI] [PubMed] [Google Scholar]
- 3.Vetter TR, Ali NM, Boudreaux AM: A case-control study of an intraoperative corneal abrasion prevention program: holding the gains made with a continuous quality improvement effort. Jt Comm J Qual Patient Saf 2012; 38: 490–6 [DOI] [PubMed] [Google Scholar]
- 4.Foroutan AR, Gheibi GH, Joshaghani M, Ahadian A, Foroutan P: Traumatic wound dehiscence and lens extrusion after penetrating keratoplasty. Cornea 2009; 28: 1097–9 [DOI] [PubMed] [Google Scholar]
- 5.Rubin DS, Parakati I, Lee LA, Moss HE, Joslin CE, Roth S: Perioperative visual Loss in spine fusion surgery: Ischemic optic neuropathy in the United States from 1998 to 2012 in the Nationwide Inpatient Sample. Anesthesiology 2016; 125: 457–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Todd MM: Good News: But Why Is the Incidence of Postoperative Ischemic Optic Neuropathy Falling? Anesthesiology 2016; 125: 445–8 [DOI] [PubMed] [Google Scholar]
- 7.Rubin DS, Matsumoto MM, Moss HE, Joslin CE, Tung A, Roth S: Ischemic Optic Neuropathy in Cardiac Surgery: Incidence and Risk Factors in the United States from the National Inpatient Sample 1998 to 2013. Anesthesiology 2017; 126: 810–821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ahmad SR, Moss HE: Update on the Diagnosis and Treatment of Idiopathic Intracranial Hypertension. Semin Neurol 2019; 39: 682–691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wall M, Kupersmith MJ, Thurtell MJ, Moss HE, Moss EA, Auinger P: The Longitudinal Idiopathic Intracranial Hypertension Trial: Outcomes From Months 6-12. Am J Ophthalmol 2017; 176: 102–107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wall M: Idiopathic intracranial hypertension. Neurol Clin 2010; 28: 593–617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huna-Baron R, Kupersmith MJ: Idiopathic intracranial hypertension in pregnancy. J Neurol 2002; 249: 1078–81 [DOI] [PubMed] [Google Scholar]
- 12.Friedman DS, Wolfs RC, O’Colmain BJ, Klein BE, Taylor HR, West S, Leske MC, Mitchell P, Congdon N, Kempen J: Prevalence of open-angle glaucoma among adults in the United States. Arch Ophthalmol 2004; 122: 532–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gayat E, Gabison E, Devys JM: Case report: bilateral angle closure glaucoma after general anesthesia. Anesth Analg 2011; 112: 126–8 [DOI] [PubMed] [Google Scholar]
- 14.Skene DJ, Arendt J: Circadian rhythm sleep disorders in the blind and their treatment with melatonin. Sleep Med 2007; 8: 651–5 [DOI] [PubMed] [Google Scholar]
- 15.Klauke S, Sondocie C, Fine I: The impact of low vision on social function: The potential importance of lost visual social cues. J Optom 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roth S, Thisted RA, Erickson JP, Black S, Schreider BD: Eye injuries after non ocular surgery: A study of 60, 965 anesthetics from 1988 to 1992. Anesthesiology 1996; 85: 1020–1027 [DOI] [PubMed] [Google Scholar]
- 17.Deljou A, Weingarten TN, Mahr MA, Sprung J, Martin DP: Postoperative Corneal Injuries: Incidence and Risk Factors. Anesth Analg 2019; 129: 737–742 [DOI] [PubMed] [Google Scholar]
- 18.Gild WM, Posner KL, Caplan RA, Cheney FW: Eye injuries associated with anesthesia. A closed claims analysis. Anesthesiology 1992; 76: 204–208 [DOI] [PubMed] [Google Scholar]
- 19.Singh RB, Khera T, Ly V, Saini C, Cho W, Shergill S, Singh KP, Agarwal A: Ocular complications of perioperative anesthesia: a review. Graefes Arch Clin Exp Ophthalmol 2021; 259: 2069–2083 [DOI] [PubMed] [Google Scholar]
- 20.Ahmed F, House RJ, Feldman BH: Corneal Abrasions and Corneal Foreign Bodies. Prim Care 2015; 42: 363–75 [DOI] [PubMed] [Google Scholar]
- 21.Yu HD, Chou AH, Yang MW, Chang CJ: An analysis of perioperative eye injuries after nonocular surgery. Acta Anaesthesiol Taiwan 2010; 48: 122–9 [DOI] [PubMed] [Google Scholar]
- 22.Segal KL, Fleischut PM, Kim C, Levine B, Faggiani SL, Banerjee S, Gadalla F, Lelli GJ Jr.: Evaluation and treatment of perioperative corneal abrasions. J Ophthalmol 2014; 2014: 901901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Batra YK, Bali IM: Corneal abrasions during general anesthesia. Anesth Analg 1977; 56: 363–5 [DOI] [PubMed] [Google Scholar]
- 24.Krupin T, Cross DA, Becker B: Decreased basal tear production associated with general anesthesia. Arch Ophthalmol 1977; 95: 107–108 [DOI] [PubMed] [Google Scholar]
- 25.Zernii EY, Golovastova MO, Baksheeva VE, Kabanova EI, Ishutina IE, Gancharova OS, Gusev AE, Savchenko MS, Loboda AP, Sotnikova LF, Zamyatnin AA Jr., Philippov PP, Senin II: Alterations in Tear Biochemistry Associated with Postanesthetic Chronic Dry Eye Syndrome. Biochemistry (Mosc) 2016; 81: 1549–1557 [DOI] [PubMed] [Google Scholar]
- 26.Aragona P, Aguennouz M, Rania L, Postorino E, Sommario MS, Roszkowska AM, De Pasquale MG, Pisani A, Puzzolo D: Matrix metalloproteinase 9 and transglutaminase 2 expression at the ocular surface in patients with different forms of dry eye disease. Ophthalmology 2015; 122: 62–71 [DOI] [PubMed] [Google Scholar]
- 27.Demirci H, Frueh BR: Palpebral spring in the management of lagophthalmos and exposure keratopathy secondary to facial nerve palsy. Ophthalmic Plast Reconstr Surg 2009; 25: 270–5 [DOI] [PubMed] [Google Scholar]
- 28.Elner VM, Hassan AS, Frueh BR: Graded full-thickness anterior blepharotomy for upper eyelid retraction. Arch Ophthalmol 2004; 122: 55–60 [DOI] [PubMed] [Google Scholar]
- 29.Sampat A, Parakati I, Kunnavakkam R, Glick DB, Lee NK, Tenney M, Eggener S, Roth S: Corneal abrasion in hysterectomy and prostatectomy: Role of laparoscopic and robotic assistance. Anesthesiology 2015; 122: 994–1001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Malafa MM, Coleman JE, Bowman RW, Rohrich RJ: Perioperative Corneal Abrasion: Updated Guidelines for Prevention and Management. Plast Reconstr Surg 2016; 137: 790e–798e [DOI] [PubMed] [Google Scholar]
- 31.Grixti A, Sadri M, Watts MT: Corneal protection during general anesthesia for nonocular surgery. Ocul Surf 2013; 11: 109–18 [DOI] [PubMed] [Google Scholar]
- 32.Drzymalski DM, Ward K, Hernandez JM, Hoot J, Au SC, Yang FC, Azocar RJ: The effect of Tegaderm™ versus EyeGard® on eyelid erythema during general anesthesia: a randomized-controlled trial. Can J Anaesth 2020; 67: 560–567 [DOI] [PubMed] [Google Scholar]
- 33.Grover VK, Kumar KV, Sharma S, Sethi N, Grewal SP: Comparison of methods of eye protection under general anaesthesia. Can J Anaesth 1998; 45: 575–7 [DOI] [PubMed] [Google Scholar]
- 34.Morris A, Bonanno L, Bennett M: Effectiveness of corneal abrasion prevention interventions for adults undergoing general anesthesia for more than one hour: a systematic review protocol. JBI Database System Rev Implement Rep 2018; 16: 1785–1790 [DOI] [PubMed] [Google Scholar]
- 35.Roth S, Tung A, Ksiazek S: Visual loss in a prone-positioned spine surgery patient with the head on a foam headrest and goggles covering the eyes: an old complication with a new mechanism. Anesth Analg 2007; 104: 1185–7 [DOI] [PubMed] [Google Scholar]
- 36.Amano Y, Sugimoto Y, Sugita M: Ocular disorders due to eyelash extensions. Cornea 2012; 31: 121–5 [DOI] [PubMed] [Google Scholar]
- 37.Colby K: Update on Corneal Transplant in 2021. JAMA 2021; 325: 1886–1887 [DOI] [PubMed] [Google Scholar]
- 38.Elder MJ, Stack RR: Globe rupture following penetrating keratoplasty: how often, why, and what can we do to prevent it? Cornea 2004; 23: 776–80 [DOI] [PubMed] [Google Scholar]
- 39.Meyer JJ, McGhee CN: Incidence, severity and outcomes of traumatic wound dehiscence following penetrating and deep anterior lamellar keratoplasty. Br J Ophthalmol 2016; 100: 1412–5 [DOI] [PubMed] [Google Scholar]
- 40.Learned DL, Gupta CK, Stec LA, Heidemann DG: Perioperative Corneal Transplant Wound Dehiscence. Anesthesiology 2016; 124: 185. [DOI] [PubMed] [Google Scholar]
- 41.Steinberg J, Eddy MT, Katz T, Fricke OH, Richard G, Linke SJ: Traumatic wound dehiscence after penetrating keratoplasty: case series and literature review. Eur J Ophthalmol 2012; 22: 335–41 [DOI] [PubMed] [Google Scholar]
- 42.Stevenson LJ, Abell RG, McGuinness MB, Vajpayee RB: Comparative Evaluation Of Clinical Characteristics And Visual Outcomes Of Traumatic And Non-Traumatic Graft Dehiscence Following Corneal Transplantation Surgery. Clin Ophthalmol 2019; 13: 2243–2249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vachon Claude A, Warner David O, Bacon Douglas R: Succinylcholine and the Open Globe: Tracing the Teaching. Anesthesiology 2003; 99: 220–223 [DOI] [PubMed] [Google Scholar]
- 44.Moreno RJ, Kloess P, Carlson DW: Effect of succinylcholine on the intraocular contents of open globes. Ophthalmology 1991; 98: 636–8 [DOI] [PubMed] [Google Scholar]
- 45.Alghamdi WM, Markoulli M, Papas EB: The effect of contact lens wear on the cellular morphology of the lid wiper area. Optom Vis Sci 2018; 95: 491–497 [DOI] [PubMed] [Google Scholar]
- 46.Weissman B, Chun MW, Barnhart LA: Corneal abrasion associated with contact lens correction of keratoconus--a retrospective study. Optom Vis Sci 1994; 71: 677–81 [DOI] [PubMed] [Google Scholar]
- 47.Berrocal MH, Acaba LA, Acaba A: Surgery for Diabetic Eye Complications. Curr Diab Rep 2016; 16: 99. [DOI] [PubMed] [Google Scholar]
- 48.Sigler EJ, Randolph JC, Charles S, Calzada JI: Intravitreal fluorinated gas preference and occurrence of rare ischemic postoperative complications after pars plana vitrectomy: a survey of the american society of retina specialists. J Ophthalmol 2012; 2012: 230596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stinson TW 3rd, Donlon JV Jr.: Interaction of intraocular air and sulfur hexafluoride with nitrous oxide: a computer simulation. Anesthesiology 1982; 56: 385–8 [DOI] [PubMed] [Google Scholar]
- 50.Naderi K, Masoero P, Karthikeyan G, Karia N, Chandra A: Warning wristbands for patients with intra-ocular gas. Eye 2020; 34: 1712–1712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wolf GL, Capuano C, Hartung J: Effect of nitrous oxide on gas bubble volume in the anterior chamber. Arch Ophthalmol 1985; 103: 418–9 [DOI] [PubMed] [Google Scholar]
- 52.Lockwood AJ, Yang YF: Nitrous oxide inhalation anaesthesia in the presence of intraocular gas can cause irreversible blindness. Br Dent J 2008; 204: 247–8 [DOI] [PubMed] [Google Scholar]
- 53.Aström S, Kjellgren D, Mönestam E, Bäcklund U: Nitrous oxide anesthesia and intravitreal gastamponade. Acta Anaesthesiol Scand 2003; 47: 361–2 [DOI] [PubMed] [Google Scholar]
- 54.Vote BJ, Hart RH, Worsley DR, Borthwick JH, Laurent S, McGeorge AJ: Visual loss after use of nitrous oxide gas with general anesthetic in patients with intraocular gas still persistent up to 30 days after vitrectomy. Anesthesiology 2002; 97: 1305–8 [DOI] [PubMed] [Google Scholar]
- 55.Hart RH, Vote BJ, Borthwick JH, McGeorge AJ, Worsley DR: Loss of vision caused by expansion of intraocular perfluoropropane (C(3)F(8)) gas during nitrous oxide anesthesia. Am J Ophthalmol 2002; 134: 761–3 [DOI] [PubMed] [Google Scholar]
- 56.Fu AD, McDonald HR, Eliott D, Fuller DG, Halperin LS, Ramsay RC, Johnson RN, Ai E: Complications of general anesthesia using nitrous oxide in eyes with preexisting gas bubbles. Retina 2002; 22: 569–74 [DOI] [PubMed] [Google Scholar]
- 57.Briggs M, Wong D, Groenewald C, McGalliard J, Kelly J, Harper J: The effect of anaesthesia on the intraocular volume of the C3F8 gas bubble. Eye (Lond) 1997; 11 (Pt 1): 47–52 [DOI] [PubMed] [Google Scholar]
- 58.Hart RH, Vote BJ, Borthwick JH, McGeorge AJ, Worsley DR: Loss of vision caused by expansion of intraocular perfluoropropane (C(3)F(8)) gas during nitrous oxide anesthesia. Am J Ophthalmol 2002; 134: 761–3 [DOI] [PubMed] [Google Scholar]
- 59.Shen Y, Drum M, Roth S: The prevalence of perioperative visual loss in the United States: a 10-year study from 1996 to 2005 of spinal, orthopedic, cardiac, and general surgery. Anesth Analg 2009; 109: 1534–45 [DOI] [PubMed] [Google Scholar]
- 60.Goldsmith MO: Occlusion of the central retinal artery following retrobulbar hemorrhage. Ophthalmologica 1967; 153: 191–6 [DOI] [PubMed] [Google Scholar]
- 61.Wray SH: The management of acute visual failure. J Neurol Neurosurg Psych 1993; 56: 234–240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Calway T, Rubin DS, Moss HE, Joslin CE, Beckmann K, Roth S: Perioperative retinal artery occlusion: Risk factors in cardiac surgery from the United States National Inpatient Sample 1998-2013. Ophthalmology 2017; 124: 189–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Grant GP, Turbin RE, Bennett HL, Szirth BC, Heary RF: Use of the Proneview Helmet System with a modified table platform for open access to the eyes during prone spine surgery. Anesth Analg 2006; 103: 499–500 [DOI] [PubMed] [Google Scholar]
- 64.Hayreh SS, Jonas JB: Optic disk and retinal nerve fiber layer damage after transient central retinal artery occlusion: an experimental study in rhesus monkeys. Am J Ophthalmol 2000; 129: 786–95 [DOI] [PubMed] [Google Scholar]
- 65.Roth S, Pietrzyk Z: Blood flow after retinal ischemia in cats. Invest Ophthalmol Vis Sci 1994; 35: 3209–3217 [PubMed] [Google Scholar]
- 66.Raphael J, Moss HE, Roth S: Perioperative Visual Loss in Cardiac Surgery. J Cardiothorac Vasc Anesth 2019; 33: 1420–1429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Daniel WT III, Kilgo P, Puskas JD, Thourani VH, Lattouf OM, Guyton RA, Halkos ME: Trends in aortic clamp use during coronary artery bypass surgery: effect of aortic clamping strategies on neurologic outcomes. The Journal of thoracic and cardiovascular surgery 2014; 147: 652–657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Glas KE, Swaminathan M, Reeves ST, Shanewise JS, Rubenson D, Smith PK, Mathew JP, Shernan SK, Durham N, Charleston S: Guidelines for the performance of a comprehensive intraoperative epiaortic ultrasonographic examination: recommendations of the American Society of Echocardiography and the Society of Cardiovascular Anesthesiologists; endorsed by the Society of Thoracic Surgeons. Anesthesia & Analgesia 2008; 106: 1376–1384 [DOI] [PubMed] [Google Scholar]
- 69.Grocott HP, Tran T: Aortic atheroma and adverse cerebral outcome: risk, diagnosis, and management options, Seminars in Cardiothoracic and Vascular Anesthesia, SAGE Publications Sage CA: Los Angeles, CA, 2010, pp 86–94 [DOI] [PubMed] [Google Scholar]
- 70.Lawton JS, Tamis-Holland JE, Bangalore S, Bates ER, Beckie TM, Bischoff JM, Bittl JA, Cohen MG, DiMaio JM, Don CW, Fremes SE, Gaudino MF, Goldberger ZD, Grant MC, Jaswal JB, Kurlansky PA, Mehran R, Metkus TS, Nnacheta LC, Rao SV, Sellke FW, Sharma G, Yong CM, Zwischenberger BA: Guidelines for Coronary Artery Revascularization: Executive Summary. Journal of the American College of Cardiology 2022; 79: 197–215 [DOI] [PubMed] [Google Scholar]
- 71.Mac Grory B, Schrag M, Biousse V, Furie KL, Gerhard-Herman M, Lavin PJ, Sobrin L, Tjoumakaris SI, Weyand CM, Yaghi S: Management of Central Retinal Artery Occlusion: A Scientific Statement From the American Heart Association. Stroke 2021; 52: e282–e294 [DOI] [PubMed] [Google Scholar]
- 72.Schrag M, Youn T, Schindler J, Kirshner H, Greer D: Intravenous Fibrinolytic Therapy in Central Retinal Artery Occlusion: A Patient-Level Meta-analysis. JAMA Neurol 2015; 72: 1148–54 [DOI] [PubMed] [Google Scholar]
- 73.Schultheiss M, Härtig F, Spitzer MS, Feltgen N, Spitzer B, Hüsing J, Rupp A, Ziemann U, Bartz-Schmidt KU, Poli S: Intravenous thrombolysis in acute central retinal artery occlusion - A prospective interventional case series. PLoS One 2018; 13: e0198114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mac Grory B, Nackenoff A, Poli S, Spitzer MS, Nedelmann M, Guillon B, Preterre C, Chen CS, Lee AW, Yaghi S, Stretz C, Azher I, Paddock J, Bakaeva T, Greer DM, Shulman JG, Kowalski RG, Lavin P, Mistry E, Espaillat K, Furie K, Kirshner H, Schrag M: Intravenous Fibrinolysis for Central Retinal Artery Occlusion: A Cohort Study and Updated Patient-Level Meta-Analysis. Stroke 2020; 51: 2018–2025 [DOI] [PubMed] [Google Scholar]
- 75.Pettersen JA, Hill MD, Demchuk AM, Morrish W, Hudon ME, Hu W, Wong J, Barber PA, Buchan AM: Intra-arterial thrombolysis for retinal artery occlusion: the Calgary experience. Canadian Journal of Neurological Sciences 2005; 32: 507–11 [PubMed] [Google Scholar]
- 76.Page PS, Khattar NK, White AC, Cambon AC, Brock GN, Rai SN, James RF: Intra-Arterial Thrombolysis for Acute Central Retinal Artery Occlusion: A Systematic Review and Meta-Analysis. Front Neurol 2018; 9: 76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ferreira D, Soares C, Tavares-Ferreira J, Fernandes T, Araújo R, Castro P: Acute phase treatment in central retinal artery occlusion: thrombolysis, hyperbaric oxygen therapy or both? J Thromb Thrombolysis 2020; 50: 984–988 [DOI] [PubMed] [Google Scholar]
- 78.Kim YS, Nam MS, Park EJ, Lee Y, Kim H, Kim SH, Cha YS: The effect of adjunctive hyperbaric oxygen therapy in patients with central retinal artery occlusion. Undersea Hyperb Med 2020; 47: 57–64 [PubMed] [Google Scholar]
- 79.Rozenberg A, Hadad A, Peled A, Dubinsky-Pertzov B, Or L, Eting E, Efrati S, Pras E, Einan-Lifshitz A: Hyperbaric oxygen treatment for non-arteritic central retinal artery occlusion retrospective comparative analysis from two tertiary medical centres. Eye (Lond) 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cugati S, Varma DD, Chen CS, Lee AW: Treatment options for central retinal artery occlusion. Current treatment options in neurology 2013; 15: 63–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Duxbury O, Bhogal P, Cloud G, Madigan J: Successful treatment of central retinal artery thromboembolism with ocular massage and intravenous acetazolamide. BMJ case reports 2014; 2014: bcr2014207943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Fieß A, Cal Ö, Kehrein S, Halstenberg S, Frisch I, Steinhorst UH: Anterior chamber paracentesis after central retinal artery occlusion: a tenable therapy? BMC Ophthalmol 2014; 14: 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Adamson R, Swenson ER: Acetazolamide Use in Severe Chronic Obstructive Pulmonary Disease. Pros and Cons. Ann Am Thorac Soc 2017; 14: 1086–1093 [DOI] [PubMed] [Google Scholar]
- 84.Karmaniolou I, Petropoulos G, Theodoraki KJCJoAJcda: Management of idiopathic intracranial hypertension in parturients: anesthetic considerations. 2011; 58: 650. [DOI] [PubMed] [Google Scholar]
- 85.Hilt H, Gramm J-J, Link J: Changes in intracranial pressure associated with extradural anaesthesia. Br J Anaesth 1986; 58: 676–680 [DOI] [PubMed] [Google Scholar]
- 86.Worrell J, Lane S: Impact of pseudotumor cerebri (idiopathic intracranial hypertension) in pregnancy: a case report. Aana j 2007; 75: 199–204 [PubMed] [Google Scholar]
- 87.Moore DM, Meela M, Kealy D, Crowley L, McMorrow R, O’Kelly B: An intrathecal catheter in a pregnant patient with idiopathic intracranial hypertension: analgesia, monitor and therapy? Int J Obstet Anesth 2014; 23: 175–8 [DOI] [PubMed] [Google Scholar]
- 88.Month RC, Vaida SJ: A combined spinal-epidural technique for labor analgesia and symptomatic relief in two parturients with idiopathic intracranial hypertension. Int J Obstet Anesth 2012; 21: 192–4 [DOI] [PubMed] [Google Scholar]
- 89.Kim K, Orbegozo M: Epidural anesthesia for cesarean section in a parturient with pseudotumor cerebri and lumboperitoneal shunt. J Clin Anesth 2000; 12: 213–5 [DOI] [PubMed] [Google Scholar]
- 90.Biousse V, Newman NJ: Ischemic optic neuropathies. N Engl J Med 2015; 372: 2428–36 [DOI] [PubMed] [Google Scholar]
- 91.Johnson LN, Arnold AC: Incidence of nonarteritic and arteritic anterior ischemic optic neuropathy. Population-based study in the state of Missouri and Los Angeles County, California. J Neuroophthalmol 1994; 14: 38–44 [PubMed] [Google Scholar]
- 92.Bajpai V, Madan S, Beri S: Arteritic anterior ischaemic optic neuropathy: An update. Eur J Ophthalmol 2021; 31: 2818–2827 [DOI] [PubMed] [Google Scholar]
- 93.Hayreh SS: Posterior ischaemic optic neuropathy: clinical features, pathogenesis, and management. Eye (Lond) 2004; 18: 1188–206 [DOI] [PubMed] [Google Scholar]
- 94.Sharma S, Kwan S, Fallano KA, Wang J, Miller NR, Subramanian PS: Comparison of Visual Outcomes of Nonarteritic Anterior Ischemic Optic Neuropathy in Patients with and without Diabetes Mellitus. Ophthalmology 2017; 124: 450–455 [DOI] [PubMed] [Google Scholar]
- 95.Beck RW, Servais GE, Hayreh SS: Anterior ischemic optic neuropathy. IX. Cup-to-disc ratio and its role in pathogenesis. Ophthalmology 1987; 94: 1503–8 [PubMed] [Google Scholar]
- 96.Cestari DM, Gaier ED, Bouzika P, Blachley TS, De Lott LB, Rizzo JF, Wiggs JL, Kang JH, Pasquale LR, Stein JD: Demographic, Systemic, and Ocular Factors Associated with Nonarteritic Anterior Ischemic Optic Neuropathy. Ophthalmology 2016; 123: 2446–2455 [DOI] [PubMed] [Google Scholar]
- 97.Arnold AC: Pathogenesis of nonarteritic anterior ischemic optic neuropathy. J Neuro-ophthalmol 2003; 23: 157–63 [DOI] [PubMed] [Google Scholar]
- 98.Pillunat LE, Anderson DR, Knighton RW, Joos KM, Feuer WJ: Autoregulation of human optic nerve head circulation in response to increased intraocular pressure. Exp Eye Res 1997; 64: 737–44 [DOI] [PubMed] [Google Scholar]
- 99.Arnold AC, Hepler RS: Flourescein angiography in acute nonarteritic anterior optic neuropathy. Am J Ophthalmol 1994; 117: 222–230 [DOI] [PubMed] [Google Scholar]
- 100.Mehrabian Z, Guo Y, Miller NR, Henderson AD, Roth S, Bernstein SL: Approaches to Potentiated Neuroprotective Treatment in the Rodent Model of Ischemic Optic Neuropathy. Cells 2021; 10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zhang C, Guo Y, Slater BJ, Miller NR, Bernstein SL: Axonal degeneration, regeneration and ganglion cell death in a rodent model of anterior ischemic optic neuropathy (rAION). Exp Eye Res 2010; 91: 286–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Aptel F, Khayi H, Pepin JL, Tamisier R, Levy P, Romanet JP, Chiquet C: Association of Nonarteritic Ischemic Optic Neuropathy With Obstructive Sleep Apnea Syndrome: Consequences for Obstructive Sleep Apnea Screening and Treatment. JAMA Ophthalmol 2015; 133: 797–804 [DOI] [PubMed] [Google Scholar]
- 103.Isayama Y, Hiramatsu K, Asakura S, Takahashi T: Posterior ischemic optic neuropathy. I. Blood supply of the optic nerve. Ophthalmologica 1983; 186: 197–203 [DOI] [PubMed] [Google Scholar]
- 104.Isayama Y, Takahashi T, Inoue M, Jimura T: Posterior ischemic optic neuropathy. III. Clinical diagnosis. Ophthalmologica 1983; 187: 141–7 [DOI] [PubMed] [Google Scholar]
- 105.Lee LA, Roth S, Todd MM, Posner KL, Polissar NL, Neradilek MB, Torner J, Newman NJ, Domino KB: The Postoperative Visual Loss Study Group. Risk factors associated with ischemic optic neuropathy after spinal fusion surgery. Anesthesiology 2012; 116: 15–24 [DOI] [PubMed] [Google Scholar]
- 106.Buono LM, Foroozan R: Perioperative posterior ischemic optic neuropathy: Review of the literature. Surv Ophathalmol 2005; 50: 15–26 [DOI] [PubMed] [Google Scholar]
- 107.Warner ME, Warner MA, Garrity JA, MacKenzie RA, Warner DO: The frequency of perioperative vision loss. Anesth Analg 2001; 93: 1417–1421 [DOI] [PubMed] [Google Scholar]
- 108.Strome SE, Hill JS, Burnstine MA, Beck J, Chepeha DB, Esclamado RM: Anterior ischemic optic neuropathy following neck dissection. Head Neck 1997; 19: 148–52 [DOI] [PubMed] [Google Scholar]
- 109.Pazos GA, Leonard DW, Blice J, Thompson DH: Blindness after bilateral neck dissection: case report and review. Am J Otolaryngol 1999; 20: 340–5 [DOI] [PubMed] [Google Scholar]
- 110.Roth S, Gillesberg I: Injuries to the Visual System and Other Sense Organs, Anesthesia and Perioperative Complications, 2nd edition. Edited by Benumof JL, Saidman LJ. St Louis, Mosby, 1999, pp 377–408 [Google Scholar]
- 111.Allashem HM, Sward DG, Sethuraman K, Matthews MK: Hyperbaric oxygen therapy for perioperative posterior ischemic optic neuropathy: a case report. Undersea Hyperb Med 2019; 46: 701–707 [PubMed] [Google Scholar]
- 112.Alexander JL, Shulman MD, Sethuraman K: Acute direct traumatic optic neuropathy treated with steroids, minocycline and hyperbaric oxygen: a case report. Undersea Hyperb Med 2019; 46: 709–712 [PubMed] [Google Scholar]
- 113.Bienert M, Plange N, Remky A, Arend KO, Kuerten D: Acute Effect of Hypervolemic Hemodilution on Retrobulbar Hemodynamics in Anterior Ischemic Optic Neuropathy. Biomed Res Int 2018; 2018: 4756313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Steigerwalt RDJ, Limoli PG, Nebbioso M: Visual field improvement in non-arteritic posterior ischemic optic neuropathy in a patient treated with intravenous prostaglandin E1 and steroids. Drug Discov Ther 2017; 11: 226–229 [DOI] [PubMed] [Google Scholar]
- 115.Foroozan R: New Treatments for Nonarteritic Anterior Ischemic Optic Neuropathy. Neurol Clin 2017; 35: 1–15 [DOI] [PubMed] [Google Scholar]
- 116.Wang MY, Brewer R, Sadun AA: Posterior ischemic optic neuropathy: Perioperative risk factors. Taiwan J Ophthalmol 2020; 10: 167–173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Stevens WR, Glazer PA, Kelley SD, Lietman TM, Bradford DS: Ophthalmic complications after spinal surgery. Spine (Phila Pa 1976) 1997; 22: 1319–24 [DOI] [PubMed] [Google Scholar]
- 118.Lee LA, Newman NJ, Wagner TA, Dettori JR, Dettori NJ: Postoperative ischemic optic neuropathy. Spine (Phila Pa 1976) 2010; 35: S105–16 [DOI] [PubMed] [Google Scholar]
- 119.Paez-Escamilla M, Abo-Zed A, Abramovitz B, Stefko ST, Waxman E: Recovery of vision after treatment of hemodialysis related bilateral optic nerve ischemia. Am J Ophthalmol Case Rep 2022; 25: 101373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Dorecka M, Miniewicz-Kurkowska J, Romaniuk D, Gajdzik-Gajdecka U, Wójcik-Niklewska B: Anterior ischemic optic neuropathy after conventional coronary artery bypass graft surgery. Med Sci Monit 2011; 17: Cs70–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Chen CS, Johnson MA, Flower RA, Slater BJ, Miller NR, Bernstein SL: A primate model of nonarteritic anterior ischemic optic neuropathy. Invest Ophthalmol Vis Sci 2008; 49: 2985–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Roth S, Dreixler J, Newman NJ: Haemodilution and head-down tilting induce functional injury in the rat optic nerve: A model for peri-operative ischemic optic neuropathy. Eur J Anaesthesiol 2018; 35: 840–847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lee LA, Deem S, Glenny RW, Townsend I, Moulding J, An D, Treggiari MM, Lam A: Effects of anemia and hypotension on porcine optic nerve blood flow and oxygen delivery. Anesthesiology 2008; 108: 864–72 [DOI] [PubMed] [Google Scholar]
- 124.Becker SM, Tumminia SJ, Chiang MF: The NEI Audacious Goals Initiative: Advancing the Frontier of Regenerative Medicine. Transl Vis Sci Technol 2021; 10: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Gokoffski KK, Lam P, Alas BF, Peng MG, Ansorge HRR: Optic Nerve Regeneration: How Will We Get There? Journal of neuro-ophthalmology : the official journal of the North American Neuro-Ophthalmology Society 2020; 40: 234–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Roth S, Raphael J: Cardiac Surgery and Ischemic Optic Neuropathy. J Cardiothorac Vasc Anesth 2021; 35: 39–40 [DOI] [PubMed] [Google Scholar]
- 127.Shapira OM, Kimmel WA, Lindsey PS, Shahian DM: Anterior ischemic optic neuropathy after open heart operations. Ann Thorac Surg 1996; 61: 660–6 [DOI] [PubMed] [Google Scholar]
- 128.Nuttall GA, Garrity JA, Dearani JA, Abel MD, Schroeder DR, Mullany CJ: Risk factors for ischemic optic neuropathy after cardiopulmonary bypass: a matched case/control study. Anesth Analg 2001; 93: 1410–6, table of contents [DOI] [PubMed] [Google Scholar]
- 129.Khera R, Angraal S, Couch T, Welsh JW, Nallamothu BK, Girotra S, Chan PS, Krumholz HM: Adherence to Methodological Standards in Research Using the National Inpatient Sample. Jama 2017; 318: 2011–2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Practice Advisory for Perioperative Visual Loss Associated with Spine Surgery 2019: An Updated Report by the American Society of Anesthesiologists Task Force on Perioperative Visual Loss, the North American Neuro-Ophthalmology Society, and the Society for Neuroscience in Anesthesiology and Critical Care. Anesthesiology 2019; 130: 12–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Le HV, Wick JB, Lafage R, Kelly MP, Kim HJ, Gupta MC, Bess S, Burton DC, Ames CP, Smith JS, Shaffrey CI, Schwab FJ, Passias PG, Protopsaltis TS, Lafage V, Klineberg EO: Surgical Factors and Treatment Severity for Perioperative Complications Predict Hospital Length of Stay in Adult Spinal Deformity Surgery. Spine (Phila Pa 1976) 2022; 47: 136–143 [DOI] [PubMed] [Google Scholar]
- 132.Du A, Saba R, Brovman EY, Greenberg P, Urman RD: A contemporary medicolegal analysis of perioperative vision loss from 2007 to 2016. Journal of Healthcare Risk Management 2020; 39: 20–27 [DOI] [PubMed] [Google Scholar]
- 133.Corda DM, Dexter F, Pasternak JJ, Trentman TL, Nottmeier EW, Brull SJ: Patients’ perspective on full disclosure and informed consent regarding postoperative visual loss associated with spinal surgery in the prone position. Mayo Clinic proceedings 2011; 86: 865–868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Spatz ES, Krumholz HM, Moulton BW: The New Era of Informed Consent: Getting to a Reasonable-Patient Standard Through Shared Decision Making. Jama 2016; 315: 2063–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Saydah SH, Gerzoff RB, Saaddine JB, Zhang X, Cotch MF: Eye Care Among US Adults at High Risk for Vision Loss in the United States in 2002 and 2017. JAMA Ophthalmology 2020; 138: 479–489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Cocanour CS: Informed consent-It’s more than a signature on a piece of paper. Am J Surg 2017; 214: 993–997 [DOI] [PubMed] [Google Scholar]
- 137.Taugher PJ: Visual loss after cardiopulmonary bypass. Am J Ophthalmol 1976; 81: 280–288 [DOI] [PubMed] [Google Scholar]
- 138.Shahian DM, Speert PK: Symptomatic visual deficits after open heart operations. Ann Thor Surg 1989; 48: 275–279 [DOI] [PubMed] [Google Scholar]
- 139.Shaw PJ, Bates D, Cartlidge NEF, French JM, Heaviside D, Julian DG, Shaw DA: Neurologic and neuropsychologic morbidity following major surgery: comparison of coronary artery bypass and peripheral vascular surgery. Stroke 1987; 18: 700–707 [DOI] [PubMed] [Google Scholar]
- 140.Myers MA, Hamilton SR, Bogosian AJ, Smith CH, Wagner TA: Visual loss as a complication of spine surgery. A review of 37 cases. Spine (Phila Pa 1976) 1997; 22: 1325–9 [DOI] [PubMed] [Google Scholar]
- 141.Katz DA, Karlin LI: Visual field defect after posterior spine fusion. Spine (Phila Pa 1976) 2005; 30: E83–5 [DOI] [PubMed] [Google Scholar]
- 142.Mione G, Pische G, Wolff V, Tonnelet R, Humbertjean L, Richard S: Perioperative Bioccipital Watershed Strokes in Bilateral Fetal Posterior Cerebral Arteries During Spinal Surgery. World Neurosurg 2016; 85: 367.e17–21 [DOI] [PubMed] [Google Scholar]
- 143.De la Garza-Ramos R, Samdani AF, Sponseller PD, Ain MC, Miller NR, Shaffrey CI, Sciubba DM: Visual loss after corrective surgery for pediatric scoliosis: incidence and risk factors from a nationwide database. Spine J 2016; 16: 516–22 [DOI] [PubMed] [Google Scholar]
- 144.Aldrich MS, Alessi AG, Beck RW, Gilman S: Cortical blindness: etiology, diagnosis, and prognosis. Annals of Neurology: Official Journal of the American Neurological Association and the Child Neurology Society 1987; 21: 149–158 [DOI] [PubMed] [Google Scholar]
- 145.Howard R, Trend P, Russell RW: Clinical features of ischemia in cerebral arterial border zones after periods of reduced cerebral blood flow. Arch Neurol 1987; 44: 934–40 [DOI] [PubMed] [Google Scholar]
- 146.Horton JC, Hoyt WF: Quadrantic visual field defects. A hallmark of lesions in extrastriate (V2/V3) cortex. Brain 1991; 114 ( Pt 4): 1703–18 [DOI] [PubMed] [Google Scholar]
- 147.Hogue CW Jr., Sundt TM 3rd, Goldberg M, Barner H, Davila-Roman VG: Neurological complications of cardiac surgery: the need for new paradigms in prevention and treatment. Semin Thorac Cardiovasc Surg 1999; 11: 105–15 [DOI] [PubMed] [Google Scholar]
- 148.Roach GW, Kanchuger M, Mangano CM, Newman M, Nussmeier N, Wolman R, Aggarwal A, Marschall K, Graham SH, Ley C, Ozanne G, Mangano DT, McSPIgroup: Multicenter Study of Perioperative Ischemia Research Group and Education Foundation Investigators. Adverse cerebral outcomes after coronary bypass surgery. N Engl J Med 1996; 335: 1857–1863 [DOI] [PubMed] [Google Scholar]
- 149.Russell RW, Bharucha N: The recognition and prevention of border zone cerebral ischaemia during cardiac surgery. Q J Med 1978; 47: 303–323 [PubMed] [Google Scholar]
- 150.Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, Biller J, Brown M, Demaerschalk BM, Hoh B, Jauch EC, Kidwell CS, Leslie-Mazwi TM, Ovbiagele B, Scott PA, Sheth KN, Southerland AM, Summers DV, Tirschwell DL: Guidelines for the Early Management of Patients With Acute Ischemic Stroke: 2019 Update to the 2018 Guidelines for the Early Management of Acute Ischemic Stroke: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke 2019; 50: e344–e418 [DOI] [PubMed] [Google Scholar]
- 151.Wareing TH, Davila-Roman VG, Barzilai B, Murphy SF, Kouchoukos NT: Management of the severely atheroschlerotic ascending aorta during cardiac operations. A strategy for detection and treatment. J Thor Cardiovasc Surg 1992; 103: 453–62 [PubMed] [Google Scholar]
- 152.Kouchoukos NT, Wareing TH, Daily BB, Murphy SF: Management of the severely atherosclerotic aorta during cardiac operations. J Card Surg 1994; 9: 490–494 [DOI] [PubMed] [Google Scholar]
- 153.Tezel G: Multifactorial Pathogenic Processes of Retinal Ganglion Cell Degeneration in Glaucoma towards Multi-Target Strategies for Broader Treatment Effects. Cells 2021; 10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Tezel G: Molecular regulation of neuroinflammation in glaucoma: Current knowledge and the ongoing search for new treatment targets. Prog Retin Eye Res 2021: 100998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Muench NA, Patel S, Maes ME, Donahue RJ, Ikeda A, Nickells RW: The Influence of Mitochondrial Dynamics and Function on Retinal Ganglion Cell Susceptibility in Optic Nerve Disease. Cells 2021; 10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Tham YC, Li X, Wong TY, Quigley HA, Aung T, Cheng CY: Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta-analysis. Ophthalmology 2014; 121: 2081–90 [DOI] [PubMed] [Google Scholar]
- 157.Vajaranant TS, Wu S, Torres M, Varma R: The changing face of primary open-angle glaucoma in the United States: demographic and geographic changes from 2011 to 2050. Am J Ophthalmol 2012; 154: 303–314.e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Vajaranant TS, Wu S, Torres M, Varma R: A 40-year forecast of the demographic shift in primary open-angle glaucoma in the United States. Invest Ophthalmol Vis Sci 2012; 53: 2464–6 [DOI] [PubMed] [Google Scholar]
- 159.Hollands H, Johnson D, Hollands S, Simel DL, Jinapriya D, Sharma S: Do findings on routine examination identify patients at risk for primary open-angle glaucoma? The rational clinical examination systematic review. Jama 2013; 309: 2035–42 [DOI] [PubMed] [Google Scholar]
- 160.Leung DYL, Tham CC: Normal-tension glaucoma: Current concepts and approaches-A review. Clin Exp Ophthalmol 2022 [DOI] [PubMed] [Google Scholar]
- 161.Wostyn P, De Groot V, Van Dam D, Audenaert K, Killer HE, De Deyn PP: The Glymphatic Hypothesis of Glaucoma: A Unifying Concept Incorporating Vascular, Biomechanical, and Biochemical Aspects of the Disease. Biomed Res Int 2017; 2017: 5123148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Wang SK, Cepko CL: Targeting Microglia to Treat Degenerative Eye Diseases. Front Immunol 2022; 13: 843558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Melgarejo JD, Maestre GE, Mena LJ, Lee JH, Petitto M, Chávez CA, Calmon G, Silva E, Thijs L, Al-Aswad LA, Terwilliger JD, De Moraes CG, Wei FF, Vanassche T, Verhamme P, Staessen JA, Zhang ZY: Normal-tension glaucomatous optic neuropathy is related to blood pressure variability in the Maracaibo Aging Study. Hypertens Res 2021; 44: 1105–1112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Berdahl JP, Allingham RR, Johnson DH: Cerebrospinal fluid pressure is decreased in primary open-angle glaucoma. Ophthalmology 2008; 115: 763–8 [DOI] [PubMed] [Google Scholar]
- 165.Ren R, Jonas JB, Tian G, Zhen Y, Ma K, Li S, Wang H, Li B, Zhang X, Wang N: Cerebrospinal fluid pressure in glaucoma: a prospective study. Ophthalmology 2010; 117: 259–66 [DOI] [PubMed] [Google Scholar]
- 166.Jasien JV, Fazio MA, Samuels BC, Johnston JM, Downs JC: Quantification of Translaminar Pressure Gradient (TLPG) With Continuous Wireless Telemetry in Nonhuman Primates (NHPs). Transl Vis Sci Technol 2020; 9: 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Heijl A, Leske MC, Bengtsson B, Hyman L, Bengtsson B, Hussein M, Early Manifest Glaucoma Trial G: Reduction of intraocular pressure and glaucoma progression: results from the Early Manifest Glaucoma Trial. Arch Ophthalmol 2002; 120: 1268–79 [DOI] [PubMed] [Google Scholar]
- 168.Jayanetti V, Sandhu S, Lusthaus JA: The Latest Drugs in Development That Reduce Intraocular Pressure in Ocular Hypertension and Glaucoma. J Exp Pharmacol 2020; 12: 539–548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Kalarn S, Le T, Rhee DJ: The role of trabeculectomy in the era of minimally invasive glaucoma surgery. Curr Opin Ophthalmol 2022; 33: 112–118 [DOI] [PubMed] [Google Scholar]
- 170.Chang CY, Chien YJ, Wu MY: Attenuation of increased intraocular pressure with propofol anesthesia: A systematic review with meta-analysis and trial sequential analysis. J Adv Res 2020; 24: 223–238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Rajan S, Krishnankutty SV, Nair HM: Efficacy of alpha2 agonists in obtunding rise in intraocular pressure after succinylcholine and that following laryngoscopy and intubation. Anesth Essays Res 2015; 9: 219–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Sator-Katzenschlager S, Deusch E, Dolezal S, Michalek-Sauberer A, Grubmüller R, Heinze G, Wedrich A: Sevoflurane and propofol decrease intraocular pressure equally during non-ophthalmic surgery and recovery. Br J Anaesth 2002; 89: 764–6 [PubMed] [Google Scholar]
- 173.Shirono Y, Takizawa I, Kasahara T, Maruyama R, Yamana K, Tanikawa T, Hara N, Sakaue Y, Togano T, Nishiyama T, Fukuchi T, Tomita Y: Intraoperative intraocular pressure changes during robot-assisted radical prostatectomy: associations with perioperative and clinicopathological factors. BMC Urol 2020; 20: 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Moriyama Y, Miwa K, Yamada T, Sawaki A, Nishino Y, Kitagawa Y: Intraocular pressure change during laparoscopic sacral colpopexy in patients with normal tension glaucoma. Int Urogynecol J 2019; 30: 1933–1938 [DOI] [PubMed] [Google Scholar]
- 175.Hirooka K, Ukegawa K, Nitta E, Ueda N, Hayashida Y, Hirama H, Taoka R, Sakura Y, Yamasaki M, Tsunemori H, Sugimoto M, Kiuchi Y: Retinal Nerve Fiber Layer Thickness Progression after Robotic-Assisted Laparoscopic Radical Prostatectomy in Glaucoma Patients. J Ophthalmol 2019; 2019: 6576140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Ackerman RS, Cohen JB, Getting REG, Patel SY: Are you seeing this: the impact of steep Trendelenburg position during robot-assisted laparoscopic radical prostatectomy on intraocular pressure: a brief review of the literature. J Robot Surg 2019; 13: 35–40 [DOI] [PubMed] [Google Scholar]
- 177.Mathew DJ, Greene RA, Mahsood YJ, Hallaji N, Vargas AMB, Jin YP, Finelli A, Parotto M, Belkin A, Trope GE, Buys YM: Preoperative Brimonidine Tartrate 0.2% Does not Prevent an Intraocular Pressure Rise During Prostatectomy in Steep Trendelenburg Position. J Glaucoma 2018; 27: 965–970 [DOI] [PubMed] [Google Scholar]
- 178.Joo J, Kim J, Lee J: Effect of Continuous Systemic Administration of Esmolol on Intraocular Pressure During Surgery in a Sustained Steep Trendelenburg Position. J Glaucoma 2017; 26: 1068–1071 [DOI] [PubMed] [Google Scholar]
- 179.Hoshikawa Y, Tsutsumi N, Ohkoshi K, Serizawa S, Hamada M, Inagaki K, Tsuzuki K, Koshimizu J, Echizen N, Fujitani S, Takahashi O, Deshpande GA: The effect of steep Trendelenburg positioning on intraocular pressure and visual function during robotic-assisted radical prostatectomy. Br J Ophthalmol 2014; 98: 305–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Borahay MA, Patel PR, Walsh TM, Tarnal V, Koutrouvelis A, Vizzeri G, Jennings K, Jerig S, Kilic GS: Intraocular pressure and steep Trendelenburg during minimally invasive gynecologic surgery: is there a risk? J Minim Invasive Gynecol 2013; 20: 819–24 [DOI] [PubMed] [Google Scholar]
- 181.Ventura LM, Golubev I, Lee W, Nose I, Parel JM, Feuer WJ, Porciatti V: Head-down posture induces PERG alterations in early glaucoma. J Glaucoma 2013; 22: 255–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Porciatti V, Feuer WJ, Monsalve P, Triolo G, Vazquez L, McSoley J, Ventura LM: Head-down Posture in Glaucoma Suspects Induces Changes in IOP, Systemic Pressure, and PERG That Predict Future Loss of Optic Nerve Tissue. J Glaucoma 2017; 26: 459–465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Anderson AP, Swan JG, Phillips SD, Knaus DA, Kattamis NT, Toutain-Kidd CM, Zegans ME, Fellows AM, Buckey JC: Acute effects of changes to the gravitational vector on the eye. J Appl Physiol (1985) 2016; 120: 939–46 [DOI] [PubMed] [Google Scholar]
- 184.Carey TW, Shaw KA, Weber ML, DeVine JG: Effect of the degree of reverse Trendelenburg position on intraocular pressure during prone spine surgery: a randomized controlled trial. Spine J 2014; 14: 2118–26 [DOI] [PubMed] [Google Scholar]
- 185.Czorlich P, Krätzig T, Kluge N, Skevas C, Knospe V, Spitzer MS, Dreimann M, Mende KC, Westphal M, Eicker SO: Intraocular pressure during neurosurgical procedures in context of head position and loss of cerebrospinal fluid. J Neurosurg 2018; 131: 271–280 [DOI] [PubMed] [Google Scholar]
- 186.Farag E, Sessler DI, Kovaci B, Wang L, Mascha EJ, Bell G, Kalfas I, Rockwood E, Kurz A: Effects of crystalloid versus colloid and the α-2 agonist brimonidine versus placebo on intraocular pressure during prone spine surgery: a factorial randomized trial. Anesthesiology 2012; 116: 807–15 [DOI] [PubMed] [Google Scholar]
- 187.Grant GP, Szirth BC, Bennett HL, Huang SS, Thaker RS, Heary RF, Turbin RE: Effects of prone and reverse trendelenburg positioning on ocular parameters. Anesthesiology 2010; 112: 57–65 [DOI] [PubMed] [Google Scholar]
- 188.Jasien JV, Samuels BC, Johnston JM, Downs JC: Effect of Body Position on Intraocular Pressure (IOP), Intracranial Pressure (ICP), and Translaminar Pressure (TLP) Via Continuous Wireless Telemetry in Nonhuman Primates (NHPs). Invest Ophthalmol Vis Sci 2020; 61: 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Roszkowska AM, Oliverio GW, Aragona E, Inferrera L, Severo AA, Alessandrello F, Spinella R, Postorino EI, Aragona P: Ophthalmologic Manifestations of Primary Sjögren’s Syndrome. Genes (Basel) 2021; 12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Singer MS, Salim S: Bilateral acute angle-closure glaucoma as a complication of facedown spine surgery. Spine J 2010; 10: e7–9 [DOI] [PubMed] [Google Scholar]
- 191.Stewart RJ, Landy DC, Lee MJ: Unilateral Acute Angle-Closure Glaucoma After Lumbar Spine Surgery: A Case Report and Systematic Review of the Literature. Spine (Phila Pa 1976) 2016; 41: E297–9 [DOI] [PubMed] [Google Scholar]
- 192.Xin C, Wang RK, Song S, Shen T, Wen J, Martin E, Jiang Y, Padilla S, Johnstone M: Aqueous outflow regulation: Optical coherence tomography implicates pressure-dependent tissue motion. Exp Eye Res 2017; 158: 171–186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Ficarrotta KR, Bello SA, Mohamed YH, Passaglia CL: Aqueous Humor Dynamics of the Brown-Norway Rat. Invest Ophthalmol Vis Sci 2018; 59: 2529–2537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Awad H, Bai M, Ramadan ME, Shabsigh A, Backes F, Craven MA, Abdel-Rasoul M, Bergese SD, Slabaugh M: The Effect of Increased Intraocular Pressure During Steep Trendelenburg Positioning in Robotic Prostatectomy and Hysterectomy on Structural and Functional Ocular Parameters. Anesth Analg 2020; 130: 975–982 [DOI] [PubMed] [Google Scholar]
- 195.Hirooka K, Ukegawa K, Nitta E, Ueda N, Hayashida Y, Hirama H, Taoka R, Sakura Y, Yamasaki M, Tsunemori H, Sugimoto M, Kakehi Y: The Effect of Steep Trendelenburg Positioning on Retinal Structure and Function during Robotic-Assisted Laparoscopic Procedures. J Ophthalmol 2018; 2018: 1027397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Mizumoto K, Gosho M, Iwaki M, Zako M: Ocular parameters before and after steep Trendelenburg positioning for robotic-assisted laparoscopic radical prostatectomy. Clin Ophthalmol 2017; 11: 1643–1650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Taketani Y, Mayama C, Suzuki N, Wada A, Oka T, Inamochi K, Nomoto Y: Transient but significant visual field defects after robot-assisted laparoscopic radical prostatectomy in deep tRendelenburg position. PLoS One 2015; 10: e0123361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Choi J, Lee JR, Lee Y, Lee KS, Na JH, Han S, Kook MS: Relationship between 24-hour mean ocular perfusion pressure fluctuation and rate of paracentral visual field progression in normal-tension glaucoma. Invest Ophthalmol Vis Sci 2013; 54: 6150–7 [DOI] [PubMed] [Google Scholar]
- 199.Sung KR, Cho JW, Lee S, Yun SC, Choi J, Na JH, Lee Y, Kook MS: Characteristics of visual field progression in medically treated normal-tension glaucoma patients with unstable ocular perfusion pressure. Invest Ophthalmol Vis Sci 2011; 52: 737–43 [DOI] [PubMed] [Google Scholar]
- 200.Cordier J, Vitte G: [Acute glaucoma after intervention for general surgery]. Bull Soc Ophtalmol Fr 1957: 143–5 [PubMed] [Google Scholar]
- 201.Amerasinghe N, Aung T: Angle-closure: risk factors, diagnosis and treatment. Prog Brain Res 2008; 173: 31–45 [DOI] [PubMed] [Google Scholar]
- 202.Roor TL, Kooijman JA, van der Ploeg JM, de Boer HD: Postoperative Acute Angle-Closure Glaucoma: A Rare but Serious Complication: A Case Report. A A Pract 2019; 12: 385–387 [DOI] [PubMed] [Google Scholar]
- 203.Kim BH, Lee EJ: Optic Disc Swelling After Intraocular Pressure Lowering Treatment in Acute Primary Angle Closure. J Glaucoma 2017; 26: e87–e89 [DOI] [PubMed] [Google Scholar]
- 204.Jaroudi M, Fadi M, Farah F, El Mollayess GM: Glycopyrrolate induced bilateral angle closure glaucoma after cervical spine surgery. Middle East Afr J Ophthalmol 2013; 20: 182–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Mihara R, Tanaka M, Nakahira J, Fujitate Y, Minami T: [Case with postoperative acute angle-closure glaucoma]. Masui 2011; 60: 972–4 [PubMed] [Google Scholar]
- 206.Yang MC, Lin KY: Drug-induced Acute Angle-closure Glaucoma: A Review. J Curr Glaucoma Pract 2019; 13: 104–109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Chandrakanth P, Chavan S, Verghese S, Gosalia H, Raman GV, Shettigar CK, Narendran V: Smartphone Gonioscopy With a Magnifying Intraocular Lens: A Cost-effective Angle Imaging Device. J Glaucoma 2022; 31: 356–360 [DOI] [PubMed] [Google Scholar]
- 208.Yang MC, Lin KY: Drug-induced Acute Angle-closure Glaucoma: A Review. Journal of current glaucoma practice 2019; 13: 104–109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.He M, Huang W, Friedman DS, Wu C, Zheng Y, Foster PJ: Slit lamp-simulated oblique flashlight test in the detection of narrow angles in Chinese eyes: the Liwan eye study. Invest Ophthalmol Vis Sci 2007; 48: 5459–63 [DOI] [PubMed] [Google Scholar]
- 210.Prum BE Jr., Herndon LW Jr., Moroi SE, Mansberger SL, Stein JD, Lim MC, Rosenberg LF, Gedde SJ, Williams RD: Primary Angle Closure Preferred Practice Pattern(®) Guidelines. Ophthalmology 2016; 123: P1–p40 [DOI] [PubMed] [Google Scholar]
- 211.Trends in prevalence of blindness and distance and near vision impairment over 30 years: an analysis for the Global Burden of Disease Study. Lancet Glob Health 2021; 9: e130–e143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.LeGates TA, Altimus CM, Wang H, Lee HK, Yang S, Zhao H, Kirkwood A, Weber ET, Hattar S: Aberrant light directly impairs mood and learning through melanopsin-expressing neurons. Nature 2012; 491: 594–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Prigge CL, Yeh PT, Liou NF, Lee CC, You SF, Liu LL, McNeill DS, Chew KS, Hattar S, Chen SK, Zhang DQ: M1 ipRGCs Influence Visual Function through Retrograde Signaling in the Retina. J Neurosci 2016; 36: 7184–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Maynard ML, Zele AJ, Kwan AS, Feigl B: Intrinsically Photosensitive Retinal Ganglion Cell Function, Sleep Efficiency and Depression in Advanced Age-Related Macular Degeneration. Invest Ophthalmol Vis Sci 2017; 58: 990–996 [DOI] [PubMed] [Google Scholar]
- 215.Lockley SW, Dressman MA, Licamele L, Xiao C, Fisher DM, Flynn-Evans EE, Hull JT, Torres R, Lavedan C, Polymeropoulos MH: Tasimelteon for non-24-hour sleep-wake disorder in totally blind people (SET and RESET): two multicentre, randomised, double-masked, placebo-controlled phase 3 trials. Lancet 2015; 386: 1754–64 [DOI] [PubMed] [Google Scholar]
- 216.O’Gara BP, Gao L, Marcantonio ER, Subramaniam B: Sleep, Pain, and Cognition: Modifiable Targets for Optimal Perioperative Brain Health. Anesthesiology 2021; 135: 1132–1152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Kim HN: Understanding of how older adults with low vision obtain, process, and understand health information and services. Inform Health Soc Care 2019; 44: 70–78 [DOI] [PubMed] [Google Scholar]
- 218.Burton MJ, Ramke J, Marques AP, Bourne RRA, Congdon N, Jones I, Ah Tong BAM, Arunga S, Bachani D, Bascaran C, Bastawrous A, Blanchet K, Braithwaite T, Buchan JC, Cairns J, Cama A, Chagunda M, Chuluunkhuu C, Cooper A, Crofts-Lawrence J, Dean WH, Denniston AK, Ehrlich JR, Emerson PM, Evans JR, Frick KD, Friedman DS, Furtado JM, Gichangi MM, Gichuhi S, Gilbert SS, Gurung R, Habtamu E, Holland P, Jonas JB, Keane PA, Keay L, Khanna RC, Khaw PT, Kuper H, Kyari F, Lansingh VC, Mactaggart I, Mafwiri MM, Mathenge W, McCormick I, Morjaria P, Mowatt L, Muirhead D, Murthy GVS, Mwangi N, Patel DB, Peto T, Qureshi BM, Salomão SR, Sarah V, Shilio BR, Solomon AW, Swenor BK, Taylor HR, Wang N, Webson A, West SK, Wong TY, Wormald R, Yasmin S, Yusufu M, Silva JC, Resnikoff S, Ravilla T, Gilbert CE, Foster A, Faal HB: The Lancet Global Health Commission on Global Eye Health: vision beyond 2020. Lancet Glob Health 2021; 9: e489–e551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Saleh GM: Consent of the blind and visually impaired: a time to change practice. The British journal of ophthalmology 2004; 88: 310–311 [DOI] [PMC free article] [PubMed] [Google Scholar]


