Abstract
The Lyme disease agent, Borrelia burgdorferi, harbors a significantly reduced genome and relies on the scavenging of critical nutrients from its tick and mammalian hosts for survival. Riboflavin salvage has been shown to be important for B. burgdorferi infection of mice, yet the contributions of riboflavin to B. burgdorferi metabolism and survival in the tick remain unknown. Using a targeted mass spectrometry approach, we confirmed the importance of bb0318, the putative ATPase component of an ABC-type riboflavin transporter, for riboflavin salvage and the production of FMN and FAD. This analysis further revealed that Δbb0318 B. burgdorferi displayed increased levels of glycerol 3-phosphate compared to the wild-type. The glycerol 3-phosphate dehydrogenase activity of GlpD was found to be FAD-dependent and the transcription and translation of glpD were significantly decreased in Δbb0318 B. burgdorferi. Finally, gene bb0318 was found to be important for maximal spirochete burden in unfed larvae and essential for survival in feeding ticks. Together, these data demonstrate the importance of riboflavin salvage for B. burgdorferi carbon metabolism and survival in ticks.
Keywords: Borrelia burgdorferi, carbon metabolism, glycerol 3-phosphate dehydrogenase, Ixodes scapularis, Lyme disease, riboflavin
1 |. INTRODUCTION
The spirochete Borrelia (Borreliella) burgdorferi is an obligate parasite and is maintained in nature in an enzootic cycle between Ixodes scapularis ticks and a variety of small mammals, birds, and reptiles. Humans are inadvertent hosts and can become infected with B. burgdorferi if bitten by an infected tick leading to Lyme disease (Coburn et al., 2021). B. burgdorferi harbors a fragmented and reduced genome lacking genes required for numerous metabolic and regulatory pathways, including genes that encode proteins for the de novo synthesis of amino acids, nucleotides, and enzyme cofactors (Casjens et al., 2012; Fraser et al., 1997). Therefore, B. burgdorferi must scavenge these critical nutrients from its host environments to survive. One of these nutrients is riboflavin, which is important for humans and bacteria alike (Averianova et al., 2020). Riboflavin is the precursor to flavin adenine mononucleotide (FAD) and flavin mononucleotide (FMN), which are cofactors required for an array of proteins to function. Flavoproteins contribute to a wide range of important biological functions including central metabolism, redox homeostasis, DNA repair, and apoptosis (Massey, 2000). Unlike many bacterial species, B. burgdorferi appears to lack the enzymatic machinery for the de novo synthesis of riboflavin (Casjens et al., 2000; Fraser et al., 1997), suggesting that the spirochete relies on the uptake of riboflavin from its hosts. Yet, little is known about the role of riboflavin in the biology of B. burgdorferi.
Using in vivo expression technology in B. burgdorferi (BbIVET), we previously identified gene, bb0318 (Ellis et al., 2014; Showman et al., 2016), which is predicted to encode a homolog of RfuB, the ATPase component of a putative riboflavin ABC-type transporter (Deka et al., 2013). Gene bb0318 is encoded on the B. burgdorferi chromosome as part of a four gene operon, bb0316-bb0319. Genes bb0316 and bb0317 are predicted to each encode a permease (Fraser et al., 1997), and bb0319 was demonstrated to encode a riboflavin binding protein, leading to the prediction that the BB0316-BB0319 transport system is involved in the uptake of riboflavin (Deka et al., 2013). Recently, it was reported that a B. burgdorferi mutant lacking genes bb0316-bb0319 demonstrates reduced sensitivity to the toxic riboflavin analog, roseoflavin, reduced uptake of radiolabeled riboflavin and reduced incorporation of radiolabeled riboflavin into FMN and FAD compared to that of wild-type B. burgdorferi (Muramatsu et al., 2021), further supporting the contribution of the transport system to riboflavin scavenge. Moreover, we and others have found that B. burgdorferi mutants lacking bb0318 are highly attenuated for infection in mice (Muramatsu et al., 2021; Showman et al., 2016), demonstrate increased sensitivity to reactive oxygen species (Ramsey et al., 2017; Showman et al., 2016) and macrophage killing (Showman et al., 2016) and are defective for growth at 25°C (Muramatsu et al., 2021). Together, these phenotypes suggest a critical role for riboflavin in B. burgdorferi biology.
Herein, we sought to understand the contribution of riboflavin to the metabolism of B. burgdorferi as well as the requirement for riboflavin scavenge during the tick phase of the enzootic cycle. In so doing, we have discovered an important link between riboflavin and B. burgdorferi carbon metabolism and that riboflavin uptake is essential for survival of the spirochete in ticks.
2 |. RESULTS
2.1 |. B. burgdorferi lacking bb0318 alone recapitulates the phenotypes of B. burgdorferi lacking the entire BB0316-BB0319 transport system
We previously demonstrated that B. burgdorferi lacking gene bb0318, which is annotated to encode the putative ATPase component of the BB0316-BB0319 ABC-type transport system, is highly attenuated for infection (Showman et al., 2016). A similar infection defect was recently reported for a B. burgdorferi mutant lacking the entire bb0316-bb0319 operon (Muramatsu et al., 2021), suggesting that the deletion of bb0318 alone may be sufficient to render the ABC transport system inactive. To examine this hypothesis further, we generated a Δbb0316-bb0319 B. burgdorferi clone and compared the behavior of the quadruple mutant to that of the single bb0318 mutant. Muramatsu et al. (2021) demonstrated that a Δbb0316-bb0319 B. burgdorferi clone has enhanced resistance to the toxic riboflavin analog roseoflavin and has a significant growth defect in BSK-II medium at 25°C. Consistent with these findings, our Δbb0316-bb0319 B. burgdorferi clone demonstrated a two log increase in resistance to roseoflavin compared to the wild-type (Figure S1a) as well as a significant growth defect at 23°C (Figure S1b). A slight growth defect was also detected for Δbb0316-bb0319 B. burgdorferi at 35°C (Figure S1c). Notably, the phenotypes of Δbb0318 B. burgdorferi paralleled all of those of Δbb0316-bb0319 B. burgdorferi (Figure S1). Together, these data indicate that deletion of bb0318 phenocopies B. burgdorferi lacking bb0316-bb0319 and support the notion that in the absence of bb0318 B. burgdorferi harbors an inactive BB0316-BB0319 ABC transport system and therefore likely reduced capacity for riboflavin uptake.
2.2 |. Δbb0318 B. burgdorferi demonstrate diminished intracellular levels of riboflavin, FMN and FAD as well as accumulation of a glycerol metabolism intermediate
Given the critical role of riboflavin and the riboflavin derived cofactors FMN and FAD for significant biological processes (Massey, 2000) and that Δbb0318 B. burgdorferi appears to harbor an inactive BB0316-BB0319 ABC-type transport system, we sought to elucidate the metabolic consequences of reduced riboflavin, FMN and FAD in Δbb0318 B. burgdorferi. To begin we aimed to validate the contribution of gene bb0318 to riboflavin uptake by B. burgdorferi using a targeted liquid chromatography–tandem mass spectrometry (LCMS) approach. Quadruplicate cultures of wild-type and Δbb0318 B. burgdorferi were grown to mid-logarithmic phase in BSK-II medium. The cells were harvested, and intracellular metabolites extracted. A broad panel of central metabolites were assayed by LCMS and the relative fold change differences in the intracellular amounts of each target metabolite between Δbb0318 and wild-type B. burgdorferi were determined. As expected, these data revealed that levels of riboflavin, FMN, and FAD were diminished in Δbb0318 B. burgdorferi compared to the wild-type (Figure 1a), further supporting the contribution of bb0318 to riboflavin uptake. Furthermore, the finding that the intracellular levels of riboflavin, FMN, and FAD in the Δbb0318 mutant were reduced but still detectable was consistent with the hypothesis that the mutant retained some ability to scavenge riboflavin and perhaps FMN and FAD via another transport system (Muramatsu et al., 2021). In addition to riboflavin and its cofactors, the targeted metabolite analysis revealed a broad metabolic disruption associated with the absence of gene bb0318 (Figure 1b and Tables S2, S3). This analysis revealed that the amino acid citrulline and hexose sugars (glucose/fructose pool) were significantly decreased in the Δbb0318 mutant compared to wild-type B. burgdorferi. The intermediates glycerol 3-phosphate, glucose 1-phosphate, fructose 1,6-bisphosphate, 6-phosphogluconate, and glucosamine 6-phosphate as well as adenine, were significantly increased in the Δbb0318 mutant compared to wild-type B. burgdorferi. Together, these data support a role for bb0318 in riboflavin salvage and indicate that gene bb0318 contributes to the regulation of the balance of 3-carbon versus 6-carbon metabolism.
FIGURE 1.
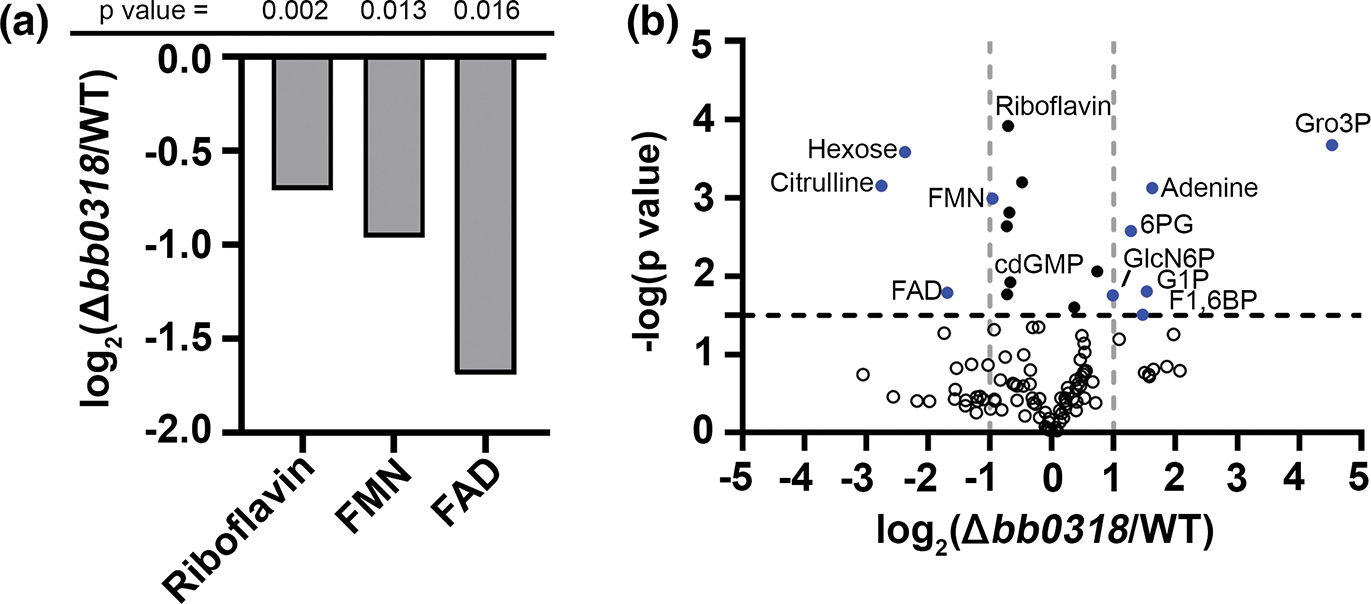
Borrelia burgdorferi lacking bb0318 have reduced intracellular levels of riboflavin, FMN, and FAD, and increased levels of glycerol 3-phosphate. Wild-type (WT) and Δbb0318 B. burgdorferi were grown to mid-logarithmic phase in BSK-II and targeted metabolomics was performed via LCMS. (a) Graph displaying the log2 fold change of Δbb0318 B. burgdorferi versus WT in intracellular levels of riboflavin, FMN, and FAD. p-values from an unpaired t-test for each metabolite are indicated across the top. (b) Volcano plot of the metabolite data set with the log2 fold change of the Δbb0318 mutant versus WT on the x-axis and the −log10 p-value from an unpaired t-test on the y-axis. A horizontal line at 1.5 (p-value = .032) corresponds to the 20% false discovery rate (FDR) cut off for the dataset by a Benjamini-Hochberg correction and vertical lines indicate a positive or negative twofold change. n = 4. Metabolites that passed the 20% FDR filter and had a log2 fold change of ≤−1 or ≥1 between Δbb0318 and WT are indicated by blue circles. Metabolites that passed the 20% FDR filter but not the fold change cut-off between Δbb0318 and WT are indicated by black circles and metabolites that fell below both thresholds are indicated by open circles. FAD, flavin adenine dinucleotide; FMN, flavin mononucleotide; cdGMP, cyclic di-GMP; GlcN6P, glucosamine 6-phosphate; 6PG, 6-phosphogluconate; F1,6BP, fructose 1,6-bisphosphate; G1P, glucose 1-phosphate; Gro3P, glycerol 3-phosphate.
2.3 |. B. burgdorferi GlpD is an FAD-dependent, peripheral membrane glycerol 3-phosphate dehydrogenase
In our analysis, glycerol 3-phosphate (Gro3P) was found to be the metabolite with the greatest fold change difference between the Δbb0318 mutant and wild-type B. burgdorferi (Figure 1b and Table S2). In B. burgdorferi, Gro3P is generated by the reduction of the glycolysis intermediate dihydroxyacetone phosphate (DHAP) by GpsA/BB0368 (Drecktrah et al., 2022) and/or phosphorylation of imported glycerol by GlpK/BB0241, a putative glycerol kinase (Schwan et al., 2003). In addition, Gro3P is believed to be converted to DHAP by a putative Gro3P dehydrogenase, GlpD/BB0243 (Fraser et al., 1997; Gherardini et al., 2021). In E. coli, glycerol 3-phosphate dehydrogenase (G3PDH) (EC 1.1.5.3) is an FAD-dependent, peripheral membrane enzyme that localizes to the cytoplasmic membrane (Schryvers et al., 1978; Walz et al., 2002). B. burgdorferi GlpD shares ~30% amino acid sequence identity with E. coli GlpD. Yet, by structural modeling and functional prediction analysis using I-TASSER (Roy et al., 2010; Yang et al., 2015; Zhang, 2008), E. coli GlpD was identified as the homolog of B. burgdorferi GlpD, consistent with previous predictions (Pappas et al., 2011). Together these data raised the possibility that B. burgdorferi GlpD is FAD-dependent. To test this, the B. burgdorferi glpD sequence was codon optimized for E. coli and recombinant B. burgdorferi GlpD was produced in E. coli and purified as an N-terminal fusion to the highly soluble E. coli NusA protein, as an approach to enhance soluble recombinant protein expression (Davis et al., 1999; De Marco et al., 2004), along with N- and C-terminal 6XHis tags. Recombinant GlpD, along with the Nus protein alone, was examined for G3PDH activity in the presence and absence of FAD. GlpD demonstrated dose-dependent, FAD-dependent G3PDH activity (Figure 2a,b). No activity was detected for the Nus protein alone (Figure 2a,b). The Abcam G3PDH assay buffer contains NAD+, under the assumption that the G3PDH enzyme being tested is NAD-dependent. GlpD was found to have little to no enzymatic activity without the addition of FAD to the reaction, suggesting that NAD+ is not a cofactor for GlpD. Furthermore, we determined the localization of the GlpD protein in the spirochete. GlpD, like the membrane protein VlsE but unlike soluble SodA, was found to be restricted to the spirochete membrane fraction (Figure 2c). To further characterize the mode of GlpD association with the spirochete membrane, the membrane fraction was washed with high salt, high urea or detergent. Peripheral membrane proteins are known to dissociate from the membrane in the presence of high salt or high urea. In contrast, integral membrane proteins may only be released from the membrane with detergent solubilization (Matsunaga et al., 2002). A trace amount of GlpD was removed from the membrane with the high salt wash but a significant amount of the protein was present in the soluble fraction following the urea wash (Figure 2d). In addition, the urea treatment resulted in immunoreactive proteins of lower molecular weight, which could represent GlpD breakdown products under this condition. Treatment of the membrane with Triton X-100 also shifted a large proportion of the GlpD protein pool to the soluble fraction, but the detergent did not solubilize the membrane completely (Figure 2d). The finding that GlpD was washed off the membrane with urea suggests that GlpD is likely a peripheral membrane protein. The association of GlpD with the membrane is unlikely to occur through electrostatic interactions as treatment with high salt only weakly affected the protein localization. Together, these data indicate that GlpD is an FAD-dependent, peripheral membrane glycerol 3-phosphate dehydrogenase and suggest that increased abundance of Gro3P in Δbb0318 B. burgdorferi may result, at least in part, from decreased GlpD activity due to diminished cofactor availability.
FIGURE 2.

Borrelia burgdorferi GlpD is an FAD-dependent, peripheral membrane glycerol 3-phosphate dehydrogenase. (a) The reducing equivalents produced by increasing amounts of recombinant GlpD protein or the Nus protein control in the presence (+FAD) or absence (−FAD) of 10 μM FAD. Error bars represent standard deviation from the mean of triplicate measures. Statistical significance was determined via an unpaired t-test, GraphPad Prism 9.0.0. (***p ≤ .005,***p < .0001, ns: not significant). (b) The glycerol 3-phosphate dehydrogenase activities of recombinant GlpD or the Nus control protein in the presence (+FAD) or absence (−FAD) of 10 μM FAD were calculated as reducing equivalents produced per minute (min) per milligram (mg) of recombinant protein (reducing equivalents/min/mg). Statistical significance was determined via a one-way ANOVA with Dunnett’s multiple comparisons test to the Nus protein control in the absence of FAD, GraphPad Prism 9.0.0. (***p < .0001). (c) Total protein lysate (T) of wild-type B. burgdorferi was fractionated into the membrane (M) and soluble (S) components using ultracentrifugation. Protein fractions from equivalent numbers of spirochetes were analyzed by immunoblot with antibodies against GlpD (αGlpD), VlsE (membrane protein) (αVlsE), or SodA (cytoplasmic protein) (αSodA). (d) The B. burgdorferi membrane fraction was washed with 500 mM NaCl (high NaCl), 3.6 M urea (Urea) or 2% Triton X-100 (Triton X-100). The samples were separated into the membrane (M) and soluble (S) components using ultracentrifugation and analyzed by immunoblot with antibodies against GlpD (αGlpD). Molecular weights are shown in kilodaltons (kDa).
2.4|. B. burgdorferi lacking bb0318 demonstrate decreased expression of the glp operon
It was recently demonstrated that the complementary activities of GlpD and GpsA control the intracellular pool of Gro3P (Drecktrah et al., 2022). Therefore, beyond decreased activity of GlpD due to FAD deficiency, we explored the possibility that the accumulation of Gro3P in the absence of bb0318 may have resulted from dysregulation of glpD or gpsA expression. The glpD gene is encoded within the four gene glycerol utilization operon (glpF, glpK, bb0242, and glpD) (Fraser et al., 1997). In addition to the putative primary transcription start site for glpF (bb0240), the first gene in the operon, putative internal transcription start sites within the operon that may serve as individual primary transcription start sites for genes glpK (bb0241), bb0242, and glpD (bb0243) have been identified (Adams et al., 2017), suggesting the possibility for multiple layers of regulation of these genes. Therefore, expression of genes glpF, glpK, and bb0242 were also included in the analysis. Reverse transcription-quantitative PCR (RT-qPCR) performed with RNA isolated from the wild-type, Δbb0318, and Δbb0318/bb0318+ B. burgdorferi revealed that the expression levels of the glp operon genes were significantly reduced in Δbb0318 B. burgdorferi compared to that of the wild-type (Figure 3a). The expression levels of all four target genes were restored to that of the wild-type in Δbb0318/bb0318+ B. burgdorferi. In contrast, the expression levels of gpsA in the Δbb0318 mutant and complement clones were slightly increased compared to that of the wild-type. We also examined the relative GlpD protein levels using fluorescent immunoblot analysis. Consistent with the gene expression data, relative GlpD protein levels were significantly reduced in Δbb0318 B. burgdorferi compared to the wild-type (Figure 3b,c). Altogether, these data suggested that the accumulation of Gro3P in Δbb0318 B. burgdorferi was due, in part, to decreased transcription of glpD, likely driven from the glp operon promoter as expression of the entire operon was reduced in the mutant, thereby leading to reduced GlpD protein levels.
FIGURE 3.

Borrelia burgdorferi lacking bb0318 demonstrates decreased expression of the glp operon. (a) RNA was extracted from wild-type (WT), Δbb0318, and Δbb0318/bb0318+ B. burgdorferi grown in BSK-II for RT-qPCR analysis of the glp operon genes and gpsA. Expression of the target genes was normalized to that of enolase, bb0337. Data are presented as relative gene expression to WT and are the average of biological triplicate samples ± standard deviation. Statistical significance was determined via a one-way ANOVA with Dunnett’s multiple comparisons test to WT (*p < .05, ** p < .01, ***p < .001, ****p < .0001). Protein lysates were generated from WT, Δbb0318, and Δbb0318/bb0318+ B. burgdorferi and analyzed by immunoblot with antibodies against GlpD (αGlpD, green) and FlaB (αFlaB, red). (b) A representative image of three biological replicates is shown. (c) Relative GlpD protein levels determined by normalization of the GlpD protein signal intensity to that of the FlaB protein. Data represent the average of biological triplicate measures ± standard deviation. Statistical analyses were performed using the one-way ANOVA with Dunnett’s multiple comparisons test to WT, GraphPad Prism 9.0.0 (**p < .01).
2.5 |. Loss of bb0318 does not affect rrp1 expression
The Hk1/Rrp1 two component system is a critical activator of glpD expression (Caimano et al., 2015; He et al., 2011; Rogers et al., 2009). Given the significant reduction in glpD expression in Δbb0318 B. burgdorferi we sought to determine if this was the result of dysregulation of rrp1. The relative expression of rrp1 in Δbb0318 B. burgdorferi was no different than that of the wild-type (Figure 4a). Fluorescent immunoblot demonstrated that Rrp1 was produced at the same relative level in the wild-type and Δbb0318 B. burgdorferi (Figure 4b). Together these data indicate that loss of bb0318 did not affect the transcription or translation of rrp1.
FIGURE 4.

Borrelia burgdorferi lacking bb0318 demonstrates wild-type levels of rrp1 expression. (a) RNA was extracted from wild-type (WT), Δbb0318, wild-type clone 5A4 (5A4-WT) and Δrrp1 (5A4-Δrrp1) grown in BSK-II for RT-qPCR analysis of rrp1. Expression of the target gene was normalized to that of enolase, bb0337. Data are presented as relative gene expression to the respective wild-type and are the average of biological triplicate samples ± standard deviation. Statistical significance was determined via an unpaired t-test, GraphPad Prism 9.0.0. (*p < .05, **p < .01, ***p < .0001, ns: not significant). (b) Protein lysates were generated from WT, Δbb0318, and 5A4-Δrrp1 B. burgdorferi and analyzed by immunoblot with antibodies against Rrp1 (αRrp1, green) and FlaB (αFlaB, red). A representative image of three biological replicates is shown. Relative Rrp1 protein levels were determined by normalization of the Rrp1 protein signal intensity to that of the FlaB protein. Data represent the average of biological triplicate measures ± standard deviation. Statistical analyses were performed using the one-way ANOVA with Dunnett’s multiple comparisons test to WT, GraphPad Prism 9.0.0 (**p < .01, ns: not significant).
2.6 |. Gene bb0318 contributes to B. burgdorferi burden in unfed ticks and is essential for B. burgdorferi survival in feeding ticks
It is well established that the glp operon is critical for maximal fitness of B. burgdorferi in unfed ticks (He et al., 2011; Pappas et al., 2011). Given the importance of the glp operon for B. burgdorferi survival in ticks, this led to the hypothesis that bb0318 contributes to B. burgdorferi persistent infection of unfed ticks. B. burgdorferi lacking bb0318 are highly attenuated for infection of mice (Muramatsu et al., 2021; Showman et al., 2016) confounding our ability to naturally infect naïve Ixodes scapularis larvae by feeding. Therefore, to test this hypothesis, naïve larvae were artificially infected by immersion with wild-type, Δbb0318, and Δbb0318/bb0318+ B. burgdorferi. In contrast to previous studies that have investigated B. burgdorferi persistence in ticks by quantifying spirochete loads in unfed nymphs following the molt, we assessed the spirochete loads in unfed larvae over a nearly 5.5-week time course. Spirochete loads in the unfed larvae were assessed by qPCR 3 days post-immersion and then weekly out to 37 days. This approach revealed no detectable bb0318-dependent differences in spirochete burden per larva at days 3 and 10 post-immersion (Figure 5a). However, at day 17 post-immersion, the average number of Δbb0318 B. burgdorferi per larva was found to be significantly reduced compared to that of wild-type and Δbb0318/bb0318+ B. burgdorferi (Figure 5a). This difference resulted from an approximate tenfold increase in the average number of wild-type and Δbb0318/bb0318+ B. burgdorferi per larva and little to no change in the number Δbb0318 B. burgdorferi per larva. This difference in spirochete burden was maintained throughout the remainder of the 37-day time course. Because spirochete burden in ticks was measured by qPCR, we wanted to confirm that at the endpoint of the time course, the ticks harbored live spirochetes and that the quantification of B. burgdorferi per larva was not based on detection of remnant DNA alone. Five cohorts of ticks per B. burgdorferi genotype were assessed at day 37 for the presence of live spirochetes by reisolation in BSK-II medium. After 7 days of incubation, the reisolation cultures were scored for the presence of live spirochetes by dark field microscopy. Five out of five tick cohorts for each B. burgdorferi clone were found to be reisolation positive, indicating that all B. burgdorferi clones resulted in persistent infection of unfed ticks. However, B. burgdorferi lacking bb0318, despite being alive, lacked the ability to undergo population expansion in this host environment.
FIGURE 5.

Gene bb0318 is important for B. burgdorferi to achieve maximal spirochete burden in unfed larvae and essential for B. burgdorferi survival in feeding ticks. Ixodes scapularis larvae were artificially infected by immersion with wild-type (WT), Δbb0318 and Δbb0318/bb0318+ B. burgdorferi. (a) B. burgdorferi were quantified by qPCR at various time points post-immersion over a 37-day time course by qPCR. Each data point represents a group of 20 larvae. (b) B. burgdorferi were quantified by qPCR 3 days post-immersion. Each data point represents a group of 10 larvae. The mean is indicated by a horizontal black line. (c) 10 days post-immersion, larvae were fed to repletion on mice. Seven days post-feeding to repletion individual fed larva were homogenized and the number of B. burgdorferi per larva determined by colony forming units counts in solid medium. The mean is indicated by a horizontal black line. Statistical significance was evaluated by one-way ANOVA with Dunnett’s multiple comparisons test to WT (*p < .05, **p < .01, ***p < .0001).
To gather additional insight into the contributions of gene bb0318 to the survival of B. burgdorferi in the tick stage of the enzootic cycle, B. burgdorferi infected larvae were fed on naïve mice. Naïve Ixodes scapularis larvae were artificially infected by immersion with wild-type, Δbb0318, and Δbb0318/bb0318+ B. burgdorferi. To confirm equal densities of B. burgdorferi within the unfed larvae, groups of ten larvae per B. burgdorferi clone were analyzed by qPCR 3 days post-immersion (Figure 5b). On day 10 post-immersion, groups of ~150 infected larvae per mouse were allowed to feed to repletion. Replete larvae were collected and groups of 20 fed larvae per clone were individually homogenized and plated in solid BSK-agarose for colony forming unit (CFU) analysis. Strikingly, no Δbb0318 B. burgdorferi infected larvae were found to carry any viable spirochetes following feeding (Figure 5c). In contrast, the wild-type and Δbb0318/bb0318+ B. burgdorferi demonstrated 80% and 50% infection rates, respectively, with up to greater than 105 spirochetes per larva (Figure 5c). These data demonstrate that bb0318 is essential for B. burgdorferi survival during the tick blood meal. Given that we and others previously established that bb0318 is critical for B. burgdorferi infection of mice (Muramatsu et al., 2021; Showman et al., 2016) and that no surviving Δbb0318 B. burgdorferi were detected in feeding ticks, infection of mice by tick transmission was not assessed in this study.
3 |. DISCUSSION
B. burgdorferi has a limited capacity for de novo synthesis of key macromolecules and is dependent on the ability to scavenge nutrients from its environment. B. burgdorferi lacks the metabolic pathways to synthesize the essential nutrient riboflavin (Fraser et al., 1997), the precursor for the cofactors FMN and FAD, important for maintenance of redox homeostasis, central metabolism, and energy production (Massey, 2000). It was recently established that the predicted riboflavin ABC-type transport system encoded by genes bb0316-bb0319 (Deka et al., 2013) contributes to riboflavin uptake (Muramatsu et al., 2021). We have presented data to indicate that Δbb0318 B. burgdorferi phenocopies B. burgdorferi lacking the entire bb0316-bb0319 operon, suggesting that deletion of bb0318 alone is sufficient to inactivate the BB0316-BB0319 riboflavin transport system. Moreover, comparative analysis of intracellular metabolite levels between Δbb0318 and wild-type B. burgdorferi using a targeted LCMS approach demonstrated diminished levels of riboflavin, FMN, and FAD. Strikingly, our analysis also revealed that Gro3P levels were significantly increased in Δbb0318 B. burgdorferi relative to the wild-type. This result led to the discovery of the contribution of the BB0316-BB0319 riboflavin transport system to the expression of the glycerol utilization gene glpD and to experimental definition of GlpD as an FAD-dependent, peripheral membrane glycerol 3-phosphate dehydrogenase. Together these findings suggested that Gro3P accumulation in Δbb0318 B. burgdorferi was the consequence of both reduced GlpD protein abundance and activity and therefore an imbalance in the GpsA/GlpD driven reciprocal interconversion of Gro3P and DHAP (Drecktrah et al., 2022) favoring the conversion of DHAP to Gro3P by GpsA. A shift toward Gro3P accumulation was recently demonstrated for a B. burgdorferi mutant lacking glpD (Drecktrah et al., 2022), providing further support for the proposed mechanism of increased levels of Gro3P in Δbb0318 B. burgdorferi.
Riboflavin, FMN, and FAD levels in Δbb0318 B. burgdorferi were reduced but not absent suggesting an alternative means by which the mutant is able to transport riboflavin and/or the riboflavin-derived cofactors, as was also postulated previously by Muramatsu et al. (2021). It is possible that B. burgdorferi harbors an as of yet unidentified second riboflavin transport system. However, this is unlikely given the severe attenuation of Δbb0318 B. burgdorferi in both ticks and mice. Alternatively, when grown in complex, nutrient-rich BSK-II medium in vitro, the high availability of riboflavin, FMN, and FAD may allow B. burgdorferi to scavenge these nutrients by a noncanonical mechanism. B. burgdorferi is unusual in its ability to transport deoxynucleotides (Jain et al., 2012; Lawrence et al., 2009). Riboflavin and the cofactors share structural similarities with nucleotides, raising the possibility that B. burgdorferi may have the potential to acquire one or more of these nutrients when present in high concentrations in vitro through a promiscuous nucleotide transporter.
Transcription of glpD and the glp operon has been shown to be regulated by a number of different mechanisms. The glycerol utilization system is positively regulated by the Hk1/Rrp1 two component system (Caimano et al., 2015; He et al., 2011; Rogers et al., 2009) as well as DksA or the stringent response under conditions of starvation (Boyle et al., 2021; Bugrysheva et al., 2015; Drecktrah et al., 2015) and negatively regulated by RpoS (Grove et al., 2017) and BosR (Hyde et al., 2006). The cyclic-di-GMP effector protein PlzA has been shown to be both a negative and positive regulator of glp expression depending on whether or not the protein is bound to the second messenger (Zhang et al., 2018). No evidence of transcriptional or translational dysregulation of rrp1 was detected in Δbb0318 B. burgdorferi. Rrp1 affects gene expression through the production of cyclic-di-GMP (Kostick et al., 2011; Rogers et al., 2009). A significant decrease in cyclic-di-GMP levels was detected in Δbb0318 B. burgdorferi compared to the wild-type (log2 fold change = −0.67, −Log[p] = 1.92) (Table S2). There are a number of possibilities for the reduced levels of cyclic-di-GMP in Δbb0318 B. burgdorferi. These include reduced diguanylate cyclase activity of Rrp1, reduced signaling through Hk1, increased phosphodiesterase activity of PdeA or PdeB, decreased GTP pools, and/or increased stringent response activity. No significant changes in the levels of GTP or ppGpp were detected in the metabolite analysis of Δbb0318 B. burgdorferi (Table S3). Future work will focus on understanding how the bb0318 gene or the riboflavin uptake system affects cyclic-di-GMP levels and the mechanism of bb0318-dependent expression of the glp operon.
The comparative metabolite analysis revealed a shift in carbon metabolism in Δbb0318 B. burgdorferi. This pattern was characterized by increased levels of fructose-1, 6 biphosphate (F1,6BP), glucose 1-phosphate (G1P), and glucosamine 6-phosphate (GlcN6P) and decreased levels of hexose in Δbb0318 B. burgdorferi relative to the wild-type. Notably, Δbb0318 B. burgdorferi did not display any changes in the energy balance of the cell or the redox status of the cell as evidenced by the lack of changes in adenosine phosphorylation and the maintenance of the NAD+ to NADH ratio. The lack of bioenergetic and redox effects in Δbb0318 B. burgdorferi was surprising considering previously observed decreases in ATP levels and polarization of the NAD+ to NADH ratio in B. burgdorferi lacking either glpD or gpsA (Drecktrah et al., 2022). The investigation of glpD and gpsA, using complete knockouts of each of these genes, concluded that the bi-directional conversions of DHAP and Gro3P were responsible for balancing the NAD+/NADH ratio of the cell and enabling redox dependent reactions, including key steps of glycolysis, to occur (Drecktrah et al., 2022). While riboflavin salvage was important for expression of the glp operon and for GlpD activity, the amount of active GlpD in Δbb0318 B. burgdorferi was likely sufficient to allow the redox status of the cell to balance under the nutrient-rich in vitro conditions examined here. Consistent with the notion that bb0318 affects numerous systems beyond GlpD and the glpD operon, the metabolic changes associated with Δbb0318 B. burgdorferi extended to numerous pathways not observed for ΔglpD B. burgdorferi (Drecktrah et al., 2022). In addition to glycolytic intermediates, the loss of bb0318 affected amino acids, nucleotides, and signaling molecules such as the cyclic-dinucleotides. The lack of changes in riboflavin, FMN, and FAD in the glpD mutant (Drecktrah et al., 2022) further emphasize the likely function of bb0318 above the glp operon.
It is important to note that the metabolomic and gene expression analyses were carried out in BSK-II medium at 35°C, where the major carbon source available to the spirochetes is glucose. While Δbb0318 B. burgdorferi appears to be able to partially compensate of the loss of bb0318 by upregulating hexose metabolism under this in vitro growth condition, this mechanism will be unavailable in hexose-restricted stages of the B. burgdorferi enzootic cycle. Under these more nutrient restrictive conditions the lower expression level of the glp operon in Δbb0318 B. burgdorferi may be inadequate to maintain redox balance contributing to slower growth and attenuation. We and others (Muramatsu et al., 2021) have established that B. burgdorferi lacking bb0318 demonstrate a significant growth defect in BSK-II medium at 23°C, suggesting severe bioenergetic effects in the absence of riboflavin uptake that are unable to be compensated for in this growth condition.
In the tick, B. burgdorferi shifts its metabolic program in favor of alternative carbon sources including glycerol, chitobiose, and N-acetyl glucosamine (Caimano et al., 2016) and has been shown to rely on GlpD and the Rrp1-regulon for maximal survival (Caimano et al., 2011; He et al., 2011; Kostick et al., 2011; Pappas et al., 2011). Given the negative effects of the bb0318 mutant on the glp operon, nonglucose carbon metabolism and growth at 23°C, we sought to determine the requirement for riboflavin scavenge during the tick phase of the enzootic cycle. Assessment of the spirochete loads in unfed larvae over a 37-day time course indicated that in the first 17 days post-immersion wild-type and Δbb0318/bb0318+ B. burgdorferi both had a doubling time of approximately 3.5 days and after this time point the spirochete population sizes did not change. The ability of B. burgdorferi to replicate in unfed ticks was found to be dependent on the bb0318 gene, and therefore, the ability to scavenge riboflavin. Interestingly, however, despite the inability of Δbb0318 B. burgdorferi to undergo population expansion in unfed ticks, these spirochetes remained viable and were reisolated from the ticks in BSK-II medium at the 37-day timepoint. Moreover, gene bb0318 was essential for B. burgdorferi survival in feeding ticks. The contribution of bb0318 to population expansion in unfed ticks and requirement for bb0318 for survival during the tick blood meal are phenotypes consistent with those of B. burgdorferi lacking glpD or rrp1 (Caimano et al., 2011, He et al., 2011, Kostick et al., 2011, Pappas et al., 2011). Riboflavin uptake by B. burgdorferi is expected to be critical for the activity of putative FAD-dependent enzymes, which are likely to contribute to spirochete survival in ticks. We demonstrated here that GlpD is an FAD-dependent G3PDH, supporting the importance of FAD availability for carbon metabolism in the tick. In addition, B. burgdorferi must overcome significant reactive nitrogen stress during tick infection (Bourret et al., 2016). Genes bb0728 (cdr) and bb0515 (trxB) each encode putative FAD-dependent flavoproteins (Muramatsu et al., 2021; Showman et al., 2016). The CoA/CoA-disulfide reductase (CoA/CoADR) and thioredoxin/thioredoxin reductase (TrxA/TrxB) systems have been proposed to contribute to mechanisms of B. burgdorferi defense against reactive nitrogen species (Bourret et al., 2016). No change in coenzyme A levels were detected in Δbb0318 B. burgdorferi compared to the wild-type and thioredoxin was not measured in our analysis. However, like BB0318, CoADR protects B. burgdorferi from reactive oxygen species (Boylan et al., 2006, 2008; Eggers et al., 2011), is important for B. burgdorferi persistence in ticks, and is required for infection of mice (Eggers et al., 2011), suggesting that reduced CoADR activity due to low FAD availability likely contributes to the phenotypes of the bb0318 mutant (Showman et al., 2016).
In sum, these data revealed that riboflavin salvage and maintenance of flavin pools intersects the GlpD protein and has consequences for carbon metabolism. Moreover, riboflavin salvage was essential for B. burgdorferi survival in Ixodes scapularis ticks. Together this work expands the understanding of the critical importance of riboflavin salvage in the biology of B. burgdorferi.
4 |. EXPERIMENTAL PROCEDURES
4.1 |. Bacterial strains and growth conditions
B. burgdorferi clones used in this study were derived from clone B31 A3–68 Δbbe02 (wild-type), which lacks plasmids cp9 and lp56, as well as the bbe02 gene on lp25 (Rego et al., 2011). The construction of the Δbb0318 B. burgdorferi was previously described. (Showman et al., 2016). The 5A4-WT and 5A4-Δrrp1 clones were kind gifts from Melissa Caimano and Justin Radolf (Caimano et al., 2015). Spirochetes were grown in liquid Barbour-Stoenner-Kelly II (BSK-II) growth medium supplemented with gelatin and 6% rabbit serum at 35°C. Individual B. burgdorferi colonies were isolated by plating in solid BSK-agarose medium. Plates were kept at 35°C with 2.5% CO2. The antibiotics kanamycin (200 μg/ml), streptomycin (50 μg/ml), and/or gentamycin (40 μg/ml) were added to the cultures when appropriate. Cloning steps were performed using Escherichia coli DH5α, grown in Luria-Bertani (LB) broth or on LB agar plates. For E. coli cultures, the antibiotics kanamycin (50 μg/ml), spectinomycin (300 μg/ml), and/or gentamicin (15 μg/ml), were used when appropriate.
4.2 |. Generation of Δbb0316-bb0319 B. burgdorferi
The bb0316-bb0319 deletion construct was engineered for in frame replacement of the operon with the promoterless spectinomycin/streptomycin resistance gene, aadA, using the PCR-based overlap extension strategy described previously (Ellis et al., 2014) and oligonucleotides 1823, 1825, 1826, 1828, 2303, and 2304 (Table S1). The allelic exchange plasmid pCR-BLUNT-Δbb0316-bb0319-aadA was verified by PCR, restriction enzyme digestion, and DNA sequence analysis. Allelic exchange plasmid DNA was transformed into B. burgdorferi A3–68Δbbe02 as described (Samuels et al., 2018). Δbb0316-bb0319 clones were verified by PCR using external oligonucleotides to the lesion and to contain the endogenous plasmid content of the parent (Elias et al., 2002; Jewett, Lawrence, et al., 2007). A single clone was selected for further experiments.
4.3 |. Roseoflavin survival assay
In biological triplicate, B. burgdorferi clones were inoculated at a starting density of 1 × 105 spirochetes/ml in BSK-II medium containing 75 μm roseoflavin (Sigma) in 0.6% DMSO or 0.6% DMSO alone. The cultures were quantified by Petroff-Hausser count under dark field microscopy following 96 h of incubation at 35°C. The percent survival of each clone was calculated as the spirochete density in the presence of roseoflavin divided by the spirochete density in the presence of vehicle alone multiplied by 100.
4.4 |. In vitro growth assay
Growth curves were performed in biological triplicate as described (Showman et al., 2016). Growth analysis at 23°C was performed over a 15-day time course. Cultures were sampled every 3 days and plated in solid medium for colony forming unit counts. Growth analysis at 35°C was performed over a 96-h time course. Cultures were sampled every 24 h and quantified by Petroff-Hausser count under dark field microscopy.
4.5 |. Metabolite extraction
Wild-type and Δbb0318 B. burgdorferi were grown to mid-logarithmic phase (~2 × 107) in BSK-II at pH 7.6. Two hundred milliliters of each culture were centrifuged at 5000g and washed twice with increasingly smaller volumes of HN buffer (50 mM Hepes, 50 mM NaCl, pH 7.4), 10 ml and 1 ml, respectively. Cells were lysed by resuspension in 500 μl of distilled water followed by boiling at 99°C for 15 min. After boiling, the suspensions were centrifuged at 12,000g for 10 min to remove cellular debris and the supernatants were collected for further extraction. Pellets were saved for total protein analysis and quantified using the BCA assay (Thermo Fisher Scientific). Approximately 500 μl of each of the supernatant was extracted with 2 volumes of 100% ethanol 1.2 ml (Cf = 70% ethanol) and 1.8 ml (Cf = 85% ethanol), respectively. The resulting extract was evaporated under a nitrogen stream at 34°C. Upon evaporation, the samples were resuspended in mobile phase A (50% methanol in LCMS grade water) and filtered through a 0.45 μm nylon membrane syringe filter (GE Healthcare). Samples were prepared in quadruplicate (n = 4 biological replicates).
4.6 |. Liquid chromatography mass spectrometry for metabolomics
Tributylamine and all synthetic molecular references were purchased from Millipore Sigma. LCMS grade water, methanol, isopropanol, and acetic acid were purchased through Fisher Scientific. All samples were separated using a Sciex ExionLC™ AC system and measured using a Sciex 5500 QTRAP® mass spectrometer. Aqueous metabolites were analyzed using a previously established ion pairing method with modification (McCloskey et al., 2015; Schwarz et al., 2021). To control for signal stability, quality control samples were injected every 10 injections. Samples were resolved with a Waters™ Atlantis T3 column (100 Å, 3 μm, 3 mm × 100 mm) and eluted via a 15 min gradient from 5 mM tributylamine, 5 mM acetic acid in 2% isopropanol, 5% methanol, 93% water (v/v) to 100% isopropanol. Each metabolite was identified by a pair of negative mode multiple reaction monitoring (MRM) pairs and a defined retention time based on previously analyzed pure standards. Heavy labeled standards were not utilized, and relative quantification was performed. Signals were integrated using MultiQuant® Software 3.0.3. Signals with greater than 50% missing values were discarded and remaining missing values were replaced with the lowest measured value in the experimental batch. A coefficient of variance cutoff of 30% was applied and all signals above were discarded. Metabolites with multiple MRMs were quantified with the higher signal to noise MRM. Filtered datasets were total sum normalized prior to analysis. Single and multivariate analysis was performed in MarkerView® Software 1.3.1. A Benjamini-Hochberg false discovery cutoff was calculated for the dataset with a false discovery rate of 20% corresponding to a p-value of .032 by unpaired t-test.
4.7 |. GlpD localization
Fractionation of B. burgdorferi lysate into soluble and membrane fractions was performed as previously described (Kuhn et al., 2021). Briefly, wild-type B. burgdorferi was grown to a density of ~1 × 108 cells/ml in BSK-II medium. Spirochetes were harvested by centrifugation at 3210g for 10 min at 20°C and washed twice with 10 ml of cold HN buffer (50 mM Hepes, 50 mM NaCl, pH 7.4). The resulting bacterial pellet was resuspended in 1 ml of cold HN buffer and 100 μl Halt Protease Inhibitor Cocktail (Thermo Fisher Scientific). Spirochetes were lysed via sonication and spun at 125,000g to separate the soluble and membrane components of the lysate. Protein samples were separated on an SDS-PAGE gel and transferred to a nitrocellulose membrane (Bio-Rad). Immunoblots were performed using rabbit anti-SodA NIH754 (1:1000) (Jewett, Byram, et al., 2007), rabbit anti-VlsE (1:1000) (Rockland Immunochemicals), and rabbit anti-GlpD (1:5000) (Drecktrah et al., 2022) antibodies. Aliquots of the membrane fraction were washed for 30 min at 4°C with 500 mM NaCl (50 mM Hepes, 500 mM NaCl, pH 7.4), 3.6 M urea or 2% Triton X-100 and spun at 125,000g to separate the soluble and membrane components. The resulting soluble fractions were dialyzed with HN buffer overnight at 4°C. Protein samples were analyzed by immunoblot using rabbit anti-GlpD (1:5000) antibody (Drecktrah et al., 2022).
4.8 |. G3PDH assay
The glpD/bb0243 open reading frame sequence was codon-optimized for translation in E. coli and the DNA synthesized by GenScript. This sequence was PCR amplified using Phusion enzyme (Thermo Scientific) and primers 2819 and 2820 (Table S1). The DNA fragment and expression vector pET43.1b were digested with restriction enzymes HindIII and XhoI, ligated and cloned in E. coli, generating a 6xhis, nus N-terminal and 6xhis C-terminal fusion construct. The fusion construct was verified by restriction digest and Sanger sequencing. The pET43.1b-glpDopt plasmid was moved to NiCo21 (DE3) E. coli (New England Biolabs) for protein induction and purification, as previously described (Kuhn et al., 2021) (Figure S2). The glycerol 3-phosphate dehydrogenase activity of recombinant Nus-GlpD and Nus alone in the presence and absence of 10 μM FAD was determined using the G3PDH colorimetric assay kit (Abcam). Twenty-five micrograms, 15 μg and 10 μg of Nus-GlpD and 25 μg of Nus alone were used in the assay. The assay was performed in triplicate according to the manufacturer’s instructions. Reducing equivalents were measured at 450 nm every 15 min over a 1-h time course at 37°C using a Biotek Cytation 5 plate reader. G3PDH activity was calculated as reducing equivalents/min/mg. Statistical significance was determined via unpaired t-test or one-way ANOVA with Dunnett’s multiple comparisons test to the Nus protein control in the absence of FAD, GraphPad Prism 9.0.0.
4.9 |. Complementation of Δbb0318 B. burgdorferi
The BB0318 open reading frame sequence was amplified from B. burgdorferi B31 clone A3 genomic DNA using Phusion enzyme (Thermo Scientific) and primers 2088 and 2089 (Table S1). The DNA fragment and shuttle vector pBSV2G flaBp (Jain et al., 2015) were digested with restriction enzymes BamHI and SalI, ligated and cloned in E. coli. The plasmid was confirmed by restriction digest and DNA sequence analysis. Plasmid pBSV2G flaBp-bb0318 was transformed into Δbb0318 B. burgdorferi. Resulting Δbb0318/bb0318+ transformants were confirmed by PCR and verified to carry the endogenous plasmid content of the parent clone (Jewett, Lawrence, et al., 2007).
4.10 |. RNA extraction and RT-qPCR
Triplicate cultures were grown for each B. burgdorferi clone in 50 ml of BSK-II. Cells were grown to late stationary phase (24 h after reaching stationary, 2.5–3 × 108 cells/ml) and pelleted by centrifugation at 3210g for 10 min at 20°C. Ten milliliters of the culture was used for RNA extraction and the remaining 40 ml was used for the generation of protein lysates (see below). RNA samples were extracted from all cultures using hot phenol-chloroform extraction and ethanol precipitation as described previously (Drecktrah et al., 2015). Samples were subjected to two DNase treatments to remove any contamination from DNA utilizing DNase I (Roche). Subsequently, 1 μg of the DNase treated samples was used to prepare cDNA using the iScript Select cDNA synthesis kit and random hexamers (Bio-Rad), following the manufacturer’s instructions. Parallel duplicate reactions without reverse transcriptase for each sample were also performed. Quantitative PCR was performed using 100 ng of cDNA per sample, iQ SYBR green supermix (Bio-rad) and primers for the target genes and constitutive control gene, bb0337 (Table S1). Gene expression was assessed by using the 2−ΔΔCTmethod, in which the gene expression of the target gene was normalized to that of the constitutive control gene, bb0337. Statistical significance was determined by unpaired t-test or one-way ANOVA with Dunnett’s multiple-comparisons test to wild-type (GraphPad Prism, version 9.0.0).
4.11 |. SDS-PAGE and fluorescent immunoblot
B. burgdorferi clones were grown to late stationary phase (24 h after reaching stationary, 2.5–3 × 108 cells/ml) in BSK-II medium. Spirochetes were harvested by centrifugation at 3210g for 10 min at 20°C and washed twice in 1 ml of cold HN buffer (50 mM Hepes, 50 mM NaCl, pH 7.4). Cell pellets were resuspended in protein sample buffer to a final density of 1 × 106 cells/μl. Total protein lysates (1 × 107cells/lane) were separated by SDS-PAGE and transferred to a PVDF membrane. Immunoblots were performed using both rabbit anti-GlpD (1:5000) (Drecktrah et al., 2022) and mouse anti-FlaB H9724 (1:200) (Barbour et al., 1986) or rabbit anti-Rrp1 (1:1000) (Bontemps-Gallo et al., 2016) and anti-FlaB, in a 1:1 solution of Odyssey blocking buffer (LI-COR Biosciences) and Tris-buffered saline, pH 7.4 and 0.1% Tween20 (TBST) followed by IRDye 800CW goat anti-rabbit IgG and IRDye 680LT goat anti-mouse IgG (H + L) secondary antibodies (LI-COR Biosciences). Immunoblots were visualized and quantified using the LI-COR Odyssey scanner and software (Image Studio version 4). Statistical significance was determined by one-way ANOVA with Dunnett’s multiple-comparisons test to wild-type (GraphPad Prism, version 9.0.0).
4.12 |. Artificial infection of larval ticks by immersion and feeding of ticks on mice
Naïve Ixodes scapularis larval ticks were purchased from the Oklahoma State University. Cohorts of ~150 larvae were dehydrated in a bell jar with saturated ammonium sulfate for 24 h. B. burgdorferi clones (WT, Δbb0318, and Δbb0318/bb0318+) were grown to 6 × 107-1 × 108 cells/ml in BSK-II medium and diluted to 2 × 107 cells/ml. Cohorts of dehydrated ticks were inoculated with B. burgdorferi cultures by immersion (Policastro & Schwan, 2003) for 1.5 h at 35°C with periodic vortexing, followed by two washes in phosphate buffered saline (PBS). Ticks were dried with Whatman paper and allowed to recover for 48 h. The plasmid contents of all B. burgdorferi clones was verified by PCR (Jewett, Lawrence, et al., 2007). Three days post-infection by immersion a subset the larval cohorts were allowed to feed to repletion on groups of 4 naïve C3H/HeN mice (Envigo) per B. burgdorferi clone, as previously described (Kuhn et al., 2021).
4.13 |. Quantification of B. burgdorferi loads in ticks
B. burgdorferi burden in unfed larvae was assessed on day 3, 10, 17, 23, 30, and 37 post-infection by immersion. Groups of 10–20 unfed larvae were pooled per B. burgdorferi clone and DNA was isolated using a NucleoSpin tissue kit (Clontech Laboratories) per the manufacturer’s instructions. Spirochete densities were determined by qPCR as previously described (Kuhn et al., 2021). Seven days post feeding to repletion, the number of B. burgdorferi per fed larva was determined by plating of individual triturated ticks in solid medium for colony forming units. Ticks were surface sterilized by multiple washes in 3% hydrogen peroxide, 70% ethanol, and sterile water. Each tick was homogenized with a sterile plastic pestle in BSK-II medium supplemented with an antibiotic cocktail of rifampicin (50 μg/ml), phosphomycin (20 μg/ml), and amphotericin B (2.5 μg/ml) (RPA cocktail). The homogenates were plated in BSK-agarose medium supplemented with RPA cocktail and the spirochete densities were obtained from colony forming unit counts. Statistical significance was determined by one-way ANOVA with Dunnett’s multiple-comparisons test to the wild-type (GraphPad Prism, version 9.0.0).
Supplementary Material
ACKNOWLEDGMENTS
We thank Adrienne Showman and Katelan Yap for technical assistance. We thank Melissa Caimano and Justin Radolf for the kind gift of the Δrrp1 and 5A4-WT B. burgdorferi. We thank Travis Jewett and the Jewett lab members for critical feedback. We also thank Dan Dulebohn for insightful discussions. Graphical abstract was created using Biorender.com. This work was supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health (R01AI099094 to MWJ) and provided by the Division of Intramural Research, National Institute of Allergy and Infectious Diseases of the National Institutes of Health (CMB and FCG). DR is the recipient of the Florida Education Fund’s McKnight Doctoral Fellowship. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
ETHICS STATEMENT
The University of Central Florida is accredited by the International Association for Assessment and Accreditation of Laboratory Animal Care. Protocols for all animal experiments were prepared according to the guidelines of the National Institutes of Health and were reviewed and approved by the University of Central Florida Institutional Animal Care and Use Committee.
SUPPORTING INFORMATION
Additional supporting information can be found online in the Supporting Information section at the end of this article.
DATA AVAILABILITY STATEMENT
The data that supports the findings of this study are available in the figures and in the supplementary material of this article.
REFERENCES
- Adams PP, Flores Avile C, Popitsch N, Bilusic I, Schroeder R, Lybecker M et al. (2017) In vivo expression technology and 5′ end mapping of the Borrelia burgdorferi transcriptome identify novel RNAs expressed during mammalian infection. Nucleic Acids Research, 45, 775–792. http://www.ncbi.nlm.nih.gov/pubmed/27913725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Averianova LA, Balabanova LA, Son OM, Podvolotskaya AB & Tekutyeva LA (2020) Production of vitamin B2 (riboflavin) by microorganisms: an overview. Frontiers in Bioengineering and Biotechnology, 8, 570828. https://www.ncbi.nlm.nih.gov/pubmed/33304888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbour AG, Hayes SF, Heiland RA, Schrumpf ME & Tessier SL (1986) A Borrelia-specific monoclonal antibody binds to a flagellar epitope. Infection and Immunity, 52, 549–554. http://www.ncbi.nlm.nih.gov/pubmed/3516878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bontemps-Gallo S, Lawrence K & Gherardini FC (2016) Two different virulence-related regulatory pathways in Borrelia burgdorferi are directly affected by osmotic fluxes in the blood meal of feeding ixodes ticks. PLoS Pathogens, 12, e1005791. https://www.ncbi.nlm.nih.gov/pubmed/27525653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourret TJ, Lawrence KA, Shaw JA, Lin T, Norris SJ & Gherardini FC (2016) The nucleotide excision repair pathway protects Borrelia burgdorferi from nitrosative stress in Ixodes scapularis ticks. Frontiers in Microbiology, 7, 1397. http://www.ncbi.nlm.nih.gov/pubmed/27656169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boylan JA, Hummel CS, Benoit S, Garcia-Lara J, Treglown-Downey J, Crane EJ 3rd et al. (2006) Borrelia burgdorferi bb0728 encodes a coenzyme A disulphide reductase whose function suggests a role in intracellular redox and the oxidative stress response. Molecular Microbiology, 59, 475–486. http://www.ncbi.nlm.nih.gov/pubmed/16390443 [DOI] [PubMed] [Google Scholar]
- Boylan JA, Lawrence KA, Downey JS & Gherardini FC (2008) Borrelia burgdorferi membranes are the primary targets of reactive oxygen species. Molecular Microbiology, 68, 786–799. https://www.ncbi.nlm.nih.gov/pubmed/18373524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle WK, Richards CL, Dulebohn DP, Zalud AK, Shaw JA, Lovas S et al. (2021) DksA-dependent regulation of RpoS contributes to Borrelia burgdorferi tick-borne transmission and mammalian infectivity. PLoS Pathogens, 17, e1009072. https://www.ncbi.nlm.nih.gov/pubmed/33600418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bugrysheva JV, Pappas CJ, Terekhova DA, Iyer R, Godfrey HP, Schwartz I et al. (2015) Characterization of the RelBbu regulon in Borrelia burgdorferi reveals modulation of glycerol metabolism by (p)ppGpp. PLoS One, 10, e0118063. https://www.ncbi.nlm.nih.gov/pubmed/25688856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caimano MJ, Drecktrah D, Kung F & Samuels DS (2016) Interaction of the Lyme disease spirochete with its tick vector. Cellular Microbiology, 18, 919–927. http://www.ncbi.nlm.nih.gov/pubmed/27147446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caimano MJ, Dunham-Ems S, Allard AM, Cassera MB, Kenedy M & Radolf JD (2015) Cyclic di-GMP modulates gene expression in Lyme disease spirochetes at the tick-mammal interface to promote spirochete survival during the blood meal and tick-to-mammal transmission. Infection and Immunity, 83, 3043–3060. http://www.ncbi.nlm.nih.gov/pubmed/25987708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caimano MJ, Kenedy MR, Kairu T, Desrosiers DC, Harman M, Dunham-Ems S et al. (2011) The hybrid histidine kinase Hk1 is part of a two-component system that is essential for survival of Borrelia burgdorferi in feeding Ixodes scapularis ticks. Infection and Immunity, 79, 3117–3130. https://www.ncbi.nlm.nih.gov/pubmed/21606185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casjens SR, Mongodin EF, Qiu WG, Luft BJ, Schutzer SE, Gilcrease EB et al. (2012) Genome stability of Lyme disease spirochetes: comparative genomics of Borrelia burgdorferi plasmids. PLoS One, 7, e33280. http://www.ncbi.nlm.nih.gov/pubmed/22432010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casjens S, Palmer N, Van Vugt R, Huang WM, Stevenson B, Rosa P et al. (2000) A bacterial genome in flux: the twelve linear and nine circular extrachromosomal DNAs in an infectious isolate of the Lyme disease spirochete Borrelia burgdorferi. Molecular Microbiology, 35, 490–516. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=10672174 [DOI] [PubMed] [Google Scholar]
- Coburn J, Garcia B, Hu LT, Jewett MW, Kraiczy P, Norris SJ et al. (2021) Lyme disease pathogenesis. Current Issues in Molecular Biology, 42, 473–518. https://www.ncbi.nlm.nih.gov/pubmed/33353871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis GD, Elisee C, Newham DM & Harrison RG (1999) New fusion protein systems designed to give soluble expression in Escherichia coli. Biotechnology and Bioengineering, 65, 382–388. https://www.ncbi.nlm.nih.gov/pubmed/10506413 [PubMed] [Google Scholar]
- De Marco V, Stier G, Blandin S & De Marco A (2004) The solubility and stability of recombinant proteins are increased by their fusion to NusA. Biochemical and Biophysical Research Communications, 322, 766–771. https://www.ncbi.nlm.nih.gov/pubmed/15336530 [DOI] [PubMed] [Google Scholar]
- Deka RK, Brautigam CA, Biddy BA, Liu WZ & Norgard MV (2013) Evidence for an ABC-type riboflavin transporter system in pathogenic spirochetes. mBio, 4, e00615–e00612. http://www.ncbi.nlm.nih.gov/pubmed/23404400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drecktrah D, Hall LS, Crouse B, Schwarz B, Richards C, Bohrnsen E et al. (2022) The glycerol-3-phosphate dehydrogenases GpsA and GlpD constitute the oxidoreductive metabolic linchpin for Lyme disease spirochete host infectivity and persistence in the tick. PLoS Pathogens, 18, e1010385. https://www.ncbi.nlm.nih.gov/pubmed/35255112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drecktrah D, Lybecker M, Popitsch N, Rescheneder P, Hall LS & Samuels DS (2015) The Borrelia burgdorferi RelA/SpoT homolog and stringent response regulate survival in the tick vector and global gene expression during starvation. PLoS Pathogens, 11, e1005160. http://www.ncbi.nlm.nih.gov/pubmed/26371761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggers CH, Caimano MJ, Malizia RA, Kariu T, Cusack B, Desrosiers DC et al. (2011) The coenzyme A disulphide reductase of Borrelia burgdorferi is important for rapid growth throughout the enzootic cycle and essential for infection of the mammalian host. Molecular Microbiology, 82, 679–697. http://www.ncbi.nlm.nih.gov/pubmed/21923763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias AF, Stewart PE, Grimm D, Caimano MJ, Eggers CH, Tilly K et al. (2002) Clonal polymorphism of Borrelia burgdorferi strain B31 MI: implications for mutagenesis in an infectious strain background. Infection and Immunity, 70, 2139–2150. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=11895980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis TC, Jain S, Linowski AK, Rike K, Bestor A, Rosa PA et al. (2014) In vivo expression technology identifies a novel virulence factor critical for Borrelia burgdorferi persistence in mice. Plos Pathogens, 10, ARTN e1004260. 10.1371/journal.ppat.1004260, 10.1371/journal.ppat.1004260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser CM, Casjens S, Huang WM, Sutton GG, Clayton R, Lathigra R et al. (1997) Genomic sequence of a Lyme disease spirochaete, Borrelia burgdorferi. Nature, 390, 580–586. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=9403685 [DOI] [PubMed] [Google Scholar]
- Gherardini FC, Dulebohn DP, Bourret TJ & Richards CR (2021) Metabolism and physiology of Borrelia. In: Radolf JD & Samuels DS (Eds.) Lyme disease and relapsing fever spirochetes: genomics, molecular biology, host interactions and disease pathogenesis. Norfolk, UK: Caister Academic Press. [Google Scholar]
- Grove AP, Liveris D, Iyer R, Petzke M, Rudman J, Caimano MJ et al. (2017) Two distinct mechanisms govern RpoS-mediated repression of tick-phase genes during mammalian host adaptation by Borrelia burgdorferi, the Lyme disease spirochete. mBio, 8, e01204–17. https://www.ncbi.nlm.nih.gov/pubmed/28830947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He M, Ouyang Z, Troxell B, Xu H, Moh A, Piesman J et al. (2011) Cyclic di-GMP is essential for the survival of the Lyme disease spirochete in ticks. PLoS Pathogens, 7, e1002133. http://www.ncbi.nlm.nih.gov/pubmed/21738477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyde JA, Seshu J & Skare JT (2006) Transcriptional profiling of Borrelia burgdorferi containing a unique bosR allele identifies a putative oxidative stress regulon. Microbiology, 152, 2599–2609. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=16946255 [DOI] [PubMed] [Google Scholar]
- Jain S, Showman AC & Jewett MW (2015) Molecular dissection of a Borrelia burgdorferi in vivo essential purine transport system. Infection and Immunity, 83, 2224–2233. http://www.ncbi.nlm.nih.gov/pubmed/25776752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain S, Sutchu S, Rosa PA, Byram R & Jewett MW (2012) Borrelia burgdorferi harbors a transport system essential for purine salvage and mammalian infection. Infection and Immunity, 80, 3086–3093. http://www.ncbi.nlm.nih.gov/pubmed/22710875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jewett MW, Byram R, Bestor A, Tilly K, Lawrence K, Burtnick MN et al. (2007) Genetic basis for retention of a critical virulence plasmid of Borrelia burgdorferi. Molecular Microbiology, 66, 975–990. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=17919281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jewett MW, Lawrence K, Bestor AC, Tilly K, Grimm D, Shaw P et al. (2007) The critical role of the linear plasmid lp36 in the infectious cycle of Borrelia burgdorferi. Molecular Microbiology, 64, 1358–1374. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=17542926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostick JL, Szkotnicki LT, Rogers EA, Bocci P, Raffaelli N & Marconi RT (2011) The diguanylate cyclase, Rrp1, regulates critical steps in the enzootic cycle of the Lyme disease spirochetes. Molecular Microbiology, 81, 219–231. https://www.ncbi.nlm.nih.gov/pubmed/21542866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn HW, Lasseter AG, Adams PP, Avile CF, Stone BL, Akins DR et al. (2021) BB0562 is a nutritional virulence determinant with lipase activity important for Borrelia burgdorferi infection and survival in fatty acid deficient environments. PLoS Pathogens, 17, e1009869. https://www.ncbi.nlm.nih.gov/pubmed/34415955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence KA, Jewett MW, Rosa PA & Gherardini FC (2009) Borrelia burgdorferi bb0426 encodes a 2′-deoxyribosyltransferase that plays a central role in purine salvage. Molecular Microbiology, 72, 1517–1529. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=19460093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massey V (2000) The chemical and biological versatility of riboflavin. Biochemical Society Transactions, 28, 283–296. https://www.ncbi.nlm.nih.gov/pubmed/10961912 [PubMed] [Google Scholar]
- Matsunaga J, Young TA, Barnett JK, Barnett D, Bolin CA & Haake DA (2002) Novel 45-kilodalton leptospiral protein that is processed to a 31-kilodalton growth-phase-regulated peripheral membrane protein. Infection and Immunity, 70, 323–334. https://www.ncbi.nlm.nih.gov/pubmed/11748198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mccloskey D, Gangoiti JA, Palsson BO & Feist AM (2015) A pH and solvent optimized reverse-phase ion-paring-LC–MS/MS method that leverages multiple scan-types for targeted absolute quantification of intracellular metabolites. Metabolomics, 11, 133801350. [Google Scholar]
- Muramatsu MK, Zhou J, Fitzgerald BL, Deka RK, Belisle JT & Norgard MV (2021) An rfuABCD-like operon and its relationship to riboflavin utilization and mammalian infectivity by Borrelia burgdorferi. Infection and Immunity, 89, e0030721. https://www.ncbi.nlm.nih.gov/pubmed/34310888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pappas CJ, Iyer R, Petzke MM, Caimano MJ, Radolf JD & Schwartz I (2011) Borrelia burgdorferi requires glycerol for maximum fitness during the tick phase of the enzootic cycle. PLoS Pathogens, 7, e1002102. http://www.ncbi.nlm.nih.gov/pubmed/21750672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Policastro PF & Schwan TG (2003) Experimental infection of Ixodes scapularis larvae (Acari: Ixodidae) by immersion in low passage cultures of Borrelia burgdorferi. Journal of Medical Entomology, 40, 364–370. http://www.ncbi.nlm.nih.gov/pubmed/12943118 [DOI] [PubMed] [Google Scholar]
- Ramsey ME, Hyde JA, Medina-Perez DN, Lin T, Gao L, Lundt ME et al. (2017) A high-throughput genetic screen identifies previously uncharacterized Borrelia burgdorferi genes important for resistance against reactive oxygen and nitrogen species. PLoS Pathogens, 13, e1006225. http://www.ncbi.nlm.nih.gov/pubmed/28212410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rego RO, Bestor A & Rosa PA (2011) Defining the plasmid-borne restriction-modification systems of the Lyme disease spirochete Borrelia burgdorferi. Journal of Bacteriology, 193, 1161–1171. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=21193609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers EA, Terekhova D, Zhang HM, Hovis KM, Schwartz I & Marconi RT (2009) Rrp1, a cyclic-di-GMP-producing response regulator, is an important regulator of Borrelia burgdorferi core cellular functions. Molecular Microbiology, 71, 1551–1573. https://www.ncbi.nlm.nih.gov/pubmed/19210621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy A, Kucukural A & Zhang Y (2010) I-TASSER: a unified platform for automated protein structure and function prediction. Nature Protocols, 5, 725–738. http://www.ncbi.nlm.nih.gov/pubmed/20360767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuels DS, Drecktrah D & Hall LS (2018) Genetic transformation and complementation. Methods in Molecular Biology, 1690, 183–200. https://www.ncbi.nlm.nih.gov/pubmed/29032546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schryvers A, Lohmeier E & Weiner JH (1978) Chemical and functional properties of the native and reconstituted forms of the membrane-bound, aerobic glycerol-3-phosphate dehydrogenase of Escherichia coli. The Journal of Biological Chemistry, 253, 783–788. https://www.ncbi.nlm.nih.gov/pubmed/340460 [PubMed] [Google Scholar]
- Schwan TG, Battisti JM, Porcella SF, Raffel SJ, Schrumpf ME, Fischer ER et al. (2003) Glycerol-3-phosphate acquisition in spirochetes: distribution and biological activity of glycerophosphodiester phosphodiesterase (GlpQ) among Borrelia species. Journal of Bacteriology, 185, 1346–1356. https://www.ncbi.nlm.nih.gov/pubmed/12562805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz B, Sharma L, Roberts L, Peng X, Bermejo S, Leighton I et al. (2021) Cutting edge: severe SARS-CoV-2 infection in humans is defined by a shift in the serum lipidome, resulting in dysregulation of eicosanoid immune mediators. Journal of Immunology, 206, 329–334. https://www.ncbi.nlm.nih.gov/pubmed/33277388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Showman AC, Aranjuez G, Adams PP & Jewett MW (2016) Gene bb0318 is critical for the oxidative stress response and infectivity of Borrelia burgdorferi. Infection and Immunity, 84, 3141–3151. http://www.ncbi.nlm.nih.gov/pubmed/27550932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walz AC, Demel RA, De Kruijff B & Mutzel R (2002) Aerobic sn-glycerol-3-phosphate dehydrogenase from Escherichia coli binds to the cytoplasmic membrane through an amphipathic alpha-helix. The Biochemical Journal, 365, 471–479. https://www.ncbi.nlm.nih.gov/pubmed/11955283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Yan R, Roy A, Xu D, Poisson J & Zhang Y (2015) The I-TASSER Suite: protein structure and function prediction. Nature Methods, 12, 7–8. http://www.ncbi.nlm.nih.gov/pubmed/25549265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y (2008) I-TASSER server for protein 3D structure prediction. BMC Bioinformatics, 9, 40. http://www.ncbi.nlm.nih.gov/pubmed/18215316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang JJ, Chen T, Yang Y, Du J, Li H, Troxell B et al. (2018) Positive and negative regulation of glycerol utilization by the c-di-GMP binding protein PlzA in Borrelia burgdorferi. Journal of Bacteriology, 200, e00243–18. https://www.ncbi.nlm.nih.gov/pubmed/30181123 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that supports the findings of this study are available in the figures and in the supplementary material of this article.


