Abstract
Once considered to be exotic species of limited synthetic utility, vinyl cations have recently been shown to be highly versatile intermediates in a variety of processes. Here, we report a method for the synthesis of aryl-substituted benzocycloheptenes and −hexenes using the hydrotriflate salt of an electron-poor pyridine as a uniquely efficient proton source for a vinyl cation mediated Friedel–Crafts cyclization. The mild conditions made possible by this reagent allowed a range of simple and functionalized alkynes bearing pendant aryl groups to serve as suitable substrates for this scalable and convenient protocol.
In efforts to develop new means of C−C bond formation, vinyl cations have recently emerged as promising synthetic intermediates in a number of valuable transformations.1 Though previously considered to be exotic species that are too unstable or difficult to generate for functional group tolerant and chemoselective transformations, more recent elucidation of their kinetic properties1b,2 and the development of new strategies for their formation3 have revealed that they are versatile intermediates that could be utilized in a variety of interesting reaction pathways, including reactions with weakly nucleophilic species, skeletal rearrangements, and C–H insertion reactions (Scheme 1A).1d
Scheme 1.
Recent strategies for the generation and utilization of vinyl cation intermediates.
In one approach, the vinyl cation is generated through the loss or abstraction of a leaving group at the vinylic position of an alkene (Scheme 1B).3a-d,4 While early studies reported the generation of vinyl cations by solvolysis of vinyl (pseudo)halides,4 more recently, other leaving groups and reaction conditions have been explored. Brewer and coworkers reported the generation of vinyl cation intermediates following the loss of N2 from a vinyl diazonium species.3a Moreover, Nelson and coworkers reported the formation of vinyl cations by abstraction of TfO− from vinyl triflates using a weakly-coordinated silylium ion or Li+.3b-d In addition, the fragmentation of vinyl iodonium and bromonium species has also been used to generate vinyl cation intermediates.5
In a second general approach, vinyl cation formation proceeds through the addition of various electrophilic species to alkynes (Scheme 1C).3e-g,6 Using copper and diaryliodonium salts, Gaunt and coworkers reported the formation of a 6–7 fused ring system through an intramolecular Friedel-Crafts cyclization via vinyl cation intermediates formed from the addition of an aryl cation equivalent to alkynes.3e Using a similar system, the Gaunt and Chen groups reported a cyclopentene synthesis through C−H insertion into a vinyl cation intermediate.6c,6d Other electrophilic species that have been employed in this approach include alkyl cations,3f,g a CF3+ equivalent,3h as well as electrophilic selenonium species.3i
Protonation of alkynes to form vinyl cations constitutes a particularly simple and attractive special case of this second general approach. However, most previous reports have employed very harshly acidic conditions (e.g., H2SO4, TfOH, Tf2NH) to effect the initial protonation of the alkyne.7 To address this limitation, Niggemann and coworkers reported a system involving Brønsted/Lewis cooperative protonation of the alkyne triple bond to achieve alkyne hydroimidation under remarkably mild conditions.8 Our own group recently reported the use of substituted pyridinium salts as proton sources for the formation of vinyl cations from alkyne substrates.9 This tunable class of Brønsted acids provides a conceptually and operationally simple approach for the generation of vinyl cations from readily available alkyne starting materials. In our initial report, our group used these reagents to prepare vinyl fluorides in a regio- and stereoselective manner through alkyne hydrofluorination. Herein, we report the synthesis and use of a related pyridinium reagent for intramolecular hydroarylation under transition-metal-free conditions through a vinyl cation Friedel−Crafts process (Scheme 1D).10 The protocol was applied to the synthesis of benzocyclohexene and benzocycloheptene derivatives, which are scaffolds of potential interest for the synthesis of pharmaceutical candidates and other bioactive compounds.11
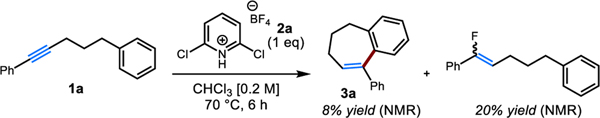
Initially, we observed that attempted hydrofluorination of 1a using our previously reported tetrafluoroborate salt 2a resulted in the formation of a mixture of hydrofluorination and hydroarylation products (Scheme 2). To probe the generality of this process, we investigated the cyclization of electronically deactivated substrate 1i. While the attempted cyclization of 1i with tetrafluoroborate salt 2a gave no desired product (Table 1, entry 1), replacement of the tetrafluoroborate counterion with triflate (2b) led to a significantly higher yield of the benzocycloheptene product (entry 2). A higher reaction temperature (entry 3) and prolonged reaction time (entry 4) led to significantly higher yields of the desired product. As was observed for our recently developed hydrofluorination method and consistent with the formation of a vinyl cation intermediate, chloroform and 1,2-dichloroethane (DCE) proved to be ideal solvents, with markedly lower yields observed when using either less polar solvents such as toluene, or more coordinating solvents such as acetonitrile (entries 5–8).1a,9 Reagents bearing other counterions were also prepared. The bistriflimide (2c, entry 10) and hexafluorophosphate (2d, entry 11) pyridinium salts also gave lower yields, presumably as a consequence of ion pairing effects.
Scheme 2.
Discovery of pyridinium-promoted hydroarylation
Table 1.
Optimization of Reaction Conditions

| |||||
|---|---|---|---|---|---|
| entry | reagent | solvent | T[°C] | time [h] |
yield[%]b |
| 1 | 2a | CHCl3 | 70 | 6 | 0c |
| 2 | 2b | CHCl3 | 70 | 6 | 14 |
| 3 | 2b | CHCl3 | 90 | 6 | 45 |
| 4 | 2b | CHCl3 | 90 | 12 | 70 |
| 5 | 2b | PhCH3 | 90 | 12 | 27 |
| 6 | 2b | PhCF3 | 90 | 12 | 50 |
| 7 | 2b | CH3CN | 90 | 12 | 13 |
| 8 | 2b | DCE | 90 | 12 | 73 (68)d |
| 9 | 2b e | DCE | 90 | 12 | 68 |
| 10 | 2c | DCE | 90 | 12 | 41 |
| 11 | 2d | DCE | 90 | 12 | 14 |
| 12 | 2b f | DCE | 90 | 12 | 39 |
Conditions: 0.1 mmol 1i, 0.1 mmol 2, 0.5 mL solvent (0.2 M).
Yield determined by 1H NMR using 1,1,2,2-tetrachloroethane as the internal standard.
No reaction.
Isolated yield from 0.2 mmol scale reaction.
Using 2b stored in the desiccator for 1 month and DCE stored on the benchtop.
0.02 mmol (20 mol %) 2b used.
Using a catalytic amount (20 mol %) of 2b gave a suboptimal yield of 39% (Table 1, entry 12), indicating that while the reagent is in principle catalytic, stoichiometric quantities are preferable in terms of synthetic utility. With the exception of triflic acid (up to 59% yield), the use of various alternative Brønsted acids in place of 2b generally gave low yields of 3i. In particular, the typical sulfonic acids MsOH and p-TsOH gave very low conversion to the desired product, in spite of the similar pKa values of these acids to the 2,6-dichloropyridinium reagents.12 While triflic acid was a competent Brønsted acid promoter, it required careful optimization of reaction time for significant yields to be obtained, with the highest yield observed at 1 h reaction time and significant decomposition by 3 h. By contrast, 2b was more forgiving with respect to reaction time, causing negligible decomposition even after 36 h, a full day after consumption of the starting material (Table 2). Considering this more robust reactivity profile and the higher yields obtained, along with the long shelf life (Table 1, entry 9), crystallinity, and ease of preparation and handling of the reagent, we selected triflate salt 2b as our Brønsted acid of choice for subsequent explorations of the substrate scope of this transformation.
Table 2.
Product Stability Experiments

| ||||
|---|---|---|---|---|
| entry | reagent | pKa (in H2O) | time [h] | yield [%]b |
| 1 | TfOH | ca. −14 | 1 | 59 |
| 2 | TfOH | ca. −14 | 3 | 20 |
| 3 | 2b | −2.6 | 12 | 73 |
| 4 | 2b | −2.6 | 36 | 72 |
| 5 | p-TsOH | −2.8 | 12 | 6 |
| 6 | MsOH | −2.6 | 12 | 9 |
| 7 | F3CCO2H | +0.3 | 12 | 0 |
Conditions: 0.1 mmol 1i, 0.1 mmol reagent, 0.5 mL DCE (0.2 M), 90 °C.
Yield determined by 1H NMR using 1,1,2,2-tetrachloroethane as the internal standard.
A range of substrates reacted successfully under our optimized conditions, allowing for the formation of an array of benzocycloheptenes (Scheme 3). Products bearing electron donating (e.g., 3f, 3m) and electron withdrawing aryl groups (e.g., 3e, 3i) on the alkyne could be synthesized, as could products bearing electronically neutral to electron withdrawing substituents (e.g., 3z) on the distal aryl ring. Several benzocyclohexene products could also be formed, including a vinyl iodide (3ag) from an aryl-tethered alkynyl iodide. In addition, the relatively mild reaction conditions allowed for the presence of potentially sensitive functional groups, including nitriles (3j, 3q), ketones (3v, 3w), esters (3e, 3i), an α,β-unsaturated ester (3t), and a phthalimide (3m). Several heterocycles, including a furan (3o), an indole (3p), a pyridone (3r), and thiophenes (3u, 3ab) were also compatible with the optimized reaction conditions. In contrast, subjection of thiophene containing substrate 1u to triflic acid (70 °C, 1 h) resulted in formation of large amounts of tarry material and very little (< 5% yield) 3u, again highlighting the unique mildness and functional group tolerance of reagent 2b.
Scheme 3.
Scope of the intramolecular Friedel−Crafts vinylation
aIn chloroform (0.2 M). bIn 1,2-dichloroethane (0.2 M). c6 h at 70 °C. d12 h at 90 °C. e48 h at 100 °C. f0.2 equiv 2c (−NTf2 salt) used in place of 2b 1,2-dichloroethane (0.1M) for 1.5 h at 90 °C gFrom alkynyl iodide. h6 h at 90 °C. iTwo isomeric products isolated from reaction with 1ag (R2=m-Cl) as the substrate.
The protocol was found to be scalable, as demonstrated by the synthesis of 3i on gram scale (64% yield) from 5 mmol of starting material. Several derivatizations of this benzocycloheptene were subsequently performed (Scheme 4). Olefin epoxidation using 3-chloroperoxybenzoic acid under mildly basic conditions gave epoxide 4 in 73% yield.13 Dibromination followed by elimination with potassium hydroxide yielded vinyl bromide 5 in 97% yield.14 Finally, fluoromethoxylation with Selectfluor (F-TEDA-BF4) and methanol gave a 4:1 mixture of diastereomers, from which trans-adduct 6 was isolated in 76% yield.15
Scheme 4.
Gram scale reaction and product derivatization
Previous mechanistic and computational studies of alkyne hydrofluorination by 2a supported the formation of vinyl cations from alkynes and electron-poor pyridinium reagents.9 To provide additional evidence for vinyl cation formation, we considered whether our pyridinium reagent could promote other processes that are characteristic of these intermediates. In light of numerous previous reports of C−H insertion into vinyl cations3b,6c,6d, we prepared substrate 7 containing a remote 3° alkyl group to investigate whether analogous reactivity was possible with putative vinyl cation intermediates generated from pyridinium reagents. Indeed, we found that hexafluorophosphate-based reagent 2d could promote the cyclization of 7 to give cyclopentene derivative 8, albeit in low yield (Scheme 5). The formation of this formal C−H insertion product provides additional evidence that vinyl cations could be generated as intermediates by protonation of an alkyne using only a moderately strong Brønsted acid.1
Scheme 5.
Observation of cyclization through C−H insertion
In summary, we have developed an operationally simple and functional group tolerant synthesis of aryl substituted benzocycloheptenes and benzocyclohexenes from simple alkyne starting materials. Compared to other Brønsted acids of similar strength and handling properties, the hydrotriflate salt of an electron-deficient pyridine was found to be a particularly effective Brønsted acidic promoter. Efforts to develop reagents for the more efficient generation of vinyl cation intermediates and to better understand the underlying structure-activity relationships are ongoing in our research group and will be reported in due course.
Research reported in this publication was supported by the National Institute of General Medical Sciences, National Institutes of Health (R35GM142945), as well as startup funding from the University of Pittsburgh. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. We thank Professor Hosea Nelson (Caltech) for helpful discussions on vinyl cation chemistry and communication of some preliminary results from the Nelson group. We also thank James Paule and Leah Raizen (Pittsburgh) for the preparation of some starting materials and other experimental assistance.
Supplementary Material
Footnotes
Electronic Supplementary Information (ESI) available: [details of any supplementary information available should be included here]. See DOI: 10.1039/x0xx00000x
Footnotes relating to the title and/or authors should appear here.
Conflicts of interest
There are no conflicts of interest to declare.
Notes and references
- 1(a).Niggemann M and Gao S, Angew. Chem. Int. Ed, 2018, 57, 16942; [DOI] [PubMed] [Google Scholar]; (b) Liu X-J, Xu Y, Tang C, Qian P-C and Ye L-W, Sci. Chin. Chem, 2022, 65, 20; [Google Scholar]; (c) Vasilyev AV, Russ. Chem. Rev, 2013, 82, 187; [Google Scholar]; (d) Stang PJ, Vinyl Cations, Academic Press, New York, 1979. [Google Scholar]
- 2.Byrne PA, Kobayashi S, Würthwein E-U, Ammer J and Mayr H, J. Am. Chem. Soc, 2017, 139, 1499. [DOI] [PubMed] [Google Scholar]
- 3.By heterolytic dissociation of a leaving group: [Google Scholar]; (a) Cleary SE, Hensinger MJ and Brewer M, Chem. Sci, 2017, 8, 6810; [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Popov S, Shao B, Bagdasarian AL, Benton TR, Zou L, Yang Z, Houk KN and Nelson HM, Science, 2018, 361, 381; [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Wigman B, Popov S, Bagdasarian AL, Shao B, Benton TR, Williams CG, Fisher SP, Lavallo V, Houk KN and Nelson HM, J. Am. Chem. Soc, 2019, 141, 9140; [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Li Z, Gandon V and Bour C, Chem. Commun, 2020, 56, 6507; by electrophilic addition to an alkyne: [DOI] [PubMed] [Google Scholar]; (e) Walkinshaw AJ, Xu W, Suero MG and Gaunt MJ, J. Am. Chem. Soc, 2013, 135, 12532; [DOI] [PubMed] [Google Scholar]; (f) Biermann U, Koch R and Metzger JO, Angew. Chem. Int. Ed, 2006, 45, 3076; [DOI] [PubMed] [Google Scholar]; (g) Fu L and Niggemann M, Chem. Eur. J, 2015, 21, 6367; [DOI] [PubMed] [Google Scholar]; (h) Ji Y-L, Lin J-H, Xiao J-C and Gu Y-C, Org. Chem. Front, 2014, 1, 1280; [Google Scholar]; (i) An S, Zhang Z, Li P, Eur. J. Org. Chem, 2021, 21, 3059; by electrochemical oxidation: [Google Scholar]; (j) Wigman B, Lee W, Wei W, Houk KN and Nelson HM, Angew. Chem. Int. Ed, 2022, 61, e202113972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4(a).Grob CA and Cseh G, Helv. Chim. Acta, 1964, 47, 194; [Google Scholar]; (b) Grob CA, Csapilla J and Cseh G, Helv. Chim. Acta, 1964, 47, 1590; [Google Scholar]; (c) Stang PJ and Summerville R, J. Am. Chem. Soc, 1969, 91, 4600; [Google Scholar]; (d) Chandy MJ and Hanack M, Tetrahedron Lett., 1975, 16, 4515; [Google Scholar]; (e) Hargrove RJ and Stang PJ, Tetrahedron, 1976, 32, 37; [Google Scholar]; (f) Stang PJ and Anderson AG, J. Am. Chem. Soc, 1978, 100, 1520. [Google Scholar]
- 5(a).Okuyama T, Acc. Chem. Res, 2002, 35, 12; [DOI] [PubMed] [Google Scholar]; (b) Zhdankin VV and Stang PJ, Chem. Rev, 2008, 108, 5299; [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Miyamoto K, Shiro M and Ochiai M, Angew. Chem. Int. Ed, 2009, 48, 8931. [DOI] [PubMed] [Google Scholar]
- 6(a).Hanack M, Angew. Chem. Int. Ed. Engl, 1978, 17, 333; [Google Scholar]; (b) Müller T, Juhasz M and Reed CA, Angew. Chem. Int. Ed, 2004, 43, 1543; [DOI] [PubMed] [Google Scholar]; (c) Zhang F, Das S, Walkinshaw AJ, Casitas A, Taylor M, Suero MG and Gaunt MJ, J. Am. Chem. Soc, 2014, 136, 8851; [DOI] [PubMed] [Google Scholar]; (d) Peng J, Chen C, Chen J, Su X, Xi C and Chen H, Org. Lett, 2014, 16, 3776; [DOI] [PubMed] [Google Scholar]; (e) Pérez-Saavedra B, Vázquez-Galiñanes N, Saá C and Fañanás-Mastral M, ACS Catal., 2017, 7, 6104; [Google Scholar]; (f) Wen L-R, Shen Q-Y, Guo W-S and Li M, Org. Chem. Front, 2016, 3, 870; [Google Scholar]; (g) Wang G, Chen C and Peng J, Chem. Commun, 2016, 52, 10277; [DOI] [PubMed] [Google Scholar]; (h) Rahman MA, Ogawa O, Oyamada J, Kitamura T, Synthesis, 2008, 23, 3755; [Google Scholar]; (i) Zalivatskaya AS, Golovanov AA, Boyarskaya IA, Kruykova MA, Khoroshilova OV, Vasilyev AV, Eur. J. Org. Chem. 2021, 2021, 2634. [Google Scholar]
- 7(a).Jacobs TL and Searles SJ Jr, J. Am. Chem. Soc, 1944, 66, 686; [Google Scholar]; (b) Noyce DS, Matesich MA, Melvyn O, Schiavelli D and Peterson PE, J. Am. Chem. Soc, 1965, 87, 2295; [Google Scholar]; (c) Zhang L and Kozmin SA, J. Am. Chem. Soc, 2004, 126, 10204; [DOI] [PubMed] [Google Scholar]; (d) Yu P. Savechenkov, Rudenko AP, Vasilyev AV and Fukin GK, Russ. J. Org. Chem, 2005, 41, 1316; [Google Scholar]; (e) Alkhafaji HMH, Ryabukhin DS, Muzalevskiy VM, Vasilyev AV, Fukin GK, Shastin AVand Nenajdenko VG, Eur. J. Org. Chem, 2013, 2013, 1132; [Google Scholar]; (f) Kaiser D, Veiros LF and Maulide N, Chem. Eur. J, 2016, 22, 4727; [DOI] [PubMed] [Google Scholar]; (g) Shibuya M, Fujita S, Abe M and Yamamoto Y, ACS Catal., 2017, 7, 2848; [Google Scholar]; (h) Zhang J, Li S, Qiao Y, Peng C, Wang X-N and Chang J, Chem. Commun, 2018, 54, 12455; [DOI] [PubMed] [Google Scholar]; (i) Takahashi I, Fujita T, Shoji N and Ichikawa J, Chem. Commun, 2019, 55, 9267; [DOI] [PubMed] [Google Scholar]; (j) Zhang C, Lv S, Wang Y, Zhang J, Wang X-N and Chang J, Org. Chem. Front, 2022, 9, 1300–1307; [Google Scholar]; (k) Zhang W, Sun J, Xiang L, Si W, Song R, Yang D and Lv J, Org. Lett, 2021, 23, 5998; [DOI] [PubMed] [Google Scholar]; (l) Xiang Y, Li Z, Wang LN and Yu Z-X, J. Org. Chem, 2018, 83, 7633. [DOI] [PubMed] [Google Scholar]
- 8(a).Schroeder S, Strauch C, Gaelings N and Niggemann M, Angew. Chem. Int. Ed, 2019, 58, 5119; [DOI] [PubMed] [Google Scholar]; (b) Chuchmareva M, Strauch C, Schröder S, Collong A, Niggemann M, Tetrahedron. Lett, 2021, 74, 153173. [Google Scholar]
- 9.Guo R, Qi X, Xiang H, Geaneotes P, Wang R, Liu P and Wang YM, Angew. Chem. Int. Ed, 2020, 59, 16651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reviews on alkyne hydroarylation: [Google Scholar]; (a) Nevado C and Echavarren AM, Synthesis 2005, 167; [Google Scholar]; (b) Kitamura T, Eur. J. Org. Chem. 2009, 2009, 1111; [Google Scholar]; (c) Wang X, Zhou L, and Lu W, Curr. Org. Chem. 2010, 14, 289; [Google Scholar]; (d) Yamamoto Y, Chem. Soc. Rev. 2014, 43, 1575. [DOI] [PubMed] [Google Scholar]
- 11(a).McCague R, Kuroda R, Leclercq G and Stoessel S, J. Med. Chem, 1986, 29, 2053; [DOI] [PubMed] [Google Scholar]; (b) Hattori K, Nagano M, Kato T, Nakanishi I, Imai K, Kinoshita T and Sakane K, Bioorg. Med. Chem. Lett, 1995, 5, 2821; [Google Scholar]; (c) Tandon VK, Singh KA, Awasthi AK, Khanna JM, Lal B and Anand N, Bioorg. Med. Chem. Lett, 2004, 14, 2867. [DOI] [PubMed] [Google Scholar]
- 12.Cook MJ, Dassanyake NL, Johnson CD, Katritzky AR and Toon TW, J. Chem. Soc., Perkin Trans. 2, 1974, 1069. [Google Scholar]
- 13.Liu W, Leischner T, Li W, Junge K and Beller M, Angew. Chem. Int. Ed, 2020, 59, 11321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alamillo-Ferrer C, Curle JM, Davidson SC, Lucas SCC, Atkinson SJ, Campbell M, Kennedy AR and Tomkinson NCO, J. Org. Chem, 2018, 83, 6728. [DOI] [PubMed] [Google Scholar]
- 15.Stavber S, Sotler-Pecan T and Zupan M, Bull. Chem. Soc. Jpn, 1996, 69, 169. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.