Abstract
A shared mechanism across species heralds the arrival of self-generated sensations, helping the brain to anticipate, and therefore distinguish, self- from externally-generated sensations. In mammals, this sensory prediction mechanism is supported by communication within a cortico-ponto-cerebellar-thalamo-cortical loop. Schizophrenia is associated with impaired sensory prediction, as well as abnormal structural and functional connections between nodes in this circuit. Despite the pons’ principal role in relaying and processing sensory information passed from the cortex to cerebellum, few studies have examined pons connectivity in schizophrenia. Here, we first briefly describe how the pons contributes to sensory prediction. We then summarize schizophrenia-related abnormalities in the cortico-ponto-cerebellar-thalamo-cortical loop, emphasizing the dearth of research on the pons relative to thalamic and cerebellar connections. We conclude with recommendations for advancing our understanding of how the pons relates to sensory prediction failures in schizophrenia.
Keywords: efference copy/corollary discharge, forward model, predictive coding, psychosis, cerebellum, thalamus
The pons, a bridge to understanding sensory prediction deficits in schizophrenia
The pons (from Latin, “bridge”) is the principal route through which information from higher cortical areas is transmitted to the cerebellum [1]. This cortico-ponto-cerebellar pathway is thought to be fundamental to sensory prediction processes that occur rapidly and outside of conscious awareness, helping the brain to anticipate and distinguish incoming sensory information as self- versus externally-generated [2]. People with schizophrenia have notable sensory prediction deficits that may relate to core schizophrenia symptoms [3,4]. Yet, few studies have directly examined the cortico-ponto-cerebellar pathway in humans (e.g., [5,6]), and the relationship between this pathway and sensory prediction abnormalities in schizophrenia remains largely unknown.
In the current opinion piece, we argue that studying the pons is a logical and necessary next step for characterizing sensory prediction deficits in schizophrenia. We first review the mechanisms associated with sensory prediction abnormalities observed in schizophrenia. Next, we provide a brief overview of the models commonly used to conceptualize sensory prediction and their hypothesized neurobiological pathways. We then summarize evidence for deficiencies in these neurobiological pathways in schizophrenia, highlighting critical research gaps. We close with recommendations for using multimodal neuroimaging and other neuroscientific approaches to study the contributions of the pons to sensory prediction abnormalities in schizophrenia.
Searching for mechanisms of aberrant sensory prediction in schizophrenia
Self-produced sensations are experienced differently from those produced by external sources [7,8]. Our brains have mechanisms that predict outcomes based on our own actions so that self-generated experiences are seen as non-alarming [3]. Importantly, these predictions compensate for delays and noise intrinsic to our sensory systems, enabling more efficient motor control [9,10]. Decades of research support the theory that sensory prediction stems from an efference copy/corollary discharge system (see Glossary), in which predicted sensations are passed through a cortico-ponto-cerebellar-thalamo-cortical loop that first prepares the brain for what is to come and subsequently compares the predicted and actual sensory feedback [11,12]. This efference copy/corollary discharge mechanism, which is preserved across species and multiple sensory systems [13], may help to reduce cognitive load by minimizing the processing of self-generated feedback [14] and is vital to normal perception and cognitive functioning [3].
Schizophrenia is associated with sensory prediction impairments [4], which may underlie misperceptions of self-generated sensations as originating from external sources instead of the self [15]. Such misperceptions can arise from a failure to suppress or attenuate brain activity in response to sensations from self-generated movements [16–18]. The misattribution of self-generated actions as being externally-generated is posited to contribute to the development and maintenance of psychotic symptoms [19]. For instance, when predicting whether tactile sensations corresponded with self-generated action, people with schizophrenia did not exhibit the same degree of attenuated activation in the secondary somatosensory cortex as seen in comparison participants [17]. Poor attenuation in secondary somatosensory cortex also correlated with more severe hallucinations.
It is possible that attenuated suppression of self-generated sensations results, at least in part, from breakdowns early in the sensory processing stream, i.e., at the level of efference copy/corollary discharge generation. For instance, in healthy individuals, greater synchrony of electroencephalography (EEG) auditory signals before speaking (i.e., pre-speech phase synchrony) correlated with better suppression of auditory responses following speech onset relative to playback of one’s recorded voice [20,21]. People with schizophrenia exhibited less pre-speech synchrony, particularly those with severe auditory hallucinations, and pre-speech synchrony was unrelated to auditory cortical suppression during vocalizing [20]. This suggests a deficiency in the mechanism that prepares the brain to expect self-generated sensations from speaking, i.e., the efference copy/corollary discharge. Similar pre-speech effects were seen in volunteers under delta-9 tetrahydrocannabinol (THC), particularly those who reported greater schizophrenia-like THC-induced symptoms [22].
The ability to correctly attribute self-generated sensory experiences is closely related to sense of agency [23]. Certain schizophrenia symptoms correspond with failures to identify oneself as the agent of one’s experience (e.g., delusions of control) while others reflect a heightened sense of agency (e.g., delusions of grandeur) [24–26]. It is theorized that deficits in sensory attribution and agency emerge from a faulty efference copy sent from the cortex to cerebellum, a transmission that depends on the pons [11,19].
Sensory prediction depends on internal models that are flexibly updated
Classical internal forward models (also called internal prediction models) posit that every action is accompanied by transmission of a motor plan (i.e., efference copy) to a forward model, which is then used to form predictions of the sensory consequences of that action (i.e., corollary discharge) [27]. Mismatches between these predictions and actual sensations produce a prediction error. When an event is caused by self-generated actions, and is therefore aligned with the internal forward model, the resulting feedback will match the prediction and yield a prediction error of zero that in turn leads to a dampening or canceling of sensation [27]; that is, the sensation is experienced as internally generated [4]. Conversely, mismatches between predictions and feedback lead to non-zero prediction errors, after which there is less (or no) dampening of sensation, and the prediction error then updates the internal forward model. In the context of schizophrenia, reduced neural attenuation following self-generated sensory feedback could reflect a dysfunction in predicting the sensory consequences of one’s own actions [4].
Sensory prediction has also been framed as a hierarchical process in which neuronal activity encodes top-down expectations of sensory experience that are sent to lower-level brain areas, and any resulting discrepancies are used to update internal models (allowing new predictions to form) [28]. In a repeating loop, bottom-up signals transmit the prediction error to higher levels within a cortical hierarchy; the higher levels respond by adjusting expectations to yield more optimal top-down predictions [29]. This processing loop, known as predictive coding, centers on the idea that the brain generates inferential models of the world to efficiently make sense of immense incoming sensory information [28]. The classic formulation of predictive coding was as a theory of cerebral cortex function [30], although animal and human studies from the last decade or so show robust evidence of prediction error responses in subcortical auditory pathways, as well as the cerebellum (e.g., [31–33]).
Using a Bayesian modeling approach, this theory can be formalized into a single framework known as Bayesian predictive coding [34]: probabilistic beliefs or predictions (priors) are integrated with observed sensory data (likelihood), prediction errors are calculated, and the resulting posterior probability (posterior) reflects the percept that is most likely, given the prior and likelihood. The prior has a certain precision that reflects its reliability. When priors are more precise, and therefore more reliable, than the incoming sensory information to which they are compared, the resulting prediction errors will receive little weight in relation to the priors, or even be ignored. Alternatively, if prediction errors are more precise, they will be favored over priors and lead to belief updating and drive new learning.
The pons plays a key role in integrating and relaying sensory information between the cortex and cerebellum
Figure 1 illustrates the hypothesized neuroanatomical and neurofunctional connections that support sensory processing of self-generated events, elaborating on elegant models of others [11,12,35]. First, motor information originating in prefrontal, motor, and pre-motor cortical areas is transmitted to the pontine nuclei via corticopontine fibers [35]. The pontine nuclei, also called the basilar pontine nuclei, pontine gray nuclei, and reticulotegmental nuclei, are the largest precerebellar nuclei [1] and the main hub through which descending signals from cortical areas are input to the cerebellum [36]. The efference copy, which is generated from the cortex, is believed to carry a corollary discharge of the expected sensation from cortex through the pontine nuclei into the middle cerebellar peduncles, contralaterally, via mossy pontocerebellar fibers [1,37]. Recent evidence has shown that the pons does not simply relay information to the cerebellum [38]. Rather, pontine nuclei have intrinsic properties that integrate and process information from separate channels, filter synaptic input, and bind information in a manner suitable for transmission to the cerebellum [39,40]. These processes are crucial both in the context of sensory processing, as discussed earlier, and in generating complex and smooth movements (see Box 1).
Figure 1.
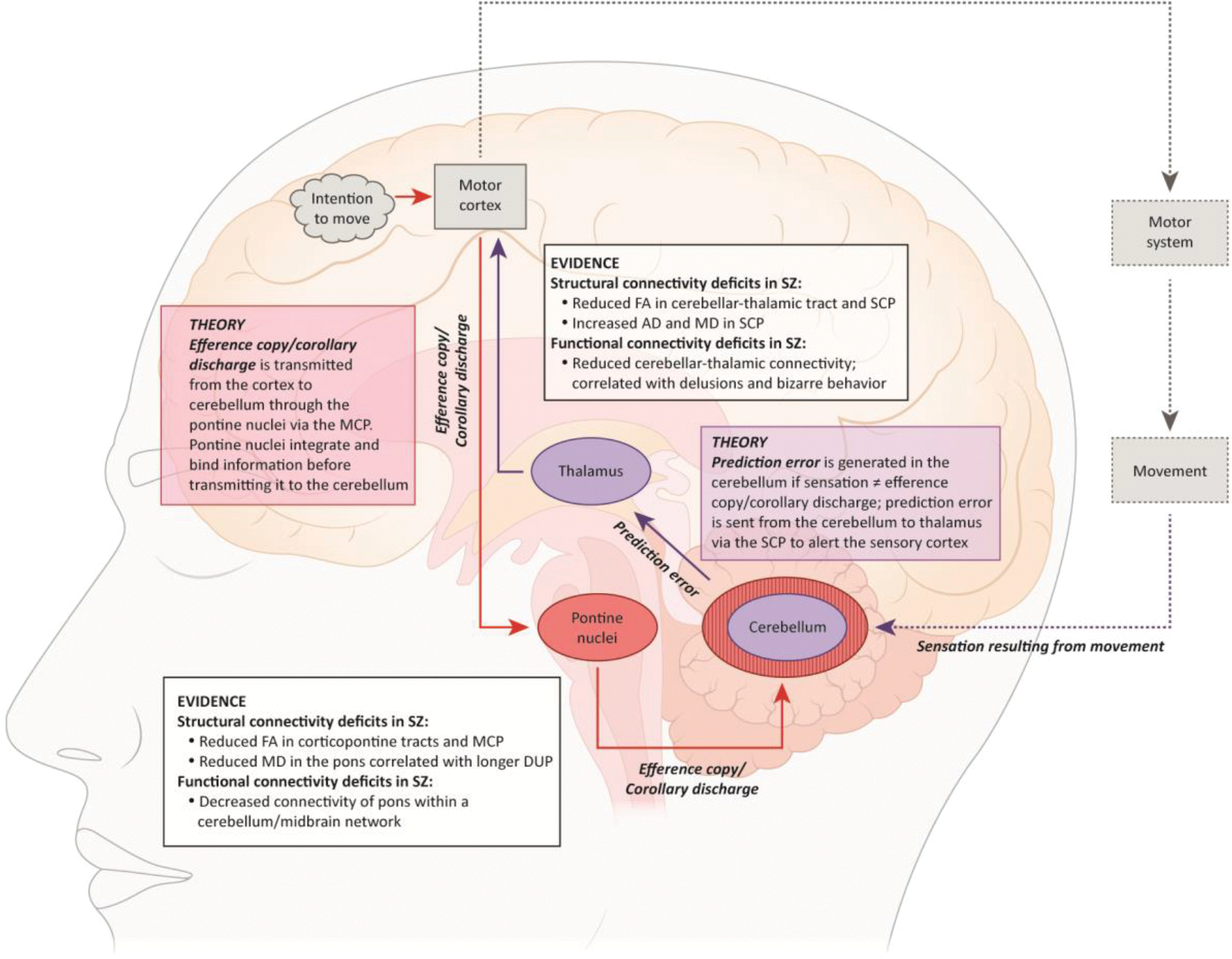
Neuroanatomical and neurofunctional model of sensory processing of self-generated events and respective alterations in schizophrenia. The circuit starts with an intention to act (or move) which initiates transmission of the motor command (i.e., plan). The expected motor plan and sensory consequences are transmitted from the cortex to the cerebellum via the pontine nuclei. The cerebellum compares the expected sensory consequences with the actual auditory, visual, and/or proprioceptive sensory feedback generated by the movement. Any discrepancies yielded between the predicted and actual sensory feedback (i.e., prediction errors) are forwarded to the thalamus and then on to several additional cortical targets (transmission strictly to the motor cortex in this figure is for illustrative purposes). Existing lines of evidence for impaired connections in schizophrenia in the afferent (red arrows from cortex to cerebellum via the pontine nuclei) and efferent (purple arrows from cerebellum to the thalamus) pathways are summarized in the “Evidence” boxes, respectively.
Abbreviations: AD, axial diffusivity; DUP, duration of untreated psychosis; FA, fractional anisotropy; MCP, middle cerebellar peduncles; MD, mean diffusivity; SCP, superior cerebellar peduncles; SZ, schizophrenia.
Box 1. The pons supports dexterous and smooth movement.
The cortico-ponto-cerebeflar pathway has been hypothesized as crucial in the evolution of complex motor functions [1], including the use of sensory feedback during movements, that is, the efference copy/corollary discharge, to control complex motor actions [41,42]. Pontine nuclei contain distinct neurons that are activated when preparing or executing movement [43]. To facilitate successful sensory-guided movement, pontine nuclei help to integrate and bind incoming sensory information in a manner appropriate for the cerebellum [44], Pontine nuclei receive extensive cortical input and are the primary input region to the cerebellum [45]; essentially, these nuclei are a bottleneck for information passed from the cortex to the cerebellum. As such, perturbing the pons can reveal what information is shared, or alternatively lost, between the cortex and cerebellum.
In mice, optogenetic inhibition of the pons disrupted the shared neural activity patterns seen in premotor (layer 5) pyramidal cells and cerebellar granule cells during a forelimb movement task, which likely reflects diminished pontine input into the cerebellum [46]. In a separate study, optogenetic pontine silencing reduced precision, accuracy, and success rate during a cued reaching task, but did not block the initiation of movement [38], That is, disrupting cortico-cerebellar communication undermined skill and dexterity while leaving gross motor functioning intact. In comparison, optogenetic silencing of the motor cortex has been found to halt the initiation and execution of reaching [47,48].
In foveated species, smooth-pursuit eye movements, which help stabilize the retinal image of a moving stimulus on the fovea. similarly depend on the cortico-ponto-cerebellar pathway [40], Robust smooth-pursuit deficits are present in schizophrenia [49], and may relate to an underlying deficiency in the efference copy/corollary discharge mechanism [9], Dorsal pontine nuclei integrate and deliver visual and non-visual eye motion signals to the cerebellum, that is, the efference copy/ corollary discharge [50]. A causal role for the dorsal pontine nuclei in smooth pursuit eye movements has been established from lesion studies [40], and could explain the smooth pursuit deficits seen in humans with pontine lesions [51]. Taken together, the pons may have a particular role in ‘fine-tuning’ motor or sensory information for the cerebellum to facilitate dexterous or smooth movement.
The pons supports dexterous and smooth movement
The cortico-ponto-cerebellar pathway has been hypothesized as crucial in the evolution of complex motor functions [1], including the use of sensory feedback during movements, i.e., the efference copy/corollary discharge, to control complex motor actions [41,42]. Pontine nuclei contain distinct neurons that are activated when preparing or executing movement [43]. To facilitate successful sensory-guided movement, pontine nuclei help to integrate and bind incoming sensory information in a manner appropriate for the cerebellum [44]. Pontine nuclei receive extensive cortical input and are the primary input region to the cerebellum [45]; essentially, these nuclei are a bottleneck for information passed from the cortex to the cerebellum. As such, perturbing the pons can reveal what information is shared, or alternatively lost, between the cortex and cerebellum.
In mice, optogenetic inhibition of the pons disrupted the shared neural activity patterns seen in premotor (layer 5) pyramidal cells and cerebellar granule cells during a forelimb movement task, which likely reflects diminished pontine input into the cerebellum [46]. In a separate study, optogenetic pontine silencing reduced precision, accuracy, and success rate during a cued reaching task, but did not block the initiation of movement [38]. That is, disrupting cortico-cerebellar communication undermined skill and dexterity, while leaving gross motor functioning intact. In comparison, optogenetic silencing of the motor cortex was found to halt the initiation and execution of reaching [47,48].
In foveated species, smooth-pursuit eye movements, which help stabilize the retinal image of a moving stimulus on the fovea, similarly depend on the cortico-ponto-cerebellar pathway [40]. Robust smooth-pursuit deficits are present in schizophrenia [49], and may relate to an underlying deficiency in the efference copy/corollary discharge mechanism [49]. Dorsal pontine nuclei integrate and deliver visual and non-visual eye motion signals to the cerebellum, i.e., the efference copy/corollary discharge [50]. A causal role for the dorsal pontine nuclei in smooth pursuit eye movements has been established from lesion studies [40], and could explain the smooth pursuit deficits seen in humans with pontine lesions [51]. Taken together, the pons may have a particular role in “fine-tuning” motor or sensory information for the cerebellum to facilitate dexterous or smooth movement.
In a parallel loop via afferent connections, the inferior cerebellar peduncles receive and process sensory information from the spinal cord and peripheral system (including muscles and joint positions) [35]. The cerebellum is believed to integrate and compare the efference copy with the actual incoming sensory information [12]. Accordingly, the cerebellum, which is sometimes termed the brain’s “comparator,” is responsible for detecting a mismatch between the predicted sensation associated with the motor command and actual sensory feedback, with mismatches yielding a prediction error [27]. It is further suggested that prediction errors are then fed back to the cerebral cortex, via the contralateral thalamus [12], through efferent connections in the superior cerebellar peduncle [52].
It is posited that the thalamus then projects this prediction error output to multiple cortical targets [12] based on known thalamic anatomical connections [53] with prefrontal [54], parietal [55], motor [55], somatosensory, and auditory cortices [56]. Respectively, these regions are involved in cognitive control [54], sense of agency [57], motor control [57], and sensory attenuation [58]. Relationships between aberrant thalamo-cortical connections and the efference copy/corollary discharge mechanism are summarized in Box 2.
Box 2. The role of the thalamus in the efference copy/corollary discharge mechanism.
Empirical studies of eye movements demonstrate how efference copy/corollary discharge mechanisms transmit via thalamic pathways [59]. In nonhuman primates, oculomotor efference copies heralding saccadic eye movements are sent along a pathway from the midbrain (specifically, the superior colliculus) to frontal eye fields via the mediodorsal thalamus [60]. These observations provide a framework for studying efference copy/corollary discharge abnormalities in humans. Preliminary findings from humans have also implicated the mediodorsal nucleus in the efference copy mechanism. More specifically, relative to control participants, a patient with a lesion to the mediodorsal nucleus showed deficits in motor and visual updating that could reflect insufficient efference copy transfer [61].
Abnormalities in the mediodorsal-frontal eye field pathway are evident in people with schizophrenia. One study used diffusion tensor imaging (DTI) to test whether structural connectivity in the mediodorsal-frontal eye fields pathway correlated with oculomotor dysfunction of the efference copy/corollary discharge mechanism in a small sample of individuals with schizophrenia who completed a trans-saccadic perceptual task [62], Individuals with schizophrenia showed reduced microstructural integrity of the aforementioned pathway; critically, reduced integrity correlated with compromised oculomotor efference copy/corollary signals and with greater positive symptoms.
Flow between the thalamus and cortex has also been linked with efference copy/corollary discharge mechanisms during auditory processing. In mice, auditory (layer 6) corticothalamic neurons can be activated by motor-related input prior to anticipated sounds during active sensing [63]. This neuronal relationship may relate to sensory attenuation of self-generated sounds in humans. Humans without history of a psychiatric condition completed a task in which tones were actively elicited via button press or passively presented [64], Using magnetoencephalography and dynamic causal modeling, the authors found that auditory sensory attenuation (i.e., the difference between the active and passive conditions) was associated with bi-directional information transfer between the thalamus, inferior parietal lobule, and auditory cortex (i.e., Heschl’s gyrus).
The role of the thalamus in the efference copy/corollary discharge mechanism
Empirical studies of eye movements demonstrate how efference copy/corollary discharge mechanisms transmit via thalamic pathways [59]. In nonhuman primates, oculomotor efference copies heralding saccadic eye movements are sent along a pathway from the midbrain (specifically, the superior colliculus) to frontal eye fields via the mediodorsal thalamus [60]. These observations provide a framework for studying efference copy/corollary discharge abnormalities in humans. Preliminary findings from humans have also implicated the mediodorsal nucleus in the efference copy mechanism. More specifically, relative to control participants, a patient with a lesion to the mediodorsal nucleus showed deficits in motor and visual updating that could reflect insufficient efference copy transfer [61].
Abnormalities in the mediodorsal-frontal eye field pathway are evident in people with schizophrenia. One study used diffusion tensor imaging (DTI) to test whether structural connectivity in the mediodorsal-frontal eye fields pathway correlated with oculomotor dysfunction of the efference copy/corollary discharge mechanism in a small sample of individuals with schizophrenia who completed a trans-saccadic perceptual task [62]. Individuals with schizophrenia showed reduced microstructural integrity of the aforementioned pathway; critically, reduced integrity correlated with compromised oculomotor efference copy/corollary signals and with greater positive symptoms.
Flow between the thalamus and cortex has also been linked with efference copy/corollary discharge mechanisms during auditory processing. In mice, auditory (layer 6) corticothalamic neurons can be activated by motor-related input prior to anticipated sounds during active sensing [63]. This neuronal relationship may relate to sensory attenuation of self-generated sounds in humans. Humans without history of a psychiatric condition completed a task in which tones were actively elicited via button press or passively presented [64]. Using magnetoencephalography and dynamic causal modeling, the authors found that auditory sensory attenuation (i.e., the difference between the active and passive conditions) was associated with bi-directional information transfer between the thalamus, inferior parietal lobule, and auditory cortex (i.e., Heschl’s gyrus).
Robust abnormalities in the efferent sensory prediction pathways in schizophrenia
In recent years, there has been growing attention to connectivity between the cortex, cerebellum, and thalamus, and how breakdowns in those network connections contribute to schizophrenia pathophysiology and symptoms [65–67]. Resting-state functional magnetic resonance imaging (rsfMRI) studies reveal cerebellar-thalamic hypo-connectivity in schizophrenia that is observed when seeding from either the thalamus (e.g., [68–70]) or the cerebellum [71,72]; but see [73]. Reduced cerebellar-thalamic functional connectivity is linked to greater delusions and bizarre behavior [69]. There is robust evidence for aberrant connectivity between the thalamus and its cortical output targets including hyper-connectivity with sensory and association cortices, in addition to hypo-connectivity with the prefrontal cortex (see [65,67] for review and meta-analysis). These aberrant thalamic connectivity patterns are linked to an array of cognitive and psychiatric symptoms [67], including delusions and hallucinations [69].
People with schizophrenia show deficits in corresponding white matter fiber tracts that form the basis of communication between the cortex, cerebellum, and thalamus. More specifically, anatomical connectivity studies using diffusion-weighted imaging (DWI) and diffusion tensor imaging (DTI) found reduced connectivity between the thalamus and prefrontal cortex ([65,74,75], and excessive connectivity between the thalamus and sensory, motor, and parietal cortices [74,76,77]. There is some evidence for impaired fiber tract integrity of the superior cerebellar peduncles in schizophrenia as indicated by reduced fractional anisotropy (FA) [78,79], as well as increased axial diffusivity (AD) and medial diffusivity (MD), and a trend towards increased radial diffusivity [80]. Others found no overall group differences in white matter integrity of this tract [81–83]; though one of these studies observed left superior cerebellar peduncle abnormalities in a small subset of schizophrenia participants who exhibit difficulty sequencing complex motor acts [83]. Mixed findings regarding the superior cerebellar peduncle should be interpreted cautiously as most studies had relatively small schizophrenia sample sizes (N ≤ 40).
Are the afferent prediction pathways through the pons also impaired in schizophrenia?
To our knowledge, only one study directly addressed functional connectivity of the pons in schizophrenia. This study reported decreased pons connectivity within a cerebellum/midbrain network among individuals with schizophrenia relative to unaffected comparison participants [84].
A recent meta-analysis of DTI studies found white matter reductions in the bilateral cortico-ponto-cerebellum tract among individuals with schizophrenia [85]. More specifically, adolescent- [86,87] and adult-onset schizophrenia [87,88] are both associated with reduced fractional anisotropy (FA) in corticopontine tracts. People with schizophrenia also exhibit lower FA in the middle cerebellar peduncle [87,89,90] (with some exceptions [80]), which is the major white matter fiber tract connecting the pons and cerebellum. Further, reduced MD in the pons was found to correlate with a longer duration of untreated psychosis [91]. Additional high-powered studies are needed to verify these tract-based results. With respect to brain morphometry, a recent study of over 27,000 participants found that people with schizophrenia had smaller pons volumes compared to unaffected comparison participants [92], though this difference was characterized by a small effect size. Conversely, a mega-analysis reported larger gray matter concentration in the pons among people with schizophrenia [93]. Contradictory results may be due to confounds associated with scanning the brainstem, such as noise, physiological artifacts [94], or overlap with neighboring brain regions that are responsible for dopamine production and could therefore be enlarged due to illness factors [93].
Impaired structural integrity in cortico-ponto-cerebellar tracts, as described above, elevates the possibility of functional deficiencies between nodes along those pathways. This follows from direct evidence that functional connectivity relationships depend on intact anatomical pathways; e.g., functional connectivity between the motor cortex and cerebellum was reduced in a small group of first-episode stroke patients with focal pontine lesions [95]. This finding is consistent with findings in mice in which optogenetic inhibition of the pons impeded cortico-cerebellar communication [46].
Possible implications of pontine abnormalities to the efference copy/corollary discharge mechanism in schizophrenia
One hypothesis is that pons deficits could interfere with processing self-generated stimuli during initial sensory prediction stages. As noted previously, schizophrenia is associated with deficits in early sensory processing [96], such as reduced synchrony of neuro-oscillations involved during speech preparation [20,21]. The pons is a vital site in early auditory pathways, projecting to the granule cell domain of the cochlear nuclei, which are the first step in the auditory pathway [97]. Auditory perceptual disturbances were documented in case reports of individuals with pontine lesions [98,99]. Acute psychotic symptoms, including paranoid hallucinations, were also seen in a case report of central pontine myelinolysis, i.e., demyelination of the central pons [100].
Abnormal pons functioning could also undermine communication between the cortex and cerebellum, thereby disrupting the fluidity and precision of movement. This dysconnectivity may help explain the fine motor and coordination abnormalities observed in schizophrenia even prior to illness onset [101]. Preliminary support comes from a small sample of first-episode psychosis participants for whom reduced pons volume correlated with greater neurological soft signs (i.e., subtle neurological abnormalities comprising deficits in sensory integration, motor coordination, and sequencing of complex motor acts) [102]. Relative to controls, people with schizophrenia also showed decreased connectivity between the cerebellum and primary motor cortex during self-paced movements [103], fostering questions as to whether a pontine abnormality is an underlying mechanism.
Bridging the gap
As described above, there is general agreement that the pons is central to the efference copy/corollary discharge system. Despite its principal role in relaying and integrating information between the cortex and cerebellum, the pons has been largely overlooked in human neuroscience, probably due to difficulties in scanning the brainstem using neuroimaging techniques such as fMRI. The brainstem is notoriously difficult to study due to its small size, the complexity of its components, its closeness to major arteries and ventricles, geometric distortions that make brainstem white matter tracts appear to be spuriously intertwined, and its susceptibility to physiological “noise” (e.g., cardiorespiratory signals) and imaging artifacts [94]. Poor contrast between gray and white matter is also particularly problematic for the pons, compared to the cerebellum, for instance, which shows much clearer separation [94]. As a related note, it should be mentioned that the cerebellum’s role in executive function and cognition has been traditionally under-appreciated as well, although attention to these cerebellar functions has been growing [104].
Recent advances in magnetic resonance imaging (MRI) technology, including improved signal-to-noise ratios from increased magnetic field strengths and scanning sequences that lower signal drop out in susceptible regions, now allow for more feasible and accurate imaging of pontine connections [94,105]. In one study, for instance, the authors were able to visibly delineate the ponto-cerebellar tract using DTI, with better visibility using 7 than 3 Tesla (T) data [106]. Another study reconstructed the cortico-ponto-cerebellar and cerebellar-thalamo-cortical structural pathways using advanced tractography approaches [6]. These methods could be applied to evaluate the integrity of these pontine connections in psychiatric disorders in which the pons and cerebellum are involved. Advances in MRI technology have also impacted the ability to capture the pons’ functional dynamics. In a study using 7T data from the Human Connectome Project [5], the authors mapped the pons’ topographical organization, identifying clusters within the pons that are temporally related to specific parcellations of the cerebral cortex. These technological improvements make it increasingly feasible to isolate and probe the feedforward connections in cortico-pontine and ponto-cerebellar pathways, thus opening the door for new areas of inquiry.
Concluding remarks
We conclude with strategies for harnessing multiple neuroimaging and neuromodulation methodologies to accelerate the field’s understanding of how the cortico-ponto-cerebellar pathway supports sensory prediction, and whether deficiencies in that pathway lead to schizophrenia symptoms (see Outstanding Questions).
Outstanding Questions Box.
Do people with schizophrenia show deficient functional connectivity between the cortex and pons and/or the pons and cerebellum? If so, do functional connectivity abnormalities correlate with deficits in the underlying structure of the corticopontine and pontocerebellar tracts? Further, does aberrant connectivity of these pontine pathways correlate with neuroimaging or behavioral assays of poor sensory prediction?
What is the relative contribution of MRI-derived metrics of cortico-ponto-cerebellar-thalamo-cortical connectivity to EEG assays of sensory prediction in characterizing clinical features of schizophrenia? Is there something unique about deficiencies in the afferent versus efferent sensory prediction pathways; in other words, do individuals who exhibit abnormal cortico-ponto-cerebellar connectivity differ in symptoms and disorder presentation from those with deficient cerebellar-thalamus-cortical connectivity?
Does acute or long-term NMDAR hypofunction induced by NMDAR antagonists, like ketamine, lead to connectivity abnormalities in the cortico-ponto-cerebellar-thalamo-cortical loop akin to connectivity abnormalities seen in schizophrenia? Could NMDAR hypofunction represent a shared mechanism for deficient connectivity in this circuit and sensory prediction abnormalities observed behaviorally or via brain imaging modalities (like EEG)?
Can non-invasive brain stimulation methods applied to the cerebellum or motor cortex be used to modulate the sensory prediction pathways that connect these regions, e.g., the cortico-ponto-cerebellar pathway? And in turn, can these methods restore deficient connectivity in cerebellar-thalamic or ponto-cerebellar pathways in schizophrenia? Does connectivity restoration equate to improvements in sensory prediction or other clinical symptoms?
One avenue is to capitalize on the excellent temporal resolution of EEG, a relative limitation of fMRI. EEG is a particularly effective complement to MRI in this context, given that fluid coordination of thought and action likely depends on rapid inter-regional processing in the cortico-ponto-cerebellar-thalamo-cortical loop [11]. Critical discoveries from EEG studies have illuminated neural signals associated with auditory sensory attenuation deficits to self-generated sounds in schizophrenia [16,18]. Combining EEG with functional connectivity would capitalize on both the respective temporal and spatial precision of these methods to reveal a more cohesive picture of this sensory prediction loop. One future direction is to test whether abnormal connectivity within the cortico-ponto-cerebellar pathway correlates with poor sensory attenuation or pre-speech synchrony indexed by EEG. Additionally, one could examine if such neural delineations are related to predictive coding, more broadly, or specific to active contexts related to self-generated actions. For example, is the cortico-ponto-cerebellar pathway needed for predictive coding during pre-attentive detection of deviant auditory stimuli (i.e., mismatch negativity) [107]. Whether cerebellar-thalamo-cortical connectivity has similar relationships with EEG measures of efference copy/corollary discharge, relative to cortico-ponto-cerebellar connectivity, would further speak to specificity of the efferent and afferent pathways.
Another promising route forward is non-invasive brain stimulation, including transcranial magnetic stimulation (TMS), as both a probe of dysfunctional cortico-ponto-cerebellar connectivity and a therapeutic intervention. When applied to the cerebellum, repetitive TMS enhanced cerebellar-prefrontal functional connectivity in people with schizophrenia [108,109]. Similarly, in a group of individuals with essential tremor, this approach was reported to restore deficient cerebellar-thalamo-cortical functional connectivity [110], although the participant group in this study was relatively small and efficacy under more stringent settings remains to be tested. Repetitive TMS to motor cortex in a small group of stroke patients also increased FA in the middle cerebellar peduncle and the contralesional superior cerebellar peduncle [111]. In this case, we speculate that the impact of motor cortex stimulation on cerebellar connections is related to the activation of mossy fibers emanating from the pons along the cortico-ponto-cerebellar pathway [112]. These lines of research raise interesting questions as to whether stimulation along the cortico-ponto-cerebellar pathway can mimic network perturbations seen in schizophrenia, and conversely, whether other stimulation patterns can restore deficient cortico-ponto-cerebellar connections to facilitate effective sensory prediction.
Beyond basic fMRI connectivity approaches, psychophysiological interactions (PPI) methods can help clarify how temporal relationships between brain regions relate to specific behavioral conditions [113]. A relevant study found that cerebellar-motor connectivity was greater when self-monitoring active versus passive hand movements that had identical sensory feedback in healthy adults [114]. One might extend this work to ascertain whether connectivity of cerebellar and motor cortex with the pons mediate the ability to distinguish self- versus externally-generated sensations, and whether deficient connectivity within that pathway relates to abnormal sensory perception in schizophrenia. TMS and PPI can be applied in concert to assess whether neuromodulation can augment the condition-specific connectivity. For instance, PPI revealed alterations in neural connectivity associated with deep memory encoding following repetitive TMS [115]. This methodological combination has applications for relating underlying network features with specific sensory prediction processes that are measurable with fMRI, such as smooth pursuit eye movements [116].
Pharmacological probes can also offer insights as to the neurotransmitter systems that underlie the cortico-ponto-cerebellar pathway and aid in effective sensory prediction. For example, N-methyl-D-aspartate receptor (NMDAR) hypofunction may underlie sensory prediction deficits seen in schizophrenia as well as the functional pathways believed to support sensory prediction. Recently it was shown that ketamine, an NMDAR antagonist, perturbed behavioral and EEG assays of sensory prediction in healthy volunteers during an audiovisual task [117]. EEG-derived measures of auditory predictive coding for healthy individuals under ketamine mirror the deficient patterns seen in participants with schizophrenia [118]. Additionally, ketamine produced significant schizophrenia-like deficits in smooth pursuit eye movements in healthy participants [119]. It is currently unknown whether NMDAR hypofunction contributes to aberrant connections between the cortex and pons. Evidence that ketamine-induce thalamo-cortical hyper-connectivity of healthy adults under ketamine was more similar to participants with schizophrenia than unaffected comparison participants lends support for pursuing this unanswered question [120].
In closing, the pons is a promising target for elucidating the pathophysiology of sensory prediction impairments in schizophrenia. Integrating neuroimaging with behavioral tasks, brain stimulation, and pharmacological probes can reveal how inter-regional relationships in the cortico-ponto-cerebellar-thalamo-cortical loop map onto specific sensory prediction mechanisms relevant to psychiatric conditions. These multimodal approaches can help bridge the gap in our understanding of the complex and multi-faceted construct that is sensory prediction.
Highlights.
A basic mechanism common to all species heralds the arrival of self-generated sensations, allowing animals to distinguish between self- and externally-generated sensations. Importantly, this mechanism is impaired in schizophrenia.
In mammals, the distinction between self- and externally-generated sensations depends on rapid and unconscious communication between regions within a cortico-ponto-cerebellar-thalamo-cortical loop.
The pons is the principal hub through which descending cortical signals relaying expected sensory experiences are transmitted to the cerebellum, where they are compared with actual sensory feedback.
Abnormal connections between various nodes in the cortico-ponto-cerebellar-thalamo-cortical loop are well-documented in schizophrenia, although relatively sparse attention has been paid to the pons, despite its centrality in this loop.
Consideration of the full cortico-ponto-cerebellar-thalamo-cortical loop is critical for characterizing sensory abnormalities in schizophrenia.
Acknowledgements
SVA was supported by a Career Development Award from the Department of Veterans Affairs (CX002355). JPYH was supported by the Department of Veterans Affairs Sierra Pacific Mental Illness Research, Education, and Clinical Centers (MIRECC). JMF was supported by funding from the National Institute of Mental Health (1R03MH121900–001) and a VA Senior Research Career Scientist award.
Glossary
- Axial diffusivity (AD)
a common diffusion tensor imaging (DTI) diffusivity metric that reflects the magnitude of water diffusion in the direction parallel to the axonal fibers. It is computed as the diffusion rate along the main axis of diffusion for white matter tracts.
- Diffusion tensor imaging (DTI)
the modeling of diffusion weighted neuroimaging data that provides detailed information about tissue microstructure such as fiber orientation, axonal density, and degree of myelination. This technique models how water diffuses, i.e., “travels”, along white matter tracts throughout the brain. It is commonly used in clinical practice and research to examine the structural connectivity of the brain.
- Diffusion weighted imaging (DWI)
a neuroimaging method based on the rate of the diffusivity of water molecules within brain tissue. It is a magnetic resonance imaging approach that is sensitive to the movement of water within the white matter architecture of the brain.
- Efference copy/corollary discharge
the efference copy is the transmission of an action’s motor plan. The corollary discharge is the formation of predictions of the sensory consequences of an action. These terms are sometimes used interchangeably within the sensory prediction literature. The efference copy/corollary discharge mechanism is present across species and sensory systems.
- Electroencephalography (EEG)
a neuroimaging method used to measure scalp-recorded electrical activity of the brain. EEG has millisecond temporal resolution and can thus detect pre-attentive cognitive or sensory processes.
- Inferior cerebellar peduncles
paired structures consisting of major white matter tracts within the cerebellum that receive and process sensory information from the cerebral cortex, spinal cord, and peripheral system.
- Fractional anisotropy (FA)
the most common diffusion tensor imaging (DTI) diffusivity metric ranging between 0 (isotropic movement of water molecules) and 1 (anisotropic movement of water molecules). FA values indicate the overall directionality of water diffusion. FA values are greater in organized white matter tracts than in cerebral spinal fluid or disorganized fibers.
- Internal forward model
a theoretical framework for sensory prediction in which a brain structure (e.g., the cerebellum) predicts the sensory consequences of motor commands and is involved in computing prediction errors by comparing sensory predictions to sensory feedback through feedforward and feedback loops. Feedfoward loops are responsible for predicting what is going to happen. Feedback loops confront the prediction with the reality. This comparison of prediction versus sensory feedback occurs in the cerebellum.
- Mean diffusivity (MD)
a common diffusion tensor imaging (DTI) diffusivity metric diffusivity metric that describes overall water diffusion and is calculated as the mean amount of diffusion in the three principal directions of the diffusion tensor. MD represents the average mobility of water molecules irrespective of any tissue-based directionality.
- Middle cerebellar peduncles
paired structures consisting of white matter fiber tracts that connect the pons with the cerebellum. Mossy fibers originate in the (contralateral) pontine nuclei and terminate in the opposite hemisphere of the cerebellum. This is the main afferent pathway connecting the cortex with the cerebellum and the largest afferent system of the cerebellum.
- Psychophysiological interaction (PPI)
a neuroimaging connectivity method for investigating task-specific changes in the relationship between activity in different brain regions. PPI identifies brain regions in which activity is dependent on the interaction between psychological factors (the task) and the physiological state (the time course of brain activity). The more recent generalized PPI approach can accommodate more than two task conditions in the model by spanning the entire experimental space (i.e., modeling all regressors in the experimental design.
- Radial diffusivity (RD)
a common diffusion tensor imaging (DTI) diffusivity metric that reflects water diffusion in the direction perpendicular to the axonal fibers. It is computed as the diffusion rate that is perpendicular to the main axis of diffusion for white matter tracts.
- Resting-state functional magnetic resonance imaging (rsfMRI)
a neuroimaging method that is used to examine intrinsic functional connectivity between brain regions or networks while no task is being performed. rsfMRI is assessed with either eyes open (fixation on a crosshair) or eyes closed. This method is particularly amenable for populations with medical and/or mental health issues, because it is not confounded by cognitive demands.
- Superior cerebellar peduncles
paired structures consisting of white matter tracts that relay cerebellar outputs via the red nucleus and the contralateral thalamus. It is the main efferent fiber pathway linking the cerebellum with the thalamus and cerebral cortex.
Footnotes
Declaration of interests
The authors declare no competing interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kratochwil CF et al. (2017) The long journey of pontine nuclei neurons: From rhombic lip to cortico-ponto-cerebellar circuitry. Front Neural Circuits 11, 1–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ford JM and Mathalon DH (2012) Anticipating the future: Automatic prediction failures in schizophrenia. Int J Psychophysiol 83, 232–239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bansal S et al. (2018) The function and failure of sensory predictions. Ann N Y Acad Sci 1426, 199–220 [DOI] [PubMed] [Google Scholar]
- 4.Randeniya R et al. (2018) Sensory prediction errors in the continuum of psychosis. Schizophr Res 191, 109–122 [DOI] [PubMed] [Google Scholar]
- 5.Karbasforoushan H et al. (2022) There is a topographic organization in human cortico-pontine connectivity. Brain Commun 4, 1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Palesi F et al. (2017) Contralateral cortico-ponto-cerebellar pathways reconstruction in humans in vivo: Implications for reciprocal cerebro-cerebellar structural connectivity in motor and non-motor areas. Sci Rep 7, 1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blakemore SJ et al. (1998) Central cancellation of self-produced tickle sensation. Nat Neurosci 1, 635–640 [DOI] [PubMed] [Google Scholar]
- 8.Blakemore SJ et al. (2000) The perception of self-produced sensory stimuli in patients with auditory hallucinations and passivity experiences: Evidence for a breakdown in self-monitoring. Psychol Med 30, 1131–1139 [DOI] [PubMed] [Google Scholar]
- 9.Shadmehr R and Krakauer JW (2008) A computational neuroanatomy for motor control. Exp Brain Res 185, 359–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davidson PR and Wolpert DM (2005) Widespread access to predictive models in the motor system: A short review. J Neural Eng 2, 313–319 [DOI] [PubMed] [Google Scholar]
- 11.Pinheiro AP et al. (2020) Cerebellar circuitry and auditory verbal hallucinations: An integrative synthesis and perspective. Neurosci Biobehav Rev 118, 485–503 [DOI] [PubMed] [Google Scholar]
- 12.Welniarz Q et al. (2021) The Forward Model: A Unifying Theory for the Role of the Cerebellum in Motor Control and Sense of Agency. Front Syst Neurosci 15, 1–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crapse TB and Sommer MA (2008) Corollary discharge across the animal kingdom. Nat Rev Neurosci 9, 587–600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pynn LK and DeSouza JFX (2013) The function of efference copy signals: Implications for symptoms of schizophrenia. Vision Res 76, 124–133 [DOI] [PubMed] [Google Scholar]
- 15.Shergill SS et al. (2005) Evidence for sensory prediction deficits in schizophrenia. Am J Psychiatry 162, 2384–2386 [DOI] [PubMed] [Google Scholar]
- 16.Roach BJ et al. (2021) Theta Phase Synchrony Is Sensitive to Corollary Discharge Abnormalities in Early Illness Schizophrenia but Not in the Psychosis Risk Syndrome. Schizophr Bull 47, 415–423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shergill SS et al. (2014) Functional magnetic resonance imaging of impaired sensory prediction in schizophrenia. JAMA Psychiatry 71, 28–35 [DOI] [PubMed] [Google Scholar]
- 18.Ford JM et al. (2001) Neurophysiological evidence of corollary discharge dysfunction in schizophrenia. Am J Psychiatry 158, 2069–2071 [DOI] [PubMed] [Google Scholar]
- 19.Thakkar KN et al. (2021) Reconciling competing mechanisms posited to underlie auditory verbal hallucinations: Mechanisms of Auditory Hallucinations. Philos Trans R Soc B Biol Sci 376, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ford JM et al. (2007) Synch before you speak: Auditory hallucinations in schizophrenia. Am J Psychiatry 164, 458–466 [DOI] [PubMed] [Google Scholar]
- 21.Chen C-MA et al. (2011) The corollary discharge in humans is related to synchronous neural oscillations. Encycl Cogn Sci 23, 2892–2904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stone JM et al. (2012) Communication breakdown: Delta-9 tetrahydrocannabinol effects on pre-speech neural coherence. Mol Psychiatry 17, 568–569 [DOI] [PubMed] [Google Scholar]
- 23.Leptourgos P and Corlett PR (2020) Embodied predictions, agency, and psychosis. Front Big Data 3, 1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Synofzik M et al. (2010) Misattributions of agency in schizophrenia are based on imprecise predictions about the sensory consequences of one’s actions. Brain 133, 262–271 [DOI] [PubMed] [Google Scholar]
- 25.Voss M et al. (2010) Altered awareness of action in schizophrenia: A specific deficit in predicting action consequences. Brain 133, 3104–3112 [DOI] [PubMed] [Google Scholar]
- 26.Garbarini F et al. (2016) Abnormal sense of agency in patients with schizophrenia: Evidence from bimanual coupling paradigm. Front Behav Neurosci 10, 1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miall RC and Wolpert DM (1996) Forward models for physiological motor control. Neural Networks 9, 1265–1279 [DOI] [PubMed] [Google Scholar]
- 28.Friston KJ (2010) The free-energy principle: A unified brain theory? Nat Rev Neurosci 11, 127–138 [DOI] [PubMed] [Google Scholar]
- 29.Picard F and Friston K (2014) Predictions, perception, and a sense of self. Neurology 83, 1112–1118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Friston KJ (2005) A theory of cortical responses. Philos Trans R Soc B Biol Sci 360, 815–836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hull C (2020) Prediction signals in the cerebellum: Beyond supervised motor learning. Elife 9, 1–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Carbajal GV and Malmierca MS (2018) The Neuronal Basis of Predictive Coding Along the Auditory Pathway: From the Subcortical Roots to Cortical Deviance Detection. Trends Hear 22, 1–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tabas A and von Kriegstein K (2021) Adjudicating Between Local and Global Architectures of Predictive Processing in the Subcortical Auditory Pathway. Front Neural Circuits 15, 1–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sterzer P et al. (2018) The Predictive Coding Account of Psychosis. Biol Psychiatry 84, 634–643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ramnani N (2006) The primate cortico-cerebellar system: Anatomy and function. Nat Rev Neurosci 7, 511–522 [DOI] [PubMed] [Google Scholar]
- 36.Huang CC et al. (2013) Convergence of pontine and proprioceptive streams onto multimodal cerebellar granule cells. Elife 2013, 1–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grimaldi G and Manto M (2012) Topography of cerebellar deficits in humans. Cerebellum 11, 336–351 [DOI] [PubMed] [Google Scholar]
- 38.Guo JZ et al. (2021) Disrupting cortico-cerebellar communication impairs dexterity. Elife 10, 1–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schwarz C and Thier P (1999) Binding of signals relevant for action: Towards a hypothesis of the functional role of the pontine nuclei. Trends Neurosci 22, 443–451 [DOI] [PubMed] [Google Scholar]
- 40.Thier P and Ilg UJ (2005) The neural basis of smooth-pursuit eye movements. Curr Opin Neurobiol 15, 645–652 [DOI] [PubMed] [Google Scholar]
- 41.Sitek KR et al. (2013) Auditory cortex processes variation in our own speech. PLoS One 8, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chang EF et al. (2013) Human cortical sensorimotor network underlying feedback control of vocal pitch. Proc Natl Acad Sci U S A 110, 2653–2658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tziridis K et al. (2009) The role of the monkey dorsal pontine nuclei in goal-directed eye and hand movements. J Neurosci 29, 6154–6166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tziridis K et al. (2012) Pontine reference frames for the sensory guidance of movement. Cereb Cortex 22, 345–362 [DOI] [PubMed] [Google Scholar]
- 45.Brodal P and Bjaalie JG (1992) Organization of the pontine nuclei. Neurosci Res 13, 83–118 [DOI] [PubMed] [Google Scholar]
- 46.Wagner MJ et al. (2019) Shared Cortex-Cerebellum Dynamics in the Execution and Learning of a Motor Task. Cell 177, 669–682.e24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Guo JZ et al. (2015) Cortex commands the performance of skilled movement. Elife 4, 1–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sauerbrei BA et al. (2020) Cortical pattern generation during dexterous movement is input-driven. Nature 577, 386–391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Thakkar KN and Rolfs M (2019) Disrupted Corollary Discharge in Schizophrenia: Evidence From the Oculomotor System. Biol Psychiatry Cogn Neurosci Neuroimaging 4, 773–781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ono S (2015) The neuronal basis of on-line visual control in smooth pursuit eye movements. Vision Res 110, 257–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sharpe JA (2008) Neurophysiology and neuroanatomy of smooth pursuit: Lesion studies. Brain Cogn 68, 241–254 [DOI] [PubMed] [Google Scholar]
- 52.Sokolov AA et al. (2017) The Cerebellum: Adaptive Prediction for Movement and Cognition. Trends Cogn Sci 21, 313–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sakai ST (2013) Cerebellar thalamic and thalamocortical projections. In Handbook of the Cerebellum and Cerebellar Disorders pp. 1–2424 [Google Scholar]
- 54.Phillips JM et al. (2021) Disentangling the influences of multiple thalamic nuclei on prefrontal cortex and cognitive control. Neurosci Biobehav Rev 128, 487–510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wilber AA et al. (2015) Cortical connectivity maps reveal anatomically distinct areas in the parietal cortex of the rat. Front Neural Circuits 8, 1–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ohga S et al. (2018) Direct relay pathways from lemniscal auditory thalamus to secondary auditory field in mice. Cereb Cortex 28, 4424–4439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Seghezzi S et al. (2019) The brain in (Willed) action: A meta-analytical comparison of imaging studies on motor intentionality and sense of agency. Front Psychol 10, 1–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rummell BP et al. (2016) Attenuation of responses to self-generated sounds in auditory cortical neurons. J Neurosci 36, 12010–12026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sherman SM (2016) Thalamus plays a central role in ongoing cortical functioning. Nat Neurosci 19, 533–541 [DOI] [PubMed] [Google Scholar]
- 60.Sommer MA and Wurtz RH (2008) Brain circuits for the internal monitoring of movements. Annu Rev Neurosci 31, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zimmermann E et al. (2020) Separate and overlapping functional roles for efference copies in the human thalamus. Neuropsychologia 147, 107558. [DOI] [PubMed] [Google Scholar]
- 62.Yao B et al. (2019) Structural thalamofrontal hypoconnectivity is related to oculomotor corollary discharge dysfunction in schizophrenia. J Neurosci 39, 2102–2113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Clayton KK et al. (2021) Auditory Corticothalamic Neurons Are Recruited by Motor Preparatory Inputs. Curr Biol 31, 310–321.e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hua L et al. (2020) Investigating cortico-subcortical circuits during auditory sensory attenuation: A combined magnetoencephalographic and dynamic causal modeling study. Hum Brain Mapp 41, 4419–4430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Giraldo-Chica M et al. (2018) Prefrontal-Thalamic Anatomical Connectivity and Executive Cognitive Function in Schizophrenia. Biol Psychiatry 83, 509–517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Moberget T and Ivry RB (2019) Prediction, psychosis, and the cerbellum. Biol Psychiatry Cogn Neurosci Neuroimaging 4, 820–831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ramsay IS (2019) An Activation Likelihood Estimate Meta-analysis of Thalamocortical Dysconnectivity in Psychosis. Biol Psychiatry Cogn Neurosci Neuroimaging 4, 859–869 [DOI] [PubMed] [Google Scholar]
- 68.Anticevic A et al. (2014) Mediodorsal and visual thalamic connectivity differ in schizophrenia and bipolar disorder with and without psychosis history. Schizophr Bull 40, 1227–1243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ferri J et al. (2018) Resting-state thalamic dysconnectivity in schizophrenia and relationships with symptoms. Psychol Med 48, 2492–2499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Xi C et al. (2020) Schizophrenia patients and their healthy siblings share decreased prefronto-thalamic connectivity but not increased sensorimotor-thalamic connectivity. Schizophr Res 222, 354–361 [DOI] [PubMed] [Google Scholar]
- 71.Collin G et al. (2011) Impaired cerebellar functional connectivity in schizophrenia patients and their healthy siblings. Front Psychiatry 2, 1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chen YL et al. (2013) Resting-state fMRI mapping of cerebellar functional dysconnections involving multiple large-scale networks in patients with schizophrenia. Schizophr Res 149, 26–34 [DOI] [PubMed] [Google Scholar]
- 73.Zhuo C et al. (2018) Altered resting-state functional connectivity of the cerebellum in schizophrenia. Brain Imaging Behav 12, 383–389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cho KIK et al. (2016) Altered thalamo-cortical white matter connectivity: Probabilistic tractography study in clinical-high risk for psychosis and first-episode psychosis. Schizophr Bull 42, 723–731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sheffield JM et al. (2020) Thalamocortical Anatomical Connectivity in Schizophrenia and Psychotic Bipolar Disorder. Schizophr Bull DOI: 10.1093/schbul/sbaa022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yao B et al. (2020) Altered thalamocortical structural connectivity in persons with schizophrenia and healthy siblings. NeuroImage Clin 28, 102370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sheffield JM et al. (2020) Thalamocortical anatomical connectivity in schizophrenia and psychotic bipolar disorder. Schizophr Bull 46, 1062–1071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Okugawa G et al. (2006) Neural disorganization in the superior cerebellar peduncle and cognitive abnormality in patients with schizophrenia: A diffusion tensor imaging study. Prog Neuro-Psychopharmacology Biol Psychiatry 30, 1408–1412 [DOI] [PubMed] [Google Scholar]
- 79.Magnotta VA et al. (2008) Investigating connectivity between the cerebellum and thalamus in schizophrenia using diffusion tensor tractography: A pilot study. Psychiatry Res 163, 193–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lee KH et al. (2019) Functional and structural connectivity of the cerebellar nuclei with the striatum and cerebral cortex in first-episode psychosis. J Neuropsychiatry Clin Neurosci 31, 143–151 [DOI] [PubMed] [Google Scholar]
- 81.Wang F et al. (2003) A diffusion tensor imaging study of middle and superior cerebellar peduncle in male patients with schizophrenia. Neurosci Lett 348, 135–138 [DOI] [PubMed] [Google Scholar]
- 82.Kanaan RAA et al. (2009) Microstructural Organization of Cerebellar Tracts in Schizophrenia. Biol Psychiatry 66, 1067–1069 [DOI] [PubMed] [Google Scholar]
- 83.Hüttlova J et al. (2014) Abnormalities in myelination of the superior cerebellar peduncle in patients with schizophrenia and deficits in movement sequencing. Cerebellum 13, 415–424 [DOI] [PubMed] [Google Scholar]
- 84.Khadka S et al. (2013) Is Aberrant Functional Connectivity A Psychosis Endophenotype? A Resting State Functional Magnetic Resonance Imaging Study. Biol Psychiatry 74, 458–466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Vitolo E et al. (2017) White matter and schizophrenia: A meta-analysis of voxel-based morphometry and diffusion tensor imaging studies. Psychiatry Res - Neuroimaging 270, 8–21 [DOI] [PubMed] [Google Scholar]
- 86.Douaud G et al. (2007) Anatomically related grey and white matter abnormalities in adolescent-onset schizophrenia. Brain 130, 2375–2386 [DOI] [PubMed] [Google Scholar]
- 87.Kyriakopoulos M et al. (2009) Effect of age at onset of schizophrenia on white matter abnormalities. Br J Psychiatry 195, 346–353 [DOI] [PubMed] [Google Scholar]
- 88.Koch K et al. (2010) Disrupted white matter integrity of corticopontine-cerebellar circuitry in schizophrenia. Eur Arch Psychiatry Clin Neurosci 260, 419–426 [DOI] [PubMed] [Google Scholar]
- 89.Kim SE et al. (2021) Impaired cerebro-cerebellar white matter connectivity and its associations with cognitive function in patients with schizophrenia. npj Schizophr 7, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Okugawa G et al. (2005) Diffusion tensor imaging study of the middle cerebellar peduncles in patients with schizophrenia. Cerebellum 4, 123–127 [DOI] [PubMed] [Google Scholar]
- 91.Filippi M et al. (2014) Patterns of brain structural changes in first-contact, antipsychotic drug-naïve patients with schizophrenia. Am J Neuroradiol 35, 30–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Elvsåshagen T et al. (2020) The genetic architecture of human brainstem structures and their involvement in common brain disorders. Nat Commun 11, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gupta CN et al. (2015) Patterns of gray matter abnormalities in schizophrenia based on an international mega-analysis. Schizophr Bull 41, 1133–1142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sclocco R et al. (2018) Challenges and opportunities for brainstem neuroimaging with ultrahigh field MRI. Neuroimage 168, 412–426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lu J et al. (2011) Focal pontine lesions provide evidence that intrinsic functional connectivity reflects polysynaptic anatomical pathways. J Neurosci 31, 15065–15071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Javitt DC and Sweet R (2015) Auditory dysfunction in schizophrenia: integrating clinical and basic features. Nat Rev Neurosci 16, 535–550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ohlrogge M et al. (2001) Projections of the Pontine Nuclei to the Cochlear Nucleus in Rats. J Comp Neurol 436, 290–303 [PubMed] [Google Scholar]
- 98.Schielke E et al. (2000) Musical hallucinations with dorsal pontine lesions. Neurology 55, 454–455 [DOI] [PubMed] [Google Scholar]
- 99.Serby MJ et al. (2013) Musical hallucinations associated with pontine lacunar lesions. J Neuropsychiatry Clin Neurosci 25, 153–156 [DOI] [PubMed] [Google Scholar]
- 100.Gopal M et al. (2017) Acute Psychosis as Main Manifestation of Central Pontine Myelinolysis. Case Rep Neurol Med 2017, 1–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Dickson H et al. (2020) Adolescent trajectories of fine motor and coordination skills and risk for schizophrenia. Schizophr Res 215, 263–269 [DOI] [PubMed] [Google Scholar]
- 102.Fritze S et al. (2019) Differential contributions of brainstem structures to neurological soft signs in first- and multiple-episode schizophrenia spectrum disorders. Schizophr Res 210, 101–106 [DOI] [PubMed] [Google Scholar]
- 103.Moussa-Tooks AB et al. (2019) Impaired effective connectivity during a cerebellar-mediated sensorimotor synchronization task in schizophrenia. Schizophr Bull 45, 531–541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Koziol LF et al. (2014) Consensus paper: The cerebellum’s role in movement and cognition. Cerebellum 13, 151–177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Henderson LA and Macefield VG (2013) Functional imaging of the human brainstem during somatosensory input and autonomic output. Front Hum Neurosci 7, 1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Straub S et al. (2019) Mapping the human brainstem: Brain nuclei and fiber tracts at 3 T and 7 T. NMR Biomed 32, 1–13 [DOI] [PubMed] [Google Scholar]
- 107.Garrido MI et al. (2009) The mismatch negativity: A review of underlying mechanisms. Clin Neurophysiol 120, 453–463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Brady RO Jr et al. (2019) Breakdown of functional connectivity in cerebellar-prefrontal network underlies negative symptoms in schizophrenia. Am J Psychiatry 176, 512–520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Basavarajua R et al. (2021) Intermittent theta burst stimulation of cerebellar vermis enhances fronto-cerebellar resting state functional connectivity in schizophrenia with predominant negative symptoms: A randomized controlled trial. Schizophr Res 238, 108–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Popa T et al. (2013) Cerebellar rTMS stimulation may induce prolonged clinical benefits in essential tremor, and subjacent changes in functional connectivity: An open label trial. Brain Stimul 6, 175–179 [DOI] [PubMed] [Google Scholar]
- 111.Li J et al. (2018) Excitatory repetitive transcranial magnetic stimulation induces contralesional cortico-cerebellar pathways after acute ischemic stroke: A preliminary DTI study. Front Behav Neurosci 12, 1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Daskalakis ZJ et al. (2004) Exploring the connectivity between the cerebellum and motor cortex in humans. J Physiol 557, 689–700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.McLaren DG et al. (2012) A Generalized Form of Context-Dependent Psychophysiological Interactions (gPPI): A Comparison to Standard Approaches. Neuroimage 61, 1277–1286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Van Kemenade BM et al. (2019) Distinct Roles for the Cerebellum, Angular Gyrus, and Middle Temporal Gyrus in Action-Feedback Monitoring. Cereb Cortex 29, 1520–1531 [DOI] [PubMed] [Google Scholar]
- 115.Vidal-Piñeiro D et al. (2014) Task-dependent activity and connectivity predict episodic memory network-based responses to brain stimulation in healthy aging. Brain Stimul 7, 287–296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Schröder R et al. (2020) Functional connectivity during smooth pursuit eye movements. J Neurophysiol 124, 1839–1856 [DOI] [PubMed] [Google Scholar]
- 117.Mohanta S et al. (2021) Predictive Feedback, Early Sensory Representations, and Fast Responses to Predicted Stimuli Depend on NMDA Receptors. J Neurosci 41, 10130–10147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kort NS et al. (2017) Role of N-Methyl-D-Aspartate Receptors in Action-Based Predictive Coding Deficits in Schizophrenia. Biol Psychiatry 81, 514–524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Steffens M et al. (2016) Effects of ketamine on brain function during smooth pursuit eye movements. Hum Brain Mapp 37, 4047–4060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Abram SV et al. (2022) Validation of ketamine as a pharmacological model of thalamic dysconnectivity across the illness course of schizophrenia. Mol Psychiatry [DOI] [PMC free article] [PubMed] [Google Scholar]


