Abstract
Darobactin A is a ribosomally synthesized, post-translationally modified peptide (RiPP) with potent and broad-spectrum anti-Gram-negative antibiotic activity. The structure of darobactin A is characterized by an ether and C–C crosslinking. However, the specific mechanism of the crosslink formation, especially the ether crosslink, remains elusive. Here, using in vitro enzyme assays, we demonstrate that both crosslinks are formed by the DarE radical S-adenosylmethionine (SAM) enzyme in an O2-dependent manner. The relevance of the observed activity to darobactin A biosynthesis was demonstrated by proteolytic transformation of the DarE product into darobactin A. Furthermore, DarE assays in the presence of 18O2 or [18O]water demonstrated that the oxygen of the ether crosslink originates from O2 and not from water. These results demonstrate that DarE is a radical SAM enzyme that uses oxygen as a co-substrate in its physiologically relevant function. Since radical SAM enzymes are generally considered to function under anaerobic environments, the discovery of a radical SAM oxygenase represents a significant change in the paradigm and suggests that these radical SAM enzymes function in aerobic cells. Also, the study revealed that DarE catalyzes the formation of three distinct modifications on DarA; ether and C–C crosslinks and α,β-desaturation. Based on these observations, possible mechanisms of the DarE-catalyzed reactions are discussed.
Graphical Abstract

INTRODUCTION
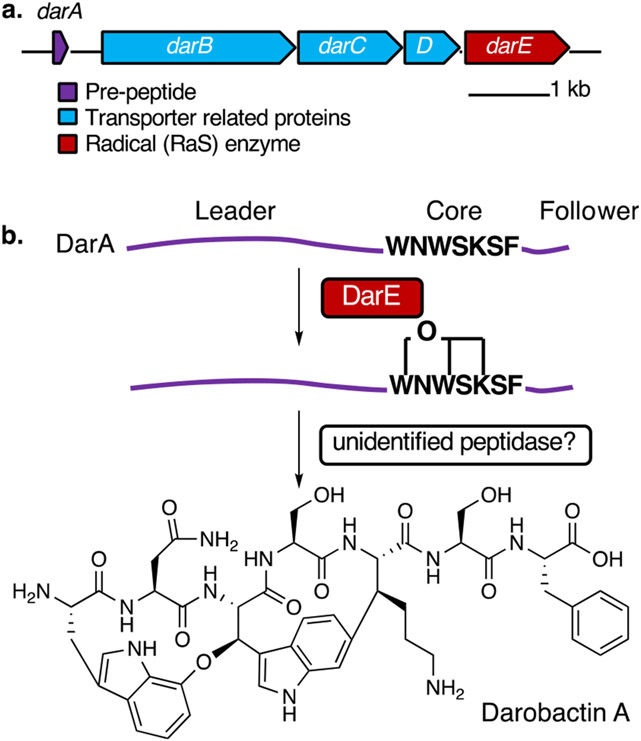
The bicyclic heptapeptide darobactin A is a ribosomally synthesized, post-translationally modified peptide (RiPP) with antibiotic activities against diverse sets of Gram-negative bacteria including many clinically important pathogens.1 Darobactin A exhibits its antibiotic activity by inhibiting BamA, a protein in the Bam complex responsible for the assembly of proteins in the outer membrane of Gram-negative bacteria.1,2 Darobactin A has a rigid β-strand conformation through the ether and C–C crosslinks on the heptapeptide core structure (W1–N2–W3–S4–K5–S6–F7, see Figure 1 for the structure), which mimics the recognition signal of native substrates and blocks the open lateral gate of BamA.2 However, the mechanism by which this unique and biologically important bicyclic structure is formed remains unknown.
Figure 1.
Darobactin A biosynthesis. (a) BGC of darobactin A. (b) Proposed biosynthetic pathway of darobactin A.
The darobactin biosynthetic gene cluster (BGC) consists of the precursor peptide-encoding darA, the transporter genes darBCD, and a radical S-adenosyl-l-methionine (SAM) enzyme gene darE (Figure 1a). Earlier studies have established that the co-expression of DarA and DarE is sufficient for the production of darobactin A in Escherichia coli1,3,4 (Figure 1b). Radical SAM enzymes form a large enzyme superfamily5 and catalyze various radical-mediated reactions.6 These enzymes harbor an oxygen-sensitive 4Fe–4S cluster to catalyze the reductive cleavage of SAM to transiently generate the 5′-deoxyadenosyl radical (5′-dA•) that then in most cases abstracts a H atom from the substrate or, in other cases, adds to the substrate to carry out various radical reactions. Since the 4Fe–4S clusters of radical SAM enzymes are oxygen-sensitive, characterization of these enzymes has been performed under strictly anaerobic conditions, and it has been largely assumed that these enzymes must function in the absence of oxygen. So far, no radical SAM enzymes have been reported to have physiological functions that require molecular oxygen as the co-substrate.
In the past decade, many radical SAM enzymes have been identified to be responsible for the maturation of RiPPs. In particular, radical SAM enzymes in the SPASM subfamily,7 characterized by the presence of auxiliary 4Fe–4S clusters (AUX) in addition to the canonical radical SAM 4Fe–4S cluster, were reported to catalyze the formation of various crosslinks during RiPP biosynthesis. Such crosslinks include C–C bonds between aromatic and aliphatic carbons,8,9 thioethers via a Cys residue,10 and an ether via Thr alcohol.11 However, all of these reported crosslinks utilize functional groups already available in the precursor peptides. In contrast, the ether ring in darobactin A is unique in that the oxygen atom of the ether must be post-translationally installed. The origin of this oxygen atom and the mechanism of its installation remain ambiguous.
Recently, when this manuscript was in preparation, Guo et al. reported in vitro characterization of Photorhabdus khanii DarE with an N-terminal His-tag.12 The authors reported the formation of DarA with an ether crosslink without the C–C crosslink as the major product and proposed water as the source of the ether oxygen. However, no evidence was provided for whether such a compound may be converted into darobactin A, and hence, the biological relevance of this observation remained unclear.
Here, we report the successful in vitro reconstitution of darobactin A biosynthesis. Importantly, our 18O-labeling study provides strong evidence that the oxygen atom of the ether crosslink is derived from molecular oxygen and not from water, making DarE the first radical SAM oxygenase. These results extend the repertoire of the reactions catalyzed by radical SAM enzymes and provide the evidence for their function under an aerobic environment.
MATERIALS AND METHODS
General.
Sodium dithionite (SDT) was purchased from Sigma-Aldrich. β-Mercaptoethanol (βME) was obtained from Calbiochem. Dithiothreitol (DTT) was obtained from Amresco. G-25 Sephadex resin was obtained from GE Healthcare. Ni-NTA agarose resin was obtained from Qiagen. Strep-Tactin XT 4Flow high-capacity resin was obtained from IBA LifeSciences. E. coli DH5α and BL21(DE3) competent cells were obtained from Invitrogen. All anaerobic experiments were carried out in an MBRAUN glovebox maintained at 10 ± 2 °C with an O2 concentration of <0.1 ppm. All anaerobic buffers were degassed on a Schlenk line and equilibrated in the glovebox overnight. All plastic devices were evacuated in the antechamber of the glovebox overnight before use. All high-performance liquid chromatography (HPLC) experiments were performed on a Hitachi L-2130 pump equipped with an L-2455 diode array detector, an L-2485 fluorescence detector, an L-2200 autosampler, and an L-2300 column oven maintained at 40 °C. UV–vis absorption spectra were determined using a U-3900 UV–vis ratio recording double-beam spectrometer (Hitachi). All mass spectra were recorded on a 6224 accurate-mass time-of-flight mass spectrometer (Agilent) equipped with a Dual ESI source, and accurate mass data were obtained by internal calibration using a secondary nebulizer to continuously deliver the reference solution. Liquid chromatography mass spectrometry (LC–MS) analysis was conducted on a 6224 TOF LC/MS system equipped with a Phenomenex Kinetex C18 EVO column (3 × 100 mm, 2.6 μm particle), monitored by a diode array detector (254 nm). Chromatography was performed with the flow rate of 0.5 mL/min using solvents A (H2O/MeOH/formic acid = 100:3:0.3) and B (H2O/MeCN/formic acid = 3:100:0.3) and the following gradient conditions: 100% A isocratic between 0 and 0.5 min and 0–50% B linear gradient between 0.5 and 8 min.
Heterologous Production of Darobactin A.
The Pseudoalteromonas luteoviolacea darA and darE genes were codon optimized for E. coli using the Java Codon Adjustment Tool (jCAT),13 and the resulting sequences were synthesized by Eurofins Genomics (Eberswalde, Germany). The resulting synthetic darA and darE genes were PCR amplified using the primer pairs darA-f/darA-r and darE-f/darE-r (Table S1) using Q5 polymerase (NEB Biolabs, New Brunswick, USA) according to the manufacturer’s manual. All PCR products were gel purified using 1 or 2% TAE agarose gels, and DNA was recovered using the Zymo Research Large Fragment DNA Recovery Kit (Zymo Research, USA) according to the manufacturer’s manual. An empty pRSFduet-1 plasmid (Novagen) was digested using NdeI/AvrII. All fragments were assembled using a homemade isothermal assembly master mix,14 and the assembled plasmids were transferred to E. coli Top10 using the standard electroporation methodology and selected on LBKan. The identity of the plasmids was corroborated by test restriction. The correctly assembled plasmid was transferred to E. coli Bap1 and E. coli Bap1 harboring pGro7 (Takara) by electroporation. E. coli Bap1 + pRSFduet-1 (empty vector) was used as a negative control.
For heterologous Darobactin production, 20 mL of LBKan was inoculated with an overnight preculture of E. coli Bap1 + pRSFduet-1, E. coli Bap1 + pRSF-darAEPse of E. coli Bap1 + pRSF-darAEPse + pGro7 (Takara) cells, and the cultures were incubated at 30 °C to an OD600 of ~0.5. Transcription was induced by the addition of isopropyl β-D-1-thiogalactopyranoside (IPTG) to a 1 mM final concentration. After 3 days of cultivation, 0.5 mL of the culture was separated into the supernatant and pellet by centrifugation, and the cleared supernatant was applied to self-packed C18 stage tips. The stage tips were washed using ddH2O, the material was eluted in 50 μL of 80:20 MeCN/H2O, and 5 μL of the concentrated supernatants were analyzed by LC–MS using a 20 μg/mL darobactin A solution as the standard.
Expression and Purification of PlDarA.
The P. luteoviolacea darA gene with the His6- and SUMO-tags fused at its 5′-end was synthesized using Genscript with the codon usage optimized for E. coli and cloned into the NcoI-BamHI site of pET16b (pET16b-SUMO-PlDarA). PlDarA was then expressed in E. coli BL21(DE3) cells harboring pET16b-SUMO-PlDarA and pGro7. The E. coli cells were grown in LB medium (50 mL) with 100 mg/L ampicillin and 30 mg/L chloramphenicol and incubated at 37 °C, 220 rpm overnight until saturation. An aliquot (30 mL) of the overnight culture then was used to inoculate 1.5 L of LB medium with the same antibiotics, which was grown at 37 °C, 220 rpm until OD600 = 0.6–0.8. Protein expression was induced with 60 mg/L IPTG, and the culture was incubated at 18 °C, 220 rpm for 20 h. The cells were harvested by centrifugation, washed with buffer A (50 mM Tris–HCl pH 7.6, 150 mM NaCl, 10% v/v glycerol), frozen with liquid nitrogen, and stored at −20 °C. Approximately 6.1 g of the wet cell paste was obtained per liter of the culture.
SUMO-PlDarA Purification.
In a typical purification, 20 g of the cell pellet was suspended and homogenized in 80 mL of buffer A supplemented with 5 mM β-ME and 0.8 mL of a protease inhibitor cocktail (100X ProteaseArrest, G-Biosciences). The cell suspension was lysed by two passages through a French pressure cell operating at 14,000 psi. The resulting lysate was cleared by centrifugation (20,000×g, 20 min, 4 °C). The supernatant was incubated with Ni-NTA agarose resin (Qiagen, 10 mL equilibrated in buffer A containing 20 mM imidazole and 1.0 μl benzonase/30 mL supernatant) for 1 h. The resin was washed with 10 volumes of buffer A with 20 mM imidazole and 3 mM β-ME, and the protein was eluted with buffer A with 400 mM imidazole and 3 mM β-ME. Fractions containing SUMO-PlDarA were exchanged into buffer A using a Sephadex G-25 column. The concentration of SUMO-PlDarA was determined by the Bradford assay using bovine serum albumin (BSA) as a standard. The resultant protein solution was frozen in liquid N2 and stored at −80 °C. Typically, 14 mg of SUMO-PlDarA was prepared from each gram of the wet cell paste. The SUMO tag of SUMO-PlDarA (100 mg) was cleaved by SUMO-protease (0.3 mg) at 0 °C for 1 h. The resulting solution was passed through Ni-NTA agarose resin (10 ml equilibrated in distilled water). The protein was eluted with distilled water. Fractions containing PlDarA were brought into an mBraun anaerobic glovebox ([O2] < 0.1 ppm) and exchanged into anaerobic water using a Sephadex G-25 column. The concentration of PlDarA was determined by HPLC.
Expression of His-PlDarE.
pRSF-His-PlDarE was created by amplifying darEPse using the primers his-darE-f/his-darE-r (Table S1) using Q5 polymerase (NEB) according to the manufacturer’s manual. The amplified gene was gel purified using 1% TAE agarose gels and the ZymoResearch Large Fragment DNA Recovery Kit according to the manufacturer’s manual. The purified fragment was then digested with BamHI/SalI and cloned into the corresponding site of pRSFduet-1. Both fragments were fused using a homemade isothermal assembly master mix,14 transferred to E. coli Top10 using the standard electroporation methodology, and selected on LBKan. The correct assembly was verified by sequencing, and the plasmid was designated as pRSF-His-PlDarE. To express His-PlDarE, E. coli Bap1 cells were transformed with pRSF-His-PlDarE and pGro7. The resulting transformant was grown in LB medium (50 mL) with 50 mg/L kanamycin and 30 mg/L chloramphenicol and incubated at 37 °C, 220 rpm overnight until saturation. An aliquot (30 mL) of the overnight culture was then used to inoculate 1.5 L of terrific broth containing 50 mg/L cysteine, 30 mg/L iron(III), and 0.5 g/L l-arabinose with the same antibiotics, which was grown at 37 °C, 220 rpm until OD600 = 0.6–0.8. Protein expression was induced with 60 mg/L IPTG, and the culture was incubated at 18 °C, 220 rpm for 20 h. The cells were harvested by centrifugation, washed with buffer A (50 mM Tris pH 7.6, 150 mM NaCl, 10% glycerol), frozen with liquid nitrogen, and stored at −20 °C. Approximately 22 g of the wet cell paste was obtained per liter of the culture.
Purification of His-PlDarE.
His-PlDarE was anaerobically purified typically from 10–30 g of the cell paste of E. coli BAP1 harboring pRSF-His-PlDarE. The cell pellet was brought into an mBraun anaerobic glovebox ([O2] < 0.1 ppm). Then, each gram of the cell pellet was suspended and homogenized in 4 mL of anaerobic buffer A supplemented with 5 mM β-ME. The cell suspension was brought out of the glovebox and lysed by two passages through a French pressure cell operating at 14,000 psi under a constant Ar flow. The resulting lysate was cleared by centrifugation (20,000×g, 20 min, 4 °C) using centrifugal tubes filled with Ar gas and brought back to the glovebox. All subsequent purification steps were carried out under strict anaerobic conditions in the glovebox ([O2] < 0.1 ppm) maintained at 10 °C. The dark brown-colored supernatant was incubated with Ni-NTA agarose resin (Qiagen, 20 mL equilibrated in buffer A containing 40 mM imidazole and 1.0 μL benzonase/30 mL supernatant) for 1 h. The resin was washed with 10 volumes of buffer A with 20 mM imidazole and 3 mM β-ME, and the protein was eluted with buffer A with 400 mM imidazole and 3 mM β-ME. Fractions containing dark brown-colored His-PlDarE were exchanged into buffer A using a Sephadex G-25 column. The concentration of His-PlDarE was determined by the Bradford assay using BSA as a standard. The amounts of Fe2+/3+ were quantified by the following published protocols.15 Typically, the preparation yielded His-PlDarE with 4.0 ± 0.8 Fe per monomer. The resulting purified His-PlDarEwas immediately used for anaerobic reconstitution carried out at 10 °C by a slow addition of 8–12 equiv FeII(NH4)2(SO4)2 and Na2S per His-PlDarE monomer over the course of 10 min. The amounts of FeII(NH4)2(SO4)2 and Na2S were adjusted based on the amounts of Fe2+/3+ associated with the as-isolated His-PlDarE. The resulting mixture was incubated for 60 min at 10 °C. The protein was then desalted using a Sephadex G-25 column equilibrated with buffer A. The amounts of Fe and sulfide were determined by the ferrozine assay.15 The resultant protein solution was frozen in liquid N2 and stored at −80 °C. Typically, 0.8 mg of His-PlDarE was prepared from each gram of the wet cell paste.
Expression and Purification of Untagged PlDarE.
The Twin-strep II tag and the Factor Xa cleavage site (MASAWSHPQ-FEKGGGSGGGSGGSAWSHPQFEKSGIEGR) were introduced to the 5′-end of the codon optimized P. luteoviolacea darE gene by PCR. The pRSF-His-PlDarE plasmid was amplified using a primer pair pRSF-strep-DarE-f/r (Table S1) and ligated with a strep-f/r oligo pair (Table S1) using the InFusion kit (Takara). The resulting plasmid pRSF-Strep-PlDarE was then used as a template to amplify the Strep-PlDarE gene using a primer pair pET-strep-PlDarE-f/r (Table S1). The resulting PCR products were digested with NdeI and HindIII and introduced into the corresponding site of pET30b to yield pET30-Strep-PlDarE. Strep-PlDarE was expressed in an identical way to His-PlDarE using E. coli Bap1 with pET30-Strep-PlDarE and pGro7. Approximately 6.9 g of the wet cell paste was obtained per liter of the culture. To purify Strep-PlDarE, the E. coli cells were lysed in the same manner as that for His-PlDarE. The cleared lysate was loaded onto the Strep-Tactin XT 4Flow high-capacity resin, the column was washed with 10 column volumes of buffer W (100 mM Tris–HCl pH 8.0, 150 mM NaCl), and the protein was eluted with buffer BXT (100 mM Tris–HCl pH 8.0, 150 mM NaCl, and 50 mM biotin). Fractions containing dark brown-colored PlDarE were exchanged into buffer W using a Sephadex G-25 column. The concentration of PlDarE was determined by the Bradford assay using BSA as a standard. The strep tag was removed by incubation with Factor Xa protease (1 μg per 1 mg protein, New England Biolabs) in buffer W supplemented with 2 mM CaCl2 at 10 °C for 4 h. Then, the Factor Xa protease was removed by Xarrest resin, and the cleaved strep tag was removed using Strep-tactin resin. The 4Fe–4S cluster was reconstituted as described above for His-PlDarE, and the resulting reconstituted PlDarE contained 12.2 ± 0.5 Fe per monomer. Typically, 2.0 mg of PlDarE was prepared from each gram of the wet cell paste.
DarE Activity Assay.
His-PlDarE or PlDarE (50 μM) was anaerobically incubated with DarA (50 μM) in the presence of SAM (1.0 mM), flavodoxin (10 μM), flavodoxin reductase (2.0 μM), NAD(P)H (1.0 mM), and oxygen (100 μM) in an airtight glass vial with a total volume of 50 μL of buffer H (20 mM HEPES pH 8.0) supplemented with 5 mM DTT for 3 h at 25 °C. The reaction was initiated by adding DarA and oxygen-saturated buffer H. After 3 h of incubation, the reaction was diluted twice and boil-quenched at 100 °C for 5 min. After the removal of the precipitate by centrifugation, an aliquot (40 μL) of the supernatant was injected into the HPLC system equipped with an XSelect Peptide CSH C18 column (Waters) equilibrated in 0.1% trifluoroacetic acid (TFA) in water. The elution was achieved with a flow rate at 1.0 mL/min using solvents A (0.1% TFA in water) and B (0.1% TFA in MeCN): 3% B for 5 min, 3–27% B for 5–15 min, and 27–30% B for 15–50 min. Chromatography was performed using the L-2455 diode array detector.
Purification of DarE Products.
A DarE reaction (1.0 ml) was performed in the conditions described above and quenched with 1 ml of buffer Q (0.2% TFA and 6% MeCN in water). After the removal of the precipitate by centrifugation, an aliquot (500 μL) of the supernatant was injected into the HPLC system and chromatographed under the above-mentioned conditions. P1 and P2 were monitored using the L-2455 diode array detector and eluted at 44 and 30 min, respectively. After combining all the fractions containing P1 or P2, TFA and MeCN were removed using a centrifugal evaporator. The resulting solution was lyophilized and redissolved in water. HRMS (ESI-TOF): m/z calcd for P1, C281H448N74O89, [M+7H]+7, 13C = 3) 899.0493; found, 899.0417; m/z calcd for P2, (C281H446N74O90, [M+7H]+7, 13C = 4) 901.1897; found, 901.1809.
Proteinase K Treatment of DarE Products.
P1 and P2 (2.5 μg) were treated with proteinase K (100 ng) in 20 μL ofbuffer K (50 mM Tris–HCl pH 8.0 and 5.0 mM CaCl2) for 1 h at 37 °C. The reaction mixture was then quenched with an equal volume of buffer Q. Precipitation was removed, and an aliquot (20 μL) of the supernatant was analyzed by HPLC. Another aliquot (5 μL) was analyzed by LC–high-resolution MS (LC–HRMS). HRMS (ESI-TOF): m/z calcd for proteinase K-digested P1, (C35H43N9O8, z = +2) 359.6696; found, 359.6689; m/z calcd for proteinase K-digested P2, (C47H55N11O12, z = +2) 483.7094; found, 483.7097.
18O2 Incorporation Experiments.
18O2-saturated buffer H was prepared by degassing buffer H on a Schlenck line followed by a refill with 18O2 gas (97% enrichment, Cambridge Isotope Laboratory). PlDarE assays with 18O2 were performed in a way identical to the assays described above, except that an aliquot (10 μL) of each reaction mixture was added to proteinase K (100 ng) and buffer K (40 μL). The resulting solution was incubated at 37 °C for 1 h and subsequently centrifuged to collect the supernatant for LC–MS analysis.
H218O Incorporation Experiments.
The PlDarE assay (0.5 mL) with H218O was performed as described above, except that HEPES buffer was prepared in 95% 18O enriched water. The final 18O enrichment in the assay mixture was ~80%. P2 was purified by HPLC and characterized as described above.
P1, P2, and 5′-dA Quantitation.
DarE assays (60 μL) were performed in the conditions described above and diluted twice with distilled water followed by boil-quenching at 100 °C for 5 min. After the removal of the precipitate by centrifugation, an aliquot (60 μL) of the supernatant was analyzed by HPLC under the above-mentioned conditions. The elution was monitored by absorption at 280 nm, and P1 and P2 were eluted at 50 and 36 min, respectively. P1 was quantified by comparing the peak areas with that of a synthetic DarA core peptide (WNWSKSF, purchased from Genscript, ε280 nm = 11.2 mM−1 cm−1). The concentration of P2 was determined using darobactin A as a standard. The concentration of darobactin A was determined by NMR in the presence of a known concentration of benzaldehyde as an internal standard. 5′-dA was quantified by analyzing the same supernatant (10 μL) using HPLC equipped with an XSelect Peptide CSH C18 column (Waters) equilibrated in 30 mM ammonium formate buffer pH 4.0 (solvent C). The elution was performed with a flow rate at 0.5 mL/min using solvent C and methanol (solvent D): 1–60% D for 0–15 min and 60–99% B for 15–16 min. Chromatography was performed at 260 nm. The concentration of 5′-dA was determined by comparing the peak areas with those of the 5′-dA standard (Sigma, ε259 nm = 15.4 mM−1 cm−1). The reported quantitation was an average of six repeats, and the errors are the standard deviation.
RESULTS
In search for the soluble expression in E. coll, we screened several DarEs from different organisms and identified that DarE from P. luteoviolacea (PlDarE) can be expressed as a soluble protein. When PlDarE was co-expressed with P. luteoviolacea DarA (PlDarA) in E. coli, darobactin A production was observed (Figure S1a). The identity of darobactin A was confirmed based on the LC–MS comparison with the structurally characterized darobactin A standard (Figure S1).
Based on the observation in E. coli, we expressed and purified PlDarE in E. coli. Initially, we used N-terminally His6-tagged PlDarE (His-PlDarE) that was purified to >90% purity (Figure S2) with the broad light absorption feature characteristic for 4Fe–4S cluster proteins (Figure S3). The as-isolated His-PlDarE had 3.6 ± 0.9 Fe per monomer, which was increased to 8.8 ± 0.3 Fe per monomer after chemical reconstitution of the cluster. Since DarE is a member of the SPASM subfamily with conserved Cys ligands for the three 4Fe–4S clusters, supported by sequence alignment (Figure S4) and an AlphaFold structural prediction (Figure S5), the theoretical Fe content of the fully loaded DarE is 12 per monomer. Thus, the observed amount of Fe likely represents only partial loading of the three clusters.
The catalytic function of His-PlDarE was assessed using recombinant PlDarA. To this end, we expressed PlDarA in E. coli as an N-terminal fusion with the His6-tag and a SUMO protein. After purification using a Ni-NTA column, the His-SUMO tag was removed by treating with the SUMO protease to prepare the full-length and untagged DarA The identity of the purified DarA was confirmed by LC–MS (Figure S6). When the purified DarA was incubated with His-PlDarE under anaerobic conditions in the presence of SAM and the flavodoxin system as a reductant, we observed the formation of a product (P0, Figure 2a). This compound exhibited a UV–vis absorption spectrum similar to that of unmodified DarA and distinct from that of darobactin A (Figure 2b). LC–HRMS analysis of P0 showed the molecular weight to be 2 Da smaller than that of DarA (Figure 2c). Therefore, we tentatively assigned P0 to be DarA with a single C–C crosslink, likely between W3 and K5.
Figure 2.
Characterization of His-PlDarE. (a) HPLC characterization of His-DarE assays. (b) UV–vis spectra of P0, DarA, and darobactin A. (c) Extracted and deconvoluted mass spectra of His-PlDarE assays. Mass spectra were extracted from the retention time that covers unmodified PlDarA and P0.
Since no ether crosslink formation was reproducibly observed with His-PlDarE even after extensive screening of the reaction conditions, we hypothesized that the N-terminal His-tag may be interfering with the DarE function. Therefore, we created an N-terminally Strep-tagged DarE with a Factor Xa cleavage site immediately preceding the first codon of DarE (Strep-PlDarE) for a traceless tag removal. This protein was expressed in E. coli in a manner similar to that of His-PlDarE and purified using a Strep-tactin resin. Subsequently, the Strep-tag was cleaved by treatment with Factor Xa protease. The resulting enzyme contained 7.5 ± 0.7 equiv of Fe. Subsequent chemical reconstitution increased the Fe content to 12.2 ± 0.5 equiv per monomer, consistent with the presence of three 4Fe–4S clusters per monomer. This result is in a sharp contrast to His-PlDarE, where we observed only ~9 Fe per monomer.
The resulting untagged PlDarE was tested for its catalytic function using PlDarA. When we investigated its activity under strictly anaerobic conditions (<0.1 ppm O2), we observed a single product (P1, Figure 3a, trace i) with a unique UV absorption band at ~350 nm (Figure 3b). P1 was produced only in the presence of SAM, NADPH, DarE, and DarA, supporting that it is produced by the radical SAM activity of DarE. Furthermore, LC–HRMS analysis revealed its molecular weight to be 2 Da smaller than that of DarA (Figure 3c). Importantly, P1 was distinct from P0 observed in the His-PlDarE reaction, and P0 was not detectable in the assays with PlDarE fully loaded with three 4Fe–4S clusters.
Figure 3.
Activity assays of PlDarE. (a) HPLC analyses of PlDarE assay in the absence of O2 (i), in the presence of O2 (ii), (ii) without SAM (iii), (ii) without NADPH (iv), (ii) without PlDarE (v), (ii) without PlDarA (vi), and the PlDarA standard (vii). (b) UV–vis absorption spectra of P1 (gray), P1 + proteinase K (green), P2 (red), P2 + proteinase K (dashed black), darobactin A (orange), and PlDarA (blue). (c) Extracted and deconvoluted mass spectra of DarE assays. Mass spectra were extracted from the retention time that covers unmodified PlDarA, P1, and P2. The mass peaks indicated with asterisks (*) have a molecular weight close to that of PlDarA with a 14 Da modification, but they were also present in the controls without SAM or NADPH and hence unlikely to be the product of the radical SAM enzyme activity.
Since we did not observe any oxygen insertion, we tested the activity of DarE in the presence of oxygen. Upon testing several assay conditions, we observed the formation of two products (Figure 3a). One of them co-migrated with P1 formed in the absence of O2, but the other product (P2) migrated at a distinct retention time. P2 was not observed when O2 was replaced with hydrogen peroxide or potassium superoxide (Figure S7). In the presence of O2, the formation of P1 and P2 accompanied the production of 5′-dA (Figure S8), supporting that their production requires the reductive SAM cleavage. The UV–vis absorption spectrum of P2 was distinct from that of P1 or DarA and agreed with that of darobactin A (Figure 3b). P2 was produced only in the presence of O2, SAM, and PlDarE (Figure 3a). In addition, P2 production was observed only when flavodoxin/flavodoxin reductase was used as a reductant and not when using either SDT or Ti(III) citrate as a reductant (Figure S7). LC–MS characterization of this species revealed its molecular weight to be 12 Da larger than that of DarA (Figure 3c), which is consistent with the presence of two crosslinks and the addition of an oxygen atom.
To better characterize P1 and P2, we purified them by HPLC (Figure S9) and digested with proteinase K. Proteinase K has broad specificity and hydrolyzes amide bonds at the C-terminus to hydrophobic (aliphatic and aromatic) amino acids. The proteinase K treatment of each of these compounds yielded only one major product with UV absorption features identical to those of the parent molecules (Figure 4a,b). Based on the quantitation of the HPLC peak before and after the proteinase K treatment, the transformation was quantitative (>90% yield). Importantly, the proteinase K digest of P2 co-migrated with darobactin A on HPLC and LC–MS (Figures 4b, and S10) and showed an identical molecular weight to darobactin A (Figure 4c). Moreover, the MS/MS fragmentation pattern agreed with darobactin A (Figure S11) with the observation of the key fragment ions characteristic to the ether crosslinking, including those with hydroxyindole (Figures S11 and S12). These observations provided strong evidence that P2 has the ether and C–C crosslinks identical to that of darobactin A, and the physiological function of DarE is the C–C and ether crosslink formation using molecular oxygen as the source of ether oxygen.
Figure 4.
Characterization of PlDarE products. Shown are HPLC chromatograms (a and b) and mass spectra (c) of P1, P2, P1 + proteinase K, P2 + proteinase K, and the darobactin A standard. (b) magnified view of (ii), (iv), and (vi) in (a). See Figure S10 for the extracted ion chromatograms and full-range MS spectra.
The molecular weight of the P1 proteinase K digest (Figure 4c, panel i) was consistent with a pentapeptide (WNWSK) from the core sequence of DarA with a loss of 2 Da. The MS/MS fragmentation pattern was consistent with the loss of two hydrogen atoms from the W3 residue (Figures S11 and S12). No fragment ions specific to a C–C crosslink were observed, and the unique UV–vis absorption band at 350 nm suggests the presence of an extended aromatic system. Therefore, although we cannot fully eliminate the possibility of a W1–W3 C–C crosslink, we characterize P1 as DarA with Cα-Cβ desaturation of W3. To test if P1 is an intermediate of the P2 formation, we incubated purified P1 with DarE. However, even after prolonged incubation (>10 h), P1 was not consumed, and no products, including P2, were observed. Based on this observation, we propose P1 as an off-pathway shunt product.
To obtain further evidence for the use of O2 as the source of ether oxygen, we performed the PlDarE assay in the presence of 18O2. The MS characterization of P2 formed in the presence of 18O2 suggested an increase of the molecular weight by 2 Da compared to that of P2 from the assay with natural isotope O2 (Figure 5a). Based on the isotope signal pattern, the 18O enrichment of P2 generated in the presence of 18O2 was calculated as 81 ± 2% (Figure S13), which likely represents the imperfect 18O2 replacement. Furthermore, when we converted P2 produced in 18O2 into darobactin A, we observed a 2 Da increase of the molecular weight of darobactin A (Figure 5b). The 18O enrichment in darobactin A was estimated to be 85 ± 3% based on the ratio of the MS signal intensities at m/z 483.71 and 484.71 [M + 2H]2+. To test if 18O in water is incorporated into P2, we performed the DarE assay in [18O]water (80% enrichment) with natural isotope O2. The resulting P2 and its proteinase K digest exhibited mass spectra indistinguishable from those of P2 formed in natural isotope water (Figure 5a,b). The comparison of the m/z 483.71 and 484.71 signal intensities in the proteinase K digest revealed no detectable level of 18O incorporation (<5%, Figure 5b). These observations together suggest that DarE incorporates one of the oxygen atoms of O2 into the ether crosslink of P2. In sum, these results demonstrate that DarE catalyzes both C–C and ether crosslinking during darobactin A biosynthesis using O2 as a native co-substrate.
Figure 5.
Characterization of P2 formed in the presence of 18O2 or [18O]water. (a) Mass spectra of P2 formed in the presence of natural isotope O2, 18O2, or [18O]water. (b) Mass spectra of proteinase K digests of P2 formed in the presence of natural isotope O2, 18O2, or [18O]water. Shown are the z = +2 ions. Calculated m/z for [18O]darobactin A = 484.7110 [M + 2H]2+; Observed m/z 484.7133 (4.8 ppm). Calculated m/z for darobactin A = 483.7089 [M + 2H]2+; Observed m/z 483.7107 (3.7 ppm) and 483.7094 (1.0 ppm). * indicates the presence of natural abundance isotope P2 due to the imperfect [18O2]O2 gas exchange (see Figure S13 for the calculation of the 18O enrichment).
To obtain mechanistic insights into the DarE catalysis, we determined the stoichiometry of the reaction. P1 was quantified using synthetic darobactin core heptapeptide (WNWSKSF) as a standard. P2 was quantified using darobactin A as a standard. The extinction coefficient of darobactin A was determined to be 2.9 cm−1 mM−1 at 280 nm, which was significantly less than that of unmodified PlDarA (11.2 cm−1 mM−1), suggesting the significant effects of the indole ring modifications on the light absorption feature. Using these standards, HPLC quantitation revealed the formation of 10.8 ± 2.9 μM P1 and 50.3 ± 8.7 μM P2 from 100 μM PlDarA, suggesting that P2 is the major product of PlDarE assays. In the same assays, 5′-dA was quantified by HPLC as 111 ± 15 μM. Thus, the ratio of P1 : P2 : 5′-dA was 0.21 ± 0.04 : 1.0 : 2.3 ± 0.5. Since the desaturation reaction likely consumes 1 equiv of SAM, the results suggest that DarE consumes 2 equiv of SAM to produce P2.
Finally, we performed the activity assays using substoichiometric amounts of PlDarE relative to PlDarA. Consequently, we found that, at least under the current assay conditions, the amounts of P1 and P2 were always substoichiometric to PlDarE (Figure S7a). Since PlDarE catalyzes both the ether and C–C crosslinking without detectable accumulation of intermediates, PlDarE is likely catalytic. Thus, the substoichiometric product formation is most likely due to a strong product inhibition, and a product release may require proteolytic cleavage of the leader peptide.
DISCUSSION
Our characterization of PlDarE revealed that this enzyme catalyzes both the ether and C–C crosslinks and produces modified DarA (P2) that can be converted into darobactin A after digestion with proteinase K. Of particular significance, we found that PlDarE uses O2 as the source of ether bond oxygen. This result is in sharp contrast to the recent proposal that N-terminally His-tagged P. khanii DarE (His-PkDarE) incorporates 18O from water into its product (DarA with a 14 Da modification). Although we cannot eliminate the possibility that PlDarE and PkDarE (61% amino acid sequence identity) catalyze the ether crosslinking in two distinct mechanisms, the reported characterization of the His-PkDarE product leaves significant ambiguity. The structure was proposed solely based on MS without showing specific fragment ions for the ether crosslink. The authors did not describe if the DarA + 14 Da species can be converted to darobactin A. No results were reported for His-PkDarE assays in the presence of O2. In addition, the His-PkDarE was partially reconstituted with only 8 Fe/DarE. Therefore, further characterizations are required to make conclusions about the mechanism of ether crosslink formation by PkDarE.
Still, the comparison of the results between His-PkDarE and His-PlDarE assays revealed an important commonality. In both cases, the major product is missing either or both of the ether and/or C–C crosslinks. His-PkDarE produced PkDarA +14 Da species as the major product and His-PlDarE produced PlDarA – 2 Da (P0). Even when the His-PlDarE assays were performed in the presence of O2, P0 remained the major product (Figure 2c). Importantly, both His-PkDarE and His-PlDarE were loaded with only 8–9 Fe per monomer. On the other hand, the untagged PlDarE was fully loaded with 12 Fe per monomer, suggesting the successful assembly of all three 4Fe–4S clusters. Therefore, the observed functional difference between His-tagged and untagged DarE is likely caused by the amount of cluster loading.
The ability of DarE to use O2 as a co-substrate in the physiologically relevant reaction is unprecedented among radical SAM enzymes. The only reported example of a radical SAM enzyme reaction with O2 is for MqnE,16 where the reaction with a substrate analogue designed to trap a radical intermediate yielded a hydroperoxide adduct. However, MqnE does not catalyze such a reaction with the native substrate and is hence unlikely to possess a specific mechanism to catalyze the hydroperoxide addition reaction. Thus, DarE’s native oxygenase activity is unprecedented and is of significant mechanistic interest.
Scheme 1 summarizes the possible mechanisms of O2-dependent ether crosslinking by DarE. Our current favorite mechanism is a reaction of O2 with the W3 Cβ radical (mechanism A). In this mechanism, DarE abstracts the Hβ atom of W3 and the resulting radical adds to O2. Subsequent transfers of an electron and a proton would yield a hydroperoxide intermediate. This reaction is analogous to that of the MqnE reaction with the radical-trapping substrate analogue. In the MqnE reaction, the stabilization of the organic radical using the substrate was critical for its reaction with O2. In the DarE catalysis, the W3 Cβ radical is stabilized by the delocalization through the indole ring, which may allow O2 to react. For the subsequent ether formation, multiple mechanisms are conceivable. For example, a nucleophilic attack of the indole ring of W1 to the hydroperoxide followed by rearomatization would yield the ether product (mechanism A1). Alternatively, a transfer of an electron and a proton would allow homolytic cleavage of the O–O bond to yield an alkoxy radical, which then attacks the indole ring of W1 (Scheme 1, mechanism A2). Subsequent oxidative quenching of the indole radical would yield the ether product.
Scheme 1.
Proposed Mechanisms of DarE-catalyzed Ether Crosslink Formation
The observation of P1 as a minor shunt product is also consistent with the formation of the W3 Cβ radical. Most likely, P1 is formed when the W3 Cβ radical is formed in the absence of O2 bound to the active site, or the AUX clusters are in the incorrect redox state. (Note: the production of P1 requires an electron acceptor, whereas the ether crosslinking requires an electron donor.) Thus, the proposed mechanism explains the P1 formation in the absence of O2 or redox mismatch (see below for the relationship between the reductant and AUX clusters). Since P1 was not converted to the ether product, we can eliminate all the mechanistic options with P1 as an intermediate including the one proposed by Guo et al.12
Tryptophan is susceptible to oxidation by reactive oxygen species (ROS),17 and 7-hydroxylation proceeds by the addition of a hydroxyl radical.17,18 Thus, it is also conceivable that the oxygen insertion by DarE proceeds via W1 oxidation using ROS such as the hydroxyl radical generated from O2 in the active site (Scheme 1, mechanism B). The resulting 7-hydroxy-W1 would be used for ether bond formation using a radical SAM chemistry either via coordination of the 7-hydroxy group to the AUX1 cluster or α,β-desaturated W3 in a way similar to what has been proposed for thioether crosslinked RiPP biosynthesis.10,19,20 In this mechanism, the P1 formation is explained by a promiscuous reactivity of DarE, in which DarE generates the W3-Cβ radical prior to the W1 oxidation. To test mechanism B, we performed DarE assays with hydrogen peroxide or potassium superoxide, potential precursors of hydroxyl radical formation from O2. However, no P2 was observed. No W1 oxidation was observed when the DarE assays were performed in the absence of SAM. Thus, while these results do not necessarily eliminate mechanism B, we currently think it is less likely.
In our in vitro assays, the ether crosslink was observed only when flavodoxin was used as a reductant. Assays with chemical reductants, such as SDT or Ti(III) citrate, did not yield P2. The observed requirement for flavodoxin is likely due to the rapid scavenging of oxygen by chemical reductants (e.g., ~700 μM s−1 with 2 mM SDT at 25 °C21) compared to flavodoxin (~0.0017 μM s−122). In addition, flavodoxin may be able to better set the redox state of the 4Fe–4S clusters for the ether bond formation. The proposed mechanisms of ether crosslinking require an injection of at least one electron (Scheme 1). While the donor of this electron is currently unknown, one of the candidates is the AUX clusters. On the other hand, the formation of C–C crosslinking between W3 and K5 would require the AUX clusters to serve as electron acceptors (see Scheme 2 and the related discussion below). Therefore, the AUX clusters of DarE would serve as both the electron donor and acceptor, in which the AUX clusters must be in an appropriate redox state in each step of catalysis without being over-reduced or oxidized. Such an adjustment may be better achieved by a more physiologically relevant reductant, flavodoxin, than by chemical reductants. In general, the role of reductase in radical SAM enzymes is not well-understood, although biological reductases were reported to be critical for the physiological function of several radical SAM enzymes, including PqqE23 and NosL.24 Our observation of the strict requirement of flavodoxin for DarE’s oxygenase activity further emphasizes the significant role of reductases in radical SAM enzyme catalysis.
Scheme 2.
Proposed Mechanisms of DarE-catalyzed C–C Crosslink Formation
DarE’s use of O2 as a co-substrate in the biologically relevant reaction is unprecedented in radical SAM enzymes and was unexpected due to the oxygen-sensitive nature of radical SAM enzymes. However, accumulating evidence suggests that radical SAM enzymes function in aerobic cells. A recent study of Dph1–Dph2 suggested that this non-canonical radical SAM enzyme in diphthamide biosynthesis is associated with an Fe-protein Dph3 that can donate Fe and repair an oxidatively damaged 4Fe–4S cluster of Dph1–Dph2.25 While the mechanism of cluster maintenance and the physiological reductase are unknown for most other radical SAM enzymes, the presence of such mechanisms in cells would allow radical SAM enzymes to function under aerobic conditions. Our discovery of DarE as a radical SAM oxygenase emphasizes that at least some radical SAM enzymes function under an aerobic cellular environment and can tolerate certain levels of O2 in the presence of the physiological reductant or 4Fe–4S cluster maintenance machinery.
In addition to the ether crosslink, DarE catalyzed the C–C crosslinking between W3 and K5. A recent report on SuiB,26 a radical SAM enzyme that catalyzes C–C crosslinking during the biosynthesis of streptide RiPPs, suggested that the C–C bond crosslinking on these RiPPs proceeds through the formation of an amino acid Cβ radical, followed by its addition to an sp2 carbon of an aromatic side chain and oxidative radical quenching.27 This mechanism is certainly a plausible option for DarE’s W3–K5 crosslinking. However, considering the distant sequence similarity of DarE and SuiB, it is also possible that DarE catalyzes the C–C crosslinking by a different mechanism. Thus, we here consider an alternative mechanism, in which the K5 Cβ radical is oxidatively quenched to yield an α,β-unsaturated peptide (Scheme 2, mechanism B). The subsequent nucleophilic attack of W3 would yield the C–C crosslink. The nucleophilic mechanism was originally proposed for radical SAM enzymes that form thioether crosslinked RiPPs,19 although limited evidence is currently available. For DarE, our observation of P1 production is consistent with DarE’s ability to catalyze the α,β-desaturation. Further study is needed to test if the α,β-desaturation is used as a mechanism of the C–C crosslink formation.
The order of C–C and ether crosslink formation may be deduced from our current observations. The formation of P1 demonstrates that DarE can generate the Cβ radical on W3 without the W3–K5 crosslinking. No W3–K5 crosslink was observed in the absence of the W1–W3 crosslink. Thus, although we cannot be exclusive, our data suggest that the W1–W3 ether crosslink precedes the W3–K5 crosslink. Future characterization of an on-pathway intermediate will provide further insights into the order of crosslink formation.
CONCLUSIONS
Our in vitro characterization of PlDarE elucidated that this enzyme is a unique dual-function enzyme that catalyzes both the ether and C–C crosslinks. The ether crosslink formation requires O2 as the source of the ether oxygen. PlDarE also catalyzed the production of an α,β-unsaturated peptide. Thus, this enzyme makes three chemically distinct modifications on the DarA peptide substrate. Future mechanistic characterization of this enzyme would reveal novel insights into the mechanism of RiPP biosynthesis by radical SAM enzymes.
Supplementary Material
ACKNOWLEDGMENTS
We acknowledge Leo Padva for cloning initial expression constructs and thank Yu Imai for providing darobactin A.
Funding
This work was supported by the Duke University School of Medicine and in part the National Institute of General Medical Sciences R01 GM112838 (to K.Y.) and by the National Institute of Allergy and Infectious Diseases R01 AI158388 (to K.L.). I Dewa M. Kresna thanks the Deutscher Akademischer Austauschdienst (DAAD) for his PhD scholarship. The German Federal Ministry of Education and Research (BMBF) and the Hesse State Ministry of Higher Education Research and Arts (HMWK) supported research in the Schaberle lab. J.R., E.d.l.M., and Y.N thank the French National Research agency for financial support through the contract ANR-20-CE44-0005. They used the platforms of the Grenoble Instruct-ERIC center (ISBG; UMS 3518 CNRS-CEA-UGA-EMBL) within the Grenoble Partnership for Structural Biology (PSB), which is supported by FRISBI (ANR-10-INBS-05-02) and GRAL and financed within the Universite Grenoble Alpes graduate school (Ecoles Universitaires de Recherche) CBH-EUR-GS (ANR-17-EURE-0003).
ABBREVIATIONS
- BGC
biosynthetic gene cluster
- RiPP
ribosomally synthesized and post-translationally modified peptide
- SAM
S-adenosylmethionine
- AA
amino acid
Footnotes
The authors declare no competing financial interest.
Supporting Information
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/jacs.2c05565.
Oligo sequence; sodium dodecyl sulfate polyacrylamide gel electrophoresis and UV–vis spectra of DarA and DarE; MS and MS/MS spectra of DarA, modified DarA, proteolyzed DarE products, darobactin A; and 5′-dA; and AlphaFold model of DarE (PDF)
Contributor Information
Hai Nguyen, Department of Biochemistry, Duke University School of Medicine, Durham, North Carolina 27710, United States.
I Dewa Made Kresna, Institute for Insect Biotechnology, Justus-Liebig-University of Giessen, 35392 Giessen, Germany.
Nils Böhringer, Institute for Insect Biotechnology, Justus-Liebig-University of Giessen, 35392 Giessen, Germany; German Center for Infection Research (DZIF), Partner Site Giessen-Marburg-Langen, 35392 Giessen, Germany.
Jeremie Ruel, University of Grenoble Alpes, CEA, CNRS, IBS, Metalloproteins Unit, F-38000 Grenoble, France.
Eugenio de la Mora, University of Grenoble Alpes, CEA, CNRS, IBS, Metalloproteins Unit, F-38000 Grenoble, France.
Jil-Christine Kramer, Institute for Insect Biotechnology, Justus-Liebig-University of Giessen, 35392 Giessen, Germany.
Kim Lewis, Antimicrobial Discovery Center, Department of Biology, Northeastern University, Boston, Massachusetts 02115, United States.
Yvain Nicolet, University of Grenoble Alpes, CEA, CNRS, IBS, Metalloproteins Unit, F-38000 Grenoble, France.
Till F. Schäberle, Institute for Insect Biotechnology, Justus-Liebig-University of Giessen, 35392 Giessen, Germany; German Center for Infection Research (DZIF), Partner Site Giessen-Marburg-Langen, 35392 Giessen, Germany; Department of Bioresources, Fraunhofer Institute for Molecular Biology and Applied Ecology, 35392 Giessen, Germany.
Kenichi Yokoyama, Department of Biochemistry, Duke University School of Medicine, Durham, North Carolina 27710, United States; Department of Chemistry, Duke University, Durham, North Carolina 27710, United States.
REFERENCES
- (1).Imai Y; Meyer KJ; Iinishi A; Favre-Godal Q; Green R; Manuse S; Caboni M; Mori M; Niles S; Ghiglieri M; et al. A new antibiotic selectively kills Gram-negative pathogens (vol 576, pg 459, 2019). Nature 2020, 580, No. E3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Kaur H; Jakob RP; Marzinek JK; Green R; Imai Y; Bolla JR; Agustoni E; Robinson CV; Bond PJ; Lewis K; et al. The antibiotic darobactin mimics a beta-strand to inhibit outer membrane insertase. Nature 2021, 593, 125. [DOI] [PubMed] [Google Scholar]
- (3).Wuisan ZG; Kresna IDM; Böhringer N; Lewis K; Schäberle TF Optimization of heterologous Darobactin A expression and identification of the minimal biosynthetic gene cluster. Metab. Eng 2021, 66, 123–136. [DOI] [PubMed] [Google Scholar]
- (4).Groß S; Panter F; Pogorevc D; Seyfert CE; Deckarm S; Bader CD; Herrmann J; Müller R Improved broad-spectrum antibiotics against Gram-negative pathogens via darobactin biosynthetic pathway engineering. Chem. Sci 2021, 12, 11882–11893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Sofia HJ; Chen G; Hetzler BG; Reyes-Spindola JF; Miller NE Radical SAM, a novel protein superfamily linking unresolved steps in familiar biosynthetic pathways with radical mechanisms: functional characterization using new analysis and information visualization methods. Nucleic Acids Res. 2001, 29, 1097–1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Broderick JB; Duffus BR; Duschene KS; Shepard EM Radical S-adenosylmethionine enzymes. Chem. Rev 2014, 114, 4229–4317 Research Support, N.I.H., Extramural Research Support, U.S. Gov’t, Non-P.H.S.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Grell TA; Goldman PJ; Drennan CL SPASM and twitch domains in S-adenosylmethionine (SAM) radical enzymes. J. Biol. Chem 2015, 290, 3964–3971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Schramma KR; Bushin LB; Seyedsayamdost MR Structure and biosynthesis of a macrocyclic peptide containing an unprecedented lysine-to-tryptophan crosslink. Nat. Chem 2015, 7, 431–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Nguyen TQN; Tooh YW; Sugiyama R; Nguyen TPD; Purushothaman M; Leow L; Hanif K; Yong RHS; Agatha I; Winnerdy FR; et al. Post-translational formation of strained cyclophanes in bacteria. Nat. Chem 2020, 12, 1042. [DOI] [PubMed] [Google Scholar]
- (10).Flühe L; Knappe TA; Gattner MJ; Schäfer A; Burghaus O; Linne U; Marahiel MA The radical SAM enzyme AlbA catalyzes thioether bond formation in subtilosin A (vol 8, pg 350, 2012). Nat. Chem. Biol 2012, 8, 737. [DOI] [PubMed] [Google Scholar]
- (11).Clark KA; Bushin LB; Seyedsayamdost MR Aliphatic Ether Bond Formation Expands the Scope of Radical SAM Enzymes in Natural Product Biosynthesis. J. Am. Chem. Soc 2019, 141, 10610–10615. [DOI] [PubMed] [Google Scholar]
- (12).Guo S; Wang S; Ma S; Deng Z; Ding W; Zhang Q Radical SAM-dependent ether crosslink in daropeptide biosynthesis. Nat. Commun 2022, 13, 2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Grote A; Hiller K; Scheer M; Munch R; Nortemann B; Hempel DC; Jahn D JCat: a novel tool to adapt codon usage of a target gene to its potential expression host. Nucleic Acids Res. 2005, 33, W526–W531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Gibson DG; Young L; Chuang R-Y; Venter JC; Hutchison CA; Smith HO Enzymatic assembly of DNA molecules up to several hundred kilobases. Nat. Methods 2009, 6, 343–345. [DOI] [PubMed] [Google Scholar]
- (15).fish WW Rapid Colorimetric Micromethod for the Quantitation of Complexed Iron in Biological Samples. Methods Enzymol. 1988, 158, 357–364. [DOI] [PubMed] [Google Scholar]
- (16).Joshi S; Fedoseyenko D; Sharma V; Nesbit MA; Britt RD; Begley TP Menaquinone Biosynthesis: New Strategies to Trap Radical Intermediates in the MqnE-Catalyzed Reaction. Biochemistry 2021, 60, 1642–1646. [DOI] [PubMed] [Google Scholar]
- (17).Jacobitz AW; Liu Q; Suravajjala S; Agrawal NJ Tryptophan Oxidation of a Monoclonal Antibody Under Diverse Oxidative Stress Conditions: Distinct Oxidative Pathways Favor Specific Tryptophan Residues. J. Pharm. Sci 2021, 110, 719–726. [DOI] [PubMed] [Google Scholar]
- (18).Solar S; Solar W; Getoff N Resolved Multisite Oh-Attack on Aqueous Tryptophan Studied by Pulse-Radiolysis. Radiat. Phys. Chem 1984, 23, 371–376. [Google Scholar]
- (19).Bruender NA; Wilcoxen J; Britt RD; Bandarian V Biochemical and Spectroscopic Characterization of a Radical S-Adenosyl-L-methionine Enzyme Involved in the Formation of a Peptide Thioether Cross-Link. Biochemistry 2016, 55, 2122–2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Grove TL; Himes PM; Hwang S; Yumerefendi H; Bonanno JB; Kuhlman B; Almo SC; Bowers AA Structural Insights into Thioether Bond Formation in the Biosynthesis of Sactipeptides. J. Am. Chem. Soc 2017, 139, 11734–11744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Tao Z; Goodisman J; Souid AK Oxygen measurement via phosphorescence: reaction of sodium dithionite with dissolved oxygen. J. Phys. Chem. A 2008, 112, 1511–1518. [DOI] [PubMed] [Google Scholar]
- (22).Mayhew SG; Foust GP; Massey V Oxidation-reduction properties of flavodoxin from Peptostreptococcus elsdenii. J. Biol. Chem 1969, 244, 803–810. [PubMed] [Google Scholar]
- (23).Barr I; Latham JA; Iavarone AT; Chantarojsiri T; Hwang JD; Klinman JP Demonstration That the Radical S-Adenosylmethionine (SAM) Enzyme PqqE Catalyzes de Novo Carbon-Carbon Cross-linking within a Peptide Substrate PqqA in the Presence of the Peptide Chaperone PqqD. J. Biol. Chem 2016, 291, 8877–8884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Sicoli G; Mouesca JM; Zeppieri L; Amara P; Martin L; Barra AL; Fontecilla-Camps JC; Gambarelli S; Nicolet Y Fine-tuning of a radical-based reaction by radical S-adenosyl-L-methionine tryptophan lyase. Science 2016, 351, 1320–1323. [DOI] [PubMed] [Google Scholar]
- (25).Zhang YG; Su D; Dzikovski B; Majer SH; Coleman R; Chandrasekaran S; Fenwick MK; Crane BR; Lancaster KM; Freed JH; et al. Dph3 Enables Aerobic Diphthamide Biosynthesis by Donating One Iron Atom to Transform a [3Fe-4S] to a [4Fe-4S] Cluster in Dph1-Dph2. J. Am. Chem. Soc 2021, 143, 9314–9319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Balo AR; Caruso A; Tao L; Tantillo DJ; Seyedsayamdost MR; Britt RD Trapping a cross-linked lysinetryptophan radical in the catalytic cycle of the radical SAM enzyme SuiB. Proc. Natl. Acad. Sci. U.S.A 2021, 118, No. e2101571118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Yokoyama K; Lilla EA C-C bond forming radical SAM enzymes involved in the construction of carbon skeletons of cofactors and natural products. Nat. Prod. Rep 2018, 35, 660–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.