Abstract
Background:
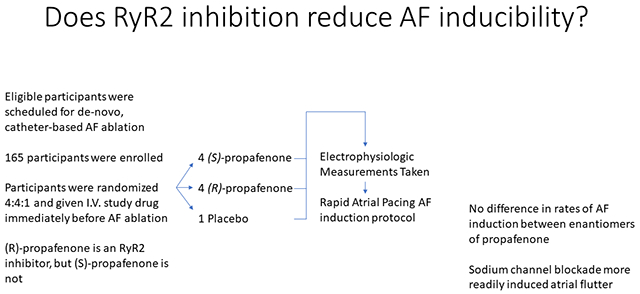
Experimental data suggest RyR2-mediated intracellular calcium leak is a mechanism for atrial fibrillation (AF), but evidence in humans is still needed. Propafenone is comprised of two enantiomers that are equally potent sodium channel blockers, however (R)-propafenone is an RyR2 inhibitor whereas (S)-propafenone is not. This study tested the hypothesis that RyR2 inhibition with (R)-propafenone prevents induction of AF compared to (S)-propafenone or placebo in patients referred for AF ablation.
Methods:
Participants were randomized 4:4:1 to a one-time intravenous dose of (R)-propafenone, (S)-propafenone, or placebo. The study drug was given at the start of the procedure and an AF induction protocol using rapid atrial pacing was performed prior to ablation. The primary endpoint was 30 seconds of AF or atrial flutter (AFL).
Results:
193 participants were enrolled and 165 (85%) completed the study protocol (median age: 63 years, 58% male, 95% paroxysmal AF). Sustained AF and/or AFL was induced in 60 participants (84.5%) receiving (R)-propafenone, 60 (80.0%) receiving (S)-propafenone group, and 12 (63.2%) receiving placebo. AFL occurred significantly more often in the (R)-propafenone (N=23, 32.4%) and (S)-propafenone (N=26, 34.7%) groups compared to placebo (N=1, 5.3%, P=0.029). There was no significant difference between (R) vs. (S)-propafenone for the primary outcome of AF and/or AFL induction in univariable (P=0.522) or multivariable analysis (P=0.199, adjusted for age and serum drug level).
Conclusion:
There is no difference in AF inducibility between (R)-propafenone and (S)-propafenone at clinically relevant concentrations. These results are confounded by a high rate of inducible atrial flutter due to sodium-channel blockade.
Clinical Trial Registration:
https://clinicaltrials.gov; Unique Identifier: NCT02710669
Keywords: atrial fibrillation, ablation, clinical trial, propafenone, calcium leak
Graphical Abstract
Introduction
Atrial fibrillation (AF) is the most prevalent cardiac arrhythmia requiring antiarrhythmic drug therapy and recent data support expanding its use based on a beneficial effect of rhythm control (ablation and antiarrhythmic drugs) early after AF diagnosis. However, a challenge with antiarrhythmic drug therapy has been the heterogeneity of underlying etiologies for AF and it could be improved by targeting therapy to underlying mechanisms. Accordingly, we seek to investigate intracellular calcium leak through the cardiac ryanodine receptor (RyR2) calcium release channel as a potential mechanism for AF risk.1–3 Experimental data from our group and others using mouse models demonstrate that pathologic RyR2-mediated calcium leak triggers paroxysmal AF4–9, but research to support its relevance in humans is still needed. Accordingly, we propose a mechanistic clinical trial using stereospecific formulations of propafenone to study whether pharmacologically targeting RyR2 will prevent initiation of AF.
Propafenone is a commonly used medication to treat atrial arrhythmias. The formulation used clinically is a 50/50 racemic mixture of the (R) and (S)-enantiomers.10 Both enantiomers inhibit the fast sodium current (INa) to a comparable degree, which has traditionally been considered its primary mechanism of action; however, (R) and (S)-propafenone also have stereospecific properties (Figure 1).10–12 (R)-propafenone is an RyR2 inhibitor but not a beta-blocker, whereas (S)-propafenone is a beta-blocker but a much weaker inhibitor of RyR2 channels.11, 12 To test the hypothesis that RyR2 inhibition prevents initiation of AF, we randomized 192 participants scheduled to undergo AF ablation to receive a one-time intravenous dose of (R)-propafenone (RyR2 inhibitor) versus (S)-propafenone (not an RyR2 inhibitor at clinically relevant concentrations)10 versus placebo to undergo an AF stimulation protocol with rapid atrial pacing using an intracardiac electrode. This was performed in the EP lab prior to AF ablation. The primary endpoint was induction of 30 seconds of AF, atrial flutter (AFL), or atrial tachycardia.13
Figure 1:
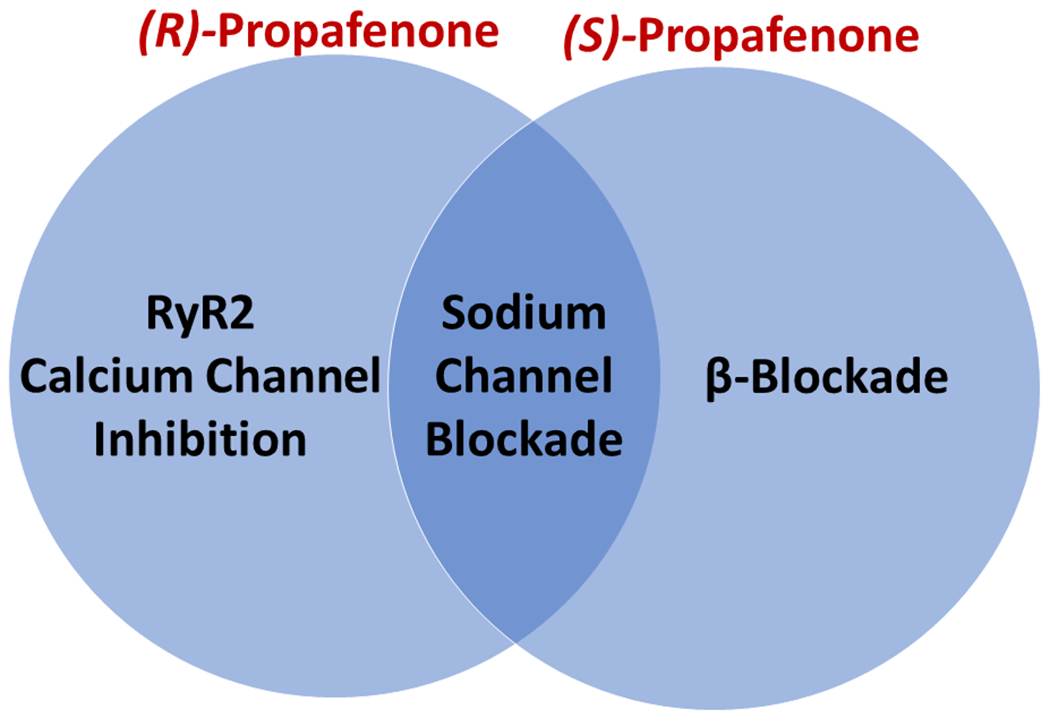
Stereospecific Properties of (R)- and (S)-Propafenone
Methods
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Study Design
The study was a single center, randomized, double-blind clinical trial. Participants were assigned to (R)-propafenone, (S)-propafenone or placebo in a 4:4:1 ratio using permuted block randomization with a randomly varying balancing interval.
Study Population
Eligible subjects were adults greater than 18 years of age who were diagnosed with AF and referred to undergo AF ablation. Major exclusion criteria were: 1) prior catheter or surgical AF ablation, 2) use of amiodarone within the preceding 3 months, 3) inability to stop antiarrhythmic medications prior to the procedure, 4) currently in AF at the time of screening, 5) individuals without a permanent pacemaker or implantable cardioverter defibrillator who had sinus node dysfunction or atrioventricular (AV) block defined as resting heart rate less than 50 beats per minute, PR interval greater than 280 ms, QRS duration greater than 120 ms, or second (Mobitz I or II) or third degree AV block, 6) concomitant use of CYP3A4 or CYP2D6 inhibitors, 7) left ventricular ejection fraction less than 40%, or 8) New York Heart Association Class III or IV heart failure symptoms. The study is registered with clinicaltrials.gov and a complete list of the eligibility criteria can be found there (NCT02710669). All participants underwent written, informed consent. The study protocol was approved by the Vanderbilt University Medical Center Institutional Review Board. A Data Safety Monitoring Board (DSMB) that included members with expertise in cardiology, electrophysiology, pharmacology, biostatistics, and ethics was established and met regularly to address study progress, safety, and data integrity.
Study Drugs
The use of (R) and (S)-propafenone for this study was performed under FDA Investigational New Drug Application #126813 (Comparison of (R) and (S)-propafenone for Prevention of Atrial Fibrillation Induction). (R) and (S)-propafenone hydrochloride were manufactured by the University of Minnesota’s Institute for Therapeutics Discovery and Development (Minneapolis, MN). Injectable formulations were compounded by the Vanderbilt Investigational Drug Services by dissolving either (R) or (S)-propafenone hydrochloride in 5% dextrose water to achieve a 2 mg/mL solution. Per instructions from the FDA, an open-label run-in phase was performed where (R) and (S)-propafenone were administered to three participants each at a dose of 1 mg/kg. The DSMB reviewed the results and determined that the 1 mg/kg dose achieved optimal levels of inducibility and safety and recommended the trial proceed using the 1 mg/kg dose rather than increasing to 2 m/kg. Dosing was based on body weight up to a maximum dose of 100 mg. It was prepared as a 100 mL solution. The matching placebo was 100 mL of D5W.
Study Protocol
Prior to the AF ablation procedure, participants followed the instructions provided by their clinical team. Antiarrhythmic medications were held for 3 days prior to the procedure. AV nodal blockers were continued due to ethical concerns that their discontinuation could increase the risk for adverse events such as AF with rapid ventricular rate, angina, or heart failure exacerbation. Upon arrival to the electrophysiology laboratory, participants underwent endotracheal intubation and were administered general anesthesia. The study drug was infused while the participant underwent sterile preparation, central venous access placement, and positioning of the intracardiac electrodes. The study drug was infused over 10 minutes and then allowed to distribute another 10 minutes prior to the start of the pacing protocol.14–16
Assessment of Surface ECG Intervals
Surface 12-lead ECG electrodes were placed in standard position. Pre-study drug ECG measurements (RR, PR, QRS, QTc) were acquired immediately prior to drug administration and post-study drug measurements were acquired 20-minutes later. All participants were in a sinus or atrial-paced rhythm. The recording systems used included St. Jude EP Workmate (Abbott Laboratories Inc, Abbott Park, IL) or GE CardioLab (General Electric Inc, Chicago, IL). ECG measurements were made using digital caliper tools at a sweep speed of 100 mm/s.
Determination of the Primary Arrhythmia Inducibility Endpoint and Assessment of AV Nodal and Atrial Conduction and Refractoriness
Unlike the surface ECG measurements, there was no measurement of intracardiac intervals (AV node conduction and refractoriness) prior to study drug administration because intracardiac electrodes were not yet positioned in the body. This was due to ethical concerns and logistical considerations regarding prolongation of the procedure time for research purposes. Twenty minutes after start of the study drug, an abbreviated electrophysiology (EP) study was performed. All participants underwent placement of a decapolar coronary sinus catheter (Webster CS Bidirectional Catheter, Biosense Webster Inc, Irvine, CA). Pacing was performed from the proximal electrode (9,10) at 20 milliamps and a pulse width of 2 ms. The primary endpoint of the study was induction of 30 seconds of AF, atrial flutter (AFL), or atrial tachycardia (AT). After the primary endpoint was met, the study protocol was complete and additional pacing/stimulation was not performed. Step 1 of the EP study was measurement of the AV block (Wenckebach) cycle length (AVBCL), AV node effective refractory period (AVN ERP) and atrial ERP (AERP). AVN ERP and AERP were measured at drive trains (S1) of 600 ms and 450 ms. Extrastimuli (S2) were introduced starting at a coupling interval of 500ms and decremented by 10ms with each pacing train. A 3-second rest period was used. Step 2 consisted of 15-beat bursts from the CS proximal electrode. The starting cycle length was 250ms, which was decremented by 10ms with each burst. A 10-second rest period was used between bursts. Step 2 was complete when 1:1 atrial capture was lost or a minimum cycle length of 180ms was reached. If the primary endpoint was not met in Step 2, Step 3 was performed. Step 3 consisted of 15-second bursts. The cycle length used for the bursts was the fastest cycle length achieved during Step 2 that maintained 1:1 atrial conduction. A 10-second rest period was used between bursts. The rest period began after completion of the pacing train, or after spontaneous termination of any non-sustained atrial arrhythmias that were induced. A total of 5 bursts were performed. If the primary endpoint was not met after 5 bursts, the study protocol was considered complete and the participant was determined to be non-inducible (Table 1).
Table 1:
Electrophysiology Study/AF Induction Protocol
| Stage | Stopping Rules | |
|---|---|---|
| Stage 1 | AV Block Cycle Length | Met Primary End Point (protocol completed) |
| AV Node ERP (S1: 600) | ||
| Atrial ERP (S1: 600) | ||
| AV Node ERP (S1: 450) | ||
| Atrial ERP (S1: 450) | ||
| Stage 2 | 15-beat Atrial Burst at 250ms | Met Primary End Point (protocol completed) OR Lost 1:1 atrial capture (proceed to Stage 3) |
| 15-beat Atrial Burst at 240ms | ||
| 15-beat Atrial Burst at 230ms | ||
| 15-beat Atrial Burst at 220ms | ||
| 15-beat Atrial Burst at 210ms | ||
| 15-beat Atrial Burst at 200ms | ||
| 15-beat Atrial Burst at 1900ms | ||
| 15-beat Atrial Burst at 180ms | ||
| Stage 3 | #1 15-second* Atrial Burst | Met Primary End Point (protocol completed) |
| #2 15-second* Atrial Burst | ||
| #3 15-second* Atrial Burst | ||
| #4 15-second* Atrial Burst | ||
| #5 15-second* Atrial Burst | ||
| End Protocol | No sustained AF/AT/AFL induced |
The cycle length used for Stage 3 was the shortest cycle length (fastest pacing) that had 1:1 atrial conduction
Statistical Analysis
Continuous variables are presented as median and inter-quartile range (median, [IQR]) and categorical variables as the frequency and percentage (N,%). Data were analyzed using a modified intention-to-treat protocol. Participants who received study drug were included and those who were withdrawn prior to study drug administration and data collection were excluded. The primary analysis focused on comparing (R) and (S)-propafenone. For data collected at multiple time points (e.g. blood pressure, electrocardiographic intervals), comparisons were analyzed using the Wilcoxon signed-rank test. For data collected at one time point, comparisons were made using the Mann-Whitney U test. Univariable and multivariable logistic and ordinal regression analyses were used to test for associations between (R) vs. (S)-propafenone with the primary and secondary outcomes of interest. Univariable analysis of the primary and secondary endpoints and the association between (R) vs. (S)-propafenone was performed with a Fisher’s exact test for binary outcomes and a Chi-square for trend for the ordinal outcomes. The primary analysis was the adjusted logistic regression of induction of 30-seconds of AF, AFL, or atrial tachycardia using (R) vs. (S)-propafenone as the primary determinant. The sample size needed for the trial was calculated to be a total of 186 participants based on the ability to detect a minimum difference of 20% between (R)-propafenone and (S)-propafenone (control) for the primary endpoint using 80% power, a 2-sided significance level of 0.05, and estimating a 50% event rate for the primary endpoint in the (S)-propafenone (control) group. To account for dropout, a total of 210 participants were planned. To avoid overfitting the regression model, a 10:1 ratio was used for the number of covariates to the number of participants reaching the primary endpoint. These were pre-specified and included the treatment assignment, age and serum drug level. Two pre-specified secondary outcomes were also included in the analysis. Due to the propensity for class IC antiarrhythmic drugs to induce AFL, a pre-specified secondary analysis used AF-only as the endpoint and excluded participants for whom AFL was induced. This model was also adjusted for treatment assignment, age, and serum drug level. An ordinal regression was also pre-specified that used as an outcome the stage at which AF/AFL/AT was induced during the AF stimulation protocol (Stages 1-3). AF/AFL induction due to the stimulation protocol resulted in physiologically missing EP study data. Data for AVBCL, AVN ERP, AERP are presented as complete case analyses with the numbers for missing data reported. Due to the high degree of AFL induction, a post-hoc multivariable logistic regression was performed for induction of AFL based on drug levels, treatment assignment, history of typical AFL and LA size. Statistical analysis was performed using Stata build 16 (StataCorp LLC, College Station, TX) and R version 3.6.2 (R Foundation for Statistical Computing, https://www.R-project.org/). Figures were created using GraphPad Prism version 5.04 (GraphPad Software, San Diego, CA).
Results
Baseline Characteristics
The study was stopped early on May 7th, 2020 by the DSMB and investigator team because enrollment in clinical trials at our institution was halted indefinitely due to the COVID-19 pandemic (since March 23rd, 2020) and at that time enough participants had been enrolled for adequate power analysis of the primary endpoints. One hundred ninety-three participants were enrolled and 192 were randomized 4:4:1 to (R)-propafenone, (S)-propafenone, or placebo, respectively. One participant withdrew consent after randomization. Seventeen participants (8.9%) were noted to be in AF on the morning of their ablation procedure prior to administration of study drug and were therefore withdrawn per protocol. An additional nine participants (4.7%) were withdrawn at the discretion of the operator. One hundred sixty-five participants (86%) completed the study protocol and were analyzed using a modified intention-to-treat analysis. Seventy-one participants received (R)-propafenone, 75 participants received (S)-propafenone and 19 participants received placebo (Figure 2). Measurement of (R)- and (S)-propafenone levels confirmed participants received the correct study drug (Supplemental Figure 1). The study population was representative of an AF ablation population at our medical center (Table 2). The median age at enrollment was 63.4 years (IQR: [56.3, 70.2]), 58% male (N=96) and predominantly White (160, 97%). One-hundred-sixteen participants (70%) were on an AV nodal blocker (beta-blocker or non-DHP calcium channel blocker) at the time of EP study. Forty-eight participants (29.1%) had a history of atrial flutter and 11 (6.7%) had previously undergone CTI ablation. The majority of participants (156, 94.6%) had paroxysmal AF.
Figure 2:
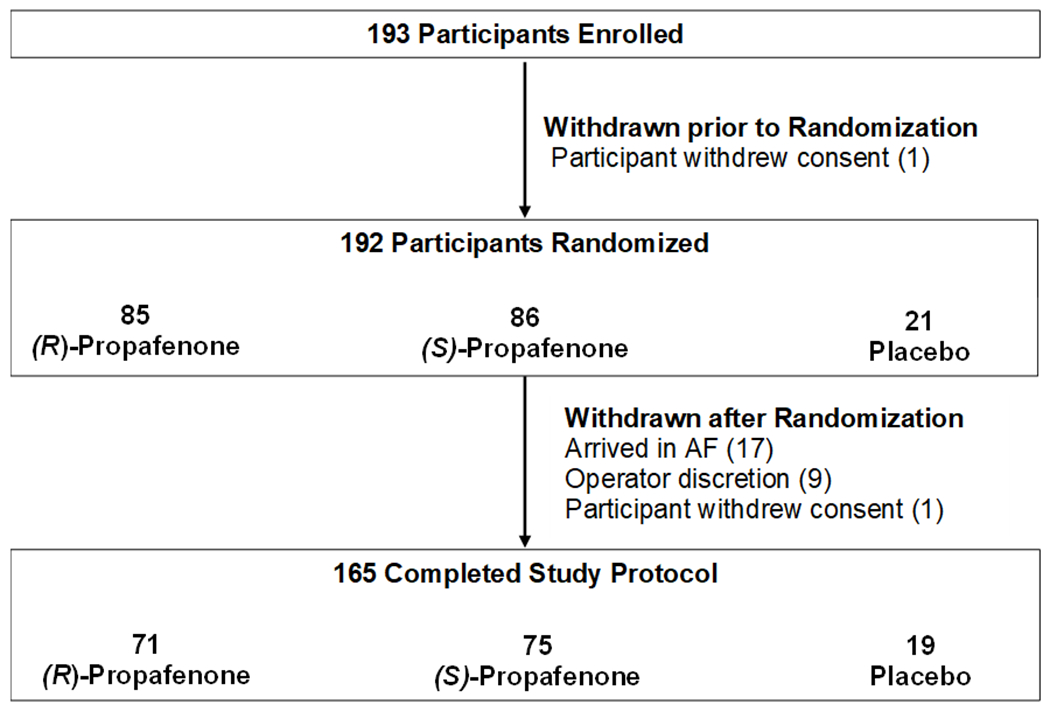
Consort Diagram
Table 2:
Demographics and Baseline Characteristics
| Overall | (R)-Propafenone | (S)-Propafenone | Placebo | |
|---|---|---|---|---|
|
| ||||
| N | 165 | 71 | 75 | 19 |
|
| ||||
| Age at enrollment | 63.4 [56.3, 70.2] | 61.8 [56.4, 68.5] | 64.9 [58.5, 70.7] | 56.3 [50.2, 70.6] |
|
| ||||
| Male | 96 (58.2%) | 38 (53.5%) | 46 (61.3%) | 12 (63.2%) |
|
| ||||
| Race | ||||
| White | 160 (97%) | 70 (98.6%) | 72 (96%) | 18 (94.7%) |
| Black | 3 (1.8%) | 1 (1.4%) | 2 (2.7%) | 0 (0%) |
| Asian | 2 (1.2%) | 0 (0%) | 1 (1.3%) | 1 (5.3%) |
| Other | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
|
| ||||
| BMI (kg/m2) | 30.1 [26.9, 34.4] | 29.3 [26.1, 33.2] | 30.4 [27.4, 35.7] | 29.6 [26.2, 35.7] |
|
| ||||
| Hypertension | 117 (70.9%) | 51 (71.8%) | 55 (73.3%) | 11 (57.9%) |
|
| ||||
| CAD | 24 (14.5%) | 11 (15.5%) | 11 (14.7%) | 2 (10.5%) |
|
| ||||
| Diabetes mellitus | 19 (11.5%) | 8 (11.3%) | 11 (14.7%) | 0 (0%) |
|
| ||||
| OSA | 41 (24.8%) | 21 (29.6%) | 18 (24%) | 2 (10.5%) |
|
| ||||
| CHF | 9 (5.5%) | 3 (4.2%) | 6 (8%) | 0 (0%) |
|
| ||||
| LVEF | ||||
| ≥50% | 164 (994%) | 70 (98.6%) | 75 (100%) | 19 (100%) |
| 40-49% | 1 (0.6%) | 1 (1.4%) | 0 (0%) | 0 (0%) |
|
| ||||
| Pacemaker/ICD | 7 (4.2%) | 2 (2.8%) | 4 (5.3%) | 1 (5.3%) |
|
| ||||
| Age at AF diagnosis | 60.0 [51.4, 66.4] | 59.2 [51.1, 65.2] | 61.3 [54.2, 67.0] | 51.5 [46.6, 68.9] |
|
| ||||
| Type of AF | ||||
| Paroxysmal | 156 (94.6%) | 63 (88.7%) | 75 (100%) | 18 (94.7%) |
| Persistent | 9 (5.4%) | 8 (11.3%) | 0 (0%) | 1 (5.3%) |
|
| ||||
| AV nodal blocker† | 116 (70%) | 44 (62%) | 48 (64%) | 14 (74%) |
|
| ||||
| Prior Class I | 83 (50.3%) | 33 (46.5%) | 40 (53.3%) | 10 (52.6%) |
|
| ||||
| Prior atrial flutter | 48 (29.1%) | 19 (26.8%) | 23 (30.7%) | 6 (31.6%) |
|
| ||||
| Prior CTI ablation | 11 (6.7%) | 5 (7.0%) | 4 (5.3%) | 2 (10.5%) |
Medians [IQR], N (%);
Includes beta-blockers and non-DHP calcium channel blockers used at the time of EP study.
Electrocardiographic and Electrophysiologic Measurements
The effect of study drug administration on ECG intervals is reported in Supplemental Tables 1 and 2 and Figure 3. Analysis of pooled data demonstrate that the RR-interval and QRS duration significantly increased with study drug administration for (R)-propafenone, (S)-propafenone, and placebo. The PR-interval significantly increased with administration of (R)- and (S)-propafenone, but not placebo. The QTc significantly increased with administration of (S)-propafenone, but not (R)-propafenone or placebo. Analysis of paired data demonstrates there was no significant difference between (R) and (S) propafenone in the magnitude of change for any of the ECG measurements, or when compared to placebo (Figure 3).
Figure 3:
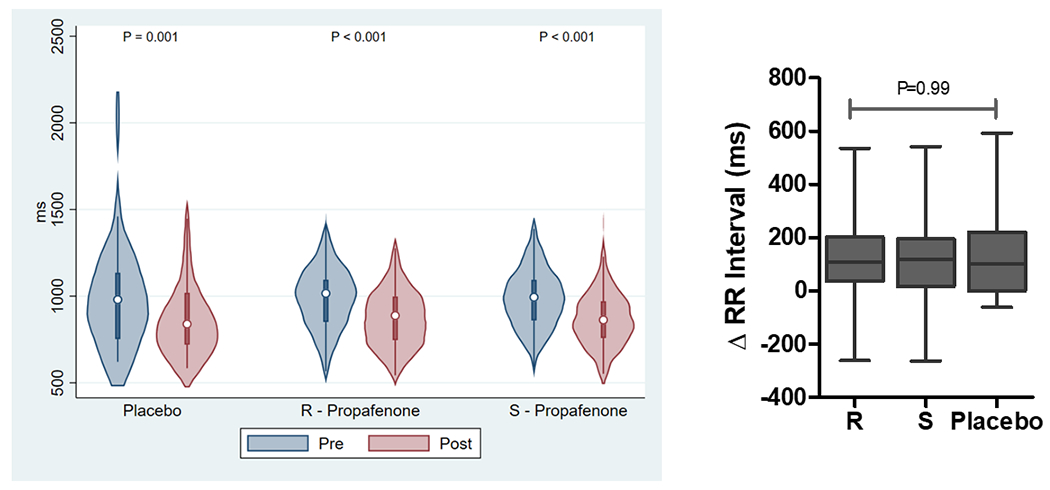
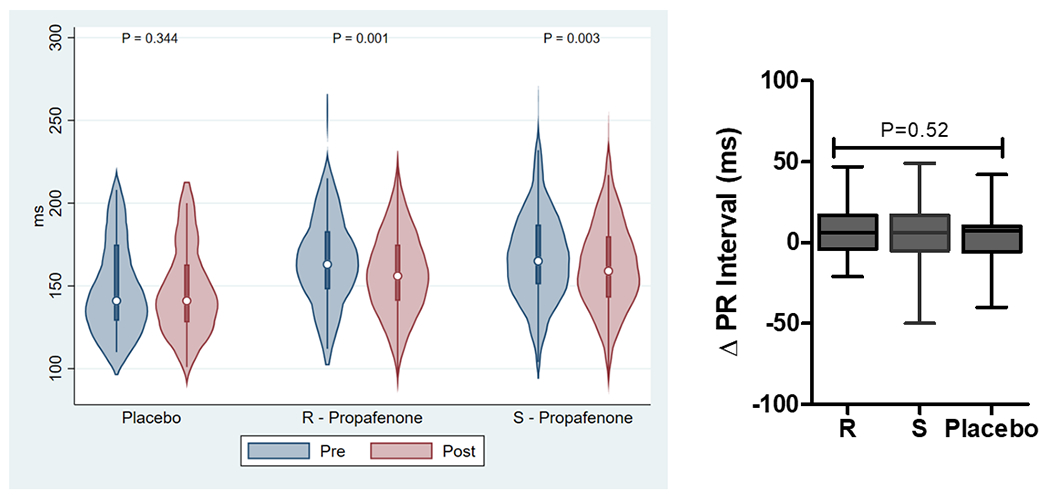
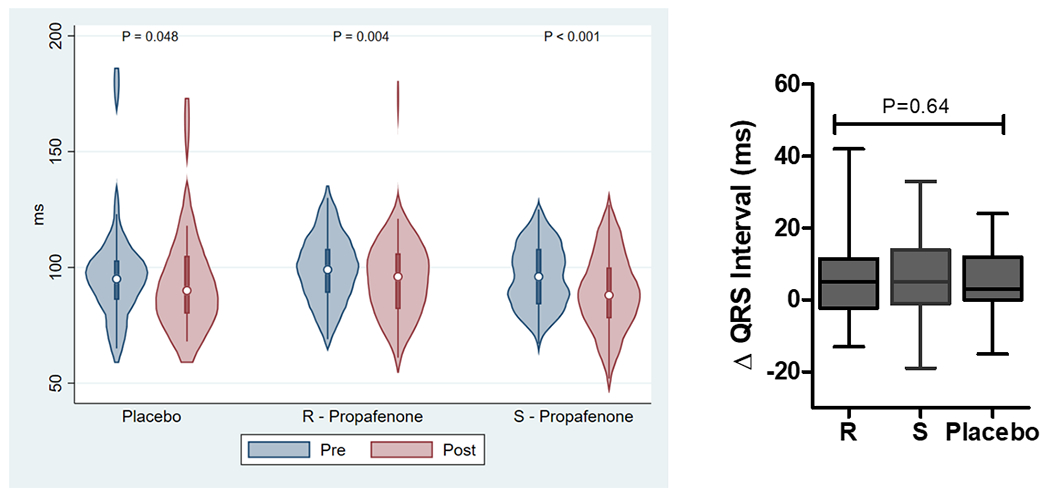
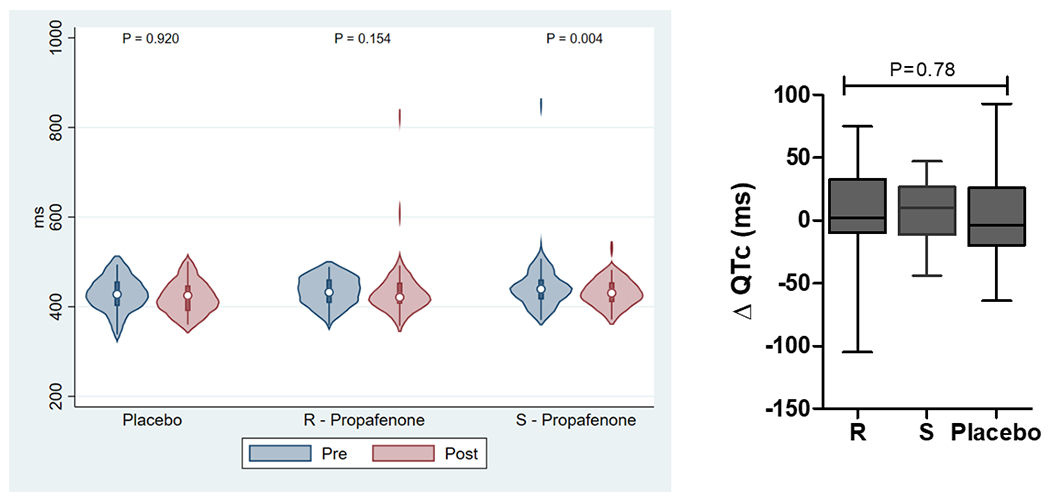
The Effect of Study Drug on ECG Intervals (RR, PR, QRS, QTc). Left figures are absolute ECG measurements pre/post study drug. Right figures are the change in ECG measurement pre/post study drug.(P-values were calculated using a Wilcoxon signed-rank test for pre/post comparisons and a Kruskal-Wallis H test for intra-group comparisons).
No clinically significant differences were noted between (R)-propafenone, (S)-propafenone, or placebo for AVBCL, AVN ERP, or AERP (drive trains of 600 ms and 450 ms used for ERP assessment; Supplemental Table 3). However, missing data for these measurements was common among participants that completed the protocol (12.7%, 21/165) limiting the assessment of these endpoints. Missing data occurred due to a variety of reasons: 1) sustained AF or AFL was induced during the EP study and therefore the primary endpoint was met and the study protocol ended (N=11), 2) the AVBCL was greater than the drive train (applies to AVN ERP only, N=2), and 3) AVN ERP was less than AERP (applies to AERP only, N=8).
Arrhythmia Induction
Sustained AF and/or AFL was induced in 132 participants (80.0%), 60 (84.5%) in the (R)-propafenone group, 60 (80.0%) in the (S)-propafenone group, and 12 (63.2%) in the placebo group (Supplemental Table 4). Fifty participants (30.3%) had only AFL induced. AFL occurred more in the (R)-propafenone (N=23, 32.4%) and (S)-propafenone (N=26, 34.7%) groups compared to placebo (N=1, 5.3%, P=0.029; Figure 4. In univariable analysis, there were no significant differences between (R) vs. (S)-propafenone for the primary outcome of AF and/or AFL induction (P = 0.522). In the secondary analysis examining AF-only, 37 (77.1%) of (R)-propafenone and 34 (69.4%) of (S)-propafenone participants had AF induced (P = 0.493). No significant differences were found in the stage of induction between the (R) and (S)-propafenone groups (P = 0.979; Chi-square for trend).
Figure 4:
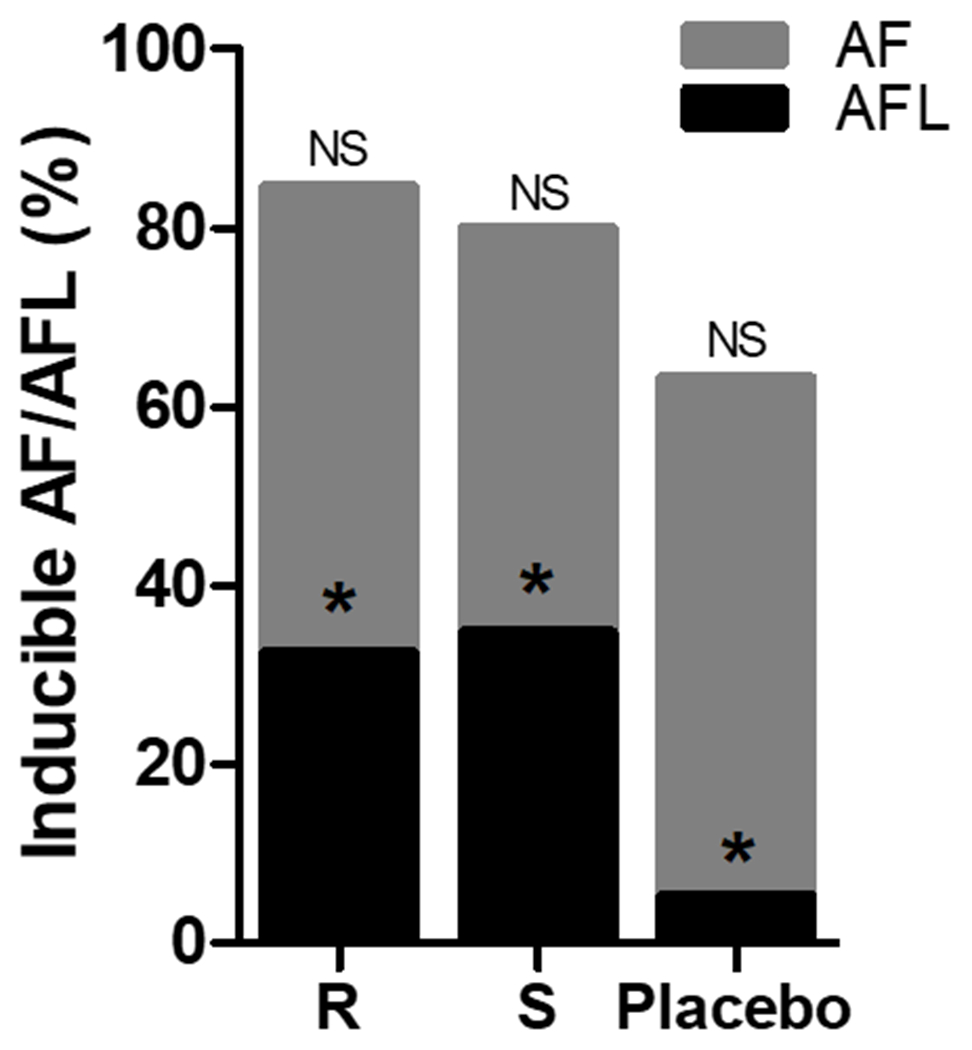
The Effect of Study Drug on AF/AFL Inducibility. There was a non-significant (NS) difference between treatment groups for the combined AF/AFL endpoint (P=0.12). *For AFL-only, P=0.02 for (R)-propafenone vs. Placebo and P=0.01 for (S)-propafenone vs. Placebo. There was no difference between (R) vs. (S)-propafenone (Fisher’s exact test).
In multivariable analysis, there was no significant association of atrial arrhythmia induction between (R)-propafenone and (S)-propafenone (OR 0.53, 95% CI: [0.21 – 1.39], P=0.199) with adjustment for age and serum drug level. Similarly, there was no significant observed difference in the secondary outcome of stage of induction (OR 1.06, 95% CI: [0.58 – 1.95], P=0.848) between (R) and (S)-propafenone. In the AF-only analysis, the observed difference in AF induction between (R) and (S)-propafenone remained non-significant in both the binary model (OR: 0.50, 95% CI: [0.18 – 1.39], P = 0.185) and the ordinal model (OR: 1.26, 95% CI: [0.57, 2.79], P=0.576).
A post-hoc analysis of AFL induction demonstrated that both (R) (OR: 8.70, 95% CI: [1.09 – 69.36], P=0.041) and (S)-propafenone (OR: 9.64, 95% CI: [1.22 – 76.42], P=0.032) were significantly associated with increased odds of AFL induction compared to placebo, after adjustment for history of typical atrial flutter and LA size. In a model adjusting for drug level, both (R) and (S)-propafenone levels were significantly associated with AFL induction; however, the interaction term (OR: 1.00, 95% CI: [1.00, 1.00], P=0.354) did not demonstrate a difference between the (R) and (S) enantiomers.
Discussion
This study successfully enrolled and randomized 192 participants into a mechanistic trial using investigational formulations of propafenone to study the effect of inhibiting intracellular RyR2-mediated calcium leak on AF inducibility. No difference was detected in the primary endpoint of AF inducibility between (R)-propafenone, which inhibits RyR2, and the active control, (S)-propafenone, a beta-blocker which has no RyR2 activity, or placebo. Both formulations of propafenone demonstrated a greater than 8-fold increase in the inducibility of AFL compared to placebo, which highlights a well-known problem with sodium channel blockade, and confounded the ability to detect any potential benefit on the inducibility of AF between treatment groups. These results: 1) leave open the question of whether RyR2 inhibition is effective for the treatment of AF in humans, and 2) do not support continued efforts to develop a stereospecific formulation of (R)-propafenone because there was no difference between it and (S)-propafenone on AF-inducibility. Future studies to investigate the potential of RyR2 inhibition for the treatment of AF should use RyR-selective drugs that do not inhibit Na channels, such as nanoparticle formulations of dantrolene (Ryanodex, Eagle Pharmaceuticals Inc., Woodcliff Lake, NH) that were not available when the trial was started, or newer RyR2-selective compounds that are in preclinical development, such as ent-verticilide.17
Our trial enrolled participants with mostly paroxysmal AF who were undergoing AF ablation and studied them at the time of their procedure. This design presents a unique opportunity to collect data on AF inducibility using a rapid atrial pacing protocol18, 19, which is a surrogate measure for a drug’s efficacy at treating AF and is similar to the rapid atrial pacing protocols used in pre-clinical animal research.20–22 We were able to enroll and complete data collection on 192 participants at a single site within 27 months (average enrollment >7 participants/month), which demonstrates the potential of this design as an efficient model for other Phase II clinical trials studying investigational drugs for AF. Advantages of this design are the large number of patients that currently undergo AF ablation facilitates recruitment. Also, administration of only a single dose of study drug and completion of data collection at one study visit avoids the need for labor-intensive long-term follow-up. However, it should be acknowledged that while AF inducibility with rapid atrial pacing has been used to evaluate the efficacy of AF ablation23, the evidence to support its use for detecting pulmonary vein triggers due to RyR2-mediated calcium release is limited to animal models6 and may not apply to humans. Another advantage of studying patients at the time of AF ablation is the opportunity to collect detailed electrophysiologic and hemodynamic data to define mechanisms, pharmacodynamics, and safety. Serum drug levels measured at the time of the post-drug EP study were within the range of published therapeutic levels for propafenone enantiomers.10, 24 The most important mechanistic observation was that AFL was induced at a significantly higher rate in the (R) and (S)-propafenone groups compared to placebo, which demonstrates the study drug distributed into cardiac tissue and the proarrhythmic effect of sodium-channel blockade on reducing conduction velocity and promoting reentry. However, there was no difference in the rate of AFL between the (R) and (S)-propafenone groups, which could be interpreted that in the setting of sodium channel blockade, RyR2 inhibition has an insignificant effect on factors promoting reentry. Alternatively, the protective effect of beta-blockade25–27 by (S)-propafenone may have offset the benefit from RyR2 blockade by (R)-propafenone, which does not have beta-blocking properties (Figure 1). It is also theoretically possible that, although (S)-propafenone exhibits little to no inhibitory activity on murine or ovine RyR2 channels, it cannot be excluded that (S)-propafenone acts differently on human RyR2 channels, which could explain the lack of difference between (R) and (S)-propafenone observed here.
The assessment of ECG intervals was confounded by a variety of factors. One was a higher degree of adrenergic stimulation during the pre-drug assessment because they were awake and unsedated, compared to the post-drug assessment when they were under stable general anesthesia. This was reflected by significantly shorter RR-intervals in all groups during the pre-drug assessment. There are also significant effects on ECG intervals due to the pharmacologic and physiologic effects of sedatives (e.g. propofol, opioids) and paralytics that were present at the post, but not pre-drug assessment. Also, AV nodal blockers were not discontinued prior to the procedure, which affects ECG interval assessment as well as a potential effect from beta-receptor blockade on AF inducibility. All of these factors contributed to no significant differences being detected for the change in ECG intervals between treatment arms (Figure 3).
Other limitations were due to the time constraint imposed by fitting the data collection protocol into the clinical workflow of AF ablation. This prohibited performing an EP study and rapid atrial pacing protocol before study drug administration, which would have enabled a baseline assessment of EP parameters and inducibility. Also, due to time constraints, we had to perform an abbreviated EP study, which entailed using standardized maximum output pacing stimulation (20 mA, 2 ms). Also, if sustained AF/AFL was induced during the EP study, the primary endpoint was determined to be met and the study complete, rather than performing cardioversion and resuming the EP study. This resulted in a higher rate of missing data for AVBCL, AVN ERP, and AERP. Also, analogous to their efficacy in CPVT28, both beta-blockers and L-type calcium channel blocker would be expected to reduce the likelihood of RyR2-mediated AF. As such, beta-blocking properties of S-propafenone and/or concomitant treatment with L-type calcium channel blockers could have contributed to the apparent lack of differences between R- and S-propafenone in our study.
Conclusions
There was no difference in AF inducibility between (R)-propafenone, which inhibits RyR2-mediated calcium leak, and (S)-propafenone, which has no RyR2 activity at clinically relevant concentrations. These results were confounded by a high rate of inducible AFL due to sodium-channel blockade by both enantiomers and possibly the protective effect of the beta-blocking properties of (S)-propafenone. Despite differences in the molecular targets of propafenone enantiomers, clinically important differences could not be demonstrated.
Supplementary Material
WHAT IS KNOWN
Experimental data suggest RyR2-mediated intracellular calcium leak is a mechanism for atrial fibrillation.
Propafenone is comprised of two enantiomers with stereospecific properties.
The (R) and (S) enantiomers are equally potent sodium channel blockers, but (R)-propafenone is also an RyR2 inhibitor, whereas (S)-propafenone is a beta-blocker.
WHAT THE STUDY ADDS
Sodium channel blockade by both (R) and (S)-propafenone both promote typical atrial flutter obscuring any difference in AF inducibility related to RyR2 inhibition.
To study the isolated effect of RyR2 inhibition in AF, an RyR2 inhibitor that doesn’t block the sodium channel is needed.
Sources of Funding:
This work was supported by National Heart, Lung and Blood Institute awards (R01HL124935, R35HL144980) to Knollmann. Dr. Shoemaker was supported by NIH K23HL127704. Other resources used in this project (REDCap) were funded by the NIH’s Clinical Translational Science Award (CTSA) to Vanderbilt (UL1 TR002243) from the National Center for Advancing Translational Sciences.
Disclosures:
Dr. Montgomery serves on the advisory board for Medtronic Inc. Dr. Kanagasundram receives speaking fees/honoraria from Biosense Webster and Janssen. Dr. Michaud receives consulting fees/honoraria from Boston Scientific, Medtronic, and St. Jude Medical and research funding from Boston Scientific and Biosense Webster. Dr. Crossley receives consulting fees/honoraria from Bayer Healthcare, Boston Scientific, Janssen Pharmaceuticals, Medtronic, and Spectranetics. Dr. Ellis receives research grants (to Vanderbilt University) unrelated to this work from Boehringer-Ingelheim Inc., Medtronic Inc., and Boston Scientific Inc. Dr. Ellis also receives consulting/advisory fees from Medtronic Inc., Abbott Medical Inc., Boston Scientific Inc., and Atricure Inc.
Nonstandard Abbreviations and Acronyms
- AF
Atrial Fibrillation
- AFL
Atrial Flutter
- RyR2
Ryanodine Receptor
References:
- 1.Vest JA, Wehrens XH, Reiken SR, Lehnart SE, Dobrev D, Chandra P, Danilo P, Ravens U, Rosen MR and Marks AR. Defective cardiac ryanodine receptor regulation during atrial fibrillation. Circulation. 2005;111:2025–32. [DOI] [PubMed] [Google Scholar]
- 2.Voigt N and Dobrev D. Cellular and molecular correlates of ectopic activity in patients with atrial fibrillation. Europace. 2012;14 Suppl 5:v97–v105. [DOI] [PubMed] [Google Scholar]
- 3.Wakili R, Voigt N, Kaab S, Dobrev D and Nattel S. Recent advances in the molecular pathophysiology of atrial fibrillation. J Clin Invest. 2011;121:2955–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Voigt N, Heijman J, Wang Q, Chiang DY, Li N, Karck M, Wehrens XH, Nattel S and Dobrev D. Cellular and molecular mechanisms of atrial arrhythmogenesis in patients with paroxysmal atrial fibrillation. Circulation. 2014;129:145–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chelu MG, Sarma S, Sood S, Wang S, van Oort RJ, Skapura DG, Li N, Santonastasi M, Muller FU, Schmitz W, et al. Calmodulin kinase II-mediated sarcoplasmic reticulum Ca2+ leak promotes atrial fibrillation in mice. J Clin Invest. 2009;119:1940–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Faggioni M, Savio-Galimberti E, Venkataraman R, Hwang HS, Kannankeril PJ, Darbar D and Knollmann BC. Suppression of spontaneous ca elevations prevents atrial fibrillation in calsequestrin 2-null hearts. Circ Arrhythm Electrophysiol. 2014;7:313–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li N, Chiang DY, Wang S, Wang Q, Sun L, Voigt N, Respress JL, Ather S, Skapura DG, Jordan VK, et al. Ryanodine receptor-mediated calcium leak drives progressive development of an atrial fibrillation substrate in a transgenic mouse model. Circulation. 2014;129:1276–1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shan J, Xie W, Betzenhauser M, Reiken S, Chen BX, Wronska A and Marks AR. Calcium leak through ryanodine receptors leads to atrial fibrillation in 3 mouse models of catecholaminergic polymorphic ventricular tachycardia. Circ Res. 2012;111:708–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sood S, Chelu MG, van Oort RJ, Skapura D, Santonastasi M, Dobrev D and Wehrens XH. Intracellular calcium leak due to FKBP12.6 deficiency in mice facilitates the inducibility of atrial fibrillation. Heart Rhythm. 2008;5:1047–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kroemer HK, Funck-Brentano C, Silberstein DJ, Wood AJ, Eichelbaum M, Woosley RL and Roden DM. Stereoselective disposition and pharmacologic activity of propafenone enantiomers. Circulation. 1989;79:1068–76. [DOI] [PubMed] [Google Scholar]
- 11.Galimberti ES and Knollmann BC. Efficacy and potency of class I antiarrhythmic drugs for suppression of Ca(2+) waves in permeabilized myocytes lacking calsequestrin. J Mol Cell Cardiol. 2011;51:760–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hwang HS, Hasdemir C, Laver D, Mehra D, Turhan K, Faggioni M, Yin H and Knollmann BC. Inhibition of cardiac Ca2+ release channels (RyR2) determines efficacy of class I antiarrhythmic drugs in catecholaminergic polymorphic ventricular tachycardia. Circ Arrhythm Electrophysiol. 2011;4:128–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Calkins H, Hindricks G, Cappato R, Kim YH, Saad EB, Aguinaga L, Akar JG, Badhwar V, Brugada J, Camm J, et al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation: Executive summary. Europace. 2018;20:157–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ganau G and Lenzi T. Intravenous propafenone for converting recent onset atrial fibrillation in emergency departments: a randomized placebo-controlled multicenter trial. FAPS Investigators Study Group. J Emerg Med. 1998;16:383–7. [DOI] [PubMed] [Google Scholar]
- 15.Bianconi L and Mennuni M. Comparison between propafenone and digoxin administered intravenously to patients with acute atrial fibrillation. PAFIT-3 Investigators. The Propafenone in Atrial Fibrillation Italian Trial. Am J Cardiol. 1998;82:584–8. [DOI] [PubMed] [Google Scholar]
- 16.Goy JJ, Metrailler JC, Humair L and de Torrente A. Restoration of sinus rhythm in atrial fibrillation of recent onset using intravenous propafenone. Am Heart J. 1991;122:1788–90. [DOI] [PubMed] [Google Scholar]
- 17.Batiste SM, Blackwell DJ, Kim K, Kryshtal DO, Gomez-Hurtado N, Rebbeck RT, Cornea RL, Johnston JN and Knollmann BC. Unnatural verticilide enantiomer inhibits type 2 ryanodine receptor-mediated calcium leak and is antiarrhythmic. Proc Natl Acad Sci U S A. 2019;116:4810–4815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leong-Sit P, Robinson M, Zado ES, Callans DJ, Garcia F, Lin D, Dixit S, Bala R, Riley MP, Hutchinson MD, et al. Inducibility of atrial fibrillation and flutter following pulmonary vein ablation. J Cardiovasc Electrophysiol. 2013;24:617–23. [DOI] [PubMed] [Google Scholar]
- 19.Oral H, Chugh A, Lemola K, Cheung P, Hall B, Good E, Han J, Tamirisa K, Bogun F, Pelosi F, Jr., et al. Noninducibility of atrial fibrillation as an end point of left atrial circumferential ablation for paroxysmal atrial fibrillation: a randomized study. Circulation. 2004;110:2797–801. [DOI] [PubMed] [Google Scholar]
- 20.Nishida K, Michael G, Dobrev D and Nattel S. Animal models for atrial fibrillation: clinical insights and scientific opportunities. Europace. 2010;12:160–72. [DOI] [PubMed] [Google Scholar]
- 21.Riley G, Syeda F, Kirchhof P and Fabritz L. An introduction to murine models of atrial fibrillation. Front Physiol. 2012;3:296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schrickel JW, Bielik H, Yang A, Schimpf R, Shlevkov N, Burkhardt D, Meyer R, Grohe C, Fink K, Tiemann K, et al. Induction of atrial fibrillation in mice by rapid transesophageal atrial pacing. Basic Res Cardiol. 2002;97:452–60. [DOI] [PubMed] [Google Scholar]
- 23.Liu H, Yuan P, Zhu X, Fu L, Hong K and Hu J. Is Atrial Fibrillation Noninducibility by Burst Pacing After Catheter Ablation Associated With Reduced Clinical Recurrence?: A Systematic Review and Meta-Analysis. J Am Heart Assoc. 2020;9:e015260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schulz M, Schmoldt A, Andresen-Streichert H and Iwersen-Bergmann S. Revisited: Therapeutic and toxic blood concentrations of more than 1100 drugs and other xenobiotics. Crit Care. 2020;24:195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sezai A, Nakai T, Hata M, Yoshitake I, Shiono M, Kunimoto S and Hirayama A. Feasibility of landiolol and bisoprolol for prevention of atrial fibrillation after coronary artery bypass grafting: a pilot study. J Thorac Cardiovasc Surg. 2012;144:1241–8. [DOI] [PubMed] [Google Scholar]
- 26.Crystal E, Connolly SJ, Sleik K, Ginger TJ and Yusuf S. Interventions on prevention of postoperative atrial fibrillation in patients undergoing heart surgery: a meta-analysis. Circulation. 2002;106:75–80. [DOI] [PubMed] [Google Scholar]
- 27.Arsenault KA, Yusuf AM, Crystal E, Healey JS, Morillo CA, Nair GM and Whitlock RP. Interventions for preventing post-operative atrial fibrillation in patients undergoing heart surgery. Cochrane Database Syst Rev. 2013:CD003611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wleklinski MJ, Kannankeril PJ and Knollmann BC. Molecular and tissue mechanisms of catecholaminergic polymorphic ventricular tachycardia. J Physiol. 2020;598:2817–2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


