Abstract
Background:
Clinical trial participants who benefit from experimental neural devices for the treatment of debilitating and otherwise treatment-resistant conditions are generally not ensured continued access to effective therapy or maintenance of devices at the conclusion of trials.
Objective/Hypothesis:
Post-trial obligations have been extensively examined in the context of drug trials, but there has been little empirical examination of stakeholder perspectives regarding these obligations in the rapidly growing field of neural device research.
Methods:
This study examined the perspectives of 44 stakeholders (i.e., 23 researchers and 21 patient-participants) involved in implantable neural device trials.
Results:
Researchers were concerned about current post-trial management, identified barriers like cost, and suggested ways to improve the system. Many patient-participants were unaware of whether they would have post-trial access, but most thought they should keep devices if beneficial, and agreed with researchers that more should be done to help them keep and maintain these neural devices.
Conclusion:
To our knowledge, this is the first in-depth examination of researcher perspectives regarding continued access to experimental neural devices and only the second such examination of patient-participant perspectives. These data can help inform future ethical and policy decisions about post-trial access to implantable neurotechnology.
Keywords: Neuroethics, Neurotechnology, Ethics, Post-trial, Deep brain stimulation, Continued access
1. Introduction
A critical ethical and practical challenge in neurotechnology development is whether, and if so, how, to ensure that participants who benefit from implantable experimental devices have continued access when clinical trials end [1–7]. The term “continued access” in the implantable neural device context refers to the opportunity to keep the device and maintain the therapy if it is beneficial. There have been several known instances of loss of access or difficulty maintaining devices, such as patients implanted with Neurovista’s seizure advisory system [8], patients who received deep brain stimulation (DBS) as part of the trials of DBS for depression [7,9], and most recently, patients losing access to maintenance and upgrades for Second Sight’s artificial vision devices [10]. These events highlight an urgent need to address this challenge. Patients can lose access to experimental neural devices for multiple reasons: device developers phase out the product to invest in a different product, device developers go out of business, trials lack plans to continue maintaining the device once the trial ends, or health insurance providers do not cover maintenance of the device because it is considered experimental [4,11].
Currently, the most trialed intracranial neurostimulation devices are DBS systems. Conventional DBS systems that provide continuous stimulation have some form of approval from the Food and Drug Administration (FDA) in the United States for Parkinson disease, dystonia, essential tremor, and obsessive compulsive disorder (OCD) [11,12]. However, DBS systems are being trialed for different indications, brain targets, and advanced paradigms such as adaptive stimulation. Adaptive DBS (aDBS) devices have the capacity to record neural activity in order to detect potential biomarkers associated with symptoms, and modulate therapeutic stimulation when relevant biomarkers are detected [13,14]. Except for NeuroPace’s RNS system for epilepsy, aDBS devices are not approved by the FDA for most indications and are currently being trialed for multiple conditions. This makes aDBS systems a helpful case study for examining ethical considerations like continued access to beneficial experimental implantable neural devices [1,15].
One caveat for many current aDBS systems is that they can be switched into a conventional DBS mode, giving study participants for which conventional DBS is considered standard of care (e.g., patients with treatment-resistant Parkinson’s disease) an opportunity, if approved by their insurance or paid out of pocket, to have access to some form of the technology, conventional or adaptive stimulation, when the trial ends. However, there is no mechanism in place that would ensure access to the experimental form of the device even if the adaptive mode were more effective for a particular participant. Thus, while researchers and participants in some aDBS trials may have more options to retain some form of the technology, aDBS trial participants still face many of the post-trial challenges encountered in other experimental neurotechnologies. For example, in aDBS trials for OCD or Tourette syndrome for which conventional DBS is not fully FDA-approved or the established standard of care, if insurance denies coverage, participants would generally be responsible for any cost associated with maintenance of the device [11].
In this study, we recruited researchers and participants who were part of experimental aDBS trials for Parkinson’s disease, dystonia, essential tremor, OCD, and Tourette syndrome to examine their perspectives on: (a) current continued access practices and policies, (b) whether study participants should be afforded continued access to effective experimental therapies after trial completion, and (c) who should pay for such continued access and why they should pay. Though there are some published studies that look at post-trial access for medical interventions [16], to our knowledge, this is the first in-depth examination of researcher perspectives regarding continued access to experimental neural devices and only the second such examination of patient-participant perspectives [7]. These data can help inform future ethical and policy decisions about post-trial access to implantable neurotechnology.
2. Methods and analysis
In-depth, semi-structured interviews were conducted with researchers (n = 23) and patient-participants (n = 21) involved in aDBS trials in the United States. The researcher recruitment and interview guide were developed as described in Muñoz et al., 2020 and Zuk et al., 2020. Researcher interviews were conducted via phone or Zoom and lasted an average of 56 min. Patient-participants were recruited based on their involvement in aDBS clinical trials, and interviewed both before surgery and approximately 6 months after surgery. The interview guide was developed based on a review of current clinical and bioethics literature, discussion with researchers, and some preliminary data from researcher interviews. Patient-participant interviews were conducted either in person, over the telephone or by Zoom, and these interviews lasted an average of 25 min.
Interviews were recorded and transcribed. The transcripts were analyzed with the aid of MAXQDA 2018 [17]. The research team developed a codebook, including codes related to continued access, that was used to identify thematic patterns in researcher and patient-participant responses. The team (PZ, KKQ, LT, GLM, MP) identified text segments in interview transcripts where a given topic was discussed. Three members of the research team (GLM, CS, MP) applied thematic discourse analysis [18] to outputs containing the aggregated segments to identify overarching themes and sub-themes. Themes identified by one member of the team were corroborated by at least one other member. This study was approved by the Baylor College of Medicine Institutional Review Board.
3. Results
We interviewed 23 researchers (response rate 82% or 23/28) with diverse disciplinary backgrounds and project roles (Table 1) and 21 patient-participants (response rate 91.3% or 21/23) diagnosed with dystonia (n = 1; 5%), essential tremor (n = 3; 14%), Parkinson’s Disease (n = 8; 38%), Tourette Syndrome (n = 4; 19%), or obsessive-compulsive disorder (n = 5; 24%). The majority of patient-participants were non-Hispanic (n = 14; 74%) and white (n = 15; 79%) (Table 2).
Table 1.
Researcher demographics.
| Researcher Demographics (n = 23) | |
|---|---|
| Total (n = 23) | |
| Gender | |
| Male | 13 (57%) |
| Female | 9 (39%) |
| Prefer not to answer | 1 (4%) |
| Race and Ethnicity | |
| Asian | 3 (13%) |
| White | 18 (78%) |
| Prefer not to answer | 2 (9%) |
| What degree(s) do you currently hold? | |
| M.D. or equivalent | 8 (35%) |
| Ph.D. or equivalent (clinical) | 3 (13%) |
| Ph.D. or equivalent (research) | 4 (17%) |
| Both M.D. and Ph.D. or equivalent (clinical) | 2 (9%) |
| Both M.D. and Ph.D. or equivalent (research) | 1 (4%) |
| B.Eng. or M.Sc. Engineering | 2 (9%) |
| B.A. or B.S. | 3 (13%) |
| Project role | |
| Clinical Trial Coordinator | 4 (17%) |
| Engineer | 5 (22%) |
| Mental Health Clinician | 4 (17%) |
| Neurologist | 5 (22%) |
| Neurosurgeon | 5 (22%) |
| Research focus | |
| Movement disorders | 6 (26%) |
| Psychiatric disorders | 8 (35%) |
| Both | 9 (39%) |
| Years of research experience (mean) | |
| Years of experience related to conventional DBS | 8.7 |
| Years of experience related to aDBS | 4.5 |
Table 2.
Patient-participant demographics.
| Patient-participant demographics (n = 21b)a | |
|---|---|
| Total (n = 21b) | |
| Gender | |
| Male | 11 (58%) |
| Female | 8 (42%) |
| Are you of Hispanic, Latino, or Spanish origin? | |
| Yes | 4 (21%) |
| No | 14 (74%) |
| No response | 1 (5%) |
| Race and Ethnicity | |
| Asian | 1 (5%) |
| White | 15 (79%) |
| Other | 3 (16%) |
| Age | |
| Mean | 50 |
| Min-Max | 24–72 |
| Condition | |
| Dystonia | 1 (5%) |
| Essential Tremor | 3 (14%) |
| Parkinson’s Disease | 8 (38%) |
| Tourette Syndrome | 4b (19%) |
| Obsessive-Compulsive Disorder | 5 (24%) |
| Total household income (before taxes) from all sources in the last year | |
| $0 to $49,999 | 6 (32%) |
| $50,000 to $99,999 | 3 (16%) |
| $100,000 to $149,999 | 5 (26%) |
| $150,000 or more | 5 (26%) |
| Source of health insurancec | |
| Employer | 1 |
| Parents or partner | 6 |
| Medicaid or other state insurance | 5 |
| Medicare | 6 |
| Private health insurance | 1 |
| Other | 1 |
All patients with essential tremor, Tourette syndrome, Parkinson’s disease, and dystonia had additional implanted hardware and no patients with OCD had additional hardware. The additional hardware was placement of cortical strips in addition to the DBS leads.
Two patients receiving aDBS for Tourette syndrome did not complete the demographics survey. All “Total” percentages are therefore calculated with n = 19 as a denominator and within-disorder percentages for Tourette syndrome calculated with n = 2 as a denominator.
Categories are not mutually exclusive as participants could select all that apply.
4. Researcher perspectives
4.1. Concerns about current management of post-trial access
More than half of researchers (n = 13; 57%) expressed concern about how post-trial access is currently managed. One researcher said: “I think it’s a huge issue that we don’t have great answers to at this point” (B003). Another stated: “[I]t seems to me not ethical to have a therapy that, say, a subset of patients really benefits from [and] then say, ‘Well, sorry. Now you can’t have this. It was just for the study’” (B004). A third researcher said: “There’s no greater shame in our world than offering a service and taking it away. That’s always what keeps researchers up at night […] I think that you have to find a way to keep it in if the patient finds it to be beneficial” (B007).
4.2. Barriers to continued access
Most researchers (n = 17; 74%) explained that there are barriers to continued access, such as cost of maintenance (n = 9; 39%): “Once they’re off the study, the cost does shift back to the patient. […] So, I mean, it is one of the unfortunate things about just the way healthcare works, but the study is only gonna be able to provide so much” (A006). Some worried that “to make researchers responsible for the cost of such a thing, a cost which would be difficult to estimate, would unnecessarily hamper the science” (B019). But others felt that “the [post-trial] maintenance support that me and my team give, it’s not a burden on us. Again, these (studies) are so small, the feasibility studies, that it hasn’t gone out of hand” (B009). Some researchers (n = 7; 30%) highlighted challenges with insurance providers since they often do not cover experimental devices or indications: “DBS is not an approved indication [for Tourette syndrome]. So, then, they [patients] end up having to go out of pocket for their surgery to maintain it. And, so, it’s an issue of giving them a glimpse of hope and then taking it away. They’ve seen what they could have and then they can’t have it again” (A003). In addition, some researchers (n = 4; 26%) noted how device manufacturers’ decisions can complicate continued access for participants. One researcher stated: “[I]t does create really nasty situations for device companies that are stuck in a situation where you have a bunch of patients, dozens of patients implanted, then the study ends, and you have the company no longer interested in seeking approval for the device, so then who’s going to pay for it” (B015)? Researchers (n = 5; 22%) also identified regulatory restrictions as a barrier to continued access, mainly because the devices or indications are not FDA approved. Finally, limited access to clinical and technical expertise (n = 4; 17%) was identified as another barrier: “Just the fact that the patient needs to keep having follow-ups with a doctor that knows how to work with DBS systems, and there aren’t very many of those. Depending on where they’re living, that might be difficult” (A005).
4.3. Researchers as advocates for continued access
Some researchers (n = 7; 30%) discussed ways in which they take concrete steps to facilitate post-trial access. This includes reducing costs for participants by charging specialized vists as regular visits (n = 4; 17%): “I think the plan […] for us [is] to see her [the patient] and service her device, and then, bill it as a neurology visit.” One researcher discussed facilitating insurance coverage for participants early on: “One way we’re trying to mitigate those issues, is try to get insurance more involved and get approval from the start” (A006). Another researcher said they choose sponsors that offer follow-up care: “In several of my trials, we’ve actually managed that issue by just running the trial at the VA. […] [F]or example, studies have been done where replacement is paid for by the study sponsor, even after the trial has ended” (B006) In addition, one researcher said that they have applied for follow-up grants that include continued access costs in the budget:
Well, most cases we have a follow-up study that looks for longer-term follow-up and budgets into that the costs of continued care or programming with this, whatever, so that is clearly the best way forward […] but I mean, you know, the follow-up study doesn’t get funded or the PI moves away, or whatever. All these things certainly happen. So, none of these are fool proof, you know?
(B015)
4.4. During consent participants May struggle to understand the implications of potentially No post-trial access
Some researchers (n = 4; 17%) were concerned that participants may not fully grasp the long-term implications of a lack of post-trial access, even though it is discussed during the informed consent process. Reflecting on how patient-participants think about post-trial access, one researcher said: “I don’t think they’re very concerned about it. Maybe they’re less concerned than they should be, because it’s a very abstract concept. They are anticipating getting a surgery, so there’s a zillion things that are going to happen between now and the end of the study” (B004). Another researcher noted: “They’re told, but I don’t think they really care in the moment. And, then, later on when they’re faced with a $20,000 bill […] because your battery died and we can’t give you a new one, then they get upset” (A003). These researchers thus suggested that the issue may have less salience for participants when they are enrolling into a research study, when the prospect of needing continued access is still in the long- or middle-term future.
4.5. Improving continued access
Most researchers (n = 19; 83%) suggested ways to improve continued access (Table 3 supplementary materials). Many (n = 13; 57%) noted that insurance providers should have a more active role. However, some questioned the feasibility of implementing this in practice: “Insurance companies doing a better job of covering things, […] that’s easy to say and hard to implement” (A005). Others (n = 8; 35%) suggested that research sponsors (e.g., NIH, device manufacturers, and VA) might provide funding for continued access by making it part of research budgets. One researcher suggested that device manufacturers could contribute to continued access by providing access to hardware. Only one researcher saw no need for any change to current practice of continued access.
5. Patient-participant perspectives
5.1. Recall of information about continued access to device maintenance
A few days to a few weeks after the consent process, participants were asked during pre-surgery interviews whether they recalled any discussions with the research team about continued access to device maintenance once the study ended. At that time, 38% (n = 8) had no recollection, 38% (n = 8) recalled such discussions, and 24% (n = 5) provided unclear or ambiguous responses. During the 6-month post-surgery interviews, 33% (n = 7) did not remember discussing continued access, but 57% (n = 12) did recall discussing the issue, and 10% (n = 2) had unclear responses. Tables 4 and 5 in supplementary materials provide a sample of pre- and post-surgery responses from participants, respectively, when asked if they recalled any discussions with the research team about continued access.
5.2. Should participants Be able to keep the device?
Participants were asked what should happen to the device at the end of the study. During pre-surgery interviews, most participants (n = 16; 76%) answered that they should be able to keep the device. One participant explained: “If it provides the relief that I’m hoping it’ll provide, I’m all up for keeping it in, you know” (Q006). No participant said that they should not be able to keep the device, but a few (n = 3; 14%) provided unclear answers, and a couple (n = 2; 10%) were not directly asked. During 6-month post-surgery interviews, most participants (n = 17; 81%), still thought they should be able to keep the device: “[A]t the end of the study, if it’s still working, and I’m still charging the battery, leave well enough alone. […] Well, if they want it back, they’re going to have to fight me for it” (S017). Similar to pre-surgery responses, none of the participants said that they should not be able to keep the device. One participant (n = 1; 5%) provided an unclear answer, and a few (n = 3; 14%) were not directly asked.
5.3. How should continued access be paid for?
Participants were also asked how they thought keeping the device functioning at the end of the study should be paid for and why.
During pre-surgery interviews, most participants stated that insurance providers (n = 14; 67%) should pay for some or all of the maintenance cost. Some participants stated that they (participants) should cover some or all of the cost (n = 6; 29%), followed by device manufacturers (n = 5; 24%), the research team (n = 3; 14%), government (n = 1; 5%), and study-related institutions like hospitals (n = 1; 5%) (Fig. 1). In addition, some participants said that participants themselves (n = 3; 14%) should not be responsible for continued access, and one participant said the research team (n = 1; 5%) should not have to incur these costs. During 6-month post-surgery interviews, there were two notable changes. First, twice as many participants (n = 10; 48%) identified device manufacturers as bearing some or all responsibility for covering continued access to device maintenance. Second, the number of participants mentioning insurance providers (n = 8; 38%) decreased by almost half.
Fig. 1.
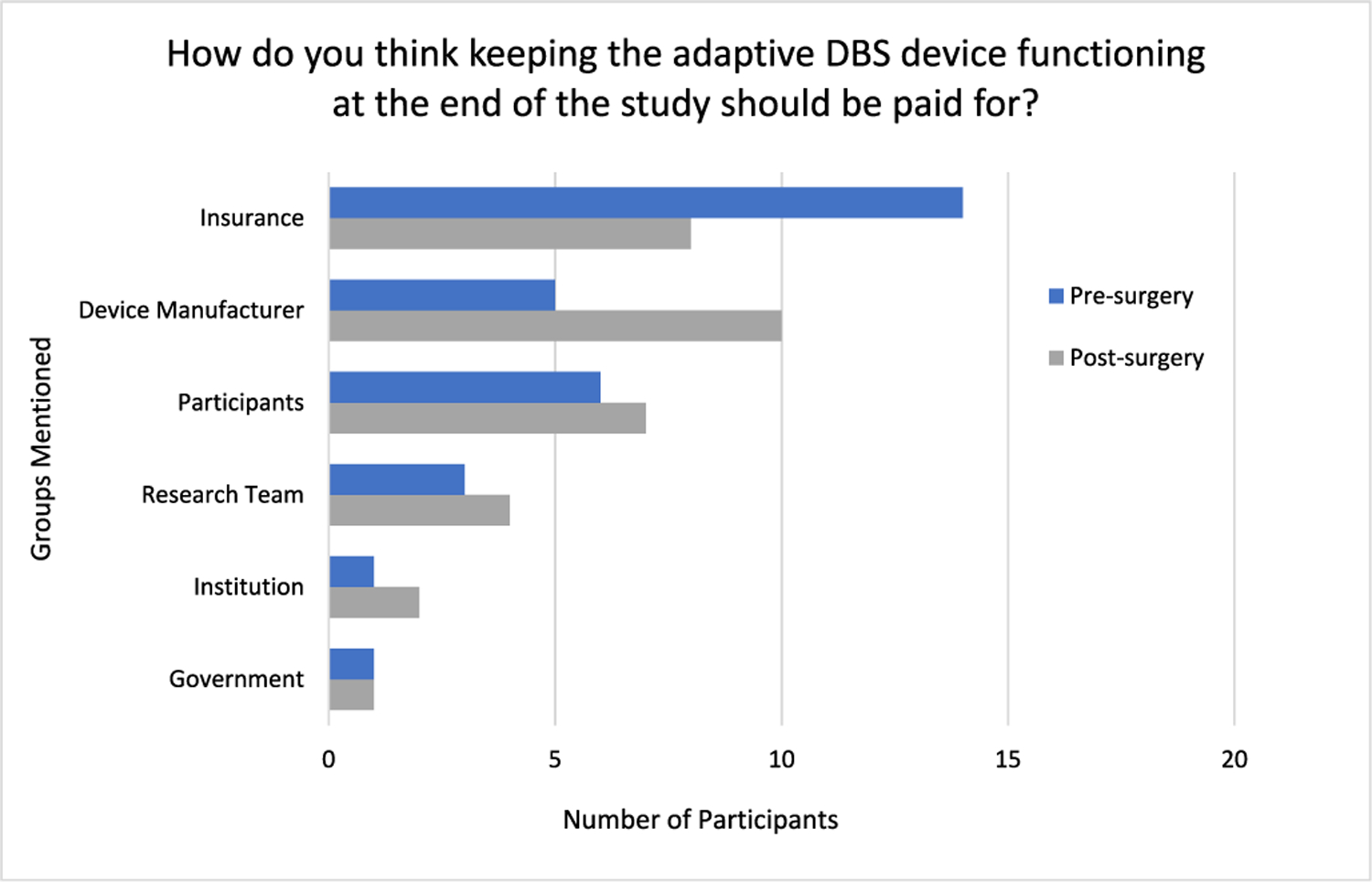
Participant perspectives on who should pay for continued access (n = 21)*
*Totals sum to >21 as some participants mentioned multiple groups as bearing some responsibility.
5.4. Why are certain groups financially responsible for continued access?
Below we highlight the different considerations participants offered as rationales for who they thought should pay for keeping the device functioning post-trial. We focus here on post-surgery responses because these are more likely to reflect participants’ views on continued access informed by their study participation. We have included participants’ pre-surgery responses in supplementary materials (Table 6).
6. Device manufacturers
Participants offered various reasons why device manufacturers should cover some or all costs of continued access, including knowledge benefit, reciprocity with participants, and a duty of non-abandonment. First, device manufacturers benefit from the knowledge obtained through study data collection, which would not be possible without participants. One participant stated: “[Institution name] in combination with [device manufacturer], because [device manufacturer] continues to benefit from data being gathered” (R026). Another participant emphasized that device manufacturers (as well as other institutions) have an obligation of reciprocity given that they benefit from the relationship: “I think I’m benefiting [device manufacturer] and the hospital and I think they should kind of both tip me and let me keep [the device]” (R017). Another participant framed this as reciprocity and a duty of non-abandonment that device manufacturers have to participants given the invaluable data they are able to collect during the study: “Yeah, I think since the company or the team or the whatever, is getting, I hope, a lot of information, I think they do have a responsibility to continue to take care for the people that have these devices. I would not expect or want to be cut loose [at] a certain definitive date” (R028). Finally, one participant thought that, although it’s not their responsibility, device manufacturers should pay for device maintenance because it would not be especially burdensome for many of them to cover some of the costs:
It would be the patient’s responsibility, but I also realize they’re a big multi-billion dollar company. And it’s not like they’re doing it for everyone. They’d just be doing it for a handful of people. So, it wouldn’t cost them that much to provide the new device, I imagine, at their cost. So, I wouldn’t say it’s a requirement or that I would expect it, but it would be really nice if they would at least cover the first couple of replacements or so, if they needed to replace them every year or two years. That would be a great help to the people participating in the trials.
(Q011).
7. Insurance
Participants’ rationale for why insurance providers should cover some cost of device maintenance was related to their perspectives about the function of health insurance. One participant stated:
I think insurance should definitely pay for that. I think that my mom, pardon the expression, pays out the [expletive] for the insurance that we have, and it’s not like they have to pay for the surgery or the study or the … I think the insurance should take some [expletive] responsibility. […] And if they would just [expletive] pay out and do what they’re supposed to do, then yeah, they need to pay for it. Sorry. […] Well whose responsibility should it be? Why are we paying for insurance?
(Q009).
Similarly, another participant noted: “I pay for health insurance, so I hope health insurance would cover it. Out of pocket costs, I would pay for them if… the device works well for me, so I would do whatever I could to keep it” (R021). Implicit in participants’ reasoning here is that if the experimental device provides therapeutic benefit, and given that no other approved treatment options have been successful, then insurance providers ought to bear some financial responsibility to facilitate device maintenance. Covering the maintenance of an experimental device found to improve a patient’s symptoms could also decrease the cost of managing the patient’s condition compared to paying for additional ineffective treatments or hospitalizations.
8. Participants
Some participants emphasized that that they themselves should bear some responsibility for post-trial expenses. One justification offered was that participants were told they would not receive continued access and had entered into the agreement, and so they ought to honor such agreements. One participant explained: “We agreed that it’s up to the patient to kind of figure that out after. So I do think it should stick that way just since that’s what we agreed to. But I know that poses some challenges, I mean that one kind of makes me nervous as far as like, will my insurance start covering it when we tell them this” (Q014)? Another participant said that participants should be responsible for continued access only if they have the means:
If you could afford it then you should be able to pay maintenance, but if you can’t then same way I got into this study, then that’s the way it should be […]. It would be kind of cruel to give a guy this much life back and snatch it away just because of a few pennies, although it’s more than a few pennies.
(R014)
9. Research teams
Some participants expressed that research teams have a responsibility towards participants whereby they ought to reciprocate the time and effort participants put into the study as well as the risks endured. One participant said:
Well, I think the study should pay for it. I guess the people in the study, they took the risk, they had everything done and I think…, whatever is needed to keep it up if it can’t be paid for by the individual person, then it should, not that it is, but it should be taken care of [by] the study even when the study ends.
(S015)
Similarly, another participant emphasized their significant contributions to the study team:
The study. […] Because it’s a lot of time and effort […] I had to go through brain surgery … After the DBS was placed, I had to come in every two weeks, and that takes up the whole day. […] Every two weeks, and then every month. And it’s just very time-consuming.
(Q008)
10. Group who should not have to pay
Several participants specifically named groups who they thought should not be responsible for costs. Some (n = 2; 10%) explained that participants should not be responsible for continued access costs because they already contribute to research by being in the study. One said: “Well, like I said, they’re in a study, and they’re trying to help not only themselves but other people, too. That’s why you get in a study, so they put themselves out to help. So, they should be helped also, quite frankly” (S017). Another participant noted that covering post-trial access costs would be an unfair burden on participants: “I don’t think the patient should. I think that would be too much” (R026). One participant noted that the research teams already operate on a fairly limited budget with grants and for this reason they should not have to cover funds for post-trial care:
I’m not going to ask Dr. [researcher name] to pay for it. I’m not going to ask the study to pay for it because it’s not like they have the money, they’re getting grants. Who else should pay for it? I didn’t decide to have OCD, I don’t think. God, I hope not. I didn’t decide to … I don’t know. I don’t know who else to [expletive] sort of blame and have them pay.
(Q009)
11. Discussion
In this study, 44 stakeholders were interviewed about their perspectives on post-trial continued access to implantable neural devices. Our results corroborate several important themes from Sankary et al., 2021’s previous study of patient-participant perspectives [7]. These include the potential for patient-participants to be focused on short-term considerations such as symptom relief rather than post-trial access, lack of patient-participant understanding of (potentially unclear) post-trial options, concern about who will be responsible for post-trial costs and related financial uncertainties, and the implications of all of this for informed consent to neural device trials. On the issue of whether participants who benefit from these devices should have continued access, researchers and patients largely agreed that those who experience benefit from devices should receive this access, and that the way this is currently managed is problematic. This finding is consistent with what many ethical experts and commentators have argued [2–4]. For example, some have argued that among other reasons because neuromodulation infrastructure and expertise is limited (also noted by researchers in this study), researchers and sponsors carrying out implantable neuromodulation have a duty of non-abandonment, which in this case would entail a longitudinal fiduciary obligation by researchers and sponsors to provide ongoing care and cover associated costs [3]. Others have argued that, especially when it comes to neuromodulation trials for treatment-resistant conditions, researchers, sponsors, device manufacturers, and insurance providers, have an obligation to take reasonable steps to facilitate post-trial access to and maintenance of devices for participants who benefit [4]. This obligation is based mainly on reciprocity for participants’ valuable contributions to the research (e.g., undertaking risks, including bodily risks associated with neurosurgery, providing data, contributing a significant amount of time for testing), and compassion towards participants who benefit, a rationale also voiced by some patients in previous qualitative work on this topic [4,7]. Moreover, participants are in the vulnerable position of not having any other effective treatment available except for the experimental device, thus, facilitating post-trial access to these patients prevents serious harm [4].
The more difficult issue seems to be how to accomplish this outcome [3–6,19]. Researchers and participants agreed that cost was a key challenge. Both groups identified insurance providers, device manufacturers, and participants themselves as having some or all responsibility to cover the costs of continued access. Pre-surgery, insurance providers were the group most frequently identified by participants as responsible for covering these costs, however this shifted post-surgery to device manufacturers. We suspect that one important reason for the shift can be attributed to participants noticing, over the course of the study, how their contributions in these trials (e.g., data collected) benefit device manufacturers. As a number of participants suggest, device manufacturers obtain invaluable information about the utility of these devices from trials, and this would not have been possible without the contributions of participants. Relatedly, the decrease in the number of participants identifying insurance providers as having responsibility to fund the costs may be tied to participants’ perception about how much device manufacturers gain actually from participants’ contributions. Most researchers held the view that if the device benefits the patient, insurance providers should cover at a minimum, part of the cost, with some justifying this view on grounds of medical necessity. One important challenge is that even if insurance covered some cost, participants might still be left with significant co-pays and co-insurance [20,21].
In addition to cost, other key issues will need to be addressed to develop and to implement appropriate policy regarding continued access to beneficial experimental neural devices for patients with otherwise treatment-resistant conditions or patients with loss of function. These issues include:
How to determine whether to provide or facilitate continued access for a particular patient (e.g., how and who determines whether the patient is benefitting from the device and whether continued access should be covered based on financial need or provided to everyone who benefits).
How long continued access should be made available (e.g., whether device manufacturers can commit to producing parts for a particular time after a trial ends).
What each entity’s (e.g., device manufacturer, institution where the research is conducted, researchers, funders) responsibility for facilitating access should be.
How to cover the cost of continued access (e.g., insurance premium device manufacturer’s and/or others pay per participant).
Many researchers expressed concern about a participants’ ability to understand and/or appreciate, during the consenting process, the implications of future access to the device. It was noted that participants may have failed to understand that even if the device is beneficial in treating their treatment-resistant condition they still may not have access to it post-trial. In fact, while the trials in which these patients participated covered post-trial access in the informed consent process, during pre-surgery interviews with participants, 38% did not remember discussions about what would happen to the device after study completion, and a similar number did not remember such information during the 6-month post-surgery interview. This suggests that a substantial number of study participants who underwent implantation of investigational neural devices were unaware that when the study ends within a few years, there may not be a mechanism for covering the cost of maintaining the device.
Yet, most participants expressed a belief that those implanted should be able to keep the device and continue therapy after completion of the study if the treatment proves beneficial. Issues with participants not being able to identify key information about a study during the informed consent process have been previously reported [7,22,23]. Understanding what will happen with an implanted neural device when a person’s participation in a clinical trial ends is ethically crucial. Further investigation would be needed to understand why participants may be having trouble focusing on post-trial issues or recalling the information, but we suspect this is partly due—as researchers suggest—to the fact that continued access is a long- or middle-term issue and that participants might be more focused on the surgical procedure for device implantation and its prospect for success. We have previously suggested that this might occur as part of what we call “treatment search fatigue,” in which patient-participants who have dealt with severe, treatment-resistant symptoms for prolonged periods of time may be more attentive to potential benefits than risks and/or especially willing to take on risks for the prospect of benefit [24]. While the consent process for these trials must cover a large amount of information, it may be helpful to highlight issues such as what will happen with the device at the end of the trial and give special attention to risks related to post-trial access. This could be done, for example, in the “concise and focused presentation of key information” required at the beginning of the informed consent process by the revised Common Rule in the U.S. Participants will need this information to render an informed decision about their participation and to prepare for the potential financial implications of maintaining the device.2
As investment in implantable neurotechnology research and development expands, more individuals will be at risk for “unsupported” implanted devices [4]. As we mentioned earlier, examples of patients losing access to or having difficulty securing maintenance and support of implantable neural devices continue to mount [7–9], with the most recent example of 350 blind participants losing access to maintenance and upgrades for visual neuroprosthetic devices [10]. There are many scenarios in which participants might lose access to experimental devices (e.g., device developers phase out the product to invest in a different product, device developers go out of business, studies may simply not have plans to continue maintaining the device once the trial ends, insurance providers do not cover the cost of maintaining the experimental device). To promote the responsible development of implantable neural devices and the well-being of patients who participate in these studies, it is essential that device manufacturers, research funders, academic and hospital institutions, researchers, and other stakeholders take urgent action to avoid these and other scenarios of loss of access. Discussions among these stakeholders ought to identify the cost and resources available to address this situation responsibly. Importantly, any action ought to include participant perspectives and voices to ensure that any proposed solution(s) is responsive to the needs and concerns of the patients who undertake the risks and burdens to make this research possible and face the possible return of treatment-resistant symptoms without access to these devices. Our study offers some data on participant perspectives about post-trial continued access to neural device research. The goal should be to prevent situations in which clinical trial participants who benefit from experimental neural devices implanted for the treatment of debilitating and otherwise treatment-refractory disorders will be stuck with the bill or lose access to the technology [3,4].
12. Limitations
Qualitative studies based on in-depth interviews aim to identify the range of responses or themes that have emerge from key stakeholder groups. Thus, although we reached theme saturation, and interviewed 44 stakeholders, we cannot claim generalizability of these findings. Moreover, management of continued access to experimental devices is influenced by the specifics of the healthcare system and medical device development regulations in the environment where the research takes place; and our sample was limited to aDBS researchers and to participants in the United States. However, to our knowledge, this is the first empirical examination of researcher perspectives regarding continued access to neural devices and the second such examination of patient-participant perspectives.
13. Conclusion
Implantable neural devices offer great promise to aid individuals with treatment-resistant conditions or loss of function. In the process of developing these devices, it is essential that device manufacturers, research funders, researchers, insurance providers, and the academic and hospital institutions work together to identify mechanisms to facilitate continued access for individuals who benefit from experimental brain implants. Our findings suggest that researchers have serious concerns about current procedures for post-trial management (or lack thereof), inclusive of the desire to keep devices functional after the trial ends if they provide benefit. Alarmingly, many participants are frequently not aware or do not comprehend what will actually happen with the device after the trial ends. A critical issue here is that patients who participate in implantable neural device research frequently have severe treatment-resistant conditions or loss of function. If these participants benefit from the investigational implanted neural devices, and ultimately lose access to them, they likely have no other viable alternatives. In part because of these challenges, researchers often take action in an attempt to facilitate continued access, but the burden of finding viable solutions should not rest solely with researchers. Solutions and improved processes should be collectively sought by the various stakeholders who stand to gain from the research. Process enhancement for post-trial access of devices should be addressed by implementing consistent policies rather than piecemeal solutions. Finally, all those involved in enabling device-based research – and especially those who are financial beneficiaries, should actively participate in process enhancement, access, and health equity initiatives.
Supplementary Material
Acknowledgements
The authors would like to thank Rebecca Hsu, Lavina Kalwani, Richa Lavingia, Amanda Merner, Demetrio Sierra-Mercado, and two anonymous reviewers.
Funding
Research for this work was supported by a BRAIN Initiative grant from the US National Institute of Mental Health of the National Institutes of Health under Award Number R01MH114854.
Footnotes
CRediT authorship contribution statement
Gabriel Lázaro-Muñoz: Conceptualization, Methodology, Validation, Formal analysis, Investigation, Writing – original draft, Supervision, Writing – review & editing, Project administration, Funding acquisition. Michelle T. Pham: Conceptualization, Methodology, Validation, Formal analysis, Writing – original draft, Writing – review & editing. Katrina A. Muñoz: Writing – review & editing, Data curation. Kristin Kostick-Quenet: Methodology, Writing – review & editing. Clarissa E. Sanchez: Formal analysis, Writing – review & editing, Data curation. Laura Torgerson: Investigation, Data curation, Writing – review & editing, Project administration. Jill Robinson: Writing – review & editing. Stacey Pereira: Methodology,Writing – review& editing. Simon Outram: Investigation, Writing – review & editing. Barbara A. Koenig: Methodology, Investigation, Writing – review & editing. Philip A. Starr: Resources, Writing – review & editing. Aysegul Gunduz: Resources, Writing – review & editing. Kelly D. Foote: Resources, Writing – review & editing. Michael S. Okun: Resources,Writing – review & editing. Wayne Goodman: Conceptualization, Methodology, Resources, Writing – review & editing, Supervision, Project administration, Funding acquisition. Amy L. McGuire: Conceptualization, Methodology, Writing – review & editing, Supervision, Project administration, Funding acquisition. Peter Zuk: Conceptualization, Methodology, Investigation, Data curation, Writing – review & editing, Project administration.
Declaration of competing interest
None.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.brs.2022.07.051.
There is also the possibility of iatrogenic harms, which we have not discussed here [25].
References
- [1].Muñoz KA, Kostick K, Sanchez C, Kalwani L, Torgerson L, Hsu R, et al. Researcher perspectives on ethical considerations in adaptive deep brain stimulation trials. Front Hum Neurosci 2020;14. 10.3389/fnhum.2020.578695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Hendriks S, Grady C, Ramos KM, Chiong W, Fins JJ, Ford P, et al. Ethical challenges of risk, informed consent, and posttrial responsibilities in human research with neural devices. JAMA Neurol 2019;76:1506. 10.1001/jamaneurol.2019.3523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Fins JJ. Deep brain stimulation, deontology and duty: the moral obligation of non-abandonment at the neural interface. J Neural Eng 2009;6:050201. 10.1088/1741-2552/6/5/050201.050201. [DOI] [PubMed] [Google Scholar]
- [4].Lázaro-Muñoz G, Yoshor D, Beauchamp MS, Goodman WK, McGuire AL. Continued access to investigational brain implants. Nat Rev Neurosci 2018;19:317–8. 10.1038/s41583-018-0004-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Sierra-Mercado D, Zuk P, Beauchamp MS, Sheth SA, Yoshor D, Goodman WK, et al. Device removal following brain implant research. Neuron 2019;103:759–61. 10.1016/j.neuron.2019.08.024. [DOI] [PubMed] [Google Scholar]
- [6].Richardson HS, Belsky L. The ancillary-care responsibilities of medical researchers: an ethical framework for thinking about the clinical care that researchers owe their subjects. Hastings Cent Rep 2004;34:25. 10.2307/3528248. [DOI] [PubMed] [Google Scholar]
- [7].Sankary LR, Zelinsky M, Machado A, Rush T, White A, Ford PJ. Exit from brain device research: a modified grounded theory study of researcher obligations and participant experiences. AJOB Neuroscience 2021. 10.1080/21507740.2021.1938293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Drew L. Like taking away a part of myself” — life after a neural implant trial. Nat Med 2020;26:1154–6. 10.1038/d41591-020-00028-8. [DOI] [PubMed] [Google Scholar]
- [9].Underwood E. Researchers grapple with the ethics of testing brain implants. Science 2017. 10.1126/science.aar3698. 1979. [DOI] [Google Scholar]
- [10].Strickland E, Harris M. Their bionic eyes are now obselete and unsupported: second Sight left users of its retinal implants in the dark. IEEE Spectrum 2022. [Google Scholar]
- [11].Rossi PJ, Giordano J, Okun MS. The problem of funding off-label deep brain stimulation. JAMA Neurol 2017;74:9. 10.1001/jamaneurol.2016.2530. [DOI] [PubMed] [Google Scholar]
- [12].Miocinovic S, Somayajula S, Chitnis S, Vitek JL. History, applications, and mechanisms of deep brain stimulation. JAMA Neurol 2013;70:163. 10.1001/2013.jamaneurol.45. [DOI] [PubMed] [Google Scholar]
- [13].Bouthour W, Mégevand P, Donoghue J, Lüscher C, Birbaumer N, Krack P. Biomarkers for closed-loop deep brain stimulation in Parkinson disease and beyond. Nat Rev Neurol 2019;15:343–52. 10.1038/s41582-019-0166-4. [DOI] [PubMed] [Google Scholar]
- [14].Krauss JK, Lipsman N, Aziz T, Boutet A, Brown P, Chang JW, et al. Technology of deep brain stimulation: current status and future directions. Nat Rev Neurol 2021;17:75–87. 10.1038/s41582-020-00426-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Outram S, Muñoz KA, Kostick-Quenet K, Sanchez CE, Kalwani L, Lavingia R, et al. Patient, caregiver, and decliner perspectives on whether to enroll in adaptive deep brain stimulation research. Front Neurosci 2021;15. 10.3389/fnins.2021.734182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Lawton J, Blackburn M, Rankin D, Werner C, Farrington C, Hovorka R, et al. Broadening the debate about post-trial access to medical interventions: a qualitative study of participant experiences at the end of a trial investigating a medical device to support type 1 diabetes self-management. AJOB Empirical Bioethics 2019;10:100–12. 10.1080/23294515.2019.1592264. [DOI] [PubMed] [Google Scholar]
- [17].Kuckartz U. Qualitative text analysis: a guide to methods, practice & using software. 1 oliver’s yard, 55 city road. London EC1Y 1SP United Kingdom: SAGE Publications Ltd; 2014. 10.4135/9781446288719. [DOI] [Google Scholar]
- [18].Boyatzis R. Transforming qualitative information: thematic analysis and code development. Thousand Oaks, CA: Sage Publications; 1998. [Google Scholar]
- [19].Grady C. Payment of clinical research subjects. J Clin Invest 2005;115:1681–7. 10.1172/JCI25694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Stroupe KT, Weaver FM, Cao L, Ippolito D, Barton BR, Burnett-Zeigler IE, et al. Cost of deep brain stimulation for the treatment of Parkinson’s disease by surgical stimulation sites. Mov Disord 2014;29:1666–74. 10.1002/mds.26029. [DOI] [PubMed] [Google Scholar]
- [21].Chen T, Mirzadeh Z, Lambert M, Gonzalez O, Moran A, Shetter AG, et al. Cost of deep brain stimulation infection resulting in explantation. Stereotact Funct Neurosurg 2017;95:117–24. 10.1159/000457964. [DOI] [PubMed] [Google Scholar]
- [22].Koenig BA. Have we asked too much of consent? Hastings Cent Rep 2014;44:33–4. 10.1002/hast.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Grady C. Enduring and emerging challenges of informed consent. N Engl J Med 2015;372:855–62. 10.1056/nejmra1411250. [DOI] [PubMed] [Google Scholar]
- [24].Zuk P, Lázaro-Muñoz G. Treatment search fatigue and informed consent. AJOB Neuroscience 2021;12:77–9. 10.1080/21507740.2020.1866115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Gilbert F, Lancelot M. Incoming ethical issues for deep brain stimulation: when long-term treatment leads to a “new form of the disease. J Med Ethics 2021;47:20–5. 10.1136/medethics-2019-106052. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


