Abstract
Background:
Opioid Use Disorder (OUD) is a serious public health problem, and the behavioral and physiological effects of opioid withdrawal can be a major impediment to recovery. Medication for OUD is currently the mainstay of treatment; however, it has limitations and alternative approaches are needed.
Objective:
The purpose of this study was to assess the effects of transcutaneous cervical vagus nerve stimulation (tcVNS) on behavioral and physiological manifestations of acute opioid withdrawal.
Methods:
Patients with OUD undergoing acute opioid withdrawal were randomly assigned to receive double blind active tcVNS (N = 10) or sham stimulation (N = 11) while watching neutral and opioid cue videos. Subjective opioid withdrawal, opioid craving, and anxiety were measured using a Visual Analogue Scale (VAS). Distress was measured using the Subjective Units of Distress Scale (SUDS), and pain was measured using the Numerical Rating Scale (NRS) for pain. Electrocardiogram signals were measured to compute heart rate. The primary outcomes of this initial phase of the clinical trial (ClinicalTrials.gov NCT04556552) were heart rate and craving.
Results:
tcVNS compared to sham resulted in statistically significant reductions in subjective opioid withdrawal (p = .047), pain (p = .045), and distress (p = .004). In addition, tcVNS was associated with lower heart rate compared to sham (p = .026). Craving did not significantly differ between groups (p = .11).
Conclusions:
tcVNS reduces behavioral and physiological manifestations of opioid withdrawal, and should be evaluated in future studies as a possible non-pharmacologic, easily implemented approach for adjunctive OUD treatment.
Keywords: Opioid use disorder, Withdrawal, Non-invasive, Vagus nerve stimulation, Sham-controlled, Double-blind
1. Introduction
Substance use is an escalating crisis in the United States, with drug overdose deaths, the majority secondary to opioids, rising over 100,000 in 2021 [1]. The standard of care for treating opioid use disorder (OUD) is medication for OUD (MOUD), including opioid receptor agonists and antagonists; however, the barriers to care are high [2-4]. “Secret shopper” studies demonstrate that fewer than 25% of patients are able to successfully access MOUD [5], and other potential barriers may prevent many patients from seeking treatment in the first place [6,7]. As many as 70% of patients with OUD will return to opioid use without medication, and even with the opioid agonist, methadone, 46% will return to use [8]. Treatment with the opioid antagonist naltrexone also requires an extended period of detoxification before initiation, which can be associated with withdrawal symptoms [9-11]. Return to use during this period may be associated with an even higher risk of relapse-related overdose and death, due to loss of tolerance [2,12,13]. New paradigms are therefore necessary for managing withdrawal in the treatment of OUD.
In 2017, the Food and Drug Administration (FDA) approved a percutaneous form of vagus nerve stimulation (VNS) for the treatment of opioid withdrawal [14]. This was based on the results of an open label trial of 73 patients with OUD, where significant reductions in opioid withdrawal symptoms were observed within 1 h [15]. This study was limited by lack of a sham stimulation control, and lack of objective measures of withdrawal including physiological measures of sympathetic nervous system activation.
We previously demonstrated that transcutaneous cervical VNS (tcVNS) reduces sympathetic [16-20] and inflammatory biomarker responses to stress [21,22], and modulates central brain regions involved in stress and emotion that have also been implicated in addictions (Fig. 1) [23-25]. Given the key role of sympathetic nervous system activation in opioid withdrawal, this suggests that tcVNS could reduce sympathetic activation and symptoms of withdrawal in patients with OUD. Therefore, the purpose of this study was to compare tcVNS to sham stimulation in patients with OUD undergoing acute withdrawal. We hypothesized that tcVNS would result in reductions in subjective withdrawal, craving, pain, and distress, as well as physiological markers such as heart rate.
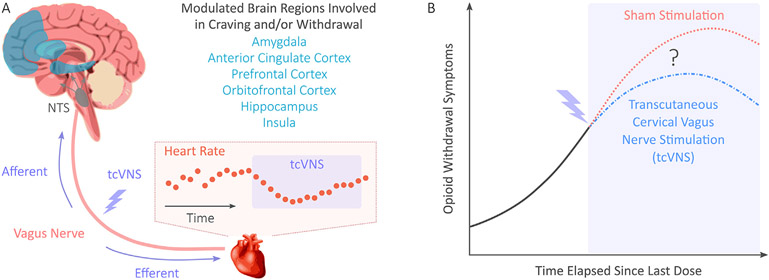
Fig. 1.
Background and research question. (A) Previous studies of transcutaneous cervical vagus nerve stimulation (tcVNS) found modulated brain activity in regions associated with opioid craving and/or withdrawal. Heart rate reductions during tcVNS were also observed. The hypothesized mechanism of action is shown, where tcVNS is believed to produce afferent signaling to the nucleus tractus solitarius (NTS), followed by neural processing and resultant efferent signaling affecting peripheral organs such as the heart. (B) In this study, we hypothesized that tcVNS would reduce opioid withdrawal symptoms in comparison to sham stimulation in a double-blind, randomized, sham-controlled pilot study.
2. Material and methods
2.1. Study cohort
To investigate the effects of tcVNS on patients with OUD experiencing opioid withdrawal, the first of two phases of a clinical trial (ClinicalTrials.gov NCT04556552), approved by the institutional review boards of Emory University (IRB00117320) and the Georgia Institute of Technology (H20203), was conducted between November 2020 and September 2021. This initial phase's primary outcomes were heart rate and craving; the second phase will evaluate the remaining UG3 outcomes specified on ClinicalTrials.gov (e.g., anterior cingulate function) in a separate sample. Please see the Supplementary Material for more details. All participants were required to meet criteria for an OUD based on the Structured Clinical Interview for DSM-5 (SCID) and be between the ages of 18 and 80 years old. Participants were excluded if currently pregnant, breastfeeding, or implanted with a device (e.g., pacemaker); or if they had a history of meningitis, traumatic brain injury, neurological disorder, loss of consciousness for greater than 1 min, schizophrenia, schizoaffective disorder, bulimia, serious medical or neurological illness, carotid atherosclerosis, or cervical vagotomy.
All participants underwent acute opioid withdrawal during the study. Withdrawal was induced by requiring abstinence from opioids for a minimum of 8 h prior to study onset. Participants were recruited for study participation prior to initiation of MOUD, in partnership with the Emory Healthcare Addiction Services and Alliance Recovery Center (Decatur, GA). The Addiction Severity Index (ASI) was administered for all participants to detail prior substance use, and for the fifth participant onwards, urine drug tests were conducted to detect recent substance use. All participants provided written informed consent after receiving a complete description of the study, and data were collected either at the Emory University School of Medicine or at Alliance Recovery Center.
2.2. Study protocol
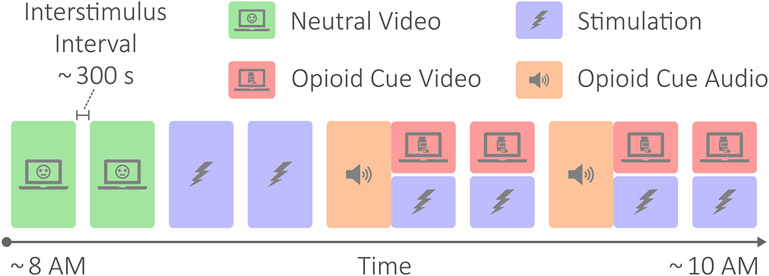
Fig. 2 outlines the study protocol. The protocol involved the patient viewing neutral videos meant to elicit neutral or positive affect for control purposes (baseline), receiving active tcVNS or sham stimulation, and hearing an opioid cue audio followed by opioid use videos meant to elicit opioid craving [26]. The neutral videos were recordings of a mailwoman describing her job, the opioid cue audio consisted of a guided breathing exercise followed by instructions to vividly recollect recent opioid use, and the opioid cue videos contained snippets of opioid use and imagery. The audio recording lasted approximately 4 min, while the video recordings were played for approximately 2 min at a time. Stimulation accompanied each of the opioid cue videos, and was also administered twice prior to any opioid cues. Stimulation occurred for exactly 2 min for each administration. All data collection took place in the morning and lasted for approximately 2 h. Participants wore masks to protect against Coronavirus Disease 2019 (COVID-19) and remained seated in a reclining chair throughout.
Fig. 2.
Protocol diagram. The protocol involved neutral videos meant to elicit neutral or positive affect for control purposes; active tcVNS or sham stimulation; and opioid cue audio and videos meant to elicit opioid craving. The protocol lasted approximately 2 h, where each protocol condition block was separated by approximately 5 min. Note that the opioid cue audios were immediately followed by opioid cue videos and stimulation.
2.3. Randomization and blinding
To maintain double blinding, participants were randomized into active tcVNS and sham stimulation groups using simple randomization. An individual not involved in recruitment, enrollment, data collection, or analysis assigned stimulation devices to participant identification numbers. The active tcVNS and sham stimulation devices were pre-numbered by the manufacturer and appeared and operated identically, differing only in stimulation waveforms. Thus, researchers and participants alike were blinded to stimulus type throughout the screening, clinical interview, and data collection. Unblinding occurred only upon active vs. sham analysis of the data.
2.4. tcVNS and sham stimulation
A handheld active tcVNS (gammaCore, electroCore, Basking Ridge, New Jersey) or sham stimulation device (gammaCore, electroCore, Basking Ridge, New Jersey) was connected to bar electrodes adhered to each participant. Each participant received only one of either active or sham stimulation, using the same device throughout the protocol. Electrode gel was applied to each electrode prior to placement to reduce skin-electrode impedance. A hook-and-loop strap was then used to fasten the bar electrodes over each participant's right carotid artery and maintain proper skin-electrode contact. Please see Fig. S1 for details.
The active tcVNS and sham stimulation devices only differed in the voltage signals delivered. The active devices produce an alternating current (AC) voltage signal consisting of five periods of a 5 kHz sinusoid, repeating every 40 ms (i.e., the device produces no signal for the remaining 39 ms of each cycle). Sham devices produce an AC biphasic square wave at a frequency of 0.2 Hz. For both devices, stimulation amplitude was adjustable using a rotary switch that ranged from 0 to 5. For the active devices, this corresponded to output voltages ranging from 0 to 26 V; for sham devices, this corresponded to voltages ranging from 0 to 4 V.
At the onset of each administration, a researcher turned the device on, keeping voltage output to 0 V until an audible beep was heard and a light emitting diode (LED) illuminated. Stimulation intensity was then increased from 0 V to the maximum level the participant could painlessly tolerate using a rolling switch. If participants felt any pain during stimulation, the intensity was reduced. Stimulation automatically stopped 120 s after administration onset. Participants were monitored and asked about undesirable side effects of stimulation after each administration.
2.5. Measurement of opioid withdrawal, craving, and pain
Opioid withdrawal symptoms were measured using two scales: a visual analog scale for withdrawal symptoms (VAS-withdrawal) and the Clinical Opiate Withdrawal Scale (COWS) [27]. After each of the eight protocol condition blocks (Fig. 2), the VAS-withdrawal was administered. Participants were asked to rate their withdrawal symptoms on a scale of 0–10, where 0 represented no symptoms at all and 10 represented the most severe experienced. The COWS includes eleven items, and the scores for each item range between 0 and 4 (or 5 for some items). The total score ranges from 0 to 48, 0 representing no withdrawal symptoms and 48 representing the worst measurable. The COWS was administered prior to and following the protocol.
Opioid craving was a primary outcome of this initial phase of the clinical trial and was measured using a visual analog scale for craving (VAS-craving). The VAS-craving ranged from 0 to 10, where 0 represented no cravings at all, and 10 represented extreme cravings. The VAS-craving was administered after each of the eight protocol condition blocks (Fig. 2).
Pain was measured using the Numerical Rating Scale (NRS) for pain. The NRS pain ranged from 0 to 10, where 0 represented no pain at all, and 10 represented the worst pain ever felt. Similar to the COWS, the NRS pain was administered prior to and following the protocol.
2.6. Measurement of distress and anxiety
Perceived distress and anxiety were measured throughout the protocol due to their roles in craving and relapse [28,29]. Distress was measured using the subjective units of distress scale (SUDS) that ranges from 0 to 100, where 0 represents complete relaxation and 100 represents the most distress ever felt [30]. Anxiety was measured using a visual analog scale for anxiety (VAS-anxiety) that ranged from 0 to 10, where 0 represents no anxiety at all and 10 represents the most ever felt. Both the SUDS and VAS-anxiety were administered after each of the eight protocol condition blocks (Fig. 2).
As in a prior study on tcVNS for patients with posttraumatic stress disorder [22], overlap was reduced between SUDS and VAS-Anxiety by using the following script when administering SUDS: “On a scale from 0 to 100, with 0 meaning ‘not at all’ and 100 meaning ‘the most ever,’ how distressed are you feeling right now?” For VAS-Anxiety, the following script was used: “On a scale from 0 to 10, 0 meaning ‘not at all’ and 10 meaning ‘the most ever,’ how anxious are you feeling right now?” Hence, unlike the typical administration of SUDS, the word “anxiety” was not mentioned and instead left for VAS-Anxiety measurements.
2.7. Measurement of heart rate
Heart rate was a primary outcome of this phase of the clinical trial and was computed from electrocardiogram (ECG) measurements. ECG was sensed using three Ag/AgCl electrodes in a lead I configuration. Using the Biopac RSPEC-R system (Biopac Systems, Goleta, CA), ECG signals were measured continuously throughout the protocol and data were acquired using the Biopac MP150 data acquisition system at a rate of 2 kHz. ECG signals were assessed for quality and “clean” R-peak to R-peak intervals (also referred to as “normal-to-normal” intervals) were extracted using the quality assessment and processing methods of prior work [31]; all the open source toolbox's default thresholds were used. An instantaneous heart rate value was then estimated for each normal-to-normal interval. This produced a heart rate time series for each participant used in subsequent analysis.
2.8. Comparing survey responses
To study tcVNS effects on the progression of perceived withdrawal, craving, pain, distress, and anxiety symptoms, the active and sham groups’ pre-stimulation to post-stimulation differences in survey scores were compared. For the VAS measures and SUDS, baseline was defined using the responses following the second neutral video, as this was the final protocol condition prior to stimulation. Differences were computed by subtracting baseline measurements from those made after the final protocol condition. For COWS and NRS-pain, differences between the post-protocol and pre-protocol measurements were computed. These differences are denoted with a Δ. Note that the post-protocol measurements of COWS and NRS-pain took place immediately after the final VAS and SUDS surveys were administered, i.e., the measurements used as minuends (when computing the Δ values) correspond to the same timepoint.
2.9. Comparing heart rate responses
Active and sham stimulation effects on heart rate were compared across all administrations. For each participant, the average heart rate during the second neutral video served as baseline. This baseline heart rate was subtracted from the average heart rate during each stimulation administration to produce a Δ heart rate value for each stimulation. Each participant's Δ heart rate values were also averaged across all stimulations to produce a single Δ heart rate value per participant for an additional active vs. sham comparison.
2.10. Statistical analysis
All statistical tests performed treated device group (active vs. sham) as the independent variable and the outcome of interest as the dependent variable. The Δ survey responses were compared using unpaired t-tests or Mann-Whitney U tests for normally and non-normally distributed variables (assessed using the Shapiro-Wilk test), respectively. The Δ heart rate responses, one per participant, were compared using an unpaired t-test. The Δ heart rate responses, one per condition, per participant, were compared using a mixed model with a random intercept per participant. These mixed models were fit using the lme4 package in R, where P values were obtained using the Satterthwaite's degrees of freedom method. All statistical tests performed were two-tailed with a significance level of .05.
Upon finding an imbalance in history of depression between the active and sham groups, additional analyses were conducted to assess the confounding effects of history of depression. Unpaired t-tests were replaced with multivariate linear models to adjust for history of depression. For the Δ heart rate mixed model, history of depression was adjusted for by including it as an additional covariate. Mann-Whitney U tests were replaced with ordinal logistic regression models to include history of depression as an additional covariate.
3. Results
3.1. Participant characteristics
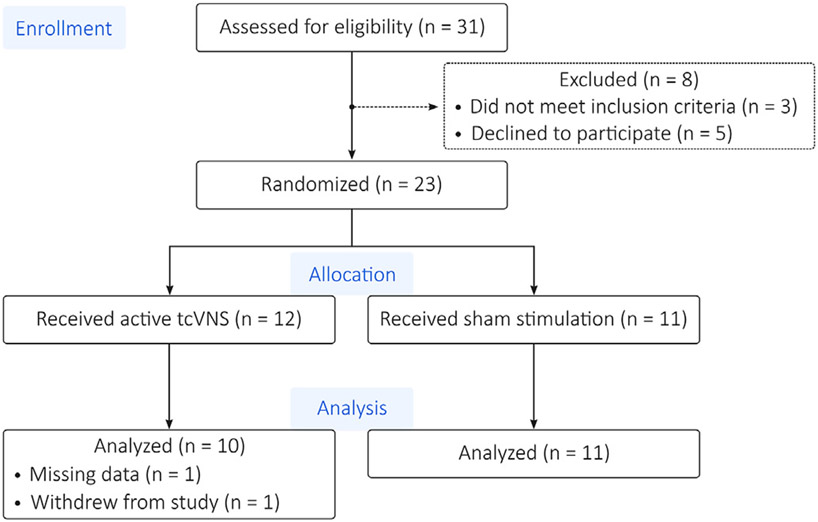
As shown in Fig. 3, of the 31 participants assessed for eligibility, 23 met inclusion criteria and agreed to participate. These 23 were randomized to exclusively receive either active or sham stimulation. One participant in the active group had unusable data, however, due to equipment malfunctions and a second participant in the active group withdrew from the study during the protocol. Therefore, this investigation considers the remaining 21 participants, 10 in the active and 11 in the sham group.
Fig. 3.
CONSORT Diagram. Of the 31 patients assessed for eligibility, 23 met inclusion criteria and agreed to participate. These 23 were randomized to exclusively receive either active or sham stimulation. One participant in the active group had unusable data due to equipment malfunctions. Another participant in the active group withdrew from the study.
Demographic data for each participant are included in Table S1 [age: 35 ± 11 years; body mass index: 28.1 ± 7.3 (mean ± standard deviation)]. Table S2 details medical history. Table 1 compares the active and sham groups’ characteristics. The sham group had more participants with a history of depression (8 participants, 73% of the sham group) than the active group (2, 20%). As shown in Table S3, the stimulation amplitudes received were 19.1 ± 5.2 V (mean ± SD) for active tcVNS and 3.9 ± 0.2 V for sham stimulation. Note these stimulation amplitudes were those delivered after all intensity adjustments were made. Table S4 details the urine drug test results, and Table S5 details drug use in the 30 days prior to study reported during completion of the ASI and/or SCID.
Table 1.
Active vs. Sham Characteristics Comparison.
| Parameter | Active (n = 10) | Sham (n = 11) |
|---|---|---|
| Age [years, mean (SD)] | 37.50 (11.4) | 33.27 (10.2) |
| Female [#, %] | 1, 10% | 5, 45.45% |
| Education [years, mean (SD)] | 12.4 (0.8) | 11.8 (1.4) |
| Studied at Alliance [#, %] | 5, 50% | 3, 27% |
| BMI [kg/m2, mean (SD)] | 26.50 (4.77) | 29.55 (9.05) |
| History of Smoking [#, %] | 9, 90% | 7, 64% |
| History of Alcohol Use [#, %] | 5, 50% | 7, 64% |
| History of Cardiovascular Disease [#, %] | 1, 10% | 1, 9% |
| History of Respiratory Disease [#, %] | 0, 0% | 1, 9% |
| History of Hematologic Disease [#, %] | 1, 10% | 0, 0% |
| History of Depression [#, %] | 2, 20% | 8, 73% |
| History of Posttraumatic Stress Disorder [#, %] | 2, 20% | 1, 9% |
| History of Anxiety Disorder [#, %] | 1, 10% | 1, 9% |
Abbreviations: SD, standard deviation.
3.2. Side effects of stimulation
No serious side effects were observed or reported. General complaints of uncomfortable muscle contraction were noted when stimulation amplitudes were initially set higher than what the participant could comfortably receive for 2 min (this only occurred in the active group). These concerns were remedied by decreasing stimulation amplitude. One participant (assigned to the active group) complained of lightheadedness after receiving stimulation. It remains unclear, however, whether this was tcVNS-induced (e.g., vasovagal syncope) or a coincidentally timed symptom of withdrawal. This lightheadedness soon subsided, and the participant continued the protocol with no further complaints.
3.3. Significant tcVNS-Induced reductions in opioid withdrawal, pain, and distress
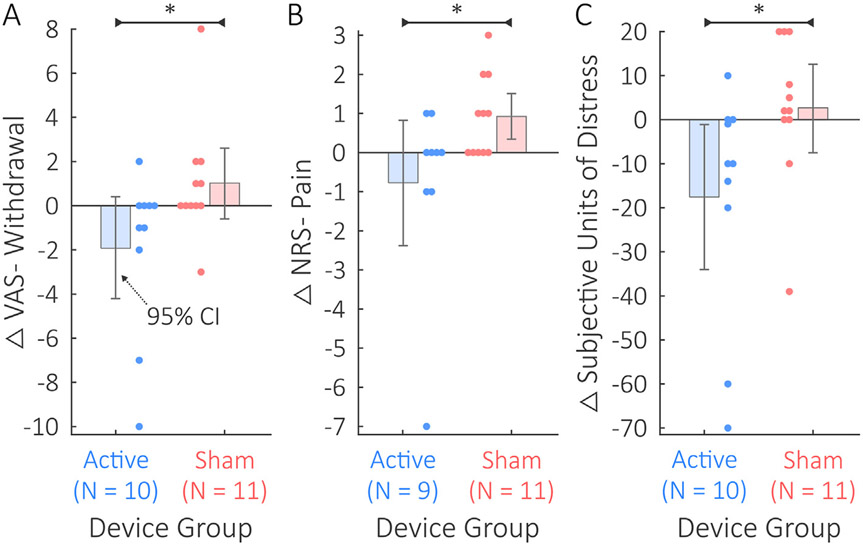
Significant differences existed between the active and sham groups in ΔVAS-withdrawal, ΔNRS-pain, and ΔSUDS (Fig. 4). The active group's ΔVAS-withdrawal scores of −1.9 ± 3.7 were significantly lower than the sham group's ΔVAS-withdrawal scores of 0.4 ± 1.0 with an effect size of f = 0.74; U = 29; p = .047 (Fig. 4A). The active group's ΔNRS-pain scores of −0.8 ± 2.4 were significantly lower than the sham group's ΔNRS-pain scores of 0.9 ± 1.0 with an effect size of f = 0.77; U = 23; p = .045 (Fig. 4B). Note that one participant in the active group was excluded from the ΔNRS-pain comparison due to missing data. The active group's ΔSUDS scores of −17.5 ± 26.5 were significantly lower than the sham group's ΔSUDS scores of 2.2 ± 5.9 with an effect size of f = 0.85; U = 17; p = .004 (Fig. 4C).
Fig. 4.
Comparison between active tcVNS and sham stimulation on self-report withdrawal, pain, and distress. Error bars depict 95% confidence intervals (CIs), Δ indicates post-protocol minus baseline changes, and * denotes p < .05. A) The active group's ΔVAS-Withdrawal scores were significantly less than for the sham group. B) The active group's Δ NRS-Pain scores were significantly lower than for the sham group. One patient who received active tcVNS was missing a post-protocol measurement of NRS-pain. C) The active group's Δ SUDS scores were significantly reduced compared to the sham group.
The differences between active and sham groups' ΔVAS-craving, ΔVAS-anxiety, and ΔCOWS were not statistically significant, though the active group's means were nominally lower for all measures. The active group's ΔVAS-craving scores (−2.2 ± 3.6) were not significantly different (d = 0.73, t = 1.66, p = .112) from the sham group's ΔVAS-craving scores (0.1 ± 2.7). The active group's ΔVAS-anxiety scores (−1.9 ± 3.5) were not significantly different (f = 0.67, U = 36, p = .175) from the sham group's ΔVAS-anxiety scores (−0.1 ± 1.8). The active group's ΔCOWS scores (0.6 ± 3.5) were not significantly different (d = 0.32, t = 0.73, p = .475) from the sham group's ΔCOWS scores (1.8 ± 4.1).
Table S6 details an item-by-item analysis of ΔCOWS. The Anxiety or Irritability item's change (i.e., ΔAnxiety or Irritability) was significantly lower (f = 0.80, U = 21.5, p = .017) for the active group (−0.6 ± 0.7) compared to the sham group (0.7 ± 1.3). No other items significantly differed between groups. Tables S7-S11 detail the baseline and post-protocol values for each of the survey responses.
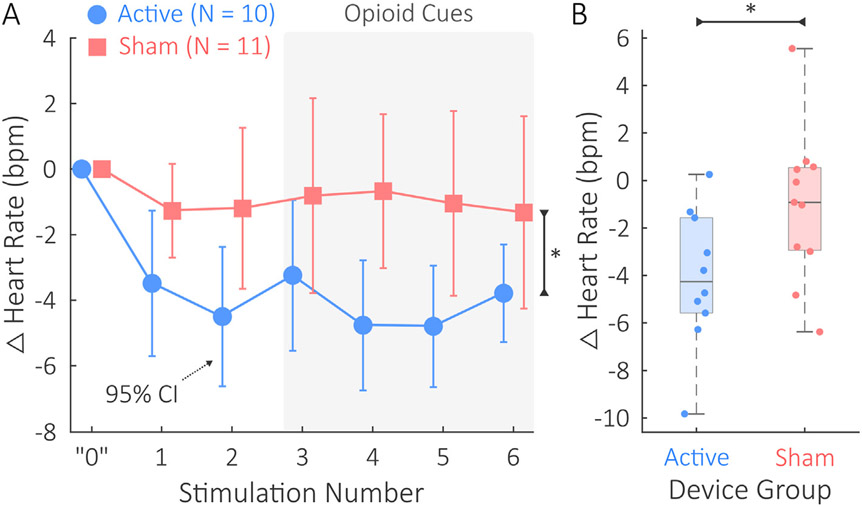
3.4. Significant tcVNS-Induced reductions in heart rate
Fig. 5 illustrates the active vs. sham comparisons in Δ heart rate responses to stimulation. Active tcVNS had a significant effect on Δ heart rate across all administrations (Fig. 5A) with an estimated decrease in 3.0 bpm for the active group compared to sham. The standardized fixed effect coefficient was β = 0.76; p = .026. Table S12 and Table S13 detail the mean heart rates themselves at baseline and during each stimulation.
Fig. 5.
Heart rate comparison between the active tcVNS and sham stimulation groups. Δ denotes changes relative to baseline. A) During all stimulation administrations, the active group's mean Δ heart rates in beats per minute (bpm) were significantly lower than the sham group's (p < .05, denoted by *). The error bars show 95% confidence intervals (CIs). The horizontal shift between active and sham data is included solely for the purpose of visualization; measurements were taken equivalently. A light gray background is used for stimulations 3–6 to highlight that opioid cues were presented to participants during this portion of the protocol. The “0” shown represents the baseline used as the initial point to compute differences from for Δ heart rate. B) The mean Δ heart rate response across all stimulations was significantly lower in the active group compared to the sham group (p < .05, denoted by *). Horizontal jitter is used to overlay the participant-specific datapoints.
Mean Δ heart rate also significantly differed between device groups (Fig. 5B). The mean Δ heart rate of the active group (−5.5 ± 3.5 bpm) was significantly lower than the mean Δ heart rate of the sham group (−1.4 ± 4.6 bpm). The effect size observed for this comparison was d = 0.99; t = 2.3; p = .035.
3.5. Post-hoc adjustments for history of depression
The results remained materially unchanged after post-hoc adjustments for history of depression. Table S14 details the linear model coefficients and standard errors before and after adjusting for history of depression. Table S15 details the ordinal logistic regression coefficients and standard errors before and after adjusting for history of depression.
4. Discussion
This double-blind, randomized, sham-controlled study of 21 patients with OUD found that tcVNS reduced both psychological and physiological symptoms of acute opioid withdrawal. In particular, statistically significant reductions in opioid withdrawal symptoms, distress, and pain were observed over the course of a 2-h protocol for the active tcVNS group, in comparison to the sham stimulation group. This was accompanied by a statistically significant decrease in heart rate during active tcVNS compared to sham stimulation, across all stimulation administrations. These findings support the potential for adjunctive use of tcVNS in mitigating acute opioid withdrawal symptoms, especially during detoxification and transitional periods (e.g., prior to MOUD) – periods of increased vulnerability to relapse where the prescription of MOUD may not be appropriate [32].
4.1. tcVNS reduced risk factors of opioid relapse
Compared to sham stimulation, active tcVNS elicited statistically significant reductions in three risk factors of relapse: withdrawal symptoms, pain, and distress. Opioid withdrawal symptoms are highly uncomfortable and are known to contribute to relapse in patients with OUD, especially during the acute phases [33-36]. After long-term use of opioids, many patients cite avoidance of withdrawal symptoms as a primary factor in continuing opioid use [33]. Pain intensification during opioid withdrawal is also associated with relapse risk [37,38]. If a patient recovering from OUD is exposed to a nociceptive stimulus, the increased pain sensitivity can motivate opioid use, and thus, perpetuate the addictive cycle [39]. Distress is another critical risk factor of return to opioid use [28,29,34]. Often, patients with OUD continue opioid use to avoid negative affective states and alleviate feelings of distress or anxiety [33,40]. Heightened levels of distress can also trigger relapse, even after lengthy periods of successful abstinence [23,41]. Reducing each of these risk factors via tcVNS may thus reduce the likelihood of relapse during early periods of withdrawal. It should be noted, however, that although the observed reductions in withdrawal symptoms, pain, and distress were statistically significant, they may not suffice in ultimately preventing a patient's relapse. Future longitudinal studies will be necessary to evaluate relapse rate as an outcome.
The active group's ΔVAS-Craving scores were 2.29 points less than the sham group's; however, this difference was not statistically significant. The reasoning for the discrepancy between statistically significant reductions in withdrawal symptoms and distress and the lack thereof for craving is unknown. We speculate, however, that subtle differences in the neurophysiological underpinnings of craving, withdrawal, and distress may explain this discrepancy. Craving is characterized by dysregulated executive function, where the predominant mediator is the prefrontal cortex [42,43]. In contrast, withdrawal is regarded as dysregulation of negative emotional states and the autonomic nervous system, where the extended amygdala, hypothalamus, and brainstem regions are believed to predominantly mediate the dysphoria and autonomic imbalance [42,43]. Notably, distress is mediated by similar regions [44]. Although these three responses share overlapping neural bases, the statistically significant survey score and heart rate reductions seem to indicate that the effects of tcVNS on executive function may not be as potent as tcVNS effects on the autonomic nervous and limbic systems. This may help explain the nonsignificant difference in ΔVAS-Craving between the active and sham groups. Functional neuroimaging studies in the context of OUD will be necessary to validate this hypothesis.
4.2. Reduced heart rate, distress, and withdrawal symptoms suggest autonomic mediation
Acute reductions in heart rate during tcVNS, as well as reductions in distress and withdrawal symptoms over the course of the protocol, indicate that the primary effects of tcVNS are mediated by the autonomic nervous system. Heart rate is mediated by both the parasympathetic and sympathetic branches of the autonomic nervous system, where decreases in sympathetic arousal and/or increases in parasympathetic activity cause heart rate to decrease [45]. Withdrawal symptoms are also a result of autonomic imbalance in favor of the sympathetic nervous system (e.g., via increased noradrenaline production in the locus coeruleus) [35]. Increased heart rate, sweating, pupil size, etc. – all symptoms of withdrawal – are a result of increased sympathetic arousal and/or decreased parasympathetic activity [46]. Distress is also associated with sympathetic arousal and reduced parasympathetic activity [47]. Therefore, perceived reductions in withdrawal symptoms and distress, along with measured reductions in heart rate, would align with the hypothesis that tcVNS effects on opioid withdrawal are autonomically mediated.
4.3. Implications for the adjunctive use of tcVNS for OUD
Based on this study's findings, the adjunctive use of tcVNS for OUD management should be evaluated in future studies. The non-invasive nature of tcVNS and its amenability to handheld administration enable on-demand and mobile use as needed. During periods of vulnerability, self-administered tcVNS could reduce the risk of opioid misuse by mitigating associated withdrawal symptoms, pain, or distress. For MOUD using opioid agonists such as buprenorphine, initial doses are typically followed by mild to moderate withdrawal symptoms [48]. These symptoms could be mitigated by tcVNS to improve treatment adherence. For individuals transitioning to treatment with an opioid antagonist, the required detoxification period results in a window of heightened vulnerability [49]. During this time window, tcVNS could be used to reduce symptom burden. Importantly, tcVNS poses minimal risk and requires minimal training for self-administration [50]. Its non-pharmacological nature allows targeting of the autonomic nervous system via the vagus nerve with less side effects that can be associated with pharmacological treatments for withdrawal such as lofexidine [51]. On the other hand, pharmacological treatments may produce stronger and more widespread effects that may be necessary in severe cases of withdrawal, even if side effects could linger for hours according to the drugs' half-lives. Future work will be necessary to evaluate these potential tradeoffs.
4.4. Limitations and future work
This study is not without limitations. Treatment with tcVNS, in comparison to sham stimulation, reduced subjective withdrawal as measured by the VAS but not clinician-observed withdrawal measured with the COWS. Other studies, however, have also noted greater effects of medication treatment on subjective versus clinician-rated withdrawal [8]. The lack of significant effects on craving and anxiety scores further limits conclusions of tcVNS efficacy. Assessing distress and anxiety separately using SUDS and VAS-Anxiety, respectively, may have resulted in confusion due to linguistic difficulties in disentangling distress and anxiety. There may have also been discrepancies between participants who treated these measures as simply differing by a scale factor of 10 (e.g., participant 7) and others who treated these as measuring differing phenomena. Compared to sham, tcVNS reduced the Anxiety or Irritability item of COWS, but no statistically significant difference existed between the groups for VAS-Anxiety. This may have been due to differing scales (VAS-Anxiety: 0–10; COWS Anxiety or Irritability: 0, 1, 2, or 4) or the inclusion of clinician judgement during COWS assessment. It may have also been helpful to make repeated measurements of COWS and/or NRS-pain after each protocol condition. At a minimum, this would have helped to standardize the baseline timepoints for all Δ computations. This study also had a small sample size, which limited statistical power and increased margin of error. Although participants were asked to confirm eight or more hours of drug abstinence prior to study initiation, no objective method was employed to confirm; the urine drug tests produced positive results for opioids taken within approximately 24–72 h prior, depending on drug half-life. The exact time of last substance use prior to the study was not recorded, limiting the contextualization of participant differences.
As this study focused on the initial phase of withdrawal, further investigations are necessary to study tcVNS use during later phases of withdrawal. Longitudinal studies during the full period of detoxification should assess whether tcVNS use ultimately leads to reduced overall symptoms and relapse rates, and increased completion of detoxification and enrollment into OUD treatment. Studying the neurophysiological effects of tcVNS, as well as additional physiological measures (e.g., blood pressure) in the context of OUD also remain compelling avenues of future work. These investigations would help to determine whether differing neurophysiological effects could explain the discrepancy between withdrawal and craving effects, as well as to evaluate the hypothesis of autonomic mediation of tcVNS effects. If this hypothesis holds true, comparative studies of tcVNS and alpha-2-adrenergic agonists such as lofexidine are warranted. These future studies could help evaluate possible tradeoffs between non-invasive neuromodulation approaches (e.g., tcVNS) and pharmacological treatments (e.g., clonidine) for opioid withdrawal.
5. Conclusions
This double-blind, randomized, sham-controlled pilot study of tcVNS is the first randomized, placebo-controlled (i.e., sham controlled) investigation of non-invasive neuromodulation in human participants with OUD [52]. The observed reductions in withdrawal symptoms, pain, and distress for the active tcVNS group compared to the sham stimulation group are therefore encouraging given they likely cannot be attributed to placebo effects. The reductions in heart rate strengthen the results, as they seem to indicate tcVNS-induced autonomic effects that counteract sympathetic arousal accompanying withdrawal. As a non-invasive, non-pharmacological therapy that poses minimal risk and is amenable to self-administration, tcVNS for the management of opioid withdrawal in individuals with OUD should be evaluated in future studies as a possible adjunctive treatment for OUD.
Supplementary Material
Acknowledgements
The authors gratefully acknowledge the Alliance Recovery Center in Decatur, GA for their invaluable assistance with recruitment efforts. The work of A. H. Gazi was supported by a National Science Foundation (NSF) Graduate Research Fellowship (DGE-2039655). This research was supported by the National Institutes of Health (NIH) (UG3 DA048502 and R01HL155711). The work of T. P. Lambert was supported by National Institute on Drug Abuse (NIDA) UG3 DA048502-01A1S2, and the work of R. Alvarado Ortega was supported by NIDA UG3 DA048502-01A1S1. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NSF, NIH, or its NIH Helping to End Addiction Long Term (HEAL) Initiative.
Abbreviations:
- OUD
Opioid Use Disorder
- VNS
Vagus Nerve Stimulation
- tcVNS
Transcutaneous Cervical Vagus Nerve Stimulation
- VAS
Visual Analogue Scale
- SUDS
Subjective Units of Distress Scale
- NRS
Numerical Rating Scale
- MOUD
Medication for Opioid Use Disorder
- SCID
Structured Clinical Interview for DSM-5
- COWS
Clinical Opiate Withdrawal Scale
- ECG
Electrocardiogram
- ASI
Addiction Severity Index
Footnotes
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: The active and sham vagus nerve stimulation devices used in this research were provided in-kind by electroCore, Inc. J. D. Bremner has received grant funding support in the past from electroCore and currently serves on the Scientific Advisory Board for Evren Technologies, Inc. All other authors report no biomedical financial interests or potential conflicts of interest.
CRediT authorship contribution statement
Asim H. Gazi: Conceptualization, Methodology, Software, Validation, Formal analysis, Investigation, Resources, Data curation, Writing – original draft, Writing – review & editing, Visualization, Funding acquisition. Anna B. Harrison: Conceptualization, Methodology, Software, Validation, Investigation, Resources, Data curation, Writing – review & editing, Visualization. Tamara P. Lambert: Methodology, Investigation, Resources, Data curation, Writing – review & editing. Malik Obideen: Methodology, Investigation, Resources, Data curation, Writing – review & editing. Parvaneh Alavi: Investigation, Resources, Data curation, Writing – review & editing. Nancy Murrah: Methodology, Resources, Writing – review & editing, Supervision. Lucy Shallenberger: Resources, Data curation, Writing – review & editing. Emily G. Driggers: Methodology, Investigation, Resources, Data curation, Writing – review & editing. Rebeca Alvarado Ortega: Investigation, Resources, Writing – review & editing. Brianna P. Washington: Investigation, Resources, Writing – review & editing. Kevin M. Walton: Conceptualization, Writing – review & editing, Supervision. Justine W. Welsh: Conceptualization, Methodology, Resources, Writing – review & editing, Supervision, Funding acquisition. Viola Vaccarino: Conceptualization, Methodology, Writing – review & editing, Supervision, Funding acquisition. Amit J. Shah: Conceptualization, Methodology, Writing – review & editing, Supervision, Funding acquisition. Yi-Lang Tang: Resources, Writing – review & editing. Rahul Gupta: Resources, Writing – review & editing. Sudie E. Back: Methodology, Resources, Writing – review & editing. Omer T. Inan: Conceptualization, Methodology, Resources, Writing – review & editing, Visualization, Supervision, Project administration, Funding acquisition. J. Douglas Bremner: Conceptualization, Methodology, Resources, Writing – original draft, Writing – review & editing, Supervision, Project administration, Funding acquisition.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.brs.2022.08.017.
References
- [1].Drug Overdose Deaths in the U.S. Top 100,000 Annually [Internet]. [cited 2022 Feb 25]. Available from:: https://www.cdc.gov/nchs/pressroom/nchs_press_releases/2021/20211117.htm. [Google Scholar]
- [2].Bailey GL, Herman DS, Stein MD. Perceived relapse risk and desire for medication assisted treatment among persons seeking inpatient opiate detoxification. J Subst Abuse Treat 2013. Sep 1;45(3):302–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Sigmon SC, Bisaga A, Nunes EV, O'Connor PG, Kosten T, Woody G. Opioid detoxification and naltrexone induction strategies: recommendations for clinical practice. Am J Drug Alcohol Abuse 2012. May;38(3):187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Volkow ND, Frieden TR, Hyde PS, Cha SS. Medication-assisted therapies — tackling the opioid-overdose epidemic. N Engl J Med 2014. May 29;370(22):2063–6. [DOI] [PubMed] [Google Scholar]
- [5].Beetham T, Saloner B, Wakeman SE, Gaye M, Barnett ML. Access to office-based buprenorphine treatment in areas with high rates of opioid-related mortality: an audit study. Ann Intern Med 2019;171(1):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Bell J, Strang J. Medication treatment of opioid use disorder. Biol Psychiatr 2020. Jan 1;87(1):82–8. [DOI] [PubMed] [Google Scholar]
- [7].Lagisetty PA, Bohnert A. Role of an accurate treatment locator and cash-only practices in access to buprenorphine for opioid use disorders. Ann Intern Med 2019;171(1):58–9. [DOI] [PubMed] [Google Scholar]
- [8].Mattick RP, Breen C, Kimber J, Davoli M. Methadone maintenance therapy versus no opioid replacement therapy for opioid dependence. Cochrane Database Syst Rev 2002. Apr 22;(4):CD002209. [DOI] [PubMed] [Google Scholar]
- [9].Krupitsky E, Nunes EV, Ling W, Illeperuma A, Gastfriend DR, Silverman BL. Injectable extended-release naltrexone for opioid dependence: a double-blind, placebo-controlled, multicentre randomised trial. Lancet (London, England) 2011;377(9776):1506–13. [DOI] [PubMed] [Google Scholar]
- [10].Tanum L, Solli Kk, Latif ZEH, Benth JŠ, Opheim A, Sharma-Haase K, et al. Effectiveness of injectable extended-release naltrexone vs daily buprenorphine-naloxone for opioid dependence: a randomized clinical non-inferiority trial. JAMA Psychiatr 2017. Dec 1;74(12):1197–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Lee JD, Friedmann PD, Kinlock TW, Nunes EV, Boney TY, Hoskinson RA, et al. Extended-Release naltrexone to prevent opioid relapse in criminal justice offenders. N Engl J Med 2016. Mar 31;374(13):1232–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Strang J, McCambridge J, Best D, Beswick T, Bearn J, Rees S, et al. Loss of tolerance and overdose mortality after inpatient opiate detoxification: follow up study. BMJ Br Med J (Clin Res Ed) 2003. May 3;326(7396):959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Sullivan M, Bisaga A, Pavlicova M, Choi CJ, Mishlen K, Carpenter KM, et al. Long-acting injectable naltrexone induction: a randomized trial of outpatient opioid detoxification with naltrexone versus buprenorphine. Am J Psychiatr 2017. May 1;174(5):459–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Zagorski N. FDA clears way for stimulation device to treat opioid withdrawal symptoms. https://doi.org/101176/appi.pn20181a16. 2017. Dec 26;53(1):1–1. [Google Scholar]
- [15].Miranda A, Taca A. Neuromodulation with percutaneous electrical nerve field stimulation is associated with reduction in signs and symptoms of opioid withdrawal: a multisite, retrospective assessment. https://doi.org/101080/0095299020171295459. 2017. Jan 2;44(1):56–63. [DOI] [PubMed] [Google Scholar]
- [16].Gurel NZ, Huang M, Wittbrodt MT, Jung H, Ladd SL, Shandhi MMH, et al. Quantifying acute physiological biomarkers of transcutaneous cervical vagal nerve stimulation in the context of psychological stress. Brain Stimul 2020. Jan 1;13(1):47–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Gurel NZ, Wittbrodt MT, Jung H, Shandhi MMH, Driggers EG, Ladd SL, et al. Transcutaneous cervical vagal nerve stimulation reduces sympathetic responses to stress in posttraumatic stress disorder: a double-blind, randomized, sham controlled trial. Neurobiol Stress 2020. Nov 1:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Gazi AH, Gurel NZ, Richardson KLS, Wittbrodt MT, Shah AJ, Vaccarino V, et al. Digital cardiovascular biomarker responses to transcutaneous cervical vagus nerve stimulation: state-space modeling, prediction, and simulation. JMIR mHealth uHealth 2020. Sep 1;8(9):e20488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Gazi AH, Sundararaj S, Harrison AB, Gurel NZ, Wittbrodt MT, Shah AJ, et al. Transcutaneous cervical vagus nerve stimulation lengthens exhalation in the context of traumatic stress. In: IEEE-EMBS international conference on biomedical and health informatics. BHI; ); 2021. p. 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Gazi AH, Sundararaj S, Harrison AB, Gurel NZ, Wittbrodt MT, Alkhalaf M, et al. Transcutaneous cervical vagus nerve stimulation inhibits the reciprocal of the pulse transit time's responses to traumatic stress in posttraumatic stress disorder. In: Annual international conference of the IEEE engineering in medicine and biology society; 2021. p. 1444–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Bremner JD, Gurel NZ, Jiao Y, Wittbrodt MT, Levantsevych OM, Huang M, et al. Transcutaneous vagal nerve stimulation blocks stress-induced activation of Interleukin-6 and interferon-γ in posttraumatic stress disorder: a double-blind, randomized, sham-controlled trial. Brain, Behav Immun - Heal 2020. Dec 1;9:100138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Bremner JD, Gurel NZ, Wittbrodt MT, Shandhi MH, Rapaport MH, Nye JA, et al. Application of noninvasive vagal nerve stimulation to stress-related psychiatric disorders. J Personalized Med 2020. Sep 1;10(3):1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Koob GF. Neurobiology of opioid addiction: opponent process, hyperkatifeia, and negative reinforcement. Biol Psychiatr 2020. Jan 1;87(1):44–53. [DOI] [PubMed] [Google Scholar]
- [24].Wittbrodt MT, Gurel NZ, Nye JA, Shandhi MMH, Gazi AH, Shah AJ, et al. Noninvasive cervical vagal nerve stimulation alters brain activity during traumatic stress in individuals with posttraumatic stress disorder. Psychosom Med 2021. Nov;83(9):969–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Wittbrodt MT, Gurel NZ, Nye JA, Ladd S, Shandhi MMH, Huang M, et al. Non-invasive vagal nerve stimulation decreases brain activity during trauma scripts. Brain Stimul 2020. Sep 1;13(5):1333–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Back SE, Gros DF, McCauley jL, Flanagan JC, Cox E, Barth KS, et al. Laboratory-induced cue reactivity among individuals with prescription opioid dependence. Addict Behav 2014;39(8):1217–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Wesson DR, Ling W. The clinical opiate withdrawal scale (COWS). J Psychoact Drugs 2003;35(2):253–9. [DOI] [PubMed] [Google Scholar]
- [28].Mantsch JR, Baker DA, Funk D, Lê AD, Shaham Y. Stress-induced reinstatement of drug seeking: 20 Years of progress. 2016 411 Neuropsychopharmacology 2015. May 15;41(1):335–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Sinha R. How does stress increase risk of drug abuse and relapse?. 2001 1584 Psychopharmacol 2001;158(4):343–59. [DOI] [PubMed] [Google Scholar]
- [30].Wolpe J, Lazarus AA. Behavior therapy techniques: a guide to the treatment of neuroses. Behavior therapy techniques: a guide to the treatment of neuroses. Elmsford, NY, US: Pergamon Press; 1966. The Commonwealth and international library. Mental health and social medicine division.). [Google Scholar]
- [31].Gazi AH, Wittbrodt MT, Harrison AB, Sundararaj S, Gurel NZ, Nye JA, et al. Robust estimation of respiratory variability uncovers correlates of limbic brain activity and transcutaneous cervical vagus nerve stimulation in the context of traumatic stress. IEEE Trans Biomed Eng 2022;69(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Bailey GL, Herman DS, Stein MD. Perceived relapse risk and desire for medication assisted treatment among persons seeking inpatient opiate detoxification. J Subst Abuse Treat 2013. Sep;45(3):302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Cicero TJ, Ellis MS. The prescription opioid epidemic: a review of qualitative studies on the progression from initial use to abuse. Dialogues Clin Neurosci 2017. Sep 1;19(3):259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Rigg KK, Ibañez GE. Motivations for non-medical prescription drug use: a mixed methods analysis. J Subst Abuse Treat 2010. Oct;39(3):236–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Kosten TR, Baxter LE. Review article: effective management of opioid withdrawal symptoms: a gateway to opioid dependence treatment. Am J Addict 2019. Feb 1;28(2):55–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Pergolizzi JV, Raffa RB, Rosenblatt MH. Opioid withdrawal symptoms, a consequence of chronic opioid use and opioid use disorder: current understanding and approaches to management. J Clin Pharm Therapeut 2020. Oct 1;45(5):892–903. [DOI] [PubMed] [Google Scholar]
- [37].Shah A, Hayes CJ, Martin BC. Characteristics of initial prescription episodes and likelihood of long-term opioid use — United States, 2006–2015. MMWR Morb Mortal Wkly Rep 2019. Mar 17;66(10):265–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Compton P, Charuvastra VC, Ling W. Pain intolerance in opioid-maintained former opiate addicts: effect of long-acting maintenance agent. Drug Alcohol Depend 2001. Jul 1;63(2):139–46. [DOI] [PubMed] [Google Scholar]
- [39].Bommersbach T, Ross DA, De Aquino JP. Perpetual hunger: the neurobiological consequences of long-term opioid use. Biol Psychiatr 2020. Jan 1;87(1):e1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Sinha R. Chronic stress, drug use, and vulnerability to addiction. Ann N Y Acad Sci 2008;1141:105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Stewart J, Blvd MW. Pathways to relapse: the neurobiology of drug- and stress-induced relapse to drug-taking. J Psychiatry Neurosci 2000;25(2):125. [PMC free article] [PubMed] [Google Scholar]
- [42].Koob GF. Neurobiology of opioid addiction: opponent process, hyperkatifeia, and negative reinforcement. Biol Psychiatr 2020. Jan 1;87(1):44–53. [DOI] [PubMed] [Google Scholar]
- [43].Koob GF, Volkow ND. Neurocircuitry of addiction. 2010 351 Neuropsychopharmacology 2009. Aug 26;35(1):217–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Godoy LD, Rossignoli MT, Delfino-Pereira P, Garcia-Cairasco N, Umeoka EH de L. A comprehensive overview on stress neurobiology: basic concepts and clinical implications. Front Behav Neurosci 2018. Jul 3;12:127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Klabunde RE. Cardiovascular physiology concepts. second ed. Philadelphia, PA: Lippincott Williams and Wilkins; 2011. [Google Scholar]
- [46].McCorry LK. Physiology of the autonomic nervous system. Am J Pharmaceut Educ 2007;71(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Ziegler MG. Psychological stress and the autonomic nervous system. In: Prim auton nerv syst. second ed.; 2004. Jan 1. p. 189–90. [Google Scholar]
- [48].Organization WH. In: Davoli M, editor. Guidelines for the psychosocially assisted pharmacological treatment of opioid dependence. WHO Press; 2009. [PubMed] [Google Scholar]
- [49].Comer SD, Sullivan MA, Yu E, Rothenberg JL, Kleber HD, Kampman K, et al. Injectable, sustained-release naltrexone for the treatment of opioid dependence: a randomized, placebo-controlled trial. Arch Gen Psychiatr 2006. Feb 1;63(2):210–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Redgrave J, Day D, Leung H, Laud PJ, Ali A, Lindert R, et al. Safety and tolerability of Transcutaneous Vagus Nerve stimulation in humans; a systematic review. Brain Stimul 2018. Nov 1;11(6):1225–38. [DOI] [PubMed] [Google Scholar]
- [51].Pergolizzi JV, Annabi H, Gharibo C, LeQuang JA. The role of lofexidine in management of opioid withdrawal. Pain Ther 2019. Jun 1;8(1):67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Young JR, Smani SA, Mischel NA, Kritzer MD, Appelbaum LG, Patkar AA. Non-invasive brain stimulation modalities for the treatment and prevention of opioid use disorder: a systematic review of the literature. https://doi.org/101080/1055088720201736756. 2020. Feb 17;38(2):186–99. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.