Abstract
Background and objectives:
The goal of this review is to describe the general features, mechanisms, technical recording factors, and clinical applications of brain evoked potentials (EPs) generated by deep brain stimulation (DBS) for Parkinson’s disease (PD).
Results:
Evoked potentials in response to DBS pulses occur on the timescale of milliseconds and are found both locally at the site of stimulation and remotely in the cortex. DBS evoked potentials arise from a complex integration of antidromic and orthodromic conduction pathway responses, and provide information valuable for understanding the mechanisms and circuits involved in symptom treatment. Furthermore, these signals may provide biomarkers for improving DBS outcomes and function. For example, evoked potentials may have utility as control signals for DBS programming or adaptive DBS. Despite their promise there are still critical gaps in our understanding of the mechanisms by which evoked potentials arise and how these signals may be measured and applied in the clinical setting. Technical challenges of recording a highly transient signal at sufficient resolution without the interference of stimulation artifact present a barrier to understanding better DBS-induced EPs.
Conclusions:
We describe the current scientific landscape of evoked potentials to facilitate and stimulate further investigation.
Keywords: DBS, Parkinson’s disease, Cortical evoked potentials, DBS local Evoked potentials
1. Introduction
Parkinson’s disease (PD) is the world’s fastest growing neurological disorder, with the prevalence expected to double to 12 million patients between 2015 and 2040 [1]. PD is characterized by loss of dopaminergic neurons in the substantia nigra pars compacta (SNc) [2], which results in motor symptoms including resting tremor, postural instability, bradykinesia, and shuffling gait [2,3]. However, PD pathology and symptom progression begins prior to the loss of dopaminergic neurons in the SNc. Early pathology begins in the medulla and olfactory bulb, mirroring the early symptoms of rapid eye movement (REM) sleep behavior disorder and decreased sense of smell [3]. Next, pathology progresses to the SNc and other midbrain and forebrain structures. This stage is associated with classic PD motor symptoms and is when PD is most commonly diagnosed. Lastly, in advanced stages, pathology spreads to the cortex, resulting in cognitive decline and hallucinations.
PD is initially managed with medications aimed at restoring dopaminergic activity in the brain. Response to levodopa, a dopamine precursor, both supports the diagnosis of PD and is the most effective medical therapy [1]. However, as the disease progresses, many patients develop side effects, fluctuations in medication response, or medication refractory tremor, at which time deep brain stimulation (DBS) can improve motor symptoms [2,3]. DBS electrodes for PD treatment are implanted in either subthalamic nucleus (STN) or globus pallidus internus (GPi). Multiple clinical trials have demonstrated that DBS is superior to medical therapy in moderate-to-severe PD. The EARLYSTIM randomized trial showed benefit at 4 years after PD diagnosis [4,5], leading to revision of the prior FDA approval in 2002 to earlier DBS in 2016.
While DBS effectively treats several motor symptoms of Parkinson’s disease, limitations remain in side effect profile, management of non-motor symptom, accurate lead placement with intraoperative testing, and selection of optimal stimulation parameters. For example, STN DBS can cause motor side effects such as speech impairment and dyskinesia [6–8]. Although DBS electrode implantation can be conducted with intraoperative patient-awake microelectrode recording and testing of symptom control and side effects to confirm accurate lead placement, patients often have anxiety and discomfort in this setting [9]. DBS electrode implantation is usually done as an awake procedure with intraoperative clinical observation during test stimulation to confirm both alleviation of motor symptoms and avoidance of side effects; however, the GALAXY randomized clinical trial concluded awake surgery does not produce better outcomes over asleep surgery [10]. Lastly, stimulation parameter selection is a time-consuming, empirical practice conducted over multiple programming sessions in a specialty clinic [11,12], and the results may be suboptimal due to the extensive parameter space, which is further enlarged by new 8 channel segmented leads [13]. Further, optimal stimulation parameters are dependent on the dynamic clinical state of the patient, delayed time to clinical benefit (i.e., up to 1–2 days), the patient’s medication status, disease progression, and sleep/wake state [11,14,15]. A snapshot of patient responses to DBS parameter changes in the clinic cannot capture these complex fluctuating changes in motor status.
Many of these challenges in DBS implementation may be improved through the use of biomarkers linked to neural activity, patient state, and symptom relief for more automated, objective programming. For example, biomarkers generated by DBS, evoked potentials (EP), have shown potential to improve programming, reveal relevant mechanisms, and identify circuits involved in DBS for PD [16–20]. Evoked potentials are generated by neural activity in response to applied stimuli and reveal information about neural connectivity and function. The transmembrane currents in activated neurons generate voltages in the tissue that can be recorded with implanted electrodes. Similar to how electrocardiograms (EKGs) provide valuable clinical insight into heart function, EPs have proven clinically useful, for example in implementation of cochlear implants [21], and the diagnostic detection and localization of nerve and brain lesions [22].
DBS generates EPs in both local (subcortical) and remote (cortical) regions of the nervous system based on neuronal and axonal stimulation (Fig. 1). DBS local evoked potentials (DLEPs), recorded at the site of stimulation, arise from the synchronous activation of neural elements near the active DBS contacts [16,18,23]. Alternatively, these locally recorded signals in STN have also been termed evoked resonant neural activity (ERNA). Cortical evoked potentials (cEPs) are also observed in response to DBS [24,25]. These EPs have various potential clinical applications [16–18,26,27] and may ultimately be used as biomarkers for DBS optimization.
Fig. 1.
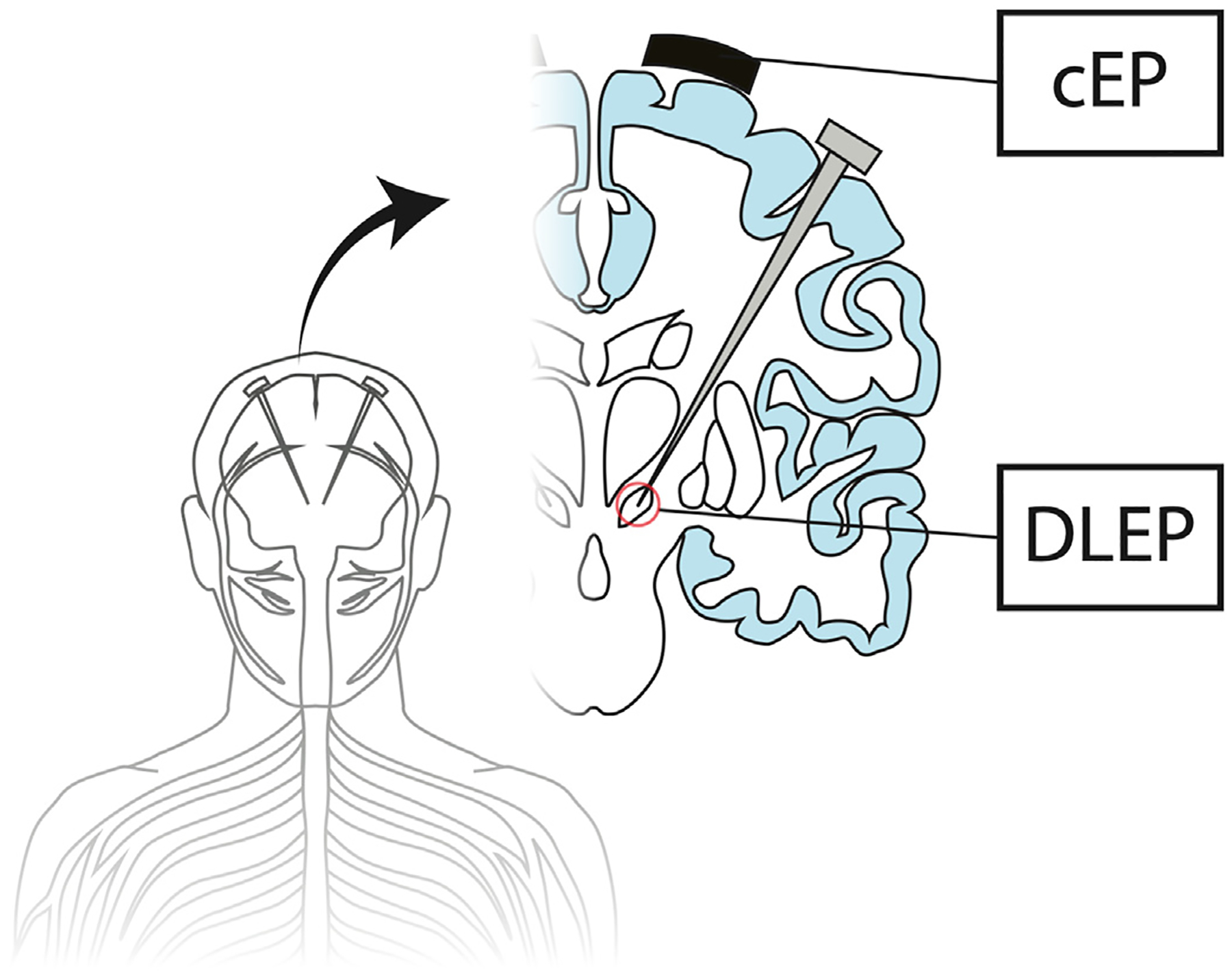
Evoked potentials can be recorded locally at the site of deep brain stimulation (DBS), i.e., DBS local evoked potentials (DLEP) or remotely at the cortex, i.e., cortical evoked potentials (cEP).
Here we review the general features, technical recording considerations, underlying neural mechanisms, and clinical applications of DBS-induced EPs. This review highlights both the promise of novel neurophysiological studies and future device and therapy advances that could be enabled by EPs.
2. Cortical evoked potentials
2.1. Features of cortical evoked potentials generated by DBS
Cortical evoked potentials (cEPs) have been recorded during DBS in both humans [24,25,28–30] and rats [19,31]. For example, STN DBS induces cEPs that vary with stimulation frequency [25]. cEPs generated by low frequency (≤20 Hz) STN DBS exhibit short- (R1, 1–3 ms), intermediate- (R2, 5–15 ms) and long- (R3, 18–25 ms) latency components [19,32] (Fig. 2). At frequencies between 5 and 50 Hz cEP amplitude showed constant latency but peak amplitude at 20 Hz [33]. Clinically relevant DBS frequencies (>100 Hz) contain interpulse intervals shorter than the latency of the R2 and R3 cEP components, and thus these components are obscured. A study investigating DBS frequency (4.5–130 Hz) found R1 peak amplitude was lowest at 130 Hz and R1 latency was greatest at 130 Hz [19]. Both Eusebio [33] and Kumaravelu [19] probed cEPs at varying stimulation frequencies: Kumaravelu (testing 4.5 Hz, 9 Hz, 50 Hz, and 130 Hz) found the maximum R1 amplitude at 9 Hz, although amplitude peaked at 20 Hz. Eusebio showed there was a constant R1 latency up to 20 Hz, but Kumaravelu’s R1 latency exhibited a nonlinear increase as the frequency approached 130 Hz. Overall, R1 amplitude and latency may reflect changing network dynamics across stimulation frequencies and R2 and R3 may vary even at interpulse intervals that obscure and overlap with them.
Fig. 2.
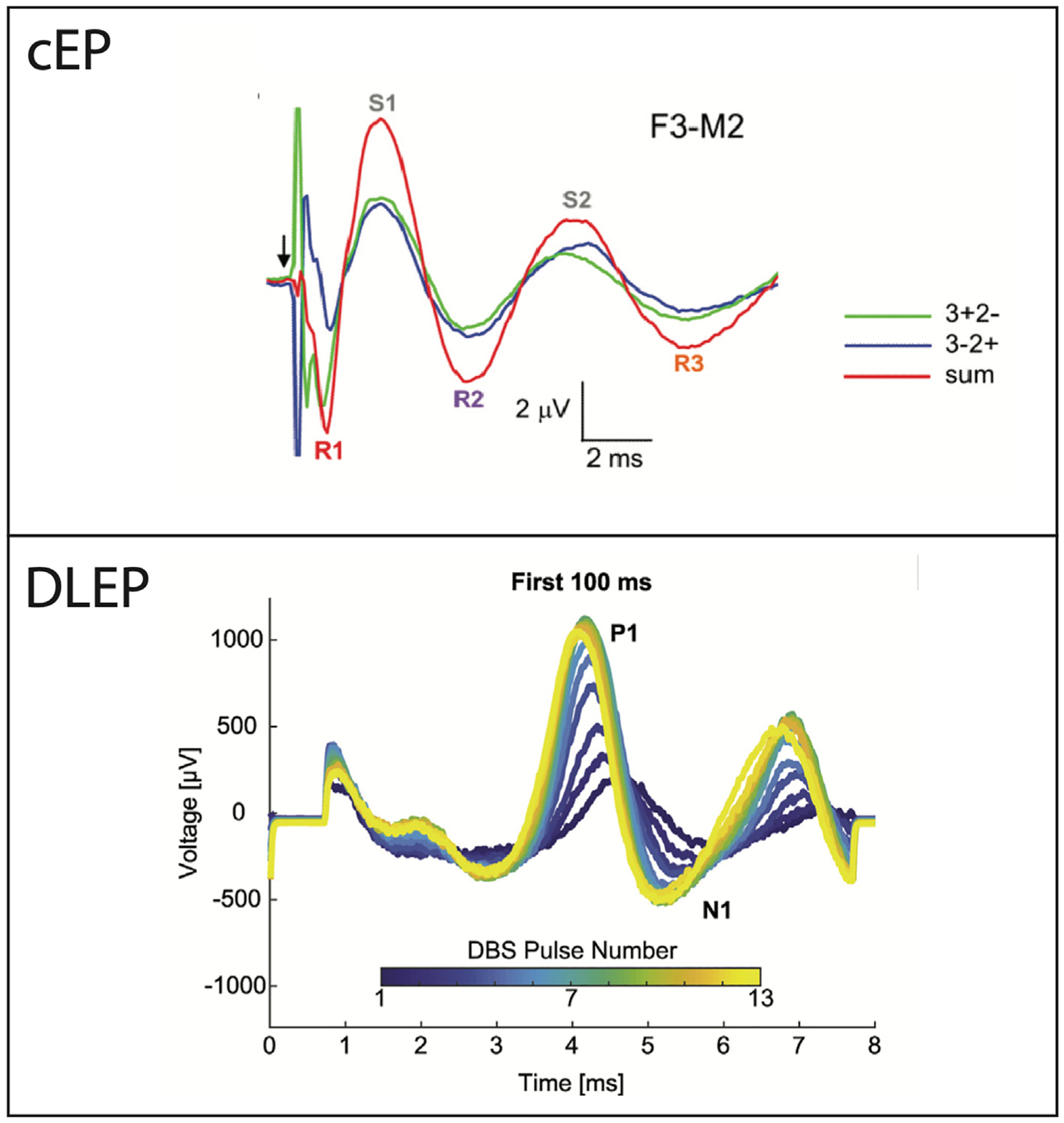
Example of evoked potential waveforms. The cEP shows that the reversal of anode and cathode contacts (i.e., blue, and green traces) reverses the stimulus artifact, but the brain response remains the same polarity. The DLEP waveform shows that the amplitude and latency evolves over the course of stimulation. Note the amplitude and timescale differences among the cEP and DLEP. This cEP waveform was adapted from Walker et al. Movement disorders: official journal of the Movement Disorder Society (2012b) 27(11), 1404–1412. The DLEP waveform was adapted from Schmidt et al. Brain stimulation (2020) 13(6), 1706–1718.
Initial cEP studies distinguished between short and long-latency components but disagreed on component properties. Ashby et al. [24] reported short-latency cEPs in nearly all subjects, while Mackinnon et al. [30] found short-latency cEPs in <50%, but that long-latency responses were more consistent. However, later studies using appropriate equipment and techniques to reduce stimulation artifact found that short latency cEPs were consistently present and less variable in latency than later peaks [32,34]. Earlier studies showed stimulus artifact confounding cEP traces up to ~5 ms [30,33].
The amplitude of cEPs is strongly dependent on the specific location of DBS electrode contacts in the brain, with maximal amplitude recorded ipsilateral to stimulation [32–34]. With STN DBS, cEP amplitude is ~ 1–5 mV and is largest when stimulating dorsal portions of the STN [30]. With thalamic ventral intermedius (VIM) DBS it is not clear how electrode location impacts cEP amplitude or latency [28,34]. The cEP is largest in M1 and premotor areas, but also present in S1 and superior parietal lobule [20] whereas latency varies based on recording contact location.
2.2. Technical aspects of recording cEPs
Cortical evoked potentials can be recorded using scalp electroencephalogram (EEG) [25,30,32,35] or directly on the brain surface (electrocorticogram, ECoG) with ipsilateral subdural strips [20,26]. ECoG strip placement is guided using intraoperative CT co-registered to the preoperative MRI with contacts typically placed over the premotor cortex, precentral gyrus (M1), postcentral gyrus (S1), and superior parietal lobe. Physiological confirmation of ECoG electrode location is confirmed using reversal of somatosensory-evoked potentials (SSEPs) generated by median nerve stimulation [36]. Animal studies often record cEPs using stainless steel screws over the region of interest [19,37].
Early studies on cEPs were limited by electrical stimulation artifacts that obscure short-latency responses [24,25,29,30]. DBS can be delivered through either monopolar stimulation (i.e., electrode contact(s) are the cathode while the implanted pulse generator (IPG) case is the anode) or bipolar configuration (i.e., separate electrode contacts are the anode and cathode). Initial studies suggested that monopolar stimulation produced larger electrical artifacts than bipolar stimulation [32,34]. To reduce artifacts, monopolar stimulation can be delivered with anode/cathode pair reversal (i.e., ± to −/+) across stimulation epochs, then averaging epochs through summing (Fig. 2). This process assumes that underlying brain responses do not reverse in polarity whereas the stimulus artifact cancels out with reversed polarities. But the brain response may also be affected by the polarity of stimulation, as suggested by modeling [38] and corroborated experimentally [39].
2.3. Mechanisms of cEP generation
The initial R1 component of STN DBS-induced cEP is thought to arise from antidromic activation of the hyperdirect pathway [19,31,40–42] (Fig. 3). The short-latency R1 component shows short chronaxies, short refractory periods, and is able to follow trains of stimuli at > 100 Hz without blocking, suggesting that this component derives from antidromic activation [24,40]. The R1 component’s ability to follow high-frequency stimulation [43] supports the hypothesis that the R1 component of cEPs is mediated by antidromic activation of the hyperdirect pathway. Further, studies in anesthetized rats report the presence of only short-latency STN-induced cEPs whereas conscious rats also exhibit intermediate- and long-latency cEPs [19,31,44]. Irwin et al. [35] similarly found R1 components preserved and R2/R3 components suppressed under anesthesia in humans. Activity that arises from polysynaptic activation is strongly suppressed by anesthesia [45] supporting that R1 is due to direct activation of fibers and antidromic propagation to cortex.
Fig. 3.
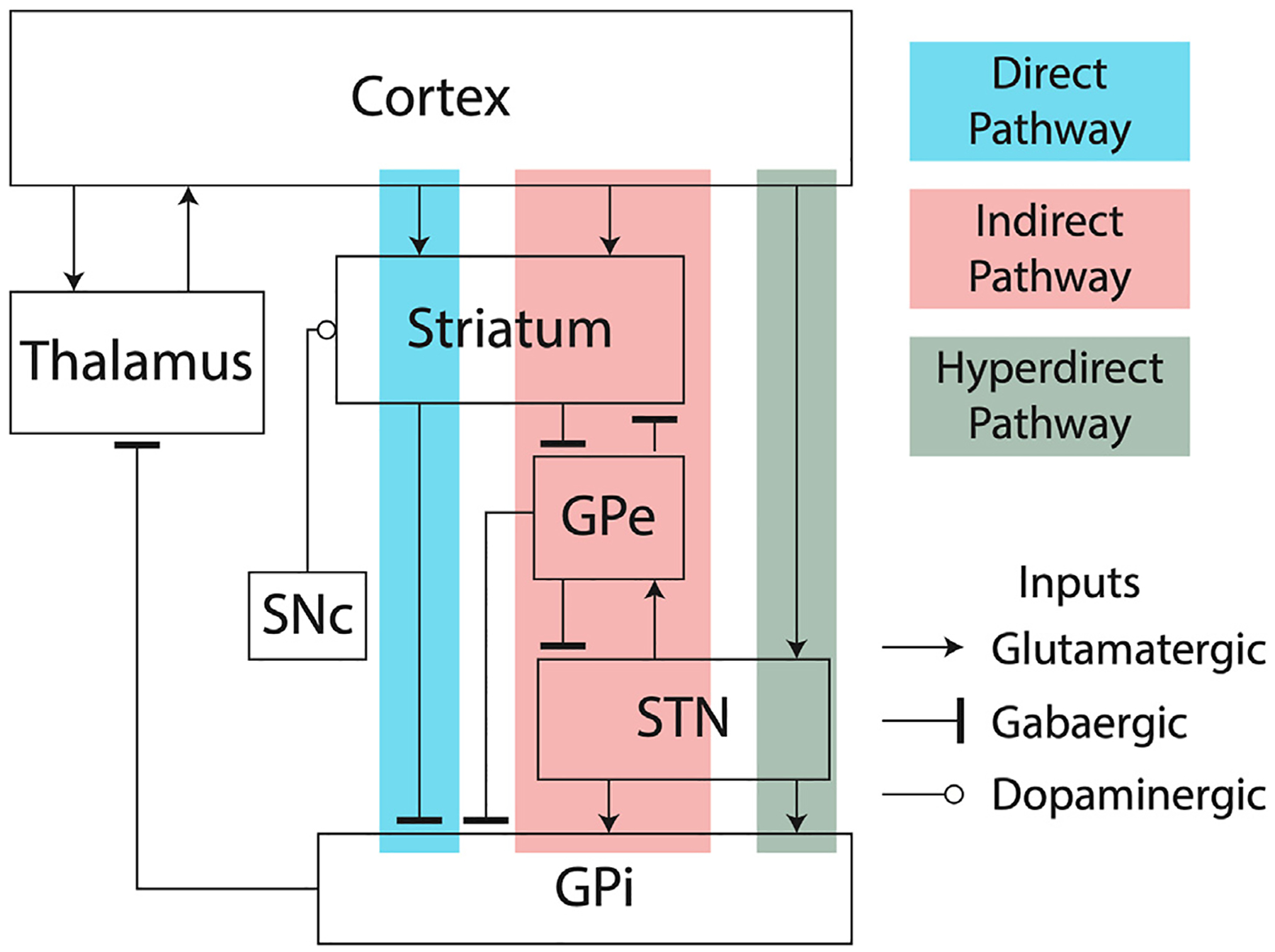
Block diagram showing the direct, indirect and hyperdirect pathways of the basal ganglia.
Li et al. [31], Gradinaru et al. [41], and Kumaravelu et al. [19] all demonstrated antidromic activation of hyperdirect pathway through electrical or optogenetic stimulation of STN. Recordings in monkeys found STN stimulation produced short-latency cEPs, but GPi stimulation, while it produced long-latency cEPs, did not produce the short latency cEPs [40]. This implies GPi stimulation does not antidromically activate cortical fibers, consistent with the anatomical knowledge that GPi is not known to have projections to or from the motor cortex [40,46].
Directional DBS leads allow greater specificity in stimulating neural circuits and improve clinical outcomes [47,48]. These leads also provide a more precise means of probing neural circuits and understanding the mechanisms of cEP generation. For example, Peeters et al. [49] measured cEP during 10 Hz DBS on directional lead contacts in patients with PD. Similar to previous works, they observed a 3 ms short-latency EP over the motor cortex during dorsolateral STN DBS, providing further evidence of engagement of the hyperdirect pathway.
Moreover, diffusion tensor imaging (DTI), a minimally invasive technique that allows visualization of fiber tracts in vivo in humans, provides further supporting evidence of the hyperdirect pathway as the mechanism of short-latency cEP generation. Brunenburg et al. [50] used DTI tractography to identify connectivity between STN and motor cortex in support of the hyperdirect pathway. Lambert et al. [51] expanded upon this neuroanatomical characterization to show three distinct clusters within human STN based on connectivity, including the hyperdirect pathway fibers to the motor cortex. Based on the length of the projections identified in tractography and the conduction velocity of cortical white matter tracts, the latencies of cEPs are consistent with direct projections from motor cortex to the STN in humans [52].
The R2 and R3 components of the cEP are hypothesized to result from orthodromic propagation through the STN-GPi-thalamic pathway [40,53,54] (Figs. 3 and 4). Supporting this theory, studies in patients undergoing STN DBS with prior GPi pallidotomies found unexpectedly small cEPs [25]. Additionally, Devergnas and Wichmann [40] described that the long-latency component of cEPs evoked by GPi stimulation occurred faster than those of STN stimulation, indicating STN stimulation potentially included orthodromic propagation through the pallidum and thalamus.
Fig. 4.
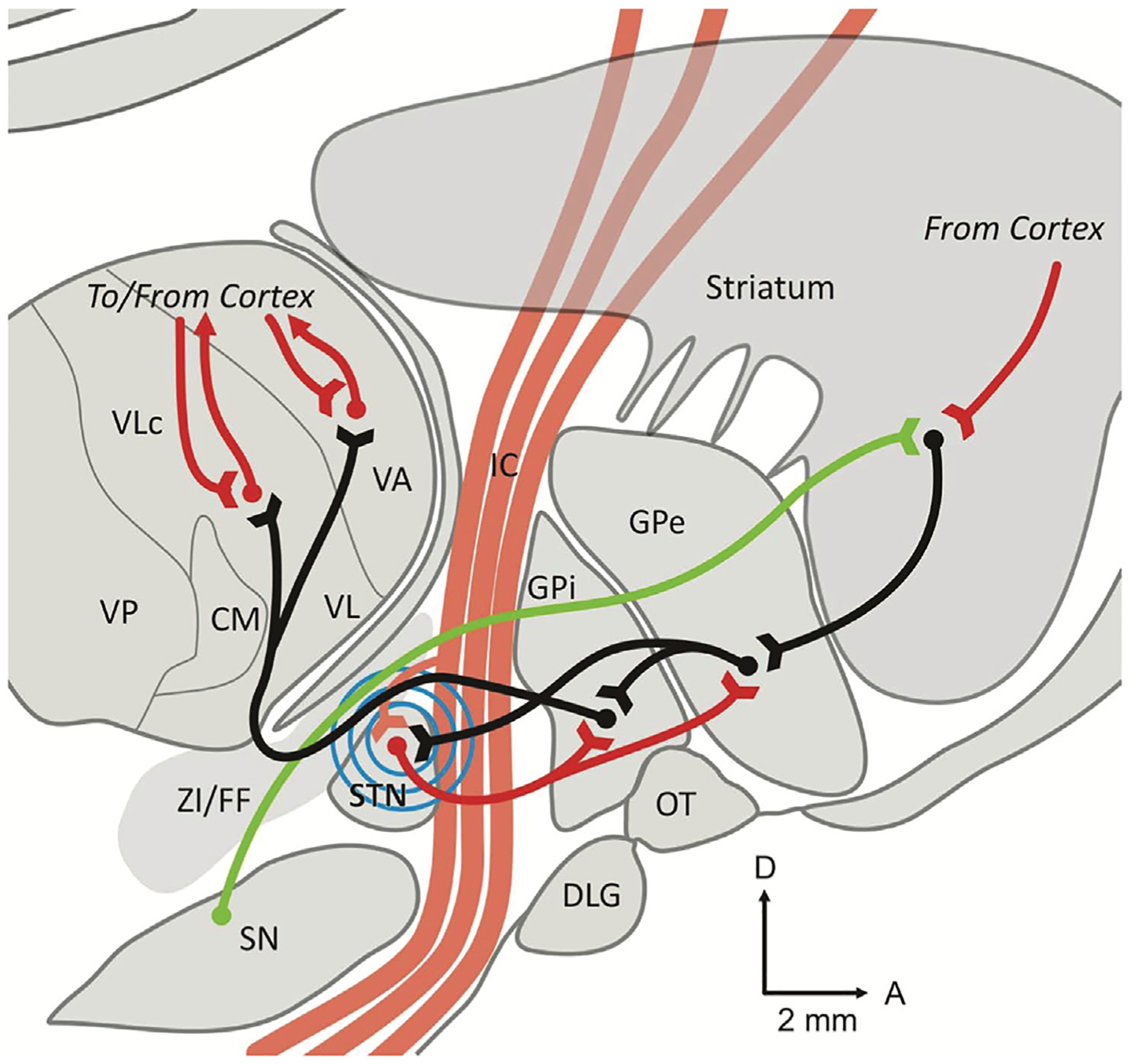
Parasagittal slice through the nonhuman primate brain. This figure shows the major anatomical pathways involved in subthalamic nucleus (STN) DBS, including the internal capsule (IC), globus pallidus internus (GPI), and globus pallidus externus (GPE). Excitatory glutamatergic connections are shown as red lines, inhibitory GABAergic connections are shown as black lines, and dopaminergic connections are shown as green lines. The blue concentric lines represent STN DBS. Abbreviations: CM centromedian nucleus of the thalamus; DLG, lateral geniculate body; FF, Fields of Forel; IC, Internal capsule; GPE, globus pallidus externus; GPI, globus pallidus internus; OT, optic tract; SN, substantia nigra; STN, subthalamic nucleus; VA, ventral anterior nucleus of the thalamus; VL, ventrolateral nucleus of the thalamus; VP, ventral posterior nucleus of the thalamus; ZI, zona incerta. This figure was reproduced from Devergnas and Wichmann Frontiers in systems neuroscience (2011) 5, 30.
Other studies refute the STN-GPi-thalamic pathway theory. Firstly, a study by Limousin et al. [55] found long-latency cEPs evoked by STN DBS occurred sooner (18–20 ms) than those evoked by GPi stimulation (25.0–25.8 ms) with other studies in agreement [56–58]. Secondly, Baker et al. [25] concluded there was no apparent difference in the morphology or topography of the cEPs from the two patients that had undergone prior GPi pallidotomies. Therefore, long-latency cEPs may be independent of basal ganglia, and a result of either cortico-cortical interactions following antidromically activated pyramidal neurons or reciprocal connections between the cortex and the thalamus [59]. In support of this, Kumaravelu’s study using a detailed model of the cortical column interconnected to the thalamus suggested the intermediate- and long-latency components of the cEP are due to activation of L5 pyramidal neurons and subsequent polysynaptic activation of L2/3 pyramidal neurons, respectively [19]. Orthodromic activation through basal ganglia-thalamus-cortex pathways was not necessary to generate the cEP components in the model.
In summary, the R1 component of the cEP arises from antidromic activation of the hyperdirect pathway and is present with stimulation of STN but not GPi. As anesthesia suppresses polysynaptic activation, R1 is present under anesthesia whereas the intermediate- and long latency components of the cEP are suppressed. The neural origins of the intermediate- and long-latency cEP components are currently an open question. There is experimental evidence for both orthodromic propagation and experimental and computational results showing these components arise through recurrent cortical connections.
2.4. Clinical applications
There are several potential clinical applications of DBS-induced cEPs. First, cEPs may guide electrode placement in awake patients since clinically effective contacts can evoke long-latency cEP components [29] which are suppressed with anesthesia [19,31,35,44]. Second, cEPs may show utility as biomarkers to quantify clinical effect and the extent of neural activation and thus could be useful in electrode selection during later programing sessions. Modeling from Kumaravelu et al. [19] showed lower intensity STN DBS evoked short-latency cEPs while higher intensities also recruited intermediate- and long-latency components. In agreement with this prediction, Kelley et al. [52] and Irwin et al. [35] observed later cEP components also increased in response to higher STN DBS voltage. Monitoring cEPs may help predict and avoid unwanted side effects. Romeo et al. [60] reported DBS at 20 Hz evoked EEG activity that predicted DBS amplitude threshold for motor side effects during postoperative stimulation at 130 Hz with positive and negative predictive values of 100% and 87%, respectively. Also of note, the pattern of cEPs seems to be largely unaffected by dopaminergic medications, but the amplitude of later components of the cEP decreased more rapidly with medication [33]. Furthermore, the barrier to using cEPs in the clinical setting is relatively low as cEPs do not require adjustments to DBS leads/IPGs currently used and can be measured non-invasively using readily available EEG technology. Together, these attributes point to cEPs as a promising biomarker to quantify the response to various STN DBS parameters including amplitude and frequency that could be advantageous for both postoperative DBS programming and as a feedback signal for adaptive DBS.
3. DBS local evoked potentials
3.1. General features of DBS local evoked potentials
Unlike remote cEPs, DBS local evoked potentials (DLEPs) are local responses recorded from the stimulating electrode with latencies as short as 0.2 ms in locations including STN, GPi, and VIM [16,17,61–63]. DBS in STN, GPi, and Vim all elicit short latency, large amplitude DLEPs that are readily detected locally by either circular or directional leads and do not require an additional sensing lead (e.g., an ECoG array) [61]. DLEPs are generally composed of two evoked responses: a short-latency evoked component (R1) and a longer latency component exhibiting oscillatory activity, primarily in STN (Fig. 2). The short-latency DLEP has a peak latency of about 0.31 ms, and an absolute and relative refractory period of 0.56 ms and 2.94 ms, respectively [61]. Additionally, R1 has a relatively large peak amplitude of ~0.54 mV, in contrast to the much smaller ~1–5 μV amplitudes of cEPs [20,35,64] and ~1–4 μV amplitude beta band activity [15,16,65] so DLEPs provide a higher signal to noise ratio (Fig. 2). Peak DLEP R1 amplitude does not differ across stimulation targets among STN, GPi, and VIM. However, peak R1 amplitude, area, and latency are altered by interstimulus interval [61].
The long-latency DLEP in STN and GPi begins ~ 4.5 ms after stimulation, with amplitudes ~ 0.24 mV [16–18,27,61–63]. Long-latency DLEPs exhibit a characteristic decaying oscillation, with higher frequency DBS at 130 Hz reducing the oscillation frequency compared to 20 Hz DBS [18]. The frequency of this oscillation also decreases with increasing DBS amplitude [18,62]. Coincidentally, lower DLEP oscillation frequencies correlate with therapeutic stimulation amplitudes [62] and reduced STN beta band power [18].
STN and GPi DBS both elicit long-latency DLEPs, but VIM DBS does not [61,63]. Paired pulse stimulation revealed short-term facilitation of long-latency DLEPs at interpulse intervals (IPIs) of 1–4 ms and 5–10 ms, coinciding with therapeutically relevant frequencies for DBS [61]. Additionally, the long-latency DLEP differed in amplitude across contacts, with the highest amplitude DLEPs recorded in dorsal STN, and higher amplitudes correlating with therapeutic efficacy [17,61].
3.2. DLEP vs ERNA
Multiple groups have independently confirmed the presence of DLEPs but with varying terminology [16–18,27,61–63]. The short-latency signal (at ~0.3 ms) was named STN evoked potentials (sEPs) and the later portion as evoked resonant neural activity (ERNA) [17,18,27,66]. A recent report has shown that the frequency of the oscillation changes between periods and the signal lacks the attractor dynamics at DBS frequency harmonics [16]. However, Awad et al. [61] found that paired pulse stimulation induced short-term facilitation suggesting resonant activity, and Ozturk et al. [67] observed evoked activity was greater at 160 Hz STN stimulation compared to 180 Hz stimulation. Only STN DBS demonstrates the later, oscillatory signal suggesting specific circuitry components are likely responsible [17,18,63,68]. Since the presence of resonance is an open question and at best is present in select target nuclei for PD, the authors have chosen to refer to signals recorded at the site of DBS stimulation as DBS local evoked potentials (DLEPs).
3.3. Recording DLEPs
Like cEPs, DLEPs may be obscured by stimulation artifacts. By employing the same stimulus inversion technique used when recording cEPs, DLEPs can be uncovered at latencies <1 ms [16,23]. However, there are larger stimulation artifacts when stimulating and recording in the same nucleus, which can saturate the recording amplifiers so that the stimulus inversion technique is not sufficient [17,63]. Similarly, other techniques used to suppress stimulus artifacts, such as curve fitting, template subtraction, and masker-probe paradigms, are inappropriate because of amplifier saturation. Template subtraction and polarity averaging additionally rely on the assumptions that the artifact shape is constant between pulses [69] and that the artifact follows the stimulus pulse polarity [70]. Further, DLEP recordings are complicated by overlapping frequency spectra between the DLEP and stimulus artifact, preventing frequency filtering. Sample-and-hold amplifiers have not shown the ability to remove artifact at the sub-millisecond level required to capture short latency DLEPs [23].
Kent and Grill [23] developed novel recording instrumentation that reduced the magnitude and duration of the artifact, increasing signal gain while preventing amplifier saturation. They employed three amplifier stages with anti-parallel input diode clamps to reduce selectively the stimulus artifact and prevent saturation. The second and third amplifier stages are bandpass filtered between 0.1 Hz and 10 kHz [23] and blanked from 20 us before the stimulus pulse to 20–500 us after the pulse [16]. A monopolar stimulation configuration with adjacent, symmetrical recording contacts (so called “sandwich sensing”) was found to minimize the size of the artifact [23,63].
As the duration of DLEPs extends beyond the interpulse interval of clinically therapeutic DBS (i.e., 7.7 ms for 130 Hz), novel stimulation paradigms are needed to investigate the multiple peaks of the damped oscillation. For example, a pause in DBS can provide a longer recording gap [71] if the omission of occasional pulses does not reduce therapeutic effect or influence DLEP characteristics. Alternatively, stimulation may be performed as short bursts (e.g., 10 pulses at 130 Hz) to probe the DLEP after the stimulation [17,71]. These paradigms may be especially useful in studying the evolution of DLEP properties over time.
3.4. Mechanism of DLEP generation
The neural elements and circuit mechanisms generating the DLEP are yet to be completely established. The STN connects to a cortico-basal ganglia-thalamo-cortical network with myriad feedback loops that may contribute to oscillatory responses [18,33,72–74]. Thus, it may be caused by network loops between STN and cortex, GPe, or even with the STN itself, although DLEPs and high frequency oscillations (HFOs) occupy similar frequency bands at about twice the stimulation rate of 130 Hz [18]. Any correlation between DLEPs and HFO could also be explained as one being the epiphenomenon of the other. However, Ozturk et al. [67] reports that the HFO persists after DBS pulse artifact and evoked waveforms were removed using a template extraction filter, although HFO did not occur when stimulating the STN at 20 Hz. These results suggest HFOs and evoked potentials may be independent of each other.
Schmidt et al. [16] provide both computer simulations and in vivo measurements to support that DLEPs arise from interactions between STN and pallidum due to quasi-periodic pallidal inhibition. The biophysical model, which included the STN, GPe, their synaptic interconnectivity, and afferent cortical-STN axons, replicated all phases of the human DLEP. Using this model, Schmidt et al. determined that the hyperdirect pathway was required to initiate DLEPS in STN DBS (Fig. 3), through excitation of cortical axon terminals and separate activation of reciprocal STN/GPe connections. The model results and simultaneous recordings in the STN and GP during STN DBS in humans both yielded the distinctive quasi-periodic inhibition of the STN every 3–4 ms characteristic of DLEPs. Showing the model-based responses are predictive of simultaneous STN and GP recordings suggests that STN and GPe interactions are sufficient to generate DLEPs (Figs. 3 and 4). Further, these oscillations are consistent with single unit recordings of pallidal units firing periodically in 3–4 ms intervals during STN DBS [75], supporting the idea that the oscillations are due to reciprocal STN/GPe connections.
3.5. Clinical applications
DLEPs have several desirable properties for clinical application. First, DLEPs have a large amplitude signal that can be reliably recorded during STN DBS, and there is potential for DLEPs to be used to guide electrode implantation within subregions of the STN [76]. DLEPs localize to the STN, are not present in white matter tracts adjacent to the STN, are greatest in the dorsal STN, where DBS is reported to be most effective [17,77], and even persist under anesthesia as they appear to likely result from monosynaptic connections [17,18].
Second, DLEPs may serve as a biomarker for DBS parameter selection. After electrode implantation, clinicians select customized, optimal parameters through trial and error, and ineffective programming can result in reduced efficacy and contribute to side effects, such as disturbances in speech, postural stability, and gait [9,78,79]. Current IPGs and directional electrodes allow for focused, selective stimulation, but also present a programming challenge. DLEP amplitude was correlated with greater therapeutic effect from DBS [17], and the DLEP oscillation frequency during DBS is modulated from ~310 Hz to ~260 Hz when stimulation reaches therapeutic levels [18] but the plateau frequency of the DLEP oscillations varies between patients. Further research is needed to investigate the correlation between DBS efficacy and the properties of DLEPS, such as the amplitude, decay rate, and oscillation frequency.
Third, since DLEPs are modulated by DBS they could be used as a feedback signal for adaptive DBS, since changes in DLEP amplitude and frequency correlate with therapeutic effect of STN DBS in Parkinson’s disease [17,18]. Additionally, DLEP frequency correlates with beta band activity [18], which is considered a biomarker for akinesia and rigidity [80,81]. Moreover, Wiest et al. [66] found the time to steady state amplitude of DLEPs significantly correlated with UPDRS score off-medication and with the difference in UPDRS score between off- and on-medication. However, DLEPs likely require a minimum sampling frequency of ~10 kHz, which is not achievable yet with any implanted DBS IPG, such as Medtronic Percept, Summit RC+S, or Activa PC+S, thus limiting immediate clinical usefulness.
4. Conclusion
Research into DBS EPs has provided important new knowledge on human central circuits involved in DBS and suggests EPs may have multiple applications in the clinical deployment of DBS. DBS has evolved greatly over the past two decades, and the next frontier in DBS technology will likely be a move toward adaptive DBS for automated, efficient, and effective treatment. Contributing to this effort, DBS EPs are promising candidates for a feedback signal in adaptive DBS. Similarly, EPs may find utility as a biomarker for DBS parameter selection, especially as the introduction of directional leads further expands the large stimulation parameter space. Furthermore, EPs may have utility in proper positioning of DBS electrodes in awake or asleep surgery.
While the current understanding of EPs in DBS provides a promising new avenue for studying DBS mechanisms, further research is needed to characterize fully and quantitatively the effects of DBS on EPs and their relation to motor symptoms. Additionally, further research is needed to understand the relationship between DLEPs and cEPs. For example, it is not clear whether DLEPs and cEPs provide redundant or complementary insight into DBS efficacy.
In addition to further research on EP mechanisms and characterization, further technical development is required to make use of EPs to optimize clinical outcomes. The challenges stem from significant limitations of current implantable recording systems. EPs have the technical challenges of large stimulation artifacts, need for high sampling frequencies (i.e., at least 10 kHz) and short latencies as barriers to clinical implementation. Additional circuitry to process and interpret these signals, especially in the case of adaptive DBS, is necessary to capitalize on the promise of EPs. As the body of EP research expands and the utility of these signals becomes clear, product development to miniaturize and internalize the equipment needed to employ these signals in the clinical setting will follow.
Acknowledgments
Preparation of this review was supported in part by NIH grants R37 NS040894, UH3 NS103468, T32 GM 007171, and by a research grant from Boston Scientific Corp.
Footnotes
Declarations of interest
Dr. Kyle Mitchell will be serving as a site principal investigator for a Medtronic sponsored clinical trial and a trial with Deep Brain Innovations. He has done consulting for Rune Labs and Boston Scientific.
References
- [1].Dorsey ER, Sherer T, Okun MS, Bloem BR. The emerging evidence of the Parkinson pandemic. J Parkinsons Dis 2018;8(s1):S3–8. 10.3233/JPD-181474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Kalia LV, Lang AE. Parkinson’s disease. Lancet Aug 29 2015;386(9996): 896–912. 10.1016/S0140-6736(14)61393-3. [DOI] [PubMed] [Google Scholar]
- [3].Armstrong MJ, Okun MS. Diagnosis and treatment of Parkinson disease: a review. JAMA Feb 11 2020;323(6):548–60. 10.1001/jama.2019.22360. [DOI] [PubMed] [Google Scholar]
- [4].Schuepbach WM, Rau J, Knudsen K, et al. Neurostimulation for Parkinson’s disease with early motor complications. Feb 14 N Engl J Med 2013;368(7): 610–22. 10.1056/NEJMoa1205158. [DOI] [PubMed] [Google Scholar]
- [5].Jakobs M, Lee DJ, Lozano AM. Modifying the progression of Alzheimer’s and Parkinson’s disease with deep brain stimulation. Neuropharmacology Jul 2020;171:107860. 10.1016/j.neuropharm.2019.107860. [DOI] [PubMed] [Google Scholar]
- [6].Baizabal-Carvallo JF, Jankovic J. Movement disorders induced by deep brain stimulation. Parkinsonism Relat. Disord Apr 2016;25:1–9. 10.1016/j.parkreldis.2016.01.014. [DOI] [PubMed] [Google Scholar]
- [7].Mahlknecht P, Akram H, Georgiev D, et al. Pyramidal tract activation due to subthalamic deep brain stimulation in Parkinson’s disease. Mov Disord Aug 2017;32(8):1174–82. 10.1002/mds.27042. [DOI] [PubMed] [Google Scholar]
- [8].Sauleau P, Raoul S, Lallement F, et al. Motor and non motor effects during intraoperative subthalamic stimulation for Parkinson’s disease. J Neurol. Apr 2005;252(4):457–64. 10.1007/s00415-005-0675-5. [DOI] [PubMed] [Google Scholar]
- [9].Hartmann CJ, Fliegen S, Groiss SJ, Wojtecki L, Schnitzler A. An update on best practice of deep brain stimulation in Parkinson’s disease. Ther Adv Neurol Disord 2019;12:1756286419838096. 10.1177/1756286419838096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Holewijn RA, Verbaan D, van den Munckhof PM, et al. General anesthesia vs local anesthesia in microelectrode recording-guided deep-brain stimulation for Parkinson disease: the GALAXY randomized clinical trial. JAMA Neurol Oct 1 2021;78(10):1212–9. 10.1001/jamaneurol.2021.2979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Hoang KB, Cassar IR, Grill WM, Turner DA. Biomarkers and stimulation algorithms for adaptive brain stimulation. Front Neurosci 2017;11:564. 10.3389/fnins.2017.00564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Lozano AM, Lipsman N, Bergman H, et al. Deep brain stimulation: current challenges and future directions. Nat Rev Neurol Mar 2019;15(3):148–60. 10.1038/s41582-018-0128-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Kuncel AM, Grill WM. Selection of stimulus parameters for deep brain stimulation. Clin Neurophysiol Nov 2004;115(11):2431–41. 10.1016/j.clinph.2004.05.031. [DOI] [PubMed] [Google Scholar]
- [14].Arlotti M, Marceglia S, Foffani G, et al. Eight-hours adaptive deep brain stimulation in patients with Parkinson disease. Neurology Mar 13 2018;90(11):e971–6. 10.1212/WNL.0000000000005121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Little S, Pogosyan A, Neal S, et al. Adaptive deep brain stimulation in advanced Parkinson disease. Ann Neurol Sep 2013;74(3):449–57. 10.1002/ana.23951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Schmidt SL, Brocker DT, Swan BD, Turner DA, Grill WM. Evoked potentials reveal neural circuits engaged by human deep brain stimulation. Brain Stimul Nov - Dec 2020;13(6):1706–18. 10.1016/j.brs.2020.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Sinclair NC, McDermott HJ, Bulluss KJ, et al. Subthalamic nucleus deep brain stimulation evokes resonant neural activity. Ann Neurol May 2018;83(5): 1027–31. 10.1002/ana.25234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Sinclair NC, McDermott HJ, Fallon JB, et al. Deep brain stimulation for Parkinson’s disease modulates high-frequency evoked and spontaneous neural activity. Neurobiol Dis Oct 2019;130:104522. 10.1016/j.nbd.2019.104522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Kumaravelu K, Oza CS, Behrend CE, Grill WM. Model-based deconstruction of cortical evoked potentials generated by subthalamic nucleus deep brain stimulation. J Neurophysiol Aug 1 2018;120(2):662–80. 10.1152/jn.00862.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Miocinovic S, de Hemptinne C, Chen W, et al. Cortical potentials evoked by subthalamic stimulation demonstrate a short latency hyperdirect pathway in humans. J Neurosci Oct 24 2018;38(43):9129–41. 10.1523/JNEUROSCI.1327-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Hoth S, Dziemba OC. The role of auditory evoked potentials in the context of cochlear implant provision. Otol Neurotol Dec 2017;38(10):e522–30. 10.1097/MAO.0000000000001480. [DOI] [PubMed] [Google Scholar]
- [22].Nuwer MR. Fundamentals of evoked potentials and common clinical applications today. Electroencephalogr Clin Neurophysiol Feb 1998;106(2):142–8. 10.1016/s0013-4694(97)00117-x. [DOI] [PubMed] [Google Scholar]
- [23].Kent AR, Grill WM. Recording evoked potentials during deep brain stimulation: development and validation of instrumentation to suppress the stimulus artefact. J Neural Eng Jun 2012;9(3):036004. 10.1088/1741-2560/9/3/036004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Ashby P, Paradiso G, Saint-Cyr JA, Chen R, Lang AE, Lozano AM. Potentials recorded at the scalp by stimulation near the human subthalamic nucleus. Clin Neurophysiol Mar 2001;112(3):431–7. 10.1016/s1388-2457(00)00532-0. [DOI] [PubMed] [Google Scholar]
- [25].Baker KB, Montgomery EB Jr, Rezai AR, Burgess R, Luders HO. Subthalamic nucleus deep brain stimulus evoked potentials: physiological and therapeutic implications. Mov Disord Sep 2002;17(5):969–83. 10.1002/mds.10206. [DOI] [PubMed] [Google Scholar]
- [26].Levinson LH, Caldwell DJ, Cronin JA, et al. Intraoperative characterization of subthalamic nucleus-to-cortex evoked potentials in Parkinson’s disease deep brain stimulation. Front Hum Neurosci 2021;15:590251. 10.3389/fnhum.2021.590251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Sinclair NC, Fallon JB, Bulluss KJ, Thevathasan W, McDermott HJ. On the neural basis of deep brain stimulation evoked resonant activity. Biomed Phys Eng Exp 2019;55. 10.1088/2057-1976/ab366e. [DOI] [Google Scholar]
- [28].Hartmann CJ, Hirschmann J, Vesper J, Wojtecki L, Butz M, Schnitzler A. Distinct cortical responses evoked by electrical stimulation of the thalamic ventral intermediate nucleus and of the subthalamic nucleus. Neuroimage Clin 2018;20:1246–54. 10.1016/j.nicl.2018.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Kuriakose R, Saha U, Castillo G, et al. The nature and time course of cortical activation following subthalamic stimulation in Parkinson’s disease. Cerebr Cortex Aug 2010;20(8):1926–36. 10.1093/cercor/bhp269. [DOI] [PubMed] [Google Scholar]
- [30].MacKinnon CD, Webb RM, Silberstein P, et al. Stimulation through electrodes implanted near the subthalamic nucleus activates projections to motor areas of cerebral cortex in patients with Parkinson’s disease. Eur J Neurosci Mar 2005;21(5):1394–402. 10.1111/j.1460-9568.2005.03952.x. [DOI] [PubMed] [Google Scholar]
- [31].Li S, Arbuthnott GW, Jutras MJ, Goldberg JA, Jaeger D. Resonant antidromic cortical circuit activation as a consequence of high-frequency subthalamic deep-brain stimulation. J Neurophysiol Dec 2007;98(6):3525–37. 10.1152/jn.00808.2007. [DOI] [PubMed] [Google Scholar]
- [32].Walker HC, Huang H, Gonzalez CL, et al. Short latency activation of cortex during clinically effective subthalamic deep brain stimulation for Parkinson’s disease. Mov Disord Jun 2012;27(7):864–73. 10.1002/mds.25025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Eusebio A, Pogosyan A, Wang S, et al. Resonance in subthalamo-cortical circuits in Parkinson’s disease. Brain Aug 2009;132(Pt 8):2139–50. 10.1093/brain/awp079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Walker HC, Huang H, Gonzalez CL, et al. Short latency activation of cortex by clinically effective thalamic brain stimulation for tremor. Mov Disord Sep 15 2012;27(11):1404–12. 10.1002/mds.25137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Irwin ZT, Awad MZ, Gonzalez CL, et al. Latency of subthalamic nucleus deep brain stimulation-evoked cortical activity as a potential biomarker for postoperative motor side effects. Clin Neurophysiol Jun 2020;131(6):1221–9. 10.1016/j.clinph.2020.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Crowell AL, Ryapolova-Webb ES, Ostrem JL, et al. Oscillations in sensorimotor cortex in movement disorders: an electrocorticography study. Brain Feb 2012;135(Pt 2):615–30. 10.1093/brain/awr332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].McConnell GC, So RQ, Hilliard JD, Lopomo P, Grill WM. Effective deep brain stimulation suppresses low-frequency network oscillations in the basal ganglia by regularizing neural firing patterns. J Neurosci Nov 7 2012;32(45): 15657–68. 10.1523/JNEUROSCI.2824-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].McIntyre CC, Grill WM. Selective microstimulation of central nervous system neurons. Ann Biomed Eng Mar 2000;28(3):219–33. 10.1114/1.262. [DOI] [PubMed] [Google Scholar]
- [39].Wang Q, Millard DC, Zheng HJ, Stanley GB. Voltage-sensitive dye imaging reveals improved topographic activation of cortex in response to manipulation of thalamic microstimulation parameters. J Neural Eng Apr 2012;9(2): 026008. 10.1088/1741-2560/9/2/026008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Devergnas A, Wichmann T. Cortical potentials evoked by deep brain stimulation in the subthalamic area. Front Syst Neurosci 2011;5:30. 10.3389/fnsys.2011.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Gradinaru V, Mogri M, Thompson KR, Henderson JM, Deisseroth K. Optical deconstruction of parkinsonian neural circuitry. Science Apr 17 2009;324(5925):354–9. 10.1126/science.1167093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Gunalan K, McIntyre CC. Biophysical reconstruction of the signal conduction underlying short-latency cortical evoked potentials generated by subthalamic deep brain stimulation. Clin Neurophysiol Feb 2020;131(2):542–7. 10.1016/j.clinph.2019.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Degos B, Deniau JM, Le Cam J, Mailly P, Maurice N. Evidence for a direct subthalamo-cortical loop circuit in the rat. Eur J Neurosci May 2008;27(10): 2599–610. 10.1111/j.1460-9568.2008.06229.x. [DOI] [PubMed] [Google Scholar]
- [44].Dejean C, Hyland B, Arbuthnott G. Cortical effects of subthalamic stimulation correlate with behavioral recovery from dopamine antagonist induced akinesia. Cerebr Cortex May 2009;19(5):1055–63. 10.1093/cercor/bhn149. [DOI] [PubMed] [Google Scholar]
- [45].Berg-Johnsen J, Langmoen IA. The effect of isoflurane on excitatory synaptic transmission in the rat hippocampus. Acta Anaesthesiol Scand May 1992;36(4):350–5. 10.1111/j.1399-6576.1992.tb03480.x. [DOI] [PubMed] [Google Scholar]
- [46].Lanciego JL, Luquin N, Obeso JA. Functional neuroanatomy of the basal ganglia. Cold Spring Harb Perspect Med Dec 1 2012;2(12):a009621. 10.1101/cshperspect.a009621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Steigerwald F, Muller L, Johannes S, Matthies C, Volkmann J. Directional deep brain stimulation of the subthalamic nucleus: a pilot study using a novel neurostimulation device. Mov Disord Aug 2016;31(8):1240–3. 10.1002/mds.26669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Dembek TA, Reker P, Visser-Vandewalle V, et al. Directional DBS increases side-effect thresholds-A prospective, double-blind trial. Mov Disord Oct 2017;32(10):1380–8. 10.1002/mds.27093. [DOI] [PubMed] [Google Scholar]
- [49].Peeters J, Boogers A, Van Bogaert T, et al. Electrophysiologic evidence that directional deep brain stimulation activates distinct neural circuits in patients with Parkinson disease. Neuromodulation Dec 18 2021. 10.1016/j.neurom.2021.11.002. [DOI] [PubMed] [Google Scholar]
- [50].Brunenberg EJ, Moeskops P, Backes WH, et al. Structural and resting state functional connectivity of the subthalamic nucleus: identification of motor STN parts and the hyperdirect pathway. PLoS One 2012;7(6):e39061. 10.1371/journal.pone.0039061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Lambert C, Zrinzo L, Nagy Z, et al. Confirmation of functional zones within the human subthalamic nucleus: patterns of connectivity and sub-parcellation using diffusion weighted imaging. Neuroimage Mar 2012;60(1):83–94. 10.1016/j.neuroimage.2011.11.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Kelley R, Flouty O, Emmons EB, et al. A human prefrontal-subthalamic circuit for cognitive control. Brain Jan 1 2018;141(1):205–16. 10.1093/brain/awx300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].McIntyre CC, Mori S, Sherman DL, Thakor NV, Vitek JL. Electric field and stimulating influence generated by deep brain stimulation of the subthalamic nucleus. Clin Neurophysiol Mar 2004;115(3):589–95. 10.1016/j.clinph.2003.10.033. [DOI] [PubMed] [Google Scholar]
- [54].Miocinovic S, Parent M, Butson CR, et al. Computational analysis of subthalamic nucleus and lenticular fasciculus activation during therapeutic deep brain stimulation. J Neurophysiol Sep 2006;96(3):1569–80. 10.1152/jn.00305.2006. [DOI] [PubMed] [Google Scholar]
- [55].Limousin P, Brown P, Marsden J, Defebvre L, Rothwell J. Evoked potentials from subthalamic nucleus, internal pallidum and thalamic stimulation in parkinsonian and postural tremor patients. Journal of Physiology-London 1998:176P–7P. [Google Scholar]
- [56].Bhanpuri NH, Bertucco M, Ferman D, et al. Deep brain stimulation evoked potentials may relate to clinical benefit in childhood dystonia. Brain Stimul Sep-Oct 2014;7(5):718–26. 10.1016/j.brs.2014.06.003. [DOI] [PubMed] [Google Scholar]
- [57].Ni Z, Kim SJ, Phielipp N, et al. Pallidal deep brain stimulation modulates cortical excitability and plasticity. Ann Neurol Feb 2018;83(2):352–62. 10.1002/ana.25156. [DOI] [PubMed] [Google Scholar]
- [58].Tisch S, Rothwell JC, Zrinzo L, Bhatia KP, Hariz M, Limousin P. Cortical evoked potentials from pallidal stimulation in patients with primary generalized dystonia. Mov Disord Jan 30 2008;23(2):265–73. 10.1002/mds.21835. [DOI] [PubMed] [Google Scholar]
- [59].Phillips CG. Actions of antidromic pyramidal volleys on single Betz cells in the cat. Q J Exp Physiol Cogn Med Sci Jan 1959;44(1):1–25. 10.1113/expphysiol.1959.sp001364. [DOI] [PubMed] [Google Scholar]
- [60].Romeo A, Dubuc DM, Gonzalez CL, et al. Cortical activation elicited by subthalamic deep brain stimulation predicts postoperative motor side effects. Neuromodulation Jun 2019;22(4):456–64. 10.1111/ner.12901. [DOI] [PubMed] [Google Scholar]
- [61].Awad MZ, Vaden RJ, Irwin ZT, et al. Subcortical short-term plasticity elicited by deep brain stimulation. Ann Clin Transl Neurol May 2021;8(5):1010–23. 10.1002/acn3.51275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Gmel GE, Hamilton TJ, Obradovic M, et al. A new biomarker for subthalamic deep brain stimulation for patients with advanced Parkinson’s disease–a pilot study. J Neural Eng Dec 2015;12(6):066013. 10.1088/1741-2560/12/6/066013. [DOI] [PubMed] [Google Scholar]
- [63].Kent AR, Swan BD, Brocker DT, Turner DA, Gross RE, Grill WM. Measurement of evoked potentials during thalamic deep brain stimulation. Brain Stimul Jan-Feb 2015;8(1):42–56. 10.1016/j.brs.2014.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Awad MZ, Irwin ZT, Vaden RJ, Guthrie BL, Walker HC. Short latency cortical evoked potentials elicited by subthalamic nucleus deep brain stimulation: commentary and results from paired pulse studies. Clin Neurophysiol Feb 2020;131(2):465–7. 10.1016/j.clinph.2019.11.015. [DOI] [PubMed] [Google Scholar]
- [65].Brittain JS, Sharott A, Brown P. The highs and lows of beta activity in cortico-basal ganglia loops. Eur J Neurosci Jun 2014;39(11):1951–9. 10.1111/ejn.12574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Wiest C, Tinkhauser G, Pogosyan A, et al. Local field potential activity dynamics in response to deep brain stimulation of the subthalamic nucleus in Parkinson’s disease. Neurobiol Dis Sep 2020;143:105019. 10.1016/j.nbd.2020.105019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Ozturk M, Viswanathan A, Sheth SA, Ince NF. Electroceutically induced subthalamic high-frequency oscillations and evoked compound activity may explain the mechanism of therapeutic stimulation in Parkinson’s disease. Commun Biol Mar 23 2021;4(1):393. 10.1038/s42003-021-01915-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Kent AR, Grill WM. Neural origin of evoked potentials during thalamic deep brain stimulation. J Neurophysiol Aug 2013;110(4):826–43. 10.1152/jn.00074.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Wagenaar DA, Potter SM. Real-time multi-channel stimulus artifact suppression by local curve fitting. J Neurosci Methods Oct 30 2002;120(2):113–20. 10.1016/s0165-0270(02)00149-8. [DOI] [PubMed] [Google Scholar]
- [70].Bahmer A, Peter O, Baumann U. Recording and analysis of electrically evoked compound action potentials (ECAPs) with MED-EL cochlear implants and different artifact reduction strategies in Matlab. J Neurosci Methods Aug 15 2010;191(1):66–74. 10.1016/j.jneumeth.2010.06.008. [DOI] [PubMed] [Google Scholar]
- [71].Thevathasan W, Sinclair NC, Bulluss KJ, McDermott HJ. Tailoring subthalamic nucleus deep brain stimulation for Parkinson’s disease using evoked resonant neural activity. Front Hum Neurosci 2020;14:71. 10.3389/fnhum.2020.00071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Herrington TM, Cheng JJ, Eskandar EN. Mechanisms of deep brain stimulation. J Neurophysiol Jan 1 2016;115(1):19–38. 10.1152/jn.00281.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].McIntyre CC, Hahn PJ. Network perspectives on the mechanisms of deep brain stimulation. Neurobiol Dis Jun 2010;38(3):329–37. 10.1016/j.nbd.2009.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Montgomery EB Jr, Gale JT. Mechanisms of action of deep brain stimulation(DBS). Neurosci Biobehav Rev 2008;32(3):388–407. 10.1016/j.neubiorev.2007.06.003. [DOI] [PubMed] [Google Scholar]
- [75].Hashimoto T, Elder CM, Okun MS, Patrick SK, Vitek JL. Stimulation of the subthalamic nucleus changes the firing pattern of pallidal neurons. J Neurosci Mar 1 2003;23(5):1916–23. 10.1523/JNEUROSCI.23-05-01916.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Soares MI, Soares-Dos-Reis R, Rosas MJ, Monteiro P, Massano J. Intraoperative microelectrode recording in Parkinson’s disease subthalamic deep brain stimulation: analysis of clinical utility. J Clin Neurosci Nov 2019;69:104–8. 10.1016/j.jocn.2019.08.021. [DOI] [PubMed] [Google Scholar]
- [77].Maks CB, Butson CR, Walter BL, Vitek JL, McIntyre CC. Deep brain stimulation activation volumes and their association with neurophysiological mapping and therapeutic outcomes. J Neurol Neurosurg Psychiatr Jun 2009;80(6): 659–66. 10.1136/jnnp.2007.126219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Koeglsperger T, Palleis C, Hell F, Mehrkens JH, Botzel K. Deep brain stimulation programming for movement disorders: current concepts and evidence-based strategies. Front Neurol 2019;10:410. 10.3389/fneur.2019.00410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Picillo M, Lozano AM, Kou N, Puppi Munhoz R, Fasano A. Programming deep brain stimulation for Parkinson’s disease: the Toronto Western Hospital algorithms. Brain Stimul May-Jun 2016;9(3):425–37. 10.1016/j.brs.2016.02.004. [DOI] [PubMed] [Google Scholar]
- [80].Kuhn AA, Kempf F, Brucke C, et al. High-frequency stimulation of the subthalamic nucleus suppresses oscillatory beta activity in patients with Parkinson’s disease in parallel with improvement in motor performance. J Neurosci Jun 11 2008;28(24):6165–73. 10.1523/JNEUROSCI.0282-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Kuhn AA, Tsui A, Aziz T, et al. Pathological synchronisation in the subthalamic nucleus of patients with Parkinson’s disease relates to both bradykinesia and rigidity. Exp Neurol Feb 2009;215(2):380–7. 10.1016/j.expneurol.2008.11.008. [DOI] [PubMed] [Google Scholar]


