Abstract
Background/purpose
Bone resorption inhibitors, such as bisphosphonates (BPs) and anti-receptor activator of nuclear factor kappa B ligand antibodies (denosumab; Dmab), are used to treat osteoporosis and effectively reduce the risk of fracture. However, medication-related osteonecrosis of the jaw (MRONJ) has been reported as a rare adverse effect. Invasive tooth extraction procedures are reportedly a factor in the development of MRONJ. In this study, we aimed to retrospectively observe and clinically examine the effect of medication status on MRONJ development after tooth extraction in patients receiving drug treatment for osteoporosis.
Materials and methods
This study was conducted among patients who visited our hospital between December 2015 and December 2021. We collected and analyzed the medical information of patients who underwent dental extractions while using osteoporosis medications, including oral and injectable BPs and Dmab.
Results
Among antiresorptive medication users, 40 patients (70 teeth) underwent extraction. The mean duration of BP/Dmab use was 40.4 months, and the mean duration of drug holiday was 6.9 months. MRONJ after tooth extraction was not seen in BP users, but we observed two cases in Dmab users. A significant difference in MRONJ development was confirmed with the use of injectable compared with oral medication administration (odds ratio=5.01).
Conclusion
The use of injectable bone resorption inhibitors was associated with a higher risk of developing MRONJ. The route of administration, duration of medication, and withdrawal period should be carefully considered to prevent MRONJ after tooth extraction.
Keywords: Bisphosphonate, Denosumab, Drug holiday, Medication-related osteonecrosis of the jaw, Tooth extraction
Introduction
Osteoporosis is defined by the National Institutes of Health as a skeletal disorder characterized by compromised bone strength, predisposing an individual to an increased risk of fracture. Bone strength reflects the integration of two main features: bone quantity and bone quality.1 The pathogenesis is an alteration in the balance of normal bone remodeling, with bone resorption exceeding bone formation, resulting in reduced bone density and decreased bone strength.2 Secondary osteoporosis (related to endocrine factors, nutrition, medicines, immobility, rheumatism, and congenital causes) is a disease presenting low bone mass that accounts for a larger proportion than primary osteoporosis.3
Bisphosphonates (BPs) have been widely used for the treatment and prevention of osteoporosis, malignancy-associated hypercalcemia, osteodynia associated with bone metastasis of a solid cancer (e.g., breast cancer), and multiple myeloma-associated bone diseases.4 The pharmacological action of BPs is to inhibit bone resorption by suppressing osteoclast function, and the efficacy of BPs has been demonstrated. Additionally, anti-receptor activator of nuclear factor kappa B ligand (RANKL) antibody has recently been used as a new treatment for osteoporosis and bone lesions caused by bone metastasis of cancer. Denosumab (Dmab) is a human IgG2 monoclonal antibody against RANKL, which inhibits bone resorption by osteoclasts.5
Since the first report of BP-related osteonecrosis of the jaw (BRONJ) in 2003,6 there have been reports of osteonecrosis of the jaw associated with Dmab and angiogenesis inhibitors, and medication-related osteonecrosis of the jaw (MRONJ) is now the commonly used terminology.4 The incidence of MRONJ in patients taking BPs for the treatment of osteoporosis is lower than that in patients with cancer.4,7,8 By route of administration, the incidence of MRONJ is estimated to be 1.04–69 per 100,000 patients per year for orally administered BPs and 0–90 per 100,000 patients per year for BP injections.9 Although the incidence of MRONJ itself is small, the number of patients with osteoporosis has been increasing in recent years owing to aging of the world's population, which represents a serious problem in the field of dentistry and oral medicine. It has been pointed out that invasive surgical procedures, such as tooth extraction, may trigger the development of MRONJ. Although tooth extraction is frequently performed in clinical dentistry, it is an invasive procedure that involves bone exposure and requires caution in patients taking bone resorption inhibitors. With regard to the development of MRONJ after tooth extraction, there is no clear information on how to use bone resorption inhibitors, how long to use them, or how long to stop using them, and there is no consensus on treatment.10,11 To fill this evidence gap, the purpose of this study was to retrospectively observe and clinically examine the effect of medication status on the development of adverse events after tooth extraction procedures in patients receiving drug treatment for osteoporosis.
Materials and methods
This study was approved by the Ethics Committee of Nihon University School of Dentistry (approval number EP20D006) and was conducted in accordance with the 1975 Declaration of Helsinki, revised in 2013. Furthermore, this retrospective study was conducted in accordance with the guidelines for observational/descriptive studies regarding enhanced reporting of observational studies in epidemiology.12 In this study, we collected clinical records of patients who visited between December 2015 and December 2021. Written informed consent was obtained from all participants.
Patient selection and data sources
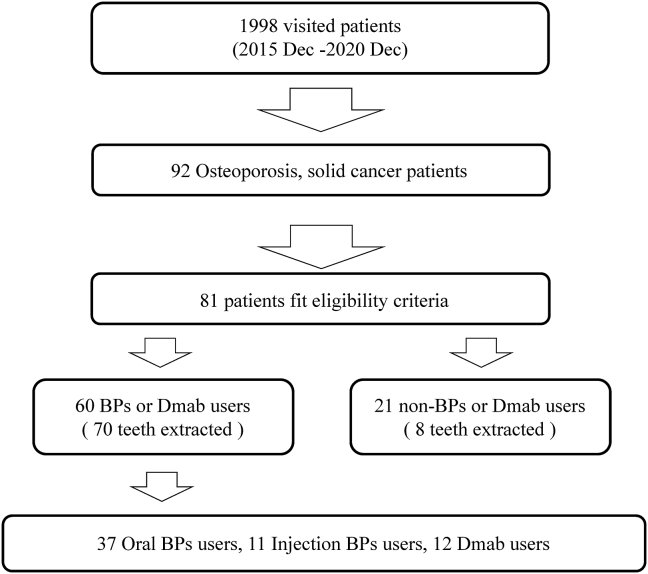
The inclusion criteria for this study were as follows: (1) patients who came to our hospital from December 2015; (2) patients who were using osteoporosis medications such as nitrogen-containing BPs, anti-RANKL antibody, selective estrogen receptor modulator (SERM), parathyroid hormone, and novel active vitamin D3; (3) patients who underwent tooth extraction based on a referral or who had already developed MRONJ at the time of the first visit. The exclusion criteria were (1) patients who did not give their consent for the extraction procedure; (2) patients with contraindications to the procedure owing to systemic diseases; (3) general systemic contraindications such as pregnancy, metabolic diseases, and immunosuppression; (4) patients who had received radiotherapy of the head and neck region in the past (Fig. 1).
Figure 1.
The experimental procedure in osteoporosis patients. BP, bisphosphonate; Dmab, denosumab.
Surgical protocol for tooth extraction
All extractions were performed by multiple oral surgeons under local anesthesia after consultation with a medical doctor. If it was difficult to extract the tooth owing to root adhesion or enlargement, root separation was performed. The granulation was carefully removed and sutured after washing with saline solution. Patients were given oral antibiotics (amoxicillin hydrate) three times a day for 3 days to prevent infection, as postoperative medication. Analgesics (diclofenac sodium, loxoprofen sodium hydrate) were used according to patients’ pain symptoms. Sutures were removed approximately 1 week after tooth extraction. The patient was followed for several weeks until primary healing of the socket was achieved, and if there were no symptoms of paralysis, healing failure, or discomfort associated with sequestrum, treatment was completed and the patient was returned to the referral clinic.
Data collection
The following information was extracted from the clinical records: date of first visit, age, sex, primary disease (osteoporosis, malignancy), type of medication, route of administration, duration of medication, information regarding drug holidays, site of tooth extraction, dental diagnosis that led to the tooth extraction, period of follow-up after tooth extraction, and resumption period of osteoporosis medication after tooth extraction. The route of drug administration was classified as oral use only and injection use (even if the drug had been used orally before or after injection, it was included in injection use). Changes from teriparatide to Dmab or BPs, or from Dmab to BPs, were defined as sequential therapy.
Statistical analysis
All statistical analyses were performed using EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan), a graphical user interface for R 2.13.0 (R Foundation for Statistical Computing, Vienna, Austria).13 The values obtained in descriptive statistics were tested for normality using the Kolmogorov–Smirnov test; the Student’s t-test or Mann–Whitney U test was also performed.
A logistic regression model was used to measure the association between the predictor and outcome variables while controlling for confounders. Statistical analysis was performed with MRONJ development as the objective variable and tooth extraction (no: 0, yes: 1), total duration of medication (≤4 years: 0, >4 years: 1), drug holiday (>2 months: 0, ≤2 months: 1), route of drug administration (oral BPs: 0, BP injection or Dmab: 1), and type of medication (BPs: 0, Dmab: 1) as the explanatory variables. P < 0.05 was considered statistically significant.
Results
Description of patients
The demographic data of patients included in this cohort study are shown in Table 1. Of 1998 patients who attended our hospital between 2015 and 2021, 92 were treated for osteoporosis, including cancer treatment follow-up. One patient lost to follow-up owing to death was excluded; finally, 81 patients who were taking osteoporosis medications met the eligibility criteria. Of this population, 60 patients had a history of BP or Dmab use (BPs/Dmab group). Other drugs (raloxifene hydrochloride, SERM, teriparatide, active vitamin D3) were used by 21 patients (“others” group). The mean age of all patients was 74.6 years, and 47 patients underwent tooth extraction. Extraction sites were observed in the mandible more than in the maxilla. The mean age of patients and the number of extracted teeth were significantly greater in the BPs/Dmab group than in the others group. The mean duration from the first visit to tooth extraction was longer in the BPs/Dmab group, but the mean duration from tooth extraction to completion was similar in both groups. Forty patients (70 teeth) underwent tooth extraction in the BPs/Dmab group (Table 2). Oral BPs alone were used in 24 patients; injectable BPs were used in seven patients, including two patients who used oral BPs before and after injection; and injectable Dmab (including sequential therapy with teriparatide) was used in nine patients. The mean duration of BP use was 51.9 months for oral BPs, 37 months for injections, and 20.3 months for Dmab; the mean duration of drug holiday was 7.7, 8.8, and 3.3 months, respectively. The diagnoses for tooth extraction were chronic apical periodontitis, chronic periodontitis, root fracture, and pericoronitis. The resumption of BPs was confirmed in 14 cases, and the mean duration to resumption was 1.5 months.
Table 1.
Characteristics of participants.
| Osteoporosis/solid cancer | Total | BP/Dmab users | Users of other drugs | P value | |
|---|---|---|---|---|---|
| Patients | 92 | 60 | 32 | ||
| (Women) | (82) | (56) | (26) | ||
| Osteoporosis medication users | 81 | Oral BPs | 37 | 21 | |
| Injection BPs | 11 | ||||
| Dmab | 12 | ||||
| a Age (years, mean ± SD) | 74.6 ± 10.8 | 77.5 ± 7.9 | 68.3 ± 13.4 | ∗∗ | |
| Median | 76 | 78 | 70 | 3.13E-05 | |
| Oral BPs | 77.1 ± 8.9 | ||||
| Injection BPs | 77.8 ± 4.0 | ||||
| Dmab | 78.5 ± 7.9 | ||||
| b Number of extracted teeth | 78 | 70 | 8 | ∗∗ | |
| (Patients) | (47) | (40) | (7) | 8.54E-05 | |
| Upper | 32 | 29 | 3 | ||
| Lower | 46 | 41 | 5 | ||
| Period of tooth extraction from first visit | 96.2 ± 96.4 days | 103.8 ± 99.8 days | 43.1 ± 42.7 days | ||
| Median | 69 | 89 | 33 | ||
| Period of follow-up after tooth extraction | 122.7 ± 182.0 days | 122.6 ± 170.0 days | 123.9 ± 268.7 days | ||
| Median | 42 | 45 | 28 | ||
| MRONJ | 11 patients | Referral clinic, 9 patients | 0 | ||
| Our clinic, 2 patients (3.3%) |
BP, bisphosphonate; Dmab, denosumab; SD, standard deviation; MRONJ, medication-related osteonecrosis of the jaw.
∗∗P < 0.01.
Student's t-test.
Mann–Whitney U test.
Table 2.
Profile of BPs and Dmab users.
| Oral BPs | Injection BPs | Dmab | Total | |
|---|---|---|---|---|
| Patients | 24 | 7 | 9 | 40 |
| Risedronate: 4 | Ibandronate: 6 | PRALIAⓇ: 8 | ||
| Minodronate: 9 | Zoledronate: 1 | RANMARKⓇ: 1 | ||
| Alendronate: 9 | ||||
| Unknown: 2 | ||||
| Age (years, mean ± SD) | 76.3 ± 8.8 | 78.3 ± 4.5 | 78.6 ± 7.3 | 77.2 ± 7.8 |
| ≤65 years (0) | 3 | 0 | 0 | 3 |
| >65 years (1) | 21 | 7 | 9 | 37 |
| Number of extracted teeth | 37 teeth | 13 teeth | 20 teeth | 70 teeth (49 cases: running number) |
| MRONJ | ||||
| At our clinic (after extraction) | 0 | 0 | 2 patients | 2 patients |
| Mean duration of medication use (months) | 51.9 | 37 | 20.3 | 40.4 |
| Range (months) | 1–212 | 4–180 | 0–66 | 1–212 |
| ≤4 years (0) | 18 cases | 7 cases | 11 cases | 36 cases |
| >4years (1) | 9 cases | 1 case | 3 cases | 13 cases |
| Mean duration of drug holiday (months) | 7.7 | 8.8 | 3.3 | 6.9 |
| Range (months) | 0–60 | 0–25 | 0–8 | 0–60 |
| >2 months (0) | 22 cases | 5 cases | 6 cases | 33 cases |
| ≤2 months (1) | 5 cases | 3 cases | 8 cases | 16 cases |
| Mean duration of resumption (months) | 1.5 | 1.5 | 1.7 | 1.5 |
| >3 weeks (0) | 6 cases | 4 cases | 3 cases | 13 cases |
| ≤3 weeks (1) | 1 case | 0 | 0 | 1 case |
BP, bisphosphonate; Dmab, denosumab; SD, standard deviation; MRONJ, medication-related osteonecrosis of the jaw.
MRONJ development after tooth extraction
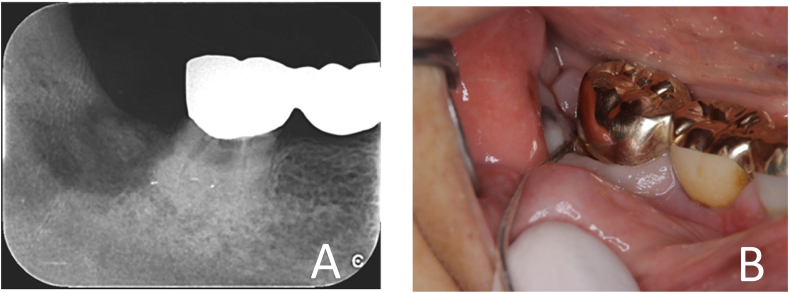
There were two cases of MRONJ after tooth extraction (1 month and 3 months after extraction) at our clinic (Fig. 2). As there were 60 BP/Dmab users, the incidence of MRONJ was 3.3%. Additionally, of the nine patients who were referred with a diagnosis of MRONJ at the first visit, five underwent extraction immediately before referral, one developed at an incompatible denture site, and three were spontaneous cases. Treatment for MRONJ included local cleansing and sequestrectomy. In most cases, no drug withdrawal was needed, and one case of interruption and resumption with BonvivaⓇ syringes for intravenous injection was observed. The results of analysis for factors associated with the development of MRONJ (Table 3) showed a significant difference only for the use of injection with respect to routes of administration (odds ratio [OR] = 5.01). Other explanatory variables were tooth extraction (OR = 0.52), total medication duration of 4 months or more (OR = 2.33), withdrawal of medication for 2 months or less (OR = 1.21), and Dmab use (OR = 2.29), none of which were significantly different.
Figure 2.
Clinical findings (woman, age 84 years). A) Tooth 48 was extracted within 3 months of the first dose of PraliaⓇ. B) MRONJ (stage 0) was diagnosed. MRONJ, medication-related osteonecrosis of the jaw.
Table 3.
Risk indicators for MRONJ according to logistic regression analysis.
| Explanatory variable | Odds ratio | [95% CI] | P value |
|---|---|---|---|
| Tooth extraction (no; 0, yes; 1) | 0.53 | [0.1220–2.270] | 0.390 |
| Total administration period (≤48 months; 0, ≥49 months; 1) | 2.33 | [0.5390–10.100] | 0.258 |
| Drug holiday (≥3 months; 0, ≤2 months; 1) | 1.21 | [0.2990–4.930] | 0.786 |
| Route of administration (oral; 0, injection; 1) | 5.01 | [1.1100–22.600] | 0.036 ∗ |
| Antiresorptive agent (BPs; 0, Dmab; 1) | 2.29 | [0.4000–13.100] | 0.352 |
BP, bisphosphonate; Dmab, denosumab; CI, confidence interval.
∗P < 0.05.
Discussion
BP preparations are analogs of pyrophosphate, a physiological inhibitor of calcification, which has an affinity for calcium-rich hydroxyapatite in bone and can synthesize a variety of derivatives.14 Third-generation BP preparations contain nitrogen and have strong activity in inhibiting bone resorption, with relative activity 10,000 times higher than that of first-generation formulations like etidronate.15 All BPs used in this study, both oral and injectable, were second- and third-generation formulations. The results of this study, in which more patients were using oral than injectable drugs, are consistent with previous reports on the use of bone resorption inhibitors in Japan.7
The overall average patient age in our study was 74.6 years, reflecting the medical situation of a super-aging society in Japan.16 The mean age in the BPs/Dmab group was more than 9 years older than the mean age in the group taking other medications, suggesting that the need for treatment of more severe osteoporosis is greater in older people. Considering that the number of teeth lost generally increases with age, it can be speculated that the difference in the number of extracted teeth between the two groups may be based on these age differences.
The risk of developing MRONJ in patients with osteoporosis who are prescribed oral BPs is reported to be 0.0004%–0.1%, increasing to 0.21% after 4 years of drug use.4,17, 18, 19, 20, 21 A recent position paper summarizes this risk as 0.01%–0.02%.7 In the present study, the incidence of MRONJ was 3.3%, which was higher than previous reports, even though 73% of BP/Dmab users had been taking their medication for less than 4 years. This is thought to be because many patients with high systemic risk are referred to our institution; thus, severe cases tend to be more concentrated at our center. The high incidence rates in this study should therefore be interpreted with caution.
Only two patients using Dmab developed MRONJ after tooth extraction at our clinic. The mean withdrawal period of Dmab users was calculated to be 3.3 months in the records, but most patients had their teeth extracted during the 6-month interval of PraliaⓇ administration. In other words, we estimated the period between the last injection and the time of tooth extraction as the withdrawal period. The main pharmacological differences between BPs and Dmab are in the distribution of these drugs in bone and their effects on progenitor cells and mature osteoclasts. The half-life of Dmab is short, approximately 1 month, and it does not induce apoptosis in osteoclasts and does not deposit in bone like BPs.5 Therefore, it can be assumed that the patients in this study underwent tooth extraction procedures when bone metabolic turnover was generally complete. However, if Dmab users tend to avoid withdrawal owing to a long interval of use, this may be a factor that induces the development of MRONJ.
The incidence of MRONJ is generally higher with injectable drugs than with oral drugs.22, 23, 24, 25 In the present regression analysis, the risk of adverse events was also higher for injectable drugs (BPs and Dmab) than for oral ones, which may reflect the stronger pharmacological effect of injectable drugs on bone resorption. The duration of medication use was the longest among oral BPs users, but there were some cases of self-discontinuation and difficulty in medication management owing to aging. The reason for the lack of significant differences in medication duration may reflect this poor compliance with oral medications, and the results should be interpreted with caution.26,27
Because BPs, unlike anti-RANKL antibodies, accumulate in bone tissue and are metabolized via bone remodeling,5 it is reasonable to estimate a withdrawal period of 3–4 months. The mean withdrawal period obtained in this study was approximately 8 months for both oral and injectable BP users. Because BRONJ was 0%, an adequate rest period before tooth extraction is suggested as one factor for clinical success. However, there is no consensus on whether short-term withdrawal of BPs before invasive dentistry prevents ONJ.28,29 Rather, BP withdrawal worsens symptoms, decreases bone mineral density, and increases the incidence of fractures in patients with osteoporosis.28,30 Considering the great benefit of fracture prevention, withdrawal of medication should be avoided.31 From another point of view, it has been pointed out that there is insufficient recognition of this lesion among professionals in the medical and dental fields, and this is important with the increasing incidence of MRONJ.29 Control of inflammatory lesions in the oral cavity is essential for the prevention of MRONJ,7,32 and it is necessary for the attending physician to encourage the patient to visit the dentist and improve oral hygiene before administering drugs.
The strength of this study is that we were able to provide evidence on the risk factors for MRONJ by focusing on tooth extraction procedures, which is a little-studied area. However, one limitation of this study is that it was difficult to determine the resumption of medication after withdrawal owing to a lack of information. More detailed analysis in future studies is necessary, with a large multicenter sample, an aim of our future research.
In conclusion, despite the limitations of the study design, we focused on patients using osteoporosis drugs in this single-center clinical study to investigate the relationship between drug withdrawal duration and MRONJ development after tooth extraction. MRONJ after dental extraction under medication control was not seen in BPs users, but we observed two cases of post-extraction MRONJ in Dmab users. Investigation of risk factors for the development of MRONJ revealed that the use of injectable preparations was associated with a higher risk of MRONJ development than use of oral preparations. Careful consideration of the duration of medication and withdrawal period when performing tooth extraction in patients with osteoporosis is important to prevent MRONJ development.
Declaration of competing interest
The authors have no conflicts of interest relevant to this article.
Acknowledgments
This study was supported by a grant from the Dental Research Center, Nihon University School of Dentistry.
References
- 1.NIH consensus development panel on osteoporosis prevention, diagnosis, and therapy, March 7–29, 2000. Highlights of the conference. South Med J. 2001;94:569–573. [PubMed] [Google Scholar]
- 2.Deal C. Future therapeutic targets in osteoporosis. Curr Opin Rheumatol. 2009;21:380–385. doi: 10.1097/BOR.0b013e32832cbc2a. [DOI] [PubMed] [Google Scholar]
- 3.Kanis J.A., Cooper C., Rizzoli R., Reginster J.Y. Scientific advisory board of the European society for clinical and economic aspects of osteoporosis (ESCEO) and the committees of scientific advisors and national societies of the international osteoporosis foundation (IOF). European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Osteoporos Int. 2019;30:3–44. doi: 10.1007/s00198-018-4704-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ruggiero S.L., Dodson T.B., Fantasia J., et al. American association of oral and maxillofacial surgeons. American association of oral and maxillofacial surgeons position paper on medication-related osteonecrosis of the jaw – 2014 update. J Oral Maxillofac Surg. 2014;72:1938–1956. doi: 10.1016/j.joms.2014.04.031. [DOI] [PubMed] [Google Scholar]
- 5.Baron R., Ferrari S., Russell R.G. Denosumab and bisphosphonates: different mechanisms of action and effects. Bone. 2011;48:677–692. doi: 10.1016/j.bone.2010.11.020. [DOI] [PubMed] [Google Scholar]
- 6.Marx R.E. Pamidronate (Aredia) and zoledoronate (Zometa) induced avascular necrosis of the jaws: a growing epidemic. J Oral Maxillofac Surg. 2003;61:1115–1117. doi: 10.1016/s0278-2391(03)00720-1. [DOI] [PubMed] [Google Scholar]
- 7.Yoneda T., Hagino H., Sugimoto T., et al. Antiresorptive agent-related osteonecrosis of the jaw: position paper 2017 of the Japanese allied committee on osteonecrosis of the jaw. J Bone Miner Metabol. 2017;35:20. doi: 10.1007/s00774-016-0810-7. [DOI] [PubMed] [Google Scholar]
- 8.Khan A.A., Morrison A., Kendler D.L., et al. International task force on osteonecrosis of the jaw. Case-based review of osteonecrosis of the jaw (ONJ) and application of the international recommendations for management from the international task force on ONJ. J Clin Densitom. 2017;20:8–24. doi: 10.1016/j.jocd.2016.09.005. [DOI] [PubMed] [Google Scholar]
- 9.Khan A.A., Morrison A., Hanley D.A., et al. International Task Force on Osteonecrosis of the Jaw. Diagnosis and management of osteonecrosis of the jaw: a systematic review and international consensus. J Bone Miner Res. 2015;30:3–23. doi: 10.1002/jbmr.2405. [DOI] [PubMed] [Google Scholar]
- 10.Dos Santos Ferreira L., Abreu L.G., Calderipe C.B., Martins M.D., Schuch L.F., Vasconcelos A.C.U. Is teriparatide therapy effective for medication-related osteonecrosis of the jaw? A systematic review and meta-analysis. Osteoporos Int. 2021;32:2449–2459. doi: 10.1007/s00198-021-06078-z. [DOI] [PubMed] [Google Scholar]
- 11.Seki K., Hagiwara Y. Peri-implantitis-induced medication-related osteonecrosis of the jaw: a case report. J Dent Sci. 2022;17:576–577. doi: 10.1016/j.jds.2021.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kuller L.H., Goldstein B.D. Suggestions for STROBE recommendations. Epidemiology. 2007;18:792–793. doi: 10.1097/EDE.0b013e3181571e16. [DOI] [PubMed] [Google Scholar]
- 13.Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013;48:452–458. doi: 10.1038/bmt.2012.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Russell R.G., Roger M.J. Bisphosphonates: from the laboratory to the clinic and back again. Bone. 1999;25:97–106. doi: 10.1016/s8756-3282(99)00116-7. [DOI] [PubMed] [Google Scholar]
- 15.Ebetino F.H., Hogan A.M., Sun S., et al. The relationship between the chemistry and biological activity of the bisphosphonates. Bone. 1999;25:97–106. doi: 10.1016/j.bone.2011.03.774. [DOI] [PubMed] [Google Scholar]
- 16.Statistics Bureau of Japan . 2020. Ministry of internal affairs and communications. Statistical handbook of Japan 2020.https://www.stat.go.jp/english/data/handbook/pdf/2020all.pdf#page=23 Available from: [Date accessed. [Google Scholar]
- 17.Sammut S., Malden N., Lopes V., Ralston S. Epidemiological study of alendronate-related osteonecrosis of the jaw in the southeast of Scotland. Br J Oral Maxillofac Surg. 2016;54:501–505. doi: 10.1016/j.bjoms.2015.10.036. [DOI] [PubMed] [Google Scholar]
- 18.Lo J.C., O'Ryan F.S., Gordon N.P., et al. Prevalence of osteonecrosis of the jaw in patients with oral bisphosphonate exposure. J Oral Maxillofac Surg. 2010;68:243–253. doi: 10.1016/j.joms.2009.03.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marx R.E., Cillo J.E., Jr., Ulloa J.J. Oral bisphosphonate-induced osteonecrosis: risk factors, prediction of risk using serum CTX testing, prevention, and treatment. J Oral Maxillofac Surg. 2007;65:2397–2410. doi: 10.1016/j.joms.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 20.Chiu W.Y., Chien J.Y., Yang W.S., Juang J.M., Lee J.J., Tsai K.S. The risk of osteonecrosis of the jaws in Taiwanese osteoporotic patients treated with oral alendronate or raloxifene. J Clin Endocrinol Metab. 2014;99:2729–2735. doi: 10.1210/jc.2013-4119. [DOI] [PubMed] [Google Scholar]
- 21.Barasch A., Cunha-Cruz J., Curro F.A., et al. Risk factors for osteonecrosis of the jaws: a case-control study from the CONDOR dental PBRN. J Dent Res. 2011;90:439–444. doi: 10.1177/0022034510397196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Black D.M., Reid I.R., Boonen S., et al. The effect of 3 versus 6 years of zoledronic acid treatment of osteoporosis: a randomized extension to the HORIZON-Pivotal Fracture Trial (PFT) J Bone Miner Res. 2012;27:243–254. doi: 10.1002/jbmr.1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Papapoulos S., Chapurlat R., Libanati C., et al. Five years of denosumab exposure in women with postmenopausal osteoporosis: results from the first two years of the FREEDOM extension. J Bone Miner Res. 2012;27:694–701. doi: 10.1002/jbmr.1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grbic J.T., Black D.M., Lyles K.W., et al. The incidence of osteonecrosis of the jaw in patients receiving 5 milligrams of zoledronic acid: data from the health outcomes and reduced incidence with zoledronic acid once yearly clinical trials program. J Am Dent Assoc. 2010;141:1365–1370. doi: 10.14219/jada.archive.2010.0082. [DOI] [PubMed] [Google Scholar]
- 25.Shibahara T. Imaging modalities for drug-related osteonecrosis of the jaw (2), Overview of the position paper on medication-related osteonecrosis of the jaw and the current status of the MRONJ in Japan. Jpn Dent Sci Rev. 2019;55:71–75. doi: 10.1016/j.jdsr.2018.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Silverman S.L., Schousboe J.T., Gold D.T. Oral bisphosphonate compliance and persistence: a matter of choice? Osteoporos Int. 2011;22:21–26. doi: 10.1007/s00198-010-1274-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Park J.H., Park E.K., Koo D.W., et al. Compliance and persistence with oral bisphosphonates for the treatment of osteoporosis in female patients with rheumatoid arthritis. BMC Muscoskel Disord. 2017;18:152. doi: 10.1186/s12891-017-1514-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Taguchi A., Shiraki M., Tsukiyama M., et al. Impact of osteonecrosis of the jaw on osteoporosis treatment in Japan: results of a questionnaire-based survey by the Adequate Treatment of Osteoporosis (A-TOP) Research Group. Calcif Tissue Int. 2015;97:542–550. doi: 10.1007/s00223-015-0045-y. [DOI] [PubMed] [Google Scholar]
- 29.Taguchi A., Shiraki M., Sugimoto T., Ohta H., Soen S., Japan Osteoporosis Society Lack of cooperation between physicians and dentists during osteoporosis treatment may increase fractures and osteonecrosis of the jaw. Curr Med Res Opin. 2016;32:1261–1268. doi: 10.1185/03007995.2016.1170005. [DOI] [PubMed] [Google Scholar]
- 30.Curtis J.R., Westfall A.O., Cheng H., Delzell E., Saag K.G. Risk of hip fracture after bisphosphonate discontinuation: implications for a drug holiday. Osteoporos Int. 2008;19:1613–1620. doi: 10.1007/s00198-008-0604-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hellstein J.W., Adler R.A., Edwards B., et al. Managing the care of patients receiving antiresorptive therapy for prevention and treatment of osteoporosis: executive summary of recommendations from the American Dental Association Council on Scientific Affairs. J Am Dent Assoc. 2011;142:1243–1251. doi: 10.14219/jada.archive.2011.0108. [DOI] [PubMed] [Google Scholar]
- 32.Otto S., Tröltzsch M., Jambrovic V., et al. Tooth extraction in patients receiving oral or intravenous bisphosphonate administration: a trigger for BRONJ development? J Cranio-Maxillo-Fac Surg. 2015;43:847–854. doi: 10.1016/j.jcms.2015.03.039. [DOI] [PubMed] [Google Scholar]