Abstract
Kinematic and kinetic changes following anterior cruciate ligament (ACL) rupture and reconstruction (ACLR) have been fundamental to the understanding of mechanical disrupted load as it contributes to the development of posttraumatic osteoarthritis. These analyses overlook the potential contribution of muscle activity as it relates to the joint loading environment. Males and females classified as non-copers present with unique knee kinematics and kinetics after ACL injury. The purpose of this study was to perform sex-specific analyses in these individuals to explore muscle activity timing during gait after ACL rupture. Thirty-nine participants (12 females, 27 males) were enrolled. Muscle activity during gait was evaluated before and after pre-operative physical therapy, and six months after ACLR. Surface electromyography data were evaluated to determine timing (e.g., the time the muscle activity begins (‘On’) and ends (‘Off’)) for seven muscles: vastus lateralis and medialis (VL, VM), lateral and medial hamstrings (LH, MH), lateral and medial gastrocnemius (LG, MG), and soleus (SOL). General linear models with generalized estimating equations detected the effects of limb and time for muscle activity timing. Males presented with more limb asymmetries before and after pre-operative PT in the VL On (p < 0.001) and Off (p = 0.007), VM On and Off (p < 0.001), and MH off (p < 0.001), but all limb differences resolved by six months post ACLR. Changes in muscle activity in males were pervasive over time in both limbs. Females presented with no interlimb differences pre-operatively, and only involved limb VL off (p = 0.027) and VM off (p = 0.003) and the LH off in both limbs (p < 0.038) changed over time. Our data indicate that inter-limb differences in muscle activity across time points and changes in muscle activity timing over the course of physical therapy were sex specific. Males presented with more inter-limb differences in muscle activity across time points, and females presented with fewer asymmetries before and after pre-operative physical therapy. These data support that sex-specific adaptations should be taken into consideration when assessing biomechanical changes after ACLR.
Keywords: Anterior cruciate ligament (ACL), Gait, Knee, Rehabilitation
1. Introduction
Aberrant knee mechanics during gait predominate in both males and females after anterior cruciate ligament (ACL) rupture, persist up to 2 years after ACL reconstruction (ALCR),(Capin et al., 2017a; Capin et al., 2017b; Capin et al., 2019) and are associated with the subsequent development of posttraumatic osteoarthritis (PTOA). The predominant theory linking aberrant mechanics to PTOA is by way of altered load (i. e., contact force) distribution within the knee joint.(Pfeiffer et al., 2019; Saxby et al., 2019; Wellsandt et al., 2016, Andriacchi et al., 2004) Alterations in knee mechanics during gait after ACLR are characterized by reduced knee joint excursion during stance (i.e., stiff knee strategy) and underloading of the medial tibiofemoral compartment of the knee joint leading to interlimb asymmetries.(Hart et al., 2016; Khandha et al., 2017; Wellsandt et al., 2016) Studies of the longitudinal kinematic and kinetic changes following injury and ACLR have been fundamental to the investigation of disrupted load as an instigator factor in the development of PTOA, but overlook the potential contribution of muscle activity to this pathogenic pathway.
Altered muscle activity patterns, like altered knee kinematics and kinetics, are a hallmark feature of gait in those post-ACL injury and contribute directly to the contact forces experienced at the articular surfaces.(Hurd and Snyder-Mackler, 2007; Rudolph et al., 2001) Muscle timing variables (i.e., onset and offset) provide insight into neuromuscular changes after ACL injury and ACLR. Further, identifying the changes in timing of muscle activity over clinically important time points may provide information to quantify neuromuscular changes over the course of rehabilitation. Knowledge of specific changes over time will deepen our understanding of biomechanical adaptations after knee joint injury and may provide specific time- and muscle group- based targets for rehabilitation intervention.
Non-copers are an ideal subgroup of ACL-injured athletes to study how muscle activity patterns may change during the course of recovery. A ‘non-coper’ is a term used to describe individuals with continued dynamic knee instability during a functional screening exam after ACL rupture.(Rudolph et al., 2001, 1998) This subgroup of ACL-injured athletes present with a stiff knee gait (i.e., reduced knee excursion and internal knee extension moment, increased muscle co-contraction) compared to better-performing ACL-deficient individuals and uninjured counterparts.(Fitzgerald et al., 2000; Rudolph et al., 2001, 1998) Non-copers initiate muscle activity of the medial gastrocnemius sooner and delay the relaxation of the lateral hamstring compared to their copers (i.e., better-performing) and uninjured counterparts.(Rudolph et al., 2001) Given that non-copers’ altered muscle activation patterns and stiff knee strategy are unsuccessful in stabilizing the knee,(Hurd and Snyder-Mackler, 2007) further exploration of sex-specific muscle activity may reflect the role muscle timing plays in the neuromuscular impairments that contribute to the persistence of asymmetric gait patterns.
Our previous work evaluating sex-specific changes in the gait mechanics of non-copers following ACL injury provided early evidence that males and females demonstrate unique kinematic and kinetic adaptations prior to and after ACLR.(Di Stasi et al., 2015) In that study, females demonstrated improved symmetry in knee mechanics after pre-operative physical therapy compared to their male counterparts.(Di Stasi et al., 2015) However, at six months after ACLR, only females demonstrated a worsening of limb asymmetries in knee excursions and internal knee extension moments.(Di Stasi et al., 2015) While these observed sex differences in gait kinematics and kinetics are an important step in understanding differential responses between males and females, the contributions of muscle activity to disrupted gait mechanics cannot be assumed without direct measurement. A sex-specific analysis on muscle activity in this same cohort enables a deeper understanding of neuromuscular impairments underlying gait asymmetries and may offer new targets for rehabilitation.
Therefore, the purpose of this study was to determine if muscle activity timing during gait corroborated previously reported sex-specific kinematic and kinetic findings of Di Stasi et al. (Di Stasi et al., 2015) in the same cohort of non-copers at three clinically important time frames (acutely after ACL rupture, after pre-operative PT, six months after ACLR). Based on previously reported gait comparisons in this patient population,(Di Stasi et al., 2015) we hypothesized that females will demonstrate a greater number of limb asymmetries at baseline that will diminish following training and return six months post-operatively, whereas males will have fewer limb asymmetries at six months post ACLR.
2. Methods
This secondary analysis includes 39 participants (12 females, 27 males) from a larger randomized controlled trial (Level II evidence).(Di Stasi et al., 2015) Athletes were included if they participated in Level I or II sports prior to injury (Hefti et al., 1993) and presented with >3 mm side-to-side difference in ACL laxity using KT-1000 (MEDmetric Corp., San Diego, CA) testing.(Daniel et al., 1985) ACL rupture was confirmed using magnetic resonance imaging. Athletes were excluded from participation in the study if they had concomitant knee ligament injuries (e.g., PCL, MCL, LCL), repairable meniscus tear, or chondral lesions > 1 cm2. The Institutional Review Board of the University of Delaware approved this study, and informed consent was obtained from all participants. Data were collected initially when participants were screened and classified as non-copers(Fitzgerald et al., 2000) (initial screening), after pre-operative physical therapy, and six months after ACLR. After the initial data collection all participants completed ten sessions of progressive pre-operative rehabilitation emphasizing restoration of symmetric knee range of motion and quadriceps strength,(Manal and Snyder-Mackler, 1996) and 18 participants received additional pre-operative perturbation training.(Fitzgerald et al., 2000) We performed a preliminary analysis to test differences in muscle timing between those who received traditional physical therapy and those who had traditional physical therapy augmented by perturbation training and found no differences, thus groups were collapsed for the purpose of this study. A second data collection occurred after pre-operative physical therapy and a third data collection occurred at six months after ACLR. All 39 participants underwent ACLR with either a hamstrings autograft or soft tissue allograft. Post-ACLR rehabilitation followed a standardized, criterion-based physical therapy protocol focusing on resolving effusion, range of motion, and strength deficits and restoring functional impairments.(Adams et al., 2012; Manal and Snyder-Mackler, 1996) Objective testing was completed throughout the course of rehabilitation to determine progress towards goals and discharge for return to sport.
2.1. Electromyography during gait analysis
An embedded six-component force plate (Model 6090, Bertec Corporation, Worthington, OH) was used to capture the stance phase of gait, and a portable electromyography (EMG) unit (Motion Labs Systems, Baton Rouge, LA) and surface electrode were used to capture muscle timing. Gait speed was controlled and maintained at ± 5 % for each participant’s mean speed. Successful trials were only included if the participant had one foot cleanly strike the force plate at a time.
Participants were first prepared for EMG with isopropyl alcohol and non-sterile gauze pads used to clean the skin prior to the application of pre-amplified stainless steel dipole surface electrodes (12 mm disk diameter, 20 mm inter-electrode spacing, input impedance > 100 mΩ, CMRR > 100 dB at 65 Hz). Electrodes were placed mid-muscle belly and parallel to muscle fibers for optimum signal collection. Data were collected from seven muscles bilaterally: vastus lateralis and medialis (VL, VM), lateral and medial hamstrings (LH, MH), lateral and medial gastrocnemius (LG, MG), and soleus (SOL). Maximal voluntary isometric contractions (MVIC) and a resting trial were collected for 4 s for each muscle group prior to walking gait trials. The EMG signals were visually inspected after each trial to verify electrode placement and adjust channel gains to avoid signal clipping. EMG data were bandpass filtered in the backpack unit from 20 to 2000 Hz, with an anti-aliasing filter of 1000 Hz and output voltage of ± 5 V. All analog data were converted to digital signals with a 16-bit A to D board. EMG data were post-processed using the Professional Version of Visual3D (C-Motion, Inc., Germantown, MD) with customized scripts created in a LabVIEW program (National Instruments, Austin, TX). Initial contact and toe-off were determined by a force plate threshold of 50 N. Five walking trials were time normalized to 100 % of stance and averaged for statistical analyses.
Time-normalized EMG data were synced with gait data. Raw EMG data were band-pass filtered from 20 to 350 Hz, corrected for DC offset, highpass filtered at 20 Hz to remove artifact, and full wave rectified. Data were low-pass filtered with a bidirectional 4th order Butterworth filter with a frequency cutoff of 20 Hz to create a linear envelope. Muscle timing variables (i.e., onset and offset) were determined in the custom LabVIEW program. Muscles were determined to be On when the respective muscle signal reached 2.5 times its mean resting value, and Off when the signal dropped below 2.5 times its mean resting value across three frames of data. Muscle timing variables (On, Off) from seven individual muscles (VL, VM, LH, MH, LG, MG, and SOL) for both limbs within males and females at each time point were confirmed using a previously validated visual inspection method.(Hodges and Bui, 1996) For reference, positive On times are relative to after initial contact with the force plate has occurred, while negative values indicate milliseconds prior to contacting the force plate.
2.2. Statistical analysis
Statistical analyses were performed using SPSS Version 26 (SPSS, IBM, Chicago, IL). Numerical EMG data were analyzed using parametric statistics; however nonparametric analyses were used when assumptions of statistical tests were violated. Alpha was set at 0.05 a priori.
2.3. Effect of limb and time within sex
Generalized linear models with generalized estimating equations were used to detect the effects of limb (involved, uninvolved) and time (baseline, after pre-operative physical therapy, six months post-ACLR) for muscle activity timing variables of interest, run separately for each sex. This statistical approach was determined a priori supported by previously published kinematic and kinetic findings from this patient population.(Di Stasi et al., 2015) Significant interactions were followed by planned post hoc pairwise comparisons to compare inter-limb differences at each time point and individual limb differences over time, adjusted for multiple comparisons using a least significant differences correction. When no significant interactions were found, significant main effects of limb or time were reported.
3. Results
No significant differences were found for age, body size, time from injury, or gait speed between male and female non-copers before pre-operative physical therapy (p ≥ 0.078) (Table 1). Results were presented as interlimb differences, involved limb changes over time, and uninvolved limb changes over time (Table 2). A summary of results can be found in Table 3.
Table 1.
Demographics of participants reported by sex.
| Males (n = 27) | Females (n = 12) | |
|---|---|---|
|
| ||
| Age (years) | 27.9 ± 10.0 | 31.8 ± 12.2 |
| Height (m) | 1.81 ± 0.06 | 1.67 ± 0.05 |
| Weight (kg) | 96.10 ± 14.14 | 78.80 ± 20.65 |
| BMI (kg/m2) | 29.7 ± 4.5 | 27.9 ± 6.6 |
| Gait Speed (m/s) | 1.51 ± 0.12 | 1.54 ± 0.12 |
| Time from injury (weeks) | 12.0 ± 10.0 | 9.0 ± 10.3 |
Values reported as mean ± standard deviation.
Abbreviations; BMI, body mass index; m, meters; kg, kilogram; s, second.
Table 2.
Muscle timing stratified by time, group, and limb: means (95 % CI); p Value represents significant limb by time interactions, negative values indicate milliseconds prior to contacting the force plate.
| Muscle timing (ms) | Pre |
Post |
6 Months |
p Value | ||||
|---|---|---|---|---|---|---|---|---|
| Inv | Uninv | Inv | Uninv | Inv | Uninv | |||
|
| ||||||||
| VL On | Female | −24.70 [−29.1, −20.3] | −20.93 [−23.9, −18.0] | −24.40 [−28.4, −20.4] | −22.87 [−25.7, −20.1] | −22.84 [−25.5, −20.2] | −22.08 [−24.3, −19.8] | p = 0.644 |
| Male | −23.2 [−24.7, −21.7] | −19.81 [−21.7, −17.9] | −21.54 [−23.4, −19.6] | −18.53 [−20.2, −16.9] | −19.28 [−21.3, −17.2] | −20.0 [−21.6, −18.4] | p < 0.001 | |
|
|
||||||||
| VL Off | Female | 48.27 [37.4, 59.1] | 39.41 [32.1, 46.7] | 45.81 [36.7, 54.9] | 40.25 [33.7, 46.8] | 34.82 [30.2, 39.4] | 35.65 [30.9, 40.4] | p = 0.027 |
| Male | 48.82 [40.7, 57.0] | 36.37 [30.4, 42.3] | 47.03 [38.8, 55.2] | 35.56 [30.1, 41.0] | 38.19 [31.2, 45.2] | 39.87 [33.1, 46.6] | p = 0.007 | |
| VM On | Female | −24.50 [−27.6, −21.4] | −20.63 [−23.2, −18.1] | −23.93 [−26.8, −21.1] | −20.97 [−23.7, −18.2] | −21.48 [−24.5, −18.4] | −20.68 [−23.0, −18.4] | p = 0.096 |
| Male | −22.04 [−24.0, −20.0] | −17.28 [−19.2, −15.4] | −21.63 [−23.4, −19.9] | −16.62 [−18.6, −14.7] | −19.37 [−21.1, −17.6] | −18.87 [−20.5, −17.2] | p < 0.001 | |
| VM Off | Female | 40.81 [31.2, 50.4] | 36.80 [30.8, 42.8] | 40.88 [29.3, 52.4] | 38.41 [29.7, 47.1] | 31.68 [25.2, 38.1] | 42.87 [30.1, 55.7] | p = 0.003 |
| Male | 45.10 [37.0, 53.2] | 33.45 [29.4, 37.5] | 37.21 [30.8, 43.6] | 29.77 [27.3, 32.2] | 35.06 [28.8, 41.4] | 31.28 [28.2, 34.3] | p < 0.001 | |
| LH On | Female | −33.92 [−27.4, −30.5] | −32.64 [−37.0, −28.3] | −34.68 [−38.8, −30.5] | −33.44 [−39.1, −27.8] | −33.76 [−37.1, −30.4] | −33.65 [−40.4, −26.9] | p = 0.844 |
| Male | −29.27 [−30.7, −27.9] | −28.85 [−31.6, −26.2] | −30.84 [−33.7, −28.0] | −31.29 [−33.6, −29.0] | −29.30 [−31.3, −27.3] | −30.52 [−32.6, −28.4] | p = 0.361 | |
| LH Off | Female | 42.83 [31.2, 54.5] | 38.32 [27.3, 49.4] | 42.01 [26.6, 57.4] | 34.23 [23.6, 44.8] | 27.22 [17.3, 37.2] | 26.6 [14.2, 39.0] | p = 0.076 |
| Male | 36.05 [28.6, 43.5] | 33.80 [25.6, 42.0] | 28.56 [21.6, 35.5] | 24.42 [18.5, 30.4] | 29.36 [22.6, 36.1] | 25.42 [19.6, 31.2] | p = 0.114 | |
| MH On | Female | −34.37 [−37.5, −31.2] | −33.84 [−37.0, −30.7] | −34.54 [−37.4, −31.7] | −33.19 [−36.2, −30.1] | −34.83 [−38.5, −31.2] | −32.82 [−36.0, −29.6] | p = 0.893 |
| Male | −31.42 [−32.9, −30.0] | −30.08 [−31.6, −28.6] | −31.27 [−32.6, −30.0] | −30.20 [−31.8, −28.6] | −30.25 [−32.0, −28.5] | −30.75 [−32.3, −29.2] | p = 0.464 | |
| MH Off | Female | 44.77 [31.6, 58.0] | 33.30 [23.6, 43.0] | 37.82 [26.1, 49.5] | 30.54 [23.1, 38.0] | 28.96 [23.5, 34.4] | 28.62 [20.7, 36.5] | p = 0.077 |
| Male | 39.70 [32.0, 47.4] | 28.88 [23.0, 34.8] | 37.78 [30.5, 45.0] | 22.52 [17.3, 27.7] | 31.52 [25.1, 38.0] | 28.03 [21.7, 34.3] | p < 0.001 | |
| LG On | Female | −5.65 [−10.4, −0.90] | −4.32 [−9.2, 0.54] | −4.92 [−10.5, 0.63] | −2.49 [−11.1, 6.1] | −1.18 [−6.7, 4.4] | −5.85 [−9.8, −1.9] | p = 0.059 |
| Male | 2.35 [−3.5, 8.2] | −1.20 [−5.5, 3.1] | 5.98 [0.38, 11.6] | 3.81 [−2.1, 9.7] | 4.30 [−2.8, 11.4] | 2.62 [−2.7, 7.9] | p = 0.546 | |
| LG Off | Female | 91.95 [87.5, 96.4] | 89.74 [87.2, 92.2] | 88.45 [86.0, 90.9] | 87.58 [84.5, 90.6] | 89.2 [85.9, 92.5] | 85.92 [81.2, 90.7] | p = 0.012 |
| Male | 88.62 [86.8, 90.5] | 88.55 [86.4, 90.7] | 89.33 [87.8, 90.8] | 88.82 [87.3, 90.3] | 89.11 [87.7, 90.5] | 89.54 [88.3, 90.8] | p = 0.901 | |
| MG On | Female | −4.38 [−9.4, 0.68] | −2.91 [−8.0, 2.2] | −2.46 [−9.3, 4.4] | 0.17 [−0.3, 7.6] | 1.68 [−5.2, 8.6] | −2.99 [−8.4, 2.4] | p = 0.374 |
| Male | 1.79 [−3.8, 7.4] | 1.98 [−3.4, 7.3] | 4.63 [−1.1, 10.3] | 8.10 [3.0, 13.1] | 8.86 [2.1, 15.7] | 7.72 [2.0, 13.4] | p = 0.113 | |
| MG Off | Female | 89.49 [84.6, 94.4] | 87.71 [85.4, 90.1] | 85.69 [83.5, 87.8] | 83.98 [81.3, 86.7] | 85.86 [83.0, 88.7] | 84.95 [79.8, 90.1] | p = 0.082 |
| Male | 86.83 [85.0, 88.6] | 87.26 [85.3, 89.3] | 87.39 [86.0, 88.8] | 86.96 [85.4, 88.5] | 87.07 [85.8, 88.4] | 87.27 [86.3, 88.2] | p = 0.926 | |
| SOL On | Female | −4.93 [−9.1, −0.79] | −5.28 [−8.5, −2.1] | −1.56 [−7.6, 4.4] | −4.56 [−8.2, −0.90] | −5.44 [−8.1, −2.8] | −6.23 [−10.2, −2.3] | p = 0.188 |
| Male | −1.69 [−5.2, 1.8] | −4.58 [−8.0, −1.1] | −0.27 [−3.1, 2.6] | −0.86 [−3.4, 1.7] | −1.26 [−4.5, 2.0] | −4.55 [−7.2, −1.9] | p = 0.021 | |
| SOL Off | Female | 91.75 [87.6, 95.9] | 90.83 [88.0, 93.6] | 89.38 [87.4, 91.4] | 87.77 [85.3, 90.2] | 87.58 [83.5, 91.7] | 86.39 [81.2, 91.6] | p = 0.634 |
| Male | 89.78 [87.8, 91.7] | 87.27 [82.4, 92.1] | 90.41 [89.4, 91.4] | 90.26 [88.4, 92.1] | 90.73 [89.1, 92.4] | 90.43 [89.4, 91.5] | p = 0.703 | |
VL, vastus lateralis; VM, vastus medialis; LH, lateral hamstrings; MH, medial hamstrings; LG, lateral gastrocnemius; MG, medial gastrocnemius; SOL, soleus.
Table 3.
Muscle timing summary of statistically significant findings.
| Males | Females | |
|---|---|---|
|
| ||
| Limb Differences | ||
| Pre | VL on/off, VM on/off, MH off | None |
| Post | VL on/off, VM on/off, MH off | None |
| 6 Months | None | None |
| Change Over Time | ||
| Inv: Pre → Post | VL on, VM off, LH off | None |
| Uninv: Pre → Post | VM off, SOL on, LH off | None |
| Inv: Post → 6 Months | VL on/off, VM on | VL off |
| Uninv: Post → 6 Months | VM on, MH off, SOL on | None |
| Inv: Pre → 6 Months | VL on/off, VM on/off, MH off, LH off | VL off, VM off, LH off |
| Uninv: Pre → 6 Months | LH off | LH off |
VL, vastus lateralis; VM, vastus medialis; LH, lateral hamstrings; MH, medial hamstrings; LG, lateral gastrocnemius; MG, medial gastrocnemius; SOL, soleus.
3.1. Males: limbs over time
Vastus Lateralis (VL):
There were significant limb by time interactions for VL On (p < 0.001) and Off (p = 0.007). The involved limb turned On earlier and Off later when compared to the uninvolved limb at both pre-operative time points (p ≤ 0.006) (Table 2, Fig. 1). No limb differences were present six months post-ACLR for VL On and Off (p ≥ 0.553). The VL on the involved limb turned On earlier at baseline when compared to both follow-up time points (p ≤ 0.049). The VL on the involved limb turn Off sooner six months post ACLR when compared to each pre-operative time point (p ≤ 0.032). VL timing on the uninvolved limb did not significantly change over time (p ≥ 0.054).
Fig. 1.
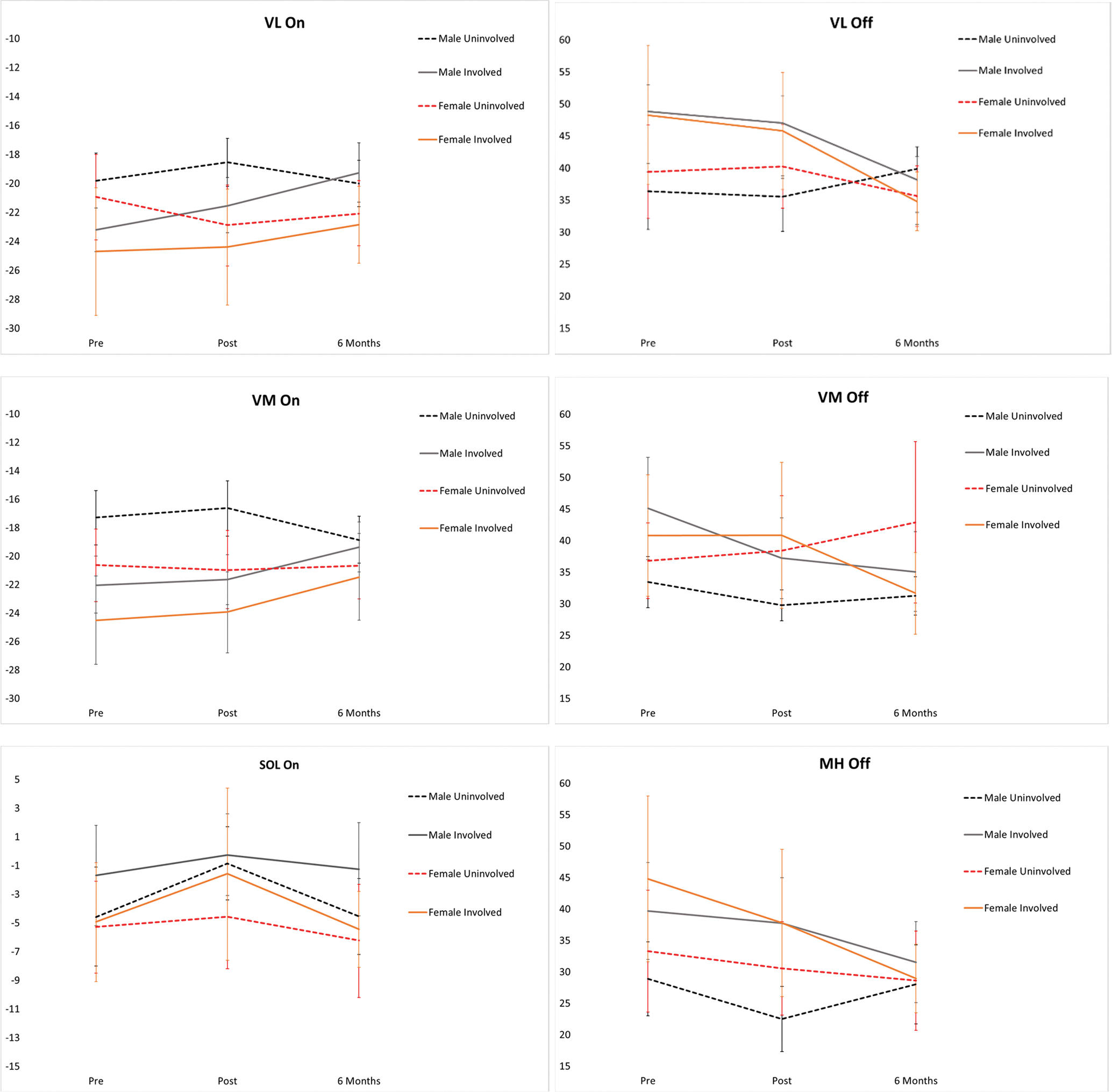
Time by limb interaction effects for males and females pre and post pre-operative physical therapy and 6 months after surgery. VL, vastus lateralis; VM, vastus medialis; LH, lateral hamstrings; MH, medial hamstrings; LG, lateral gastrocnemius; MG, medial gastrocnemius; SOL, soleus. *indicates significant interaction effects for sex and limb.
Vastus Medialis (VM):
There was a significant limb by time interaction for VM On (p < 0.001) and VM Off (p < 0.001). The involved limb turned On earlier and Off later compared to the uninvolved limb at each pre-operative PT time point (p ≤ 0.006). No limb differences were seen at six months for VM On or Off (p ≥ 0.127). The VM on the involved limb turned On earlier at six months post ACLR when compared to both pre-operative timepoints (p ≤ 0.033). The VM of the involved limb turned Off later at baseline when compared to both follow-up time points (p ≤ 0.035), The VM of the uninvolved limb turned On earlier at six months when compared to after pre-operative PT (p = 0.041). The VM of the uninvolved limb turned Off earlier after pre-operative PT compared to baseline (p = 0.046).
Lateral Hamstring (LH):
No significant limb by time interactions were found for LH On (p ≥ 0.172) No significant limb by time interaction was seen for LH Off (p = 0.361), however there was a main effect of time such that LH turned Off later at baseline when compared to both follow-up time points (p ≤ 0.028).
Medial Hamstring (MH):
No significant interactions or main effects were found for MH On (p ≥ 0.286). There was a significant time by limb interaction was found for MH Off (p < 0.001). The MH of the involved limb turned Off later than the uninvolved limb at both pre-operative time points (p < 0.001). The MH of the involved limb turned Off earlier at six months when compared to baseline (p = 0.034); however, the uninvolved limb turned Off later at six months compared to after pre-operative PT (p = 0.012).
Lateral Gastrocnemius (LG) and Medial Gastrocnemius (MG):
No significant limb by time interactions or main effects were found for LG and MG On or Off (p ≥ 0.088).
Soleus (SOL):
There was a significant time by limb interaction found for SOL On (p = 0.021) but not for SOL Off (p = 0.703). There were no limb differences for SOL On (p ≥ 0.077). The SOL of the uninvolved limb turned On earlier following pre-operative PT when compared to both baseline and six months post-ACLR (p ≤ 0.014). The involved SOL On did not change over time (p ≥ 0.383).
3.2. Females: Limbs over time
Vastus Lateralis (VL):
No significant interactions or main effects were found for VL On (p > 0.201). There was a significant time by limb interaction for VL Off (p = 0.027). There were no limb differences for VL Off (p ≥ 0.195). The involved limb turned Off earlier at six months compared to both pre-operative time points (p ≤ 0.015) (Table 2, Fig. 1).
Vastus Medialis (VM):
No significant interactions or main effects were found for VM On (p ≥ 0.096). There was a time and limb interaction for VM Off (p = 0.003). There were no limb differences for the VM Off (p ≥ 0.054). The VM of the involved limb turned Off earlier at six months after ACLR than at baseline (p = 0.014).
Lateral Hamstring (LH), Medial Hamstring (MH), Medial Gastrocnemius (MG) and Soleus (SOL):
There was a main effect of time for LH Off such that the LH turned Off earlier at six months compared to both pre-operative timepoints (p = 0.013). There was no significant interactions for On or Off for LH, MH, MG or SOL muscles activity (p ≥ 0.076). There were no significant main effects for LH On, MH On and Off, MG On and Off, or SOL On and Off (p ≥ 0.057).
Lateral Gastrocnemius (LG):
No significant interactions or main effects were found for LG On (p ≥ 0.059). There was a limb by time interaction found for LG Off (p = 0.012). There were no limb differences for LG Off (p ≥ 0.239). LG On and Off did not change over time in either limb (p ≥ 0.180).
4. Discussion
The purpose of this secondary analysis was to evaluate muscle timing changes in males and females after ACL injury and reconstruction. Our aim was to determine whether muscle activity corroborated previously reported sex-specific kinematic and kinetic findings in the same group of 39 non-copers after ACL injury at three clinically important time points. In the current study, females and males demonstrated unique patterns of muscle activity, however these limb behaviors did not follow similar patterns to those seen in their kinematics and kinetics,(Di Stasi et al., 2015) and did not support our hypotheses that females would demonstrate more asymmetries than males following ACLR.
Our exploratory analyses elucidated how muscle activity during gait may be influenced by surgical reconstruction and rehabilitation, and identified that female participants were walking with relatively symmetric muscle activity-six month-post operatively, a time that they are often beginning to engage in higher level running, agility, and sports-specific tasks to prepare for return to sport. In these females at six months, the involved limb quadriceps (VL and VM) Off time was significantly earlier compared to the baseline timepoint. These changes in muscle activity timing of the females suggest adaptations in the involved limb’s quadriceps activity, whereas the lateral hamstring activity was changing similarly in both limbs across time. Our current study’s findings are different from the kinematic and kinetic findings in the same population,(Di Stasi et al., 2015) where females presented with asymmetrical movement patterns at baseline, demonstrated improvements in gait symmetry after pre-operative physical therapy, but then became increasingly asymmetric in joint excursions and knee joint moment at the six month time point.
Males in this cohort presented with different quadriceps and hamstring muscle activity between limbs prior to ACLR (pre- and post-training), whereas muscle timing did not differ between limbs pre-operatively in females (Table 2). This group of males demonstrated prolonged quadriceps and hamstring activity in the involved limb pre-operatively. It is possible this prolonged muscle activity in the involved knee prior to surgery is a compensatory strategy for stabilization, as non-copers are characterized by dynamic instability at the knee joint.(Hurd and Snyder-Mackler, 2007; Khandha et al., 2019; Rudolph et al., 2001) Six months after ACLR, the interlimb differences in quadriceps muscle timing and medial hamstrings off time identified prior to surgery resolved. Quadriceps activity shortened in length over time and became more like the quadriceps muscle timing on the uninvolved limb. The lateral and medial hamstrings followed a similar pattern of prolonged activity prior to surgery, suggesting a co-contraction stabilization strategy. The impact of increased muscle co-contraction on joint health is not fully understood, as some studies have suggested quadriceps and hamstrings co-contraction increases tibiofemoral joint compression, (Tsai et al., 2012) while others show no increase in medial compartment contact forces.(Khandha et al., 2019) Achieving a shorter duration of muscle activity in males post-operatively may be indicative of more muscular control of the knee joint during gait.(McGibbon and Krebs, 2004; Rudolph et al., 2001).
While there was an indication of improvement in our sample with pre-operative physical therapy, asymmetries were prevalent in males despite pre-operative physical therapy. This may suggest that the 5-week rehabilitation program was not enough for males to respond, or perhaps ACLR, a restoration of passive stability, was necessary to restore limb symmetry in male’s muscle activity. Continued gait asymmetries in muscle timing for males is consistent with literature supporting persistent gait asymmetries for up to 2 years after surgery.(Capin et al., 2019; Ito et al., 2021) Additionally, during a landing task, male athletes after ACLR demonstrated longer preimpact EMG duration in both the knee flexors and extensors compared to an uninjured control group. (Rocchi et al., 2020) These findings support our data suggesting changes in motor control strategies of the involved limb in male athletes. Other studies have shown similar results of early muscle onset in individuals after ACLR during functional tests,(Gokeler et al., 2010; Theisen et al., 2016) however no previous studies have explored sex-specific muscle timing during gait longitudinally over the course of rehabilitation.
Muscle activation findings did not corroborate the kinematic and kinetic asymmetries reported in this same cohort of non-copers.(Di Stasi et al., 2015) Combined data from these studies suggest both sexes have asymmetric gait biomechanics, but primarily males may have asymmetric muscle activity. While gait adaptations may be beneficial in the short-term (e.g., increase stability, reduce demand on the quadriceps), there may be negative long-term consequences, such as the subsequent development of post-traumatic osteoarthritis,(Hart et al., 2016; Wellsandt et al., 2016) to asymmetric gait becoming a learned pattern. Further research is required to understand how muscle activity in males after pre-operative physical therapy may relate to uninjured individuals. Without data from a matched, uninjured cohort, we cannot discern whether changes in muscle timing were induced by rehabilitation, time alone, or the combination of both. However, we note that for both males and females, muscle activity timing changed in a way that appears closer to that of an uninjured gait pattern (i.e., shortened over time) and therefore may be improving.
Our previous study of joint kinematics and kinetics identified large gait asymmetries in only the women at six months, including lower internal knee extension moments and knee joint excursions on the involved limb.(Di Stasi et al., 2015) The adaptations related to the timing of the muscle activity and inter-limb comparisons in the current study seem to follow a different pattern, suggesting that identifying meaningful sex-specific changes in muscle activity is an important step in further understanding the biomechanical profile during walking after ACLR. A recent systematic review of knee muscle activity in patients after ACL injury found that collapsed across sexes, individuals after ACL rupture found both increased and reduced muscle timing activity of the quadriceps, and increased activity of the hamstrings during gait.(Shanbehzadeh et al., 2017) Our results, taken with these data, suggest individuals after ACL rupture, regardless of sex, show seemingly different neuromuscular strategies when assessing electromyography than kinematics and kinetics. Though these data may seem conflicting, it points to the gap in evidence in understanding changes in muscle activity over the course of rehabilitation. Our electromyography data deepen our understanding of gait changes after ACLR and may provide potential sex-specific targets for neuromuscular intervention. Biomechanical changes in walking after ACLR have been linked to the development of osteoarthritis,(Hart et al., 2016; Pfeiffer et al., 2019; Wellsandt et al., 2016) and the mechanics of walking may have a role in the progression of osteoarthritis in otherwise uninjured individuals.(Andriacchi et al., 2004).
A growing body of evidence suggests females are at an increased risk for poor outcomes after ACLR.(Paterno et al., 2012; Shelbourne et al., 2009) Females have an increased risk of contralateral ACL injury compared to males,(Shelbourne et al., 2009) and young females athletes in particular who return to pivoting and cutting sports after ACLR have a 15-fold greater risk of an ACL injury than healthy participants.(Paterno et al., 2012) Similarly, an analysis of the Multicenter Orthopaedic Outcomes Network (MOON) cohort found that males were more likely than females to return to soccer after ACLR.(Kaeding et al., 2014) Our data suggest sex differences are also seen in gait, however in our cohort muscle activity asymmetries are more prevalent in males than females early after injury. While beyond the scope of this current study, additional work is needed to further understand this sex-specific muscle activity. Disrupted gait patterns may affect the distribution of load in the knee joint and may impact the ability for cartilage to accommodate load. (Palmieri-Smith and Thomas, 2009) Sex-specific gait differences may need to be considered through rehabilitation following ACL rupture and reconstruction in both biomechanical and functional outcomes when considering optimizing gait after ACLR.
There are limitations to consider when interpreting the results of this study. Our sample is limited to a subgroup of ACL injuries, non-copers, which limits the generalizability of our results. This study was not powered to directly compare males and females, so we cannot make any direct comparisons between sexes. Likewise, the small sample size and multiple comparisons including seven muscles performed increases the risk of type I error. Meniscal status (i.e., partial meniscectomies or meniscal repairs) was not considered in our analysis, which may have influenced results. Finally, our study did not include healthy controls, which limits our understanding of how these results compare to muscle activity patterns of uninjured individuals.
5. Conclusion
Sex-specific differences in muscle activity in non-copers after ACLR did not parallel those of published kinematic and kinetic changes.(Di Stasi et al., 2015) Our data indicate that male non-copers presented with more inter-limb differences in muscle activity across time points, and refuting our hypothesis, females presented with fewer asymmetries before and after pre-operative physical therapy. As these results contradict previous kinematic and kinetic findings of limb differences between sexes, continued research may be necessary to further understand sex-specific adaptations in muscle activity. A deeper understanding of how males’ and females’ muscle activity differs will expand our understanding of biomechanical changes after ACL rupture and ACLR and may contribute to the development of clinical interventions targeting resultant aberrant gait mechanics. Future work should consider the effects of sex on biomechanical changes after ACL injury and reconstruction, specifically in respect to changes in the timing of muscle activity.
Acknowledgements
Funding was provided by the National Institutes of Health (NIH) R01-AR048212 and F31-AR078580. EKA received support through the Foundation for Physical Therapy Research PODS II Scholarship. The authors would like to acknowledge and thank Drs. Greg Seymour and Wendy Hurd, Martha Callahan and the Delaware Rehabilitation Institute, and Dr. Airelle Giordano and the University of Delaware Physical Therapy Clinic.
Footnotes
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
CRediT authorship contribution statement
Elanna Arhos: Conceptualization, Data curation, Formal analysis, Writing-original draft. Stephanie Di Stasi: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Writing-reviewing & editing. Erin Hartigan: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Writing-reviewing & editing. Lynn Snyder-Mackler: Conceptualization, Funding acquisition, Project administration, Resources, Writing- reviewing & editing.
References
- Adams D, Logerstedt DS, Hunter-Giordano A, Axe MJ, Snyder-Mackler L, 2012. Current concepts for anterior cruciate ligament reconstruction: a criterion-based rehabilitation progression. J. Orthop. Sports Phys. Ther. 42, 601–614. 10.2519/jospt.2012.3871 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andriacchi TP, Mündermann A, Smith RL, Alexander EJ, Dyrby CO, Koo S, 2004. A framework for the in vivo pathomechanics of osteoarthritis at the knee. Ann. Biomed. Eng. 32, 447–457. [DOI] [PubMed] [Google Scholar]
- Capin JJ, Khandha A, Zarzycki R, Manal K, Buchanan TS, Snyder-Mackler L, 2017a. Gait mechanics and second ACL rupture: Implications for delaying return-to-sport. J. Orthop. Res. 35, 1894–1901. 10.1002/jor.23476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capin JJ, Zarzycki R, Arundale A, Cummer K, Snyder-Mackler L, 2017b. Report of the primary outcomes for gait mechanics in men of the ACL-SPORTS trial: secondary prevention with and without perturbation training does not restore gait symmetry in men 1 or 2 years after ACL reconstruction. Clin. Orthop. Relat. Res. 475, 2513–2522. 10.1007/s11999-017-5279-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capin JJ, Zarzycki R, Ito N, Khandha A, Dix C, Manal K, Buchanan TS, Snyder-Mackler L, 2019. Gait mechanics in women of the ACL-SPORTS randomized control trial: interlimb symmetry improves over time regardless of treatment group. J. Orthop. Res. Accepted. 10.1002/jor.24314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel DM, Malcom LL, Losse G, Stone ML, Sachs R, Burks R, 1985. Instrumented measurement of anterior laxity of the knee. J. Bone Jt. Surg. - Ser. A. 10.2106/00004623-198567050-00006. [DOI] [PubMed] [Google Scholar]
- Di Stasi SL, Hartigan EH, Snyder-Mackler L, 2015. Sex-specific gait adaptations prior to and up to six months after ACL reconstruction. J Orthop Sport. Phys Ther 45, 207–214. 10.2519/jospt.2015.5062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald G, Axe M, Snyder-Mackler L, 2000. A decision-making scheme for returning patients to high-level activity with nonoperative treatment after anterior cruciate ligament rupture. Knee Surgery. Sport. Traumatol. Arthrosc. 8, 76–82. [DOI] [PubMed] [Google Scholar]
- Gokeler A, Hof AL, Arnold MP, Dijkstra PU, Postema K, Otten E, 2010. Abnormal landing strategies after ACL reconstruction. Scand. J. Med. Sci. Sports 20, e12–e19. 10.1111/J.1600-0838.2008.00873.X. [DOI] [PubMed] [Google Scholar]
- Hart HF, Culvenor AG, Collins NJ, Ackland DC, Cowan SM, Machotka Z, Crossley KM, 2016. Knee kinematics and joint moments during gait following anterior cruciate ligament econstruction: a systematic review and meta-analysis. Br. J. Sports Med. 50, 597–612. 10.1136/bjsports-2015-094797. [DOI] [PubMed] [Google Scholar]
- Hefti E, Müller W, Jakob RP, Stäubli HU, 1993. Evaluation of knee ligament injuries with the IKDC form. Knee Surgery Sport. Traumatol. Arthrosc. 1, 226–234. 10.1007/BF01560215. [DOI] [PubMed] [Google Scholar]
- Hodges P, Bui B, 1996. A comparison of computer-based methods for the determination of onset of muscle contraction using electromyography. Electroencephalogr. Clin. Neurophysiol. 101, 511–519. [DOI] [PubMed] [Google Scholar]
- Hurd WJ, Snyder-Mackler L, 2007. Knee instability after acute ACL rupture affects movement patterns during the mid-stance phase of gait. J. Orthop. Res. 25, 1369–1377. 10.1002/jor.20440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito N, Capin JJ, Arhos EK, Khandha A, Buchanan TS, Snyder-Mackler L, 2021. Sex and mechanism of injury influence knee joint loading symmetry during gait 6 months after ACLR. J Orthop Res 39, 1123–1132. 10.1002/jor.24822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaeding CC, Pedroza A, Reinke E, Huston LJ, Spindler KP, 2014. Risk factors and predictors of subsequent ACL injury after ACL reconstruction: prospective analysis of 2801 primary ACL reconstructions. Orthop. J. Sport. Med. doi: 10.1177/2325967114S00046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khandha A, Manal K, Wellsandt E, Capin J, Snyder-Mackler L, Buchanan TS, 2017. Gait mechanics in those with/without medial compartment knee osteoarthritis 5 years after anterior cruciate ligament reconstruction. J. Orthop. Res. 35, 625–633. 10.1002/jor.23261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khandha A, Manal K, Capin J, Wellsandt E, Marmon A, Snyder-Mackler L, Buchanan TS, 2019. High muscle co-contraction does not result in high joint forces during gait in anterior cruciate ligament deficient knees. J. Orthop. Res. 10.1002/jor.24141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manal TJO, Snyder-Mackler L, 1996. Practice guidelines for anterior cruciate ligament rehabilitation: a criterion-based rehabilitation progression. Oper. Tech. Orthop. 6, 190–196. 10.1016/S1048-6666(96)80019-X. [DOI] [Google Scholar]
- McGibbon CA, Krebs DE, 2004. Discriminating age and disability effects in locomotion: neuromuscular adaptations in musculoskeletal pathology. J. Appl. Physiol. 96, 149–160. 10.1152/japplphysiol.00422.2003. [DOI] [PubMed] [Google Scholar]
- Palmieri-Smith RM, Thomas AC, 2009. A neuromuscular mechanism of posttraumatic osteoarthritis associated with ACL injury. Exerc. Sport Sci. Rev. 37, 147–153. 10.1097/JES.0b013e3181aa6669. [DOI] [PubMed] [Google Scholar]
- Paterno MV, Rauh MJ, Schmitt LC, Ford KR, Hewett TE, 2012. Incidence of contralateral and ipsilateral Anterior Cruciate Ligament (ACL) injury after primary ACL reconstruction and return to sport. Clin. J. Sport Med. 10.1097/JSM.0b013e318246ef9e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeiffer SJ, Spang J, Nissman D, Lalush D, Wallace K, Harkey MS, Pietrosimone LS, Schmitz R, Schwartz T, Blackburn T, Pietrosimone B, 2019. Gait mechanics and T1ρ MRI of tibiofemoral cartilage 6 months after ACL reconstruction. Med. Sci. Sports Exerc. 51, 630–639. 10.1249/MSS.0000000000001834. [DOI] [PubMed] [Google Scholar]
- Rocchi JE, Labanca L, Laudani L, Minganti C, Mariani PP, Macaluso A, 2020. Timing of muscle activation is altered during single-leg landing tasks after anterior cruciate ligament reconstruction at the time of return to sport. Clin. J. Sport Med. 30 10.1097/JSM.0000000000000659. [DOI] [PubMed] [Google Scholar]
- Rudolph KS, Eastlack ME, Axe MJ, Snyder-Mackler L, 1998. 1998 Basmajian Student Award Paper Movement patterns after anterior cruciate ligament injury: a comparison of patients who compensate well for the injury and those who require operative stabilization. J. Electromyogr. Kinesiol. 10.1016/S1050-6411(97)00042-4. [DOI] [PubMed] [Google Scholar]
- Rudolph KS, Axe MJ, Buchanan TS, Scholz JP, Snyder-Mackler L, 2001. Dynamic stability in the anterior cruciate ligament deficient knee. Knee Surgery. Sport. Traumatol. Arthrosc. 9, 62–71. [DOI] [PubMed] [Google Scholar]
- Saxby DJ, Bryant AL, Van Ginckel A, Wang Y, Wang X, Modenese L, Gerus P, Konrath JM, Fortin K, Wrigley TV, Bennell KL, Cicuttini FM, Vertullo C, Feller JA, Whitehead T, Gallie P, Lloyd DG, 2019. Greater magnitude tibiofemoral contact forces are associated with reduced prevalence of osteochondral pathologies 2–3 years following anterior cruciate ligament reconstruction. Knee Surgery Sport. Traumatol. Arthrosc. 27, 707–715. 10.1007/s00167-018-5006-3. [DOI] [PubMed] [Google Scholar]
- Shanbehzadeh S, Mohseni Bandpei MA, Ehsani F, 2017. Knee muscle activity during gait in patients with anterior cruciate ligament injury: a systematic review of electromyographic studies. Knee Surgery. Sport. Traumatol. Arthrosc. 25, 1432–1442. 10.1007/S00167-015-3925-9/TABLES/2. [DOI] [PubMed] [Google Scholar]
- Shelbourne KD, Gray T, Haro M, 2009. Incidence of subsequent injury to either knee within 5 years after anterior cruciate ligament reconstruction with patellar tendon autograft. Am. J. Sports Med. 10.1177/0363546508325665. [DOI] [PubMed] [Google Scholar]
- Theisen D, Rada I, Brau A, Gette P, Seil R, 2016. Muscle activity onset prior to landing in patients after anterior cruciate ligament injury: a systematic review and meta-analysis. PLoS ONE 11. 10.1371/JOURNAL.PONE.0155277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai LC, McLean S, Colletti PM, Powers CM, 2012. Greater muscle co-contraction results in increased tibiofemoral compressive forces in females who have undergone anterior cruciate ligament reconstruction. J. Orthop. Res. 10.1002/jor.22176. [DOI] [PubMed] [Google Scholar]
- Wellsandt E, Gardinier ES, Manal K, Axe MJ, Buchanan TS, Snyder-Mackler L, 2016. Decreased knee joint loading associated with early knee osteoarthritis after anterior cruciate ligament injury. Am. J. Sports Med. 44, 143–151. 10.1007/BF00612995. [DOI] [PMC free article] [PubMed] [Google Scholar]


