Abstract
Background/purpose
Both subjective and objective evaluations are required to assess taste function. Evaluation of taste function has important clinical significances in patients with burning mouth syndrome (BMS) due to pain-taste interactions. The purpose of this study was to investigate the relationship between subjective and objective taste evaluations in patients with taste disorders based on the presence of BMS.
Materials and methods
Fifty–one patients with taste disturbances were included. The patients completed questionnaires on subjective taste sensations. The taste strip test was performed to examine objective taste function. The patients were divided into two groups: subjects with BMS (n = 24, 3 males and 21 females) and without BMS (n = 27, 8 males and 19 females).
Results
Significant differences were not observed in age, age distribution, and gender distribution between the groups. There were no significant differences in self-reported taste abilities based on the presence of BMS. However, the taste strip test showed higher correct answer rates for bitterness (P = 0.027) in the patients with BMS. In addition, a significant difference (P = 0.034) was observed in the distribution of objective types of taste disorders between the groups. A significant correlation between the subjective and objective evaluation results was observed only in patients with BMS.
Conclusion
In patients with taste disorders, patients with BMS had significant correlations between subjective and objective evaluations and different distributions in the types of taste disorders compared with those without BMS. The presence or absence of BMS should be evaluated in the diagnosis and management of taste disorders.
Keywords: Burning mouth syndrome, Subjective taste sensations, Taste disorders, Taste strip test
Introduction
Taste sensation is important in life. Taste disturbances affect food intake, reduce overall body function, and can result in a deteriorated quality of life. The process of taste perception is initiated in the taste buds by chemical stimuli and arrives at the central nervous system through the facial, glossopharyngeal, and vagus nerves.
Variable factors are associated with the process of taste perception. Systemic factors such as neurological diseases, endocrinological problems, psychiatric diseases, nutritional deficiencies, extreme stress, or medications have been known to affect taste function. Olfactory dysfunction is also associated with decreased taste function.1 In addition, oral conditions such as oral candidiasis, hyposalivation, mucositis, or burning mouth syndrome (BMS), are associated with taste disturbances. BMS is a chronic oral pain disorder presenting with burning, aching, itching, stinging, or numb sensations on the oral mucosal surfaces, which is often accompanied with taste disturbances and xerostomia.2 These symptoms could result from many local and systemic factors, termed secondary type of BMS. The primary type of BMS is considered a trigeminal neuropathy with peripheral and/or central mechanisms.
The number of patients complaining of taste disturbances is increasing due to increased life expectancy, multimorbidity and polypharmacy, increased expectations for quality of life, and the increased complexity of society. However, the diagnostic procedures for these patients have not been fully standardized. The simple and easy way to assess patients is using a focused questionnaire regarding the type and severity of taste disturbances. However, such self-reports have limitations that may not exactly reflect the condition. Thus, objective taste function tests such as electrogustometry (EGM), the whole-mouth method using taste solutions, the filter paper disk method, or the taste strip method should also be used.3 Each of these methods has strengths and weaknesses. EGM is easy to use but does not evaluate taste function based on taste qualities.4 The whole-mouth method using taste solutions is appropriate for qualitative evaluation but is relatively time-consuming and maintaining a consistent quality of fresh taste solution is difficult.5 The filter paper disk method can be used to evaluate each regional area, however, this test is also lengthy and disadvantageous because it is difficult to administer to the elderly.6 The taste strip method using commercial strips impregnated with different concentrations of tastants is used to conduct both qualitative and quantitative evaluations. This method has been validated and has the advantage of being simple to implement.7
In clinical practice, patients who visit the dental clinic with a complaint of taste disturbances are often included in the diagnosis of BMS. In many previous studies, more than 50% of patients with BMS reportedly had taste disturbances,8, 9, 10 especially discomforts from bitter or metallic taste.10 Pathophysiological connections between oral burning sensations and taste disturbances have been suggested in terms of peripheral nerve interactions and involvement of the central nervous system.11 Thus, the expressed characteristics of taste disturbances could differ in patients with taste disorders based on the presence or absence of BMS. In a previous study, the difference in taste disturbances between patients with and without burning mouth (BM) symptoms was investigated; patients with BM symptoms reported they recognize taste qualities better and showed lower EGM thresholds than patients without BM symptoms.12 However, results from taste function tests using each taste quality are also required to understand the taste problems of patients based on the presence or absence of BMS.
To increase the efficiency and accuracy of the diagnostic process in patients with taste disturbances, information on the relationship between self-reports and objective test results is necessary. In several studies, the relationship between the questionnaire and the taste function test results using EGM,12 taste solution,13 or taste strip test14 was reported. However, this relationship in terms of BMS as an influencing factor using taste function tests allowing both qualitative and quantitative evaluation has not yet been reported.
Thus, this study aimed to investigate the relationship between subjective taste sensations represented in questionnaires and objective taste strip test results in patients with taste disorders, especially to explore differences based on the presence or absence of BMS.
Materials and methods
Subjects
This was a retrospective study in which the electronic medical records of patients who visited the Department of Oral Medicine, Seoul National University Dental Hospital with taste disturbances as a chief complaint from June 2018 to December 2020 were reviewed. The research protocol was reviewed in compliance with the Helsinki Declaration and approved by the Institutional Review Board (IRB) of the Seoul National University Dental Hospital (#ERI21006) on 26 Feb., 2021. The IRB authorized an exemption of informed consent from the subjects.
The inclusion criteria were as follows: patients who complained of taste disturbances as a chief complaint with or without BM symptoms and were 18 years of age or older. The exclusion criteria were as follows: patients who could not answer a questionnaire and/or had communication problems or were pregnant. This study included 51 patients (59.5 ± 14.3 years of age), 11 males (54.8 ± 19.0 years of age) and 40 females (60.7 ± 12.4 years of age).
Assessment of taste disturbances
Examination procedures
All the patients had a radiographic examination with panoramic radiography and answered a questionnaire regarding taste disturbances and BM symptoms. To investigate other factors that could affect taste sensation, the salivary flow rate test, the “Candida detector (Kamemizu Chemical Ind., Osaka, Japan)” test, and the laboratory blood tests were done. The validated taste strip test using “Taste Strips” (Burghart, Wedel, Germany) was conducted to examine the actual taste ability of patients. All patients were interviewed by one doctor (HSK).
Questionnaire
The questionnaire for patients with taste disorders consisted of four parts:15 (1) General information: demographics, treatment history of taste problem, and drinking and smoking habits; (2) Medical history: listing of the nasal problems including allergy, nasal obstruction, rhinorrhea, sinusitis, and postnasal drip, history of otolaryngologic surgery, major illnesses, endocrine problems, mental disorders, and medications within 5 years before developing taste disturbances; (3) Olfactory symptoms: the current smell sensation and nasal problems; and (4) Taste symptoms: the current taste sensation, taste recognition ability, abnormal taste sensations, presence of oral pain, and the suspected causes associated with the occurrence of taste problems.
Additional questions were asked when the patients reported the experience of BM symptoms (Supplementary Table S1).12,16 The patients were asked to answer the intensity of BM symptoms such as burning, aching, stinging, itching, and numbness and were evaluated using a visual analog scale (VAS) ranging from 0 (no symptoms) to 10 (worst severity imaginable). The highest VAS score among them was considered VAS A. To evaluate the triad symptoms related to BMS, the intensities of taste disturbances and xerostomia were also evaluated with VAS and defined as VAS B and VAS C, respectively.
Regarding symptoms of taste disorders, the patients were asked to answer the following statements (Supplementary Table S1).13,15 First, they were asked to choose either “recognize easily (score 2)”, “recognize somewhat (score 1)”, or “recognize not at all (score 0)” to the following statements used in previous studies:12,13,15 “I can detect sweetness in cocoa, cakes, or candies”, “I can detect salt in chips, or salted nuts”, “I can detect sourness in vinegar, pickles, or lemon”, and “I can detect bitterness in coffee, beer, or tonic water”. The patients were then asked to choose types of taste disorders they had from the following statements: “I feel the taste was distorted (dysgeusia)”, “I sensed the taste even though there was nothing in the mouth (phantogeusia)”, “I feel the taste was exaggerated (hypergeusia)”, “I feel the taste was decreased (hypogeusia)”, “I cannot feel the taste totally (ageusia)”, and “I feel the taste normally (normogeusia)”. Based on the answers regarding types of taste disorders, the patients were categorized into subjective hypogeusia and/or dysgeusia. The subjective hypogeusia group included patients who chose hypogeusia. In addition, the subjective dysgeusia group included patients who chose dysgeusia, phantogeusia, or hypergeusia. When patients chose normogeusia only or ageusia only, they were asked again based on their complaints and the answers regarding each taste quality and the types of taste disorder were confirmed.
Clinical and laboratory tests for the contributing factors
To determine factors that may contribute to taste disorders, clinical and laboratory tests were performed. First, whole salivary flow rates at both unstimulated and stimulated conditions were measured.9 The patients were asked to collect unstimulated whole saliva (UWS) in a polypropylene tube by spitting for 10 min. For the stimulated whole saliva (SWS), the patients were asked to chew the paraffin wax for 2 min and swallow the secreted saliva. Then, the flow rate of SWS while chewing the paraffin wax was measured for 5 min. The salivary flow rate was recorded as mL/min. Hyposalivation was considered as a contributing factor when the flow rate of UWS was <0.1 mL/min or <0.7 mL/min for SWS.
The “Candida detector” was used to evaluate the presence of oral candidiasis. After sampling from the tongue dorsum using a swab, the culture medium coated with the sample was incubated at 37 °C for 48 h. The patients were diagnosed with oral candidiasis when the number of colonies exceeded 103 CFU/mL according to the manufacturer's instructions.
For the blood tests, the following parameters were examined to investigate systemic factors that could affect taste sensations:9 complete blood count with leukocyte differential count, erythrocyte sedimentation rate, blood chemistry tests (calcium, phosphorus, blood glucose, and kidney and liver function tests), thyroid function tests, and levels of ferritin, vitamin B12, folate, zinc, and magnesium.
Measurement of taste abilities
The taste function test using “Taste Strips” was conducted to measure the taste ability of the patients.7 Filter papers, with a tip area of 2 cm2 impregnated with taste solutions, were placed on the middle of the tongue. Eighteen taste strips, four concentrations each for sweet, salty, sour, and bitter, and two blanks, were given to each patient in a pseudo-randomized sequence.17 The concentrations used for the taste strips were as follows: sweet: 0.4, 0.2, 0.1, 0.05 g/mL sucrose; salty: 0.25, 0.1, 0.04, 0.016 g/mL sodium chloride; sour: 0.3, 0.165, 0.09, 0.05 g/mL citric acid, and bitter: 0.006, 0.0024, 0.0009, 0.0004 g/mL quinine hydrochloride. After placement of each strip, the patients had to report the taste quality they tasted among sweet, salty, sour, bitter, and no taste, and rinse with water before testing the next taste strip.
Each correct answer was given a score of 1 point. The maximum score was 16 and blanks were not counted for the score. Patients with scores <9 were considered hypogeusia according to the manufacturer's instructions. In addition, patients who had wrong answers at the strongest or second stronger concentration for one or more taste qualities were considered dysgeusia.
Analysis of taste disorder etiologies
Variable factors are potentially associated with taste disorders and the identification of probable etiologies in each patient was complicated procedures due to the complexity of etiological factors and their possible interactions. However, the primary etiology in individual patients had to be estimated by carefully evaluating the obtained data from history taking including the questionnaire, clinical examinations, and laboratory findings. Several guidelines were used to assess etiological factors. First, if the taste problems started in relation to a specific event such as taking medication, stress, or anxiety, the event was presumed to be the primary cause although the patient already had other local or systemic factors including nutrient deficiency or continuously taking medications. Second, BMS was considered the primary cause and psychological factors, hyposalivation, or hyposmia as the secondary cause when the patient complained of BM symptoms not accompanied with any oral mucosal lesions and abnormalities in blood examinations. Third, oral candidiasis was not considered a primary contributing factor when the taste disturbances were not alleviated after antifungal treatments. Fourth, diabetes mellitus was not considered a primary contributing factor when the blood glucose level was controlled with medical treatment or the diagnosis of diabetes did not coincide with the occurrence of taste disturbances. Fifth, when the patient with hyposalivation (flow rate of UWS <0.1 mL/min or SWS <0.7 mL/min) complained of hyposmia, only hyposmia confirmed with olfactory function tests in the otolaryngology department was considered the primary cause instead of hyposalivation. Otherwise, hyposmia was considered the secondary cause. Sixth, the nutrient deficiency based on blood test results was considered a primary factor instead of other factors such as hyposmia and hyposalivation.
All available data from the included patients were carefully reviewed. Finally, patients were divided into two groups to examine the difference based on the presence of BMS: patients with BMS and without BMS. The patients who had BM symptoms such as burning, aching, itching, stinging, or numb sensations without observable intraoral abnormalities, oral candidiasis, and blood test abnormalities were classified as having BMS18 and the other patients were classified as not having BMS, including having the etiologies of psychological problems, nutrient deficiency, medications, and other factors.
Statistical analysis
Because the data were not normally distributed except for age, parametric or non-parametric tests were used appropriately. The Student's t-test or Mann–Whitney U test was used to identify differences between the classified groups. The chi-squared test or Fisher's exact test was used to compare the distribution of variables. In addition, the Spearman's correlation analysis with the Bonferroni's correction was used to examine correlations between variables. P-values < 0.05 were considered statistically significant.
Results
Patient characteristics
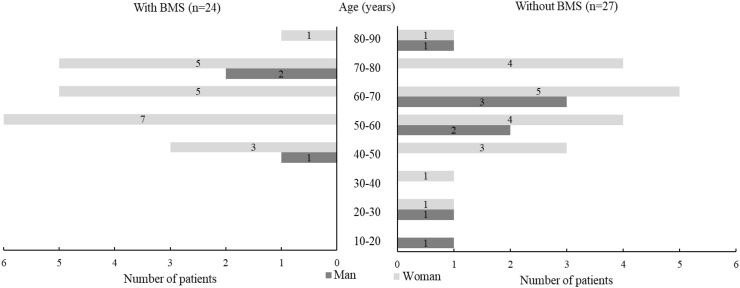
Based on the suggested etiologies, the patients were classified into BMS, psychological problems, nutrient deficiency, medications, and other conditions (including hyposmia, hyposalivation, oral candidiasis, parotitis, inflammation of oral mucosa, surgery of otitis media, and idiopathy; Supplementary Table S2). The patients were then reclassified into two groups to compare the characteristics of taste disturbances based on the presence or absence of BMS: patients with or without BMS. The patients with BMS group consisted of 24 individuals (47.1%, 61.2 ± 11.1 years of age) and included 3 males (12.5%, 60.3 ± 13.7 years of age) and 21 females (87.5%, 61.3 ± 10.6 years of age). The patients without BMS group consisted of 27 individuals (52.9%, 57.9 ± 16.5 years of age) and included 8 males (29.6%, 52.8 ± 20.3 years of age) and 19 females (70.4%, 60.1 ± 14.0 years of age). Significant differences were not observed in age, age distribution, and gender distribution between the groups (P = 0.417, P = 0.664, and P = 0.138, respectively). However, patients with BMS showed a typical age distribution of BMS (83.3% > 50 years of age) and patients without BMS showed a wider age distribution (Fig. 1).
Figure 1.
Age and gender distribution of patients with taste disorders based on the presence of BMS. Statistical significance was not observed in age (P = 0.417), age distribution (P = 0.664), and gender distribution (P = 0.138) between patients with and without BMS. BMS, burning mouth syndrome.
Subjective evaluation of taste disorders
Self-reported taste abilities
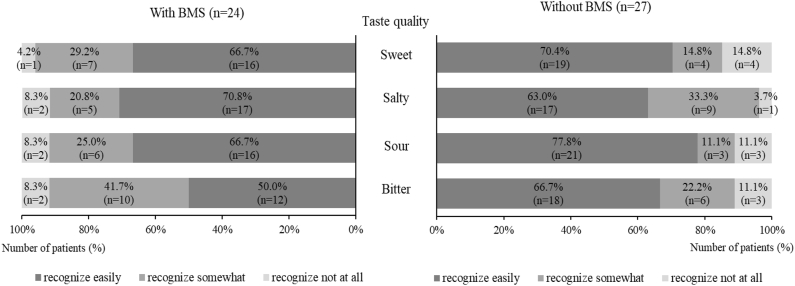
Fig. 2–1 shows the distributions of self-reported scores for the four basic taste qualities based on the presence of BMS. The percentage of patients who answered “not at all recognized” was higher in patients without BMS than with BMS in all taste qualities except saltiness (11.1–14.8% vs. 4.2–8.3%). However, statistical differences were not observed between the two groups in all taste qualities (sweet, P = 0.313; salty, P = 0.567; sour, P = 0.431; bitter, P = 0.378).
Figure 2–1.
Distribution of subjective scores for taste quality in patients with taste disorders based on the presence of BMS. Statistical significance was not observed (P > 0.05) in the distribution of subjective scores for each taste quality between patients with and without BMS.
Regarding mean subjective scores, significant differences were not observed between the two groups in all taste qualities (sweet, P = 1.000; salty, P = 0.740; sour, P = 0.523; bitter, P = 0.363; Table 1).
Table 1.
The mean subjective scores and taste strip test scores for each taste quality based on the presence of BMS.
| Median (IQR) Mean |
With BMS (n = 24) | Without BMS (n = 27) | Significance between the groups (P) | Total (n = 51) | |
|---|---|---|---|---|---|
| Subjective scores | Sweet | 2.0 (1.0–2.0) 1.63 |
2.0 (1.0–2.0) 1.56 |
1.000 | 2.0 (1.0–2.0) 1.59 |
| Salty | 2.0 (1.0–2.0) 1.63 |
2.0 (1.0–2.0) 1.59 |
0.740 | 2.0 (1.0–2.0) 1.61 |
|
| Sour | 2.0 (1.0–2.0) 1.58 |
2.0 (2.0–2.0) 1.67 |
0.523 | 2.0 (1.0–2.0) 1.63 |
|
| Bitter | 1.5 (1.0–2.0) 1.42 |
2.0 (1.0–2.0) 1.56 |
0.363 | 2.0 (1.0–2.0) 1.49 |
|
| Sum | 7.0 (4.3–8.0) 6.25 |
7.0 (5.0–8.0) 6.37 |
0.784 | 7.0 (5.0–8.0) 6.31 |
|
| Taste strip test scores | Sweet | 2.5 (1.0–3.0) 2.29 |
2.0 (1.0–3.0) 2.07 |
0.580 | 2.0 (1.0–3.0) 2.18 |
| Salty | 2.5 (1.0–3.0) 2.25 |
3.0 (2.0–3.0) 2.44 |
0.610 | 3.0 (2.0–3.0) 2.35 |
|
| Sour | 2.0 (0.0–3.0) 1.75 |
1.0 (0.0–3.0) 1.48 |
0.498 | 2.0 (0.0–3.0) 1.61 |
|
| Bitter | 3.0 (2.0–4.0) 2.75 |
2.0 (1.0–3.0) 2.07 |
0.105 | 3.0 (1.0–4.0) 2.39 |
|
| Sum | 10.5 (6.0–12.0) 9.04 |
7.0 (6.0–10.0) 8.07 |
0.276 | 8.0 (6.0–12.0) 8.53 |
|
BMS, burning mouth syndrome.
Subjective scores: self-reported scores of recognition ability for each taste quality with three levels (0 = not at all recognized, 1 = somewhat recognized, and 2 = easily recognized).
Taste strip test scores: the number of correct answers in four concentrations each for sweet, salty, sour, and bitter (0–4).
Sum: the sum of all subjective or taste stirp test scores for the four taste qualities (0–8 and 0–16, respectively).
Statistical significance in the subjective scores and taste strip test scores for each taste quality was not observed between patients with and without BMS (P > 0.05).
All data were presented as the median (interquartile range, IQR) and mean. The Mann–Whitney U test was used.
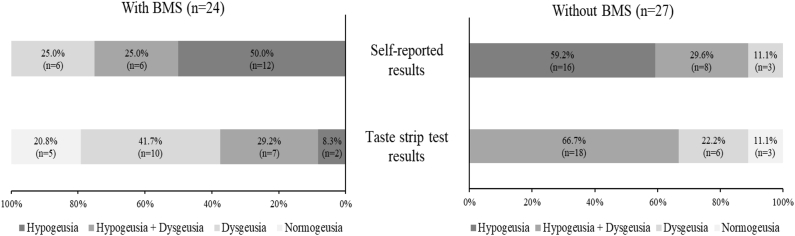
Self-reported types of taste disorders
All subjects were classified as hypogeusia and/or dysgeusia (Fig. 2–2). In both groups, most patients chose hypogeusia only (with BMS; 50.0%, n = 12, without BMS; 59.2%, n = 16), followed by both hypogeusia and dysgeusia, and dysgeusia only. However, significant difference was not observed in the distribution of self-reported types of taste disorders between the two groups (P = 0.441).
Figure 2–2.
The percentages of taste disorder types derived from the self-reported and taste strip test results based on the presence of BMS. The significant difference was observed only in the distribution of objective taste disorder types based on the taste strip test results between the groups (P = 0.034). BMS, burning mouth syndrome.
Taste strip test
Objective taste abilities
Regarding the mean taste strip scores for each taste quality (Table 1), the sum of taste strip scores was on the borderline between normal range and hypogeusia in patients with BMS and was lower than normal range in patients without BMS (the low 10th percentile score in normal healthy population: 9).17 However, statistically significant differences were not observed for any taste qualities.
The percentages of correct answers in the taste strip test are presented in Table 2. The percentage of the correct answer in all taste qualities tended to increase as the tastant concentration increased in both groups. A significant difference in the percentages of correct answers between the two groups was observed only in the second-lowest concentration of bitterness (with BMS, 75.0%; without BMS, 44.4%, P = 0.027).
Table 2.
The percentages of correct answers in the taste strip test for each taste quality based on the presence of BMS.
| Taste quality | Concentration level | With BMS (n = 24) |
Without BMS (n = 27) |
Significance between the groups | ||
|---|---|---|---|---|---|---|
| n | % | n | % | (P) | ||
| Sweet | 4 | 20 | 83.3 | 20 | 74.1 | 0.422 |
| 3 | 16 | 66.7 | 16 | 59.3 | 0.585 | |
| 2 | 13 | 54.2 | 14 | 51.9 | 0.869 | |
| 1 | 5 | 20.8 | 6 | 22.2 | 0.904 | |
| Salty | 4 | 19 | 79.2 | 22 | 81.5 | 0.835 |
| 3 | 13 | 54.2 | 16 | 59.3 | 0.714 | |
| 2 | 13 | 54.2 | 17 | 63.0 | 0.524 | |
| 1 | 9 | 37.5 | 11 | 40.7 | 0.813 | |
| Sour | 4 | 17 | 70.8 | 15 | 55.6 | 0.260 |
| 3 | 14 | 58.3 | 14 | 51.9 | 0.642 | |
| 2 | 8 | 33.3 | 9 | 33.3 | 1.000 | |
| 1 | 3 | 12.5 | 2 | 7.4 | 0.656 | |
| Bitter | 4 | 21 | 87.5 | 21 | 77.8 | 0.473 |
| 3 | 20 | 83.3 | 16 | 59.3 | 0.060 | |
| 2 | 18 | 75.0 | 12 | 44.4 | 0.027∗ | |
| 1 | 7 | 29.2 | 6 | 22.2 | 0.570 | |
| Blank 1 | 21 | 87.5 | 21 | 77.8 | 0.473 | |
| Blank 2 | 21 | 87.5 | 23 | 85.2 | 0.811 | |
BMS, burning mouth syndrome.
Significant difference was observed in the correct answer percentages only in the second-lowest concentration of bitterness between patients with and without BMS.
The chi-squared test or the Fisher's exact test was used.
∗P < 0.05.
Objective types of taste disorders
Most subjects were classified as objective hypogeusia and/or dysgeusia (Fig. 2–2). In the with BMS group, most patients were classified as dysgeusia only (41.7%, n = 10) followed by both hypogeusia and dysgeusia (29.2%, n = 7). Conversely, in the without BMS group, most patients were classified as both hypogeusia and dysgeusia (66.7%, n = 18) followed by dysgeusia only (22.2%, n = 6). Five patients (20.8%) with BMS and 3 patients (11.1%) without BMS showed normal test results (sum scores ≥9 and answered all taste qualities correctly at the strongest or second stronger concentration). The distribution of taste disorder types showed a statistically significant difference based on the presence of BMS (P = 0.034).
Salivary flow rates
The mean flow rate of UWS was 0.34 ± 0.18 mL/min in patients with BMS and 0.26 ± 0.24 mL/min in patients without BMS. The mean flow rate of SWS was 1.19 ± 0.67 mL/min in patients with BMS and 1.23 ± 0.70 mL/min in patients without BMS. The flow rate of SWS could not be measured in 3 patients without BMS who could not chew the paraffin due to denture wearing (n = 2) or temporomandibular disorders (n = 1). Significant differences were not observed in the flow rates of UWS and SWS based on the presence of BMS (P = 0.084 and P = 0.741, respectively) (Supplementary Table S3).
Significant correlations were not observed between both types of salivary flow rates and the subjective taste scores regardless of the presence of BMS. However, a significant correlation was found between the salivary flow rates and the taste strip test scores only in patients without BMS, between the flow rate of UWS and the sweetness score (rs = 0.509, P = 0.007). Conversely, significant correlations were not observed between both types of salivary flow rates and the taste strip test scores in patients with BMS (data not shown). Significant correlations were also not found between both types of salivary flow rates and the VAS scores of triad symptoms associated with BMS (BM symptoms, taste disturbances, and xerostomia) in patients with BMS (data not shown).
Correlations between subjective scores and taste strip test scores
Correlations between the subjective scores and the corresponding taste strip test scores in the patients are shown in Table 3. In patients with BMS, significant correlations were observed in sum scores (rs = 0.516, P = 0.009). However, significant correlations of any taste qualities were not observed in patients without BMS. Most correlation levels between the subjective scores and the objective taste strip test scores in patients with BMS were higher than in patients without BMS (0.158–0.516, 0.152–0.301, respectively). In total, significant correlations were also observed in sum scores (rs = 0.359, P = 0.009).
Table 3.
Correlation coefficients (rs) between subjective scores and taste strip test scores for each taste quality based on the presence of BMS.
| rs (P) | With BMS (n = 24) | Without BMS (n = 27) | Total (n = 51) |
|---|---|---|---|
| Sweet (S-T) | 0.422 (0.040) | 0.152 (0.449) | 0.270 (0.056) |
| Salty (S-T) | 0.366 (0.079) | 0.238 (0.231) | 0.293 (0.037) |
| Sour (S-T) | 0.158 (0.460) | 0.199 (0.320) | 0.159 (0.265) |
| Bitter (S-T) | 0.419 (0.041) | 0.301 (0.128) | 0.321 (0.022) |
| Sum (S-T) | 0.516∗ (0.009) | 0.198 (0.321) | 0.359∗ (0.009) |
BMS, burning mouth syndrome.
S, subjective scores: self-reported scores of recognition ability for each taste quality with three levels (0 = not at all recognized, 1 = somewhat recognized, and 2 = easily recognized).
T, taste strip test scores: the number of correct answers in four concentrations each for sweet, salty, sour, and bitter (0–4).
Sum: the sum of all subjective or taste stirp test scores for the four taste qualities (0–8 and 0–16, respectively).
The Spearman's correlation analysis with the Bonferroni's correction was used.
∗P < 0.01.
Regarding the VAS for the symptom triad (VAS A, the severity of BM symptoms; VAS B, the severity of taste disturbances; VAS C, the severity of xerostomia) in patients with BMS, all VAS scores did not show any significant correlations with the subjective and objective scores for all taste qualities. There were no significant correlations among the VAS A, VAS B, and VAS C, either (data not shown).
Diagnostic usefulness of the questionnaire compared with the taste strip test
The diagnostic usefulness of the questionnaire based on the subjective types of taste disorders was determined (Table 4). The sensitivity of subjective hypogeusia showed the highest value regardless of the presence of BMS (0.89) followed by the specificity of both hypogeusia and dysgeusia (0.71–0.89). The positive predictive values (PPVs) were higher in patients without BMS (0.67–0.91) than with BMS (0.17–0.67) and the highest value (0.91) was for dysgeusia in patients without BMS. Conversely, the negative predictive values (NPVs) were higher in patients with BMS (0.25–0.83) than without BMS (0.13–0.44).
Table 4.
Accuracy of self-reported questionnaire compared with the taste strip test results based on the presence of BMS.
| With BMS (n = 24) |
Without BMS (n = 27) |
Total (n = 51) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Hypogeusia |
Dysgeusia |
Hypogeusia and dysgeusia |
Hypogeusia |
Dysgeusia |
Hypogeusia and dysgeusia |
Hypogeusia |
Dysgeusia |
Hypogeusia and dysgeusia |
|
| (n = 9) | (n = 17) | (n = 7) | (n = 18) | (n = 24) | (n = 18) | (n = 27) | (n = 41) | (n = 25) | |
| Sensitivity | 0.89 | 0.47 | 0.14 | 0.89 | 0.42 | 0.44 | 0.89 | 0.44 | 0.36 |
| Specificity | 0.33 | 0.43 | 0.71 | 0.11 | 0.67 | 0.89 | 0.25 | 0.50 | 0.77 |
| PPV | 0.44 | 0.67 | 0.17 | 0.67 | 0.91 | 0.89 | 0.57 | 0.78 | 0.60 |
| NPV | 0.83 | 0.25 | 0.67 | 0.33 | 0.13 | 0.44 | 0.67 | 0.18 | 0.56 |
BMS, burning mouth syndrome; PPV, positive predictive value; NPV, negative predictive value.
The patients with taste strip test scores <9 were considered hypogeusia.
The patients who had incorrect answers at the strongest or second stronger concentrations for one or more taste qualities were considered dysgeusia.
Hypogeusia group included patients diagnosed as hypogeusia only and both hypogeusia and dysgeusia.
Dysgeusia group included patients diagnosed as dysgeusia only and both hypogeusia and dysgeusia.
Discussion
In the present study, significant differences based on the presence of BMS were observed not in subjective taste sensations but in the objective taste strip test results. The taste strip test results showed that patients with BMS had better taste recognition ability in bitterness and showed different distributions in the types of taste disorders compared with those without BMS. The significant correlations between the subjective and objective scores were observed only in patients with BMS. Subjective judgment of hypogeusia showed relatively high sensitivity values regardless of the presence of BMS. Subjective judgment of dysgeusia had high PPVs only in patients without BMS.
The results of the present study reflected the differences in the etiopathophysiologies of taste disorders based on the presence of BMS. The patients without BMS had various etiologies such as hyposmia, malnutrition, medications, psychological problems, and idiopathies as in previous studies.1,19 However, when accompanied with BMS, neuropathies such as taste–pain interactions, peripheral fiber degeneration, chorda tympani nerve hypofunction, and changes of grey matter concentration were suggested as pathophysiological mechanisms.20, 21, 22 The higher prevalence of dysgeusia in patients with BMS could be explained by these differences.12
The significantly higher correct answer rates in bitterness in patients with BMS compared with patients without BMS support the results of previous studies indicating that dysgeusia of bitterness is common in patients with BMS.10,11 This phenomenon might be associated with the central pathways of bitterness perception mediated with the glossopharyngeal nerve,22 which could be disinhibited by damage of the chorda tympani nerve in patients with BMS.23 Notably, although there was no statistical significance, patients with BMS had more difficulty in recognizing saltiness than patients without BMS. The higher thresholds of saltiness in patients with BMS was suggested in previous studies.22,24,25 Although the results of those studies were limited due to the comparison with healthy controls, the results of the present study could be partially supported by those studies. An animal study26 reporting that increased NaCl thresholds were related with the chorda tympani nerve hypofunction suggested as BMS pathophysiology also supports the results of our study.
The correlation levels between subjective evaluations using questionnaires and objective evaluation using the taste strip test were low in the present study, which is in agreement with previous studies in which the limitations in subjective assessments were reported.12, 13, 14,27 However, the significant correlations between subjective and objective evaluations were observed only in patients with BMS, unlike the results of our previous study in which EGM was used instead of the taste strip test.12 These differences may be due to the different evaluation methods used to test objective taste function. The EGM thresholds only correlated with the perception of saltiness among the four taste qualities,3,4 which were relatively less sensitive in patients with BMS. Furthermore, the correlations between taste function tests, including between EGM and the taste strip test, were weak.3 Therefore, the results from the taste strip test may be more appropriate for exploring the relationship between subjective and objective evaluations rather than EGM because each taste quality can be evaluated in the taste strip test.
The presence of significant correlation between the subjective and objective scores in the sum scores, but not between the severity (VAS B) of taste disturbances and objective scores, showed the importance of mentioning each taste quality rather than a single comprehensive question when evaluating taste function. A significant correlation was not observed between the severities of BM symptoms (VAS A) and taste disturbances (VAS B), and between the severity (VAS A) of BM symptoms and both the subjective and objective taste scores. Due to the pain-taste interactions in BMS pathophysiology,20,24,28 further studies are necessary to investigate this lack of correlations.
The sensitivity, specificity, PPV, and NPV of the questionnaire used in the present study to define subjective types of taste disturbances were generally low. Limitations of questionnaires in detecting taste disturbances have been suggested in previous studies13,27 in which both subjective and objective taste abilities regarding the four basic taste qualities were evaluated. The studies used the ordinal scales for subjective taste ability of each taste quality, however, data transformation into dichotomous variables to calculate sensitivity, specificity, PPV, and NPV resulted in low values and dysgeusia was not considered in those studies. Compared with the previous studies,13,27 higher sensitivity values for hypogeusia were observed in the present study regardless of the presence of BMS. Therefore, the questionnaire might be helpful in detecting patients with hypogeusia. However, appropriate objective taste function tests should be accompanied in the diagnosis because subjective evaluations were relatively inaccurate as evidenced by low PPVs. In addition, further development of the questionnaire, including both qualitative and quantitative assessments, is needed to increase the reliability of the subjective evaluation.
The present study had several limitations. First, this was a retrospective study based on an electronic chart review. Second, limitations existed in accurately identifying the causes of taste disorders in every and each patient due to the complexities of factors associated with taste disturbances. Third, only one type of objective test was used to evaluate taste function. However, valuable results were obtained regarding the relationship between subjective and objective evaluations in patients with taste disorders, especially based on the presence or absence of BMS.
In conclusion, patients with taste disorders with BMS had significant correlations between subjective and objective evaluations and different distributions in the types of taste disorders compared with those without BMS. The presence or absence of BMS should be evaluated in the diagnosis and management of taste disorders due to differences in pathophysiology.
Declaration of competing interest
The authors have no conflicts of interest relevant to this article.
Acknowledgements
This research was supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare (No. HI18C0302) and the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (No. 2019R1A2C1002437).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jds.2022.04.027.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Zang Y., Han P., Burghardt S., Knaapila A., Schriever V., Hummel T. Influence of olfactory dysfunction on the perception of food. Eur Arch Otorhinolaryngol. 2019;276:2811–2817. doi: 10.1007/s00405-019-05558-7. [DOI] [PubMed] [Google Scholar]
- 2.Chiang C.P., Wu Y.H., Wu Y.C., Chang J.Y., Wang Y.P., Sun A. Anemia, hematinic deficiencies, hyperhomocysteinemia, and serum gastric parietal cell antibody positivity in 884 patients with burning mouth syndrome. J Formos Med Assoc. 2020;119:813–820. doi: 10.1016/j.jfma.2019.10.013. [DOI] [PubMed] [Google Scholar]
- 3.Kang M.G., Choi J.H., Kho H.S. Relationships between gustatory function tests. Oral Dis. 2020;26:830–837. doi: 10.1111/odi.13291. [DOI] [PubMed] [Google Scholar]
- 4.Tomita H., Ikeda M. Clinical use of electrogustometry: strengths and limitations. Acta Otolaryngol Suppl. 2002;(546):27–38. doi: 10.1080/00016480260046391. [DOI] [PubMed] [Google Scholar]
- 5.Yamauchi Y., Endo S., Sakai F., Yoshimura I. A new whole-mouth gustatory test procedure. 1. Thresholds and principal components analysis in healthy men and women. Acta Otolaryngol Suppl. 2002;(546):39–48. doi: 10.1080/00016480260046409. [DOI] [PubMed] [Google Scholar]
- 6.Berling K., Knutsson J., Rosenblad A., von Unge M. Evaluation of electrogustometry and the filter paper disc method for taste assessment. Acta Otolaryngol. 2011;131:488–493. doi: 10.3109/00016489.2010.535850. [DOI] [PubMed] [Google Scholar]
- 7.Landis B.N., Welge-Luessen A., Brämerson A., et al. Taste Strips" - a rapid, lateralized, gustatory bedside identification test based on impregnated filter papers. J Neurol. 2009;256:242–248. doi: 10.1007/s00415-009-0088-y. [DOI] [PubMed] [Google Scholar]
- 8.Kim Y., Kim H.I., Kho H.S. Characteristics of men and premenopausal women with burning mouth symptoms: a case-control study. Headache. 2014;54:888–898. doi: 10.1111/head.12338. [DOI] [PubMed] [Google Scholar]
- 9.Kim M.J., Kim J., Kho H.S. Comparison between burning mouth syndrome patients with and without psychological problems. Int J Oral Maxillofac Surg. 2018;47:879–887. doi: 10.1016/j.ijom.2018.02.001. [DOI] [PubMed] [Google Scholar]
- 10.Scala A., Checchi L., Montevecchi M., Marini I., Giamberardino M.A. Update on burning mouth syndrome: overview and patient management. Crit Rev Oral Biol Med. 2003;14:275–291. doi: 10.1177/154411130301400405. [DOI] [PubMed] [Google Scholar]
- 11.Imamura Y., Shinozaki T., Okada-Ogawa A., et al. An updated review on pathophysiology and management of burning mouth syndrome with endocrinological, psychological and neuropathic perspectives. J Oral Rehabil. 2019;46:574–587. doi: 10.1111/joor.12795. [DOI] [PubMed] [Google Scholar]
- 12.Park Y.J., Kim M.J., Kho H.S. Relationships between subjective taste sensations and electrogustometry findings in patients with taste disorders. Int J Oral Maxillofac Surg. 2021;50:522–529. doi: 10.1016/j.ijom.2020.07.007. [DOI] [PubMed] [Google Scholar]
- 13.Soter A., Kim J., Jackman A., Tourbier I., Kaul A., Doty R.L. Accuracy of self-report in detecting taste dysfunction. Laryngoscope. 2008;118:611–617. doi: 10.1097/MLG.0b013e318161e53a. [DOI] [PubMed] [Google Scholar]
- 14.Welge-Lüssen A., Dörig P., Wolfensberger M., Krone F., Hummel T. A study about the frequency of taste disorders. J Neurol. 2011;258:386–392. doi: 10.1007/s00415-010-5763-5. [DOI] [PubMed] [Google Scholar]
- 15.Deems D.A., Doty R.L., Settle R.G., et al. Smell and taste disorders, a study of 750 patients from the university of Pennsylvania smell and taste center. Arch Otolaryngol Head Neck Surg. 1991;117:519–528. doi: 10.1001/archotol.1991.01870170065015. [DOI] [PubMed] [Google Scholar]
- 16.Kim M.J., Kim J., Kho H.S. Treatment outcomes and related clinical characteristics in patients with burning mouth syndrome. Oral Dis. 2021;27:1507–1518. doi: 10.1111/odi.13693. [DOI] [PubMed] [Google Scholar]
- 17.Mueller C., Kallert S., Renner B., et al. Quantitative assessment of gustatory function in a clinical context using impregnated "taste strips. Rhinology. 2003;41:2–6. [PubMed] [Google Scholar]
- 18.Headache Classification Committee of the International Headache Society (IHS) The international classification of headache disorders. Cephalalgia. 2018;38:1–211. doi: 10.1177/0333102417738202. [DOI] [PubMed] [Google Scholar]
- 19.Henkin R.I., Levy L.M., Fordyce A. Taste and smell function in chronic disease: a review of clinical and biochemical evaluations of taste and smell dysfunction in over 5000 patients at the Taste and Smell Clinic in Washington, DC. Am J Otolaryngol. 2013;34:477–489. doi: 10.1016/j.amjoto.2013.04.006. [DOI] [PubMed] [Google Scholar]
- 20.Kolkka-Palomaa M., Jääskeläinen S.K., Laine M.A., Teerijoki-Oksa T., Sandell M., Forssell H. Pathophysiology of primary burning mouth syndrome with special focus on taste dysfunction: a review. Oral Dis. 2015;21:937–948. doi: 10.1111/odi.12345. [DOI] [PubMed] [Google Scholar]
- 21.Sinding C., Gransjøen A.M., Schlumberger G., Grushka M., Frasnelli J., Singh P.B. Grey matter changes of the pain matrix in patients with burning mouth syndrome. Eur J Neurosci. 2016;43:997–1005. doi: 10.1111/ejn.13156. [DOI] [PubMed] [Google Scholar]
- 22.Siviero M., Teixeira M.J., de Siqueira J.T., Siqueira S.R. Somesthetic, gustatory, olfactory function and salivary flow in patients with neuropathic trigeminal pain. Oral Dis. 2010;16:482–487. doi: 10.1111/j.1601-0825.2010.01660.x. [DOI] [PubMed] [Google Scholar]
- 23.Nasri-Heir C., Gomes J., Heir G.M., et al. The role of sensory input of the chorda tympani nerve and the number of fungiform papillae in burning mouth syndrome. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2011;112:65–72. doi: 10.1016/j.tripleo.2011.02.035. [DOI] [PubMed] [Google Scholar]
- 24.Formaker B.K., Frank M.E. Taste function in patients with oral burning. Chem Senses. 2000;25:575–581. doi: 10.1093/chemse/25.5.575. [DOI] [PubMed] [Google Scholar]
- 25.Siviero M., Teixeira M.J., Siqueira J.T., Siqueira S.R. Central mechanisms in burning mouth syndrome involving the olfactory nerve: a preliminary study. Clinics. 2011;66:509–512. doi: 10.1590/S1807-59322011000300026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Golden G.J., Ishiwatari Y., Theodorides M.L., Bachmanov A.A. Effect of chorda tympani nerve transection on salt taste perception in mice. Chem Senses. 2011;36:811–819. doi: 10.1093/chemse/bjr056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gent J.F., Goodspeed R.B., Zagraniski R.T., Catalanotto F.A. Taste and smell problems: validation of questions for the clinical history. Yale J Biol Med. 1987;60:27–35. [PMC free article] [PubMed] [Google Scholar]
- 28.Su N., Poon R., Liu C., Dewan C., Darling M., Grushka M. Pain reduction in burning mouth syndrome (BMS) may be associated with selective improvement of taste: a retrospective study. Oral Surg Oral Med Oral Pathol Oral Radiol. 2020;129:461–467. doi: 10.1016/j.oooo.2020.02.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.