Abstract
Background/purpose
Several brands of calcium silicate-based cements (CSBCs) are currently marketed. Here we compared physicochemical and biological properties of new products Ortho MTA (BioMTA), Retro MTA (BioMTA), and EZ-Seal (Ezekiel) to widely used ProRoot MTA (Dentsply Tulsa).
Materials and methods
CSBCs were analyzed by X-ray diffractometry and examined by scanning electron microscopy. Elemental composition was determined by energy dispersive spectroscopy. Particle size was measured by particle size analyzer. Human stem cells from apical papilla (SCAPs) were incubated with eluates from CSBCs. Survival of SCAP cells was evaluated with MTT assay. The Alizarin red S stain was used to identify calcified nodules formed in SCAP cultures. The effects of CSBC eluates on SCAP proliferation and migration were examined using an in-vitro scratch “wound-healing” assay.
Results
All CSBC specimens showed similar X-ray diffraction patterns. The average particle size of EZ-Seal was smaller than ProRoot MTA, Ortho MTA, and Retro MTA (P < 0.001). The least cytotoxicity of eluates was found for EZ-Seal. In the Alizarin red S staining test, calcified nodules were observed in cultures with ProRoot MTA, Ortho MTA, and Retro MTA, however, no calcified nodules were observed in cultures with EZ-Seal. SCAP proliferation and migration capacity in presence of EZ-Seal was higher than with ProRoot MTA, Ortho MTA, and Retro MTA (P < 0.001).
Conclusion
EZ-Seal has a smaller average particle size and a better cytocompatibility than all other examined CSBCs.
Keywords: Calcium silicate-based endodontic cement, Mineral trioxide aggregate, Physicochemical properties, Biocompatibility, in vitro “wound-healing” assay
Introduction
Mineral Trioxide Aggregate (MTA) has been successfully used as material for endodontic applications such as perforation repair, apical microsurgery, and vital pulp therapy.1,2 MTA is currently also a main material of choice for barriers placed in regenerative endodontic procedures.3,4 MTA is biocompatible and has the potential to induce differentiation of dental pulp stem cells into odontoblast-like cells and promote formation of hard tissues.5,6
Recently, new calcium silicate based endodontic cements (CSBCs) have emerged as MTA alternatives.7,8 These products appear promising as they have been shown to overcome some shortcomings of MTA, such as long setting time, tooth discoloration and handling difficulty.9, 10, 11, 12 However, physicochemical and biological characteristics of these materials have not been comprehensively studied.
Previous publications on CSBCs have been focused on biological aspects of this material class,5,6,13 while other studies separately reported on their physicochemical properties.11,14,15 It should be considered, however, that the biological performance of CSBCs may be related to their physicochemical characteristics.
Among new CSBC products, Ortho MTA™ (BioMTA, Seoul, Korea) was reported to have no effect on tooth shade because of its very low heavy metal content.16,17 Retro MTA™ (BioMTA) was shown to have very short setting time that improved its clinical performance,13 while EZ-Seal™ (Ezekiel, Taean-gun, Chungcheongnam-do, Korea) was proclaimed to have improved handling characteristics because of addition of a natural polymer.8
To the best of our knowledge, no such studies have been published that would examine the newly launched CSBCs, such as Ortho MTA™, Retro MTA™ and EZ-Seal™.
The goal of this study was to investigate both the physicochemical and biological characteristics of the newly launched CSBCs, while comparing them to properties of widely used ProRoot MTA™ (Dentsply, Tulsa Dental, Tulsa, OK, USA).1,2,6,12,16, 17, 18, 19, 20, 21
Materials and methods
Four CSBCs were selected for this study: ProRoot MTA™, Ortho MTA™, Retro MTA™ and EZ-seal™.
Physicochemical characteristics
X-ray diffractometry (XRD)
Powder X-ray diffraction analyzer (Model Ultima IV, Rigaku, Woodlands, TX, USA) was used to obtain information on a degree of crystallinity in the samples. XRD was performed on the powder state of four CSBCs. Samples were scanned at 40 kV and 40 mA, with a scan speed of 3°/min over the 2θ range of 2–40°.
Scanning electron microscopy (SEM) and energy dispersive spectroscopy (EDS)
The elemental composition of four CSBCs was determined using SEM (Tescan VEGA II LSU, Tescan Orsay Holding, Brno-Kohoutovice, Czech Republic), which was coupled to EDS (Quantax 200; Bruker AXS, Madison, WI, USA). Samples were sputter-coated with platinum (9 nm) using a turbomolecular pumped coater Quorum Q150T ES (Quorum Technologies, Laughton, UK).
Particle size analysis
For particle size analysis, a particle size analyzer (Microtrac FLEX, Microtrac, Largo, FL, USA) was used. Four CSBCs were dispersed in water by sonication and analyzed using a light-scattering method.
Biological characteristics
Isolation of cells from apical papilla (SCAPs) and preparation of CSBC specimens
This study was approved by the Institutional Review Board of the University (processes #16–128 and #18–35). The SCAPs were obtained from human premolars extracted as part of orthodontic treatment. Informed consent was signed by the patient. SCAPs were cultured in sterile α-MEM (Thermofisher Scientific, Waltham, MA, USA) supplemented with 1% glutamine, 1% antibiotics and 10% human serum. Cultured SCAPs were harvested by 0.25% trypsin at passage 3 or 7.
Polypropylene (Thermofisher Scientific) cylindrical molds were prepared with a diameter of 6 mm and height of 2 mm and were disinfected by exposure to UV light for 30min. CSBCs were mixed according to manufacturer's instructions and allowed to set for 24 h. Each disc was placed in a separate well of a 24-well plate and immersed in a fresh growth medium for 24 h at 37 °C. The extraction was performed in general accordance with ISO 10993-12.22
Cytotoxicity evaluation
Cytotoxicity of four CSBC eluates was determined using MTT (3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyltetrazolium Bromide) assays.22 The SCAPs were seeded at a density of 1x104 cells per well in 24-well plates in the growth culture medium. The growth medium was removed, and the cells were exposed to the CSBC eluates (500 μl/well) for 24 h. Dilutions of 1:1, 1:2, 1:4, and 1:8 were prepared by addition of a fresh growth medium. SCAP cultures incubated in the culture medium without eluate served as negative controls. After 24 h, MTT solution was added, removed after 4 h, and 20% dimethyl sulfoxide solution (DMSO; Sigma–Aldrich, St Louis, MO, USA) was added. Absorbance was measured at 540 nm using a VersaMax Microplate Reader (Molecular Devices, Sunnyvale, CA, USA). The MTT assay was performed in triplicate. Proportion of surviving cells after exposure to eluate's dilution was ascertained as ratio of eluate's reading divided by reading for a negative control.
Alizarin red S staining
SCAPs were plated in 12-well plates. The osteogenic culture medium was used (StemPro®, Gibco, Waltham, MA, USA) with osteogenesis supplement (StemPro® osteogenesis supplement, Gibco, Waltham, MA, USA). For 21 days, the osteogenic culture medium containing one of the four types of CSBC eluates (dilution 1:8) was replaced every other day. Osteogenic culture medium without any CSBC eluate served as a negative control. After 21 days of incubation, the medium was aspirated, the wells were washed with PBS, and the cells were fixed with 4% paraformaldehyde. Fixed cells were stained with 40 mM Alizarin red S (ScienceCell, Calsbad, CA, USA) for 10 min. Each well was photographed under a microscope equipped with a digital camera.
In vitro scratch “wound-healing” assay
The effects of CSBC eluates on SCAP migration were examined using an in-vitro scratch “wound-healing” assay by the method used previously in this lab.23 SCAPs were seeded into 24-well plates. A simulated wound was inflicted by making a scratch across the monolayer of cells using a sterile plastic pipette tip. Subsequently, cells were washed with PBS to remove cell debris and incubated with CSBC eluates for up to 24 h to allow cell migration back into the cell-free area. Photographs of the scratch area were taken, Image J (NIH, Bethesda, MD, USA) was used to quantify areas covered with migrated cells. Cell-occupied area was measured in pixels.
Statistical evaluation
The average particle sizes of four CSBCs were compared with Kruskal Wallis and Mann–Whitney U tests with Bonferroni's correction. Two-way ANOVA with Tukey's test was used to compare survival of SCAPs exposed to four kinds of CSBC eluates. One-way ANOVA followed by Tukey's test was performed to compare migration of SCAPs into cell-free areas in presence of four kinds of CSBS eluates diluted 1:8. IBM SPSS statistics 25 software (IBM Corp, Armonk, NY, USA) was used for statistical analysis.
Results
Physicochemical characteristics
X-ray diffractometry
XRD analysis showed similar diffraction patterns for all specimens examined (Fig. 1). The highest peaks were shown near 2θ of 27.5° in both ProRoot MTA and Ortho MTA. Retro MTA and EZ-seal showed similar patterns, highest peaks appeared near 28°.
Figure 1.
XRD pattern of ProRoot MTA (A), Ortho MTA (B), Retro MTA (C), and EZ-Seal (D). Note main peak near 28° for all materials.
Scanning electron microscopy and energy dispersive spectroscopy analysis
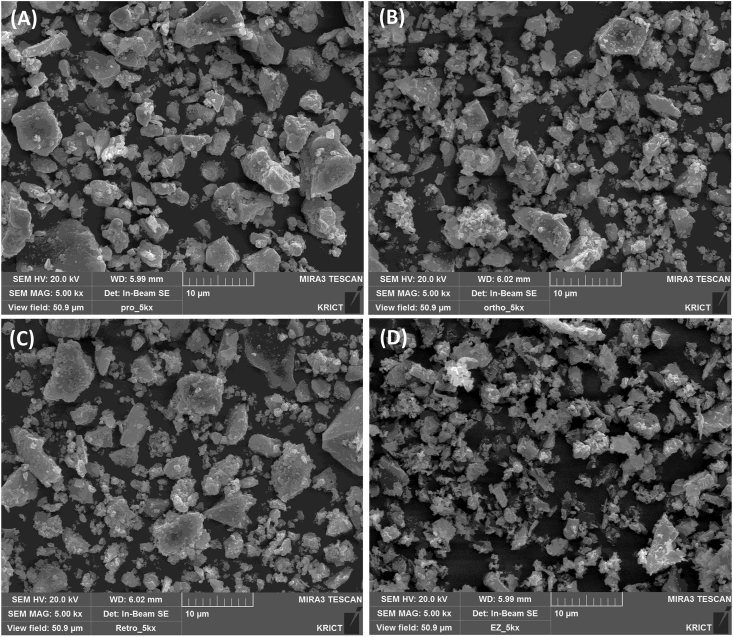
ProRoot MTA, Ortho MTA, and Retro MTA showed multiple aggregates of polygonal particles with various sizes (less than 1 μm to more than 30 μm). The EZ-Seal demonstrated more uniform and smaller particle size than other three kinds of CSBCs (Fig. 2).
Figure 2.
Representative SEM images of ProRoot MTA (A), Ortho MTA (B), Retro MTA (C), and EZ-Seal (D). ProRoot MTA (A), Ortho MTA (B), Retro MTA (C) have polygonal particles with various sizes. EZ-Seal (D) consists of smaller and more uniform particles. Original magnification ×5000.
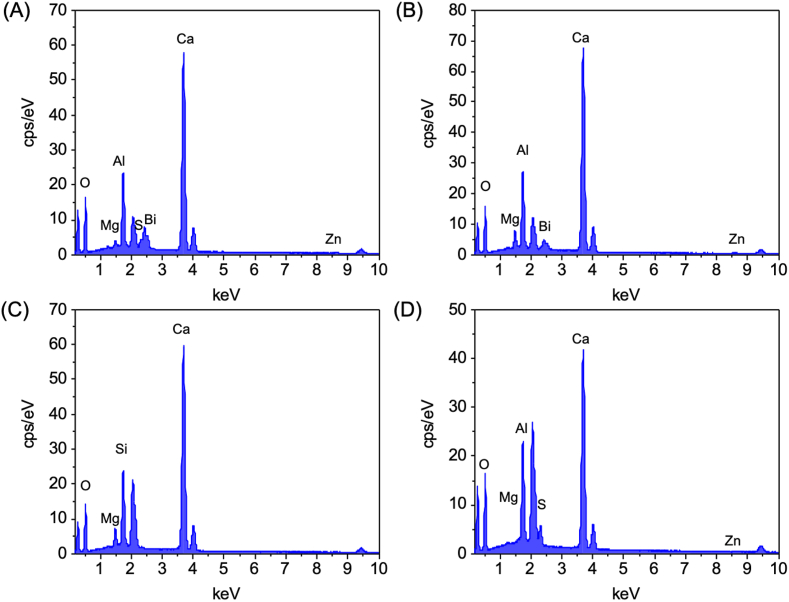
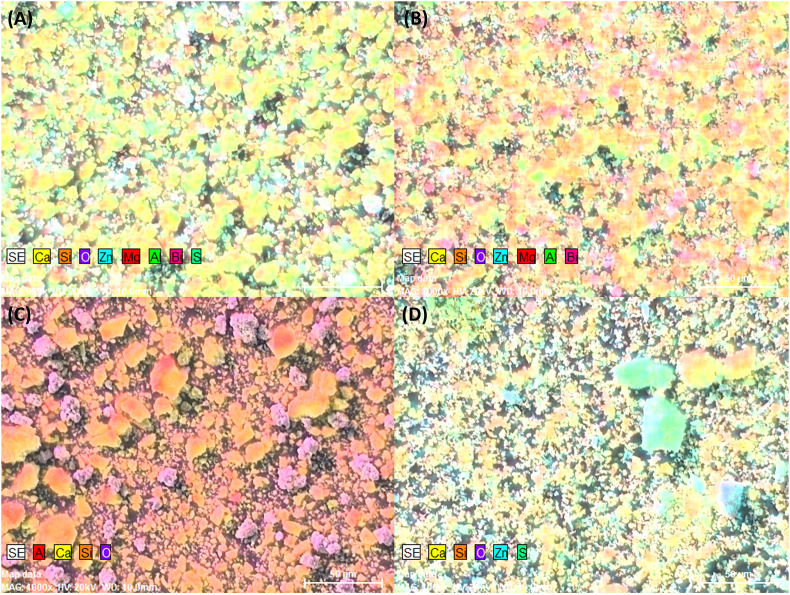
EDS showed that all four CSBCs were mainly composed of calcium, silica, and oxide. Additionally, ProRoot MTA contained Bi, Zn, Al, Mg, S. Ortho MTA contained Bi, Zn, Al, Mg. Retro MTA contained Al, and EZ-Seal contained Zn and S (Figure 3, Figure 4).
Figure 3.
EDS analysis shows elemental composition of ProRoot MTA (A), Ortho MTA (B), Retro MTA (C), and EZ-Seal (D).
Figure 4.
EDS mapping of ProRoot MTA (A), Ortho MTA (B), Retro MTA (C), and EZ-Seal (D). Four calcium silicate-based cements commonly contained Ca, Si, and O. In addition, ProRoot MTA (A) contained Bi, Zn, Al, Mg, S.; Ortho MTA (B) contained Bi, Zn, Al, Mg.; Retro MTA (C) contained Al.; EZ-Seal (D) contained Zn and S.
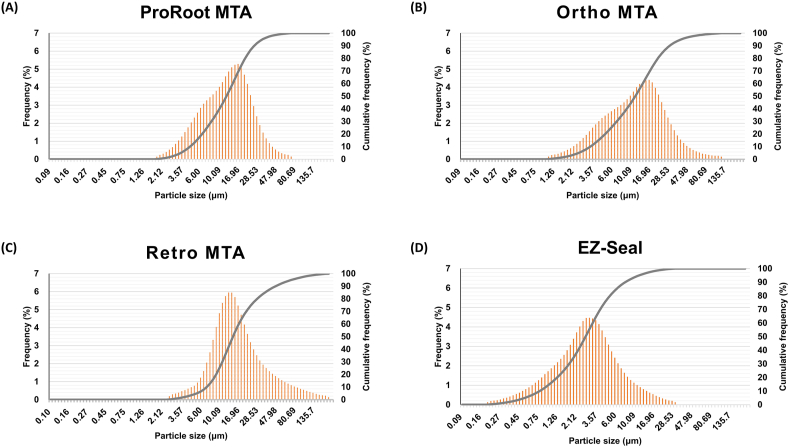
Particle size analysis
Cumulative percentages of particles less than 10 μm were 35.69%, 40.36%, 20.10%, and 92.3% for ProRoot MTA, Ortho MTA, Retro MTA, and EZ-Seal, respectively. Particle diameter ranged 1.783–73.99 μm, 1.06–124.4 μm, 2.521–209.3 μm, and 0.187–31.11 μm for ProRoot MTA, Ortho MTA, Retro MTA, and EZ-Seal, respectively (Fig. 5). The peak values of the particle size distribution were at 12.31 μm, 11.59 μm 14.72 μm and 2.696 μm, respectively. The average particle size of EZ-seal was smaller than ProRoot MTA, Ortho MTA, and Retro MTA (P < 0.001). There were no significant differences among ProRoot MTA, Ortho MTA, and Retro MTA.
Figure 5.
Particle size distribution of ProRoot MTA (A), Ortho MTA (B), Retro MTA (C), and EZ-Seal (D). Shown are histograms and cumulative function.
Biological characteristics
Cytotoxicity evaluation
There was no significant difference in cell survival among four CSBCs when SCAPs were exposed to 1:1 dilution of eluates. Overall, cell survival increased as concentrations of eluates decreased.
After 24 h incubation in 1:2, 1:4, and 1:8 eluate dilutions, EZ-Seal presented with a superior SCAP cell survival in comparison with ProRoot MTA (1:2 dilution P = 0.01, 1:4 dilution P = 0.009, and 1:8 dilution P = 0.02). Cell survival in media containing eluates of ProRoot MTA, Ortho MTA and Retro MTA did not significantly vary in respect to dilutions of eluates (Fig. 6).
Figure 6.
Survival of SCAPs after 24-h incubation with eluates of ProRoot MTA, Ortho MTA, Retro MTA, and EZ-Seal when compared to control. The results show mean ± standard deviation of three parallel experiments performed in triplicate. Different letters represent significant differences between groups in each dilution of eluate. Analysis of variance followed by Tukey's post hoc test (P < 0.05).
Alizarin red S staining
No calcified nodules were formed in control conditions (Fig. 7A) and cell cultures with EZ-Seal (Fig. 7E). In the presence of ProRoot MTA (Fig. 7B), Ortho MTA (Fig. 7C), and Retro MTA (Fig. 7D) calcified nodules were produced.
Figure 7.
Representative images of calcium nodule formation (stained by Alizarin red S) in SCAP control cultures or in cultures exposed to CSBC eluates for 21 days. (A) Control, (B) ProRoot MTA, (C) Ortho MTA, (D) Retro MTA, and (E) EZ-Seal. Original magnification ×400.
In vitro scratch “wound-healing” assay
Areas covered by migrated cells at 12 h cultures with ProRoot MTA, Ortho MTA, and Retro MTA eluates (dilution 1:8) were not different from that of control, while cultures with EZ-seal eluate showed a significantly greater migrated cell area than with ProRoot MTA eluate (P = 0.044) (Fig. 8). Twenty-four hours after the scratch, cultures with EZ-seal eluate showed even greater migrated cell area than in control cultures (P < 0.001), and in cultures with eluates from ProRoot MTA (P < 0.001), Ortho MTA (P < 0.001), and Retro MTA (P < 0.001) (Fig. 8).
Figure 8.
Cell migration ability (%) according to in vitro scratch “wound healing” assay. Different letters represent significant difference between groups in each incubation period. Analysis of variance followed by Tuckey's post hoc test (P < 0.05).
Discussion
The aim of this study was to correlate physicochemical properties of four CSBCs with biologic responses of SCAP cells exposed to CSBC eluates. Physical properties were different among the tested CSBCs, the EZ-seal mostly consists of small particles. According to Wei et al.,24 composite material with nano-sized calcium silicate (0.1–0.35 μm) demonstrated lower contact angles, i.e., greater hydrophilicity compared to composite with micro-sized calcium silicate (20–50 μm particle size). In that study,24 cell attachment as well as proliferation on the composite with nano-sized calcium silicate were superior, which could be attributed to the more hydrophilic surface of nano-sized calcium silicate. In the present study, the particle size of EZ-Seal ranged from 0.187 to 31.11 μm, and most particles (92.3%) were less than 10 μm in size. Conversely, for ProRoot MTA, Ortho MTA and Retro MTA, particles over 10 μm accounted for more than half of each cement's particles (Fig. 5). SCAPs cultured with EZ-Seal presented superior cell viability than with ProRoot MTA (Fig. 6). Likewise, cell migration areas in wound healing assay were the largest in the EZ-Seal group. (Fig. 8).
It may be speculated that small particle size of EZ-Seal might have facilitated rapid hydration of the EZ-Seal cement with water during storage in the medium and therefore, resulted in a more intense release of calcium ions.24,25 Further studies are recommended on the ionic concentration in the eluates of the CSBCs and whether the particle size affect the physical properties such as setting reaction, flow, and film thickness.
Both ProRoot MTA and Ortho MTA exhibited a sharp peak at 2θ of 27.5° representing tricalcium silicate. However, ProRoot MTA had small amount of metal content such as magnesium, zinc, and aluminum. ProRoot MTA, Ortho MTA, and Retro MTA had aluminum, while EZ-Seal did not. EZ-Seal contained calcium, silica, oxide, zinc, and sulfur. For Retro MTA and EZ-Seal, zirconium oxide was used as radiopacifying agent. A peak for zirconium oxide was identified from XRD analysis, consistent with XRD result of Biodentine (Septodont) which contained zirconium oxide.26 Bismuth oxide has been added to ProRoot MTA and Ortho MTA to improve the radiopacity of CSCBs. XRD results demonstrated that peaks for Bismuth Oxide were observed at 2θ = 27.2° and 33° (Fig. 1), according to previous studies.27,28 Kim et al. reported that bismuth oxide reduced cell viability up to 24 h, but not from 48 h, at which time a similar cell viability to pure Portland cement was noted.29 Potentially water-soluble metal salts contributed to cytotoxicity of eluates,30 and this fact may be one of the reasons that for example bismuth oxide has been replaced with zirconium oxide as radiopacifier. It is possible that various cement components get into the eluate, bind or do not bind to serum proteins, and adhere to the plastic surface of the dish, thus changing its topography and resembling the microenvironment around the applied cement.30,31 Future studies need to focus on analysis of eluate's components and their modes of biological effects on a grown SCAP monolayer.
In our SCAP cultures, Ortho MTA, Retro MTA, and EZ-Seal eluates were less cytotoxic than ProRoot MTA after 24 h of incubation. Several previous studies have revealed excellent biocompatibility of Ortho MTA and Retro MTA.32, 33, 34, 35 There has been no cell culture-based study has on cytotoxicity of EZ-Seal. EZ-Seal exhibited superior cell survival in presence of eluates diluted more than 1:1 after 24 h in culture. While cell viability at the 1:1 dilution of EZ-Seal eluate was comparable to other CSBCs. It is speculated that cytotoxic components in the eluate of EZ-Seal are less concentrated than in other CSBCs. A water-soluble polymer, contained in EZ-Seal to improve handling characteristics, did not interfere with cytocompatibility of EZ-Seal.
Proliferation and migration of cells is important for repair of wounded tissue.23,36 To address this issue, a scratch “wound-healing” assay was used in our study to functionally evaluate cytotoxicity of the most diluted CSBC eluates (dilution 1:8) on proliferation and migration of cultured SCAPs. SCAPs exposed to ProRoot MTA, Ortho MTA, and Retro MTA eluates proliferated similarly to the control. However, in presence of EZ-Seal eluate, cells proliferated more rapidly. This finding is interesting and has not been reported before. EZ-Seal may contain both inhibitory and stimulatory components, and the function of the inhibitory component may have been weakened in 1:8 dilution. A future study is needed on identification and quantification of components of CSBCs that modulate cell proliferation.
Alizarin red S was used to detect calcified nodules in SCAP cultures. Calcified nodules were formed in cultures with ProRoot MTA, Ortho MTA, and Retro MTA eluates. When applied as a pulp capping agent, a dentine bridge was formed four weeks after placing ProRoot MTA in a rat study.37 Four weeks after partial pulpotomy in a dog, complete calcified barrier formation was observed in a half of cases with Retro MTA and in 69% of cases with ProRoot MTA.38 When partial pulpotomy was performed in human teeth, 55% of cases with ProRoot MTA showed a complete bridge formation with normal pulp morphology, while 55% of cases with Retro MTA presented discontinuous bridge formation with pulp disorganization.39 According to Kang et al.,40 ProRoot MTA and Ortho MTA induced complete calcific barrier formation with less pulpal inflammation after pulpotomy in a dog. Development of calcified nodules may be related to cytotoxicity of eluates; indeed, inhibition of proliferation may increase probability of osteogenic differentiation and formation of calcified nodules.
In this study differences in some physicochemical and biological characteristics of three newly developed CSBCs (Ortho MTA, Retro MTA and EZ-Seal) were studied and compared to ProRoot MTA. EZ-Seal showed the smallest average particle size, and stimulated SCAP proliferation and migration, decreased potential to formation of calcified nodules when compared to ProRoot MTA, Ortho MTA, and Retro MTA eluates. EZ-Seal might be a promising new material for indications such as vital pulp therapy. These findings need to be confirmed by a clinical study.
Collectively, the four investigated CSBCs, Ortho MTA™, Retro MTA™, ProRoot MTA™ and EZ-Seal™, are similar in material composition but different in particle size. The smaller mean particles are found for EZ-SealTM, which in turn has less mineral production but higher migration potential. Under the conditions of this in vitro study, biologic responses of CSBCs can be connected to differences in physical properties.
Declaration of competing interest
The authors have no conflicts of interest relevant to this study.
Acknowledgements
Funding via the University of the Pacific Research program is gratefully acknowledged.
References
- 1.Torabinejad M., Parirokh M., Dummer P.M.H. Mineral trioxide aggregate and other bioactive endodontic cements: an updated overview - part II: other clinical applications and complications. Int Endod J. 2018;51:284–317. doi: 10.1111/iej.12843. [DOI] [PubMed] [Google Scholar]
- 2.Parirokh M., Torabinejad M., Dummer P.M.H. Mineral trioxide aggregate and other bioactive endodontic cements: an updated overview - part I: vital pulp therapy. Int Endod J. 2018;51:177–205. doi: 10.1111/iej.12841. [DOI] [PubMed] [Google Scholar]
- 3.Silujjai J., Linsuwanont P. Treatment outcomes of apexification or revascularization in nonvital immature permanent teeth: a retrospective study. J Endod. 2017;43:238–245. doi: 10.1016/j.joen.2016.10.030. [DOI] [PubMed] [Google Scholar]
- 4.Aly M.M., Taha S.E.E., El Sayed M.A., Youssef R., Omar H.M. Clinical and radiographic evaluation of Biodentine and mineral trioxide aggregate in revascularization of non-vital immature permanent anterior teeth (randomized clinical study) Int J Paediatr Dent. 2019;29:464–473. doi: 10.1111/ipd.12474. [DOI] [PubMed] [Google Scholar]
- 5.Chang S.W., Lee S.Y., Ann H.J., Kum K.Y., Kim E.C. Effects of calcium silicate endodontic cements on biocompatibility and mineralization-inducing potentials in human dental pulp cells. J Endod. 2014;40:1194–1200. doi: 10.1016/j.joen.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 6.Chang S.W., Lee S.Y., Kum K.Y., Kim E.C. Effects of ProRoot MTA, Bioaggregate, and Micromega MTA on odontoblastic differentiation in human dental pulp cells. J Endod. 2014;40:113–118. doi: 10.1016/j.joen.2013.09.036. [DOI] [PubMed] [Google Scholar]
- 7.BioMTA. Ortho MTA. 2017. http://www.biomta.com/shop/eng/product_1.php [Google Scholar]
- 8.Ez-Seal Ezekiel. 2017. http://www.ezekiel.co.kr/03_ezseal/ezseal/html
- 9.Nandini S., Ballal S., Kandaswamy D. Influence of glass-ionomer cement on the interface and setting reaction of mineral trioxide aggregate when used as a furcal repair material using laser Raman spectroscopic analysis. J Endod. 2007;33:167–172. doi: 10.1016/j.joen.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 10.Chang K.C., Chang C.C., Huang Y.C., Chen M.H., Lin F.H., Lin C.P. Effect of tricalcium aluminate on the physicochemical properties, bioactivity, and biocompatibility of partially stabilized cements. PLoS One. 2014;9 doi: 10.1371/journal.pone.0106754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chang S.W. Chemical characteristics of mineral trioxide aggregate and its hydration reaction. Restor Dent Endod. 2012;37:188–193. doi: 10.5395/rde.2012.37.4.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dawood A.E., Parashos P., Wong R.H.K., Reynolds E.C., Manton D.J. Calcium silicate-based cements: composition, properties, and clinical applications. J Investig Clin Dent. 2017;8 doi: 10.1111/jicd.12195. [DOI] [PubMed] [Google Scholar]
- 13.Chung C.J., Kim E., Song M., Park J.W., Shin S.J. Effects of two fast-setting calcium-silicate cements on cell viability and angiogenic factor release in human pulp-derived cells. Odontology. 2016;104:143–151. doi: 10.1007/s10266-015-0194-5. [DOI] [PubMed] [Google Scholar]
- 14.Chang S.W., Shon W.J., Lee W., Kum K.Y., Baek S.H., Bae K.S. Analysis of heavy metal contents in gray and white MTA and 2 kinds of portland cement: a preliminary study. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010;109:642–646. doi: 10.1016/j.tripleo.2009.12.017. [DOI] [PubMed] [Google Scholar]
- 15.Ha W.N., Kahler B., Walsh L.J. The influence of particle size and curing conditions on testing mineral trioxide aggregate cement. Acta Biomater Odontol Scand. 2016;2:130–137. doi: 10.1080/23337931.2016.1239181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kum K.Y., Zhu Q., Safavi K., Gu Y., Bae K.S., Chang S.W. Analysis of six heavy metals in Ortho mineral trioxide aggregate and ProRoot mineral trioxide aggregate by inductively coupled plasma-optical emission spectrometry. Aust Endod J. 2013;39:126–130. doi: 10.1111/j.1747-4477.2012.00349.x. [DOI] [PubMed] [Google Scholar]
- 17.Chang S.W., Baek S.H., Yang H.C., et al. Heavy metal analysis of ortho MTA and ProRoot MTA. J Endod. 2011;37:1673–1676. doi: 10.1016/j.joen.2011.08.020. [DOI] [PubMed] [Google Scholar]
- 18.Faraco I.M., Jr., Holland R. Response of the pulp of dogs to capping with mineral trioxide aggregate or a calcium hydroxide cement. Dent Traumatol. 2001;17:163–166. doi: 10.1034/j.1600-9657.2001.170405.x. [DOI] [PubMed] [Google Scholar]
- 19.Torabinejad M., Hong C.U., Pitt Ford T.R., Kaiyawasam S.P. Tissue reaction to implanted super-EBA and mineral trioxide aggregate in the mandible of Guinea pigs: a preliminary report. J Endod. 1995;21:569–571. doi: 10.1016/s0099-2399(06)80987-8. [DOI] [PubMed] [Google Scholar]
- 20.Song J.S., Mante F.K., Romanow W.J., Kim S. Chemical analysis of powder and set forms of portland cement, gray ProRoot MTA, white ProRoot MTA, and gray MTA-angelus. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;102:809–815. doi: 10.1016/j.tripleo.2005.11.034. [DOI] [PubMed] [Google Scholar]
- 21.Comin-Chiaramonti L., Cavalleri G., Sbaizero O., Comin-Chiaramonti P. Crystallochemical comparison between portland cements and mineral trioxide aggregate (MTA) J Appl Biomater Biomech. 2009;7:171–178. [PubMed] [Google Scholar]
- 22.Gaudin A., Tolar M., Peters O.A. Cytokine production and cytotoxicity of calcium silicate-based sealers in 2- and 3-dimensional cell culture models. J Endod. 2020;46:818–826. doi: 10.1016/j.joen.2020.03.011. [DOI] [PubMed] [Google Scholar]
- 23.Gaudin A., Tolar M., Peters O.A. Lipoxin A(4) attenuates the inflammatory response in stem cells of the apical papilla via ALX/FPR2. Sci Rep. 2018;8:8921. doi: 10.1038/s41598-018-27194-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wei J., Heo S.J., Kim D.H., Kim S.E., Hyun Y.T., Shin J.W. Comparison of physical, chemical and cellular responses to nano- and micro-sized calcium silicate/poly(epsilon-caprolactone) bioactive composites. J R Soc Interface. 2008;5:617–630. doi: 10.1098/rsif.2007.1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Land G., Stephan D. The influence of nano-silica on the hydration of ordinary portland cement. J Mater Sci. 2011;47:1011–1017. [Google Scholar]
- 26.Camilleri J. Hydration characteristics of Biodentine and Theracal used as pup capping materials. Dent Mater. 2014;30:709–715. doi: 10.1016/j.dental.2014.03.012. [DOI] [PubMed] [Google Scholar]
- 27.Belío-Reyes I.A., Bucio L., Cruz-Chavez E. Phase composition of ProRoot mineral trioxide aggregate by X-Ray Powder Diffraction. J Endod. 2009;35:875–878. doi: 10.1016/j.joen.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 28.Islam I., Chng H.K., Yap A.U.J. X-ray diffraction analysis of mineral trioxide aggregate and Portland cement. Int Endod J. 2006;39:220–225. doi: 10.1111/j.1365-2591.2006.01077.x. [DOI] [PubMed] [Google Scholar]
- 29.Kim E.C., Lee B.C., Chang H.S., Lee W., Hong C.U., Min K.S. Evaluation of the radiopacity and cytotoxicity of portland cements containing bismuth oxide. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008;105:e54–e57. doi: 10.1016/j.tripleo.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 30.Li J., Liu Y., Zhang Y., et al. Biophysical and biochemical cues of biomaterials guide mesenchymal stem cell behaviors. Front Cell Dev Biol. 2021;9:640388. doi: 10.3389/fcell.2021.640388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shi M., Song W., Han T., et al. Role of the unfolded protein response in topogrpahy-induced osteogenic differentiation in rat bone marrow mesenchymal stem cells. Acta Biomater. 2017;54:175–185. doi: 10.1016/j.actbio.2017.03.018. [DOI] [PubMed] [Google Scholar]
- 32.Koh E.T., Torabinejad M., Pitt Ford T.R., Brady K., McDonald F. Mineral trioxide aggregate stimulates a biological response in human osteoblasts. J Biomed Mater Res. 1997;37:432–439. doi: 10.1002/(sici)1097-4636(19971205)37:3<432::aid-jbm14>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 33.Kum K.-Y., Yoo Y.-J., Chang S.W. Chemical constitution, morphological characteristics, and biological properties of ProRoot mineral trioxide aggregate and Ortho mineral trioxide aggregate. J Korean Dent Sci. 2013;6:41–49. [Google Scholar]
- 34.Kim M., Yang W., Kim H., Ko H. Comparison of the biological properties of ProRoot MTA, OrthoMTA, and endocem MTA cements. J Endod. 2014;40:1649–1653. doi: 10.1016/j.joen.2014.04.013. [DOI] [PubMed] [Google Scholar]
- 35.Souza L.C., Yadlapati M., Dorn S.O., Silva R., Letra A. Analysis of radiopacity, pH and cytotoxicity of a new bioceramic material. J Appl Oral Sci. 2015;23:383–389. doi: 10.1590/1678-775720150065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Main C., Mirzayan N., Shabahang S., Torabinejad M. Repair of root perforations using mineral trioxide aggregate: a long-term study. J Endod. 2004;30:80–83. doi: 10.1097/00004770-200402000-00004. [DOI] [PubMed] [Google Scholar]
- 37.Kim D.H., Jang J.H., Lee B.N., et al. Anti-inflammatory and mineralization effects of ProRoot MTA and Endocem MTA in studies of human and rat dental pulps in vitro and in vivo. J Endod. 2018;44:1534–1541. doi: 10.1016/j.joen.2018.07.012. [DOI] [PubMed] [Google Scholar]
- 38.Lee H., Shin Y., Kim S.O., Lee H.S., Choi H.J., Song J.S. Comparative study of pulpal responses to pulpotomy with ProRoot MTA, RetroMTA, and TheraCal in dogs' teeth. J Endod. 2015;41:1317–1324. doi: 10.1016/j.joen.2015.04.007. [DOI] [PubMed] [Google Scholar]
- 39.Bakhtiar H., Aminishakib P., Ellini M.R., et al. Dental pulp response to RetroMTA after partial pulpotomy in permanent human teeth. J Endod. 2018;44:1692–1696. doi: 10.1016/j.joen.2018.07.013. [DOI] [PubMed] [Google Scholar]
- 40.Kang C.M., Hwang J., Song J.S., Lee J.H., Choi H.J., Shin Y. Effects of three calcium silicate cements on inflammatory response and mineralization-inducing potentials in a dog pulpotomy model. Materials. 2018;11:899. doi: 10.3390/ma11060899. [DOI] [PMC free article] [PubMed] [Google Scholar]