Abstract
Background
Androgen-deprivation therapy (ADT) historically represented the milestone for the treatment of metastatic hormone-sensitive prostate cancer (mHSPC). Recently, combining androgen receptor-targeted agents (ARTA) or docetaxel with ADT significantly improved clinical outcomes in this setting. The efficacy of the combined use of an ARTA with docetaxel and ADT (triplet), however, was unknown, and often conflicting data derived from subgroup analysis of randomized phase III trials. In order to better define the benefits and risks of the triplet in mHSPC, we carried out a systematic review and meta-analysis of available clinical trials.
Methods
A literature search with no data restriction using Medline/PubMed, the Cochrane Library, and American Society of Clinical Oncology/European Society for Medical Oncology (ASCO/ESMO) Meeting abstracts was carried out up to April 2022. The meta-analysis was conducted following the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) statements. Overall survival (OS) was the primary endpoint; progression-free survival (PFS) and safety were secondary endpoints. For OS and PFS, summary hazard ratios (HRs) were calculated; for safety, risk ratio (RR) was assessed. Random- or fixed-effects models were used, depending on studies heterogeneity.
Results
Five randomized clinical trials fulfilled the prespecified inclusion criteria. The triplet significantly improved OS (fixed-effect, HR = 0.74; P < 0.00001) and PFS (fixed-effect; HR = 0.50 for clinical PFS, HR = 0.49 for radiological PFS; P < 0.0001) compared with docetaxel plus ADT. We did not show heterogeneity between treatment efficacy and the disease burden, metachronous versus synchronous presentation, concomitant versus sequential strategy. Compared with docetaxel + ADT, the triplet did not increase the risk of adverse events (AEs) (RR = 1.00, P = 0.27 for any-grade AEs; RR = 1.13, P = 0.14 for severe AEs), except for severe hypertension (RR = 1.73, P = 0.001).
Conclusions
Emerging evidence supports the combination of an ARTA plus docetaxel and ADT in mHSPC patients. Given the availability of several strategies in this setting, clinical characteristics and drug safety profile may help clinicians select the appropriate treatment for mHSPC patients who are more likely to benefit from treatment intensification.
Key words: darolutamide, abiraterone, docetaxel, prostate cancer, mHSPC, meta-analysis
Highlights
-
•
The addition of ARTA or docetaxel to ADT improved survival in mHSPC.
-
•
Recently, the combination of ARTA plus docetaxel and ADT (triplet) versus docetaxel plus ADT has been investigated.
-
•
The triplet prolonged OS and PFS compared with docetaxel plus ADT and represents a potential practice-changing treatment.
-
•
Clinical characteristics and drug safety profile may help clinicians to select the appropriate treatment for mHSPC.
Introduction
Prostate cancer is the most frequently diagnosed solid tumor worldwide and the second leading cause of cancer-related death among the male population. Most patients are diagnosed in the localized setting; however, at least 30% will develop metastatic disease after local treatment. Moreover, 8% of patients are diagnosed with de novo metastatic hormone-sensitive prostate cancer (mHSPC).1
For decades, androgen-deprivation therapy (ADT) was the cornerstone of treatment and the control arm of many studies in the mHSPC setting. The duration of response to ADT usually lasts no longer than 12-24 months, however, after which all men virtually switch to castration-resistant disease.1, 2, 3, 4, 5 In the last decade, there has been a paradigm shift in the treatment of mHSPC. Since 2015, the CHAARTED, LATITUDE, and STAMPEDE phase III trials led to the approval of docetaxel and abiraterone, in combination with ADT, resulting in a survival advantage compared with ADT.2, 3, 4, 5 Subsequently, the ARCHES, ENZAMET, and TITAN trials showed the efficacy of enzalutamide and apalutamide in this setting.6, 7, 8, 9, 10 Subgroup analyses showed that the survival benefit of docetaxel in the CHAARTED trial was limited to patients with a high-volume disease (defined as the presence of four or more bone lesions of whom at least one beyond vertebral column and pelvis, and/or visceral metastases), whereas the new androgen receptor targeted agents (ARTA) improved survival regardless of disease burden.6, 7, 8, 9, 10, 11, 12 The intensification of first-line therapy combining an ARTA to docetaxel and ADT (triplet) was already explored in subgroup analysis of ARCHES, ENZAMET, and TITAN trials.6, 7, 8, 9, 10 No definitive results could be drawn, however, given the small number of patients in the subgroups. Recently, two large randomized phase III trials—PEACE-1 and ARASENS—explored the efficacy of the triplet as first-line treatment intensification for mHSPC patients.13,14 Here we carried out a meta-analysis of randomized clinical trials (RCTs) to better define the benefit achieved with the use of the triplet in mHSPC.
Materials and methods
Data retrieval strategies
This study aims to carry out a meta-analysis of RCTs exploring the efficacy of combining a new ARTA plus docetaxel and ADT in patients with mHSPC. A literature search with no data restriction using Medline/PubMed, the Cochrane Library, and American Society of Clinical Oncology/European Society for Medical Oncology (ASCO/ESMO) Meeting abstracts to identify relevant studies, including those still unpublished in extenso, was carried out up to 10 April 2022 (Supplementary Table S1, available at https://doi.org/10.1016/j.esmoop.2022.100575). A crosscheck reference from review articles and relevant studies on the same topic was carried out to confirm all possible pertinent trials retrieval. The search criteria were limited to phase II and III RCTs. The review was not registered.
Inclusion criteria
Two independent reviewers screened the studies according to specific selection and exclusion criteria. The inclusion/exclusion decisions regarding contentious studies were made in consultation with the corresponding author. The studies included in the meta-analysis had to fulfill the following inclusion criteria: (i) participants with mHSPC; (ii) ARTA (abiraterone, enzalutamide, apalutamide, or darolutamide) in combination with docetaxel and ADT in the experimental arm; (iii) the presence of a control arm for comparison (ADT plus docetaxel); and (iv) overall survival (OS) as primary or secondary endpoint expressed as the hazard ratio (HR) and progression-free survival (PFS), both radiographic (rPFS) and clinical (cPFS), as primary or key secondary outcome expressed as the HR, and major toxicities expressed as risk ratio (RR). The following exclusion criteria were used: (i) studies with insufficient data; (ii) animal studies; (iii) sample size per arm <10 participants; and (iv) non-randomized studies. No language restriction was applied.
Data extraction
Two authors independently extracted the relevant data, including the name of the first author, publication year, patient demographics (i.e. age, number, and drug administered), median follow up, median treatment duration, study design (i.e. the type of blinding, the type of control, the methods for randomization), survival outcomes expressed as HRs for OS and PFS, and number of patients who experienced toxicity.
Quality assessment and statistical analysis
Considering the nature of evaluated studies (all randomized), we preferred to assess the study quality using the Jadad 5-item scale.15 The final score ranged from 0 to 5. In case of disagreements, a consensus was achieved in discussion with the corresponding author. The publication bias or sensitivity analysis has not been carried out because of the low number of included trials.
Statistical analysis
The statistical analyses were carried out with Revman 5.3. The summary estimates were generated using a fixed-effects model (Mantel–Haenszel method) or a random-effects model (DerSimonian–Laird method) depending on the absence or presence of heterogeneity.16,17 Statistical heterogeneity was assessed with the Q-test and the I2 statistic. I2 values of 25%, 50%, and 75% were considered to indicate low, moderate, and high heterogeneity, respectively.18 When P < 0.1 and I2 < 50%, the fixed-effects model was used; otherwise, the random-effects model was used. For the time-to-event variables, including OS and PFS, HRs with 95% confidence intervals (CIs) were calculated for each study. For the rate of adverse events (AEs) (dichotomous variable), the RR with 95% CIs was calculated for each study. A subgroup analysis was carried out to highlight differences between disease burden (high volume versus low volume), treatment strategy (concomitant versus sequential start of docetaxel + ADT and ARTA), synchronous versus metachronous disease. For all the statistical analyses, a value of P < 0.05 was regarded as statistically significant, and all tests were two-sided. No correction for multiplicity was applied.
Results
Search results
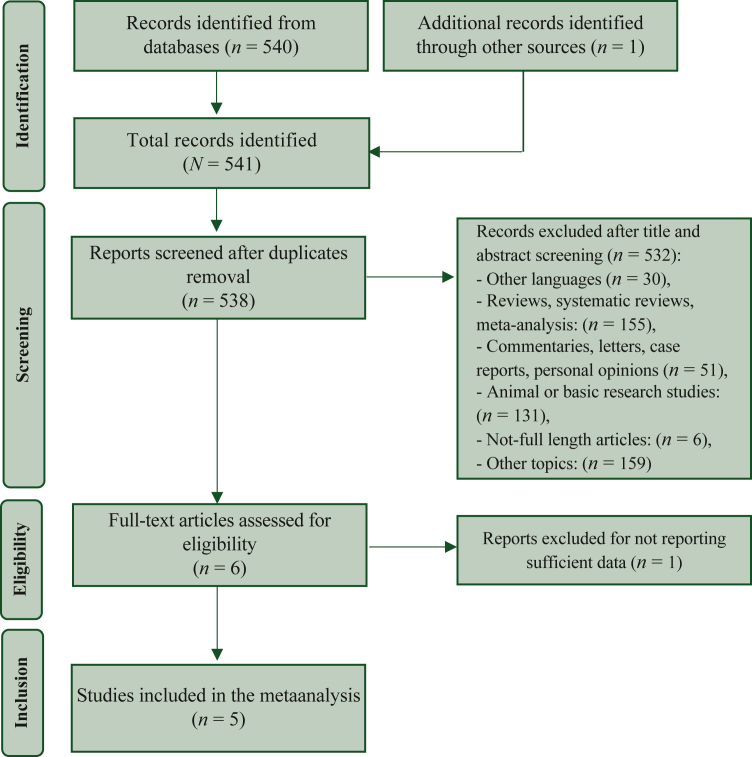
The electronic search identified 541 studies from databases and conference abstracts. After duplicate removal, 538 papers were screened. Among them, 532 papers were excluded as not English language, not RCTs, preclinical papers, or different topics. At the end of the selection process, five studies fulfilled the inclusion criteria and were included in the meta-analysis.6,8,9,13,14 The Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) flow chart summarizing the process of selection is shown in Figure 1.
Figure 1.
Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) flow chart of the selection process.
Characteristics of the included studies
All selected studies were phase III, double-blind, RCTs. The experimental arm included enzalutamide (n = 2), abiraterone (n = 1), darolutamide (n = 1), apalutamide (n = 1) plus docetaxel and ADT. The control arm was placebo (PBO) + docetaxel + ADT. In the ARASENS, PEACE-1, and ENZAMET trials, the ARTA/PBO was started concomitant to docetaxel and ADT; in the ENZAMET trial, up to two cycles of docetaxel were allowed before ARTA/PBO was started.8,13,14 In the TITAN and ARCHES trials, ARTA/PBO was administered to patients with no evidence of progression after a maximum of six cycles of docetaxel + ADT.6,9 The main characteristics of the included studies are listed in Table 1. The median Jadad score was 5, confirming a good level of quality.
Table 1.
Characteristics of the included studies
| Trial | First author | Year | Experimental arm | Control arm | Other treatments allowed | Primary endpoint(s) | Age, years (range) | Age (years) median (IQR) | Volume of metastatic disease, n (%) |
OS HR (95% CI) | rPFS HR (95% CI) | cPFS HR (95% CI) | Median follow-up, months | Jadad score | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Low | High | ||||||||||||||
| TITAN | Chi KN et al. | 2019 | Apalutamide + ADT | Placebo + ADT | Docetaxel for a maximum of 6 cycles before randomization | rPFS, OS | Exp 69 (45-94) Ctrl 68 (43-90) |
0.67 (0.51-0.89) | 0.48 (0.39-0.60) | 44.0 | 5 | ||||
| ENZAMET | Davis ID et al. | 2019 | Enzalutamide + ADT | ADT (NSAA) | Docetaxel for a maximum of 6 cycles, up to 2 cycles were permitted before randomization. | OS | Exp 69.2 (63.2- 74.5) Ctrl 69.0 (63.6-74.5) |
Exp 272 (48.3) Ctrl 265 (47.2) |
Exp 291 (51.7) Ctrl 297 (52.8) |
0.67 (0.52-0.86) | 0.40 (0.33-0.49) | 34.0 | 3 | ||
| ARCHES | Armstrong AJ et al. | 2019 | Enzalutamide + ADT | Placebo + ADT | Docetaxel for a maximum of 6 cycles before randomization | rPFS | Exp 70 (46-92) Ctrl 70 (42-92) |
0.81 (0.53-1.25) | 0.39 (0.30-0.50) | 44.6 | 5 | ||||
| PEACE-1 | Fizazi K et al. | 2021 | Abiraterone + docetaxel + ADT | Docetaxel + ADT | Radiotherapy to the primary tumor (for low metastatic burden) | rPFS, OS | Exp 66 (60-70) Ctrl 66 (59-70) |
Exp 131 (37) Ctrl 123 (35) |
Exp 224 (63) Ctrl 232 (65) |
0.75 (0.59-0.95) | 0.50 (0.34-0.74) | 0.50 (0.40-0.63) | 52.0 | 1 | |
| ARASENS | Smith MR et al. | 2022 | Darolutamide + docetaxel + ADT | Placebo + docetaxel + ADT | OS | Exp 67 (41-89) Ctrl 67 (42-86) |
0.68 (0.57-0.80) | Exp 43.7 Ctrl 42.4 |
5 | ||||||
ADT, androgen deprivation therapy; CI, confidence interval; cPFS, clinical progression-free survival; Ctrl, control arm; Exp, experimental arm; HR, hazard ratio; IQR, interquartile range; NSAA, non-steroidal anti-androgen drug; OS, overall survival; rPFS, radiographic progression-free survival.
Overall, 2836 patients were treated, 1415 in the experimental arm and 1421 in the control arm. The median age at diagnosis of the included patients ranged from 66 to 70 years.
All the studies reported the data of OS, which was the primary endpoint in four out of five studies.8,9,13,14 OS was defined as the time from treatment starting to patients’ death. In three studies, rPFS was explored as the primary endpoint (in two cases, as a co-primary endpoint with OS).6,9,13 Two studies reported the data for cPFS.8,13 PFS was defined as the time from randomization to clinical or radiographic disease progression, or patients’ death, whichever occurred first.
OS of ARTA plus docetaxel and ADT versus docetaxel plus ADT
Data for OS were available from all five studies and 2836 patients. Among them, 1415 received the experimental treatment and 1421 were treated in the control arm.6, 7, 8, 9, 10,13,14
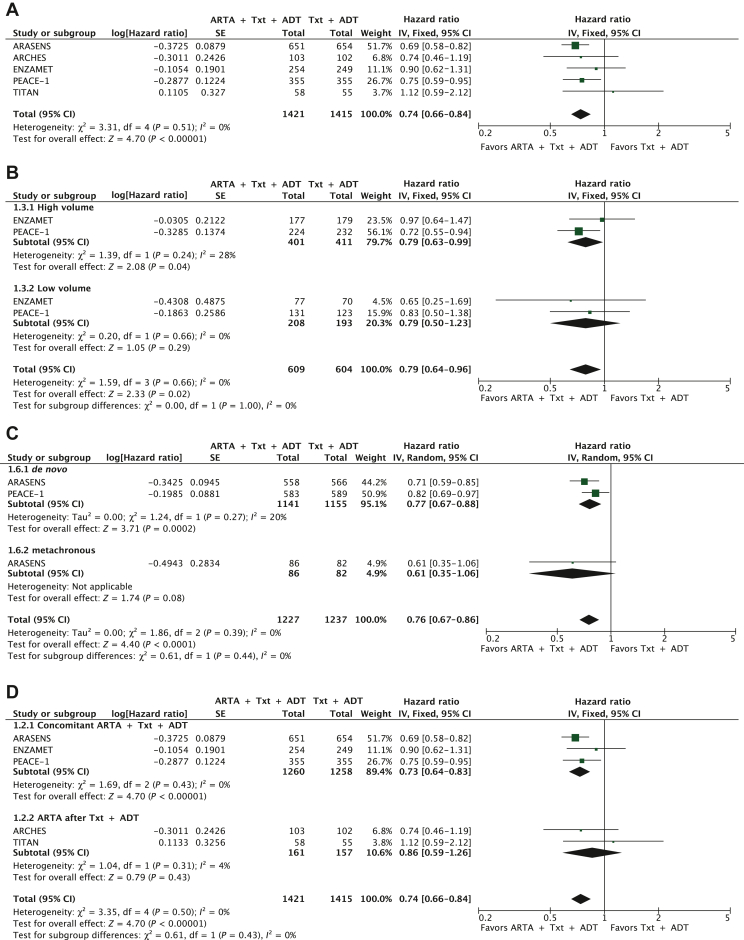
The pooled HR showed that adding an ARTA to docetaxel and ADT significantly reduced the risk of death compared with PBO plus docetaxel and ADT (HR: 0.74; 95% CI 0.66-0.84; P < 0.00001; I2 = 0%, P = 0.51) (Figure 2A).
Figure 2.
ARTA plus docetaxel and ADT versus docetaxel plus ADT: OS (A); subgroup analysis for disease burden—high versus low volume (B); subgroup analysis for synchronous versus metachronous disease (C); subgroup analysis for concomitant versus sequential strategy (D).
ADT, androgen deprivation therapy; ARTA, androgen receptor targeted agent; CI, confidence interval; df, degrees of freedom; OS, overall survival; SE, standard error; Txt, docetaxel.
We conducted subgroup analyses of OS for disease burden, synchronous/metachronous disease, and treatment strategy.
Two studies reported the pooled data for the disease burden, including 822 high-volume and 401 low-volume patients.8,13 The OS benefit from the addition of ARTA to docetaxel and ADT was confirmed in the high-volume disease (HR: 0.79; 95% CI 0.63-0.99, P = 0.04; I2 = 28%, P = 0.24). The benefit was uncertain in the low-volume population (HR: 0.79; 95% CI 0.50-1.23, P = 0.29; I2 = 0%, P = 0.66). No significant differences were detected, however, between the two subgroups (P = 1.00) (Figure 2B).
We subsequently analyzed the OS effect between groups with synchronous/de novo and metachronous disease.13,14 The OS improvement was more evident in the 1141 de novo patients treated with the triplet than the 1155 receiving the doublet (HR: 0.76, 95% CI 0.67-0.88, P = 0.0002; I2 = 20%, P = 0.27). The benefit was less clear in the metachronous disease (HR: 0.61, 95% CI 0.35-1.06), although a small number of patients were analyzed (n = 168), without significant differences between the groups (P = 0.44) (Figure 2C).
Finally, we divided the studies between concomitant and sequential strategies. The OS benefit from adding an ARTA to docetaxel and ADT was confirmed in the concomitant strategy (HR: 0.73; 95% CI 0.64-0.83, P < 0.00001; I2 = 0%, P = 0.43), representing a higher weight in the total analysis. The benefit was not clear in the sequential treatment (HR: 0.86; 95% CI 0.59-1.26, P = 0.43; I2 = 4%, P = 0.31), with fewer treated patients. No differences were detected, however, between the two subgroups (P = 0.43) (Figure 2D).
PFS of ARTA plus docetaxel and ADT versus docetaxel plus ADT
rPFS data were available in three studies and a total of 1028 patients.6,7,9,10,13 The combination of an ARTA, docetaxel and ADT significantly prolonged rPFS compared with docetaxel plus ADT (HR: 0.50; 95% CI 0.42-0.60; P < 0.00001; fixed-effects) (Supplementary Figure S1A, available at https://doi.org/10.1016/j.esmoop.2022.100575). Two studies reported cPFS, for a total of 1224 patients.8,13 The combination of an ARTA, docetaxel and ADT significantly prolonged cPFS compared with docetaxel plus ADT (HR: 0.49; 95% CI 0.41-0.58; P < 0.00001; fixed-effects) (Supplementary Figure S1B, available at https://doi.org/10.1016/j.esmoop.2022.100575).
Safety profile of ARTA plus docetaxel and ADT versus docetaxel plus ADT
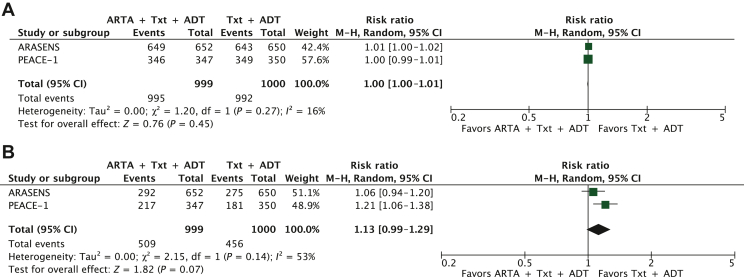
The safety data of the triplet versus the doublet were available in two studies.13,14 The triplet did not worsen the risk of any grade (P = 0.45) and severe AEs (P = 0.07) compared with the doublet (Figure 3A and B).
Figure 3.
Safety profile ARTA plus docetaxel and ADT versus docetaxel plus ADT for (A) all-grade adverse events (AEs) and (B) severe AEs.
ADT, androgen deprivation therapy; ARTA, androgen receptor targeted agent; CI, confidence interval; df, degrees of freedom; M-H, Mantel-Haenszel; Txt, docetaxel.
An increased risk of severe hypertension was associated with the triplet rather than the doublet (Supplementary Figure S2A, available at https://doi.org/10.1016/j.esmoop.2022.100575). The risk of other severe AEs such as neutropenia, febrile neutropenia, and hepatotoxicity was not increased (Supplementary Figure S2B and C, available at https://doi.org/10.1016/j.esmoop.2022.100575).
Discussion
The results of our meta-analysis show that the combination of an ARTA (enzalutamide, abiraterone, apalutamide, darolutamide) with docetaxel and ADT significantly improves OS over docetaxel and ADT in men with mHSPC, with a reduction of 26% of the risk of death (HR: 0.74; P < 0.00001). Similarly, adding an ARTA to docetaxel and ADT meaningfully prolongs cPFS and rPFS compared with docetaxel plus ADT, halving the risk of progression. The strength of these results is represented by the high homogeneity between the included studies for both PFS and OS data. We are dealing with another potential practice-changing treatment after the upfront docetaxel or ARTA as single agents, which already improved mOS to >5 years.
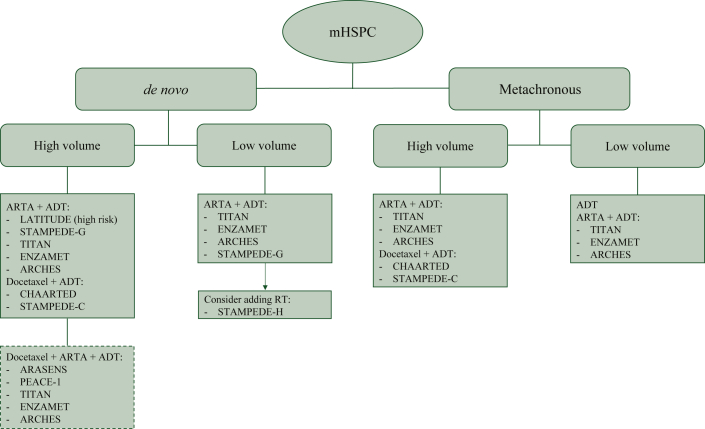
We did not show heterogeneity between treatment efficacy and the disease burden. Our meta-analysis is not powered to exclude any interaction, however, and this subgroup analysis is affected by the limited number of trials with available data. In 2015, the CHAARTED trial showed an OS benefit when docetaxel was added to ADT in patients with high-volume disease.2 Conversely, low-volume patients had a similar benefit with ADT alone or combined with docetaxel.19,20 The definition of disease burden was different in the LATITUDE trial that enrolled patients with high-risk mHSPC, defined as the presence of at least one of the following factors: Gleason score of ≥8, at least three bone lesions, or measurable visceral metastases.5 Further analyses demonstrated that ARTAs are also effective in high-volume mHSPC.6, 7, 8, 9, 10,12 The results of our meta-analysis support evidence of the efficacy of first-line treatment intensification with the triplet in high-volume mHSPC (Figure 4). The best management of low-volume patients, however, still remains a topic of debate. Given that the subgroup stratification by tumor burden was available only for two out of five studies included in our analysis, further investigations are needed.8,13 Considering the low number of treated patients and the events recorded in the low-volume subgroup (77/401), we wonder if this population gains advantages mainly from docetaxel or if the larger part of the benefit derives from the ARTA plus ADT. Effectively, whereas the group of high-volume disease largely drove the survival benefit in the CHAARTED trial, the studies with ARTA showed that these agents could be indifferently used to treat either high- or low-volume patients.2,5, 6, 7, 8, 9, 10,12 Furthermore, given the long survival of low-volume patients even with ADT alone, this option could be considered for patients with minimal disease, such as nodal involvement, or also chosen for frail patients with comorbidities that are not eligible for an ARTA (Figure 4). In the ARASENS trial, there was a minimal number of patients with limited disease to nodes only (n = 39), in which the triplet did not seem to determine a significant survival advantage (HR: 0.65, 95% CI 0.19-2.25). This result was in contrast with the subgroup characterized by heavy bony involvement (n = 1037) that achieved greater benefit from the triplet than the doublet (HR: 0.67, 95% CI 0.55-0.81).14
Figure 4.
First-line therapy options for metastatic hormone-sensitive prostate cancer (mHSPC) patients. Dotted lines indicate future treatments possibly available after studies of docetaxel plus ARTA plus ADT.
ADT, androgen deprivation therapy; ARTA, androgen receptor targeted agent; RT, radiotherapy.
In addition to the disease burden, the therapeutic algorithm should consider other clinical parameters for better patient stratification. Still, limited information is available regarding synchronous (de novo) versus metachronous diseases, which have been characterized as two different clinical subgroups having a proper prognosis, with OS ranging from 3 years in patients with de novo mHSPC (diagnosed as metastatic) to 8 years in metachronous mHSPC patients, developing metastases after initial presentation with localized disease.21 In the ARASENS trial, the benefit of the triplet was more relevant in patients with synchronous disease but not in the—small—subgroup of patients developing metachronous metastases.14 PEACE-1, which included only de novo patients, confirmed this benefit.13 The category of de novo mHSPC, however, encompasses a broad spectrum of heterogeneous clinical patterns, ranging from asymptomatic patients with an indolent disease to symptomatic patients with high Gleason scores and extensive bone or visceral involvement. Docetaxel previously demonstrated a benefit in de novo high-volume but not in low-volume mHSPC, differently from ARTA that provided a clinically meaningful benefit in de novo metastatic patients regardless of disease volume.4,5,19,20,22 Therefore, the addition of chemotherapy to ARTA and ADT could be of interest for de novo metastatic high-volume patients. In the metachronous setting, there is a lack of survival advantage for low-volume patients treated with docetaxel; on the contrary, ARTAs are associated with a survival advantage both in the high- and the low-volume setting.22,23 Hence, ADT alone or ARTA plus ADT seem reasonable options for these patients (Figure 4).
Regarding the optimal sequence of chemotherapy, ARTA and ADT, the survival advantage was more significant when the drugs were concurrently started. ARTAs directly affect the androgen receptor signaling: for example, abiraterone stops the steroid-to-androgens conversion and inhibits intratumoral steroidogenesis. Docetaxel indirectly abrogates androgen receptor translocation through the inhibition of tubulin polymerization.24 A reasonable hypothesis is that the simultaneous use of drugs with complementary mechanisms of action might potentiate their efficacy. As enzalutamide, apalutamide, and darolutamide inhibit androgen receptor translocation, however, their effect could be weakened if a previous inhibition is started by prior docetaxel.25 Of note, ARCHES and TITAN trials were not initially designed to compare ARTA plus docetaxel and ADT with docetaxel plus ADT, and data derived from small subgroups. Therefore, a longer follow up will better clarify the real advantage of the triple combination over docetaxel plus ADT in case of sequential treatment.6, 7, 8, 9, 10
A still unanswered question is represented by the role of radiotherapy (RT) to the primary tumor in the metastatic setting, addressed as a potentially effective treatment, particularly in oligometastatic or low-burden HSPC.26, 27, 28, 29 Apart from a PFS improvement, neither the HORRAD nor the STAMPEDE trials evidenced an OS benefit, except for selected patients with low-burden disease.26, 27, 28 In two out of four arms of the PEACE-1 trial, RT to the prostate (74 Gy) was allowed with or without abiraterone after docetaxel completion, but no interaction was found between RT and abiraterone for OS improvement in the overall population (P = 0.86); however, the predefined number of events for analysis in low-volume disease has not been reached, and further results are expected.13 In the meantime, RT should be limited to patients with low-volume disease at diagnosis (Figure 4).
Regarding available safety data, the addition of ARTA to docetaxel and ADT did not increase the risk of any-grade and severe AEs, except for the higher risk of severe hypertension typical of ARTA.4,5 A longer follow-up will test the hypothesis of whether the combination of the three agents would also combine AEs. This observation will also raise the real advantage of administering the triplet, especially in those patients with relevant comorbidities. In general, the safety profile of ARTAs seems more tolerable than docetaxel, even if a direct comparison has never been made.2, 3, 4, 5, 6, 7, 8, 9, 10 For example, severe neutropenia occurred regardless of ARTA, interesting up to one-third of patients treated with docetaxel.13,14 Of note, in the ARASENS trial, the authors reported that AEs progressively decreased when docetaxel was stopped.14 The safety profile of the agents, together with patients’ general conditions and comorbidities, may be helpful in the treatment selection.
Our systematic review and meta-analysis has several limitations. First and foremost, the analysis relies on trials’ results and not on individual patients’ data. Over the strategy of combining docetaxel and ARTA, other differences exist among populations included in the selected studies. For example, the ENZAMET trial allowed the administration of nonsteroidal anti-androgens; in the ARASENS, PEACE-1, and TITAN trials, luteinizing hormone-releasing hormone (LH-RH) agonists or antagonists were administered; in ARCHES, all hormonal agents were allowed.6, 7, 8, 9, 10,13,14 Variables such as the site of metastases, metachronous versus synchronous disease, prostate-specific antigen values, and Gleason score should be more intensively explored. Moreover, patients in worse general conditions were not enrolled in the included trials; indeed, we cannot confidently state that the results reflect real-world data and are easily transferable in current clinical practice. Some trials are still ongoing, with results to be updated; therefore, a longer follow-up and more complete data will confirm the benefit in terms of efficacy and the safety profile. Finally, given the design of the included studies and the control arm, the role of adding docetaxel to ARTA versus ARTA plus ADT has not been investigated.
Conclusions
The addition of an ARTA to docetaxel and ADT significantly prolongs survival compared with docetaxel and ADT in patients with mHSPC and should be adopted in daily clinical practice. Given the availability of several strategies in this setting, clinical and biological characteristics, drugs safety profile, and costs may help clinicians select the appropriate therapy for mHSPC patients who are more likely to benefit from treatment intensification.
Acknowledgements
The authors are very grateful to Maria Renata Marrone for graphic editing and Mauro Maiorano for language editing.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Disclosure
None declared.
Supplementary data
References
- 1.Siegel R.L., Miller K.D., Fuchs H.E., Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72:7–33. doi: 10.3322/caac.21708. [DOI] [PubMed] [Google Scholar]
- 2.Sweeney C.J., Chen Y.-H., Carducci M., et al. Chemohormonal therapy in metastatic hormone-sensitive prostate cancer. N Engl J Med. 2015;373:737–746. doi: 10.1056/NEJMoa1503747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.James N.D., Sydes M.R., Clarke N.W., et al. Addition of docetaxel, zoledronic acid, or both to first-line long-term hormone therapy in prostate cancer (STAMPEDE): survival results from an adaptive, multiarm, multistage, platform randomised controlled trial. Lancet. 2016;387:1163–1177. doi: 10.1016/S0140-6736(15)01037-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.James N.D., de Bono J.S., Spears M.R., et al. Abiraterone for prostate cancer not previously treated with hormone therapy. N Engl J Med. 2017;377:338–351. doi: 10.1056/NEJMoa1702900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fizazi K., Tran N., Fein L., et al. Abiraterone plus prednisone in metastatic, castration-sensitive prostate cancer. N Engl J Med. 2017;377:352–360. doi: 10.1056/NEJMoa1704174. [DOI] [PubMed] [Google Scholar]
- 6.Armstrong A.J., Szmulewitz R.Z., Petrylak D.P., et al. ARCHES: a randomized, phase III study of androgen deprivation therapy with enzalutamide or placebo in men with metastatic hormone-sensitive prostate cancer. J Clin Oncol. 2019;37:2974–2986. doi: 10.1200/JCO.19.00799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Armstrong A.J., Iguchi T., Azad A.A., et al. Final overall survival (OS) analysis from ARCHES: a phase III, randomized, double-blind, placebo (PBO)-controlled study of enzalutamide (ENZA) + androgen deprivation therapy (ADT) in men with metastatic hormone-sensitive prostate cancer (mHSPC) Ann Oncol. 2021;32:S1283–S1346. [Google Scholar]
- 8.Davis I.D., Martin A.J., Stockler M.R., et al. Enzalutamide with standard first-line therapy in metastatic prostate cancer. N Engl J Med. 2019;381:121–131. doi: 10.1056/NEJMoa1903835. [DOI] [PubMed] [Google Scholar]
- 9.Chi K.N., Agarwal N., Bjartell A., et al. Apalutamide for metastatic, castration-sensitive prostate cancer. N Engl J Med. 2019;381:13–24. doi: 10.1056/NEJMoa1903307. [DOI] [PubMed] [Google Scholar]
- 10.Chi K.N., Chowdhury S., Bjartell A., et al. Apalutamide in patients with metastatic castration-sensitive prostate cancer: final survival analysis of the randomized, double-blind, phase III TITAN study. J Clin Oncol. 2021;39(20):2294–2303. doi: 10.1200/JCO.20.03488. [DOI] [PubMed] [Google Scholar]
- 11.Botrel T.E.A., Clark O., Lima Pompeo A.C., et al. Efficacy and safety of combined androgen deprivation therapy (ADT) and docetaxel compared with ADT alone for metastatic hormone-naive prostate cancer: a systematic review and meta-analysis. PLoS One. 2016;11 doi: 10.1371/journal.pone.0157660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hoyle A.P., Ali A., James N.D., et al. Abiraterone in “high-” and “low-risk” metastatic hormone-sensitive prostate cancer. Eur Urol. 2019;76:719–728. doi: 10.1016/j.eururo.2019.08.006. [DOI] [PubMed] [Google Scholar]
- 13.Fizazi K., Foulon S., Carles J., et al. Abiraterone plus prednisone added to androgen deprivation therapy and docetaxel in de novo metastatic castration-sensitive prostate cancer (PEACE-1): a multicentre, open-label, randomised, phase 3 study with a 2 × 2 factorial design. Lancet. 2022;399(10336):1695–1707. doi: 10.1016/S0140-6736(22)00367-1. [DOI] [PubMed] [Google Scholar]
- 14.Smith M.R., Hussain M., Saad F., et al. Darolutamide and survival in metastatic, hormone-sensitive prostate cancer. N Engl J Med. 2022;386:1132–1142. doi: 10.1056/NEJMoa2119115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jadad A.R., Moore R.A., Carroll D., et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17:1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 16.DerSimonian R., Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 17.Cochran W.G. The combination of estimates from different experiments. Biometrics. 1954;10:101–129. [Google Scholar]
- 18.Higgins J.P., Thompson S.G., Deeks J.J., Altman D.G. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sweeney C., Chen Y., Liu G., et al. Long term efficacy and QOL data of chemohormonal therapy in low and high volume hormone naïve metastatic prostate cancer: E3805 CHAARTED trial. Ann Oncol. 2016;27(suppl 6):243–265. [Google Scholar]
- 20.Kyriakopoulos C.E., Chen Y.H., Carducci M.A., et al. Chemohormonal therapy in metastatic hormone-sensitive prostate cancer: long-term survival analysis of the randomized phase III E3805 CHAARTED trial. J Clin Oncol. 2018;36:1080–1087. doi: 10.1200/JCO.2017.75.3657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Francini E., Gray K.P., Xie W., et al. Time of metastatic disease presentation and volume of disease are prognostic for metastatic hormone sensitive prostate cancer (mHSPC) Prostate. 2018;78:889–895. doi: 10.1002/pros.23645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gravis G., Boher J.M., Chen Y.H., et al. Burden of metastatic castrate naive prostate cancer patients, to identify men more likely to benefit from early docetaxel: further analyses of CHAARTED and GETUG-AFU15 studies. Eur Urol. 2018;73:847–855. doi: 10.1016/j.eururo.2018.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sweeney C.J., Martin A.J., Stockler M.R., et al. Overall survival of men with metachronous metastatic hormone-sensitive prostate cancer treated with enzalutamide and androgen deprivation therapy. Eur Urol. 2021;80:275–279. doi: 10.1016/j.eururo.2021.05.016. [DOI] [PubMed] [Google Scholar]
- 24.Darshan M.S., Loftus M.S., Thadani-Mulero M., et al. Taxane-induced blockade to nuclear accumulation of the androgen receptor predicts clinical responses in metastatic prostate cancer. Cancer Res. 2011;71:6019–6029. doi: 10.1158/0008-5472.CAN-11-1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rice M.A., Malhotra S.V., Stoyanova T. Second-generation antiandrogens: from discovery to standard of care in castration resistant prostate cancer. Front Oncol. 2019;9:801. doi: 10.3389/fonc.2019.00801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boevé L.M.S., Hulshof M.C.C.M., Vis A.N., et al. Effect on survival of androgen deprivation therapy alone compared to androgen deprivation therapy combined with concurrent radiation therapy to the prostate in patients with primary bone metastatic prostate cancer in a prospective randomised clinical trial: data from the HORRAD trial. Eur Urol. 2019;75:410–418. doi: 10.1016/j.eururo.2018.09.008. [DOI] [PubMed] [Google Scholar]
- 27.Parker C.C., James N.D., Brawley C.D., et al. Radiotherapy to the primary tumour for newly diagnosed, metastatic prostate cancer (STAMPEDE): a randomised controlled phase 3 trial. Lancet. 2018;392:2353–2366. doi: 10.1016/S0140-6736(18)32486-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Burdett S., Boevé L.M., Ingleby F.C., et al. Prostate radiotherapy for metastatic hormone-sensitive prostate cancer: a STOPCAP systematic review and meta-analysis. Eur Urol. 2019;76:115–124. doi: 10.1016/j.eururo.2019.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sydes M.R., Spears M.R., Mason M.D., et al. Adding abiraterone or docetaxel to long-term hormone therapy for prostate cancer: directly randomised data from the STAMPEDE multi-arm, multi-stage platform protocol. Ann Oncol. 2018;29:1235–1248. doi: 10.1093/annonc/mdy072. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.