Abstract
Background
The presence of KRASG12C mutation in metastatic colorectal cancer (mCRC) correlates with poor outcome. Although different selective inhibitors are under clinical development, the optimal treatment remains uncertain. Thus, we conducted a retrospective analysis in a large cohort of patients with KRASG12C mCRC treated in 12 Italian oncology units.
Patients and methods
Patients with unresectable mCRC harboring KRASG12C mutation receiving a first-line chemotherapy doublet or triplet between 2011 and 2021 were included in the study. Evaluation of overall response rate (ORR), progression-free survival (PFS) and overall survival (OS) analysis was carried out.
Results
A total of 256/6952 (3.7%) patients with mCRC displayed KRASG12C mutation; of these, 111 met the inclusion criteria. The ORR of first-line therapy was 38.7% (43/111). Median PFS (mPFS) was 9 months [95% confidence interval (CI) 7.5-10.5 months]. After progression, only 62% and 36% of the patients are fit to receive second or third lines of treatment, with limited clinical benefit. Median OS (mOS) was 21 months (95% CI 17.4-24.6 months). In patients receiving first-line triplet chemotherapy, ORR was 56.3% (9/16), mPFS was 13 months (95% CI 10.3-15.7 months) and mOS was 32 months (95% CI 7.7-56.3 months). For irinotecan-based doublets, ORR was 34.5 (10/29), mPFS was 9 months (95% CI 6.4-11.6 months) and mOS was 22 months (95% CI 16.0-28.0 months). With oxaliplatin-based doublets ORR was 36.4% (24/62), mPFS was 7 months (95% CI 4.6-9.4 months) and mOS was 18 months (95% CI, 13.6-22.4 months).
Conclusion
Patients with KRASG12C-mutant mCRC had a disappointing response to standard treatments. Within the limitations of a retrospective study, these results suggest that first-line chemotherapy intensification with FOLFOXIRI is a valid option in fit patients.
Key words: mCRC, KRASG12C mutation, chemotherapy, first line treatment, real-world data
Highlights
-
•
KRASG12C mutation is rare and occurs in 3.7% of the study population.
-
•
The presence of KRASG12C mutation is correlated with an aggressive disease, with reduced response to chemotherapy.
-
•
Only 62% and 36% of patients with KRASG12C-mutant mCRC are fit to receive second or third lines of treatment, respectively.
-
•
The use of chemotherapy triplets is associated with improved outcomes compared with chemotherapy doublets.
Introduction
Treatment of metastatic colorectal cancer (mCRC) has deeply changed over the last two decades.1 For patients with RAS/BRAF wild type tumors the addition of anti-epidermal growth factor receptor (EGFR) monoclonal antibodies (mAbs) significantly improved therapeutic efficacy and led to an overall survival (OS) of 30-40 months.2 While the presence of BRAFV600E mutation confers a major aggressiveness, the use of target therapies may change the natural history of the disease.3
Moreover, immunotherapy has revolutionized the treatment of microsatellite instability high (MSI-H) mCRC with patients experiencing long-lasting responses.4,5
For many years, RAS mutations have been considered a negative prognostic biomarker that confers resistance to anti-EGFR mAbs and is correlated with poor prognosis.6 Only recently, the development of specific KRASG12C inhibitors represented a light at the end of the tunnel.7,8 The presence of KRASG12C is rare (2%-4%), however, and so far, merely few and heterogenous retrospective series have evaluated the impact of KRASG12C on the response to chemotherapy and as a prognostic biomarker, with discordant results.9, 10, 11, 12, 13, 14, 15, 16 KRASG12C tumors were associated with a shorter OS compared with non-KRASG12C tumors.9, 10, 11, 12, 13 Other studies reported that KRASG12C mutation conferred resistance with reduced overall response rate (ORR) to first-line chemotherapy doublets plus bevacizumab compared with different KRAS mutations, but did not affect progression-free survival (PFS) or OS.14 Finally, another study reported no difference in terms of response and OS across KRASG12C and other KRAS-mutant mCRC.15 Furthermore, due to the retrospective nature of those studies, the small number of patients included in the studies and the setting and/or the type of chemotherapy regimens (mono-chemotherapy versus chemotherapy doublets, post-operative chemotherapy versus palliative treatment), the best treatment strategy is still unknown.
Therefore, while novel anti-KRASG12C drugs are moving from bench to bedside, there are still different open questions including how we can optimize the available treatments for these patients, and which is the natural course of the disease. For this reason, we conducted a retrospective analysis on a large cohort of patients, with unresectable mCRC, who received an intensive first-line treatment at 12 specialized Italian oncology units.
Materials and methods
The aim of this study was to investigate the impact of KRASG1C mutation on the response to chemotherapy and on the outcome in patients with unresectable mCRC.
Medical records of patients with mCRC referred to 12 Italian oncology units from January 2011 to December 2021 were evaluated. Main inclusion criteria were (i) patients with KRASG12C-mutant mCRC; (ii) availability of clinicopathological characteristics, treatment patterns and outcomes; and (iii) patients should have received an intensive fist-line treatment, such as irinotecan- or oxaliplatin-based chemotherapy doublet or triplet. Exclusion criteria were (i) patients with no KRASG12C-mutant mCRC; (ii) patients with KRASG12C-mutant mCRC but with missing clinical information; (iii) patients unfit for systemic therapy or who received single agent chemotherapy; (iv) patients with mCRC who received up-front surgery for metastatic disease; and (v) MSI-H mCRC receiving treatment with immune checkpoint inhibitors. Evaluation of KRAS mutational status was carried out on formalin-fixed paraffin-embedded samples from primary tumors or metastasis assessed at local centers according to international approved standard methods.
Information regarding treatment patterns including the type of first-, second- and third-line chemotherapy was collected (Supplementary Tables S1 and S2, available at https://doi.org/10.1016/j.esmoop.2022.100567).
Descriptive statistics were used for clinicopathological features (Table 1). Response rate was assessed according to international guidelines. OS was defined as time from diagnosis of metastasis and death or last follow-up. PFS was defined as the time from treatment initiation to time of disease progression, death or last follow-up for patients alive without progression. Survival outcomes were calculated using the Kaplan–Meier method. Statistical analyses were conducted by using the SPSS package (v23).
Table 1.
Patients characteristics
| Characteristics | N = 111 |
|---|---|
| Age | |
| Years | 65 (40-80) |
| Sex, n (%) | |
| F | 45 (40.5) |
| M | 66 (59.5) |
| Performance status, n (%) | |
| 0 | 64 (57.7) |
| 1 | 43 (38.7) |
| 2 | 4 (3.6) |
| Tumor location, n (%) | |
| Rectum | 32 (28.8) |
| Right colon | 34 (30.6) |
| Left colon | 45 (40.5) |
| Primary tumor resection, n (%) | |
| Yes | 67 (60.4) |
| No | 44 (39.6) |
| Grading, n (%) | |
| 1 | 4 (3.6) |
| 2 | 41 (37) |
| 3 | 20 (18) |
| NA | 46 (41.4) |
| Mucinous histology, n (%) | |
| Yes | 18 (16.2) |
| No | 79 (71.2) |
| NA | 14 (12.6) |
| Microsatellite instability, n (%) | |
| MSI | 0 (0) |
| MSS | 61 (55) |
| NA | 50 (45) |
| Metastatic disease at initial diagnosis, n (%) | |
| Yes | 73 (65.8) |
| No | 38 (34.2) |
| Number of metastatic sites, n (%) | |
| <3 | 79 (71.2) |
| ≥3 | 32 (28.8) |
| Liver metastasis, n (%) | |
| Yes | 71 (64) |
| No | 40 (36) |
| Lung metastasis, n (%) | |
| Yes | 46 (41.4) |
| No | 65 (58.6) |
| Peritoneal metastasis, n (%) | |
| Yes | 21 (18.9) |
| No | 90 (81.1) |
| Nodes metastasis, n (%) | |
| Yes | 19 (17.1) |
| No | 92 (82.9) |
| CEA levels, n (%) | |
| <5 | 18 (16.2) |
| ≥5 | 68 (61.3) |
| NA | 25 (22.5) |
| Type of first-line treatment, n (%) | |
| Oxaliplatin-based doublet | 66 (59.5) |
| Irinotecan-based doublet | 29 (26.1) |
| FOLFOXIRI triplet | 16 (14.4) |
| Antiangiogenic use in combination with first-line chemotherapy, n (%) | |
| Yes | 80 (66.1) |
| No | 31 (33.9) |
CEA, carcinoembryonic antigen; F, female; M, male; MSI, microsatellite instability: MSS, microsatellite stable. NA, not available.
The study was conducted in accordance with the precepts of Good Clinical Practice and Declaration of Helsinki and was approved by the ethics committees of each participating institution.
Results
Medical records of 6952 patients with mCRC were evaluated; 256 (3.7%) displayed KRASG12C mutation (Supplementary Figure S1, available at https://doi.org/10.1016/j.esmoop.2022.100567). Of these, 111 patients with unresectable mCRC met the inclusion criteria and were included in the analysis (Supplementary Figure S1, available at https://doi.org/10.1016/j.esmoop.2022.100567). Baseline characteristics of the population are reported in Table 1. The median age was 65 years (range, 40-80 years) with a slight prevalence of male (66, 59.5%) compared with female (45, 40.5%). Most patients had a good performance status (PS) according to the Eastern Cooperative Oncology Group (ECOG); PS 0 was observed in 66/111 patients (57.7%), PS 1 in 43/111 (38.7%) and PS 2 only in 4 patients (3.6%). Forty-five (40.5%) patients had left-sided tumors, 32 (28.8%) to the rectal primary and 34 (30.6%) had a right-sided CRC. Primary tumor resection was carried out in approximately two-thirds of the cases (67/111, 60.4%). Only four tumors were well differentiated (3.6%). No MSI-H tumors were included in the study population; however, it should be noticed that patients with MSI-H tumors receiving first-line immunotherapy were excluded. Most of the patients had a metastatic disease at diagnosis (73/111, 65.8%). In line with previous findings, liver (71/111, 64%) and lung (46/111; 41.4%) were the most frequent metastatic sites.10,13,15
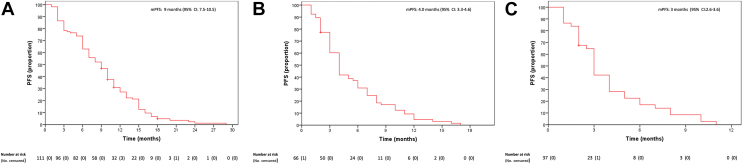
Patients with mCRC harboring KRASG12C mutation displayed disappointing responsiveness to chemotherapy. Of the 111 patients who received first-line chemotherapy, 3/111 (2.7%) had a complete response, 40 (36%) a partial response (PR), 42 (37.8%) stable disease (SD) and 26 (23.4%) progressive disease (PD) as best response (Table 2). Consequently, the ORR was 38.7% and disease control rate (DCR) (76.6%). Median PFS (mPFS) was 9 months [95% confidence interval (CI) 7.5-10.5 months] (Figure 1). Median OS (mOS) was 21 months (95% CI 17.4-24.6 months) (Figure 2). After progression, only 62% and 36% of the patients were able to receive a second and third line of treatment, respectively (Supplementary Tables S2, available at https://doi.org/10.1016/j.esmoop.2022.100567). Of 66 patients who received a second-line treatment, 62 had a measurable response; 5 (8.1%) experienced PR, 26 patients (41.9%) SD and 31 patients (50%) PD. ORR was 8.1%, with DCR 50% (Table 2). mPFS was 4.0 months (95% CI 3.3-4.6 months) (Figure 1). Only 37 patients received a third-line treatment. For 35 patients with available response evaluation, 10 patients (28.6%) obtained SD and 25 patients (71.4%) PD as best response (Table 2). mPFS was 3 months (95% CI 2.6-3.6 months) (Figure 1).
Table 2.
Response to first-, second- and third-line of therapies and according to the type of first-line treatment
| CR | PR | SD | PD | ORR | DCR | |
|---|---|---|---|---|---|---|
| Response in different line of treatments | ||||||
| First line (n = 111) | 3 (2.7%) | 40 (36%) | 42 (37.8%) | 26 (23.4%) | 43 (38.7%) | 85 (76.6%) |
| Second line (n = 62) | 0 (0%) | 5 (8.1%) | 26 (41.9%) | 31 (50%) | 5 (8.1%) | 31 (50%) |
| Third line (n = 35) | 0 (0%) | 0 (0%) | 10 (28.6%) | 25 (71.4%) | 0 (0%) | 10 (28.6%) |
| Response according to the type of first-line treatment | ||||||
| Irinotecan-based chemotherapy doublet (n=29) | 0 (0%) | 10 (34.5%) | 11 (37.9%) | 8 (27.6%) | 10 (34.5%) | 21 (72.41%) |
| Oxaliplatin-based chemotherapy doublet (n=66) | 2 (3.0%) | 22 (33.3%) | 26 (39.4%) | 16 (24.2%) | 24 (36.36%) | 50 (75.75%) |
| Chemotherapy triplet (n=16) | 1 (6.3%) | 8 (50%) | 5 (31.3%) | 2 (12.5%) | 9 (56.25%) | 14 (87.5%) |
CR, complete response; DCR, disease response rate; ORR, overall response rate; PD, progressive disease; PR, partial response; SD, stable disease.
Figure 1.
Progression-free survival of (A) first-, (B) second- and (C) third-line therapies.
CI, confidence interval; mPFS, median progression-free survival; PFS, progression-free survival.
Figure 2.
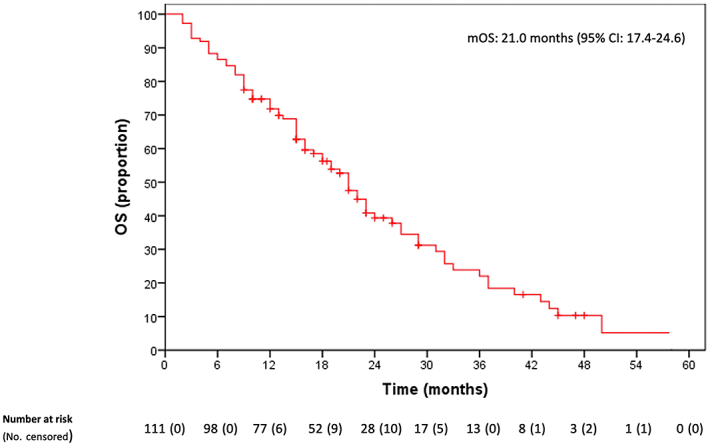
Overall survival.
CI, confidence interval; mOS: median overall survival; OS, overall survival.
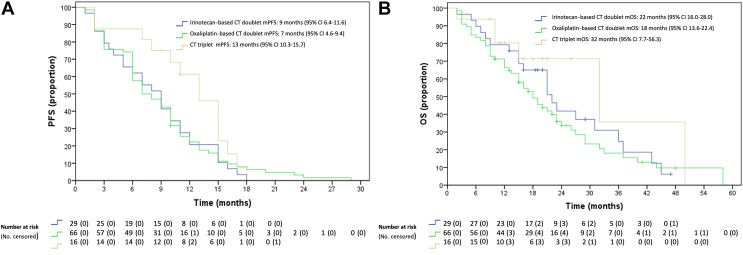
Since after disease progression to first-line treatment, the efficacy of further treatments was very limited, we evaluated the patterns of response in patients receiving first-line oxaliplatin- or irinotecan-based chemotherapy doublets or triplets (Supplementary Tables S1, available at https://doi.org/10.1016/j.esmoop.2022.100567). In the subgroup of patients receiving first-line folinic acid, 5-fluorouracil, oxaliplatin and irinotecan (FOLFOXIRI)-based triplets, ORR was 56.3% (9/16), whereas it was 36.4% (24/66) for patients receiving oxaliplatin-based doublets and it was 34.5% (10/29) for patients treated with irinotecan-based doublets (Table 2). A longer PFS with nearly statistically significant P value was observed in patients receiving chemotherapy triplets. The mPFS of patients receiving first-line FOLFOXIRI-based triplets was 13 months (95% CI 10.3-15.7 months) compared with an mPFS of 9 months (95% CI 6.4-11.6 months) in the irinotecan-based doublet group [hazard ratio (HR), 0.53; 95% CI 0.27-1.039; P = 0.065] and as compared with mPFS 7 months (95% CI 4.6-9.4 months) in the oxaliplatin-based doublet group (HR, 0.58; 95% CI 0.31-1.06; P = 0.075) (Figure 3). Similarly, an improved OS was observed in the patient group receiving chemotherapy triplets. In fact, mOS was 32 months (95% CI 7.7-56.3 months) compared with an mOS of 22 months (95% CI 16.0-28.0 months) (HR, 0.58; 95% CI 0.22-1.54 months; P = 0.275) and mOS of 18 months (95% CI 13.6-22.4 months) (HR, 0.52; 95% CI 0.22-1.26; P = 0.136) for patients treated with irinotecan- and oxaliplatin-based chemotherapy doublets, respectively. Finally, we evaluated the impact of adding bevacizumab to chemotherapy on treatment response. A total of 80 out of 111 patients received chemotherapy plus bevacizumab and 31 out of 111 underwent treatment without antiangiogenic drugs.
Figure 3.
(A) Median progression-free survival and (B) overall survival according to the type of first-line therapy.
CI, confidence interval; CT, chemotherapy; mPFS, median progression-free survival, OS, overall survival.
No difference in terms of ORR was observed between the two groups (ORR 38.75% versus 38.7% for patients treated with or without bevacizumab) (Supplementary Tables S3, available at https://doi.org/10.1016/j.esmoop.2022.100567). The addition of bevacizumab to chemotherapy compared with chemotherapy alone, however, was associated with a statistically significant improvement in mPFS 10 months (95% CI 8.6-11.4 months) versus 6 months (95% CI 3.7-8.3 months) (HR, 0.48; 95% CI 0.31-0.75; P = 0.001) and mOS 23 months (95% CI 19.8-26.2 months) versus 15 months (95% CI 9.1-20.9) (HR, 0.51; 95% CI 0.31-0.82; P = 0.005) (Supplementary Figure S2, available at https://doi.org/10.1016/j.esmoop.2022.100567).
Discussion
Treatment of mCRC is rapidly evolving thanks to better understanding of tumor biology.17 For more than a decade, different KRAS mutations were considered to have the same value as mechanisms of resistance to anti-EGFR therapies.14,18 This concept has changed in the past few years due to the discovery of selective KRASG12C-mutant inhibitors, that proved clinical activity in various tumor types, including non-small-cell lung cancer, CRC, endometrial and pancreatic cancer.19, 20, 21, 22 The percentage of KRASG12C-mutant mCRC is low, however, and the prognostic and predictive role of this mutation is still debated and poorly understood.9, 10, 11, 12, 13, 14, 15, 16
In this scenario, to clarify the impact of KRASG12C mutation on the outcome of patients treated with chemotherapy, we conducted a retrospective study on a large population of patients with unresectable mCRC who were treated with intensive chemotherapy regimens in 12 Italian institutions. We confirm that the occurrence of KRASG12C mutation is rare, with 256 positive tumors out of 6952 (3.7%). This result is in line with previous findings by Nassar and colleagues,16 who reported KRASG12C mutation in 3.2% of patients (234/7402) with CRC. A similar percentage was reported in an Asian study (2.8%, 45/1632) by Chida and colleagues.13
Notably, due to the selection which excluded patients not treated with doublet or triplet chemotherapy, the study population has comparable characteristics with patients enrolled in clinical trial.23,24 Nevertheless, in this cohort of mCRC patients, clinical efficacy of any line of therapy was reduced compared with non-KRASG12C mutant mCRC.1 These results highlight the biologic and clinical aggressiveness of this disease. In fact, in a KRAS-mutant mCRC population, an ORR of ∼50% could be expected in fit patients who are treated with chemotherapy doublets with or without bevacizumab.25,26 In the present study, we report an ORR of 38.7% for first-line treatments, thus indicating that KRASG12C mutation could define resistance to chemotherapy. Similar results were reported by Giampieri and colleagues,14 that compared the ORR of KRASG12C-mutant (15 patients) with other KRAS-mutant (105 patients) with mCRC receiving chemotherapy doublets plus bevacizumab. Remarkably, with the limitation of a study with a very small number of patients, the ORR was significantly inferior in the KRASG12C group (27% versus 52%, P = 0.017).
Of note, in the present study we observe that after disease progression, only 62% of patients are able to receive a second-line treatment and only 36% of them a third-line therapy. This percentage is significantly lower compared with other reports, in which most of the patients with mCRC receive a second line of treatment and about two-thirds a further line.27 Furthermore, the activity of further lines of treatments was limited compared with clinical trial results and with real-life data of mCRC patients.1,28, 29, 30, 31, 32, 33 In this respect, here we report an ORR of only 8.1% in second line with one out two patients experiencing PD as best response. Even worse results were obtained by third-line treatments with no response and most of the patients (71.4%) with progression of disease at the first radiological evaluation.
Therefore, novel therapeutic strategies are urgently required for this mCRC group of patients with a mostly chemoresistant disease. In this regard, signals of clinical activity of sotorasib, a selective KRASG12C inhibitor, were recently reported.7 In the CodeBreaK100 phase 1 trial, the safety of sotorasib was assessed in 62 heavily pretreated patients with KRASG12C-mutant mCRC. Six patients (10%) experienced PR, whereas 45 patients (73%) had SD as best response. mPFS was 4 months (95% CI 2.8-4.2 months) and mOS was 10.6 months (95% CI 7.7-15.6 months). There is strong preclinical evidence that combining anti-EGFR blockade could enhance the efficacy of KRASG12C inhibition.34 At the ESMO 2021 Annual Congress, the preliminary results of the KRYSTAL-1 trial investigating adagrasib as monotherapy or in combination with cetuximab in chemorefractory patients with KRASG12C-mutant mCRC were presented.8 Interestingly, among 28 patients who received the combined treatment, the ORR was 43% (12/28, including 2 unconfirmed PR) and DCR was 100%. Based on these findings, an increasing number of clinical trials investigating different KRASG12C inhibitors alone or in combination with other drugs in refractory mCRC are currently ongoing.35 Considering the limited treatment options and the promising results of KRASG12C target therapies, if available, the enrollment of such patients in clinical trials should be highly recommended.
While this class of drugs is still under clinical development, however, which is the best treatment strategy for this subset of patients in clinical practice? Considering that the maximum clinical benefit was derived by first-line treatment, we conducted a subgroup analysis to evaluate if the type of first-line regimen could impact on the course of disease. To our knowledge, although with the limitations of a retrospective multicenter analysis in 12 Italian centers, the present study reports the largest analysis so far in patients with KRASG12C-mutant mCRC who were treated with an intensive first-line therapy, including chemotherapy triplets. In this respect, in other similar studies, results with FOLFOXIRI-based regimens have not been described.9,12, 13, 14, 15 In the subgroup of patients receiving FOLFOXIRI-based therapies, a numerical increase in objective responses was observed (ORR, 56.25%) compared with irinotecan-based (34.5%) or oxaliplatin-based (36.4%) doublets. This finding was accompanied by a numerically better PFS in patients who were treated with chemotherapy triplets. These results should be interpreted with caution due the limits of a retrospective analysis in a real-life setting with a relatively small number of patients. Based on the results of the TRIBE and TRIBE-2 phase III clinical trials, FOLFOXIRI plus bevacizumab is considered as one of the standards of care in the first-line treatment of fit mCRC patients.36,37 Interestingly, a meta-analysis of individual patient data from five randomized clinical trials confirmed that FOLFOXIRI plus bevacizumab clinical efficacy was also retained in KRAS-mutant mCRC patients; however, at the cost of increased toxicity.38 Thus, there is a rationale to intensify the first-line treatment in fit patients with a KRASG12C-mutant mCRC. This hypothesis deserves to be confirmed by further analysis. The use of bevacizumab in combination with chemotherapy is considered a standard of care for the treatment of patients with RAS-mutant mCRC.1 It should be noticed, however, that in daily practice not all patients receive bevacizumab, due to the existence of contraindications, comorbidities or investigator choice. In our study, approximately two-thirds of the patients were treated with an antiangiogenic drug in combination with chemotherapy; conversely one-third were treated only with doublet or triplet chemotherapy. The use of bevacizumab resulted in improved mPFS and mOS. It should be considered, however, that these results could be affected by the difference in patient characteristics. Our results are in line with previous findings and support the use of bevacizumab in combination with chemotherapy, if feasible, for patients with KRASG12C-mutant mCRC.1,23,24
Conclusion
Patients with KRASG12C-mutant mCRC have a very aggressive disease with reduced response to standard treatments. After progression to first-line therapy, the efficacy of subsequent treatments is limited. Therefore, target therapies with selective KRASG12C inhibitors are urgently needed. Although the results reported here should be taken as hypothesis-generating findings and must be further validated, an intensified first-line therapy with FOLFOXIRI plus bevacizumab might currently represent a valid treatment option for selected, fit patients with KRASG12C-mutant mCRC.
Acknowledgements
We thank the Gruppo Oncologico dell’Italia Meridionale (GOIM).
Funding
None declared.
Disclosure
DC travel support from Sanofi and Bristol Myers Squibb (BMS). CP reports outside the submitted work personal fees for advisory role, speaker engagements and travel and accommodation expenses from Amgen, Astellas, AstraZeneca, Bayer, BMS, Clovis Oncology, Ipsen, Janssen, Incyte, Merck Serono, Merck Sharp & Dohme (MSD), Novartis, Roche and Sanofi. AZ speaker bureau: Amgen, Bayer, Servier, Pierre Fabre, Merck Serono, MSD, AstraZeneca. RBo honoraria: Novartis, AstraZeneca, Sanofi, Amgen, Roche, Pfizer, Janssen-Cilag, BMS. Consulting or advisory role: Novartis, Bayer, AstraZeneca, Sanofi, Amgen, Roche, Pfizer, Janssen-Cilag, BMS. Speakers’ bureau: AstraZeneca, Sanofi, Novartis, Bayer, Amgen, Roche, Pfizer, Janssen-Cilag, BMS. MDM honoraria BMS, MSD, Pfizer, Takeda Pharmaceuticals, AstraZeneca, Janssen Pharmaceuticals, Mediolanum Farmaceutici, Eisai. Consulting or advisory role: AstraZeneca, MSD, Pfizer, Takeda Pharmaceuticals, Janssen Pharmaceuticals, Mediolanum Farmaceutici, Eisai. AP reported receiving advisory board fees from GlaxoSmithKline, Servier, Pharmamar. RG reported receiving fees from Amgen and Servier and advisory board fees from Amgen, Servier, Bayer and Merck Serono. RBe consulting or advisory role: Lilly, Boehringer Ingelheim, Amgen, AstraZeneca, Novartis, Roche, GlaxoSmithKline, Eisai, MSD Oncology. BAM travel support from BMS. TPL has served as speaker for Servier. NN has served as an advisor and speaker for MSD, Qiagen, Biocartis, Incyte, Roche, BMS, Merck, Thermo Fisher, Boehringer Ingelheim, AstraZeneca, Sanofi, Eli Lilly and Bayer. AA has served as advisory and speaker for Amgen and Servier. ADS has served as speaker for Amgen. TT has served as advisor and speaker for Roche, Merck Serono, Sanofi, Servier, Novartis, Bayer. EMar has served as advisor and speaker for AstraZeneca, Amgen, Bayer, Merck Serono, Roche, Sanofi, Servier, Pierre Fabre. FC has served as advisor and speaker for Roche, Amgen, Merck Serono, Pfizer, Sanofi, Bayer, Servier, BMS, Cellgene, Lilly. Received institutional research grants from Bayer, Roche, Merck Serono, Amgen, AstraZeneca, Takeda. FeDV has served as advisor and speaker for BMS, Amgen, Cellgene, Lilly, Roche. EMai has served as advisor and speaker for AstraZeneca, Eli Lilly, Servier, Sanofi Genzyme, Roche, Merck, Eisai, Pfizer. All other authors have declared no conflicts of interest.
Data Sharing
The data collected for this study could be available in a de-identified form after reasonable request.
Supplementary Data
References
- 1.Ciardiello F., Ciardiello D., Martini G., Napolitano S., Tabernero J., Cervantes A. Clinical management of metastatic colorectal cancer in the era of precision medicine. CA Cancer J Clin. 2022;72:372–401. doi: 10.3322/caac.21728. [DOI] [PubMed] [Google Scholar]
- 2.Martinelli E., Ciardiello D., Martini G., et al. Implementing anti-epidermal growth factor receptor (EGFR) therapy in metastatic colorectal cancer: challenges and future perspectives. Ann Oncol. 2020;31(1):30–40. doi: 10.1016/j.annonc.2019.10.007. [DOI] [PubMed] [Google Scholar]
- 3.Tabernero J., Grothey A., Van Cutsem E., et al. Encorafenib plus cetuximab as a new standard of care for previously treated BRAFV600E-mutant metastatic colorectal cancer: updated survival results and subgroup analyses from the BEACON study. J Clin Oncol. 2021;39(4):273–284. doi: 10.1200/JCO.20.02088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.André T., Shiu K.K., Kim T.W., et al. Pembrolizumab in microsatellite-instability-high advanced colorectal cancer. N Engl J Med. 2020;383(23):2207–2218. doi: 10.1056/NEJMoa2017699. [DOI] [PubMed] [Google Scholar]
- 5.Lenz H.J., Van Cutsem E., Luisa Limon M., et al. First-line nivolumab plus low-dose ipilimumab for microsatellite instability-high/mismatch repair-deficient metastatic colorectal cancer: the phase II CheckMate 142 study. J Clin Oncol. 2022;40(2):161–170. doi: 10.1200/JCO.21.01015. [DOI] [PubMed] [Google Scholar]
- 6.Porru M., Pompili L., Caruso C., Biroccio A., Leonetti C. Targeting KRAS in metastatic colorectal cancer: current strategies and emerging opportunities. J Exp Clin Cancer Res. 2018;37(1):57. doi: 10.1186/s13046-018-0719-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fakih M.G., Kopetz S., Kuboki Y., et al. Sotorasib for previously treated colorectal cancers with KRASG12C mutation (CodeBreaK100): a prespecified analysis of a single-arm, phase 2 trial. Lancet Oncol. 2022;23(1):115–124. doi: 10.1016/S1470-2045(21)00605-7. [DOI] [PubMed] [Google Scholar]
- 8.Weiss J., Yaeger R.D., Johnson M.L., et al. LBA6 KRYSTAL-1: Adagrasib (MRTX849) as monotherapy or combined with cetuximab (Cetux) in patients (Pts) with colorectal cancer (CRC) harboring a KRASG12C mutation. Ann Oncol. 2021;32:S1294. [Google Scholar]
- 9.Henry J.T., Coker O., Chowdhury S., et al. Comprehensive clinical and molecular characterization of KRASG12C-mutant colorectal cancer. JCO Precis Oncol. 2021;5 doi: 10.1200/PO.20.00256. PO.20.00256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schirripa M., Nappo F., Cremolini C., et al. KRAS G12C metastatic colorectal cancer: specific features of a new emerging target population. Clin Colorectal Cancer. 2020;19(3):219–225. doi: 10.1016/j.clcc.2020.04.009. [DOI] [PubMed] [Google Scholar]
- 11.Ottaiano A., Normanno N., Facchini S., et al. Study of Ras mutations’ prognostic value in metastatic colorectal cancer: STORIA analysis. Cancers (Basel) 2020;12(7):1919. doi: 10.3390/cancers12071919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fakih M., Tu H., Hsu H., et al. Real-world study of characteristics and treatment outcomes among patients with KRAS p.G12C-mutated or other KRAS mutated metastatic colorectal cancer. The Oncologist. 2022;27(8):663–674. doi: 10.1093/oncolo/oyac077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chida K., Kotani D., Masuishi T., et al. The prognostic impact of KRAS G12C mutation in patients with metastatic colorectal cancer: a multicenter retrospective observational study. Oncologist. 2021;26(10):845–853. doi: 10.1002/onco.13870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Giampieri R., Lupi A., Ziranu P., et al. Retrospective comparative analysis of KRAS G12C vs. other KRAS mutations in mCRC patients treated with first-line chemotherapy doublet + bevacizumab. Front Oncol. 2021;11:736104. doi: 10.3389/fonc.2021.736104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Osterlund E., Ristimäki A., Kytölä S., et al. KRAS-G12C mutation in one real-life and three population-based Nordic cohorts of metastatic colorectal cancer. Front Oncol. 2022;12:826073. doi: 10.3389/fonc.2022.826073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nassar A.H., Adib E., Kwiatkowski D.J. Distribution of KRASG12C somatic mutations across race, sex, and cancer type. N Engl J Med. 2021;384(2):185–187. doi: 10.1056/NEJMc2030638. [DOI] [PubMed] [Google Scholar]
- 17.Di Nicolantonio F., Vitiello P.P., Marsoni S., et al. Precision oncology in metastatic colorectal cancer - from biology to medicine. Nat Rev Clin Oncol. 2021;18(8):506–525. doi: 10.1038/s41571-021-00495-z. [DOI] [PubMed] [Google Scholar]
- 18.Martini G., Ciardiello D., Vitiello P.P., et al. Resistance to anti-epidermal growth factor receptor in metastatic colorectal cancer: what does still need to be addressed? Cancer Treat Rev. 2020;86:102023. doi: 10.1016/j.ctrv.2020.102023. [DOI] [PubMed] [Google Scholar]
- 19.Hong D.S., Fakih M.G., Strickler J.H., et al. KRASG12C inhibition with sotorasib in advanced solid tumors. N Engl J Med. 2020;383(13):1207–1217. doi: 10.1056/NEJMoa1917239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Skoulidis F., Li B.T., Dy G.K., et al. Sotorasib for lung cancers with KRAS p.G12C mutation. N Engl J Med. 2021;384(25):2371–2381. doi: 10.1056/NEJMoa2103695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ou S.I., Jänne P.A., Leal T.A., et al. First-in-human phase I/IB dose-finding study of adagrasib (MRTX849) in patients with advanced KRASG12C solid tumors (KRYSTAL-1) J Clin Oncol. 2022;40(23):2530–2538. doi: 10.1200/JCO.21.02752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jänne P.A., Riely G.J., Gadgeel S.M., et al. Adagrasib in non-small-cell lung cancer harboring a KRASG12C mutation. N Engl J Med. 2022;387(2):120–131. doi: 10.1056/NEJMoa2204619. [DOI] [PubMed] [Google Scholar]
- 23.Hurwitz H., Fehrenbacher L., Novotny W., et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350(23):2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 24.Van Cutsem E., Rivera F., Berry S., et al. Safety and efficacy of first-line bevacizumab with FOLFOX, XELOX, FOLFIRI and fluoropyrimidines in metastatic colorectal cancer: the BEAT study. Ann Oncol. 2009;20(11):1842–1847. doi: 10.1093/annonc/mdp233. [DOI] [PubMed] [Google Scholar]
- 25.Tang W., Ren L., Liu T., et al. Bevacizumab plus mFOLFOX6 versus mFOLFOX6 alone as first-line treatment for RAS mutant unresectable colorectal liver-limited metastases: the BECOME randomized controlled trial. J Clin Oncol. 2020;38(27):3175–3184. doi: 10.1200/JCO.20.00174. [DOI] [PubMed] [Google Scholar]
- 26.Saltz L.B., Clarke S., Díaz-Rubio E., et al. Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: a randomized phase III study. J Clin Oncol. 2008;26(12):2013–2019. doi: 10.1200/JCO.2007.14.9930. [DOI] [PubMed] [Google Scholar]
- 27.Tampellini M., Di Maio M., Baratelli C., et al. Treatment of patients with metastatic colorectal cancer in a real-world scenario: probability of receiving second and further lines of therapy and description of clinical benefit. Clin Colorectal Cancer. 2017;16(4):372–376. doi: 10.1016/j.clcc.2017.03.019. [DOI] [PubMed] [Google Scholar]
- 28.Van Cutsem E., Tabernero J., Lakomy R., et al. Addition of aflibercept to fluorouracil, leucovorin, and irinotecan improves survival in a phase III randomized trial in patients with metastatic colorectal cancer previously treated with an oxaliplatin-based regimen. J Clin Oncol. 2012;30(28):3499–3506. doi: 10.1200/JCO.2012.42.8201. [DOI] [PubMed] [Google Scholar]
- 29.Bennouna J., Sastre J., Arnold D., et al. Continuation of bevacizumab after first progression in metastatic colorectal cancer (ML18147): a randomised phase 3 trial. Lancet Oncol. 2013;14(1):29–37. doi: 10.1016/S1470-2045(12)70477-1. [DOI] [PubMed] [Google Scholar]
- 30.Lavacchi D., Roviello G., Giommoni E., et al. Aflibercept plus FOLFIRI as second-line treatment for metastatic colorectal cancer: a single-institution real-life experience. Cancers (Basel) 2021;13(15):3863. doi: 10.3390/cancers13153863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grothey A., Van Cutsem E., Sobrero A., et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet. 2013;381(9863):303–312. doi: 10.1016/S0140-6736(12)61900-X. [DOI] [PubMed] [Google Scholar]
- 32.Mayer R.J., Van Cutsem E., Falcone A., et al. Randomized trial of TAS-102 for refractory metastatic colorectal cancer. N Engl J Med. 2015;372(20):1909–1919. doi: 10.1056/NEJMoa1414325. [DOI] [PubMed] [Google Scholar]
- 33.Martinelli E., Sforza V., Cardone C., et al. Clinical outcome and molecular characterisation of chemorefractory metastatic colorectal cancer patients with long-term efficacy of regorafenib treatment. ESMO Open. 2017;2(3):e000177. doi: 10.1136/esmoopen-2017-000177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Amodio V., Yaeger R., Arcella P., et al. EGFR blockade reverts resistance to KRASG12C inhibition in colorectal cancer. Cancer Discov. 2020;10(8):1129–1139. doi: 10.1158/2159-8290.CD-20-0187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Patelli G., Tosi F., Amatu A., et al. Strategies to tackle RAS-mutated metastatic colorectal cancer. ESMO Open. 2021;6(3):100156. doi: 10.1016/j.esmoop.2021.100156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cremolini C., Loupakis F., Antoniotti C., et al. FOLFOXIRI plus bevacizumab versus FOLFIRI plus bevacizumab as first-line treatment of patients with metastatic colorectal cancer: updated overall survival and molecular subgroup analyses of the open-label, phase 3 TRIBE study. Lancet Oncol. 2015;16(13):1306–1315. doi: 10.1016/S1470-2045(15)00122-9. [DOI] [PubMed] [Google Scholar]
- 37.Cremolini C., Antoniotti C., Rossini D., et al. Upfront FOLFOXIRI plus bevacizumab and reintroduction after progression versus mFOLFOX6 plus bevacizumab followed by FOLFIRI plus bevacizumab in the treatment of patients with metastatic colorectal cancer (TRIBE2): a multicentre, open-label, phase 3, randomised, controlled trial. Lancet Oncol. 2020;21(4):497–507. doi: 10.1016/S1470-2045(19)30862-9. [DOI] [PubMed] [Google Scholar]
- 38.Cremolini C., Antoniotti C., Stein A., et al. Individual patient data meta-analysis of FOLFOXIRI plus bevacizumab versus doublets plus bevacizumab as initial therapy of unresectable metastatic colorectal cancer. J Clin Oncol. 2020;38(28):3314–3324. doi: 10.1200/JCO.20.01225. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.