Abstract
Background
The role of both hormonal contraception and pregnancy on the outcomes of desmoid-type fibromatosis (DF) is debatable.
Materials and methods
In the present study, we selected female patients of childbearing age from the prospective ALTITUDES cohort. The primary study endpoint was event-free survival (EFS), with an event defined as relapse or progression. We estimated the risk of events according to the use of hormonal contraception [estrogen–progestin (EP) and progestin] and pregnancy status using multivariate time-dependent models, controlling for major confounders.
Results
A total of 242 patients (median age, 34.7 years) were included in the present study. The abdominal wall was the most common tumor site (51%). Patients were managed by active surveillance (80%) or surgery (20%). Pregnancy occurred within 24 months before, at the time of, and after DF diagnosis in 33%, 5%, and 10% of the cases, respectively. Exposure to hormonal contraception was documented within 24 months before, at the time of, and after diagnosis in 44%, 34%, and 39% of the cases, respectively. The 2-year EFS was 75%. After adjusting for DF location, tumor size, front-line treatment strategy, and hormonal contraception, we observed an increased risk of events occurring at 24 months after pregnancy [hazard ratio (HR) = 2.09, P = 0.018]. We observed no statistically significant association between the risk of events and current EP exposure (HR = 1.28, P = 0.65), recent EP exposure (within 1-24 months, HR = 1.38, P = 0.39), current progestin exposure (HR = 0.81, P = 0.66), or recent progestin exposure (HR = 1.05, P = 0.91).
Conclusions
In our study, a recent history of pregnancy was associated with an increased risk of progression/relapse in patients with newly diagnosed DF, whereas hormonal contraception did not demonstrate an association with progression/relapse.
Key words: desmoid-type fibromatosis, hormonal contraception, hormone-dependency tumor, outcome, pregnancy
Highlights
-
•
The role of hormonal contraception or pregnancy on DF outcomes is debatable.
-
•
We estimated the risk of progression or relapse according to the use of hormonal contraception and pregnancy status.
-
•
We found that recent exposure to hormonal contraception was not associated with DF outcomes.
-
•
A recent pregnancy was associated with a statistically significant increase in progression/relapse.
-
•
Abdominal wall DF outcomes showed similar findings as other DF outcomes.
Introduction
Desmoid-type fibromatosis (DF) is a rare, ubiquitous non-metastasizing malignancy that occurs in less than six individuals per one million population per year in France.1 Most cases occur among women, and the peak incidence is at ∼40-45 years of age.1 At the pathological level, DF comprises a monoclonal proliferation of fibroblast-like cells forming a locally aggressive soft tissue tumor. The accumulation of β-catenin via deregulation of the adenomatous polyposis coli (APC)/Wnt/β-catenin pathway is a molecular driver of DF. Two mutually exclusive mechanisms have been described: somatic mutations of catenin β1 (85%-90% of cases) and germline APC mutations (10%-15% cases). Other driving molecular alterations are uncommon.
DF is rarely multifocal, but local recurrence after surgery is observed in ∼40%-60% of cases. Outcomes are unpredictable and span a range from spontaneous regression to life-threatening tumor progression. Since the tumor course and risk of local relapse are unpredictable, the potential harms and benefits of surgery have to be weighed carefully. Therefore, in recent years, the recommended initial management strategy has shifted from attempted large en bloc resection to active surveillance. Only progressive or symptomatic DF cases are actively treated, mainly by systemic treatment.2 DF is a slowly growing tumor, and most relapses or progressions occur within 24 months after DF diagnosis.1
For decades, DF has been regarded as a possible hormone-dependent malignancy based on the following indirect arguments: expression of estrogen receptors in DF,3, 4, 5, 6, 7 the predominance of female patients with this condition,1,2 peak incidence at the childbearing age,1,2 literature reporting diagnosis or relapse after pregnancy (especially in cases of abdominal wall DF),8 and observed tumor shrinkage with the use of certain anti-estrogen agents or after delivery.9,10 The treating physicians, patients, and families are frequently uncomfortable with this possible hormonal dependency in DF and face a daily dilemma with regard to contraindications for hormonal contraception and the risks of worsening DF due to a new pregnancy. This open question has become especially relevant in the context of active surveillance. In the absence of strong evidence, recent guidelines remain vague about this critical concern.2
Hence, in the current prospective cohort of female patients with incident cases of confirmed DF, we aimed to answer the following three questions:
-
(1)
Does exposure to hormonal contraception increase the risk of progression or relapse in DF?
-
(2)
Do pregnancies increase the risk of progression or relapse in DF?
-
(3)
Do these possible associations persist after considering major known prognostic factors (tumor location, tumor size, and therapeutic strategy)?
Materials and methods
Study overview
ALTITUDES is a prospective nationwide clinical–biological cohort of patients with incident DF diagnosed between January 2016 and February 2021 and confirmed by central pathological review.1
The study inclusion criteria were as follows: (i) incident cases of DF diagnosed in France, (ii) diagnosis confirmed by a pathology review conducted by the French Sarcoma Group, (iii) affiliated to National Health Insurance, and (iv) the provision of signed informed consent by the patient or his or her legal guardians (both parents’ signatures were required in adolescent patients).
The study exclusion criteria were as follows: (i) deprivation of liberty and (ii) patients who were unable or unwilling to provide consent. The ALTITUDES study does not include specific therapeutic interventions, but instead provides prospective, observational data collection and biobanking (i.e. tumor samples, blood samples, and baseline imaging scans). The ALTITUDES study is purely descriptive and hypothesis-generating, and there was no formal sample size calculation.
For the present study, we selected female patients aged 16-45 years at the time of DF diagnosis who were diagnosed with unifocal DF. The enrolled patients were treated with initial surgery or active surveillance as the initial treatment approach. For the present analysis, we excluded patients who had received hormonal therapy for reasons other than contraception (e.g. for adjuvant treatment of breast cancer). All data were collected prospectively, with the exception of the precise nature of hormonal contraception, as that required retrospective data collection during the brief planned visits or phone interviews.
This study was approved by the ethics committee at CPP Nord-Ouest I (the French governmental organization for the protection of persons in biomedical research; 3 December 2015) and by the French Drug Agency (ANSM; 20 November 2015). This study was conducted in accordance with the principles of the Declaration of Helsinki and written informed consent was obtained from each patient. This investigation was registered under the following clinical trial registration number: NCT02867033.
Endpoints
As previously published by us,11,12 the primary endpoint evaluated in this study was event-free survival (EFS). The events considered in this study were local relapse after complete (R0/R1) resection, disease progression (according to the local investigator/evaluating physician) during active surveillance, or death. Data and EFS were reported in both therapeutic groups (i.e. the active surveillance and surgery groups) and for the overall study population.
Hormonal contraception and pregnancy exposure
We used an exposure assessment method considering the duration of successive hormonal exposures (i.e. pregnancy and hormonal contraception). To explore the association between the risk of events and hormonal exposures, we evaluated hormonal exposures as factors varying over time (rather than as fixed factors defined at baseline).13 We observed peak event occurrence between 6 and 18 months after pregnancy (Supplementary Table S1, available at https://doi.org/10.1016/j.esmoop.2022.100578). Therefore, the present study considered contraception exposure and pregnancies occurring within 24 months before or after DF diagnosis. All dates of estrogen–progestin (EP) and progestin contraception were collected, as well as the dates of conception and the outcomes of pregnancies (birth, miscarriage, or abortion) before and after the diagnosis of DF.
The time from the initial diagnosis of DF to the date of the registered event or date of the last follow-up was split into different periods for each patient and each exposure type (pregnancy, EP contraception, or progestin contraception): exposure, post-exposure, and no exposure. The period of exposure corresponded to treatment duration for contraception or pregnancy duration. The post-exposure period was defined as the period following the end of each exposure type. The ‘no-exposure’ period corresponded to no exposure at all, or periods before exposure or >24 months after exposure.
Statistical analysis
The EFS was tabulated using the Kaplan–Meier method. We modeled the risk of events associated with the different periods of hormonal exposure (pregnancy and hormonal contraception) using a Cox model incorporating time-dependent covariates in order to estimate the hazard ratios (HRs) associated with each study period (exposure, post-exposure, or no exposure).13 As no events were observed during pregnancy, we pooled the status of ‘pregnancy’ with the status of ‘post-pregnancy’ (i.e. ≤24 months), which is hereinafter termed ‘recent history of pregnancy’.
Associations between hormonal exposure (pregnancy, EP contraception, and progestin contraception) were evaluated using univariate analysis, followed by multivariate analysis, controlling for the main known prognostic factors of EFS: front-line approach (surgery versus active surveillance), tumor location (abdominal wall versus other sites), and tumor size. To illustrate these results, we generated EFS curves estimated using the multivariate models.
For each type of hormonal exposure, we evaluated the association between recent history of hormonal exposure (the period of exposure and the 24-month post-exposure period versus no exposure) and EFS according to the front-line treatment strategy, tumor site, and tumor size by including an interaction term in the multivariate models. We illustrated these subgroup analyses using forest plots.
All estimates are reported with their 95% confidence intervals (95% CIs). Analyses were carried out with a two-sided α level of 5%. Statistical analyses were conducted using STATA/SE statistical software (v.15.1; StataCorp, LP, College Station, TX).
Results
Overall, 628 patients were enrolled in the ALTITUDES study. Among them, 242 (38.7%) met the eligibility criteria for the present analysis (Supplementary Figure S1, available at https://doi.org/10.1016/j.esmoop.2022.100578). The study population is described in Table 1 and Supplementary Table S1, available at https://doi.org/10.1016/j.esmoop.2022.100578. Included patients were managed by active surveillance (80%) and surgery (20%). Among the 49 patients treated with surgery, information on the quality of surgery was available in 28 cases, which included 14 R0 resections, 10 R1 resections, and 4 R2 resections. Patient characteristics did not differ between those treated with surgery and those undergoing active surveillance (see Supplementary Table S1, available at https://doi.org/10.1016/j.esmoop.2022.100578).
Table 1.
Patient demographic and clinical characteristics
| Characteristics | Total, N= 242 |
|---|---|
| Age at inclusion (years) | |
| Median (min-max) | 34.7 (18.0-46.3) |
| Age at diagnosis (years) | |
| Median (min-max) | 34.1 (17.3-45.8) |
| 16-26, n (%) | 28 (11.6) |
| 26-36, n (%) | 124 (51.2) |
| 36-46, n (%) | 90 (37.2) |
| Tumor site (MD = 1), n (%) | |
| Abdominal wall | 124 (51.5) |
| Limbs | 22 (9.1) |
| Other site | 95 (39.4) |
| Tumor size (mm) (MD = 2)a | |
| Median (min-max) | 47 (4-530) |
| ≤50, n (%) | 138 (57.5) |
| >50, n (%) | 102 (42.5) |
| ECOG PS at baseline (MD = 35) | |
| 0 | 197 (95.2) |
| 1 | 10 (4.8) |
| CTNNB1 mutation (MD = 7) | |
| No, n (%) | 24 (10.2) |
| Yes, n (%) | 211 (89.8) |
The percentages may not sum up to 100% because of rounding.
ECOG PS, Eastern Cooperative Oncology Group performance status; MD, missing data.
We considered tumor size as a binary variable using the median as the cut-off value (≤50 mm versus >50 mm) owing to the large range of values for tumor size detected in the present study.
Figure 1A and Supplementary Table S2, available at https://doi.org/10.1016/j.esmoop.2022.100578, illustrate the history of pregnancy in the enrolled study patients. Most patients had had at least one pregnancy before the diagnosis of DF (187, 77.3%); 87 patients (36.0%) had had a pregnancy within 24 months before DF diagnosis, including 12 cases of pregnancy at the time of DF diagnosis (5.0%). There was a significantly greater number of DF located in the abdominal wall in patients with a recent history of pregnancy [62/87 (71.3%) versus 62/155 (40.3%), P < 0.0001]. The distribution of a previous history of pregnancy in patients diagnosed with DF did not differ according to the front-line management strategy (Supplementary Table S1, available at https://doi.org/10.1016/j.esmoop.2022.100578). In 25 cases (10.3%), pregnancy occurred after the diagnosis of DF (9 among the 49 patients who underwent initial surgery and 16 among the 193 patients undergoing active surveillance, 18.4% versus 8.3%, P = 0.04; Supplementary Table S2, available at https://doi.org/10.1016/j.esmoop.2022.100578). Overall, a total of 101 patients (41.7%) had a pregnancy during the study period (i.e. the time period spanning from 24 months before DF diagnosis until the time of relapse/progression or the time of the last follow-up). The history of pregnancy for these 101 patients is illustrated using a swimmer plot (Supplementary Figure S2, available at https://doi.org/10.1016/j.esmoop.2022.100578). As expected, the occurrence of pregnancy in the study period was associated with patient age: 6/28 (21.4%) at 16-26 years, 63/124 (50.8%) at 26-36 years, and 32/90 (35.6%) at 36-46 years (P = 0.006).
Figure 1.
Sankey alluvial diagrams. (A) Number of pregnant patients over time. (B) Number of patients with hormonal contraception exposure. DF, desmoid-type fibromatosis; diag, diagnosis.
Supplementary Table S3, available at https://doi.org/10.1016/j.esmoop.2022.100578, and Figure 1B illustrate the distribution of exposure to hormonal contraception in the study population. Overall, 124 (51.2%) of the enrolled patients had no exposure to hormonal contraception during the study period; 135 (55.8%) had no exposure to hormonal contraception within the 24 months before DF diagnosis; 160 (66.1%) had no exposure to hormonal contraception at the time of DF diagnosis; and 148 (61.2%) had no exposure to hormonal contraception following DF diagnosis. This did not differ according to the front-line management strategy (Supplementary Table S3, available at https://doi.org/10.1016/j.esmoop.2022.100578).
The median follow-up time was 23.9 months (95% CI 21.3-25.3 months; range, 0.4-59.7 months). An event (relapse or progression) was reported in 52 patients. There were no reported deaths. The EFS probabilities at 2 and 3 years were 75.2% (95% CI 68.3% to 80.8%) and 70.9% (95% CI 63.1% to 77.5%), respectively (Supplementary Figure S3, available at https://doi.org/10.1016/j.esmoop.2022.100578).
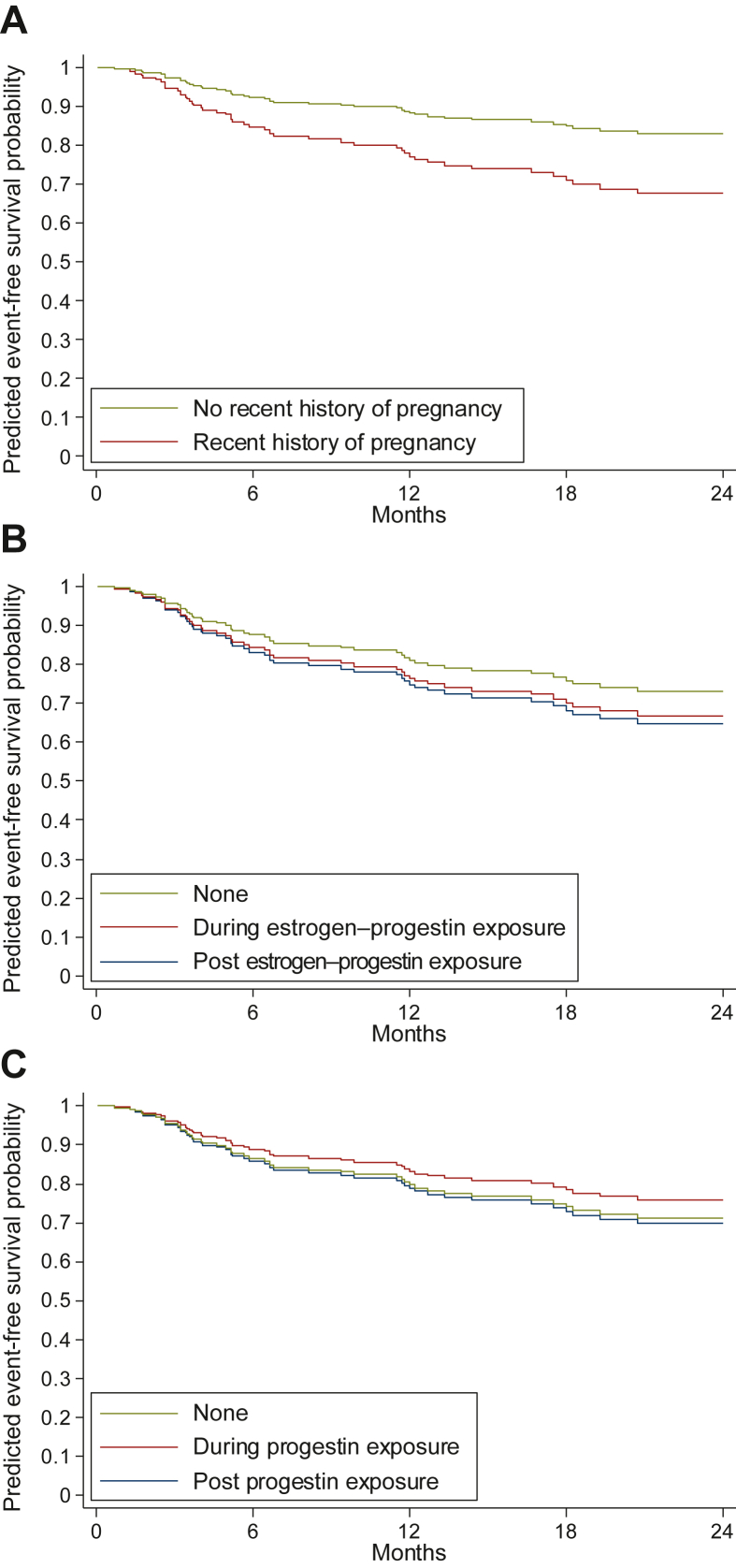
There were no events (relapse or progression) reported during pregnancy. However, 21 events occurred in the 24 months following pregnancy. When controlling for the tumor characteristics (tumor size and tumor site), front-line approach, and hormonal contraception (Table 2), the HR for EFS associated with a recent history of pregnancy was 2.09 (95% CI 1.14-3.86, P = 0.018) as compared to the ‘no pregnancy’ periods. This is illustrated in the predicted EFS curves provided in Figure 2A.
Table 2.
Prognostic factor analysis for event-free survival (242 patients, 52 events)
| Characteristics | No. of events | Univariate analysis |
Multivariate analysisa |
||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | ||
| Recent history of pregnancyb,c | 0.036 | 0.018 | |||||
| None | 31 | 1 | 1 | ||||
| Pregnancy/post-pregnancy (≤24 months) | 21 | 1.82 | 1.04-3.18 | 2.09 | 1.14-3.86 | ||
| Exposure to estrogen–progestinc | 0.63 | 0.65 | |||||
| None | 39 | 1 | 1 | ||||
| During exposure | 4 | 0.97 | 0.34-2.71 | 0.95 | 1.28 | 0.44-3.75 | 0.65 |
| Post-exposure (1-24 months) | 9 | 1.42 | 0.69-2.94 | 0.34 | 1.38 | 0.66-2.90 | 0.39 |
| Exposure to progestinc | 0.71 | 0.90 | |||||
| None | 42 | 1 | 1 | ||||
| During exposure | 5 | 0.73 | 0.29-1.85 | 0.51 | 0.81 | 0.31-2.11 | 0.66 |
| Post-exposure (1-24 months) | 5 | 1.23 | 0.48-3.13 | 0.66 | 1.05 | 0.41-2.71 | 0.91 |
| Tumor site | 0.79 | 0.31 | |||||
| Abdominal wall | 26 | 0.93 | 0.54-1.60 | 0.74 | 0.41-1.33 | ||
| Other site | 26 | 1 | 1 | ||||
| Tumor size (mm)d | 0.17 | 0.24 | |||||
| ≤50 | 25 | 1 | 1 | ||||
| >50 | 27 | 1.46 | 0.85-2.52 | 1.39 | 0.80-2.43 | ||
| Front-line approach | 0.013 | 0.012 | |||||
| Surgery | 5 | 1 | 1 | ||||
| Active surveillance | 47 | 3.20 | 1.27-8.07 | 3.32 | 1.30-8.49 | ||
95% CI, 95% confidence interval; HR, hazard ratio; No. of events, number of events (relapse or progression).
The multivariate model includes all variables listed in the table. For time-dependent variables, dates defining the periods of exposure and post-exposure for the three different types of hormonal exposure were ordered to define a status for each sub-period.
Recent history of pregnancy, exposure to estrogen–progestin contraception, and exposure to progestin contraception were used as time-dependent covariates. For each of these variables, we modeled the risk of events associated with the three different periods of hormonal exposure: the period designated as ‘during exposure’ corresponds to treatment duration (contraception) or pregnancy duration (pregnancy), ‘post-exposure’ corresponds to the 24 months after the end of contraception/pregnancy, ‘none’ corresponds to no exposure at all, or to periods before exposure or >24 months after exposure.
As no event was observed during pregnancy in the present study, we pooled the pregnancy status of ‘during pregnancy’ with the pregnancy status of ‘post-pregnancy’. Details of risk during pregnancy and during the four semesters of the 24-month post-pregnancy period are provided in Supplementary Table S1, available at https://doi.org/10.1016/j.esmoop.2022.100578.
We considered tumor size as a binary variable using the median as the cut-off value (≤50 mm versus >50 mm) owing to the large range of values for tumor size detected in the present study. We observed a non-monotonic relationship between tumor size and event risk.
Figure 2.
Event-free survival (EFS) curves according to hormonal exposure. (A) EFS curves according to recent history of pregnancy. (B) EFS curves according to exposure to estrogen–progestin contraception. (C) EFS curves according to exposure to progestin contraception. (D) Adjusted curves of EFS estimated from the multivariable Cox models including recent history of pregnancy, exposure to estrogen–progestin contraception, exposure to progestin contraception, tumor site, tumor size, and the first-line approach.
Moreover, as detailed in Supplementary Table S1, available at https://doi.org/10.1016/j.esmoop.2022.100578, the risk of events was shown to be higher 6-18 months after pregnancy. As illustrated in Supplementary Figure S4, available at https://doi.org/10.1016/j.esmoop.2022.100578, the risk associated with a recent history of pregnancy appeared homogeneous across subgroups when considering tumor size (interaction test, P = 0.65) and the front-line treatment approach (P = 0.56). This risk of relapse/progression did not statistically differ according to DF location (abdominal wall versus other locations; P = 0.08).
When considering EP or progestin contraception, we did not observe any association between current or recent exposure and the risk of events on univariate analysis or multivariate analysis controlling for tumor size (≤50 versus >50 mm), tumor site (abdominal wall versus other sites), front-line approach, other hormonal exposures, and pregnancy (Table 2, Figure 2B and C). We also did not observe any statistically significant heterogeneity across subgroups (all interaction test P values were >0.14, data not shown).
Discussion
Our study provides new evidence regarding DF and pregnancy within a multivariate analysis taking into account both (i) the main known prognostic factors of EFS (tumor location, tumor size, and front-line strategy) and (ii) the duration of periods of exposure to pregnancy. We found that abdominal wall DF occurred more frequently in patients with a recent history of pregnancy. Abdominal wall DF typically represents ∼30% of DF cases.1,2 However, in our series, abdominal wall DF represented >50% of DF cases, likely because of the selection criteria used in this study (i.e. sex and childbearing potential).
We confirmed that a recent pregnancy was associated with a high risk of the progression or relapse of DF, with an adjusted HR of 2.1. In addition, we observed that the events mainly occurred just after delivery. No event was documented during pregnancy, and the peak of progression/relapse occurred 6-18 months after pregnancy. We also found that a recent history of pregnancy was associated with a statistically significant increase of events, regardless of the DF site (abdominal versus non-abdominal; Supplementary Figure S4, available at https://doi.org/10.1016/j.esmoop.2022.100578). Moreover, the interaction was of marginal significance (P = 0.08; Supplementary Figure S4, available at https://doi.org/10.1016/j.esmoop.2022.100578) with respect to tumor location. A larger study would be needed to confirm an increased risk of progression for extra-abdominal tumors.
The association between pregnancy and DF outcomes has previously been explored in retrospective studies focusing on abdominal wall DF. Fiore et al., in a multicenter study, demonstrated that one-third of recently diagnosed DF that were still in place during pregnancy increased in size during the course of the pregnancy or (mainly) just after delivery.8 Furthermore, ∼25% of the DF cases that were still in place progressed during subsequent pregnancies.8 Fiore et al. also showed that most cases of DF progressing during pregnancy or after delivery did not require treatment and instead decreased in size during active surveillance.8
Data reported within the literature as well as in the present study demonstrated that a recent pregnancy was associated with an increased risk of the progression or relapse of recently diagnosed DF. Several hypotheses could explain this relationship. Pregnancy implies the secretion of several hormones and growth factors (mainly estrogen, progesterone, human chorionic gonadotropin, prolactin, and oxytocin). If DF is a hormone-dependent tumor, pregnancy-related hormones may drive tumor progression. Pathological analysis found that ∼30% of DF expressed estrogen receptors (only subunit β estrogen receptors have been detected).4,6,14 Other biological factors such as metabolic changes and inherent inflammation can also explain the progression observed during pregnancy. Healing of traumatized soft tissue and the inherent secretion of growth factors also comprise possible explanations of disease progression during pregnancy, as the occurrence of numerous case reports of DF after surgery or trauma is well known.15 Cesarean delivery or episiotomy may also be potential triggers.
In the context of pregnancy, the stretching and enlargement of the abdominal wall can explain the progression seen during pregnancy and within 2 years following delivery. In the other models, preclinical data suggested that the Wnt/β-catenin pathway (i.e. a deregulated DF pathway) was involved in response to mechanical stress, such as bone formation.14 This pathway is independent of estrogen/progesterone secretion. This mechanical hypothesis could explain the observed relationship between the deregulated Wnt/β-catenin pathway, pregnancy, and DF progression after pregnancy. The ‘stretched abdominal wall’ hypothesis is appealing for explaining the increased risk of progression in abdominal wall DF, but is less convincing in terms of explaining the increased risk of events in non-abdominal wall DF after pregnancy. Our study stresses that post-pregnancy progression also occurs in non-abdominal wall DF. Reasons explaining the relation between DF progression/relapse and pregnancy are likely many and complex, and should be elucidated in future studies.
In contrast, we found that a recent history of hormonal therapy did not influence the outcomes of newly diagnosed DF. To the best of our knowledge, no prior published study has explored this association. In our series, <50% of patients received hormonal contraception, and this exposure decreased after DF diagnosis. This probably reflects that both physicians and DF patients are frequently uncomfortable with the issue of hormonal contraception.
The present study had several limitations. Firstly, because of limited sample size, the sub-group analyses must be interpreted with caution (e.g. association with pregnancy in non-abdominal wall DF). Like any study assessing the effect of an exposure, the memorization bias must be taken into account particularly with regard to past contraceptive exposure. In this study, the primary endpoint was EFS, which includes both relapses and progressions. Relapses and progressions, which could be challenging to recognize in DF, have previously been reported by investigators in the absence of central confirmation. Moreover, it is known that some DF progressions occurring during pregnancy and after delivery are transient and may not require treatment.8 In the present study, only 25 patients became pregnant following a diagnosis of DF. We strongly recommend that future studies investigate this question more thoroughly. Finally, we did not analyze the impact of different dosages of hormonal contraception and cannot rule out that the effect of hormonal contraception on EFS may differ according to these dosages.
Conclusions
The present study provides evidence informing this sparsely investigated topic. Nevertheless, we are far from having sufficiently robust data to inform management guidelines. Additional confirmation is needed through multi-institutional studies. We therefore propose a cautious attitude to interpreting the current research, and note that this approach is debatable. More specifically, we cannot claim that exposure to hormonal contraception after DF is safe, and stress that further follow-up and additional data are required. Conversely, we cannot totally contraindicate hormonal contraception in this population. Pragmatically, we suggest withholding hormonal contraception at the time of DF diagnosis. Following this, if the DF is stable or spontaneously decreases in size, we suggest reintroducing hormonal contraception. Regarding pregnancy and DF, we suggest avoiding pregnancy within 2 years of being diagnosed with DF. Nevertheless, pregnancy is not formally contraindicated in this condition and instead requires careful follow-up at a specialized medical center.
Acknowledgements
The authors would like to thank the patients and families for their participation in this study; the staff members involved in study management, in particular Emilie Decoupigny and Marie Vanseymortier from the sponsorship unit at Centre Oscar Lambret (Lille University); all investigators and their teams who participated in the study; the patient advocacy group ‘SOS Desmoïde’; Guillemette Antoni, a biostatistician from CESP (INSERM Villejuif), for her advice; Françoise Bonichon for her help in the literature research; and the data-managers from Centre de Traitement des Données du Cancéropôle Nord-Ouest (CTD-CNO), who oversaw the study data management; the CTD-CNO clinical research platform was funded by the French National Cancer Institute (INCa) and ‘La Ligue Nationale Contre le Cancer’.
Funding
This work was supported by a personal grant from a donor (S. Wisnia), the Ligue Nationale Contre le Cancer (PRE2016.LCC/NP), Intersarc (funded by the French National Cancer Institute, INCA), and SOS Desmoïde (no grant number). These funders had no role in the design, conduct, or reporting of this work.
Disclosure
The authors have declared no conflicts of interest.
Data sharing
The findings of this study are available from the corresponding author upon reasonable request.
Supplementary data
References
- 1.Penel N., Coindre J.M., Bonvalot S., et al. Management of desmoid tumours: a nationwide survey of labelled reference centre networks in France. Eur J Cancer. 2016;58:90–96. doi: 10.1016/j.ejca.2016.02.008. [DOI] [PubMed] [Google Scholar]
- 2.Desmoid Tumor Working Group The management of desmoid tumours: a joint global consensus-based guideline approach for adult and paediatric patients. Eur J Cancer. 2020;127:96–107. doi: 10.1016/j.ejca.2019.11.013. [DOI] [PubMed] [Google Scholar]
- 3.Santti K., Ihalainen H., Rönty M., et al. Estrogen receptor beta expression correlates with proliferation in desmoid tumors. J Surg Oncol. 2019;119:873–879. doi: 10.1002/jso.25407. [DOI] [PubMed] [Google Scholar]
- 4.Santos G.A.C., Cunha I.W., Rocha R.M., et al. Evaluation of estrogen receptor alpha, estrogen receptor beta, progesterone receptor, and cKIT expression in desmoids tumors and their role in determining treatment options. Biosci Trends. 2010;4:25–30. [PubMed] [Google Scholar]
- 5.Deyrup A.T., Tretiakova M., Montag A.G. Estrogen receptor-β expression in extraabdominal fibromatoses: an analysis of 40 cases. Cancer. 2006;106:208–213. doi: 10.1002/cncr.21553. [DOI] [PubMed] [Google Scholar]
- 6.Leithner A. Immunohistochemical analysis of desmoid tumours. J Clin Pathol. 2005;58:1152–1156. doi: 10.1136/jcp.2005.026278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ishizuka M., Hatori M., Dohi O., et al. Expression profiles of sex steroid receptors in desmoid tumors. Tohoku J Exp Med. 2006;210:189–198. doi: 10.1620/tjem.210.189. [DOI] [PubMed] [Google Scholar]
- 8.Fiore M., Coppola S., Cannell A.J., et al. Desmoid-type fibromatosis, and pregnancy: a multi-institutional analysis of recurrence and obstetric risk. Ann Surg. 2014;259:973–978. doi: 10.1097/SLA.0000000000000224. [DOI] [PubMed] [Google Scholar]
- 9.Fiore M., Colombo C., Radaelli S., et al. Hormonal manipulation with toremifene in sporadic desmoid-type fibromatosis. Eur J Cancer. 2015;51:2800–2807. doi: 10.1016/j.ejca.2015.08.026. [DOI] [PubMed] [Google Scholar]
- 10.Skapek S.X., Anderson J.R., Hill D.A., et al. Safety and efficacy of high-dose tamoxifen and sulindac for desmoid tumor in children: results of a Children’s Oncology Group (COG) phase II study. Pediatr Blood Cancer. 2013;60:1108–1112. doi: 10.1002/pbc.24457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Penel N., Le Cesne A., Bonvalot S., et al. Surgical versus non-surgical approach in primary desmoid-type fibromatosis patients: a nationwide prospective cohort from the French Sarcoma Group. Eur J Cancer. 2017;83:125–131. doi: 10.1016/j.ejca.2017.06.017. [DOI] [PubMed] [Google Scholar]
- 12.Penel N., Bonvalot S., Bimbai A.M., et al. Lack of prognostic value of CTNNB1 mutation profile in desmoid-type fibromatosis. Clin Cancer Res. 2022 doi: 10.1158/1078-0432.CCR-21-4235. [DOI] [PubMed] [Google Scholar]
- 13.Kleinbaum D.G., Klein M. Survival Analysis. Statistics for Biology and Health. Springer; New York, NY: 2012. Extension of the Cox proportional hazards model for time-dependent variables; pp. 241–288. [Google Scholar]
- 14.Liedert A., Nemitz C., Haffner-Luntzer M., Schick F., Jakob F., Ignatius A. Effects of estrogen receptor and Wnt signaling activation on mechanically induced bone formation in a mouse model of postmenopausal bone loss. Int J Mol Sci. 2020;21:8301. doi: 10.3390/ijms21218301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Muneer M., Badran S., Zahid R., Abelmageed A., AlDulaimi M.M. Recurrent desmoid tumor with intra-abdominal extension after abdominoplasty: a rare presentation. Am J Case Rep. 2019;20:953–956. doi: 10.12659/AJCR.916227. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.