Abstract
Introduction
Ovarian cancer is the most lethal gynecologic malignancy. Although treatment with hyperthermic intraperitoneal chemotherapy (HIPEC) has shown promising results, its role remains elusive. The aim of this study was to assess the comprehensive randomized evidence for the use versus non-use of HIPEC in primary and recurrent ovarian cancer.
Materials and methods
The Medline, Embase and Cochrane databases, as well as the European Society for Medical Oncology (ESMO) and American Society of Clinical Oncology (ASCO) conference abstracts of the last 5 years, were scrutinized in January 2022 for randomized, controlled trials that studied the use of HIPEC in ovarian cancer. Overall survival (OS), disease-free survival (DFS) and progression-free survival, as well as post-operative morbidity were the outcomes of interest. This study was reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) reporting guideline.
Results
Six randomized, controlled trials that randomized 737 patients were included in our analysis; of these, four studies (519 patients) were in primary and two (218 patients) in recurrent settings. In primary ovarian cancer, the combination of HIPEC with interval cytoreductive surgery (CRS) and neoadjuvant chemotherapy significantly improved the 5-year OS [393 patients, risk ratio (RR) = 0.77; 95% confidence interval (CI) 0.67-0.90; P value = 0.001] and DFS (hazard ratio = 0.60; 95% CI 0.41-0.87; P value = 0.008) compared with standard treatment alone. In the absence of neoadjuvant chemotherapy, the use of HIPEC + CRS was not associated with any survival advantage (126 patients, 4-year OS, RR = 0.93; 95% CI 0.57-1.53; P value = 0.781), but the sample size was smaller in this subset. Use of HIPEC in recurrent ovarian cancer did not provide any survival advantage (5-year OS: 218 patients, RR = 0.85; 95% CI 0.45-1.62; P value = 0.626). The risk for grade ≥3 adverse events was similar between HIPEC and no HIPEC (RR = 1.08; 95% CI 0.98-1.18; P value = 0.109).
Conclusions
In primary ovarian cancer the combination of HIPEC with interval CRS and neoadjuvant chemotherapy is a safe option that significantly improved 5-year OS and DFS. Its use in other settings should continue to be considered investigational.
Key words: ovarian cancer, hyperthermic intraperitoneal chemotherapy, HIPEC, cytoreductive surgery, primary CRS, interval CRS
Highlights
-
•
Addition of HIPEC to a complete cytoreductive surgery could be a valid treatment option for advanced ovarian cancer.
-
•
HIPEC following neoadjuvant chemotherapy significantly increases 5-year overall survival in primary advanced ovarian cancer.
-
•
HIPEC following neoadjuvant chemotherapy significantly increases disease-free survival in primary advanced ovarian cancer.
-
•
HIPEC is a safe treatment option in ovarian cancer.
Introduction
Ovarian cancer is the most lethal gynecologic malignancy. In 2022, it is estimated that 19 880 new cases of ovarian cancer will be diagnosed in the United States and 12 810 deaths will occur from this disease.1 Approximately 75% of ovarian cancer patients are diagnosed in the advanced setting, when the disease has already predominantly spread in the peritoneal cavity.2 Thus, the research for potentially curative therapeutic strategies is essential.
In primary stage III ovarian cancer the importance of radical reduction of the comprehensive tumor burden with cytoreductive surgery (CRS) is established and has been proved to provide curative, overall and progression-free survival (PFS) benefits.3, 4, 5, 6 Accordingly, the optimal therapy for primary advanced (stage II-IV) ovarian cancer consists of CRS followed by adjuvant platinum-based chemotherapy and biological treatments.7,8
The theory that intraperitoneal chemotherapy could be effective against microscopic seeding, tumor cell entrapment and peritoneal carcinomatosis in ovarian cancer paved the way for several randomized trials, some of which showed beneficial results, while also being accompanied by toxicities.9, 10, 11, 12, 13 The 2021 guidelines suggest intraperitoneal chemotherapy, as an option, only for optimally debulked (<1 cm residual disease), stage II-III selected epithelial cancer types.8 Hyperthermic intraperitoneal chemotherapy (HIPEC) holds a series of advantages over intraperitoneal chemotherapy, since it requires a single administration that is being delivered at a special timing, following a complete CRS, when all peritoneal surfaces are exposed to the chemotherapy, while also leveraging the effect of hyperthermia (41-43°C for 30-120 min) to increase the cytotoxicity of chemotherapeutic drugs.14, 15, 16 Of note, complete cytoreduction remains the cornerstone of advanced ovarian cancer treatment and the potential additional benefit for the use of HIPEC following CRS as combination therapy has arisen as an interesting concept that has yet to be clarified.
Currently, the plethora of meta-analyses studying the efficacy of HIPEC in gynecological malignancies rely mainly on cohort studies.17, 18, 19, 20 We therefore carried out a systematic review in order to scrutinize and estimate the comprehensive available level I evidence for use versus no use of HIPEC in advanced primary and recurrent ovarian cancer.
Materials and methods
Search and selection
The Medline, Cochrane and Embase databases, as well as European Society for Medical Oncology (ESMO) and American Society of Clinical Oncology (ASCO) congress abstracts were systematically searched in January 2022 for randomized, controlled trials (RCTs) comparing the use of HIPEC treatment with any other therapy in patients with ovarian cancer. The search in Medline was updated in April 2022. The search algorithm contained: (ovarian) AND (neoplasm∗ OR cancer∗ OR tumor∗) AND (HIPEC OR IPHP OR IHC OR CHPP OR hyperthermic OR hyperthermic intraperitoneal chemotherapy OR hyperthermic intraperitoneal perfusion OR intraperitoneal hyperthermic chemoperfusion OR continuous hyperthermic peritoneal perfusion) AND (random∗). This study was reported in accordance to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) reporting guideline.21
Two independent investigators (PF, NF) screened all studies identified in our search by titles and abstracts for eligibility. Any article identified as having the potential to fulfill our inclusion criteria underwent full-text evaluation. If agreement on eligibility was not reached between the two investigators, a third investigator (DM) was involved to evaluate the article. Database searches were supplemented by using citation analysis of those eligible. The eligibility was defined by the PICO framework: Population (P): patients with primary or recurrent ovarian cancer; Intervention (I): Hyperthermic Intraperitoneal Chemotherapy (HIPEC); Comparison (C): treatment without HIPEC; Outcomes (O): Overall survival, disease-free survival, Progression-free survival, Post-operative morbidity. Studies that did not fulfill the abovementioned criteria, such as cohort and case-control studies, or were not published in English language were excluded.
Data extraction
The data extraction was carried out by two authors (PF, GM) who filled in a pre-piloted extraction form independently. Any disagreement was resolved by consensus. Multiple records reporting on the same trial were excluded. In case of double reporting data in conference abstracts and article publications, only the data from the publication, the highest level of evidence was taken into account. The data extraction included: first author, year of publication, chronological period of the study recruitment, study population, number of patients, experimental and control arm therapy details, HIPEC technique, median follow-up, median overall survival (OS), median progression-free survival (PFS) in months, survival and PFS rates, number of deaths and number of disease progression incidences, as well as rates of grade ≥3 adverse events occurrence after surgery (according to National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.0).
Outcomes
The primary outcome of interest is the OS of patients with primary advanced and recurrent ovarian cancer treated with HIPEC versus treatment without HIPEC, which is also measured at different time points of follow-up (1-, 2, -3, 4-, 5-year OS). Secondary outcomes are disease-free survival (DFS) for primary and PFS for recurrent ovarian cancer measured at consecutive time points, as well as post-operative morbidity.
Statistical analysis and risk of bias
Risk ratios (RRs) of OS were characterized by the proportion of patient deaths as indicated from each study’s reported survival rates, whereas those for DFS and PFS were calculated according to the proportion of disease progression events. The rates of post-operative morbidity in the two groups were used to calculate the corresponding RR. Because the rates of interest were minimally reported in the articles, Engauge Digitizer (https://markummitchell.github.io/engauge-digitizer/) was used to calculate the OS and PFS rates from the Kaplan–Meier curves at 1, 2, 3, 4 and 5 years. Additionally, a heterogeneity P value was calculated to assess the differences by year. These findings were compared with the corresponding data when they were directly reported in the articles and showed complete validity (± no more than one patient). When the rates were directly reported in the articles, then these estimates were taken into consideration for the analysis. As expected, this technique of measuring the rates at different time points was not feasible when assessing conference abstracts, so in this case we only considered the measures of interest that were directly mentioned. For each outcome, a random-effects model was used, utilizing the inverse variance method, to compare the RRs and 95% confidence intervals (CIs) between patients who did and did not receive HIPEC. An RR <1 will indicate a protective impact of HIPEC, whereas an RR >1 will indicate reduction in OS or PFS from the use of HIPEC compared with no use. Statistical heterogeneity was assessed using the I2 statistic. Statistical significance was indicated from a P value <0.05. Due to the small number of included trials, we did not perform an evaluation of small study effects and publication bias. All statistical analyses were carried out in Stata version 14 (Stata Corp, College Station, TX). Risk of bias of the included randomized studies (except from the conference abstract due to lack of information) was assessed by two authors (PF, GM) using RoB 2 tool: a revised Cochrane risk of bias tool for randomized trials.
Results
The review flow chart is described in Figure 1. Electronic searches identified 162 hits in Medline, 360 in Embase and 170 in Cochrane. These hits were screened by title and abstract, complemented by additional screening of ESMO congress 2017-2021 and ESMO gynecological cancers 2021 congress abstracts, as well as ASCO 2017-2022 and ASCO gynecologic oncology/women cancer 2018-2021 congress abstracts. After full-text evaluation of 11 articles, the search ultimately yielded a total of six eligible studies, five RCTs published in peer-reviewed journals22, 23, 24, 25, 26 and one RCT from conference abstract.27 Five studies were excluded due to not being randomized28,29 and due to researching outcomes unrelated to our analysis.30, 31, 32 The eligible studies were conducted in South Korea,22 Spain,23 USA,24,27 the Netherlands,25 Greece26 and three of them were multicenter.22,24,25 Overall 737 patients were randomized across six trials; among these four studies (519 patients)22,23,25,27 concerned the experimental use of HIPEC in primary ovarian cancer and two (218 patients) its investigational use in recurrent settings. Table 1 summarizes the characteristics of the included RCTs. All studies were randomized in 1 : 1 fashion, five studies described the type of randomization,22, 23, 24, 25, 26 three studies described the method of randomization concealment22,23,26 and four studies provided details for withdrawals.22, 23, 24, 25 Risk of bias assessed by the RoB 2 Cochrane tool33 is provided in Supplementary Figure S1, available at https://doi.org/10.1016/j.esmoop.2022.100586. Overall, two trials in primary ovarian cancer indicated a low risk of bias22,25 and one raised some concerns due to a lack of information for the evaluation of selection of the reported results.23 In recurrent ovarian cancer both trials presented a high risk of bias. The study by Zivanovic et al.24 showed deviations from the protocol with regards to the intended statistical analyses, while the study by Spiliotis et al.26 raised concerns regarding the statistical analyses and reporting of outcomes, as also described elsewhere.34
Figure 1.
Review flow chart.
ASCO, American Society of Clinical Oncology; EMSO, European Society for Medical Oncology.
Table 1.
Characteristics of RCTs that used HIPEC in patients with ovarian cancer
| Author | Year of publication | Recruitment period | HIPEC group (n) | Control group (n) | Experimental arm |
Control arm | Median follow-up (months) | Median OS (months) |
Median PFS (months) |
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HIPEC drug | Duration (min) | Temp (°C) | Chemotherapy | H | C | H | C | |||||||
| Primary ovarian cancer | ||||||||||||||
| Lim (NACT)22 | 2022 | 2010-2016 | 34 | 43 | Cisplatin 75 mg/m2 | 90 | 41.5 | Carboplatin and paclitaxel (+NACT) | Carboplatin and paclitaxel (+NACT) | 69.4 | 61.8/48.2 | 17.4/15.4 | ||
| Lim22 | 2022 | 2010-2016 | 58 | 49 | Cisplatin 75 mg/m2 | 90 | 41.5 | Carboplatin and paclitaxel | Carboplatin and paclitaxel | 69.4 | 71.3/— | 23.9/29.7 | ||
| Campos23 | 2022 | 2012-2018 | 35 | 36 | Cisplatin 75 mg/m2 | 60 | 42-43 | Carboplatin and paclitaxel (+NACT) | Carboplatin and paclitaxel (+NACT) | 32 | 52/45 | 18/12 | ||
| Van Driel25 | 2018 | 2007-2016 | 122 | 123 | Cisplatin 100 mg/m2 | 90 | 40 | Carboplatin and paclitaxel (+NACT) | Carboplatin and paclitaxel (+NACT) | 56.4 | 45.7/33.9 | 14.2/10.7 | ||
| Diaz-Montes27 | 2018 | 2014-2018 | 10 | 9 | Carboplatin 800 mg/m2 | 90 | — | Carboplatin and paclitaxel | I.V. paclitaxel/i.p. cisplatin/i.p. paclitaxel | — | — | — | ||
| Recurrent ovarian cancer | ||||||||||||||
| Zivanovic24 | 2021 | 2014-2019 | 49 | 49 | Carboplatin 800 mg/m2 | 90 | 41-43 | Carboplatin and paclitaxel or gemcitabine or doxorubicin | Carboplatin and paclitaxel or gemcitabine or doxorubicin | 39.5 | 52.5/59.7 | 12.3/15.7 | ||
| Spiliotis26 | 2014 | 2006-2013 | 60 | 60 | Cisplatin 100 mg/m2 and paclitaxel 175 mg/m2 or doxorubicin 35 mg/m2 and (paclitaxel 175 mg/m2 or mitomycin 15 mg/m2) | 60 | 42.5 | Systemic chemotherapy | Systemic chemotherapy | — | 26.7/13.4 (mean) | — | ||
C, control group; H, HIPEC group; HIPEC, hyperthermic intraperitoneal chemotherapy; NACT, neoadjuvant chemotherapy; OS, overall survival; PFS, progression-free survival; RCTs, randomized controlled trials.
HIPEC in primary ovarian cancer
Four trials22,23,25,27 studied the use of HIPEC in primary ovarian cancer including a total of 519 patients (259 in intervention and 260 in control group). In two of these studies, CRS and HIPEC were preceded by neoadjuvant chemotherapy,23,25 and in another one of these studies22 only a subset (42%) of patients randomized received neoadjuvant treatment. Thus, a total of 393 patients received neoadjuvant chemotherapy before allocation in the experimental HIPEC arm (191 patients) or the control arm (202 patients). Patients in the trial by Lim et al.22 who did not receive neoadjuvant chemotherapy before arm allocation were separately analyzed (primary CRS group). The trial described in the conference abstract by Diaz-Montes et al.,27 that contained only 19 patients with primary ovarian cancer (10 in the HIPEC group and 9 in the control group), was also included in the subgroup analysis of primary ovarian cancer with primary cytoreduction and it provided efficacy data regarding only 4-year OS and DFS. Thus, a total of only 126 patients received primary CRS with HIPEC (68 patients) or without HIPEC (58 patients).
Primary outcome
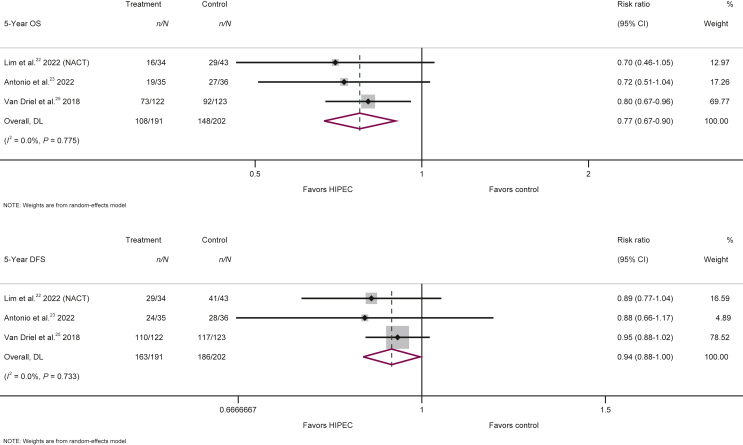
Among the 393 patients who underwent neoadjuvant chemotherapy followed by interval CRS, the use of HIPEC significantly improved 5-year OS (RR = 0.77; 95% CI 0.67-0.90; P = 0.001) compared with interval cytoreduction and neoadjuvant chemotherapy without HIPEC (Figure 2, Table 2). Nonetheless, this survival benefit was not evident in the small group of patients (126) who did not receive neoadjuvant chemotherapy before their allocation in the investigated arms (RR = 1.14; 95% CI 0.69-1.88; P = 0.599) (Table 2, Supplementary Figure S2, available at https://doi.org/10.1016/j.esmoop.2022.100586).
Figure 2.
Forest plots of 5-year overall survival (OS) and 5-year disease-free survival (DFS) for the use versus no use of HIPEC in the treatment of primary ovarian cancer with interval cytoreduction following neoadjuvant chemotherapy (POC, interval CRS + NACT).
CI, confidence interval; HIPEC, hyperthermic intraperitoneal chemotherapy.
Table 2.
Risk ratios of overall survival and disease-free and progression-free survival in different time-points
| Year |
OS, DFS/PFS |
RR |
95% Confidence interval |
P value |
|---|---|---|---|---|
| POC, interval CRS + NACT (n = 3)22,23,25 | ||||
| 1-Year | OS | 0.61 | 0.31-1.22 | 0.162 |
| 2-Year | OS | 0.64 | 0.44-0.92 | 0.017 |
| 3-Year | OS | 0.72 | 0.57-0.93 | 0.011 |
| 4-Year | OS | 0.76 | 0.64-0.91 | 0.003 |
| 5-Year | OS | 0.77 | 0.67-0.90 | 0.001 |
| 1-Year | DFS | 0.58 | 0.36-0.93 | 0.024 |
| 2-Year | DFS | 0.83 | 0.74-0.93 | 0.001 |
| 3-Year | DFS | 0.89 | 0.82-0.96 | 0.005 |
| 4-Year | DFS | 0.94 | 0.86-1.03 | 0.177 |
| 5-Year | DFS | 0.94 | 0.88-1.00 | 0.038 |
| POC, primary CRS (n = 2)22,27 | ||||
|---|---|---|---|---|
| 1-Yeara | OS | 0.84 | 0.12-5.78 | 0.864 |
| 2-Yeara | OS | 0.84 | 0.32-2.24 | 0.735 |
| 3-Yeara | OS | 0.77 | 0.38-1.60 | 0.489 |
| 4-Year | OS | 0.92 | 0.51-1.65 | 0.768 |
| 5-Yeara | OS | 1.14 | 0.69-1.88 | 0.599 |
| 1-Yeara | DFS | 1.69 | 0.74-3.85 | 0.212 |
| 2-Yeara | DFS | 1.17 | 0.77-1.76 | 0.465 |
| 3-Yeara | DFS | 1.06 | 0.76-1.48 | 0.715 |
| 4-Year | DFS | 0.93 | 0.57-1.53 | 0.781 |
| 5-Yeara | DFS | 1.10 | 0.86-1.41 | 0.446 |
| Recurrent OC (n = 2)24,26 | ||||
|---|---|---|---|---|
| 1-Year | OS | 0.29 | 0.08-1.10 | 0.068 |
| 2-Year | OS | 1.22 | 0.32-4.71 | 0.769 |
| 3-Year | OS | 0.59 | 0.16-2.20 | 0.428 |
| 4-Yearb | OS | 1.50 | 0.87-2.60 | 0.147 |
| 5-Year | OS | 0.85 | 0.45-1.62 | 0.626 |
| 1-Yearb | PFS | 1.32 | 0.84-2.06 | 0.228 |
| 2-Yearb | PFS | 1.11 | 0.91-1.36 | 0.319 |
| 3-Yearb | PFS | 1.18 | 1.01-1.38 | 0.042 |
| 4-Yearb | PFS | 1.18 | 1.01-1.38 | 0.042 |
| 5-Yearb | PFS | 1.18 | 1.01-1.38 | 0.042 |
| Grade ≥3 adverse events (n = 5) | ||||
|---|---|---|---|---|
| POC | 1.08 | 0.98-1.18 | 0.120 | |
| ROCb | 1.20 | 0.57-2.51 | 0.629 | |
| Overall | 1.08 | 0.98-1.18 | 0.109 | |
CRS, cytoreductive surgery; DFS, disease-free survival; NACT, neoadjuvant chemotherapy; OC, ovarian cancer; OS, overall survival; PFS, progression-free survival; POC, primary ovarian cancer; RR, Risk ratio; ROC, recurrent ovarian cancer.
Results only from the subset of Lim et al.22
Results only from Zivanovic et al.24
Secondary outcomes
Time point analyses for 1-, 2-, 3-, 4- and 5-year OS indicates that the OS benefits of HIPEC among patients who received neoadjuvant chemotherapy and interval CRS became statistically significant from the second year with a reduction of 36% in the risk for death that gradually shortened during the follow-up time to 23% at the fifth year, but CIs of the associations in the different years overlapped (heterogeneity P value = 0.913) (Table 2, Supplementary Figure S2, available at https://doi.org/10.1016/j.esmoop.2022.100586). When time point analyses for DFS are considered, the benefits of HIPEC among patients who received neoadjuvant chemotherapy and interval CRS are statistically significant directly from the first year with a reduction of 42% in the risk of recurrence or death that steadily diminished to 27% and 21% in the second and third year, respectively, before stabilizing at 6%-7% at the fourth and fifth year, respectively, of follow-up (heterogeneity P value = 0.040). Thus, HIPEC managed to drastically diminish the risk of recurrence at a short time window, but maintained only a part of it at longer follow-up (Table 2, Supplementary Figure S3, available at https://doi.org/10.1016/j.esmoop.2022.100586).
The summary hazard ratio for DFS in primary advanced ovarian cancer treated with interval CRS following neoadjuvant chemotherapy with and without HIPEC was hazard ratio = 0.60 (95% CI 0.41-0.87; P = 0.008, n = 3) (Supplementary Figure S4, available at https://doi.org/10.1016/j.esmoop.2022.100586). When the smaller group of patients, who directly received CRS (primary CRS group) were studied, no significant impact on OS or DFS could be documented at any time point of the analyses (1-, 2-, 3-, 4- and 5-year) (Table 2, Supplementary Figures S2 and S3, available at https://doi.org/10.1016/j.esmoop.2022.100586).
HIPEC in recurrent ovarian cancer
Two trials24,26 studied the use of HIPEC in recurrent ovarian cancer including a total of 218 patients (109 in intervention and 109 in control group). No OS benefit was evidenced for the use versus no use of HIPEC at any time point analyzed and despite the fact that some beneficial effect may be argued at the first year of follow-up, this benefit did not reach statistical significance (RR = 0.29; 95% CI 0.08-1.10; P = 0.068). (Table 2, Supplementary Figure S2, available at https://doi.org/10.1016/j.esmoop.2022.100586).
Regarding PFS in patients with recurrent ovarian cancer, the data for risk analyses were available only from the Zivanovic et al.5, 24 trial. Despite the fact that the number of patients analyzed was small (98 patients randomized, 49 patients in each arm) it is evidenced that the use of HIPEC may be associated with an 18% increase in the risk of disease progression or death, that reached statistical significance and stabilized from the third year of follow-up (Table 2, Supplementary Figure S5, available at https://doi.org/10.1016/j.esmoop.2022.100586). Nonetheless, considering the small number of patients included in the trial, no firm conclusion can be driven in this setting, and the use of HIPEC is to be considered experimental unless new data will be available from future trials.
Toxicities
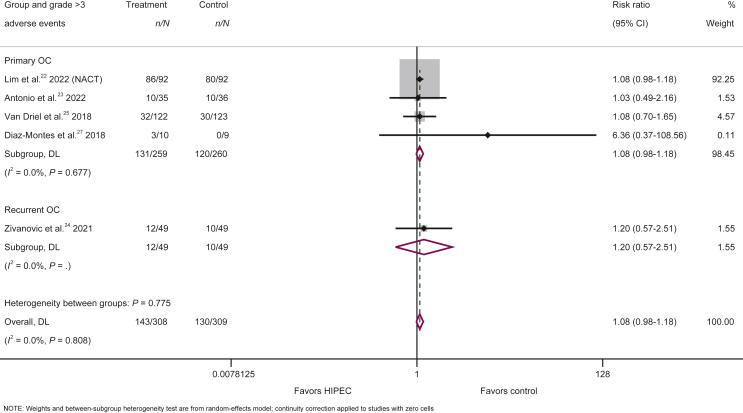
Five studies22, 23, 24, 25,27 with a total of 617 patients (308 in intervention and 309 in control group) provided data on grade ≥3 adverse events, the most common of which can be summarized in the following: electrolyte disturbance, anemia, decrease of neutrophils, decrease of white blood cells, abdominal pain, infection and ileus. The risk for occurrence of grade ≥3 adverse events (of any type) was similar between the intervention and control groups (143/308 patients in the HIPEC group and 130/309 patients in the control group), with a non-significant trend against the use of HIPEC (any setting considered) (RR = 1.08; 95% CI 0.98-1.18; P = 0.109) (Figure 3, Table 2). More specifically, the risk of grade ≥3 adverse events in primary ovarian cancer (RR = 1.08; 95% CI 0.98-1.18; P = 0.120, n = 4) (Figure 3, Table 2) did not significantly differ from the risk in recurrent ovarian cancer (RR = 1.20; 95% CI 0.57-2.51; P = 0.629, n = 1) (Figure 3, Table 2). Regarding post-operative mortality, three studies reported zero post-operative deaths in both groups,22,24,27 one study reported one death in each group23 and one study reported one death only in the control group,25 indicating that the use of HIPEC does not negatively affect post-operative mortality compared with treatment without HIPEC.
Figure 3.
Forest plot of the risk for occurrence of grade ≥3 adverse events from the use versus no use of HIPEC in the treatment of primary ovarian cancer and recurrent ovarian cancer.
CI, confidence interval; HIPEC, hyperthermic intraperitoneal chemotherapy; NACT, neoadjuvant chemotherapy; OC, ovarian cancer.
Discussion
A recent umbrella review of meta-analyses, involving data mainly from cohort studies, highlighted that HIPEC research in ovarian cancer seems promising and underscored the need of evidence from prospective randomized trials.35
This is the first systematic review and meta-analysis that depicts the available level I evidence data for the use versus no use of HIPEC in patients with ovarian cancer. Only randomized trials were included, many of which were recently published, and the two different settings of advanced primary ovarian cancer and recurrent ovarian cancer were separately analyzed, both for safety and efficacy.
In primary advanced ovarian cancer treated with interval CRS following neoadjuvant chemotherapy, our analyses on approximately 400 randomized patients showed that the use of HIPEC can significantly improve OS and DFS, and with a durable long-term (at 5 years) reduction of 23% in the risk of death when compared with treatment without HIPEC.
Our analyses also suggested that in advanced primary ovarian cancer, the use of upfront HIPEC immediately after CRS may not provide some additional OS or DFS benefits. Only ∼100 randomized patients were included in the analyses, however, and thereafter we cannot exclude the presence of outcome differences between its use versus non-use will be revealed in future larger RCTs. Of note, in a 2019 meta-analysis of cohort studies, HIPEC significantly improved OS and PFS in patients with primary ovarian cancer compared with patients who did not receive HIPEC,17 thus our analyses from available randomized data shrink the magnitude of potential survival benefit (if any) for HIPEC use in this setting. Nonetheless, it should be highlighted that currently there is no published RCT that exclusively studies the use of HIPEC in primary ovarian cancer after CRS without neoadjuvant chemotherapy, and that the analyzed randomized patients in our study for this setting represent a subset of patients from the study by Lim et al.,22 where HIPEC was used both upfront and after as neoadjuvant treatment. Consequently results in this setting should be interpreted with caution until larger, properly planned, multicenter randomized studies are available.
The reason why use of HIPEC after neoadjuvant chemotherapy provides some benefit whereas its upfront use does not, may stem from the possibility of patients/drug selection in the neoadjuvant phase. Firstly, potentially chemoresistant patients are not eligible candidates to undergo upfront surgery due to extreme regional disease extension, as well as comorbidities, and patients who progressed or remained stable after induction chemotherapy would probably never undergo CRS and would have never been randomized. In this vision, only chemosensitive patients will receive HIPEC with drug sensitivity having already been tested in the neoadjuvant regimens. Secondly, for those patients resistant to neoadjuvant regimens but fit for surgery, the use of HIPEC after neoadjuvant treatment failure will provide the opportunity to switch the drugs to other non-cross-resistant chemotherapeutical compounds during the hyperthermic treatment.
It may also be speculated that the potential benefits of neoadjuvant chemotherapy include higher rates of complete cytoreduction, reduced blood loss during surgery and post-operative morbidity, better quality of life, as well as testing the response to upfront therapy.36 A meta-analysis, however, reported inferior survival for the neoadjuvant chemotherapy approach.37 Two later randomized trials came to contradict this result.38, 39, 40 Nonetheless, it is implied that neoadjuvant chemotherapy does not offer any clear benefit and should be reserved for patients with advanced ovarian cancer who are not deemed eligible for upfront surgery due to poor performance status, comorbidities, old age or low probability of complete cytoreduction and would potentially benefit from its combination with interval debulking surgery.8
Regarding the use of HIPEC in recurrent ovarian cancer, our study indicates that the available randomized evidence from ∼200 patients analyzed is not solid enough, thus no firm conclusion can be made and the data are stated here only as a point of reference. Zivanovic et al.24 and Spiliotis et al.26 both studied the effect of CRS plus HIPEC on the treatment of recurrent ovarian cancer. The two studies reported contradictory results, where Zivanovic et al. did not support the use of HIPEC, whereas Spiliotis et al. presented data in favor of HIPEC. While critically appraising these results, it is crucial to keep in mind the limitations mentioned by Zivanovic et al., as well as the drawbacks of the Spiliotis et al. trial,41 which could be partially expected since it was the earliest published RCT. A 2020 meta-analysis of cohort studies comparing the use versus no use of HIPEC in recurrent ovarian cancer reported that HIPEC in addition to CRS and chemotherapy significantly improved OS.18 It should be pointed out, however, that the influence on the outcomes of patients with recurrent ovarian cancer is multifactorial, depending on the time interval from the last chemotherapy, the disease-free interval, the success of cytoreduction and the residual disease, the existence of peritoneal carcinomatosis synchronously to the recurrence, the performance status, as well as the potential accompanying gene mutations.42, 43, 44, 45 Larger data from randomized patients in properly planned, multicenter studies are thereafter needed before giving firm conclusions. Nonetheless, a cautious approach is to be kept in scheduling future trials in this clinical setting, since randomized data from the analysis of Zivanovic et al.24 evidenced a statistical 18% increase in the risk of disease progression or death among patients receiving HIPEC.
About 20% of primary ovarian tumors are naturally platinum-resistant and most recurrences will develop resistance over time.6 Even though Zivanovic et al.24 exclusively included platinum-sensitive patients, Spiliotis et al.26 recruited and independently analyzed platinum-resistant patients with recurrent ovarian cancer. Results for this population were encouraging, but they should be weighted by the real limitations of the study. Nonetheless, a series of cohort studies have also been supportive for the use of HIPEC in addition to optimal CRS in this population that is characterized by poorer prognosis.46, 47, 48 Moving forward, properly planned randomized trials are essential in order to clarify the possible benefits of HIPEC in chemoresistant ovarian cancer and potentially introduce a reliable therapeutic approach for this specific patient population.
HIPEC has constantly been criticized for the relatively high rates of complications and toxicities with potential risk for death and long-term severe sequels from the procedure, overshadowing HIPEC potential benefits.49, 50, 51 Two retrospective studies, each involving >1100 patients, with >20 years of experience, reported that HIPEC was associated with grade ≥3 morbidity rates of 9.6% and 20% and 30-day mortality rates of 1.5% and 2.2%, respectively.52,53 The most common complications reported were anastomotic leaks, bowel perforations, hematological complications and infections. Conversely, our results (from 617 randomized patients) indicate at level I evidence that HIPEC is a safe therapeutic modality, in any setting of ovarian cancer, with a similar risk of grade ≥3 adverse events and risk of early death events compared with cytoreduction without HIPEC. The observed absence in toxicity between HIPEC use versus no use may probably stem from the fact that the scrutinized randomized studies were carried out in institutions with long HIPEC experience. Data from literature suggest that the rate of post-operative morbidity and mortality may be correlated with the experience of the clinical centers that perform the technique.46,52,53 Thus, in experienced centers, HIPEC should be considered safe both for clinical practice and research applications.
Our study has limitations. The designs of the included RCTs were not homogenous, the patient accrual was not satisfactory and very little information for survival and PFS rates were directly reported in the articles. Thus, we relied on calculations assisted by a software program to make full use of all the available randomized evidence. Moreover, although we consider the distinguishing we made in the trial by Lim et al.22 scientifically necessary, as well as needed for a side by side comparison of treatments that have not been compared until now, neoadjuvant chemotherapy was given in selected patients which may have been a source of bias. Furthermore, although the inclusion of information from conference abstracts contains well-known caveats due to the lack of the available information, any level I evidence on such uncharted topics should not be neglected. Currently 22 RCTs are examining the effectiveness of HIPEC and CRS in the management of primary or recurrent ovarian cancer, however, and their results are eagerly awaited, especially since some of them (NCT03772028, NCT04280185, NCT03842982, NCT01376752) are expected to recruit a large number of participants ranging from 200 to 540 patients.54 The true bottleneck of HIPEC research and subsequently of the interpretation of the results of published trials is the sub-optimal study protocols and the not complete presentation of the data. The heterogeneity in HIPEC protocols was evident in our study, since three trials used cisplatin,22,23,25 two trials used carboplatin24,27 and Spiliotis et al.26 used more than one drug as the HIPEC regimen.
Conclusion
Overall, promising current evidence exists for the use of HIPEC following interval CRS with neoadjuvant chemotherapy for primary ovarian cancer and more RCT evidence is needed to evaluate the use of HIPEC in other settings. Complete cytoreduction followed by systemic chemotherapy remains the treatment mainstay. Among patients who cannot undergo upfront CRS and need neoadjuvant chemotherapy, the use of CRS followed by HIPEC is a proper option and is now documented to be effective and safe.
Acknowledgments
Funding
None declared.
Disclosure
The authors have declared no conflicts of interest.
Data sharing
All data generated or analysed during this study are included in this published article (and its supplementary information files).
Supplementary data
References
- 1.Siegel R.L., Miller K.D., Fuchs H.E., Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72(1):7–33. doi: 10.3322/caac.21708. [DOI] [PubMed] [Google Scholar]
- 2.Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66(1):7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 3.Meigs J.V. The Macmillan Company; New York: 1934. Tumors of the Female Pelvic Organs. [Google Scholar]
- 4.Griffiths C.T. Surgical resection of tumor bulk in the primary treatment of ovarian carcinoma. Natl Cancer Inst Monogr. 1975;42:101–104. [PubMed] [Google Scholar]
- 5.Hoskins W.J., Bundy B.N., Thigpen J.T., Omura G.A. The influence of cytoreductive surgery on recurrence-free interval and survival in small-volume stage III epithelial ovarian cancer: a Gynecologic Oncology Group study. Gynecol Oncol. 1992;47(2):159–166. doi: 10.1016/0090-8258(92)90100-w. [DOI] [PubMed] [Google Scholar]
- 6.Bristow R.E., Tomacruz R.S., Armstrong D.K., Trimble E.L., Montz F.J. Survival effect of maximal cytoreductive surgery for advanced ovarian carcinoma during the platinum era: a meta-analysis. J Clin Oncol. 2002;20(5):1248–1259. doi: 10.1200/JCO.2002.20.5.1248. [DOI] [PubMed] [Google Scholar]
- 7.Ledermann J.A., Raja F.A., Fotopoulou C., Gonzalez-Martin A., Colombo N., Sessa C. Newly diagnosed and relapsed epithelial ovarian carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013;24(suppl 6):vi24–vi32. doi: 10.1093/annonc/mdt333. [DOI] [PubMed] [Google Scholar]
- 8.Armstrong D.K., Alvarez R.D., Bakkum-Gamez J.N., et al. Ovarian Cancer, Version 2.2020, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2021;19(2):191–226. doi: 10.6004/jnccn.2021.0007. [DOI] [PubMed] [Google Scholar]
- 9.Armstrong D.K., Bundy B., Wenzel L., et al. Intraperitoneal cisplatin and paclitaxel in ovarian cancer. N Engl J Med. 2006;354(1):34–43. doi: 10.1056/NEJMoa052985. [DOI] [PubMed] [Google Scholar]
- 10.Alberts D.S., Liu P.Y., Hannigan E.V., et al. Intraperitoneal cisplatin plus intravenous cyclophosphamide versus intravenous cisplatin plus intravenous cyclophosphamide for stage III ovarian cancer. N Engl J Med. 1996;335(26):1950–1955. doi: 10.1056/NEJM199612263352603. [DOI] [PubMed] [Google Scholar]
- 11.Markman M., Bundy B.N., Alberts D.S., et al. Phase III trial of standard-dose intravenous cisplatin plus paclitaxel versus moderately high-dose carboplatin followed by intravenous paclitaxel and intraperitoneal cisplatin in small-volume stage III ovarian carcinoma: an intergroup study of the Gynecologic Oncology Group, Southwestern Oncology Group, and Eastern Cooperative Oncology Group. J Clin Oncol. 2001;19(4):1001–1007. doi: 10.1200/JCO.2001.19.4.1001. [DOI] [PubMed] [Google Scholar]
- 12.Tewari D., Java J.J., Salani R., et al. Long-term survival advantage and prognostic factors associated with intraperitoneal chemotherapy treatment in advanced ovarian cancer: a gynecologic oncology group study. J Clin Oncol. 2015;33(13):1460–1466. doi: 10.1200/JCO.2014.55.9898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Walker J.L., Brady M.F., Wenzel L., et al. Randomized trial of intravenous versus intraperitoneal chemotherapy plus bevacizumab in advanced ovarian carcinoma: an NRG Oncology/Gynecologic Oncology Group Study. J Clin Oncol. 2019;37(16):1380–1390. doi: 10.1200/JCO.18.01568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zivanovic O., Abramian A., Kullmann M., et al. HIPEC ROC I: a phase I study of cisplatin administered as hyperthermic intraoperative intraperitoneal chemoperfusion followed by postoperative intravenous platinum-based chemotherapy in patients with platinum-sensitive recurrent epithelial ovarian cancer. Int J Cancer. 2015;136(3):699–708. doi: 10.1002/ijc.29011. [DOI] [PubMed] [Google Scholar]
- 15.Hettinga J.V., Lemstra W., Meijer C., et al. Mechanism of hyperthermic potentiation of cisplatin action in cisplatin-sensitive and -resistant tumour cells. Br J Cancer. 1997;75(12):1735–1743. doi: 10.1038/bjc.1997.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hettinga J.V., Konings A.W., Kampinga H.H. Reduction of cellular cisplatin resistance by hyperthermia--a review. Int J Hyperthermia. 1997;13(5):439–457. doi: 10.3109/02656739709023545. [DOI] [PubMed] [Google Scholar]
- 17.Wu Q., Wu Q., Xu J., et al. Efficacy of hyperthermic intraperitoneal chemotherapy in patients with epithelial ovarian cancer: a meta-analysis. Int J Hyperthermia. 2019;36(1):562–572. doi: 10.1080/02656736.2019.1612101. [DOI] [PubMed] [Google Scholar]
- 18.Cianci S., Riemma G., Ronsini C., et al. Hyperthermic intraperitoneal chemotherapy (HIPEC) for ovarian cancer recurrence: systematic review and meta-analysis. Gland Surg. 2020;9(4):1140–1148. doi: 10.21037/gs-20-335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bouchard-Fortier G., Cusimano M.C., Fazelzad R., et al. Oncologic outcomes and morbidity following heated intraperitoneal chemotherapy at cytoreductive surgery for primary epithelial ovarian cancer: a systematic review and meta-analysis. Gynecol Oncol. 2020;158(1):218–228. doi: 10.1016/j.ygyno.2020.03.034. [DOI] [PubMed] [Google Scholar]
- 20.Kim S.I., Cho J., Lee E.J., et al. Selection of patients with ovarian cancer who may show survival benefit from hyperthermic intraperitoneal chemotherapy: a systematic review and meta-analysis. Medicine. 2019;98(50) doi: 10.1097/MD.0000000000018355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Page M.J., McKenzie J.E., Bossuyt P.M., et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lim M.C., Chang S.J., Park B., et al. Survival after hyperthermic intraperitoneal chemotherapy and primary or interval cytoreductive surgery in ovarian cancer: a randomized clinical trial. JAMA Surg. 2022;157(5):374–383. doi: 10.1001/jamasurg.2022.0143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cascales Campos A., González Gil A., Gil Gómez E., et al. Cytoreductive surgery with or without HIPEC after neoadjuvant chemotherapy in ovarian cancer: a phase 3 clinical trial. Ann Surg Oncol. 2022;29(4):2617–2625. doi: 10.1245/s10434-021-11087-7. [DOI] [PubMed] [Google Scholar]
- 24.Zivanovic O., Chi D.S., Zhou Q., et al. Secondary cytoreduction and carboplatin hyperthermic intraperitoneal chemotherapy for platinum-sensitive recurrent ovarian cancer: an MSK team ovary phase II study. J Clin Oncol. 2021;39(23):2594–2604. doi: 10.1200/JCO.21.00605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van Driel W.J., Koole S.N., Sikorska K., et al. Hyperthermic intraperitoneal chemotherapy in ovarian cancer. N Engl J Med. 2018;378(3):230–240. doi: 10.1056/NEJMoa1708618. [DOI] [PubMed] [Google Scholar]
- 26.Spiliotis J., Halkia E., Lianos E., et al. Cytoreductive surgery and HIPEC in recurrent epithelial ovarian cancer: a prospective randomized phase III study. Ann Surg Oncol. 2015;22(5):1570–1575. doi: 10.1245/s10434-014-4157-9. [DOI] [PubMed] [Google Scholar]
- 27.Diaz-Montes T.P., El-Sharkawy F., Gushchin V., Ryu H.S., Sittig M., Sardi A.J.G.O. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy as initial treatment of ovarian, fallopian tube, and primary peritoneal cancer: preliminary results of a phase II randomized clinical trial. Gynecol Oncol. 2018;149:35. [Google Scholar]
- 28.Xu Z., Ge X., Zhang T., Shi Y., Sun M. Efficacy of neoadjuvant chemotherapy combined with intraperitoneal hyperthermic chemotherapy in advanced ovarian cancer. J BUON. 2020;25(2):772–778. [PubMed] [Google Scholar]
- 29.Zhang T., Pan Q., Xiao S., Li L., Xue M. Docetaxel combined with intraperitoneal hyperthermic perfusion chemotherapy and hyperthermia in the treatment of advanced ovarian cancer. Oncol Lett. 2016;11(5):3287–3292. doi: 10.3892/ol.2016.4414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koole S.N., Kieffer J.M., Sikorska K., et al. Health-related quality of life after interval cytoreductive surgery with or without hyperthermic intraperitoneal chemotherapy (HIPEC) in patients with stage III ovarian cancer. Eur J Surg Oncol. 2021;47(1):101–107. doi: 10.1016/j.ejso.2019.05.006. [DOI] [PubMed] [Google Scholar]
- 31.Xie F., Van Bocxlaer J., Colin P., et al. PKPD modeling and dosing considerations in advanced ovarian cancer patients treated with cisplatin-based intraoperative intraperitoneal chemotherapy. AAPS J. 2020;22(5):96. doi: 10.1208/s12248-020-00489-2. [DOI] [PubMed] [Google Scholar]
- 32.Koole S.N., Bruijs L., Fabris C., et al. Central radiology assessment of the randomized phase III open-label OVHIPEC-1 trial in ovarian cancer. Int J Gynecol Cancer. 2020;30(12):1928–1934. doi: 10.1136/ijgc-2020-001825. [DOI] [PubMed] [Google Scholar]
- 33.Sterne J.A.C., Savović J., Page M.J., et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 34.Batista T.P. Comment on: surgery and HIPEC in recurrent epithelial ovarian cancer: a prospective randomized phase III study. Ann Surg Oncol. 2017;24(suppl 3):630. doi: 10.1245/s10434-017-6151-5. [DOI] [PubMed] [Google Scholar]
- 35.Souadka A., Essangri H., Majbar M.A., et al. Hyperthermic intraperitoneal chemotherapy and cytoreductive surgery in ovarian cancer: an umbrella review of meta-analyses. Front Oncol. 2022;12 doi: 10.3389/fonc.2022.809773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pecorelli S., Odicino F., Favalli G. Interval debulking surgery in advanced epithelial ovarian cancer. Best Pract Res Clin Obstet Gynaecol. 2002;16(4):573–583. doi: 10.1053/beog.2002.0302. [DOI] [PubMed] [Google Scholar]
- 37.Bristow R.E., Chi D.S. Platinum-based neoadjuvant chemotherapy and interval surgical cytoreduction for advanced ovarian cancer: a meta-analysis. Gynecol Oncol. 2006;103(3):1070–1076. doi: 10.1016/j.ygyno.2006.06.025. [DOI] [PubMed] [Google Scholar]
- 38.Vergote I., Tropé C.G., Amant F., et al. Neoadjuvant chemotherapy or primary surgery in stage IIIC or IV ovarian cancer. N Engl J Med. 2010;363(10):943–953. doi: 10.1056/NEJMoa0908806. [DOI] [PubMed] [Google Scholar]
- 39.Kehoe S., Hook J., Nankivell M., et al. Primary chemotherapy versus primary surgery for newly diagnosed advanced ovarian cancer (CHORUS): an open-label, randomised, controlled, non-inferiority trial. Lancet. 2015;386(9990):249–257. doi: 10.1016/S0140-6736(14)62223-6. [DOI] [PubMed] [Google Scholar]
- 40.Makar A.P., Tropé C.G., Tummers P., Denys H., Vandecasteele K. Advanced ovarian cancer: primary or interval debulking? Five categories of patients in view of the results of randomized trials and tumor biology: primary debulking surgery and interval debulking surgery for advanced ovarian cancer. Oncologist. 2016;21(6):745–754. doi: 10.1634/theoncologist.2015-0239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Harter P., Reuss A., Sehouli J., Chiva L., du Bois A. Brief report about the role of hyperthermic intraperitoneal chemotherapy in a prospective randomized phase 3 study in recurrent ovarian cancer from spiliotis et al. Int J Gynecol Cancer. 2017;27(2):246–247. doi: 10.1097/IGC.0000000000000864. [DOI] [PubMed] [Google Scholar]
- 42.Coleman R.L., Spirtos N.M., Enserro D., et al. Secondary surgical cytoreduction for recurrent ovarian cancer. N Engl J Med. 2019;381(20):1929–1939. doi: 10.1056/NEJMoa1902626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chi D.S., McCaughty K., Diaz J.P., et al. Guidelines and selection criteria for secondary cytoreductive surgery in patients with recurrent, platinum-sensitive epithelial ovarian carcinoma. Cancer. 2006;106(9):1933–1939. doi: 10.1002/cncr.21845. [DOI] [PubMed] [Google Scholar]
- 44.Harter P., du Bois A., Hahmann M., et al. Surgery in recurrent ovarian cancer: the Arbeitsgemeinschaft Gynaekologische Onkologie (AGO) DESKTOP OVAR trial. Ann Surg Oncol. 2006;13(12):1702–1710. doi: 10.1245/s10434-006-9058-0. [DOI] [PubMed] [Google Scholar]
- 45.Harter P., Hahmann M., Lueck H.J., et al. Surgery for recurrent ovarian cancer: role of peritoneal carcinomatosis: exploratory analysis of the DESKTOP I Trial about risk factors, surgical implications, and prognostic value of peritoneal carcinomatosis. Ann Surg Oncol. 2009;16(5):1324–1330. doi: 10.1245/s10434-009-0357-0. [DOI] [PubMed] [Google Scholar]
- 46.Bakrin N., Bereder J.M., Decullier E., et al. Peritoneal carcinomatosis treated with cytoreductive surgery and Hyperthermic Intraperitoneal Chemotherapy (HIPEC) for advanced ovarian carcinoma: a French multicentre retrospective cohort study of 566 patients. Eur J Surg Oncol. 2013;39(12):1435–1443. doi: 10.1016/j.ejso.2013.09.030. [DOI] [PubMed] [Google Scholar]
- 47.Cotte E., Glehen O., Mohamed F., et al. Cytoreductive surgery and intraperitoneal chemohyperthermia for chemoresistant and recurrent advanced epithelial ovarian cancer: prospective study of 81 patients. World J Surg. 2007;31(9):1813–1820. doi: 10.1007/s00268-007-9146-8. [DOI] [PubMed] [Google Scholar]
- 48.Classe J.M., Glehen O., Decullier E., et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for first relapse of ovarian cancer. Anticancer Res. 2015;35(9):4997–5005. [PubMed] [Google Scholar]
- 49.Cashin P.H., Graf W., Nygren P., Mahteme H. Cytoreductive surgery and intraperitoneal chemotherapy for colorectal peritoneal carcinomatosis: prognosis and treatment of recurrences in a cohort study. Eur J Surg Oncol. 2012;38(6):509–515. doi: 10.1016/j.ejso.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 50.Verwaal V.J., van Ruth S., de Bree E., et al. Randomized trial of cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy and palliative surgery in patients with peritoneal carcinomatosis of colorectal cancer. J Clin Oncol. 2003;21(20) doi: 10.1200/JCO.2003.04.187. 3737-343. [DOI] [PubMed] [Google Scholar]
- 51.Huang C.Q., Feng J.P., Yang X.J., Li Y. Cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy improves survival of patients with peritoneal carcinomatosis from colorectal cancer: a case-control study from a Chinese center. J Surg Oncol. 2014;109(7):730–739. doi: 10.1002/jso.23545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Passot G., Vaudoyer D., Villeneuve L., et al. What made hyperthermic intraperitoneal chemotherapy an effective curative treatment for peritoneal surface malignancy: A 25-year experience with 1,125 procedures. J Surg Oncol. 2016;113(7):796–803. doi: 10.1002/jso.24248. [DOI] [PubMed] [Google Scholar]
- 53.Moran B., Cecil T., Chandrakumaran K., Arnold S., Mohamed F., Venkatasubramaniam A. The results of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in 1200 patients with peritoneal malignancy. Colorectal Dis. 2015;17(9):772–778. doi: 10.1111/codi.12975. [DOI] [PubMed] [Google Scholar]
- 54.Search of Hipec in. https://www.clinicaltrials.gov/ct2/results?cond=OVARIAN+CANCER&term=HIPEC&cntry=&state=&city=&dist= www.clinicaltrials.gov. Available at. Accessed July 10, 2022.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.