Abstract
Background
Immune checkpoint inhibitor (ICI) therapy has improved patient survival in advanced cancers; however, the efficacy of ICIs in elderly patients is still elusive. This study assessed the efficacy of ICIs in elderly patients with advanced cancer in terms of overall survival (OS) and progression-free survival (PFS).
Materials and methods
We carried out a systematic review and identified 30 head-to-head phase II/III randomized controlled trials that compared immunotherapy with the standard of care in advanced solid tumor patients. The data on patients younger or over 65 years of age were indexed from PubMed-Medline, Embase, and Scopus and obtained for meta-analysis. The subgroup analyses were stratified by primary tumor type, line of treatment, or type of immunotherapy, and a meta-regression analysis was carried out after adjusting for all other variables.
Results
The study included 17 476 patients, comprising 58% (10 119) younger (<65 years old) and 42% (7357) elderly (≥65 years old) patients. The hazard ratio (HR) for OS was 0.77 [95% confidence interval (CI) 0.70-0.85] and 0.77 (95% CI 0.70-0.85) in the younger and elderly groups, respectively, suggesting similar efficacies of ICIs in these two age groups. The subgroup analyses revealed no significant relationship between age and treatment outcomes, except for the PFS benefit in younger patients with melanoma than in elderly patients (HR 0.44 in younger patients versus 0.65 in elderly patients, P = 0.04). These results were further supported by meta-regression analysis, which showed no statistically significant difference in OS (P = 0.954) and PFS (P = 0.555) between the two age groups.
Conclusions
The findings suggest that age-associated impairments of the immune system did not affect the efficacy of ICIs in elderly patients compared to younger patients. Therefore, the choice of ICIs for elderly patients can be considered, regardless of chronological age.
Key words: age, immunosenescence, immunotherapy, meta-analysis, meta-regression
Highlights
-
•
We evaluated the efficacy of ICI in 17 476 patients, comprising 58% younger and 42% elderly patients.
-
•
Meta-analysis resulted in the comparable efficacy of ICI between younger and older age groups.
-
•
Further, meta-regression analysis showed no significant difference in OS and PFS.
-
•
Our study suggests that chronological age does not lead to immunosenescence in response to ICI in immune-oncology.
Introduction
Immune evasion is one of the hallmarks of cancer, and targeting the immune checkpoint protein on T cells that hamper immune activation has shown promising clinical efficacy in various cancers.1, 2, 3 Initially, immune checkpoint inhibitors (ICIs) that target cytotoxic T-lymphocyte antigen-4 (CTLA-4), such as ipilimumab and programmed cell death protein 1 (PD-1) and programmed death-ligand 1 (PD-L1), including pembrolizumab and nivolumab (anti-PD-1) and durvalumab (anti-PD-L1), have gained wide attention as the standard of treatment across a wide range of tumors, most notably in lung cancer and melanoma.4 Furthermore, anti-PD-1 and anti-PD-L1 agents, including atezolizumab and avelumab, have been approved in metastatic and recurrent solid tumors.4,5
Despite the feasibility of ICIs, little is known about the effect of age on the efficacy of ICIs.6 The immune system weakens with aging, a phenomenon known as immunosenescence.7 Age-associated decline in immune functions includes reduced T-cell activation, decreased interleukin 2, and increased myeloid-derived suppressor cells and regulatory T cells.8, 9, 10, 11, 12 Several pre-clinical studies have shown that aging is associated with impaired antitumor immune responses.7, 8, 9, 10, 11, 12
Recently, two retrospective studies have reported the efficacy of immunotherapy in elderly patients,13,14 and showed that patients aged >60 years had a better response to anti-PD-1. Further, these studies have also shown that the resistance to pembrolizumab dropped by 13% with every 10-year increase in the age of patients. In another single-center retrospective cohort study, older patients with melanoma retained responsiveness to ICIs, such as pembrolizumab, nivolumab, and ipilimumab.13 Contrary to these results, a recent meta-analysis by Elias et al. demonstrated no difference in the hazard ratio (HR) of multiple tumors treated with PD-1 and PD-L1 inhibitors across age groups.14
As described, these reports report conflicting conclusions. Therefore, to address the discrepancies and have a consensus on the role of age in response to immunotherapy, we carried out a comprehensive meta-analysis and subgroup and meta-regression analyses of ICIs using the data obtained through an updated search and including the trials of anti-CTLA-4.
Materials and methods
Search strategy and selection criteria
We conducted the meta-analysis and meta-regression analysis following the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA).15 A checklist of PRISMA is presented in Supplementary Table S1, available at https://doi.org/10.1016/j.esmoop.2022.100577. The study protocol had been registered with PROSPERO (CRD42019127199).16
A comprehensive literature search was conducted in PubMed-Medline, Embase, and Scopus to identify the relevant articles. The dates searched were from the inception of each database to 31 December 2018. Abstracts and presentations were also reviewed from significant conference proceedings, including the American Society of Clinical Oncology (ASCO) and European Society for Medical Oncology (ESMO) from 2010 to 31 December 2018.
Search terms included the following keywords: ‘CTLA-4’, ‘cytotoxic T-lymphocyte-associated protein 4’, ‘ipilimumab’, ‘tremelimumab’, ‘PD-1’, ‘programmed death receptor 1’, ‘immune checkpoint inhibitor’, ‘pembrolizumab’, ‘nivolumab’, ‘avelumab’, ‘durvalumab’, ‘atezolizumab’. Randomized, controlled phase II/III trials with immunotherapy alone or in combination with other non-immunotherapy treatments to specifically address the role of age in immunotherapy were included. Single-arm phase I trials and trials that compared immunotherapy regimens were excluded.17
Two investigators (JBL and HSK) independently searched the databases, extracted data from the studies, and conducted independent verifications to resolve discrepancies.
Data analysis
We identified the studies by the first author, year of publication, study design, phase, line of therapy, underlying malignancy, and type of immunotherapies (anti-CTLA-4, anti-PD-L1, anti-PD-1). Only trials with solid tumors were included, wherein those with hematologic malignancy were excluded from the analysis. Data of metastatic and recurrent solid tumors with the palliative aim of treatment were included. Studies that included immunotherapy in adjuvant settings were excluded. Additional information, including mean age and median follow-up time, was included.
We utilized data of HR with 95% confidence interval (CI) of progression-free survival (PFS) and overall survival (OS) by age. Elderly patients were defined as ≥65 years old. In addition, studies with a cut-off age value of ≥75 years were included in the further analysis. Studies that did not report HR by age group18 or excluded elderly patients were not included in the analysis.19,20 To analyze the effect of age on immunotherapy, we carried out a subgroup analysis by tumor types [non-small-cell lung cancer (NSCLC), melanoma, other tumor sites], lines of therapy (first line and beyond), and types of immunotherapy (anti-CTLA-4, anti-PD-1, anti-PD-L1).
Quality of evidence
We used the Cochrane highly sensitive search strategies to identify randomized trials. The five-point Jadad ranking system was used to assess the methodological quality of studies to identify whether the study was (i) randomized, (ii) double-blinded, and (iii) included description of dropouts and withdrawals.21 A score of 0-2 was considered low, whereas a score of 3-5 was defined as high quality.
Statistical analysis
The patients were divided into two groups—younger age group: patients aged <65 years and elderly group: patients aged ≥65 years. A meta-analysis was carried out to identify the effect of immunotherapy on OS and PFS, and HR with 95% CI was estimated in two groups. PFS was defined as the time from the start of palliative immunotherapy to the date of progression or death. OS was defined as the period of diagnosis of the metastatic or recurrent solid tumor until the date of last follow-up or death. Random-effects models were used to summarize log (HR) and its variances for each age group (<65 years old and ≥65 years old). The between-study variance in the random-effects model was estimated using the DerSimonian–Laird method, and the studies were considered substantially heterogeneous when Higgins’ I2 >50%.22 We tested the heterogeneity between the two age groups to assess the differences in immunotherapy efficacy. The forest plot was used to visualize and summarize the HR and 95% CIs for each study and the aggregated estimates from the random-effects model.
We further conducted the subgroup analyses to determine possible sources of heterogeneity of the effects of immunotherapy efficacy. Tumor types (NSCLC, melanoma, other tumor sites), lines of therapy (first line and beyond), and types of immunotherapy (anti-CTLA-4, anti-PD-1, anti-PD-L1) were used for subgroup analyses. Funnel plots were used to assess the presence of publication bias. Meta-regression was also carried out to investigate the effect of age differences on immunotherapy efficacy after adjusting for the other factors and the result was summarized using the bubble plot.
All statistical analyses were carried out using the R environment (http://www.r-project.org; release version 3.5.2) with meta-package.23,24 A P value <0.05 was considered statistically significant.
Results
Patient characteristics
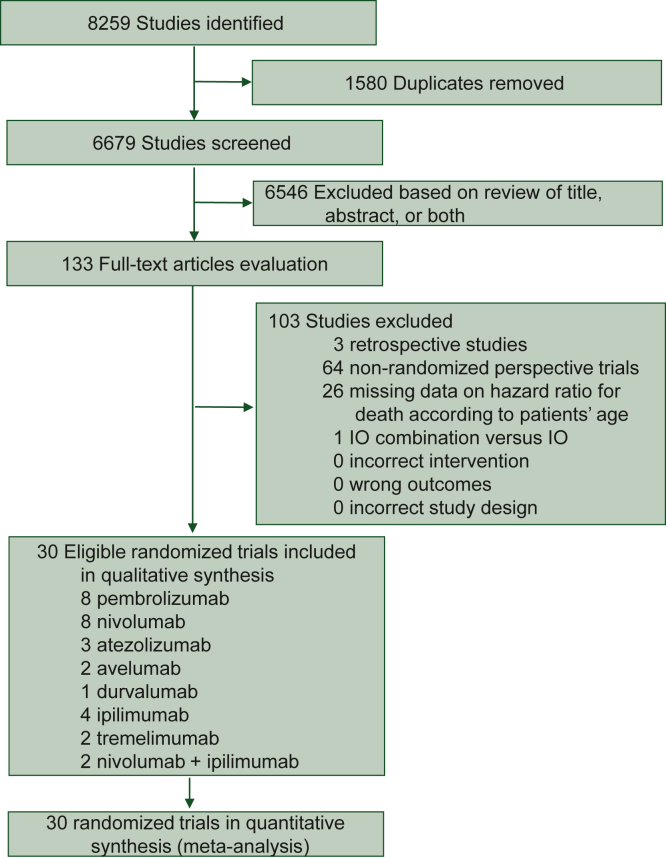
Our initial database search retrieved a total of 8259 references, of which 133 potentially relevant articles that fulfilled the inclusion criteria were evaluated in this study (Figure 1). We identified a total of 30 randomized controlled trials (RCTs) that reported the data of OS (25 RCTs) and PFS (14 RCTs) suitable for further analysis (Figure 1, Supplementary Tables S2 and S3, available at https://doi.org/10.1016/j.esmoop.2022.100577).3,25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53
Figure 1.
Study selection flow diagram.
IO, immunotherapy.
Among the 30 trials included in the meta-analysis, 16 trials used PD-1 inhibitors (8 used nivolumab and 8 used pembrolizumab), 6 used PD-L1 inhibitors [avelumab (n = 2), atezolizumab (n = 3), durvalumab (n = 1)], and 6 used anti-CTLA-4 [tremelimumab (n = 2), ipilimumab (n = 4), and combination of nivolumab with ipilimumab (n = 2)]. Most of the trials compared immunotherapy versus standard of care (SOC), whereas seven trials compared immunotherapy and SOC versus SOC.
Of the 30 RCTs, 28 were conducted as phase III trials, 1 as phase II trial,50 and 1 as phase II/III trial.44 Fourteen RCTs were conducted in first-line settings, and the remaining 16 RCTs evaluated treatment after the previous failure to systemic therapies. NSCLC and melanoma accounted for 39% and 20%, respectively. Twelve of the trials were done with patients with NSCLC, six trials for melanoma, three trials for gastric or gastroesophageal cancer, and two trials for small-cell lung cancer, clear-cell renal cell carcinoma, head and neck squamous cell carcinoma (HNSCC), and one trial for breast cancer, urothelial cancer, and mesothelioma, respectively (Supplementary Tables S2 and S3, available at https://doi.org/10.1016/j.esmoop.2022.100577).
The median age of patients was 63 years, and median follow-up ranged from 5.1 months to 54 months. Of the 17 476 patients evaluated in this study, 58% (n = 10 119) were younger (<65 years old) and 42% (n = 7357) were older (>65 years old). Most trials distributed patients equally among age groups, except for the earlier trials, and those evaluated certain malignancies, such as HNSCC and breast cancers, which included a lower proportion of elderly patients.
Risk of bias
The mean Jadad score was 3.8 (range, 3-5). No trials had a low-quality score. Of the 30 RCTs, 12 RCTs were double-blinded. Information regarding randomization, blinding, and accounting is presented in Supplementary Table S4, available at https://doi.org/10.1016/j.esmoop.2022.100577.
Role of age in immunotherapy
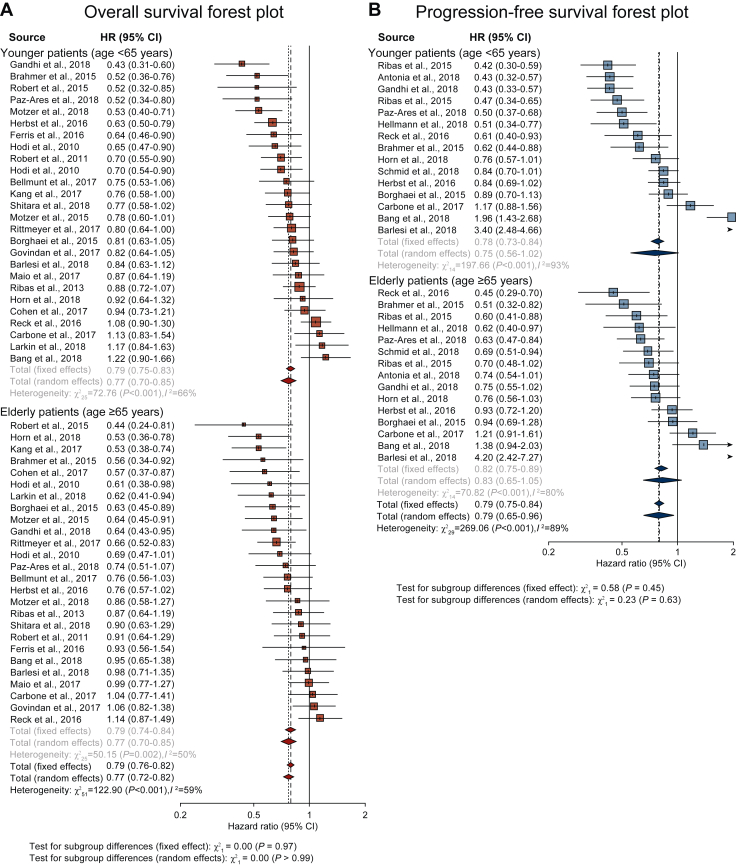
The HRs of studies based on random-effects models are represented in Figure 2. The meta-analysis demonstrated no statistically significant difference in PFS and OS for patients across age groups who received immunotherapy. The HR for OS was 0.77 (95% CI 0.70-0.85) in the younger age group and 0.77 (95% CI 0.70-0.85) in the older age group, suggesting that elderly patients showed comparable efficacy to ICIs as younger patients (Table 1). The HR for PFS was 0.75 (95% CI 0.57-0.99) in the younger age group and 0.82 (95% CI 0.67-1.01) in the older age group (Table 2). The overall estimated HR for PFS and OS were 0.79 (95% CI 0.63-0.98, P = 0.60) and 0.76 (95% CI 0.70-0.82, P = 0.99), respectively (Tables 1 and 2).
Figure 2.
Forest plots of (A) overall survival (OS) and (B) progression-free survival (PFS) by age <65 years and ≥65 years.
CI, confidence interval; HR, hazard ratio.
Table 1.
Subgroup analysis of immunotherapy efficacy in terms of overall survival (OS) between younger age (<65 years) and older age (≥65 years)
| Variables | Number of studies | Number of participants |
Pooled HR (95% CI) |
Test for difference |
||||
|---|---|---|---|---|---|---|---|---|
| All | Age <65 years | Age ≥65 years | All | Age <65 years | Age ≥65 years | P value | ||
| All | 26 | 14 511 | 8344 | 6167 | 0.76 (0.70-0.82) | 0.77 (0.70-0.85) | 0.77 (0.70-0.85) | 0.99 |
| Primary disease site | ||||||||
| NSCLC | 10 | 6008 | 3139 | 2869 | 0.74 (0.65-0.84) | 0.71 (0.61-0.84) | 0.78 (0.68-0.90) | 0.39 |
| Melanoma | 6 | 2792 | 1828 | 964 | 0.71 (0.58-0.86) | 0.76 (0.63-0.92) | 0.72 (0.60-0.87) | 0.68 |
| Other tumors | 10 | 5711 | 3377 | 2334 | 0.80 (0.72-0.89) | 0.83 (0.72-0.97) | 0.78 (0.65-0.94) | 0.61 |
| Type of immunotherapy | ||||||||
| Anti-PD-1/PD-L1 antibody | 18 | 9421 | 5256 | 4165 | 0.74 (0.67-0.81) | 0.77 (0.68-0.87) | 0.71 (0.64-0.79) | 0.36 |
| Anti-CTLA-4 antibody | 7 | 4243 | 2564 | 1679 | 0.82 (0.74-0.92) | 0.82 (0.71-0.94) | 0.93 (0.80-1.07) | 0.23 |
| Combination treatment | 1 | 847 | 524 | 323 | — | — | — | — |
| Line of treatment | ||||||||
| 1 | 11 | 7094 | 3994 | 3100 | 0.73 (0.64-0.85) | 0.74 (0.62-0.89) | 0.81 (0.69-0.95) | 0.49 |
| ≥2 | 15 | 7417 | 4350 | 3067 | 0.77 (0.71-0.83) | 0.79 (0.71-0.87) | 0.74 (0.66-0.83) | 0.45 |
CI, confidence interval; CTLA-4, cytotoxic T-lymphocyte antigen-4; HR, hazard ratio; NSCLC, non-small-cell lung cancer; PD-1, programmed cell death protein 1; PD-L1, programmed death-ligand 1.
Table 2.
Subgroup analysis of immunotherapy efficacy in terms of progression-free survival (PFS) between younger age (<65 years) and older age (≥65 years)
| Variables | Number of studies | Number of participants |
Pooled HR (95% CI) |
Test for difference |
||||
|---|---|---|---|---|---|---|---|---|
| All | Age <65 years | Age ≥65 years | All | Age <65 years | Age ≥65 years | P value | ||
| All | 15 | 7606 | 4298 | 3308 | 0.79 (0.63-0.98) | 0.75 (0.57-0.99) | 0.82 (0.67-1.01) | 0.60 |
| Primary disease site | ||||||||
| NSCLC | 10 | 5211 | 2764 | 2447 | 0.79 (0.58-1.05) | 0.75 (0.52-1.08) | 0.85 (0.64-1.12) | 0.60 |
| Melanoma | 2 | 716 | 404 | 312 | 0.53 (0.45-0.64) | 0.44 (0.35-0.56) | 0.65 (0.50-0.85) | 0.04∗ |
| Other tumors | 3 | 1676 | 1130 | 546 | 1.02 (0.64-1.63) | 1.07 (0.63-1.80) | 0.88 (0.66-1.30) | 0.57 |
| Type of immunotherapy | ||||||||
| Anti-PD-1/PD-L1 antibody | 14 | 7280 | 4142 | 3138 | 0.80 (0.64-1.02) | 0.77 (0.58-1.03) | 0.84 (0.68-1.03) | 0.65 |
| Anti-CTLA-4 antibody | 0 | — | — | — | — | — | — | — |
| Combination treatment | 1 | 326 | 156 | 170 | — | — | — | — |
| Line of treatment | ||||||||
| 1 | 7 | 3652 | 2044 | 1608 | 0.68 (0.54-0.86) | 0.66 (0.50-0.86) | 0.72 (0.58-0.89) | 0.63 |
| ≥2 | 8 | 3954 | 2254 | 1700 | 0.90 (0.62-1.31) | 0.85 (0.53-1.36) | 0.95 (0.68-1.33) | 0.70 |
CI, confidence interval; CTLA-4, cytotoxic T-lymphocyte antigen-4; HR, hazard ratio; NSCLC, non-small-cell lung cancer; PD-1, programmed cell death protein 1; PD-L1, programmed death-ligand 1.
P < 0.05.
In addition, we analyzed different age cut-offs (age <65, age ≥65, and age ≥75) as shown in Supplementary Figure S1, available at https://doi.org/10.1016/j.esmoop.2022.100577. The subgroup difference among three different age groups based on random-effects models was not statistically significant in terms of OS (P = 0.095) and PFS (P = 0.613), respectively.
Next, we evaluated the HR of clinical trials, which revealed positive results and changed the SOC. We identified trials with positive outcomes in terms of OS (16 out of 26 trials) and PFS (12 out of 15 trials), respectively (Supplementary Figure S2, available at https://doi.org/10.1016/j.esmoop.2022.100577). The HR for OS was 0.67 (95% CI 0.64-0.71) in the younger age group and 0.68 (95% CI 0.62-0.74) in the older age group, showing a comparable efficacy to ICIs between different age groups (P = 0.85; Supplementary Table S5, available at https://doi.org/10.1016/j.esmoop.2022.100577). The HR for PFS was also comparable between the two age groups (HR for PFS, 0.60 versus 0.71, P = 0.11; Supplementary Table S6, available at https://doi.org/10.1016/j.esmoop.2022.100577).
Subgroup analysis
The subgroup analysis was carried out according to primary tumor type (NSCLC, melanoma, and others), line of treatment (first line and subsequent), and type of immunotherapy (anti-PD-1 or anti-PD-L1, anti-CTLA-4, combination of both) (Figure 2, Tables 1 and 2). Regarding the primary tumor types, OS data were available from 10 trials for the NSCLC subgroup, 5 trials for the melanoma subgroup, and 11 trials for the other tumors subgroup, respectively. The analysis demonstrated no relationship between age and treatment outcome when classified according to primary tumor type.
Next, we evaluated the effects of the type of ICIs on OS. OS data were available from 18 trials for the anti-PD-1/anti-PD-L1 subgroups and 7 trials for the anti-CTLA-4 subgroup. The HR for OS in the anti-PD-1/anti-PD-L1 subgroup was 0.77 (95% CI 0.68-0.87) in the younger age group and 0.71 (95% CI 0.64-0.79) in the older age group. In the anti-CTLA-4 subgroup, the HR for OS was 0.82 (95% CI 0.71-0.94) in the younger age group and 0.93 (95% CI 0.80-1.07) in the older age group. These findings revealed no relationship between age and treatment outcome when classified according to the type of immunotherapy.
OS data were available from 11 trials for the first-line treatment and 15 for the subsequent treatment. In the first-line treatment subgroup, the HR for OS was 0.74 (95% CI 0.62-0.89) in the younger age group and 0.81 (95% CI 0.69-0.95) in the older age group. In the subsequent treatment subgroup, the HR for OS was 0.79 (95% CI 0.71-0.87) in the younger age group and 0.74 (95% CI 0.66-0.83) in the older age group. Like the primary tumor type and type of immunotherapy, the line of treatment subgroup analysis revealed comparable antitumor response to ICIs in elderly patients as younger patients.
PFS data were obtained from 10 trials for the NSCLC subgroup, 2 trials for the melanoma subgroup, and 3 trials for the other tumors subgroup. For NSCLC, the HR for PFS was 0.75 (95% CI 0.52-1.08) in the younger age group and 0.85 (95% CI 0.64-1.12) in the older age group. For other tumors, the HR for PFS was 1.07 (95% CI 0.63-1.80) in the younger age group and 0.88 (95% CI 0.66-1.30) in the older age group. Of note, younger patients with melanoma (<65 years old) had significant PFS benefits from immunotherapy (HR 0.44, 95% CI 0.35-0.56) than older patients (≥65 years) (HR 0.65, 95% CI 0.50-0.85, P = 0.04).
PFS data were obtained from 14 trials for the anti-PD-1/anti-PD-L1 subgroup analysis. The HR for PFS was 0.77 (95% CI 0.58-1.03) in the younger age group and 0.84 (95% CI 0.68-1.03) in the older age group. PFS data were obtained from seven trials for the first-line treatment subgroup and eight trials for the subsequent treatment subgroup. In the first line, the HR for PFS was 0.66 (95% CI 0.50-0.86) in the younger age group and 0.72 (95% CI 0.58-0.89) in the older age group. In the subsequent treatment subgroup, the HR for PFS was 0.85 (95% CI 0.53-1.36) and 0.95 (95% CI 0.68-1.33), respectively. Comprehensively, none of the subgroups except the melanoma subgroup showed significant differences for PFS between the younger and older patients.
Meta-regression
The meta-regression analysis showed no significant difference in the efficacy of immunotherapy between the two age groups (Table 3). After adjusting for all other variables, there was no statistically significant difference in OS (P = 0.954) and PFS (P = 0.555) according to the age group. In contrast, line of therapy (P = 0.015) and tumor types (NSCLC, P = 0.018; other tumors, P = 0.005) showed a significant difference in meta-regression. There was no significant difference in terms of OS (P = 0.852) and PFS (P = 0.101) in clinical trials with positive outcomes and changed the SOC, respectively (Supplementary Table S7, available at https://doi.org/10.1016/j.esmoop.2022.100577). Additionally, in the meta-regression analysis with different age cut-offs (age <65, age ≥65, and age ≥75 years), there was no statistically significant difference in OS (P = 0.958 in the age group ≥65 years; P = 0.073 in the age group ≥75 years) and PFS (P = 0.073 in the age group ≥65 years; P = 0.744 in the age group ≥75 years) according to the age group as shown in Supplementary Table S8, available at https://doi.org/10.1016/j.esmoop.2022.100577.
Table 3.
Meta-regression analysis to evaluate impact of covariates on the overall survival and progression-free survival
| Covariates | Overall survival |
Progression-free survival |
||
|---|---|---|---|---|
| Log HR estimate (CI bound) | P value | Log HR estimate (CI bound) | P value | |
| Age group | −0.004 (−0.144 to 0.136) | 0.954 | 0.099 (−0.229 to 0.427) | 0.555 |
| Line of therapy | 0.010 (−0.147 to 0.167) | 0.901 | −0.463 (−0.836 to −0.089) | 0.015∗ |
| Tumor type (NSCLC) | 0.052 (−0.157 to 0.261) | 0.624 | 0.639 (0.112 to 1.166) | 0.018∗ |
| Tumor type (other tumors) | 0.120 (−0.073 to 0.312) | 0.223 | 0.917 (0.284 to 1.550) | 0.005∗∗ |
| Type of immunotherapy | 0.068 (−0.084 to 0.220) | 0.382 | −0.064 (−0.427 to 0.298) | 0.729 |
CI, confidence interval; HR, hazard ratio; NSCLC, non-small-cell lung cancer.
P < 0.05.
P < 0.01.
Discussion
Our study aimed at determining whether age-associated changes in the immune system affect responses to immunotherapy. The analyses demonstrated no significant differences in PFS or OS among younger and elderly patients. Subgroup analyses of tumor type, line of treatment, and type of immunotherapy showed PFS benefit only in younger patients with melanoma than in older patients. Melanoma is a highly immunogenic tumor,54 and the observed PFS benefits in younger patients with melanoma could be attributed to the decline of function of immune cells.1,55
Pre-clinical studies have shown that PD-L1 expressions increase with aging,56,57 and higher PD-L1 expressions correlate with better responses to immunotherapy.58 Contrary to the multiple pre-clinical studies implicating immunosenescence in immunotherapy outcomes,12 our study showed that age has no role in predicting responses to immunotherapy. This is similar to the conclusions drawn by Elias et al.14 In their study, the meta-analysis of nine RCTs17,37,42,44,46, 47, 48, 49,59 suggested that PD-1 and PD-L1 inhibitors are comparable between younger (<65 years old) and older patients (≥65 years old).
This study reports meta-regression and more comprehensive analysis of ICIs using the data obtained from 30 RCTs with updated search (included recently published 17 trials3,25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36,38, 39, 40, 41) and the inclusion of 6 trials of anti-CTLA-4.29,35,41,45,52,53 Furthermore, data collected from >17 000 patients and robust quality of assessment by Jadad score added strength to our analysis. However, our study excluded two studies. One study (KEYNOTE-006) compared pembrolizumab to ipilimumab in patients with melanoma, since both arms included immunotherapy agents. The other trial, phase II POPLAR study59 that tested atezolizumab versus docetaxel in previously treated NSCLC, was excluded because we included the phase III OAK study37 of the same regimen in the meta-analysis.
Recently, two meta-analyses addressed the efficacy of ICIs between age <75 and ≥75 years.60,61 The meta-analysis by Nie et al. was based on eight trials involving patients treated with anti-PD-1/PD-L1.60 The study showed that except for melanoma, anti-PD-1 and PD-L1 did not benefit elderly patients aged ≥75 years in terms of PFS and OS. Another meta-analysis of 34 studies by Huang et al. compared cancer patients treated with anti-PD-1/PD-L1 and anti-CTLA-4 based on cut-off for both 65 and 75 years old.61 Similarly, no difference in PFS and OS was seen between the <65- and ≥65-year age groups. Concordant with these findings, our findings demonstrated no significant differences in PFS or OS benefits between the age groups. For patients aged <75 years, there was a significant improvement in OS. In terms of PFS, patients aged <75 years tended to have favorable PFS but were not statistically significant.
This study has several limitations. Firstly, the age cut-off of age 65 years is not predictive of determining immunosenescence. Although there is no consensus, most studies chose 65 years as the cut-off value for age. We showed that different age cut-off value (≥75 years) was not associated with the efficacy of ICIs in terms of OS and PFS, but a limited number of trials were available for patients aged ≥75 years. Further studies employing the emerging data on ICIs that include ≥75 years elderly population are warranted. Secondly, other factors such as comorbidities associated with age could be the factors of confounding variables and should be addressed as well. Furthermore, there was no information regarding toxicity profiles across age groups. Meta-analysis of incidence and severity of adverse events across age groups may help assess the tolerability of immunotherapy in elderly patients.
Conclusion
The meta-analysis, followed by subgroup and meta-regression analyses conducted in this study, showed that immunotherapy is effective across age groups. The choice of ICIs for elderly patients can be considered, regardless of chronological age. Future studies including older patients (≥75 years of age) and biomarkers for immunosenescence may help provide further insights into understanding the role of immunotherapy in the geriatric group.
Funding
This work was supported in part by the National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT) [grant number 2022R1A2C4001879 to HSK]. This research was also supported by the Bio & Medical Technology Development Program of the NRF and funded by the Korean government (MSIT) [grant number 2022M3A9F3016364 to HSK] and a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea [grant number: HI22C0353 to HSK].
Disclosure
The authors have declared no conflicts of interest.
Data sharing
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Contributor Information
M. Lee, Email: MLEE1004@yuhs.ac.
H.S. Kim, Email: modeerfhs@yuhs.ac.
Supplementary data
References
- 1.Grolleau-Julius A., Harning E.K., Abernathy L.M., Yung R.L. Impaired dendritic cell function in aging leads to defective antitumor immunity. Cancer Res. 2008;68(15):6341–6349. doi: 10.1158/0008-5472.CAN-07-5769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vinay D.S., Ryan E.P., Pawelec G., et al. Immune evasion in cancer: mechanistic basis and therapeutic strategies. Semin Cancer Biol. 2015;35(suppl):S185–S198. doi: 10.1016/j.semcancer.2015.03.004. [DOI] [PubMed] [Google Scholar]
- 3.Govindan R., Szczesna A., Ahn M.-J., et al. Phase III trial of ipilimumab combined with paclitaxel and carboplatin in advanced squamous non-small-cell lung cancer. J Clin Oncol. 2017;35(30):3449–3457. doi: 10.1200/JCO.2016.71.7629. [DOI] [PubMed] [Google Scholar]
- 4.Vaddepally R.K., Kharel P., Pandey R., Garje R., Chandra A.B. Review of indications of FDA-approved immune checkpoint inhibitors per NCCN guidelines with the level of evidence. Cancers (Basel) 2020;12(3) doi: 10.3390/cancers12030738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Qin S., Xu L., Yi M., Yu S., Wu K., Luo S. Novel immune checkpoint targets: moving beyond PD-1 and CTLA-4. Mol Cancer. 2019;18(1):155. doi: 10.1186/s12943-019-1091-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Talarico L., Chen G., Pazdur R. Enrollment of elderly patients in clinical trials for cancer drug registration: a 7-year experience by the US Food and Drug Administration. J Clin Oncol. 2004;22(22):4626–4631. doi: 10.1200/JCO.2004.02.175. [DOI] [PubMed] [Google Scholar]
- 7.Aw D., Silva A.B., Palmer D.B. Immunosenescence: emerging challenges for an ageing population. Immunology. 2007;120(4):435–446. doi: 10.1111/j.1365-2567.2007.02555.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Raynor J., Lages C.S., Shehata H., Hildeman D.A., Chougnet C.A. Homeostasis and function of regulatory T cells in aging. Curr Opin Immunol. 2012;24(4):482–487. doi: 10.1016/j.coi.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brenchley J.M., Karandikar N.J., Betts M.R., et al. Expression of CD57 defines replicative senescence and antigen-induced apoptotic death of CD8+ T cells. Blood. 2003;101(7):2711–2720. doi: 10.1182/blood-2002-07-2103. [DOI] [PubMed] [Google Scholar]
- 10.Meloni F., Morosini M., Solari N., et al. Foxp3 expressing CD4+ CD25+ and CD8+CD28− T regulatory cells in the peripheral blood of patients with lung cancer and pleural mesothelioma. Hum Immunol. 2006;67(1):1–12. doi: 10.1016/j.humimm.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 11.Daste A., Domblides C., Gross-goupil M., et al. Immune checkpoint inhibitors and elderly people: a review. Eur J Cancer. 2017;82:155–166. doi: 10.1016/j.ejca.2017.05.044. [DOI] [PubMed] [Google Scholar]
- 12.Kugel C.H., Douglass S.M., Webster M.R., et al. Age correlates with response to anti-PD1, reflecting age-related differences in intratumoral effector and regulatory T-cell populations. Clin Cancer Res. 2018;24(21):5347–5356. doi: 10.1158/1078-0432.CCR-18-1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ibrahim T., Mateus C., Baz M., Robert C. Older melanoma patients aged 75 and above retain responsiveness to anti-PD1 therapy: results of a retrospective single-institution cohort study. Cancer Immunol Immunother. 2018;67(10):1571–1578. doi: 10.1007/s00262-018-2219-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Elias R., Giobbie-Hurder A., Rahma O.E. Efficacy of PD-1 and PD-L1 inhibitors in older adults: a meta-analysis. J Clin Oncol. 2017;35(suppl 15) doi: 10.1186/s40425-018-0336-8. e21544-e21544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Page M.J., McKenzie J.E., Bossuyt P.M., et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Booth A., Clarke M., Dooley G., et al. The nuts and bolts of PROSPERO: an international prospective register of systematic reviews. Syst Rev. 2012;1(1):2. doi: 10.1186/2046-4053-1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Robert C., Schachter J., Long G.V., et al. Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med. 2015;372(26):2521–2532. doi: 10.1056/NEJMoa1503093. [DOI] [PubMed] [Google Scholar]
- 18.Socinski M.A., Jotte R.M., Cappuzzo F., et al. Atezolizumab for first-line treatment of metastatic nonsquamous NSCLC. N Engl J Med. 2018;378(24):2288–2301. doi: 10.1056/NEJMoa1716948. [DOI] [PubMed] [Google Scholar]
- 19.Kwon E.D., Drake C.G., Scher H.I., et al. Ipilimumab versus placebo after radiotherapy in patients with metastatic castration-resistant prostate cancer that had progressed after docetaxel chemotherapy (CA184-043): a multicentre, randomised, double-blind, phase 3 trial. Lancet Oncol. 2014;15(7):700–712. doi: 10.1016/S1470-2045(14)70189-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Beer T.M., Kwon E.D., Drake C.G., et al. Randomized, double-blind, phase III trial of ipilimumab versus placebo in asymptomatic or minimally symptomatic patients with metastatic chemotherapy-naive castration-resistant prostate cancer. J Clin Oncol. 2017;35(1):40–47. doi: 10.1200/JCO.2016.69.1584. [DOI] [PubMed] [Google Scholar]
- 21.Olivo S.A., Macedo L.G., Gadotti I.C., Fuentes J., Stanton T., Magee D.J. Scales to assess the quality of randomized controlled trials: a systematic review. Phys Ther. 2008;88(2):156–175. doi: 10.2522/ptj.20070147. [DOI] [PubMed] [Google Scholar]
- 22.Higgins J.P.T., Thompson S.G. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 23.R Core Team . R Foundation for Statistical Computing; Vienna, Austria: 2017. R: A Language and Environment for Statistical Computing. [Google Scholar]
- 24.Schwarzer G. Meta: an R package for meta-analysis. R News. 2007;7:40–45. [Google Scholar]
- 25.Gandhi L., Rodríguez-Abreu D., Gadgeel S., et al. Pembrolizumab plus chemotherapy in metastatic non–small-cell lung cancer. N Engl J Med. 2018;378(22):2078–2092. doi: 10.1056/NEJMoa1801005. [DOI] [PubMed] [Google Scholar]
- 26.Barlesi F., Vansteenkiste J., Spigel D., et al. Avelumab versus docetaxel in patients with platinum-treated advanced non-small-cell lung cancer (JAVELIN Lung 200): an open-label, randomised, phase 3 study. Lancet Oncol. 2018;19(11):1468–1479. doi: 10.1016/S1470-2045(18)30673-9. [DOI] [PubMed] [Google Scholar]
- 27.Paz-Ares L., Luft A., Vicente D., et al. Pembrolizumab plus chemotherapy for squamous non–small-cell lung cancer. N Engl J Med. 2018;379(21):2040–2051. doi: 10.1056/NEJMoa1810865. [DOI] [PubMed] [Google Scholar]
- 28.Antonia S.J., Villegas A., Daniel D., et al. Overall survival with durvalumab after chemoradiotherapy in stage III NSCLC. N Engl J Med. 2018;379(24):2342–2350. doi: 10.1056/NEJMoa1809697. [DOI] [PubMed] [Google Scholar]
- 29.Hellmann M.D., Ciuleanu T.-E., Pluzanski A., et al. Nivolumab plus ipilimumab in lung cancer with a high tumor mutational burden. N Engl J Med. 2018;378(22):2093–2104. doi: 10.1056/NEJMoa1801946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Horn L., Mansfield A.S., Szczęsna A., et al. First-line tezolizumab plus chemotherapy in extensive-stage small-cell lung cancer. N Engl J Med. 2018;379(23):2220–2229. doi: 10.1056/NEJMoa1809064. [DOI] [PubMed] [Google Scholar]
- 31.Larkin J., Minor D., D’Angelo S., et al. Overall survival in patients with advanced melanoma who received nivolumab versus investigator’s choice chemotherapy in CheckMate 037: a randomized, controlled, open-label phase III trial. J Clin Oncol. 2018;36(4):383–390. doi: 10.1200/JCO.2016.71.8023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schmid P., Adams S., Rugo H.S., et al. Atezolizumab and nab-paclitaxel in advanced triple-negative breast cancer. N Engl J Med. 2018;379(22):2108–2121. doi: 10.1056/NEJMoa1809615. [DOI] [PubMed] [Google Scholar]
- 33.Shitara K., Özgüroğlu M., Bang Y.J., et al. Pembrolizumab versus paclitaxel for previously treated, advanced gastric or gastro-oesophageal junction cancer (KEYNOTE-061): a randomised, open-label, controlled, phase 3 trial. Lancet. 2018;392(10142):123–133. doi: 10.1016/S0140-6736(18)31257-1. [DOI] [PubMed] [Google Scholar]
- 34.Bang Y.J., Ruiz E.Y., Van Cutsem E., et al. Phase III, randomised trial of avelumab versus physician’s choice of chemotherapy as third-line treatment of patients with advanced gastric or gastro-oesophageal junction cancer: primary analysis of JAVELIN Gastric 300. Ann Oncol. 2018;29(10):2052–2060. doi: 10.1093/annonc/mdy264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Motzer R.J., Tannir N.M., McDermott D.F., et al. Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. N Engl J Med. 2018;378(14):1277–1290. doi: 10.1056/NEJMoa1712126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Carbone D.P., Reck M., Paz-Ares L., et al. First-line nivolumab in stage IV or recurrent non-small-cell lung cancer. N Engl J Med. 2017;376(25):2415–2426. doi: 10.1056/NEJMoa1613493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rittmeyer A., Barlesi F., Waterkamp D., et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet. 2017;389(10066):255–265. doi: 10.1016/S0140-6736(16)32517-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cohen E.E., Mehra R., Burtness B., et al. LBA45_PR - Pembrolizumab (pembro) vs standard of care (SOC) for recurrent or metastatic head and neck squamous cell carcinoma (R/M HNSCC): phase 3 KEYNOTE-040 trial. Ann Oncol. 2017;28(suppl 5):v605–v649. [Google Scholar]
- 39.Bellmunt J., de Wit R., Vaughn D.J., et al. Pembrolizumab as second-line therapy for advanced urothelial carcinoma. N Engl J Med. 2017;376(11):1015–1026. doi: 10.1056/NEJMoa1613683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kang Y.-K., Boku N., Satoh T., et al. Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;390(10111):2461–2471. doi: 10.1016/S0140-6736(17)31827-5. [DOI] [PubMed] [Google Scholar]
- 41.Maio M., Scherpereel A., Calabrò L., et al. Tremelimumab as second-line or third-line treatment in relapsed malignant mesothelioma (DETERMINE): a multicentre, international, randomised, double-blind, placebo-controlled phase 2b trial. Lancet Oncol. 2017;18(9):1261–1273. doi: 10.1016/S1470-2045(17)30446-1. [DOI] [PubMed] [Google Scholar]
- 42.Ferris R.L., Blumenschein G., Fayette J., et al. Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N Engl J Med. 2016;375(19):1856–1867. doi: 10.1056/NEJMoa1602252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Reck M., Rodríguez-Abreu D., Robinson A.G., et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med. 2016;375(19):1823–1833. doi: 10.1056/NEJMoa1606774. [DOI] [PubMed] [Google Scholar]
- 44.Herbst R.S., Baas P., Kim D.W., et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2016;387(10027):1540–1550. doi: 10.1016/S0140-6736(15)01281-7. [DOI] [PubMed] [Google Scholar]
- 45.Reck M., Luft A., Szczesna A., et al. Phase III randomized trial of ipilimumab plus etoposide and platinum versus placebo plus etoposide and platinum in extensive-stage small-cell lung cancer. J Clin Oncol. 2016;34(31):3740–3748. doi: 10.1200/JCO.2016.67.6601. [DOI] [PubMed] [Google Scholar]
- 46.Brahmer J., Reckamp K.L., Baas P., et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015;373(2):123–135. doi: 10.1056/NEJMoa1504627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Borghaei H., Paz-Ares L., Horn L., et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373(17):1627–1639. doi: 10.1056/NEJMoa1507643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Robert C., Long G.V., Brady B., et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372(4):320–330. doi: 10.1056/NEJMoa1412082. [DOI] [PubMed] [Google Scholar]
- 49.Motzer R.J., Escudier B., McDermott D.F., et al. Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med. 2015;373(19):1803–1813. doi: 10.1056/NEJMoa1510665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ribas A., Puzanov I., Dummer R., et al. Pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory melanoma (KEYNOTE-002): a randomised, controlled, phase 2 trial. Lancet Oncol. 2015;16(8):908–918. doi: 10.1016/S1470-2045(15)00083-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ribas A., Kefford R., Marshall M.A., et al. Phase III randomized clinical trial comparing tremelimumab with standard-of-care chemotherapy in patients with advanced melanoma. J Clin Oncol. 2013;31(5):616–622. doi: 10.1200/JCO.2012.44.6112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hodi F.S., O’Day S.J., McDermott D.F., et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Robert C., Thomas L., Bondarenko I., et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med. 2011;364(26):2517–2526. doi: 10.1056/NEJMoa1104621. [DOI] [PubMed] [Google Scholar]
- 54.Maio M. Melanoma as a model tumour for immuno-oncology. Ann Oncol. 2012;23(suppl 8):viii10–viii14. doi: 10.1093/annonc/mds257. [DOI] [PubMed] [Google Scholar]
- 55.Min H., Montecino-Rodriguez E., Dorshkind K. Effects of aging on early B- and T-cell development. Immunol Rev. 2005;205(1):7–17. doi: 10.1111/j.0105-2896.2005.00263.x. [DOI] [PubMed] [Google Scholar]
- 56.Henson S.M., Macaulay R., Riddell N.E., Nunn C.J., Akbar A.N. Blockade of PD-1 or p38 MAP kinase signaling enhances senescent human CD8+ T-cell proliferation by distinct pathways. Eur J Immunol. 2015;45(5):1441–1451. doi: 10.1002/eji.201445312. [DOI] [PubMed] [Google Scholar]
- 57.Lages C.S., Lewkowich I., Sproles A., Wills-Karp M., Chougnet C. Partial restoration of T-cell function in aged mice by in vitro blockade of the PD-1/ PD-L1 pathway. Aging Cell. 2010;9(5):785–798. doi: 10.1111/j.1474-9726.2010.00611.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Patel S.P., Kurzrock R. PD-L1 expression as a predictive biomarker in cancer immunotherapy. Mol Cancer Ther. 2015;14(4):847–856. doi: 10.1158/1535-7163.MCT-14-0983. [DOI] [PubMed] [Google Scholar]
- 59.Fehrenbacher L., Spira A., Ballinger M., et al. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. Lancet. 2016;387(10030):1837–1846. doi: 10.1016/S0140-6736(16)00587-0. [DOI] [PubMed] [Google Scholar]
- 60.Nie R.-C., Chen G.-M., Wang Y., et al. Efficacy of anti-PD-1/PD-L1 monotherapy or combinational therapy in patients aged 75 years or older: a study-level meta-analysis. Front Oncol. 2021;11(430) doi: 10.3389/fonc.2021.538174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Huang X.Z., Gao P., Song Y.X., et al. Efficacy of immune checkpoint inhibitors and age in cancer patients. Immunotherapy. 2020;12(8):587–603. doi: 10.2217/imt-2019-0124. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.